ABSTRACT
Co-administration of vaccines could be an efficient strategy to increase vaccination uptake and reduce the number of clinic visits. This randomized controlled study aimed to evaluate the immunogenicity and safety of enterovirus 71 (EV71) vaccine co-administered with measles-mumps-rubella (MMR) vaccine and live-attenuated Japanese encephalitis vaccine (LA-JEV). A total of 372 healthy infants were randomly assigned in a 1:1:1 ratio to receive simultaneous administration of EV71 vaccine (dose 1) and MMR on d 0 and EV71 vaccine (dose 2) and LA-JEV on d 30 (Group 1); administration of MMR and LA-JEV on d 0 and 30, respectively (Group 2); or administration of doses 1 and 2 of EV71 vaccine on d 0 and 30, respectively (Group 3). The non-inferiority analysis of the seroconversion for EV71 neutralizing antibody after vaccination was the primary outcome. According to per protocol set, antibody response against EV71, measles, mumps, rubella, and Japanese encephalitis (JE) virus was similar regardless of administration schedule. After vaccination, the seroconversion rate of EV71 neutralizing antibody in Group 1 (107 [97.27%] of 110) was non-inferior to that in Group 3 (109 [97.32%] of 112; difference – 0.05% [95% CI – 5.38 to 5.21]). The incidences of adverse reactions were 62.60% (77/123) in Group 1, 54.84% (68/124) in Group 2, and 37.70% (46/122) in Group 3, and most of them were mild to moderate in severity. No vaccine-related serious adverse events were reported. In total, the co-administration of combined EV71 vaccine with MMR and LA-JEV showed no interference with antibody response and demonstrated good safety profiles.
KEYWORDS: Co-administration, EV71 vaccine, measles-mumps-rubella vaccine, live-attenuated Japanese encephalitis vaccine, immunogenicity, safety
Introduction
Enterovirus 71 (EV71) is a small, positive-sense, single-stranded RNA virus that belongs to the Enterovirus genus of the Picornaviridae family, which was first isolated in 1969.1–3 EV71 infection is associated with a wide spectrum of diseases, including hand, foot, and mouth disease (HFMD), herpangina, neurological signs, and nonspecific illnesses.4–6 As one of the major causative agents of HFMD, it mainly affects infants and young children under 5 y of age.7,8 From 2008 to 2017, a total of 17.9 million HFMD cases were reported in China.9 In spite of most of the HFMD cases being mild or moderate, some severe HFMD cases can lead to severe central nervous system pathology and other complications.10 It is worth noting that about 70% of the severe HFMD cases and approximately 90% of the fatal HFMD cases caused by EV71 infection, which has become a serious threat to public health and economic burden acrosssome Asian countries.6,11–13 In this light, it is important to develop efficacious vaccines to prevent EV71 infection.
As of 2018, three EV71 vaccines has been available in China for the prevention of EV71 infection, including a vero cell–based inactivated, alum-adjuvant EV71 vaccine developed by Sinovac Biotech,14 which has been demonstrated to have a high safety and efficiency in protecting infants aged 6–35 months from EV71-associated HFMD in a series of trials.15–17 The results of sero-epidemiological study showed that the maternal EV71 antibodies waned with age after birth and reached the lowest level at 6 months of age.18 Therefore, the Chinese Center for Disease Control and Prevention recommends that EV71 vaccination targets susceptible children aged 6 months or older and encourages the completion of vaccination procedures before the age of 12 months, so as to play a protective role as soon as possible.19 However, there are already 14 vaccines in national immunization schedules that need to be administered during the same period, so the time window for separate two-dose of EV71 vaccine is very limited. As one of the effective measures to solve this dilemma, simultaneous administration is defined as inoculating more than one recommended vaccine through different syringes at different anatomical sites during the same visit.20 Although co-administration of vaccines can increase vaccination uptake and reduce the number of clinic visits, it also raises concerns about the immunogenicity and safety of the vaccines involved, as simultaneous vaccination may impair the immune responses.21
In China, the busy vaccination schedule for the first two years of life has prompted the development of co-administration of vaccines with a view to increase vaccination uptake.20 Measles-mumps-rubella (MMR) vaccine and live-attenuated Japanese encephalitis vaccine (LA-JEV), both of which are included in the country’s Expanded Program on Immunization (EPI) schedule, have been recommended as routine vaccines. The current study aims to evaluate the immunogenicity and safety of simultaneous administration of EV71 vaccine with MMR and LA-JEV.
Materials and methods
Study design and participants
This study was a phase 4, single-center, open-label, randomized controlled trial conducted in Hanbin District, Ankang City, Shaanxi Province, China, from July 2020 to November 2020.
Eligible participants were healthy infants aged 8 months or older. Main exclusion criteria included prior receipt of any EV71, measles, mumps, rubella, or Japanese encephalitis vaccine; history of HFMD, measles, mumps, rubella, or Japanese encephalitis (JE); allergy or serious adverse reactions to any study vaccine components; receipt of blood products within the previous 3 months; and receipt of any live attenuated vaccines within the previous 4 weeks or of any inactivated vaccines within the previous 7 d.
After enrollment, all subjects were randomly assigned into three groups at a ratio of 1:1:1. In the co-administration group (Group 1), infants received two injections at different injection sites during the same visit, one dose each of EV71 vaccine and MMR on d 0 and one dose each of EV71 vaccine and LA-JEV on d 30. In the group where each EPI vaccine was administered separately (Group 2), infants received one dose of MMR on d 0 and one dose of LA-JEV on d 30. In the group where two doses of EV71 vaccine were administered separately (Group 3), infants received the first dose on d 0 and the second dose on d 30.
Vaccines for each group were labeled with random numbers, based on a predefined block size. Every subject was assigned a randomization code in chronological order and received the vaccine labeled with the same code.
Written informed consent was obtained from each infants’ parent or guardian before enrollment. The trial was conducted in accordance with Good Clinical Practice standards and the Declaration of Helsinki. This study was approved by the ethical review committee of the Shaanxi Provincial Center for Disease Control and Prevention (SXSCDCIRB2019-001). The trial was registered with ClinicalTrials.gov (NCT04111432).
Study vaccines
Vero cell–based inactivated alum-adjuvanted EV71 vaccine (lot 201801005; Sinovac Biotech, Beijing) was packaged in a syringe with at least 3.0 Efficacy Unit (EU) of neutralization antibody titer for EV71 per dose (0.5 mL/vial). MMR vaccine (lot 201810120; Shanghai Institute of Biological Products) was packaged in a penicillin bottle containing a median cell culture infective dose (CCID50) of ≥103.0 for measles virus, ≥104.3 for mumps virus and ≥103.0 for rubella virus in a total volume of 0.5 mL/vial. LA-JEV (lot 201806A114-2; Chengdu Institute of Biological Products) was packaged in a penicillin bottle contains not less than 5.4 log plaque-forming units (PFU) of live JE virus in a total volume of 0.5 mL/vial. All vaccines were tested and approved for lot release by the Chinese National Institute for Food and Drug Control (CNIFDC).
Immunogenicity assessment
Blood samples were collected from all participants on d 0 (before the first vaccination) and d 60 (30 d after the second vaccination). After collection, serum samples were isolated and stored at −20°C until assayed. The neutralizing antibody against EV71 and JE was detected in the Sinovac Biotech and Shaanxi Provincial Center for Disease Control and Prevention, respectively, and the detection methods were microcytopathogenic effect assay and microneutralization test (Supplementary method). Measles, mumps, and rubella IgG antibody concentrations were detected by an enzyme-linked immunosorbent assay (ELISA) kit produced by Virion\Serion (Wurzburg, Germany) in Shaanxi Provincial Center for Disease Control and Prevention.
The immunogenicity endpoints included seroconversion rate, geometric mean titer (GMT)/geometric mean concentration (GMC). For EV71 vaccine, the seroconversion rate was defined as the percentage of participants with a reciprocal neutralizing antibody titer of either (1) <1:8 before vaccination and ≥1:8 after vaccination or (2) ≥1:8 before vaccination and at least a fourfold increase after vaccination. For LA-JEV, the seroconversion rate was defined as the percentage of participants with a reciprocal neutralizing antibody titer of either (1) <1:5 before vaccination and ≥1:5 after vaccination or (2) ≥1:5 before vaccination and at least a fourfold increase after vaccination. The seropositivity of measles, mumps, and rubella was defined as the concentration of measles IgG antibody ≥200 mIU/mL, mumps IgG antibody ≥100 U/mL and rubella IgG antibody ≥20 IU/mL. Seroconversion was defined as a change from seronegative to seropositive or at least a fourfold increase in IgG antibody concentration after vaccination.
Safety assessment
Immediate adverse events (AEs) were observed on site in 30 minutes after each vaccination. A diary card was given to parents or guardians to record solicited local or systemic AEs occurring within 14 d (or 7 d for Group 3), unsolicited AEs and SAE occurring within 30 d after each dose. Solicited systemic AEs included fever, allergic reaction, fatigue, irritability, decreased appetite, nausea/vomiting, and diarrhea; solicited local AEs included pain, induration, redness, swelling, rash, and pruritus. Face-to-face or telephone visits were assigned on the 7th day or 14th day, and 30th day after each vaccination to assure completeness and accuracy. The causal relationship between adverse events and vaccination was judged by the investigators.
Statistical analysis
The sample size was estimated using NCSS-PASS, version 11.0 based on a non-inferiority design.22 We assumed a 95% proportion of seroconversion against EV71 after administration of EV71 vaccine alone. A sample size of 95 individuals per group was required for a power (calculated as 1 − β) of 0 · 80 with a one-sided α of 0 · 025 and a non-inferiority margin of – 10% with a Z test with pooled variance. With an estimated dropout rate of ≤20%, a total of 372 participants finally were recruited in this trial.
The comparison of demographic characteristics was performed for the full analysis set (FAS). The immunogenicity analysis was conducted in the per-protocol set (PPS), including infants who met eligibility criteria, complied with the protocol and had immunogenicity results before and after vaccination. Safety analysis was performed for the safety set (SS), which included infants who received at least one vaccination. Categorical variables were reported as frequency and percentage, continuous variables were reported as means and standard deviation. The Student's t-test was used for comparison of continuous data, and χ2 test/Fisher’s exact test was used for comparison of categorical data. A p-value of less than 0.05 was considered statistically significant. All analyses were done with SAS, version 9.4 (SAS Institute Inc, Cary, North Carolina, USA).
Results
Study subjects
The study process is shown in Figure 1. Between July 26, 2019, and Aug 25, 2019, a total of 379 infants were screened, of whom 372 were enrolled and randomly allocated to three groups in a 1:1:1 ratio. In total, 369 (99.19%, 369/372) participants received the first dose and were included in the safety population. Also, 352 (94.62%, 352/372) received the second dose and 338 (90.86%, 338/372) were included in the per-protocol population for immunogenicity analysis. The demographic characteristics of the participants were similar in terms of ethnicity, age, height, and weight among the three groups (Table 1).
Figure 1.
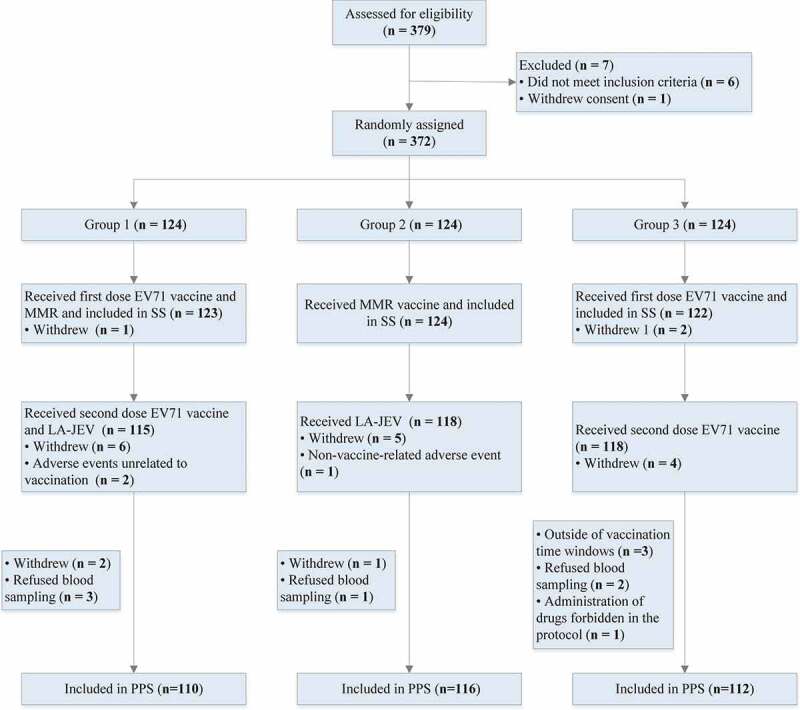
Trial profile.
Table 1.
Baseline characteristics of the study participants in the full analysis set
| Characteristic | Group 1 (n = 123) | Group 2 (n = 124) | Group 3 (n = 122) | P value |
|---|---|---|---|---|
| Male, no. (%) | 59 (47.97) | 66 (53.23) | 66 (54.10) | .582 |
| Ethnic Han, no. (%) | 123 (100.00) | 123 (99.19) | 121 (99.18) | .775 |
| Age, months | 8.30 ± 0.57 | 8.37 ± 0.72 | 8.39 ± 0.73 | .891 |
| Height, cm | 71.19 ± 2.68 | 70.38 ± 2.42 | 70.68 ± 2.67 | .095 |
| Weight, kg | 8.88 ± 0.99 | 8.82 ± 0.94 | 9.00 ± 1.06 | .408 |
Data are no. (%) of participants or mean ± SD. Study groups were as follows: simultaneous receipt of enterovirus 71 (EV71) vaccine (dose 1) and measles-mumps-rubella vaccine (MMR) on d 0 and EV71 vaccine (dose 2) and live-attenuated Japanese encephalitis vaccine (LA-JEV) on d 30 (Group 1); receipt of MMR and LA-JEV on d 0 and 30, respectively (Group 2); or receipt of doses 1 and 2 of EV71 vaccine on d 0 and 30, respectively (Group 3).
Immunogenicity
At baseline, the seropositivity and GMT of neutralizing antibody against EV71 showed no differences between Group 1 and Group 3. After vaccination, 109 (99.09%) of 110 infants in the Group 1 and 112 (100%) of 112 infants in the Group 3 developed neutralizing antibodies against EV71 (P = .495), yielding a non-inferior proportion of seroconversion of 97 · 27% in Group 1 (107 of 110 infants) and of 97 · 32% in Group 3 (109 of 112 infants; difference – 0.05% [95% CI – 5.38 to 5.21]). Similarly, the GMTs of antibody against EV71 were comparable between Group 1 and Group 3 (96.86 vs 101.25; P = .901) (Tables 2 and 3).
Table 2.
Seropositive and seroconversion rates of enterovirus 71 (EV71), measles, mumps, rubella, and Japanese encephalitis (JE) antibody pre and post vaccination, per-protocol sets
| Variable | Group 1 |
Group 2 or Group 3 |
P value | ||
|---|---|---|---|---|---|
| n (%) | 95% CI | n (%) | 95% CI | ||
| EV71 neutralizing antibody (N) | 110 | 112 | |||
| Seropositive pre-vaccination | 4 (3.64) | (3.52,6.89) | 6 (5.36) | (5.18,8.50) | .768 |
| Seropositive post-vaccination | 109 (99.09) | (95.04,99.98) | 112 (100) | (96.76,100.00) | .495 |
| Seroconversion | 107 (97.27) | (92.24,99.43) | 109 (97.32) | (92.37,99.44) | 1.000a |
| Measles IgG antibody (N) | 110 | 116 | |||
| Seropositive pre-vaccination | 0 (0.00) | (0.00,3.37) | 0 (0.00) | (0.00,3.21) | - |
| Seropositive post-vaccination | 108 (98.18) | (93.59,99.78) | 115 (99.14) | (95.29,99.98) | .963 |
| Seroconversion | 108 (98.18) | (93.59,99.78) | 115 (99.14) | (95.29,99.98) | .963 |
| Mumps IgG antibody (N) | 110 | 116 | |||
| Seropositive pre-vaccination | 2 (1.82) | (1.76,5.13) | 3 (2.59) | (2.51,5.71) | 1.000 |
| Seropositive post-vaccination | 107 (97.27) | (92.24,99.43) | 111 (95.69) | (90.23,98.59) | .777 |
| Seroconversion | 106 (96.36) | (90.95,99.00) | 109 (93.79) | (87.96,97.54) | .402 |
| Rubella IgG antibody (N) | 110 | 116 | |||
| Seropositive pre-vaccination | 0 (0.00) | (0.00,3.37) | 2 (1.72) | (1.66,4.87) | .498 |
| Seropositive post-vaccination | 105 (95.45) | (89.71,98.51) | 112 (96.55) | (91.41,99.05) | .935 |
| Seroconversion | 105 (95.45) | (89.71,98.51) | 110 (94.83) | (89.08,98.08) | .827 |
| JE neutralizing antibody (N) | 110 | 116 | |||
| Seropositive pre-vaccination | 6 (5.45) | (2.03,11.49) | 3(2.59) | (0.54,7.37) | - |
| Seropositive post-vaccination | 96 (87.27) | (79.57,92.86) | 103(88.79) | (81.60,93.90) | .725 |
| Seroconversion | 90 (81.82) | (73.33,88.53) | 100 (86.21) | (78.57,91.91) | .368 |
Study groups were as follows: simultaneous receipt of enterovirus 71 (EV71) vaccine (dose 1) and measles-mumps-rubella vaccine (MMR) on d 0 and EV71 vaccine (dose 2) and live-attenuated Japanese encephalitis vaccine (LA-JEV) on d 30 (Group 1); receipt of MMR and LA-JEV on d 0 and 30, respectively (Group 2); or receipt of doses 1 and 2 of EV71 vaccine on d 0 and 30, respectively (Group 3).
aNon-inferiority was achieved, as the lower bound of the two-sided 95% CI was > − 10%.
Table 3.
Geometric mean titer (GMT)/concentration (GMC) of enterovirus 71 (EV71), measles, mumps, rubella, and Japanese encephalitis (JE) antibody pre and post vaccination, per-protocol sets
| Group 1 |
Group 2 or Group 3 |
||
|---|---|---|---|
| Variable | GMT/GMC (95% CI) | GMT/GMC (95% CI) | P value |
| EV71 neutralizing antibody (N) | 110 | 112 | |
| Pre-vaccination GMT | 4.13 (4.00,4.27) | 4.18 (4.03,4.33) | .539 |
| Post-vaccination GMT | 96.86 (75.67,123.99) | 101.25 (78.22,131.06) | .901 |
| Measles IgG antibody (N) | 110 | 116 | |
| Pre-vaccination GMC | 35.49 (32.24,39.06) | 38.39 (35.44,41.57) | .087 |
| Post-vaccination GMC | 2982.53 (2545.55,3494.53) | 2797.91 (2371.76,3300.64) | .260 |
| Mumps IgG antibody (N) | 110 | 116 | |
| Pre-vaccination GMC | 30.85 (28.03,33.95) | 30.27 (27.40,33.43) | .703 |
| Post-vaccination GMC | 532.63 (457.18,620.53) | 424.89 (367.10,491.78) | .035 |
| Rubella IgG antibody (N) | 110 | 116 | |
| Pre-vaccination GMC | 2.62 (2.45,2.80) | 2.89 (2.65,3.15) | .146 |
| Post-vaccination GMC | 79.56 (68.58,92.30) | 75.70 (66.16,86.61) | .415 |
| Japanese encephalitis neutralizing antibody (N) | 110 | 116 | |
| Pre-vaccination GMT | 2.60 (2.52,2.68) | 2.55 (2.49,2.60) | .273 |
| Post-vaccination GMT | 7.12 (6.32,8.01) | 7.33 (6.55,8.20) | .688 |
Study groups were as follows: simultaneous receipt of enterovirus 71 (EV71) vaccine (dose 1) and measles-mumps-rubella vaccine (MMR) on d 0 and EV71 vaccine (dose 2) and live-attenuated Japanese encephalitis vaccine (LA-JEV) on d 30 (Group 1); receipt of MMR and LA-JEV on d 0 and 30, respectively (Group 2); or receipt of doses 1 and 2 of EV71 vaccine on d 0 and 30, respectively (Group 3). GMT: geometric mean titer; GMC: geometric mean concentration.
Before vaccination, seropositivity and GMC/GMT of antibodies against measles, mumps, rubella, and JE showed no differences between Group 1 and Group 2. After vaccination, the seropositivity rates for measles, rubella, and mumps and JE antibodies in Group 1 were 98.18%, 97.27%, 95.45%, and 97.27%, respectively; in Group 2 were 99.14%, 95.69%, 96.55%, and 88.79%, respectively. The seropositivity rates of measles, mumps, rubella, and JE antibodies were not significantly different between Group 1 and Group 2. Participants in Group 1 and those in Group 2 also showed no difference in the frequency of seroconversion against measles (98.18% vs 99.14%; P = .963), mumps (96.36% vs 93.79%; P = .402), rubella (95.45% vs 94.83%; P = .827), and JE (81.82% vs 86.21%; P = .512). Similarly, the GMCs of measles, mumps, and rubella IgG antibodies, and the GMT of JE neutralizing antibodies in Group 1 were generally similar to those in Group 2 (Tables 2 and 3).
Safety
The incidences of local and systemic adverse reactions (ARs) are presented in Table 4. All vaccination schedules were well tolerated in the three groups. Any ARs within 30 d after vaccination occurred in 77 (62.60%) of 123 participants in Group 1, 68 (54.84%) of 124 in Group 2 and 46 (37.70%) of 122 in Group 3. There was no statistically significant difference between Group 1 and Group 2 (P = .245), but it was higher than the Group 3 (P < .001). Most ARs were grade 1 and grade 2 in severity, and the incidence of grade 3 ARs were 4.88% (6/123) in Group 1, 5.65% (7/124) in Group 2 and 3.28% (4/122) in Group 3, respectively (Supplementary Table S1). In addition, the incidence of adverse reactions after the first dose was higher than that after the second dose (Supplementary Table S2).
Table 4.
Adverse reactions (ARs) reported following any vaccination
| Adverse reactions | Group 1 (N = 123) | Group 2 (N = 124) | Group 3 (N = 122) | Total (N = 369) |
P value |
||
|---|---|---|---|---|---|---|---|
| Three groups | Group 1 vs 2 | Group 1 vs 3 | |||||
| Solicited | 77 (62.60) | 68 (54.84) | 45 (36.89) | 190 (51.49) | <.001 | .245 | <.001 |
| Local | 6 (4.88) | 6 (4.84) | 7 (5.74) | 19 (5.15) | .915 | ||
| Redness | 5 (4.07) | 4 (3.23) | 7 (5.74) | 16 (4.34) | .560 | ||
| Induration | 1 (0.81) | 2 (1.61) | 4 (3.28) | 7 (1.90) | .320 | ||
| Pruritus | 3 (2.44) | 1 (0.81) | 0 (0.00) | 4 (1.08) | .230 | ||
| Swelling | 0 (0.00) | 0 (0.00) | 3 (2.46) | 3 (0.81) | .036 | 1.000 | .122 |
| Rash | 1 (0.81) | 1 (0.81) | 0 (0.00) | 2 (0.54) | 1.000 | ||
| Systemic | 75 (60.98) | 66 (53.23) | 43 (35.25) | 184 (49.86) | <.001 | .248 | <.001 |
| Fever | 68 (55.28) | 59 (47.58) | 32 (26.23) | 159 (43.09) | <.001 | .253 | <.001 |
| Diarrhea | 25 (20.33) | 18 (14.52) | 17 (13.93) | 60 (16.26) | .356 | ||
| Irritability | 10 (8.13) | 6 (4.84) | 7 (5.74) | 23 (6.23) | .570 | ||
| Decreased appetite | 9 (7.32) | 3 (2.42) | 3 (2.46) | 15 (4.07) | .108 | ||
| Nausea/vomiting | 6 (4.88) | 3 (2.42) | 3 (2.46) | 12 (3.25) | .576 | ||
| Allergy | 4 (3.25) | 4 (3.23) | 1 (0.82) | 9 (2.44) | .407 | ||
| Fatigue | 3 (2.44) | 1 (0.81) | 3 (2.46) | 7 (1.90) | .577 | ||
| Unsolicited | 1 (0.81) | 1 (0.81) | 2 (1.64) | 4 (1.08) | .700 | ||
| Cough | 1 (0.81) | 0 (0.00) | 2 (1.64) | 3 (0.81) | .219 | ||
| Abdominal distention | 0 (0.00) | 1 (0.81) | 0 (0.00) | 1 (0.27) | 1.000 | ||
| Overall | 77 (62.60) | 68 (54.84) | 46 (37.70) | 191 (51.76) | <.001 | .245 | <.001 |
Data are no. (%) of participants. Participants can have more than 1 reported AR. Study groups were as follows: simultaneous receipt of enterovirus 71 (EV71) vaccine (dose 1) and measles-mumps-rubella vaccine (MMR) on d 0 and EV71 vaccine (dose 2) and live-attenuated Japanese encephalitis vaccine (LA-JEV) on d 30 (Group 1); receipt of MMR and LA-JEV on d 0 and 30, respectively (Group 2); or receipt of doses 1 and 2 of EV71 vaccine on d 0 and 30, respectively (Group 3).
Most ARs were solicited, with only one participant (0.81%) in Group 1, one (0.81%) in Group 2, and two (1.64%) in Group 3 reporting unsolicited ARs (P = .700). Redness (4.34%, 16/369) was the most common local symptom. The most common systemic ARs were fever (43.09%, 159/369) and diarrhea (16.26%, 60/369). Except for a higher incidence of injection site swelling and fever in Group 1 and Group 2 than that in Group 3, there were no significant differences in the incidence of other solicited and unsolicited symptoms among the three groups (Table 4).
Throughout the trial, 32 serious adverse events were reported by a total of 18 participants: 4 (3.25%) of 123 in Group 1, 6 (4.84%) of 124 in Group 2, 8 (6.56%) of 122 in Group 3, without significant difference (P = .448). All serious adverse events were considered not related to vaccination.
Discussion
In this phase 4, open-label, single-center, randomized controlled study, we found that co-administration of EV71 vaccine with MMR and LA-JEV did not adversely impair immune responses to any of the vaccine antigens. The high rates of seroconversion and high antibody titer against EV71 found in the co-administration group and EV71 vaccination alone group provide reassurance that infants will be protected from EV71 infection, whether the EV71 vaccine is given alone or co-administered with MMR and LA-JEV. The seropositive post-vaccination and seroconversion rates of JE neutralizing antibody were relatively low when compared with other published results, which may be related to different laboratory detection methods. However, there were no significant differences observed in the seroconversion rate or GMT of JE neutralizing antibody in the co-administration group versus non-co-administration group, which indicated that the simultaneous administration did not affect the immune responses of JE antibody. Taken together, these data support the simultaneous administration of EV71 vaccine with those two EPI vaccines, which not only avoid the extra cost and inconvenience associated with an additional clinic visit, but also may increase the vaccine uptake.
The safety profile of co-administration group was higher than that of the EV71 vaccination alone group and was slightly higher than that in the MMR/LA-JEV vaccination alone group, but this trend was not considered a statistically significant difference. Both MMR and LA-JEV are EPI vaccines, which have been in use for decades in China and are considered safe. The vero cell–based inactivated alum-adjuvanted EV71 vaccine (Sinovac Biotech) also has been demonstrated to have significant safety and efficiency in protecting infants aged 6–35 months from EV71-associated HFMD in a series of trials,15,16,23 and was licensed for infants by the China Food and Drug Administration in 2015. In the present study, compared with the EV71 vaccination alone group, the other two groups reported a higher frequency of ARs, but most of them were mild or moderate intensity and resolved within a few days. This difference was mainly related to the higher incidence of fever after the first dose of vaccination in the co-administration group and MMR/LA-JEV vaccination alone group. The underlying mechanism may be caused by the measles antigen component in the MMR vaccine.24 The virus strain (191 strain) in measles vaccine still has a certain degree of toxicity.25 A small number of infants will have transient high fever after vaccination, but the prognosis is good, which is significantly different from measles infection.
Our results are consistent with a randomized study done in 2017,20 which showed simultaneous administration of EV71 vaccine with the recombinant hepatitis B vaccine and group A meningococcal polysaccharide vaccine did not affect EV71 neutralizing antibody seroconversion proportion compared with an EV71 vaccine only group. In addition, the investigators showed that the immunogenicity and safety of the recombinant hepatitis B vaccine and group A meningococcal polysaccharide vaccine in the co-administered group was not inferior to separate administration of each vaccine. Although those two EPI vaccines are different from this study, the consistency of the findings is reassuring.
This study has some limitations. First, a few enrolled participants withdrew or did not visit the clinic during the scheduled window, which led to the reduction of the sample size for analysis, although the final sample size was large enough to support the non-inferiority test. Another potential limitation of this trial is that it was conducted in a single center, and most of the participants were Han ethnicity. Although we cannot be sure of the differences in vaccine immune responses among different populations, we believe that further studies involving people of different ethnic backgrounds are needed. Third, since we chose only one laboratory method for testing, this may make it impossible to compare with other studies. Finally, a further limitation is the open-label design. This is unlikely to affect immunogenicity results because the laboratory personnel are blinded but could theoretically bias the safety assessment.
In conclusion, co-administration of EV71 vaccine with MMR and LA-JEV did not show significant interference in antibody responses and demonstrated good safety profiles. This provides convincing evidence that concomitant administration can be a reliable alternative and a good supplement to conventional planned vaccination program. Co-administration of these vaccines can reduce the number of needed immunization visits and economic costs, save parents and health workers’ time, and alleviate the pain of infants. Furthermore, simultaneous administration has the potential of increasing the coverage of vaccination and of protecting an increased number of infants from the morbidity and mortality associated with these diseases, so it can be considered for further promotion and application.
Supplementary Material
Acknowledgments
We would like to express our thanks to the staff of the Hanbin District (Ankang city) Center for Disease Control and Prevention for their efforts in the implementation of the study site.
Funding Statement
This work was supported by Sinovac Biotech Co. Ltd.
Disclosure statement
WY, YX, and LW are employees of Sinovac Biotech Co., Ltd. All other authors declare no conflict of interest.
Author contributions
All authors participated either in the design (SZ, LW, XL), implementation (XL, WY, CZ, HW, RW, QD, YH, YX) or analysis and interpretation of data (SZ, LW, XL, WY, CZ, QD) of the study, as well as in the development of this manuscript. All authors had full access to the data and granted their final approval of the manuscript before submission.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.2010428.
References
- 1.Ooi MH, Wong SC, Lewthwaite P, Cardosa MJ, Solomon T.. Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol. 2010;9(11):1097–105. doi: 10.1016/S1474-4422(10)70209-X. [DOI] [PubMed] [Google Scholar]
- 2.Chang PC, Chen SC, Chen KT.. The current status of the disease caused by enterovirus 71 infections: epidemiology, pathogenesis, molecular epidemiology, and vaccine development. Int J Environ Res Public Health. 2016;13(9). doi: 10.3390/ijerph13090890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmidt NJ, Lennette EH, Ho HH. An apparently new enterovirus isolated from patients with disease of the central nervous system. J Infect Dis. 1974;129(3):304–09. doi: 10.1093/infdis/129.3.304. [DOI] [PubMed] [Google Scholar]
- 4.Lum LC, Wong KT, Lam SK, Chua KB, Goh AY. Neurogenic pulmonary oedema and enterovirus 71 encephalomyelitis. Lancet. 1998;352(9137):1391. doi: 10.1016/s0140-6736(05)60789-1. [DOI] [PubMed] [Google Scholar]
- 5.Sun LM, Zheng HY, Zheng HZ, Guo X, He JF, Guan DW, Kang M, Liu Z, Ke CW, Li JS, et al. An enterovirus 71 epidemic in Guangdong Province of China, 2008: epidemiological, clinical, and virogenic manifestations. Jpn J Infect Dis. 2011;64(1):13–18. [PubMed] [Google Scholar]
- 6.Solomon T, Lewthwaite P, Perera D, Cardosa MJ, McMinn P, Ooi MH. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect Dis. 2010;10(11):778–90. doi: 10.1016/S1473-3099(10)70194-8. [DOI] [PubMed] [Google Scholar]
- 7.Esposito S, Principi N. Hand, foot and mouth disease: current knowledge on clinical manifestations, epidemiology, aetiology and prevention. Eur J Clin Microbiol Infect Dis. 2018;37(3):391–98. doi: 10.1007/s10096-018-3206-x. [DOI] [PubMed] [Google Scholar]
- 8.Koh WM, Bogich T, Siegel K, Jin J, Chong EY, Tan CY, Chen MI, Horby P, Cook AR. The epidemiology of hand, foot and mouth disease in Asia: a systematic review and analysis. Pediatr Infect Dis J. 2016;35(10):e285–e300. doi: 10.1097/INF.0000000000001242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J. Trend of epidemics and variation of pathogens of hand, foot and mouth disease in China: a dynamic series analysis, 2008-2017. Chin J Epidemiol. 2019;40(2):147–54. doi: 10.3760/cma.j.0254-6450.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Gu W, Zeng G, Hu YM, Hu YS, Zhang Y, Hu YL, Wang Y, Li JX, Zhu FC. A comparative analysis of immunogenicity and safety of an enterovirus 71 vaccine between children aged 3-5 years and infants aged 6-35 months. Expert Rev Vaccines. 2018;17(3):257–62. doi: 10.1080/14760584.2018.1430572. [DOI] [PubMed] [Google Scholar]
- 11.Wang XF, Lu J, Liu XX, Dai T. Epidemiological features of hand, foot and mouth disease outbreaks among Chinese preschool children: a meta-analysis. Iran J Public Health. 2018;47:1234–43. [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng YM, Jit M, Wu JT, Yang J, Leung K, Liao QH, Yu HJ. Economic costs and health-related quality of life for hand, foot and mouth disease (HFMD) patients in China. PLoS One. 2017;12(9):e0184266. doi: 10.1371/journal.pone.0184266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee MS, Tseng FC, Wang JR, Chi CY, Chong P, Su IJ. Challenges to licensure of enterovirus 71 vaccines. PLoS Negl Trop Dis. 2012;6(8):e1737. doi: 10.1371/journal.pntd.0001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yi EJ, Shin YJ, Kim JH, Kim TG, Chang SY. Enterovirus 71 infection and vaccines. Clin Exp Vaccine Res. 2017;6(1). doi: 10.7774/cevr.2017.6.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu FC, Xu WB, Xia JL, Liang ZL, Liu Y, Zhang XF, Tan XJ, Wang L, Mao QY, Wu YM, et al. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. N Engl J Med. 2014;370(9):818–28. doi: 10.1056/NEJMoa1304923. [DOI] [PubMed] [Google Scholar]
- 16.Li YP, Liang ZL, Xia JL, Wu JY, Wang L, Song LF, Mao QY, Wen SQ, Huang RG, Hu YS, et al. Immunogenicity, safety, and immune persistence of a novel inactivated human enterovirus 71 vaccine: a phase II, randomized, double-blind, placebo-controlled trial. J Infect Dis. 2014;209(1):46–55. doi: 10.1093/infdis/jit429. [DOI] [PubMed] [Google Scholar]
- 17.Li JX, Song YF, Wang L, Zhang XF, Hu YS, Hu YM, Xia JL, Li J, Zhu FC. Two-year efficacy and immunogenicity of Sinovac enterovirus 71 vaccine against hand, foot and mouth disease in children. Expert Rev Vaccines. 2016;15(1):129–37. doi: 10.1586/14760584.2016.1096782. [DOI] [PubMed] [Google Scholar]
- 18.Yang B, Wu P, Wu JT, Lau EHY, Leung GM, Yu H, Cowling BJ. Seroprevalence of enterovirus 71 antibody among children in China: a systematic review and meta-analysis. Pediatr Infect Dis J. 2015;34(12):1399–406. doi: 10.1097/INF.0000000000000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chinese Center for Disease Control and Prevention (CDC) . Technical guidelines for the use of inactivated enterovirus 71 vaccines. Beijing,China: Immunization planning center; 2016. June 6 [accessed 2021 Sept 20]. https://www.chinacdc.cn/zxdt/201606/t20160608_131032.html. [Google Scholar]
- 20.Zhang ZW, Liang ZL, Zeng J, Zhang JK, He P, Su JL, Zeng YM, Fan RF, Zhao D, Ma WJ, et al. Immunogenicity and safety of an inactivated enterovirus 71 vaccine administered simultaneously with hepatitis B vaccine and group a meningococcal polysaccharide vaccine: a phase 4, open-label, single-center, randomized, noninferiority trial. J Infect Dis. 2019;220(3):392–99. doi: 10.1093/infdis/jiz129. [DOI] [PubMed] [Google Scholar]
- 21.Yang XM. A review of combined immunization: current research situation and its promising future. Chin J Epidemiol. 2020;41(1):120–26. doi: 10.3760/cma.j.0254-6450.2020.01.022. [DOI] [PubMed] [Google Scholar]
- 22.Stucke K, Kieser M. A general approach for sample size calculation for the three-arm ‘gold standard’ non-inferiority design. Stat Med. 2012;31(28):3579–96. doi: 10.1002/sim.5461. [DOI] [PubMed] [Google Scholar]
- 23.Hu YM, Wang X, Wang JZ, Wang L, Zhang YJ, Chang L, Liang ZL, Xia JL, Dai QG, Hu YL, et al. Immunogenicity, safety, and lot consistency of a novel inactivated enterovirus 71 vaccine in Chinese children aged 6 to 59 months. Clin Vaccine Immunol. 2013;20(12):1805–11. doi: 10.1128/CVI.00491-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu Y, Lu YZ, Tao H, Wang B, Wang Y, Wang SQ, Qin J, Xu WQ. Safety and immunogenicity of domestic combined live attenuated measles, mumps and rubella vaccine. Chin J Biol. 2011;24(6):707–10. doi: 10.13200/j.cjb.2011.06.88.chuy.003. [DOI] [Google Scholar]
- 25.Xu HJ, Li W, Xia JH, Tao H, Fang HH, Li YJ, Chu Y, Ma FB, Xu WQ, Wang SQ. Adverse reaction and immunogenicity induced by domestic freeze-dried live attenuated measles-mumps-rubella combined vaccine. Chin J Biol. 2008;21(12):1111–14. doi: 10.13200/j.cjb.2008.12.84.xuhj.011. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


