ABSTRACT
Patients highly vulnerable for COVID-19 infection have been proposed to take priority for vaccination. However, vaccine hesitancy is usually more prevalent in these patients. Investigation around modifiable contributors of vaccine hesitancy plays a pivotal role in the formulation of coping strategies. We aimed to evaluate the impact of vaccine misconception in patients with lung cancer or pulmonary ground-glass opacity (GGO). A web-based questionnaire was constructed based on a qualitative interview with 15 patients and reviewed by a multidisciplinary expert panel. Six Likert five-scale questions were used to generate a score of vaccine misconception (SoVM), which ranged from 0 to 24 points, with a higher score indicating a higher level of misconception. A total of 61.6% (324/526) patients responded to our questionnaire. A higher proportion of low willingness patients (n = 173), compared to high willingness patients (n = 151), disagreed that cancer patients should be prioritized for COVID-19 vaccination (82.1% vs. 50.3%, p < .001) and perceived themselves to have contraindications (45.7% vs. 15.9%, p < .001). The mean SoVM was significantly lower in the high willingness group than the low willingness group (9.9 vs. 13.0, p < .001). Among the unvaccinated patients, the SoVM increased as the willingness to be vaccinated decreased (p < .0001). In multivariable logistic regression, patients with higher SoVM (OR 0.783, 95% CI 0.722–0.848), being female (OR 0.531, 95% CI 0.307–0.918) or diagnosed with lung cancer (OR 0.481, 95% CI 0.284–0.814) were independently associated with a lower willingness to be vaccinated against COVID-19. Receiver operating characteristic curve suggested that a SoVM of 11 yielded the best discrimination for predicting the willingness to receive COVID-19 vaccine (AUC = 0.724). The study findings reveal that patient misconception significantly contributes to vaccine hesitancy and needs to be addressed by evidence-based education tailored to their specific concerns.
KEYWORDS: COVID-19, vaccine, vaccine hesitancy, misconception, cancer, pulmonary ground-glass opacity, patient education, vaccination campaign
Introduction
Patients with malignancy were reported to be highly vulnerable to severe COVID-19-related complications, given their older age, immunocompromised state, and multiple comorbidities.1,2 In this context, academic consortiums have proposed them as the priority candidates for COVID-19 vaccination,3,4 although there is currently a scarcity of trial data in this specific population. Facing this uncertainty, vaccination guidelines or agencies may provide only vague instructions and leave the decision of vaccination to the patients themselves, including those with potential precancerous diseases in active follow-up. A strong association between low willingness to be vaccinated and concerns about the safety and efficacy of vaccines has been reported by multiple studies.5,6 Other predictors or contributors to vaccine hesitancy include age, gender, educational level, financial status, vaccine literacy, and perceived risk of infection, etc.6 Although vaccine hesitancy has been thoroughly researched in the general population, other underlying unclassifiable reasons are less clear in cancer patients who have demonstrated a higher hesitancy rate,7 especially in patients with tumors in the respiratory system. Compared to the general population, we noticed that many cancer or precancerous patients usually pay more attention to the potential impact of vaccines on their cancer or indeterminate tumor, than the general adverse effects of vaccination. Despite their popularity in this subpopulation, these concerns, such as exacerbation of tumors provoked by COVID-19 vaccines, are currently not supported by evidence. These potential misconceptions may accentuate the existing vaccine hesitancy in these patients, which can hamper the efforts to promote herd immunity to COVID-19, and should not be ignored when preparing for the third wave of COVID-19 attack.
The course of nationwide inoculation of COVID-19 vaccines is rapidly advancing in China, 8 with the rate of adverse events being as low as 11.86/100,000.9 Two recent publications on The Lancet Oncology have also confirmed the short-term safety of COVID-19 vaccine in cancer patients when compared to healthy controls.10,11As the COVID-19 vaccine is relatively safe, readily available and free of charge in China, other modifiable contributors to vaccine hesitancy in this specific population need to be identified and addressed accordingly to increase the rate of vaccine acceptance.
To the best of our knowledge, no previous study on vaccine hesitancy was conducted in patients with lung cancer or pulmonary ground-glass opacity (GGO). These diseases are highly prevalent globally and can serve as good representatives for patients with cancers or pre-cancerous diseases. Investigation and evaluation of their perspective and acceptance of COVID-19 vaccines are of great value in breaking down the obstacles of increasing vaccine coverage in this subpopulation. The current study aimed to identify modifiable contributors to COVID-19 vaccine hesitancy in patients with lung cancer or pulmonary GGO, to help formulate coping strategies to advance the vaccination campaign.
Materials and methods
Study design
This cross-sectional survey study enrolled a consecutive cohort of 526 patients with lung cancer or pulmonary GGO who visited the thoracic surgical clinic in Guangdong Provincial People’s Hospital from March to May 2021. An online questionnaire was constructed based on a qualitative interview with an initial cohort of 15 patients, and then reviewed and revised by an expert panel formed by surgeons, public health specialists, statistician, patient representatives and their caregivers. The link of questionnaire was sent to the patients on WeChat after obtaining their informed consent to participate in this study. Completion of the questionnaire did not result in any benefit or financial compensation for respondents. This study was approved by the Research Ethics Committee of Guangdong Provincial People’s Hospital (KY-Q-2021-087-01), and was conducted according to the guideline of the Declaration of Helsinki.
Construction of a score of vaccine misconception
Vaccine misconception is defined as the beliefs contradicted to or not supported by any high-level evidence and expert consensus at the current stage. Vaccine hesitancy due to misconception was commonly observed in thoracic clinics. To further clarify this phenomenon, researchers performed a semi-constructed qualitative interview with 15 lung cancer and GGO patients, and collected 12 most common concerning questions of different aspects from these patients. All questions were then reviewed by the expert panel, and six Likert five-scale questions were finally formulated to evaluate the level of misconception to COVID-19 vaccination (Figure 1(a)). These items fell into four different aspects, regarding their knowledge of the benefits of vaccination (Question 1), adverse outcome of vaccination (Questions 2&3), indications for vaccination (Questions 4&5), and necessity of vaccination campaign (Question 6). The answers to Likert scale questions were then used to construct a score of vaccine misconception (SoVM) for every patient. The SoVM ranged from 0 to 24, where “totally disagree,” “disagree,” “neither agree or disagree,” “agree,” “totally agree” were designated as 0 to 4 points, respectively. A higher SoVM indicated a higher level of misconception.
Figure 1.
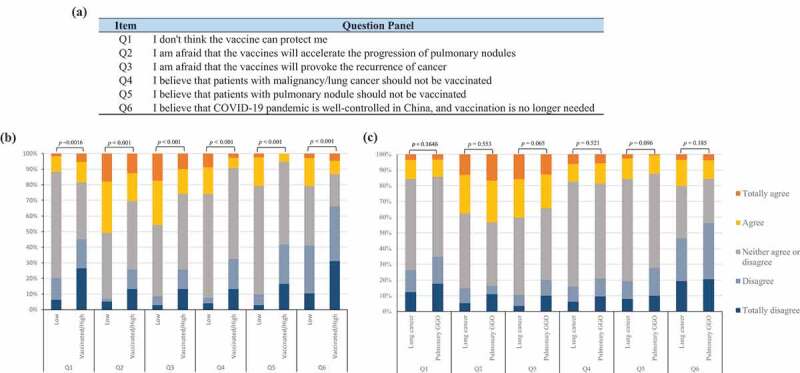
Perceptions to COVID-19 vaccination in patients with lung cancer or pulmonary group glass opacity. (a) Panel of six Likert five-scale questions; (b) subgroup analysis based on willingness; (c) subgroup analysis based on diagnoses. The Wilcoxon Rank Sum Test tested differences between the groups.
Statistical analysis
For the sake of comparison, patients were classified into two groups for comparison based on their diagnosis (lung cancer versus pulmonary GGO) or willingness for vaccination (high willingness versus low willingness). Demographic and clinical data except for age were summarized as frequency and percentage. A two-sided t-test was used to compare continuous variables. Chi-squared (χ2) test or Fisher's exact test was used to compare categorical variables when appropriate. Differences of answers to Likert scale questions and the SoVM between groups were compared by Wilcoxon or Kruskal–Wallis rank sum test. Internal consistency of SoVM was examined using Cronbach’s α. Principal component analysis was used to examine the internal-structural validity of the SoVM. All variables that were found to have a statistically significant association with levels of willingness were included in a multivariate logistic regression analysis using the “Enter” procedure. The predicting value of the SoVM was investigated using the receiver operating characteristic (ROC) curve and measured by the area under the curve (AUC). A two-sided p < .05 was considered to indicate a statistically significant difference. All analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA). Figures were generated using the Microsoft Excel 2016 and GraphPad Prism software (Version 8.0).
Sample size
As few studies investigated the vaccine misconception before, there was a lack of previous knowledge on the difference of the novel SoVM among patients with different levels of willingness. Therefore, a convenient sample size was used in the current study, with a post-hoc power calculated by two-sample t-test. Based on the current results (N1 = 173, N2 = 151, μ1 = 13.00, μ2 = 9.87, α = 0.05), a post-hoc power of >0.99 was yielded, which indicated an adequate power of our study to detect a difference between groups.
Results
Sociodemographic characteristics and willingness for COVID-19 vaccination
In total, 61.6% (324/526) patients responded to our online questionnaire. Baseline demographics and characteristics of patients are summarized in Table 1. Among them, 114 (35.2%) patients had lung cancers while the others had solitary or multiple pulmonary GGO. Lung cancer group had a significantly higher proportion of males than the GGO group (41.2% vs. 23.3%, p < .001). Otherwise, they were similar regarding age, education level and comorbidities (with all p > .05). Only 19.1% (62/324) of participants had been vaccinated against COVID-19, and they were classified as high willingness group along with the unvaccinated patients who stated their acceptance of (63/324) or intention to receive (26/324) the COVID-19 vaccine. Patients who stated their refusal (20/324) or hesitancy (153/324) for COVID-19 vaccine were categorized as low willingness group. Male patients or GGO patients were more likely to have a higher willingness for COVID-19 vaccines than female patients or lung cancer patients, respectively (with both p < .001). Participants in this survey were generally well educated, with more than 80% participants possessing a high school diploma or above. However, no statistically significant difference in education level was found in the group comparisons (p = .297 by diagnosis and p = .819 by willingness).
Table 1.
Characteristics of patients with lung cancer or pulmonary ground-glass opacity (GGO)
| Subgroup analysis by diagnosis (n, %) |
Subgroup analysis by willingness (n, %) |
|||||
|---|---|---|---|---|---|---|
| GGO | Lung cancer | P | High | Low | P | |
| Age (Mean±SD) | 49.6 ± 9.9 | 50.1 ± 9.7 | 0.706 | 50.4 ± 9.2 | 49.3 ± 10.4 | 0.295 |
| Gender | <0.001 | <0.001 | ||||
| Male | 49 (23.3) | 47 (41.2) | 62 (41.1) | 34 (19.7) | ||
| Female | 161 (76.7) | 67 (58.8) | 89 (58.9) | 139 (80.3) | ||
| Education level | 0.297 | 0.819 | ||||
| Middle school diploma or below | 24 (11.4) | 23 (20.2) | 20 (13.3) | 27 (15.6) | ||
| High school diploma or equivalent | 96 (45.7) | 44 (38.6) | 67 (44.4) | 73 (42.2) | ||
| College diploma and above | 90 (42.9) | 47 (41.2) | 64 (42.4) | 73 (42.2) | ||
| Comorbidities | ||||||
| Hx of allergy to drug/food | 14 (6.7) | 12 (10.5) | 0.222 | 14 (9.3) | 12 (6.9) | 0.4402 |
| Hx of allergy to vaccine/s | 0 | 0 | − | 0 | 0 | − |
| Hypertension | 35 (16.7) | 13 (11.4) | 0.203 | 20 (13.3) | 28 (16.2) | 0.457 |
| Diabetes mellitus | 7 (3.3) | 5 (4.4) | 0.759* | 9 (5.2) | 3 (2.0) | 0.126* |
| Coronary artery diseases | 3 (1.4) | 1 (0.9) | 1.000* | 2 (1.2) | 2 (1.3) | 1.000* |
| Chronic dermatosis | 10 (4.8) | 3 (2.6) | 0.555* | 6 (4.0) | 7 (4.1) | 0.974 |
| COPD | 0 | 0 | − | 0 | 0 | − |
| Chronic communicable diseases | 6 (2.9) | 9 (7.9) | 0.039 | 7 (4.6) | 8 (4.6) | 0.996 |
| Hematological malignancy | 0 | 0 | − | 0 | 0 | − |
| Immunodeficiency diseases | 1 (0.5) | 0 | 1.000* | 0 | 1 (0.6) | 1.000* |
| Other Hx of malignancy | 24 (11.4) | 9 (7.9) | 0.315 | 12 (8.0) | 21 (12.1) | 0.013 |
| Diagnosis | − | <0.001 | ||||
| GGO | 210 (100) | 0 | 113 (74.8) | 97 (56.1) | ||
| Lung cancer | 0 | 114 (100) | 38 (25.2) | 76 (43.9) | ||
| Willingness for vaccination | <0.001 | − | ||||
| Completely refuse | 6 (2.9) | 14 (12.3) | − | 20 (7.6) | ||
| Hesitant | 91 (43.3) | 62 (54.4) | − | 153 (58.4) | ||
| Acceptable | 40 (19.0) | 23 (20.2) | 63 (41.7) | − | ||
| Intend to receive | 18 (8.6) | 8 (7.0) | 26 (17.2) | − | ||
| Already vaccinated | 55 (26.2) | 7 (6.1) | 62 (41.1) | − | ||
*Fisher’s exact test. GGO, ground-glass opacity; COPD, chronic obstructive pulmonary disease; SD, standard deviation; Hx, history.
Willingness-related perspective to COVID-19 vaccination
The willingness and perspective to COVID-19 vaccination among patients with lung cancer or pulmonary GGO were investigated and summarized in Table 2. The high willingness group paid more attention to the vaccine itself, in terms of efficacy (33.1% vs. 16.2%, p < .001) and safety (52.3% vs. 40.5%, p = .033), than the low willingness group. More low willingness patients voted “No” to whether they agree that cancer patients should be prioritized for COVID-19 vaccination than vaccinated patients (82.1% vs. 50.3%, p < .001). It is worth noting that a significantly higher proportion of low willingness patients perceived themselves to be contraindicated for COVID-19 vaccination (45.7% vs. 15.9%, p < .001), but this misconception to vaccination guideline showed no relationship to the diagnoses of patients (Lung cancer group vs. GGO group, 31.0% vs. 33.3%, p = .660). Although doctors, vaccination guidelines, news, research data and prophylactic workers were all important information sources of COVID-19 vaccination for participants, the two most trustful sources for patients to assess their eligibility for COVID-19 vaccination were doctors and research data, rather than prophylactic workers or vaccination instructions.
Table 2.
COVID-19-vaccination-related questions for patients with lung cancer or pulmonary ground-glass opacity (GGO)
| Subgroup analysis by diagnosis (n, %) |
Subgroup analysis by willingness (n, %) |
|||||
|---|---|---|---|---|---|---|
| GGO | Lung cancer | P | High | Low | P | |
| Which of the following are the major concerns that impact your willingness to receive COVID-19 vaccination? (multiple response question) | ||||||
| I believe that I am contraindicated for COVID-19 vaccination | 65 (31.0) | 38 (33.3) | 0.660 | 24 (15.9) | 79(45.7) | <0.001 |
| I was told by prophylactic worker that I am not eligible for COVID-19 vaccination | 22 (10.5) | 17 (14.9) | 0.241 | 21 (13.9) | 18(10.4) | 0.3338 |
| Efficacy of vaccines | 56 (26.7) | 22 (19.3) | 0.139 | 50 (33.1) | 28(16.2) | <0.001 |
| Safety of vaccines | 107 (51.0) | 42 (36.8) | 0.015 | 79 (52.3) | 70(40.5) | 0.033 |
| Accessibility of vaccines | 37 (17.6) | 8 (7.0) | 0.008 | 26 (17.2) | 19(11.0) | 0.105 |
| Do you agree that cancer patients should be prioritized for COVID-19 vaccination? | 0.503 | <0.001 | ||||
| Yes | 66 (31.4) | 40 (35.1) | 75 (49.7) | 31 (17.9) | ||
| No | 144 (68.6) | 74 (64.9) | 76 (50.3) | 142 (82.1) | ||
| Which of the following are your information sources of COVID-19 vaccination? (multiple response question) | ||||||
| Prophylactic worker | 53 (25.2) | 27 (23.7) | 0.757 | 50 (33.1) | 30 (17.3) | 0.001 |
| Doctor | 85 (40.5) | 54 (47.4) | 0.231 | 62 (41.1) | 77 (44.5) | 0.532 |
| Vaccination guidelines/instructions | 70 (33.3) | 49 (43.0) | 0.085 | 61 (40.4) | 58 (33.5) | 0.201 |
| Research data | 45 (21.4) | 29 (25.4) | 0.412 | 35 (23.2) | 39 (22.5) | 0.892 |
| News | 97 (46.2) | 62 (54.4) | 0.159 | 67 (44.4) | 92 (53.2) | 0.114 |
| Friends/families | 43 (20.5) | 19 (16.7) | 0.405 | 32 (21.2) | 30 (17.3) | 0.379 |
| Others | 3 (1.4) | 3 (2.6) | 0.443 | 2 (1.3) | 4 (2.3) | 0.689* |
| Which of the following are the most trustful information source for assessing your eligibility for COVID-19 vaccines? | ||||||
| Prophylactic worker | 23 (11.0) | 10 (8.8) | 0.536 | 22 (14.6) | 11 (6.4) | 0.015 |
| Doctor | 77 (36.7) | 49 (43.0) | 0.265 | 55 (36.4) | 71 (41.0) | 0.395 |
| Vaccination guidelines/instructions | 36 (17.1) | 8 (7.0) | 0.011 | 26 (17.2) | 18 (10.4) | 0.074 |
| Research data | 57 (27.1) | 39 (34.2) | 0.183 | 37 (24.5) | 59 (34.1) | 0.059 |
| News | 10 (4.8) | 3 (2.6) | 0.351 | 6 (4.0) | 7 (4.1) | 0.974 |
| Friends/families | 7 (3.3) | 3 (2.6) | 0.727 | 5 (3.3) | 5 (2.9) | 1.000* |
| Others | 0 | 2 (1.8) | 0.123* | 0 | 2 (1.2) | 0.501* |
| SoVM Score (Mean±SD) | 11.3 ± 4.0 | 12.0 ± 3.7 | 0.09 | 9.9 ± 4.2 | 13.0 ± 3.0 | <0.001 |
*Fisher’s exact test. GGO, ground-glass opacity.
Score of vaccine misconception
We further examined the patients’ perception of COVID-19 vaccination using six Likert five-scale questions. Interestingly, a significantly different picture was found between the high willingness and low willingness groups, while the lung cancer group and pulmonary GGO group showed no obvious discrepancies (Figure 1(b,c)). More patients in the low willingness group feared the potential harmful outcome of vaccines to their cancer or indeterminate tumor, such as provoking cancer recurrence or progression of GGO (both p < .001). Insufficient knowledge of the scientific consensus that patients with cancer or indeterminate tumor are eligible for COVID-19 vaccination was observed in the majority of participants. Despite this, the high willingness group still demonstrated a better understanding of the vaccination indication (p < .001). Besides, the low willingness group presented a stronger suspicion of the vaccine efficacy (Question 1, p = .0016) and a higher proportion of incorrect cognition regarding anti-epidemic strategies (Question 6, p < .001).
All answers mentioned above were assigned with a predefined score and summed up to generate the SoVM. Given the SoVM did not satisfy the assumption of normal distribution (Shapiro–Wilk test p < .001), it was presented as median (Md) with 25% percentile to 75% percentile (IQR) in the analyses and interpretation below. As shown in Figure 2, patients with pulmonary GGO had a similar distribution of SoVM to patients with lung cancers (Figure 2(a), p = .226), with a median SoVM of 12 and IQRs ranged from 9 to 14 and 10 to 15, respectively. However, patients who had been vaccinated (Md = 10, IQR 5–12) demonstrated a significant lower SoVM than the unvaccinated patients (Md = 12, IQR 11–15) (Figure 2(b), p < .0001). Among the unvaccinated patients, the median SoVMs gradually increased from 9 (IQR 8–12) to 16 (IQR 12.5–18) as the willingness to be vaccinated decreased (Figure 2(c), p < .0001). Taken as a whole, the high willingness patients had a significantly lower mean SoVM than the low willingness patients (9.9 vs. 13.0, p < .001, Table 2). In multivariable logistic regression analysis (Table 3), female patients (OR 0.531, 95% CI, 0.307–0.918), patients with higher SoVM (OR 0.783, 95%CI, 0.722–0.848), and lung cancer patients (OR 0.481, 95%CI, 0.284–0.814) were independently associated with lower willingness to be vaccinated against COVID-19. ROC analysis suggested a SoVM of 11 yields the best discrimination for prediction of the willingness to receive the vaccine (Sensitivity = 57.0%, Specificity = 76.3%, AUC = 0.724) (Figure 3).
Figure 2.
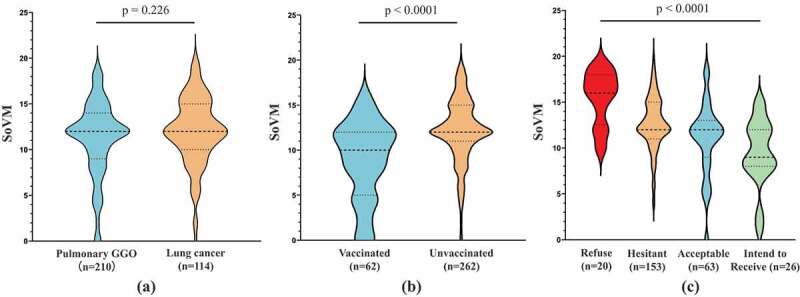
Comparisons of score of vaccine misconception (SoVM) in patients with different characteristics. (a) Difference of SoVM between pulmonary GGO and lung cancer groups; (b) difference of SoVM between vaccinated and unvaccinated groups; (c) difference of SoVM among unvaccinated patients with different level of willingness to receive COVID-19 vaccines. Dotted lines in violin plots indicates the median and quartiles. Differences between the groups were compared by the Wilcoxon or Kruskal-Wallis rank sum test.
Table 3.
Multivariable logistic regression analyses of influencing factors of vaccination willingness
| Variables | Coefficient | Standard error | χ2 | Adjusted odd ratio | 95% Confidence interval | P-value | |
|---|---|---|---|---|---|---|---|
| Intercept | 3.4057 | 0.5318 | 41.0108 | <0.0001 | |||
| Gender | −0.6333 | 0.2796 | 5.13 | 0.531 | 0.307 | 0.918 | 0.0235 |
| Diagnosis | −0.7319 | 0.2682 | 7.446 | 0.481 | 0.284 | 0.814 | 0.0064 |
| SoVM | −0.245 | 0.041 | 35.7538 | 0.783 | 0.722 | 0.848 | <0.0001 |
Vaccination willingness is defined as a dichotomous dependent variable (high or low willingness) in regression analysis. SoVM, score of vaccine misconception.
Figure 3.
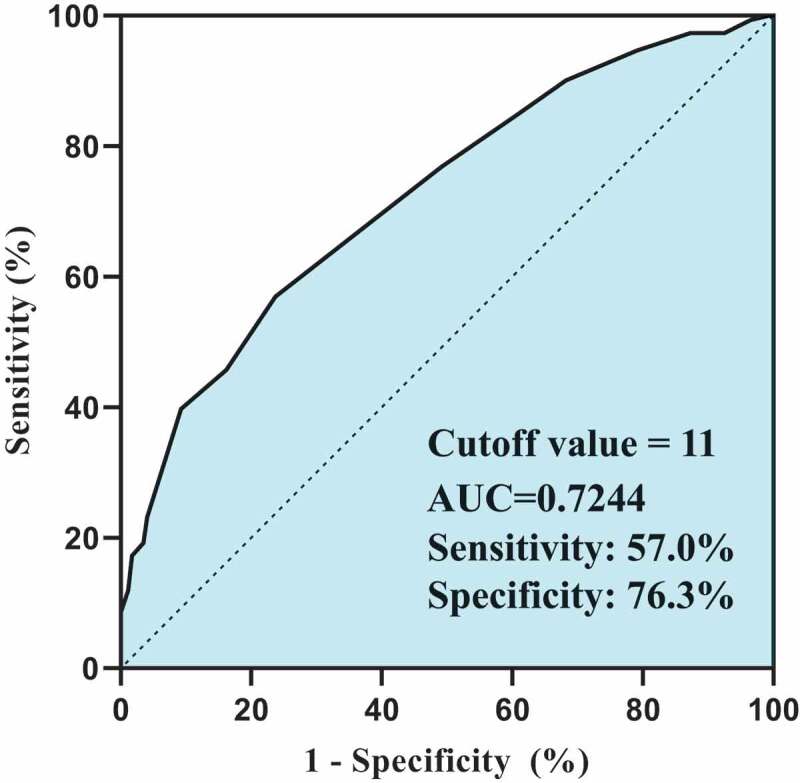
Receiver operating characteristic curve analysis of SoVM. Performance of SoVM to discriminate different levels of willingness to receive the vaccine.
Internal consistency and factor structure of SoVM
Cronbach’s α for the SoVM was 0.75, indicating an acceptable reliability for this exploratory study. A two-factor oblique solution yielded a clear structure with a clinically sound assignment of the items to two components, explaining 67.0% of the variance. Component 1 can be summarized as the disease-related misconception, including the SoVM items “adverse outcome of vaccination” and “indications of vaccination” (Questions 2 to 5), which explains 46.3% of the variance in overall vaccine misconception. Component 2 can be concluded as the general misconception, including the SoVM items “knowledge to the benefit of vaccination” and “necessity of vaccination campaign” (Question 1 and 6), which explains 20.7% of the variance in overall vaccine misconception.
Discussion
Vaccine hesitancy, which refers to delay in acceptance or refusal of vaccination despite availability of vaccination services,12 remains one of the primary obstacles to bring the COVID-19 pandemic to an end. The contributors to vaccine hesitancy usually varied from population to population, which need to be addressed in a way that is tailored to their sociodemographic characteristics and specific concerns.13–16 Knowledge of these contributors through data analysis would help to guide the development of such strategies to advance the vaccination campaign. However, contributors reported in previous studies, such as age, gender, educational level, and financial status, were mainly non-modifiable elements.6 Identification of modifiable contributors would be more useful for alleviating vaccine hesitancy. The current study explored the potential association between vaccine hesitancy and misconception of COVID-19 vaccine in patients with lung cancer or pulmonary GGO. Results presented in this study may serve as a reference for mitigating vaccine hesitancy in patients with cancer or pre-cancerous disease.
In our study, both the lung cancer and pulmonary GGO groups demonstrated a considerable high rate of vaccine hesitancy (66.7% vs. 46.2%), which are significantly higher than the results reported in other comparable studies.17,18 This discrepancy might derive from the vague inoculation instruction for cancer patients, insufficient education for this specific subpopulation and the fact that COVID-19 pandemic was well controlled in China. These factors played important roles in generating vaccination misconceptions, which ultimately exacerbated the existing vaccine hesitancy. In the current study, comparative analyses suggested that the lung cancer group and pulmonary GGO group had a good homogeneity in both dimensions of sociodemographic and vaccination-related perspectives (Table 1, Table 2 and Figure 1(c)). Therefore, these two groups of patients were taken as a whole in the analysis of vaccine misconception and willingness of inoculation. A novel but simple score to measure potential misconceptions, abbreviated as SoVM and covers four different aspects of beliefs, was established and validated by the current study in patients with lung cancer or pulmonary GGO. SoVM was found to be one of the independent influencing factors for willingness by multivariable logistic regression (OR 0.783, 95% CI, 0.722–0.848, p < .0001), adjusted by gender and diagnosis (Table 3). A higher SoVM predicted lower willingness to get COVID-19 vaccines in lung cancer or GGO patients. As is shown in Figure 3, ROC analysis suggested a moderate accuracy of SoVM to discriminate patients with different willingness to receive the vaccine (AUC = 0.724). However, the relatively low sensitivity (57.0%) and the acceptable specificity (76.3%) indicated the necessity to incorporate additional predictors.
Although unexplained interactions between vaccination and cancer, either positive or negative, have been reported in a few studies,19,20 no high-level evidence with considerable sample size can be found to draw a convincing conclusion. Therefore, the belief that COVID-19 vaccine could accelerate the progression of pulmonary GGO or provoke lung cancer recurrence can be defined as a misconception in a certain sense. As suggested by the SoVM (Figure 2), unvaccinated patients have a significantly higher level of misconception than the vaccinated patients (SoVMMd = 12 vs. 10, p < .0001). Given that vaccine inoculation may be delayed in some unvaccinated patients due to non-personal reasons, the willingness to receive vaccines may vary among the unvaccinated patients and warrants further investigation. In this study, the unvaccinated patients who stated their refusal (SoVMMd = 16, IQR 13.5–18) or hesitancy (SoVMMd = 12, IQR 11–15) for COVID-19 vaccines also showed a significantly higher level of misconception than those with a higher willingness (p < .0001). A previous study also revealed similar misconceptions to COVID-19 vaccines that exacerbated the vaccine hesitancy in patients with celiac disease. It was reported that 27.2% of hesitant patients declared that their underlying diseases influenced their willingness for COVID-19 vaccination, and one out of every five patients felt themselves to be more vulnerable to adverse events because of celiac disease.13 Therefore, patient education to address vaccination-related concerns should be evidence-based and tailored to the specific population. Among general communities, lower education level was found to be associated with lower willingness to receive COVID-19 vaccine in many previous studies. 6,13 In contrast, we found no significant impact by education level in our study. This discrepancy could be partially explained by the dominant impact from the underlying diseases, as the patients usually have similar knowledges to these diseases regardless of their education levels. Although the short-term safety of COVID-19 vaccines in cancer patients had been confirmed in several studies,10,11 misunderstanding can still exist for a long time due to lag phase of interpretation and transmission of first-hand data to the public. Obviously, no data is available at the current stage to answer the questions about long-term effects of COVID-19 vaccine on lung cancer or pulmonary GGO. We, therefore, launched a corresponding prospective cohort study (CoVac-Lung), which is registered with ClinicalTrials.gov (NCT04894682) and is under active recruitment. We also call for more prospective studies to continuously address these unanswered research questions with reliable scientific evidence 21 for the efforts to finally eradicate SARS-CoV-2.
The results of this study were bolstered by its methodological strengths, including the involvement of a qualitative interview and an expert panel in the construction of our questionnaire. This procedure strengthened the validity and reliability of our survey. We also established a novel score that can detect misconceptions to COVID-19 vaccination with good sensitivity. Future studies can be directed to examine whether tailored patient education can lower the SoVM and increase their willingness to receive COVID-19 vaccines. Nonetheless, our study may be limited by its relatively small sample size, as the morbidity of lung cancer and GGO is very high in China. The web-based nature of the survey may also result in selection bias. Older patients who are less familiar with the internet may be less likely to complete the questionnaire, which may have resulted in a relatively low response rate of our survey and limited the generalizability of study results in the elderly. Besides, given the context of provincial tertiary hospital, more than 80% of enrolled patients possessing a high school diploma or above in our study, which may limit the power to distinguish the influence of education levels. Additionally, local government’s policy and social atmosphere can influence the willingness to vaccine inoculation. This might also limit the generalizability of the results in other regions due to the involvement of a single institution.
Conclusion
To conclude, our study results suggest that vaccine hesitancy may be exacerbated by misconception or specific concerns to underlying diseases in patients with lung cancer or pulmonary GGO. Evidence-based patient education is an indispensable element that is urgently required in order to mitigate vaccine hesitancy and to advance the vaccination campaign, especially in the countries where vaccine supplies are no longer a speed-limiting factor.
Supplementary Material
Acknowledgments
The authors thank all the patients and their caregivers for participating in our qualitative interview, expert panel review and the survey study.
Funding Statement
This study was funded by the 2020 Guangdong Provincial Special Project for Popularization of Science and Technology Innovation [grant number: 2020A1414070007].
Author Contributions
Concept and design: W.Z. and G.Q.; Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: W.Z., J.Z., Z.L., P.W., and C.Z.; Critical revision of the manuscript for important intellectual content: G.Q., W.Z., Q.S. and R.C.; Obtained funding: G.Q.; Supervision: Q.S., and G.Q.
Institutional review board statement
This study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Guangdong Provincial People’s Hospital (approval number: KY-Q-2021-087-01).
Informed consent statement
Online informed consents were obtained from all subjects involved in the study.
Disclosure statement
All authors declare no conflict of interest.
Data availability statement
All anonymous data are available from corresponding author (guibinqiao@126.com) on reasonable request.
Supplementary Material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1992212.
References
- 1.Kuderer NM, Choueiri TK, Shah DP, Shyr Y, Rubinstein SM, Rivera DR, Shete S, Hsu CY, Desai A, De Lima Lopes G Jr., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907–18. doi: 10.1016/S0140-6736(20)31187-9.32473681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee LY, Cazier JB, Angelis V, Arnold R, Bisht V, Campton NA, Chackathayil J, Cheng VW, Curley HM, Fittall MW, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919–26. doi: 10.1016/S0140-6736(20)31173-9.32473682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corti C, Crimini E, Tarantino P, Pravettoni G, Eggermont AMM, Delaloge S, Curigliano G.. SARS-CoV-2 vaccines for cancer patients: a call to action. Eur J Cancer. 2021;148:316–27. doi: 10.1016/j.ejca.2021.01.046.33770576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giesen N, Sprute R, Ruthrich M, Khodamoradi Y, Mellinghoff SC, Beutel G, Lueck C, Koldehoff M, Hentrich M, Sandherr M, et al. 2021 update of the AGIHO guideline on evidence-based management of COVID-19 in patients with cancer regarding diagnostics, viral shedding, vaccination and therapy. Eur J Cancer. 2021;147:154–60. doi: 10.1016/j.ejca.2021.01.033.33676266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson HS, Manning M, Mitchell J, Kim S, Harper FWK, Cresswell S, Johns K, Pal S, Dowe B, Tariq M, et al. Factors associated with racial/ethnic group-based medical mistrust and perspectives on COVID-19 vaccine trial participation and vaccine uptake in the US. JAMA Netw Open. 2021;4(5):e2111629. doi: 10.1001/jamanetworkopen.2021.11629.34042990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reno C, Maietti E, Fantini MP, Savoia E, Manzoli L, Montalti M, Gori D. Enhancing COVID-19 vaccines acceptance: results from a survey on vaccine hesitancy in Northern Italy. Vaccines (Basel). 2021;9(4). doi: 10.3390/vaccines9040378.33924534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barriere J, Gal J, Hoch B, Cassuto O, Leysalle A, Chamorey E, Borchiellini D. Acceptance of SARS-CoV-2 vaccination among French patients with cancer: a cross-sectional survey. Ann Oncol. 2021;32(5):673–74. doi: 10.1016/j.annonc.2021.01.066.33529740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen S-T A total of 84.529 million doses of COVID-19 vaccines have been inoculated nationwide. People’s Daily. 2021. accessed 2021 Jun 13. http://www.gov.cn/xinwen/2021-06/12/content_5617312.htm.
- 9.Interpretation of Adverse Reaction Surveillance Information of National COVID-19 Vaccination . Chinese center for disease control and prevention 2021. accessed 2021 Jun 13. http://www.chi-nacdc.cn/jkzt/ymyjz/ymyjjz_6758/202105/t20210528_230908.html
- 10.Monin L, Laing AG, Munoz-Ruiz M, McKenzie DR, Del Molino Del Barrio I, Alaguthurai T, Domingo-Vila C, Hayday TS, Graham C, Seow J, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765–78. doi: 10.1016/S1470-2045(21)00213-8.33930323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waissengrin B, Agbarya A, Safadi E, Padova H, Wolf I. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol. 2021;22(5):581–83. doi: 10.1016/S1470-2045(21)00155-8.33812495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacDonald NE; Hesitancy SWGoV . Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33(34):4161–64. doi: 10.1016/j.vaccine.2015.04.036.25896383. [DOI] [PubMed] [Google Scholar]
- 13.Costantino A, Topa M, Roncoroni L, Doneda L, Lombardo V, Stocco D, Gramegna A, Costantino C, Vecchi M, Elli L. COVID-19 vaccine: a survey of hesitancy in patients with celiac disease. Vaccines (Basel). 2021;9(5). doi: 10.3390/vaccines9050511.34065654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dzieciolowska S, Hamel D, Gadio S, Dionne M, Gagnon D, Robitaille L, Cook E, Caron I, Talib A, Parkes L, et al. Covid-19 vaccine acceptance, hesitancy, and refusal among Canadian healthcare workers: a multicenter survey. Am J Infect Control. 2021;49(9):1152–57. doi: 10.1016/j.ajic.2021.04.079.33930516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puteikis K, Mameniskiene R. Factors associated with COVID-19 vaccine hesitancy among people with epilepsy in Lithuania. Int J Environ Res Public Health. 2021;18(8):4374. doi: 10.3390/ijerph18084374.33924140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yurttas B, Poyraz BC, Sut N, Ozdede A, Oztas M, Ugurlu S, Tabak F, Hamuryudan V, Seyahi E. Willingness to get the COVID-19 vaccine among patients with rheumatic diseases, healthcare workers and general population in Turkey: a web-based survey. Rheumatol Int. 2021;41(6):1105–14. doi: 10.1007/s00296-021-04841-3.33779780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelkar AH, Blake JA, Cherabuddi K, Cornett H, McKee BL, Cogle CR. Vaccine enthusiasm and hesitancy in cancer patients and the impact of a webinar. Healthcare (Basel). 2021;9(3). doi: 10.3390/healthcare9030351.33808758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villarreal-Garza C, Vaca-Cartagena BF, Becerril-Gaitan A, Ferrigno AS, Mesa-Chavez F, Platas A, Platas A. Attitudes and factors associated with COVID-19 vaccine hesitancy among patients with breast cancer. JAMA Oncol. 2021;7(8):1242. doi: 10.1001/jamaoncol.2021.1962.34110371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angrini M, Varthaman A, Garcia-Verdugo I, Sallenave JM, Alifano M, Cremer I. To vaccinate or not: influenza virus and lung cancer progression. Trends Cancer. 2021;7(7):573–76. doi: 10.1016/j.trecan.2021.02.006.33712391. [DOI] [PubMed] [Google Scholar]
- 20.Chen KY, Wu SM, Liu JC, Lee KY. Effect of annual influenza vaccination on reducing lung cancer in patients with chronic obstructive pulmonary disease from a population-based cohort study. Medicine (Baltimore). 2019;98(47):e18035. doi: 10.1097/MD.0000000000018035.31764822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korompoki E, Gavriatopoulou M, Kontoyiannis DP. COVID-19 vaccines in patients with Cancer – a welcome addition, but there is need for optimization. JAMA Oncol. 2021;7(8):1113. doi: 10.1001/jamaoncol.2021.1218.3398337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All anonymous data are available from corresponding author (guibinqiao@126.com) on reasonable request.


