ABSTRACT
Pregnant persons are at increased risk of severe illness from COVID-19. The first COVID-19 vaccines in the U.S. were authorized for emergency use in December 2020 and pregnant persons were eligible and could get vaccinated despite scarce safety data in this population. To monitor the safety of COVID-19 vaccination during pregnancy, four surveillance systems are used by the Centers for Disease Control and Prevention (CDC). The Vaccine Adverse Event Reporting System is a national, passive system that captures reports of potential adverse events. V-safe is a novel, active system that uses text messaging and web-based surveys to provide health check-ins after vaccination; and enrolls eligible v-safe participants in the v-safe pregnancy registry. The Vaccine Safety Datalink is a collaboration between the CDC and nine integrated health care organizations which performs near-real time surveillance and traditional epidemiologic studies on pregnant vaccine recipients. The CDC is committed to timely and comprehensive monitoring of COVID-19 vaccine safety in pregnancy.
KEYWORDS: Adverse events, epidemiology, coronavirus, COVID-19, mRNA vaccines, SARS-COV-2, adenovirus type 26, pregnancy, surveillance, vaccine safety
Background
Pregnant and recently pregnant persons infected with COVID-19 have an increased risk of severe illness that results in intensive care unit admission, mechanical ventilation, and death compared with non-pregnant women of reproductive age.1–3 Pregnant persons with COVID-19 are at increased risk of preterm birth and may be at risk of other adverse pregnancy outcomes compared with pregnant persons without COVID-19. Among COVID-19 vaccines authorized for emergency use in the United States by the Food and Drug Administration (FDA), the first two COVID-19 vaccines use messenger ribonucleic acid (mRNA), and a third uses a recombinant replication-incompetent adenovirus type 26 (Ad26) vector to stimulate an immune response; these vaccines were authorized in mid-December 2020 (mRNA), and the end of February 2021 (Ad26), respectively.4–7 However, data on safety of these vaccines in pregnancy were limited because pregnant persons were excluded from clinical trial enrollment and also discontinued from receiving additional doses of the vaccine if found to be pregnant during the studies.8,9 COVID-19 vaccination is recommended for all people 12 years and older, including people who are pregnant,1,10–12 The CDC and the FDA leveraged existing and implemented new vaccine safety monitoring systems to capture information about vaccination during pregnancy and to better understand the safety profiles of COVID-19 vaccines in pregnancy.
Currently, the CDC uses four systems to monitor the safety of COVID-19 vaccines in pregnancy: 1) the Vaccine Adverse Event Reporting System (VAERS), which is the front-line, national, spontaneous surveillance system that receives reports of adverse events (AEs) following vaccination in the United States;13 2) v-safe, a new smartphone-based, after-vaccination health checker system that uses text messaging and web-based surveys for people who receive COVID-19 vaccines;14 3) v-safe pregnancy registry which collects detailed pregnancy and medical history information from v-safe participants who report being pregnant around the time of vaccination; and 4) the Vaccine Safety Datalink (VSD) which is a large linked database system used for active surveillance and traditional epidemiologic research.15 In this review, we describe how these complementary systems are used to monitor the safety of COVID-19 vaccines in pregnant persons in the United States in a comprehensive manner, including the types of analyses used to detect potential safety signals and their follow-up.
Vaccine Adverse Event Reporting System (VAERS)
VAERS is a national passive vaccine safety surveillance system, implemented in 1990 and co-administered by the CDC and the FDA that receives spontaneous reports of AEs from healthcare providers, vaccine recipients, manufacturers, and other reporters following vaccination.13 Vaccine manufacturers are required, by law, to report AEs that come to their attention, and healthcare providers are required to report AEs that are considered a contraindication to further doses of vaccine and those specified in the VAERS table of reportable events following vaccination.13 VAERS data are monitored in real time to detect new, unusual, or rare vaccine AEs as well as increases in known AEs. This allows for the relatively rapid detection of these AEs, which may then be additionally evaluated in more robust surveillance systems or be assessed in epidemiological studies. Monitoring of AEs in VAERS is especially important whenever a new vaccine is licensed and recommended for use in the US population, as was the case with the administration of novel COVID-19 vaccines under the FDA’s emergency use authorization (EUA) provision. The VAERS report form (Figure 1) collects information on age, sex, pregnancy status, vaccines administered, the AE experienced, medical conditions at the time of vaccination and medical history and allows for uploading detailed medical documentation. Strengths of VAERS include its broad national scope and its ability to rapidly detect and evaluate reported AEs enabling further focused investigative efforts and hypothesis generation (Table 1). VAERS is also available to the public as a searchable database.13
Figure 1.
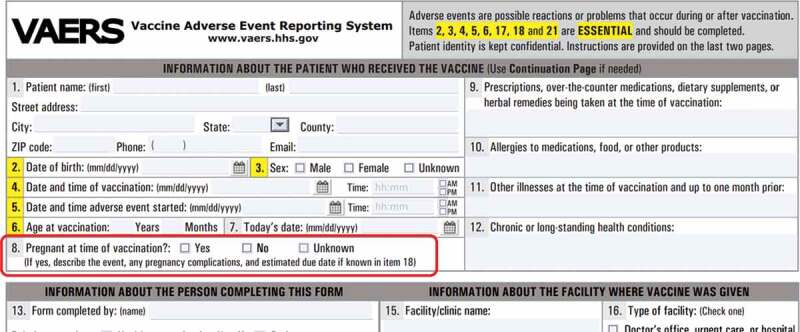
Part of a VAERS form showing specific question on pregnancy status (inside red rectangle).
Table 1.
Mechanisms for CDC COVID −19 vaccine safety monitoring among pregnant persons
| System/Project | Vaccine Adverse Event Reporting System (VAERS) | V-safe | V-safe pregnancy registry | Vaccine Safety Datalink (VSD) |
|---|---|---|---|---|
| Collaboration | CDC and FDA | CDC | CDC | CDC and 9 Integrated Healthcare Plans |
| Description | VAERS is the U.S. frontline spontaneous reporting system that detects potential vaccine safety problems. It serves as the nation’s early warning system; can rapidly detect concerns and generate hypotheses for further safety studies. Anyone can report possible vaccine adverse events to VAERS. Enhanced monitoring, involving review of all reports and associated medical records, is being conducted to review adverse events reported following COVID-19 vaccines administration to pregnant people. |
V-safe is a new CDC smartphone-based active surveillance system developed for the COVID-19 vaccination program; enrollment is voluntary. V-safe sends text messages to participants with weblinks to online surveys that assess for adverse reactions and health status during a post-vaccination follow-up period Surveys also include questions about pregnancy status at time of vaccination and about whether there has been a positive pregnancy test result following vaccination | Persons self-identifying as pregnant in v-safe may then be contacted by phone, and if they meet inclusion criteria, offered enrollment in the v-safe pregnancy registry. For persons who choose to enroll, the pregnancy registry phone-based survey collects detailed information about the participant, including medical and obstetric history, pregnancy complications, birth outcomes, and obstetric and pediatric healthcare provider contact information for medical records; infants are followed through the first three months of life. Data are analyzed in near real-time and include descriptive analyses and clinical and medical record review of adverse pregnancy and birth outcomes. | VSD is a large linked electronic health record-based database system used for epidemiologic research on vaccine safety and for active, near real-time monitoring of new vaccines and seasonal influenza vaccines. Both near real-time monitoring and traditional epidemiologic studies are being used to monitor the safety of COVID-19 vaccines in pregnancy. |
| Strengths |
|
|
|
|
| Limitations |
|
|
|
|
Signs and symptoms of AEs reported to VAERS are coded by trained personnel and entered into a database using the Medical Dictionary for Regulatory Activities (MedDRA), a clinically validated, internationally standardized medical terminology.16 A VAERS report may be assigned one or more MedDRA preferred terms (PT). A PT is a distinct descriptor for a symptom, sign, disease, diagnosis, therapeutic indication, investigation, surgical, or medical procedure, and medical, social, or family history characteristic.16 Reports are further classified as serious as per the U.S. Code of Federal Regulations if one of the following is reported: death, life-threatening illness, hospitalization or prolongation of hospitalization, permanent disability, or a congenital anomaly.17 Typically, in response to serious reports following non-COVID-19 vaccines, trained clinicians will contact the person who filed the VAERS report to request additional information, including medical records and autopsy reports when appropriate. With the urgent need to monitor the safety of the newly authorized COVID-19 vaccines, medical records are being requested for certain pre-specified conditions (e.g. pregnancy) or reported AEs, in addition to serious reports. All submitted VAERS reports specifying pregnant persons who received a COVID-19 vaccine are being identified and their medical records requested for review by clinicians, irrespective of whether they are categorized as “serious.”
Types of analysis for monitoring the safety of COVID-19 vaccines in VAERS in pregnant persons
Reports of AEs in pregnant persons receiving COVID-19 vaccination are first identified through a targeted search strategy. One or more of four types of analyses may be applied: 1) automated analysis of VAERS data; 2) analysis of data from clinical reviews; 3) calculation of crude reporting rates; and 4) data mining.
Automated analyses
These analyses describe report characteristics such as the overall number of reports, types of reporters, proportions of serious versus non-serious reports, trimester of pregnancy when vaccinated, specific COVID-19 vaccine brands received, and preferred terms (PTs).
Clinical review
Clinical review of reports and accompanying medical records are performed to 1) evaluate for unusual or unexpected reporting of AEs following vaccination of pregnant persons (e.g., more than expected fever), 2) identify events of particular concern related to pregnancy (e.g., spontaneous abortion, stillbirth); and 3) evaluate data mining signals (signal assessment). Through review of medical records, clinicians verify that the VAERS search correctly identified the clinical event of interest, characterize the completeness and quality of reports, their clinical and laboratory features, and assess for any potential risk factors (e.g., co-administration of vaccines, underlying health conditions).
Crude reporting rates
Incidence or prevalence of specific AEs are important to calculate and compare to published background rates of the AEs to indicate a possible safety concern. By virtue of its design to facilitate rapid, passive reporting of AEs, VAERS does not have comprehensive data on the number of vaccines administered nationally. However, it is possible to calculate crude reporting rates of AEs by using information collected from the manufacturer or through other surveillance systems as a proxy denominator for the number of doses of vaccine distributed and/or administered nationally. Because underreporting of AEs is an important limitation in VAERS, these calculated rates would be expected to be below the background rates for the conditions studied. Currently, there is no available data about the number of COVID-19 vaccines administered to pregnant persons, thus limiting the ability to calculate crude reporting rates of AEs in pregnant persons.
Data mining
Since AE incidence or prevalence rates cannot be directly calculated from VAERS data, two techniques have been developed as a means of signal detection (i.e., identifying disproportionately high reporting of AEs). These include: 1) calculating proportional reporting ratios (PRR)18 and 2) Empirical Bayesian (EB) data mining.19–21 The PRR is a statistic used to compare the proportions of AEs reported for a specific vaccine type (e.g., quadrivalent inactivated influenza vaccine) with the proportions of AEs reported in VAERS for the same vaccine type in the previous year, or other groups of vaccines or other comparisons.18 In the setting of the COVID-19 pandemic, a comparison could be made between proportions of AEs reported following COVID-19 vaccination and proportions reported following other vaccines in the VAERS database.
Empirical Bayesian (EB) data mining20,21 is used to identify AEs reported more frequently than expected following a specific brand of COVID-19 vaccine (e.g., Pfizer-BioNTech) in the VAERS database. Reports of specific AE’s after receipt of a particular brand or type of COVID-19 vaccine are compared with similar reports from any/all other vaccines types in the VAERS database. A vaccine-adverse event pairing “signals” when a statistical threshold is reached; this occurs when the vaccine-event pair is reported at least twice as frequently as would be expected based on the lower bound of the 95% confidence interval surrounding the EB geometric mean [EB05] >2.19–21 Reports containing PTs that exceed the data mining threshold may be reviewed to characterize and verify the signal.
Limitations of VAERS
Limitations include over- or under-reporting, biased reporting, inconsistency in quality and completeness of reports, and lack of a denominator (Table 1).13 Publicity around AEs may lead to over-reporting (stimulated reporting).22 VAERS generally cannot assess causality between receipt of a vaccine and a reported AE; it is not possible to calculate the incidence or prevalence of an AE nor estimate an increase in risk of the AE.13
V-safe and v-safe pregnancy registry
V-safe is an active safety monitoring system established by CDC specifically for the COVID-19 vaccination program.23 V-safe participants voluntarily self-enroll via a link on their smartphone; volunteers receive text reminders that include individualized links to complete short web-based surveys (Figure 2). V-safe enrollees are asked to complete daily surveys for the first 7 days after vaccination, then weekly through 6 weeks post-vaccination and then at 3, 6, and 12 months. The health check-in interval prompts reset when a person records anotherdose of vaccine. During the first week after vaccination, enrollees are asked to complete surveys about possible local injection site and systemic reactions and to rank them as mild, moderate, or severe. In addition, enrollees are asked health impact status; if they missed work, were unable to perform normal daily activities, or received care from a medical professional because of reported symptoms or health conditions. Enrollees who report seeking medical care may be contacted via telephone and encouraged to complete a VAERS report. Surveys also include questions about pregnancy status at time of vaccination (initial survey for each dose) and about whether they have had a positive pregnancy test result following vaccination (on the 3- and 6-week surveys as well as the 3-, 6-, and 12-month surveys); these questions are included for persons who do not register their sex as male and allow for identification of persons who may be eligible for enrollment in the v-safe pregnancy registry.24
Figure 2.
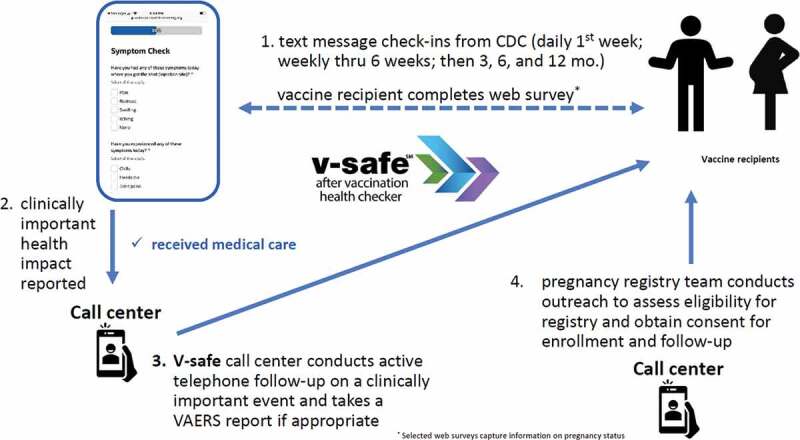
V-safe surveillance system reporting structure.
Data collected in v-safe are used to assess local and systemic reactogenicity for self-identified pregnant persons and to compare their reports to those from nonpregnant persons. Additionally, reported sociodemographic information such as age and race/ethnicity can be considered during these analyses as well as COVID vaccine type. Details about v-safe methodology are available on the CDC website.23
V-safe pregnancy registry
V-safe pregnancy registry collects additional health information from v-safe participants who report being pregnant around the time of vaccination.14 Inclusion criteria for the v-safe pregnancy registry include COVID-19 vaccination during pregnancy or in the periconception period (30 days before last menstrual period [LMP] through 14 days after LMP) (Figure 3). Pregnant persons who meet inclusion criteria during an initial screening contact are offered an opportunity to enroll in the registry. People who choose to enroll in the registry are contacted several times throughout their pregnancy. During these check-ins, they are asked questions about their pregnancy and medical history. Participants are also asked if they are willing to consent to a review of their medical records to get more detailed and comprehensive information about their pregnancies. Participants are contacted postpartum and again once infants reach 3 months of age to complete maternal and infant follow-up surveys. Data collected by phone and through abstraction of data from medical or laboratory records include 1) maternal demographic and health variables: age, race/ethnicity, laboratory test results, reports from prenatal imaging, chronic, or acute medical conditions; 2) pregnancy exposures: infections, medication, and substance use; 3) pregnancy outcome: miscarriage, stillbirth, elective termination, live birth; 4) pregnancy/delivery/post-partum complications: diabetes, preeclampsia, preterm delivery, hemorrhage, infection; and 5) infant outcomes through 3 months of life: date of delivery, gestational age, sex, birthweight, and other anthropomorphic measurements, occurrence of structural birth defects and results from screening tests and exams.
Figure 3.
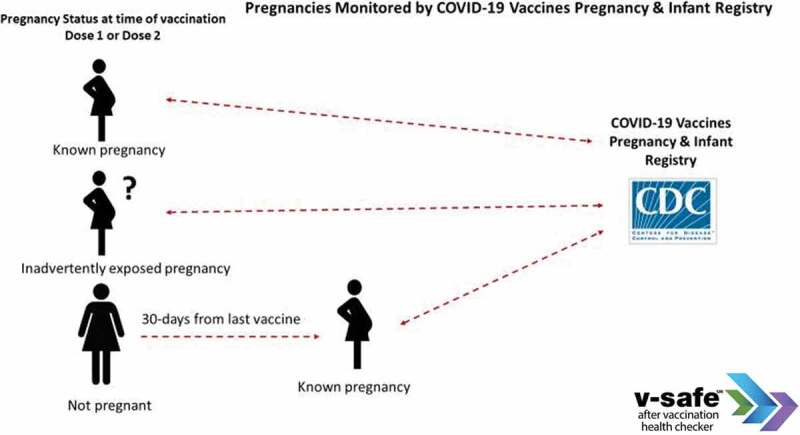
Relative pregnancy timing inclusion criteria for v-safe pregnancy registry enrollment.
Types of analysis for monitoring the safety of COVID-19 vaccines in v-safe in pregnant persons
Data from the pregnancy registry are analyzed in near real-time and include descriptive analyses of rates of follow-up among pregnant persons and any reported adverse outcomes. Specifically, proportions and rates of fetal demise (spontaneous abortion and stillbirth), pregnancy and postpartum complications (hypertensive disorders, gestational diabetes, intrauterine growth restriction, fever), adverse birth outcomes (preterm delivery, small for gestational age, low birth weight, neonatal intensive care unit stays, neonatal deaths, major birth defects) and infant hospitalizations are calculated. These proportions and rates are compared to published background rates or estimates seen in other safety monitoring systems. Adverse events may be analyzed in aggregate if rare (e.g., major cardiac defects). Any potential safety signals identified will be verified with clinical review of medical records and compared to other surveillance systems, such as VAERS, and, for birth defects, the CDC’s Metropolitan Atlanta Congenital Defects Program or the Texas Birth Defects Registry.25 Persistent signals will be evaluated in more robust data systems (such as the Vaccine Safety Datalink) that have denominator data of pregnant persons who received COVID-19 vaccines and comparison groups of pregnant persons who did not receive COVID-19 vaccines.
Details about v-safe pregnancy registry methodology are available on CDC’s website.14,23,24
Limitations of v-safe and v-safe pregnancy registry
Participation in v-safe and the v-safe pregnancy registry is voluntary. Information about enrolling in the v-safe program is included on the EUA fact sheets for health care providers and patients and is available on the CDC’s website. While all jurisdictions have been encouraged to provide v-safe information sheets to vaccine recipients, this information is not uniformly available at all vaccination locations. Thus, there may be differences between the v-safe population and those on which published background rates of certain outcomes are based. These differences may exist for demographic and clinical characteristics associated with pregnancy and neonatal outcomes. However, such comparisons are helpful to provide a crude sense of whether there are any unexpected safety signals in these early data. Additionally, as with all participant-reported surveillance systems, participant’s mistakes in completion of v-safe health surveys can result in misclassification of pregnancy status; as a result, data for participant-reported local and systemic reactions among pregnant persons to the v-safe platform may include some reports from nonpregnant persons. Also, estimates in the pregnancy registry may not reflect true post-vaccination proportions of spontaneous abortions among those truly at risk of this outcome because participants might have been vaccinated after the period of greatest spontaneous abortion risk in the first trimester.
Vaccine Safety Datalink (VSD)
The Vaccine Safety Datalink (VSD) is a collaborative project between CDC and nine integrated health care organizations originally established in 1990 (Figure 4).15 The VSD uses large, linked administrative datasets containing demographic and medical information, such as age, sex, race/ethnicity, health plan enrollment, vaccinations, hospitalizations, emergency room and outpatient encounters, mortality data, pregnancy and birth data, in order to evaluate vaccines and potential AEs using both traditional and novel epidemiologic methods. Vaccination data on the VSD population comes from a combination of electronic medical records and state immunization registries. VSD electronic data can be supplemented with medical records review when necessary for verifying diagnoses and capturing information unavailable through standard datasets. VSD data covers approximately 3% of the US population, translating to over 12 million individuals per year, including approximately 125,000 pregnant persons a year and a birth cohort of approximately 100,000 a year. Furthermore, the VSD has developed and validated a pregnancy episode algorithm (PEA) that uses electronic data, including diagnosis and procedure codes, to identify pregnancy start dates, end dates, and outcomes.26 The algorithm was originally created using international classification of diseases 9th revision, clinical modification (ICD-9-CM) codes and has been updated to use ICD-10-CM codes and to dynamically identify ongoing pregnancies and pregnancy outcomes in near real time. VSD electronic data from the PEA is then used to link pregnant mothers and their infants. Furthermore, the VSD has created and validated an electronic algorithm that can identify major structural birth defects in infants.27 Traditional epidemiologic studies (such as cohort and case–control studies), descriptive studies (such as vaccine coverage during pregnancy, rare events), and pregnancy-specific methods studies can all be conducted using VSD data.28–31 Both recommended and non-recommended vaccines during pregnancy can be studied for a variety of AEs in the pregnant person (acute adverse events, adverse pregnancy outcomes and adverse birth outcomes) and in the infant. The large cohort of pregnant persons and their infants, along with standardized vaccination information and medical encounter information, validated algorithms identifying pregnancies and major structural birth defects, and the potential for supplementing this information with medical record review, allows for a rich data source in which the safety of vaccines administered during pregnancy can be rigorously monitored in pregnant persons and their infants.
Figure 4.
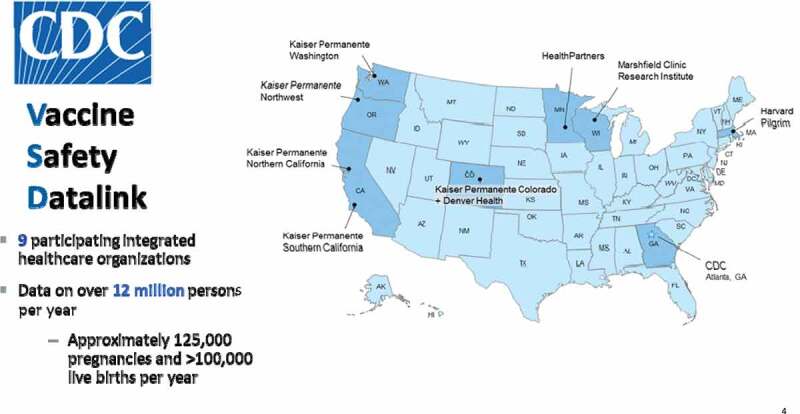
Vaccine safety datalink sites.
Monitoring the safety of maternal COVID-19 vaccines in VSD
Three large studies in pregnant persons following COVID-19 vaccine have been initiated using VSD data that include both rapid and long-term assessments. The first study estimates vaccine coverage in pregnancy using the dynamic pregnancy algorithm to develop weekly counts of vaccination.32 Weekly rates are be calculated by age, race/ethnicity, and vaccine type.32 This weekly analysis will be followed by a more in-depth analysis on a quarterly basis. The second study is a large, multi-year comprehensive study looking at acute adverse outcomes, pregnancy events, adverse birth outcomes, such as preterm delivery and small for gestational age, and infant outcomes in the first year of life, including birth defects, mortality, hospitalizations, and infant growth and development. The assessment of acute outcomes in pregnancy (such as medically attended fevers and local reactions) will be performed at regular intervals. The third large COVID-19 safety study in pregnant persons that has been initiated is evaluating pregnancy losses after maternal COVID-19 vaccines. This will evaluate spontaneous abortion and stillbirths following COVID-19 vaccines and will include medical record abstraction and adjudication for confirmation of outcome and outcome dates. This study will include interim monitoring of these outcomes followed by a larger, more extensive case-controlled study.
Limitations of VSD
The VSD population largely consists of an insured population, which may not be generalizable to the entire population of the United States (Table 1). COVID-19 vaccine roll-out has varied widely in various parts of the country, as has uptake among pregnant persons. For this reason, evaluation of many acute medically attended outcomes that may be rare will take time to be adequately powered for interim analyses. Not all outcomes are well suited for real-time analyses because of long risk windows or because they are very nonspecific, and therefore may need to be studied using a more traditional retrospective methodology that can take longer to complete. Furthermore, vaccines administered within the VSD catchment area but outside of the VSD healthcare system (e.g., pharmacies) may not be captured. However, many VSD sites are linked to state registries, which broadens the vaccination capture.
Comment
During the COVID-19 pandemic, evidence emerged that pregnant and recently pregnant persons with COVID-19 were at increased risk of severe illness, including illness that results in intensive care unit admission, mechanical ventilation, and death, compared to non-pregnant women of reproductive age.3 Because of this increased risk of negative outcomes, pregnant persons were identified as a group that could benefit from the COVID-19 vaccines despite the very limited safety data on COVID-19 vaccines in pregnancy. Four systems are being used for monitoring COVID-19 vaccine safety during pregnancy: 1) VAERS, 2) the v-safe surveillance system, 3) the v-safe pregnancy registry and 4) VSD. The first three provided the earliest maternal safety data which was found to be reassuring with no major safety issues of concern identified for the mRNA vaccines.33 However, most of the pregnancies in these systems are ongoing. Additional follow-up is needed, particularly among those vaccinated in the first and second trimesters of pregnancy. The early findings filled a gap on the safety of these vaccines. As more pregnant people get vaccinated and pregnancies come to completion, more complete data will be available and signal detection for rare events may be possible. Furthermore, large linked database systems such as the VSD can evaluate rates and risks for specific adverse events, including those initially detected in surveillance systems. Together these systems work to rapidly and comprehensively study the safety of COVID-19 vaccines among pregnant persons.
During monitoring of adverse events (AEs) after a new vaccine, such as the COVID-19 vaccines, there may be discordant results between different systems. This may be especially true during the initial monitoring periods when vaccine doses are accumulating, and rare adverse events are underpowered. When this occurs, it is important to consider the strengths and limitations of the system in which it was noted and consider if the signal was noted elsewhere (e.g., pre-authorization trials, other surveillance, and database systems). Furthermore, it is important to consider the biologic plausibility of the vaccine causing the AE and include a risk benefit analysis (i.e., number of cases from vaccine compared to number of cases prevented from disease). As a follow-up, further studies and specific research questions can provide a more definitive answer over time. If on the other hand, multiple systems using different methods show similar findings, that lends stronger evidence that a safety monitoring finding is confirmed.
Despite the different methodologies of the systems used, each with its unique set of strengths and limitations, all four systems are complementary and serve a critical role in monitoring the safety of COVID-19 vaccines administered to pregnant persons. The findings from enhanced maternal safety monitoring following COVID-19 vaccination in these four systems will inform federal agencies, healthcare providers, domestic and international immunization partners, and the public on the safety of these new vaccines.
Acknowledgments
We are specially thankful to Michael McNeill, Anne Hause, Christina Banister, and Eric Weintraub for careful review of the manuscript. We also thank the staff of the Immunization Safety Office, General Dynamics Information Technology, Oracle, and all deployers to v-safe and the v-safe pregnancy registry for their work and dedication to public health during the COVID-19 pandemic. We are especially thankful to the participants in all of our vaccine surveillance systems, without whose reports and participation we would be unable to provide timely information on vaccine safety.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
References
- 1.Centers for Disease Control and Prevention . Information about COVID-19 Vaccines for People who Are Pregnant or Breastfeeding. [accessed 2021 August 30] https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html
- 2.Centers for Disease Control and Prevention . Science Brief: evidence used to update the list of underlying medical conditions that increase a person’s risk of severe illness from COVID-19. Scientific Evidence for Conditions that Increase Risk of Severe Illness | COVID-19 | CDC. [PubMed]
- 3.Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, Debenham L, Llavall AC, Dixit A, Zhou D, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Food and Drug Administration . Fact sheet for healthcare providers administering vaccine (vaccination providers): emergency use authorization (EUA) of the PfizerBioNTech COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). 2021. [Accessed 2021 August 30]. https://www.fda.gov/media/144413/download.
- 5.Comirnaty (COVID-19 Vaccine, mRNA) FDA approval letter . [Accessed 2021 August 30]. https://www.fda.gov/media/151710/download.
- 6.Food and Drug Administration . Fact sheet for healthcare providers administering vaccine (vaccination providers): emergency use authorization (EUA) of the Moderna COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). 2021. [Accessed 2021 August 30]. https://www.fda.gov/media/144637/download
- 7.Food and Drug Administration . Fact sheet for healthcare providers administering vaccine (vaccination providers) emergency use authorization (EUA) of the Janssen COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). 2021. [Accessed 2021 August 30]. https://www.fda.gov/media/146304/download.
- 8.Taylor MM, Kobeissi L, Kim C, Amin A, Thorson AE, Bellare NB, Brizuela V, Bonet M, Kara E, Thwin SS, et al. Inclusion of pregnant women in COVID-19 treatment trials: a review and global call to action. Lancet Glob Health. 2021;9(3):e366–e371. doi: 10.1016/S2214-109X(20)30484-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beigi RH, Krubiner C, Jamieson DJ, Lyerly AD, Hughes B, Riley L, Faden R, Karron R.. The need for inclusion of pregnant women in COVID-19 vaccine trials. Vaccine. 2021;39(6):868–70. doi: 10.1016/j.vaccine.2020.12.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, McClung N, Campos-Outcalt D, Morgan RL, Mbaeyi S, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine — United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1922–24. doi: 10.15585/mmwr.mm6950e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, McClung N, Campos-Outcalt D, Morgan RL, Mbaeyi S, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine — United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69(5152):1653–56. doi: 10.15585/mmwr.mm695152e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rasmussen SA, Kelley CF, Horton JP, Jamieson DJ.. Coronavirus Disease 2019 (COVID-19) Vaccines and Pregnancy: what Obstetricians Need to Know. Obstet Gynecol. 2021. Mar 1;137(3):408–14. doi: 10.1097/AOG.0000000000004290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015;33(36):4398–405. doi: 10.1016/j.vaccine.2015.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention . V-safe COVID-19 Vaccine Pregnancy Registry. [Accessed 2021 August 30]. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/vsafepregnancyregistry.html.
- 15.McNeil MM, Gee J, Weintraub ES, Belongia EA, Lee GM, Glanz JM, Nordin JD, Klein NP, Baxter R, Naleway AL, et al. The vaccine safety datalink: successes and challenges monitoring vaccine safety. Vaccine. 2014;32(42):5390–98. doi: 10.1016/j.vaccine.2014.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medical dictionary for regulatory activities . [Accessed 2021 August 30]. https://www.meddra.org/.
- 17.21 CFR Part 600.80 . Postmarketing reporting of adverse experiences. Fed Regist 1997;7:52252–53. [Google Scholar]
- 18.Evans SJ, Waller PC, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. 2001. Oct-Nov;10(6):483–86. doi: 10.1002/pds.677. [DOI] [PubMed] [Google Scholar]
- 19.DuMouchel W. Bayesian data mining in large frequency tables, with an application to the FDA spontaneous reporting system. Am Stat. 1999;53:177–90. [Google Scholar]
- 20.Szarfman A, Machado SG, O’Neill RT. Use of screening algorithms and computer systems to efficiently signal higher-than-expected combinations of drugs and events in the US FDA’s spontaneous reports database. Drug Saf. 2002;25(6):381–92. doi: 10.2165/00002018-200225060-00001. [DOI] [PubMed] [Google Scholar]
- 21.Banks D, Woo EJ, Burwen DR, Perucci P, Braun MM, Ball R. Comparing data mining methods on the VAERS database. Pharmacoepidemiol Drug Saf. 2005;14(9):601–09. doi: 10.1002/pds.1107. [DOI] [PubMed] [Google Scholar]
- 22.Moro PL, Broder K, Zheteyeva Y, Revzina N, Tepper N, Kissin D, Barash F, Arana J, Brantley MD, Ding H, et al. Adverse events following administration to pregnant women of influenza A (H1N1) 2009 monovalent vaccine reported to the Vaccine Adverse Event Reporting System. Am J Obstet Gynecol. 2011;205(5):473.e1–473.e9. doi: 10.1016/j.ajog.2011.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vsafe after vaccination health checker . [Accessed 2021 August 30]. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/vsafe.html.
- 24.Vsafe pregnancy registry protocol . [Accessed 2021 August 30]. https://www.cdc.gov/vaccinesafety/pdf/V-safe-Protocol-508.pdf.
- 25.Metropolitan Atlanta Congenital Defects Program (MACDP) . [Accessed 2021 August 30]. https://www.cdc.gov/ncbddd/birthdefects/macdp.html.
- 26.Naleway AL, Gold R, Kurosky S, Riedlinger K, Henninger ML, Nordin JD, Kharbanda EO, Irving S, Cheetham TC, McCarthy NL. Identifying pregnancy episodes, outcomes, and mother-infant pairs in the Vaccine Safety Datalink. Vaccine. 2013. Jun 12;31(27):2898–903. doi: 10.1016/j.vaccine.2013.03.069. [DOI] [PubMed] [Google Scholar]
- 27.Kharbanda EO, Vazquez-Benitez G, DeSilva MB, Spaulding AB, Daley MF, Naleway AL, Irving SA, Klein NP, Tseng HF, Jackson LA et al. Developing algorithms for identifying major structural birth defects using automated electronic health data. Pharmacoepidemiol Drug Saf. 2021. Feb;30(2):266–74. doi: 10.1002/pds.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naleway AL, Kurosky S, Henninger ML, Gold R, Nordin JD, Kharbanda EO, Irving S, Craig Cheetham T, Nakasato C et al. Vaccinations given during pregnancy, 2002-2009: a descriptive study. Am J Prev Med. 2014. Feb;46(2):150–57. doi: 10.1016/j.amepre.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Kharbanda EO, Vazquez-Benitez G, Lipkind H, Naleway AL, Klein NP, Cheetham TC, Hambidge SJ, Vellozzi C, Nordin JD. Receipt of pertussis vaccine during pregnancy across 7 Vaccine Safety Datalink sites. Prev Med. 2014. Oct;67:316–19. doi: 10.1016/j.ypmed.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 30.Myers TR, McCarthy NL, Panagiotakopoulos L, Omer SB. Estimation of the Incidence of Guillain-Barré Syndrome During Pregnancy in the United States. Open Forum Infect Dis. 2019. Mar 15;6(3):ofz071. doi: 10.1093/ofid/ofz071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vazquez-Benitez G, Kharbanda EO, Naleway AL, Lipkind H, Sukumaran L, McCarthy NL, Omer SB, Qian L, Xu S, Jackson ML et al. Risk of preterm or small-for-gestational-age birth after influenza vaccination during pregnancy: caveats when conducting retrospective observational studies. Am J Epidemiol. 2016. Aug 1;184(3):176–86. doi: 10.1093/aje/kww043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Razzaghi H, Meghani M, Pingali C, Crane B, Naleway A, Weintraub E, Kenigsberg TA, Lamias MJ, Irving SA, Kauffman TL, et al. COVID-19 Vaccination Coverage Among Pregnant Women During Pregnancy — eight Integrated Health Care Organizations, United States, December 14, 2020–May 8, 2021. MMWR Morb Mortal Wkly Rep. doi: 10.15585/mmwr.mm7024e2externalicon. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimabukuro TT, Kim SY, Myers TR, Moro PL, Oduyebo T, Panagiotakopoulos L, Marquez PL, Olson CK, Liu R, Chang KT, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021. Apr 21:NEJMoa2104983. doi: 10.1056/NEJMoa2104983. [DOI] [PMC free article] [PubMed] [Google Scholar]


