ABSTRACT
Vaccine-associated hypermetabolic lymphadenopathy (VAHL) has been reported as a common post-vaccination side effect, especially with mRNA-based COVID-19 vaccines. Most VAHL cases present normal or enlarged regional lymph nodes close to the injection site, usually with mild-moderate FDG (18 F-Fluorodeoxyglucose) uptake on FDG positron emission tomography (PET)/CT. Here, we describe the case of a 33-year-old woman with past history of Classic Hodgkin Lymphoma (CHL) who underwent follow-up FDG PET/CT 3 days (d) after the first dose of the adenovirus-vectored Oxford-AstraZeneca COVID-19 vaccine. FDG PET/CT showed unexpected small hypermetabolic cervical and abdominal lymph nodes in the same location as at the onset of the disease, suggesting radiological relapse. Considering temporal relationship and other cases of VAHL, a new image was performed 2 months later, which revealed decreased lymph nodes and normalization of FDG uptake. This case illustrates that the possibility of a false-positive should always be considered by physicians in this new context, even when hypermetabolic lymph nodes appear far from the vaccination site.
KEYWORDS: Lymphadenopathy, VAHL, Oxford-Astrazeneca vaccine, FDG PET/CT, Hodgkin Lymphoma
Introduction
Worldwide vaccination programs against COVID-19 pandemic started in late 2020. Different types of vaccines have obtained the U.S. Food and Drug Administration (FDA) and the European Medical Agency (EMA) emergency use authorization (EUA). The Oxford-AstraZeneca COVID-19 vaccine is an adenovirus-vectored vaccine containing SARS-CoV-2 DNA.1 Despite the obvious benefit of vaccination, some side effects have been described. Benign lymphadenopathy is a relatively common post-vaccine side effect reported with all the COVID-19 vaccines currently available.2 Most of these adenopathies appear close to the injection site and often show increased transient fluorodeoxyglucose (FDG) uptake on positron emission tomography (PET)/CT.3 The incidence of vaccine-associated hypermetabolic lymphadenopathy (VAHL) seems lower with DNA-based vaccines than with mRNA-based vaccines.4 Interpreting FDG PET/CT images in this new context may be difficult, especially in patients with malignant diseases.5
Clinical case
A 33-year-old woman was diagnosed with Classic Hodgkin Lymphoma (CHL) Ann Arbor Stage IIIB in December 2018. After six ABVD (adriamycin, bleomycin, vinblastine, dacarbazine) cycles, fluorodeoxyglucose (FDG) PET/CT confirmed complete metabolic response (CMR), showing some residual subcentimetric abdominal lymph nodes. The patient also suffered from anxiety disorder since CHL diagnosis and due to this fact, she underwent follow-up FDG PET/CT in March 2021. FDG PET/CT revealed small left cervical nodes and enlargement of previously known abdominal lymph nodes with increased FDG uptake (Figure 1). Relapse of disease was the first radiological suspicion. The patient was completely asymptomatic and physical examination was unremarkable. Increased erythrocyte sedimentation rate (ESR) was the only abnormal laboratory value. Further history revealed that the patient had received the first dose of the Oxford-Astrazeneca COVID-19 vaccine 3 d prior to FDG PET/CT. Considering the vaccination history, reported cases of VAHL and the disparity between radiological and clinical presentation, we decided to repeat FDG PET/CT 8 weeks later, which showed normometabolic lymph nodes of normal size, compatible with CMR (Figure 2).
Figure 1.
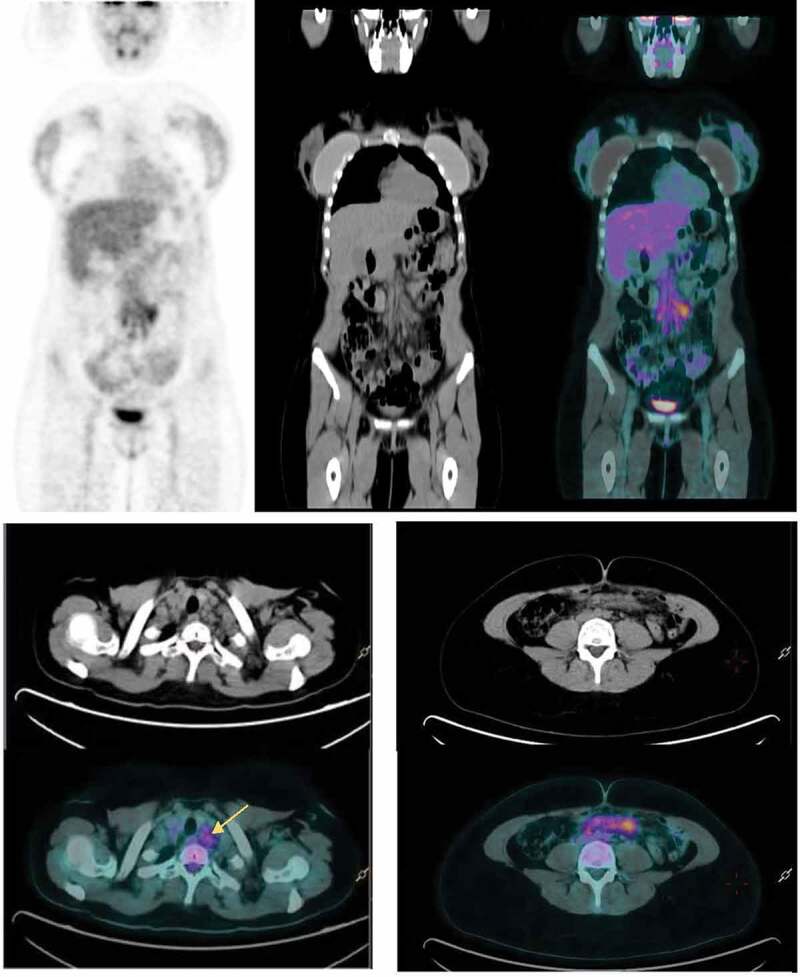
(a) Surveillance FDG PET/CT performed 3 d after the first dose of the Oxford-AstraZeneca COVID-19 vaccine shows FDG avidity in abdominal lymph nodes. (b) Increased FDG uptake in left lower cervical lymph nodes (SUVmax 3.5 g/ml). (c) Subcentimetric mesenteric lymph nodes with moderate FDG uptake 3 d (SUV max 4.7 g/ml).
Figure 2.
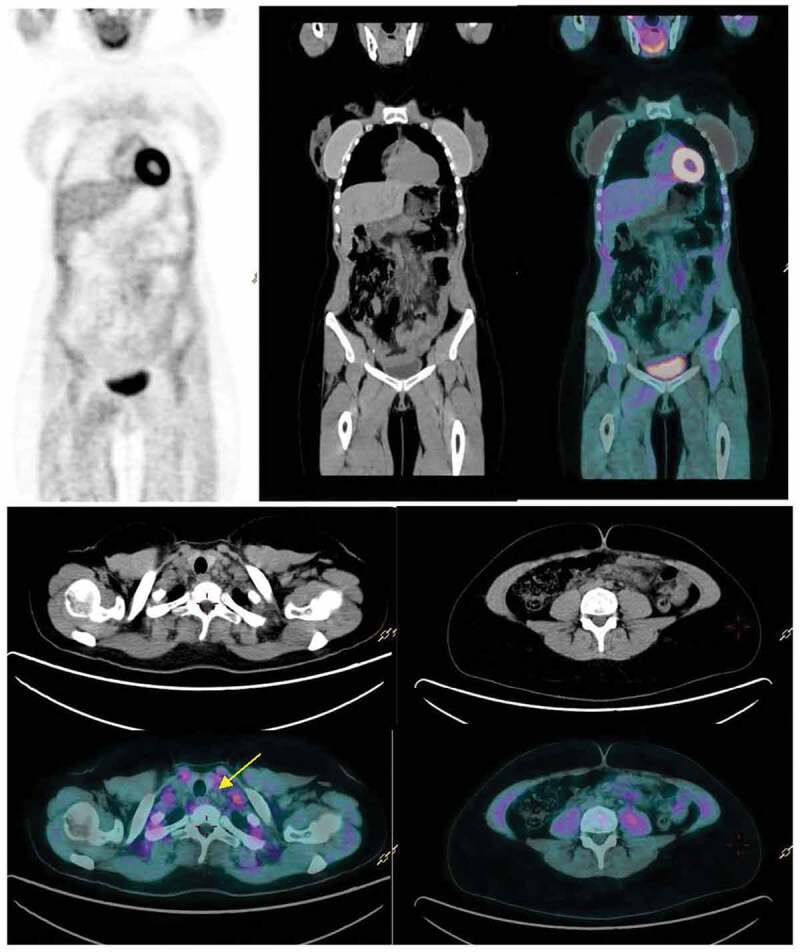
(a,b,c) Follow-up FDG PET/CT 8 weeks after vaccination reveals normalization of FDG uptake in cervical (yellow arrow) and abdominal lymph nodes.
Discussion
Reactive adenopathies have been described in general population after different viral vaccines such as H1N1 Influenza and human papillomavirus (HPV), among others.6,7 During the last months, similar findings have been observed with virtually all the COVID-19 vaccines approved or used under EUA, especially with mRNA vaccines (0.3% and 1.1% in Pfizer-BioNTech and Moderna safety clinical trials, respectively).8,9 The real-world incidence of post-vaccination lymphadenopathy seems higher.10 In patients with hematologic malignancies undergoing FDG PET/CT after mRNA vaccines, the incidence of VAHL has been reported up to 34.9%, with a clear relationship with seroconversion.11 Most of these adenopathies appear near to the vaccine administration site (usually deltoid muscle), affecting predominantly ipsilateral axillary, cervical and supraclavicular lymph nodes and frequently with avid FDG uptake on PET/CT. Deltoid musculature can also present hypermetabolic activity.2 VAHL can be detected from 24 h to some weeks later after vaccination and the degree of FDG uptake varies depending on the days since the administration.3 Other vaccine side effects like fever, headache, chills and muscle or joint pain are frequent, suggesting altogether a strong systemic inflammatory response. The interpretation of FDG PET/CT findings in this new setting is challenging, particularly in oncohematological patients, where this imaging modality is commonly used for disease staging and evaluation of treatment response.12,13
In this case, our patient was administered the first dose of the Oxford-AstraZeneca COVID-19 vaccine following the current recommendations of the Spanish Vaccination Strategy for school and high-school teachers in February 2021. FDG PET/CT was requested because of severe anxiety disorder secondary to the hematological diagnosis, despite not being recommended in routine Hodgkin Lymphoma follow-up after achieving CMR.14 On FDG PET/CT, lymph nodes were small with mild-moderate FDG uptake, according to other VAHL cases recently reported.15,16 We made an exhaustive literature search of COVID-19 vaccine-associated lymphadenopathy, but we did not find cases with hypermetabolic infradiaphragmatic lymph nodes. Given the temporal evolution and the evidence of systemic inflammatory response after COVID-19 vaccines, lymph nodes were concluded to be reactive to vaccination.
In this context, some recommendations have been proposed to facilitate image evaluation, such as indicating vaccination history (date and injection site) or administering the vaccine in the arm contralateral to a unilateral cancer.17 Some radiological findings on CT may translate a benign process, like small size or preserved fatty hilum, but VAHL morphology is very variable.2,3 A comparison with the baseline image may be helpful, but in our case was confusing, due to the hypermetabolism of residual abdominal lymph nodes. Radiologists and Nuclear Medicine physicians recommend to coordinate vaccine administration or to delay FDG PET/CT at least 4–6 weeks after vaccination when clinically possible.18
Conclusions
To our knowledge, this is the first case with hypermetabolic adenopathic enlargement away from the COVID-19 vaccine injection site and in a location where disease was initially diagnosed. Consideration of clinical presentation and vaccination history is mandatory to accurately interpret radiological findings in this new setting. Follow-up FDG PET/CT in patients with CHL who achieve CMR must be restricted to a clinical suspicion of relapse in order to avoid false-positive results.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, Bellamy D, Bibi S, Bittaye M, Clutterbuck EA, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–78. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keshavarz P, Yazdanpanah F, Rafiee F, Mizandari M.. Lymphadenopathy following COVID-19 vaccination: imaging findings review. Acad Radiol. 2021:S1076-6332(21)00196–3. Published online May 1. doi: 10.1016/j.acra.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McIntosh LJ, Bankier AA, Vijayaraghavan GR, Licho R, Rosen MP.. COVID-19 vaccination-related uptake on FDG PET/CT: an emerging dilemma and suggestions for management. AJR Am J Roentgenol. 2021;217(4):975–83. Published online March 1. doi: 10.2214/AJR.21.25728. [DOI] [PubMed] [Google Scholar]
- 4.Abou-Foul AK, Ross E, Abou-Foul M. George AP. P-257 Cervical lymphadenopathy following COVID-19 vaccine: clinical characteristics and implications for head and neck cancer services. Oral Oncol. 2021;118:20. doi: 10.1016/S1368-8375(21)00540-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Özütemiz C, Krystosek LA, Church AL, Chauhan A, Ellermann JM, Domingo-Musibay E, Steinberger D. Lymphadenopathy in COVID-19 vaccine recipients: diagnostic dilemma in oncologic patients. Radiology. 2021;300(1):E296–E300. doi: 10.1148/radiol.2021210275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burger IA, Husmann L, Hany TF, Schmid DT, Schaefer NG. Incidence and intensity of F-18 FDG uptake after vaccination with H1N1 vaccine. Clin Nucl Med. 2011;36(10):848–53. doi: 10.1097/RLU.0b013e3182177322. [DOI] [PubMed] [Google Scholar]
- 7.Studdiford J, Lamb K, Horvath K, Altshuler M, Stonehouse A. Development of unilateral cervical and supraclavicular lymphadenopathy after human papilloma virus vaccination. Pharmacotherapy. 2008;28(9):1194–97. doi: 10.1592/phco.28.9.1194. [DOI] [PubMed] [Google Scholar]
- 8.Local reactions, systemic reactions, adverse events, and serious adverse events: Janssen COVID-19 vaccine (J&J) | CDC. Published March 2, 2021. [accessed 2021 July 17]. https://www.cdc.gov/vaccines/covid-19/info-by-product/janssen/reactogenicity.html.
- 9.Reactions and adverse events of the pfizer-BioNTech COVID-19 vaccine | CDC. Published May 14, 2021. [accessed 2021 July 17]. https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html.
- 10.Cohen D, Krauthammer SH, Wolf I, Even-Sapir E. Hypermetabolic lymphadenopathy following administration of BNT162b2 mRNA Covid-19 vaccine: incidence assessed by [18F]FDG PET-CT and relevance to study interpretation. Eur J Nucl Med Mol Imag. 2021;48(6):1854–63. doi: 10.1007/s00259-021-05314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen D, Hazut Krauthammer S, Cohen YC, Perry C, Avivi I, Herishanu Y, Even-Sapir E. Correlation between BNT162b2 mRNA Covid-19 vaccine-associated hypermetabolic lymphadenopathy and humoral immunity in patients with hematologic malignancy. Eur J Nucl Med Mol Imag. 2021;48(11):3540–49. Published online May 8. doi: 10.1007/s00259-021-05389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah S, Wagner T, Nathan M, Szyszko T. COVID-19 vaccine-related lymph node activation - patterns of uptake on PET-CT. BJR Case Rep. 2021;7(3):20210040. doi: 10.1259/bjrcr.20210040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu G, Lu Y. COVID-19 mRNA vaccination-induced lymphadenopathy mimics lymphoma progression on FDG PET/CT. Clin Nucl Med. 2021;46(4):353–54. doi: 10.1097/RLU.0000000000003597. [DOI] [PubMed] [Google Scholar]
- 14.Eichenauer DA, Aleman BMP, André M, Federico M, Hutchings M, Illidge T, Engert A, Ladetto M. Hodgkin lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv19–iv29. doi: 10.1093/annonc/mdy080. [DOI] [PubMed] [Google Scholar]
- 15.Nawwar AA, Searle J, Singh R, Lyburn ID. Oxford-AstraZeneca COVID-19 vaccination induced lymphadenopathy on [18F]Choline PET/CT-not only an FDG finding. Eur J Nucl Med Mol Imag. 2021;48(8):2657–58. doi: 10.1007/s00259-021-05279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nawwar AA, Searle J, Hopkins R, Lyburn ID. False-positive axillary lymph nodes on FDG PET/CT resulting from COVID-19 immunization. Clin Nucl Med. 2021. Published online April 21. doi: 10.1097/RLU.0000000000003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.SBI-recommendations-for-managing-axillary-adenopathy-post-COVID-vaccination.pdf . [accessed 2021 July 17]. https://www.sbi-online.org/Portals/0/Position%20Statements/2021/SBI-recommendations-for-managing-axillary-adenopathy-post-COVID-vaccination.pdf.
- 18.Becker AS, Perez-Johnston R, Chikarmane SA, Chen, MM, El Homsi, M, Feigin, KN, Gallagher, KM, Hanna, EY, Hicks, M, Ilica, AT, et al. Multidisciplinary recommendations regarding post-vaccine adenopathy and radiologic imaging: radiology scientific expert panel. Radiology. 2021:210436. Published online February 24. doi: 10.1148/radiol.2021210436. [DOI] [PMC free article] [PubMed] [Google Scholar]


