ABSTRACT
Epidemiologic data regarding the risk of Guillain-Barré syndrome (GBS) following herpes zoster (HZ) are limited. We conducted a self-controlled case series analysis using two large national data sources to evaluate the risk of GBS following HZ among U.S. adults. We analyzed medical claims from the IBM® MarketScan® Commercial Claims and Encounters (persons 18–64 years during 2010–2018) and Centers for Medicare and Medicaid Services Medicare (persons ≥65 years during 2014–2018) databases. HZ cases were defined as persons with an outpatient claim with a primary or secondary ICD-9 or ICD-10 diagnostic code for HZ. GBS cases were defined as persons with an inpatient claim with a principle diagnostic code for GBS and an associated procedural code. We compared the rates of GBS following HZ in the 1–42-day risk window versus primary (100–365-day) or secondary (43–99-day) control windows. We identified 489,516 persons 18–64 years of age and 650,229 persons ≥65 years of age with HZ, among whom 11 and 41, respectively, developed GBS 1–365 days following HZ. The risk of GBS following HZ was increased during the risk window as compared to the primary control window for both groups, with a rate ratio of 6.3 (95% CI, 1.8–21.9) for those 18–64 years and 4.1 (95% CI, 1.9–8.7) for those ≥65 years. This study provides new and methodologically rigorous epidemiologic support for an association between HZ and GBS, and useful context regarding the benefits versus potential risks of zoster vaccination.
KEYWORDS: Zoster, shingles, Guillain-Barré syndrome, recombinant zoster vaccine, Shingrix, MarketScan, Medicare
Introduction
Herpes zoster (HZ), or shingles, is a localized, usually painful, cutaneous eruption that results from reactivation of latent varicella-zoster virus (VZV). HZ affected approximately one million people in the United States each year in the pre-vaccine era, and it was estimated that one in three Americans will be affected in their lifetimes.1,2 The incidence, severity of disease, and potential complications of HZ (including the most common complication of postherpetic neuralgia, or PHN) increase with age.1–3 Individuals who are immunocompromised or immunosuppressed are also at increased risk of HZ and related complications.2,4,5
In the United States, two vaccines are licensed by the U.S. Food and Drug Administration (FDA) and recommended by the Advisory Committee on Immunization Practices (ACIP) for the prevention of HZ and related complications. Zoster vaccine live (ZVL, Zostavax®) has been recommended for use in immunocompetent adults ≥60 years of age since 2006.2,6,7 In October 2017, FDA licensed, and ACIP preferentially recommended, recombinant zoster vaccine (RZV, Shingrix®) for use in immunocompetent adults ≥50 years of age.8 In July 2021, FDA expanded the indication for use of RZV, and in October 2021, ACIP recommended RZV for the prevention of HZ and related complications in adults aged ≥19 years who are or will be immunodeficient or immunosuppressed due to disease or therapy.9,10 Zostavax is no longer available for use in the United States, as of November 18, 2020.11
RZV post-licensure safety monitoring has been conducted by the Centers for Disease Control and Prevention (CDC), FDA, and collaborators through the Vaccine Adverse Events Reporting System (VAERS) and the Vaccine Safety Datalink (VSD). Self-limited systemic signs and symptoms and local reactions were commonly reported in VAERS; however, serious reported adverse events have been rare.12,13 A statistical signal for Guillain-Barré syndrome (GBS) was detected in VSD rapid cycle analysis (RCA) in January 2019; however, results of the final chart-confirmed analysis indicated that there was insufficient evidence to determine if there is an increased risk of GBS after RZV.14 FDA, CDC, and collaborators conducted additional safety assessment studies using Centers for Medicare and Medicaid Services (CMS) Medicare data to further explore the potential risk of GBS after RZV administration. An initial cohort analysis comparing post-vaccination GBS rates between RZV recipients and historical ZVL controls among persons 65 years or older identified an elevated rate ratio following RZV administration,15 and results from follow-up self-controlled case series analyses confirmed an increased risk of GBS during the 42 days after RZV administration.16 To date, two case reports of GBS with onset within 10 days after RZV administration have been documented in the literature.17,18
GBS is a rare immune-mediated disease of the peripheral nerves.19,20 In the United States, the estimated annual incidence of GBS across all ages is 1–2/100,000 persons.20–24 Risk factors for GBS include increasing age, male gender, immunocompromised status, recent viral and bacterial infections [strongest evidence for herpesviruses such as cytomegalovirus (CMV) and Epstein-Barr virus (EBV), as well as influenza virus, hepatitis E virus, Zika virus, Campylobacter jejuni, and Mycoplasma pneumoniae], and some vaccinations (e.g., some influenza and rabies vaccines).19,25–28 A possible temporal association between HZ and GBS has been documented in a small number of case reports.29–32 One population-based cohort study using Taiwan’s National Health Insurance Research Database found 0.03% of patients developed GBS in the two months following HZ and that the adjusted hazard of GBS during the follow-up period was over 18 times greater for patients with HZ than those without HZ.33 To date, there have been no epidemiologic studies of an association between HZ and GBS in other settings (e.g., the United States) or using other methods.
In this study, we evaluate the risk of GBS following HZ among adults using a self-controlled case series analysis of healthcare claims data from two large national data sources in the United States. This study aims to strengthen the epidemiologic understanding of the risk of GBS following HZ, which may help clarify the benefits versus potential risks of zoster vaccination.
Methods
Study design
We used a self-controlled risk interval study design, a subset of the self-controlled case series method, to assess for increases in GBS risk following HZ. These methods compare risks during prespecified risk and control windows within individuals and eliminate effects of time-invariant confounders.34–39 We made the assumption that time-varying variables would have minimal effect during the risk and control windows. We compared the risk of GBS during a 42-day risk window (days 1–42 after HZ, a similar risk window used in vaccine safety studies examining risk of GBS) versus a 266-day primary control window (100–365 days after HZ), assessing risk as incidence (i.e., number of episodes). We also evaluated a 57-day secondary control window (43–99 days after HZ) (Figure 1). To better assure that any signal suggesting association between HZ and GBS was not due to artifact, we looked for associations between HZ and four unrelated control outcomes for which we expected no signals, i.e., negative controls. We also evaluated GBS as a proportion of all inpatient admissions and ICU admissions, in effect, using all possible conditions as a single, robust negative control. Only cases who experienced both HZ and GBS were included in the analysis.
Figure 1.
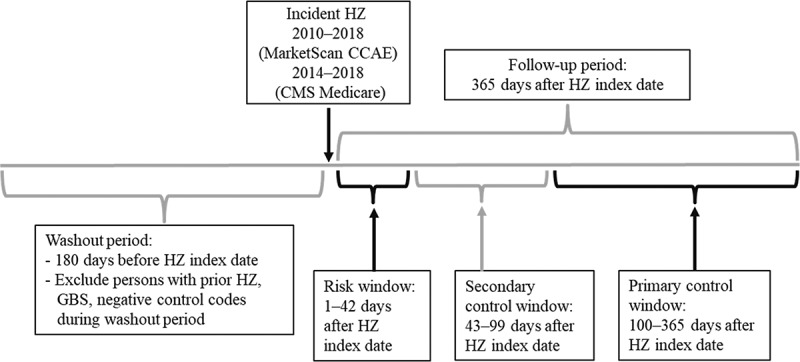
Timeline for self-controlled case series analysis assessing risk of Guillain-Barré syndrome (GBS) following herpes zoster (HZ) using MarketScan Commercial Claims and Encounters (CCAE) and Centers for Medicare and Medicaid Services (CMS) Medicare data, United States, 2010–2018.
Data sources
We analyzed healthcare claims from two sources, specifically the 2010–2018 IBM® MarketScan® Commercial Claims and Encounters (CCAE) databases and the 2014–2018 100% Medicare fee-for-service (FFS) claims databases available through the CMS Virtual Research Data Center. The MarketScan CCAE databases include healthcare claims for approximately 30–50 million persons covered by employer-sponsored insurance each year from all U.S. states. The CMS Medicare databases include a cohort of approximately 50–60 million Medicare beneficiaries annually, with 16–23 million enrolled in Medicare Parts A (hospital insurance), B (outpatient medical insurance), and D (prescription drug coverage) FFS. The MarketScan CCAE and CMS Medicare databases contain demographic data, insurance coverage, inpatient and outpatient diagnostic codes, and outpatient prescription claims. These healthcare claims databases are de-identified; therefore, upon review, this study was not considered human subjects research by CDC and thus did not require CDC Institutional Review Board approval.
Study population
From the MarketScan CCAE databases, we included all persons 18–64 years of age during 2010–2018 for whom outpatient pharmaceutical claims data were available. From the CMS Medicare databases, we included all persons ≥65 years of age during 2014–2018 with enrollment in Medicare Parts A, B, and D. We did not include beneficiaries enrolled in Medicare Part C (managed care plans) since complete medical and drug claims data were not available for this population.
Study definitions
Herpes zoster
We identified HZ cases using outpatient claims with International Classification of Diseases, 9th and 10th Revisions, Clinical Modification (ICD-9-CM and ICD-10-CM) diagnostic codes for HZ (053.xx/B02.xx) in the primary or secondary diagnostic position, excluding cases in which the first HZ code was a PHN code (053.12–053.13/B02.2x) and those with a claim for administration of any zoster vaccine within one day of the HZ claim. HZ vaccination was defined based on either Current Procedural Terminology (CPT) codes or National Drug Codes (NDC). We included only patients enrolled from 180 days before to 365 days after the HZ diagnosis and with no HZ claims during the prior 180-day period to ensure we identified an incident case. We defined the HZ index date as the date of the first HZ claim during the study period. We defined the washout period as the 180-day period prior to the HZ index date, and the follow-up period as the 365-day period after the HZ index date.
Although not included in the HZ case definition, we also evaluated outpatient antiviral use during the 0–42 days after HZ. Antiviral medications (acyclovir, valacyclovir, famciclovir) were identified using the NDC from the outpatient pharmaceutical data.
GBS
We defined GBS cases using (1) an inpatient claim with GBS as the principal diagnosis code (ICD-9-CM code 357.0 or ICD-10-CM code G61.0) and (2) at least one inpatient claim for any of the following procedures typically included in the diagnostic workup for patients with GBS within 45 days of the GBS onset date: lumbar puncture, electromyography, or nerve conduction study (Table SA1). GBS cases had to be enrolled continuously from 180 days before to 365 days after the HZ index date and have no GBS claims during the prior 180-day period before the HZ index date. The GBS onset date was defined as the date of the first GBS-coded inpatient claim during the study follow-up period, 2010–2018 in the MarketScan data and 2014–2018 in the CMS Medicare data. To better understand the characteristics of HZ-mediated GBS, we assessed the following outcomes among GBS patients: duration of hospitalization, hospitalization in the ICU, intubation, and death in the hospital (Table SA1). ICU admissions were identified using revenue codes 0200–0209.
Selected antecedent infections and RZV vaccination
To minimize the potential for confounding from infections with the strongest association with GBS (i.e., CMV, EBV, influenza virus, hepatitis E virus, Zika virus, Campylobacter jejuni, and Mycoplasma pneumoniae), we excluded persons diagnosed with these selected infectious diseases within 42 days prior to GBS onset. We used ICD-9-CM or ICD-10-CM diagnostic codes from inpatient and outpatient claims to define these diseases in any diagnostic position (Table SA1). Given the previously noted increased risk of GBS during the 42 days after RZV administration, we also excluded persons who received RZV during the 42 days prior to GBS onset.
Negative controls
We preselected the following controls because they were not expected to be associated with HZ, and they are similar to GBS in that they typically have an acute onset, frequently result in hospitalization, and have a low rate of reoccurrence: inpatient appendicitis, nephrolithiasis, cholecystitis, and inpatient and outpatient fractures of the upper limb. Inpatient admissions for appendicitis, nephrolithiasis, and cholecystitis were identified by ICD-9 and ICD-10 codes in the principle diagnostic code, and inpatient and outpatient fractures of the upper limb were identified based on ICD-9 and ICD-10 codes in any diagnostic position (Table SA1).
Statistical analysis
Data were analyzed using SAS 9.4 statistical software (SAS Institute, Inc, Cary, NC). We used conditional Poisson regression to compare rates and proportions of hospital admissions in the specified risk versus control windows, and the proc genmod procedure to calculate relative incidence, 95% confidence intervals, and p-values. To compare proportions of GBS hospitalizations versus all hospitalizations, GBS ICU admissions versus all ICU admissions, as well as hospitalizations for negative controls versus all hospitalizations in the cohort, we used generalized estimating equation methods for the analysis of repeated data since the same enrollees may have contributed data for multiple hospital admissions.
Results
We identified a total of 489,516 persons 18–64 years of age with HZ from the 2010–2018 MarketScan CCAE databases, among whom 11 developed GBS during the 1–365 days after HZ. We identified a total of 650,229 persons ≥65 years of age with HZ from the 2014–2018 CMS Medicare databases, among whom 41 developed GBS during the 1–365 days after HZ (Figure SA1). The characteristics of these patients with both HZ and GBS, and data on their GBS hospitalizations, are shown in Table 1. Race/ethnicity are not included in MarketScan data, and those ≥65 years were 93% white (data not shown due to small cell sizes for other races). There were no intubations following hospitalization for GBS in those 18–64 years, while some of those ≥65 years were intubated (data not shown due to small cell sizes). There were no deaths identified for either age group. Fifty-five percent of those 18–64 years, and 78% of those ≥65 years, were prescribed an antiviral treatment within the first 42 days after HZ. For those 18–64 years, we observed a higher proportion of GBS cases within the first 42 days after HZ compared to the primary control window. For those ≥65 years we observed a higher proportion of GBS cases within the risk window and secondary control window compared to the later primary control window.
Table 1.
Characteristics of persons with herpes zoster (HZ) and Guillain-Barré syndrome (GBS) identified in MarketScan Commercial Claims and Encounters (CCAE) and Centers for Medicare and Medicaid Services (CMS) Medicare data, United States, 2010–2018a
| MarketScan CCAE |
CMS Medicare |
|||
|---|---|---|---|---|
| 11 |
41 |
|||
| # GBS Cases | # | % | # | % |
| Year | ||||
| 2010–2013 | 4 | 36% | – | |
| 2014–2018 | 7 | 64% | 41 | 100% |
| Age group (years) | ||||
| 18–49 | 5 | 45% | – | |
| 50–64 | 6 | 55% | – | |
| 65–69 | – | DNSb | – | |
| 70–79 | – | 25 | 61% | |
| 80–89 | – | DNS | – | |
| 90–99 | – | DNS | – | |
| Gender | ||||
| Male | 2 | 18% | 19 | 46% |
| Female | 9 | 82% | 22 | 54% |
| Outcomes | ||||
| Duration GBS hospitalization [median (range), days] | 6 (3–19) | 9 (1–27) | ||
| ICU | 4 | 36% | 21 | 51% |
| Antiviral treatment | ||||
| 0–7 days after HZ | 6 | 55% | 30 | 73% |
| 0–42 days after HZ | 6 | 55% | 32 | 78% |
| Interval between HZ and GBS | ||||
| Same day | 0 | 0% | 0 | 0% |
| 1–42 days after HZ | 5 | 45% | 11 | 27% |
| 43–99 days after HZ | 1 | 9% | 13 | 32% |
| 100–180 days after HZ | 0 | 0% | DNS | – |
| 181–240 days after HZ | 1 | 9% | DNS | – |
| 241–365 days after HZ | 4 | 36% | 11 | 27% |
aMarketScan CCAE data from 2010–2018; CMS Medicare data from 2014–2018.
bDNS = Data not shown (unable to publish cells sizes <11 with CMS Medicare data).
The relative incidence of GBS was increased during the 42-day risk window as compared to the primary control window (100–365 days after HZ) both for those 18–64 years (6.3; 95% CI, 1.8–21.9, p = .0035) and those ≥65 years (4.1; 95% CI, 1.9–8.7, p = .0003) (Table 2). The risk of GBS cases admitted to the ICU was increased during the 42-day risk window as compared to the primary control window both for those 18–64 years (6.3; 95% CI, 0.9–45.0, p = .0649) and those ≥65 years (6.3; 95% CI, 2.4–16.9, p = .0002), although this was only statistically significant for those ≥65 years (data not shown due to small cell sizes).
Table 2.
Self-controlled case series analysis results for Guillain-Barré syndrome (GBS) and negative controls following herpes zoster (HZ) using MarketScan Commercial Claims and Encounters (CCAE) and Centers for Medicare and Medicaid Services (CMS) Medicare data, United States, 2010–2018a
| Risk Window |
Control Windows |
|||||
|---|---|---|---|---|---|---|
| Number of HZ cases |
Cases 1–42 days after HZ |
Primary (Cases 100–365 days after HZ) |
Secondary (Cases 43–99 days after HZ) |
|||
| Group | # | # | # | Rate Ratio | # | Rate Ratio |
| MarketScan CCAE, 2010–2018, 18–64 years (N = 489,516 HZ cases) | ||||||
| GBS | 11 | 5 | 5 | 6.3 (1.8–21.9); p = .0035 | 1 | 6.8 (0.8–58.1); p = .0805 |
| Negative controls | ||||||
| Appendicitis | 281 | 38 | 202 | 1.2 (0.8–1.7); p = .3219 | 41 | 1.3 (0.8–2.0); p = .3083 |
| Nephrolithiasis | 214 | 26 | 159 | 1.0 (0.7–1.6); p = .8685 | 29 | 1.2 (0.7–2.1); p = .4676 |
| Cholecystitis | 443 | 49 | 332 | 0.9 (0.7–1.3); p = .6592 | 62 | 1.1 (0.7–1.6); p = .7139 |
| Fractures upper limb | 3,994 | 463 | 2,898 | 1.0 (0.9–1.1); p = .8140 | 633 | 1.0 (0.9–1.1); p = .9042 |
| CMS Medicare, 2014–2018, ≥65 years (N = 650,229 HZ Cases) | ||||||
| GBS | 41 | 11 | 17 | 4.1 (1.9–8.7); p = .0003 | 13 | 1.1 (0.5–2.6); p = .7356 |
| Negative controls | ||||||
| Appendicitis | 316 | 37 | 225 | 1.0 (0.7–1.5); p = .8188 | 54 | 0.9 (0.6–1.4); p = .7334 |
| Nephrolithiasis | 279 | 44 | 194 | 1.4 (1.0–2.0); p = .0301 | 41 | 1.5 (1.0–2.2); p = .0832 |
| Cholecystitis | 1,496 | 221 | 1,054 | 1.3 (1.1–1.5); p = .0001 | 221 | 1.4 (1.1–1.6); p = .0013 |
| Fractures upper limb | 12,375 | 1,560 | 8,959 | 1.1 (1.0–1.2); p = .0004 | 1,856 | 1.1 (1.1–1.2); p = .0001 |
aMarketScan CCAE data from 2010–2018; CMS Medicare data from 2014–2018.
Sensitivity analysis
We also evaluated GBS hospitalizations as a proportion of all hospital admissions during the risk versus the primary control window, and found GBS hospitalization rates continued to be increased both for those 18–64 years (3.72; 95% CI, 1.12–12.45, p = .0325) and those ≥65 years (2.93; 95% CI, 1.44–5.94, p = .0029) (Table 3). Though numbers were small, similar patterns were observed for GBS cases admitted to the ICU when evaluated as an incidence rate or a proportion of all ICU admissions (data not shown due to small cell sizes). Findings were again only statistically significant for those ≥65 years (3.63; 95% CI, 1.43–9.20, p = .0066).
Table 3.
(a) Proportion of Guillain-Barré syndrome (GBS) inpatient admissions (IP) over all admissions, and (b) Proportion of negative control diagnoses (dx) inpatient admissions over all admissions, using MarketScan Commercial Claims and Encounters (CCAE) (18–64 years) and Centers for Medicare and Medicaid Services (CMS) (≥65 years) Medicare data, United States, 2010–2018a
| Risk window |
Primary control window |
Secondary control window |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (1–42 days after HZ) |
(100–365 days after HZ) |
(43–99 days after HZ) |
||||||||||||
| Age (years) | N (persons) | # IP | # GBS IP/ Negative control dx | % GBS IP | # IP | # GBS IP | % GBS IP | Relative Risk (95% CI) | p-Value | # IP | # GBS IP | % GBS IP | Relative Risk (95% CI) | p-Value |
| (a) Risk of GBS: GBS in cohort as proportion of all inpatient admissions | ||||||||||||||
| 18–64 | 30,748 | 5,932 | 6 | 0.10% | 29,458 | 8 | 0.03% | 3.72 (1.12–12.45) | .0325 | 6,273 | 1 | 0.02% | 6.35 (0.73–55.46) | .0946 |
| ≥65 | 140,619 | 36,541 | 22 | 0.06% | 165,386 | 34 | 0.02% | 2.93 (1.44–5.94) | .0029 | 34,255 | 20 | 0.06% | 1.03 (0.52–2.05) | .9299 |
| (b) Risk of selected negative controls: Negative control diagnoses in cohort as proportion of all inpatient admissions | ||||||||||||||
| 18–64, Appendicitis | 30,748 | 5,932 | 41 | 0.69% | 29,458 | 206 | 0.70% | 0.99 (0.70–1.39) | .9465 | 6,273 | 41 | 0.65% | 1.06 (0.68–1.64) | .8019 |
| 18–64, Nephrolithiasis | 30,748 | 5,932 | 29 | 0.49% | 29,458 | 179 | 0.61% | 0.80 (0.52–1.25) | .3309 | 6,273 | 30 | 0.48% | 1.02 (0.60–1.75) | .9358 |
| 18–64, Cholecystitis | 30,748 | 5,932 | 49 | 0.83% | 29,458 | 341 | 1.16% | 0.71 (0.53–0.96) | .0267 | 6,273 | 62 | 0.99% | 0.83 (0.57–1.22) | .3455 |
| 18–64, Fractures Upper Limb | 30,748 | 5,932 | 11 | 0.19% | 29,458 | 84 | 0.29% | 0.65 (0.35–1.22) | .1796 | 6,273 | 21 | 0.33% | 0.55 (0.27–1.15) | .1121 |
| ≥65, Appendicitis | 140,619 | 36,541 | 39 | 0.11% | 165,386 | 230 | 0.14% | 0.77 (0.54–1.09) | .1340 | 34,255 | 54 | 0.16% | 0.68 (0.45–1.03) | .0672 |
| ≥65, Nephrolithiasis | 140,619 | 36,541 | 44 | 0.12% | 165,386 | 205 | 0.12% | 0.97 (0.70–1.35) | .8625 | 34,255 | 45 | 0.13% | 0.92 (0.60–1.39) | .6810 |
| ≥65, Cholecystitis | 140,619 | 36,541 | 232 | 0.63% | 165,386 | 1,117 | 0.68% | 0.94 (0.81–1.09) | .398 | 34,255 | 236 | 0.69% | 0.92 (0.77–1.11) | .3792 |
| ≥65, Fractures Upper Limb | 140,619 | 36,541 | 156 | 0.43% | 165,386 | 843 | 0.51% | 0.74 (0.62–0.89) | .0590 | 34,255 | 188 | 0.55% | 0.78 (0.62–0.97) | .0277 |
aMarketScan CCAE data from 2010–2018; CMS Medicare data from 2014–2018.
Additionally, we evaluated the risk of inpatient appendicitis, nephrolithiasis, and cholecystitis, and inpatient and outpatient upper limb fractures as negative controls and found risks vs. the primary control window of 0.9–1.2 for those 18–64 years and 1.0–1.4 for those ≥65 years (Table 2). Further, negative control cases were distributed across the risk and control windows. When we evaluated hospital admissions for these conditions as a proportion of all hospitalizations in the cohort, rates were not increased in the risk as compared to the control windows (Table 3B).
Discussion
We found an increased risk of GBS 1–42 days after HZ compared to the primary control window (100–365 days after HZ), across both the 18–64-year and ≥65-year age groups, when analyzed as incidence and as a proportion of all inpatient admissions. Though an elevated risk of GBS following HZ was observed in both age groups, the relative risk was higher for the younger population. Whereas this might be most simply explained by a higher background rate of GBS in those ≥65 years, we cannot rule out that there might be some biological causes of an increased risk of GBS in this age group. Compared to those 18–64 years of age, there was evidence of more severe GBS following HZ (e.g., longer duration of hospitalization, higher percentage admitted to the ICU, higher percentage intubated) among those ≥65 years. It also appeared that the elevated risk of GBS may extend into the window of 43–99 days after HZ for the older group. The reason for this is unclear, although it has been noted that the estimated latency period for GBS following HZ ranges from two days to two months33 and it also seems biologically plausible that age could modify the interval of risk interval elevation.
This study provides new and methodologically rigorous epidemiologic support for an association between HZ and GBS. Kang, Sheu, and Lin33 found a significantly increased risk of GBS during the two-month period after an episode of HZ and noted that HZ patients with GBS were more likely to have had a recent infection than those without GBS. Exclusion criteria applied in this study controlled for diagnosed viral and bacterial infections noted in the literature to have the strongest association with GBS. Additionally, we used self-controlled case series methods to evaluate the risk of GBS following HZ where every case serves as its own control, eliminating some of the bias inherent in other observational designs. These methods are well-established and eliminate confounding by time-invariant factors (e.g., gender, underlying medical conditions). For this study, we used large national databases that include medical and pharmacy claims over a 5 to 9-year period. Inclusion of negative controls strengthened the study findings given the relative risks for all negative controls clustered around the null effect of RR = 1 (range 0.9–1.4), and when evaluating the proportion of negative control inpatient admissions over all inpatient admissions the rates were not increased. The null findings for the negative controls refute a hypothesis that spurious factors may lead to increased likelihood of admission for painful acute conditions in the weeks following HZ.
While this study supports an epidemiologic temporal association between HZ and GBS, an underlying causal mechanism requires additional research. Proposed mechanisms for antecedent infections triggering GBS include autoimmune-mediated responses29 and molecular mimicry.19,27 Kang, Sheu, and Lin33 also noted that it is unknown if antivirals prescribed as treatment for HZ can prevent the onset of GBS. Given the small numbers identified in this study, we were not able to adequately assess the potential effect of antiviral treatment on the risk of GBS following HZ. This warrants further investigation. We were also unable to evaluate whether possible seasonality of GBS may affect the results. There has been variable evidence on whether there is seasonal variation in GBS.26,40
This study has several limitations. Despite the use of large national databases, GBS is a rare outcome and we identified only a small number of cases that met the study criteria. Therefore, there were insufficient numbers to examine the risk of GBS following HZ by demographic or treatment factors, seasonality, or by yearly trends, or to fully examine the risks among GBS cases admitted to the ICU. Although we excluded prior Shingrix vaccination and selected antecedent infections with the strongest evidence as risk factors for GBS, we cannot exclude the possibility that GBS may have been related to causes other than HZ. Further study of the timing of GBS following HZ is warranted. It is also important to note that, although it is a large database, MarketScan is a convenience sample (i.e., not nationally representative). There is also the potential for miscoding/misclassification bias in both healthcare claims data sources. We were unable to validate GBS, HZ, or the negative control diagnoses, or account for different types of GBS, as we did not have access to medical records. Additionally, we estimated the GBS and HZ onset dates using the first hospital admission date for GBS and first outpatient visit for HZ and were unable to validate with chart review; incorrect onset dates may potentially lead to misclassification of GBS cases occurring in the risk or control windows.
In conclusion, we found a substantially increased risk of GBS in the 1–42-day window after HZ compared to the primary control window of 100–365 days after HZ, across adult age groups, in two different administrative data sources. This is the first epidemiologic evidence published using U.S. data demonstrating that HZ is associated with GBS, and our findings build on those of Kang, Sheu, and Lin.33 Future study will be needed to show whether this association can provide pathophysiological insight into a potential association between RZV and GBS. Regardless, our results provide useful context with which to interpret the benefits versus potential risks of zoster vaccination and assessments of RZV vaccination should account for the potential indirect benefit of preventing GBS.
Supplementary Material
Acknowledgments
The authors would like to thank Division of Viral Diseases staff and ACIP Herpes Zoster Work Group members for helpful feedback on this study.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, US Department of Health and Human Services.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1985890
References
- 1.Insinga RP, Itzler RF, Pellissier JM, Saddier P, Nikas AA.. The incidence of herpes zoster in a United States administrative database. J Gener Intern Med. 2005;20:748–53. doi: 10.1111/j.1525-1497.2005.0150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harpaz R, Ortega-Sanchez IR, Seward JF; Advisory Committee on Immunization Practices (ACIP) . Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2008;57(RR–5):1–30. [PubMed] [Google Scholar]
- 3.Harpaz R, Leung JW.. The epidemiology of herpes zoster in the United States during the era of varicella and herpes zoster vaccines: changing patterns among older adults. Clin Infect Dis. 2019;69(2):341–44. doi: 10.1093/cid/ciy953. [DOI] [PubMed] [Google Scholar]
- 4.Kawai K, Yawn BP. Risk factors for herpes zoster: a systematic review and meta-analysis. Mayo Clin Proc. 2017;92:1806–21. doi: 10.1016/j.mayocp.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 5.McKay SL, Guo A, Pergam SA, Dooling K. Herpes zoster risk in immunocompromised adults in the United States: a systematic review. Clin Infect Dis. 2020;71(7):e125–34. doi: 10.1093/cid/ciz1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention . Update on herpes zoster vaccine: licensure for persons aged 50 through 59 years. MMWR Morb Mortal Wkly Rep. 2011;60:1528. [PubMed] [Google Scholar]
- 7.Hales CM, Harpaz R, Ortega-Sanchez I, Bialek SR. Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep. 2014;63:729–31. [PMC free article] [PubMed] [Google Scholar]
- 8.Dooling KL, Guo A, Patel M, Lee GM, Moore K, Belongia EA, Harpaz R. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103–08. doi: 10.15585/mmwr.mm6703a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Food and Drug Administration. Shingrix US package insert, Revised: 07/2021. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2021. https://www.fda.gov/media/108597/download. [Google Scholar]
- 10.Anderson TC. Work Group interpretation of the Evidence to Recommendations Framework regarding use of RZV in immunocompromised adults, considerations for use, and proposed policy options. October 2021 Advisory Committee on Immunization Practices (ACIP) Meeting. 2021. https://www.cdc.gov/vaccines/acip/meetings/slides-2021-10-20-21.html. [Google Scholar]
- 11.Centers for Disease Control & Prevention. What everyone should know about Zostavax. 2020. [accessed 2020 Dec 24]. https://www.cdc.gov/vaccines/vpd/shingles/public/zostavax/. [Google Scholar]
- 12.Hesse EM, Shimabukuro TT, Su JR, Hibbs BF, Dooling KL, Goud R, Lewis P, Ng CS, Cano MV. Postlicensure safety surveillance of recombinant zoster vaccine (Shingrix) — United States, October 2017–June 2018. MMWR Morb Mortal Wkly Rep. 2019;68(4):91–94. doi: 10.15585/mmwr.mm6804a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su JR. Update on post-licensure safety monitoring of recombinant zoster vaccine (RZV, Shingrix). October 2020 Advisory Committee on Immunization Practices (ACIP) Meeting. 2020. https://www.cdc.gov/vaccines/acip/meetings/slides-2020-10.html. [Google Scholar]
- 14.Nelson JC. Vaccine Safety Datalink (VSD) update on post-licensure safety monitoring of recombinant zoster vaccine (RZV, Shingrix). October 2020 Advisory Committee on Immunization Practices (ACIP) Meeting. 2020. https://www.cdc.gov/vaccines/acip/meetings/slides-2020-10.html. [Google Scholar]
- 15.Shimabukuro T. Update on post-licensure safety monitoring of recombinant zoster vaccine (RZV, Shingrix). June 2019 Advisory Committee on Immunization Practices (ACIP) Meeting. 2019. https://www.cdc.gov/vaccines/acip/meetings/slides-2020-10.html. [Google Scholar]
- 16.Goud R, Lufkin B, Duffy J, Whitaker B, Wong H-L, Liao J, Lo A-C, Parulekar S, Agger P, Anderson SA, et. al. Risk of Guillain-Barré Syndrome following Recombinant Zoster Vaccine in Medicare. JAMA Internal Medicine. November 2021. doi: 10.1001/jamainternmed.2021.6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yadav R, Hundley D, Cation L. Severe Guillain-Barré syndrome following Shingrix vaccine administration. J Neurol Neurosci. 2019;10(4):301. doi: 10.36648/2171-6625.10.4.301. [DOI] [Google Scholar]
- 18.Tripathi A, Dharapak P, Laskova V. A case of Guillain-Barré syndrome (GBS) following zoster vaccination. J Gener Intern Med. 2020;35:S341–42. [Google Scholar]
- 19.Hughes RA, Cornblath DR. Guillain-Barré syndrome. Lancet. 2005;366:1653–66. doi: 10.1016/S0140-6736(05)67665-9. [DOI] [PubMed] [Google Scholar]
- 20.Sejvar JJ, Baughman AL, Wise M, Morgan OW. Population incidence of Guillain-Barré syndrome: a systematic review and meta-analysis. Neuroepidemiology. 2011;36:123–33. doi: 10.1159/000324710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beghi E, Kurland LT, Mulder DW, Wiederholt WC, Guillain-Barre syndrome . Clinicoepidemiologic features and effect of influenza vaccine. Arch Neurol. 1985;42:1053–57. doi: 10.1001/archneur.1985.04060100035016. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan JE, Schonberger LB, Hurwitz ES, Katona P. Guillain-Barré syndrome in the United States, 1978–1981: additional observations from the national surveillance system. Neurology. 1983;33:633–37. doi: 10.1212/WNL.33.5.633. [DOI] [PubMed] [Google Scholar]
- 23.Shui IM, Rett MD, Weintraub E, Marcy M, Amato AA, Sheikh SI, Ho D, Lee GM, Yih WK, Vaccine Safety Datalink Research Team . Guillain-Barré syndrome incidence in a large United States cohort (2000–2009). Neuroepidemiology. 2012;39:109–15. doi: 10.1159/000339248. [DOI] [PubMed] [Google Scholar]
- 24.Leung J, Sejvar JJ, Soares J, Lanzieri TM. Guillain-Barré syndrome and antecedent cytomegalovirus infection, USA 2009–2015. Neurol Sci. 2020;41(4):885–91. doi: 10.1007/s10072-019-04156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haber P, Sejvar J, Mikaeloff Y, DeStefano F. Vaccines and Guillain-Barré syndrome. Drug Saf. 2009;32(4):309–23. doi: 10.2165/00002018-200932040-00005. [DOI] [PubMed] [Google Scholar]
- 26.McGrogan A, Madle GC, Seaman HE, De Vries CS. The epidemiology of Guillain-Barré syndrome worldwide. A systematic literature review. Neuroepidemiology. 2009;32:150–63. doi: 10.1159/000184748. [DOI] [PubMed] [Google Scholar]
- 27.Willison HJ, Jacobs BC, Van Doorn PA. Guillain-Barré syndrome. Lancet. 2016;388:717–27. doi: 10.1016/S0140-6736(16)00339-1. [DOI] [PubMed] [Google Scholar]
- 28.Counotte MJ, Egli-Gany D, Riesen M, Abraha M, Porgo TV, Wang J, Low N. Zika virus infection as a cause of congenital brain abnormalities and Guillain-Barré syndrome: from systematic review to living systematic review. F1000Research. 2018;7:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hart IK, Kennedy PGE. Guillain-Barré syndrome associated with herpes zoster. Postgrad Med J. 1987;63:1087–88. doi: 10.1136/pgmj.63.746.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanders EACM, Peters ACB, Gratana JW, Hughes RAC. Guillain-Barré syndrome after varicella-zoster infection. J Neurol. 1987;234:437–39. doi: 10.1007/BF00314094. [DOI] [PubMed] [Google Scholar]
- 31.Rabbani MU, Gupta D. Guillain Barré syndrome following herpes zoster: a case report and review of literature. Jpn J Med. 1990;29(4):397–98. doi: 10.2169/internalmedicine1962.29.397. [DOI] [PubMed] [Google Scholar]
- 32.Roccatagliata L, Uccelli A, Murialdo A. Guillain–Barré syndrome after reactivation of varicella–zoster virus. N Engl J Med. 2001;344:65–66. [DOI] [PubMed] [Google Scholar]
- 33.Kang J-H, Sheu -J-J, Lin H-C. Increased risk of Guillain-Barré syndrome following recent herpes zoster: a population-based study across Taiwan. Clin Infect Dis. 2010;51(5):525–30. doi: 10.1086/655136. [DOI] [PubMed] [Google Scholar]
- 34.Farrington CP. Relative incidence estimation from case series for vaccine safety evaluation. Biometrics. 1995;51:228–35. doi: 10.2307/2533328. [DOI] [PubMed] [Google Scholar]
- 35.Weldeselassie YG, Whitaker HJ, Farrington CP. Use of the self-controlled case-series method in vaccine safety studies: review and recommendations for best practice. Epidemiol Infect. 2011;139:1805–17. doi: 10.1017/S0950268811001531. [DOI] [PubMed] [Google Scholar]
- 36.Hua W, Sun G, Dodd CN, Romio SA, Whitaker HJ, Izurieta HS, Black S, Sturkenboom MCJM, Davis RL, Deceuninck G, et al. A simulation study to compare three self-controlled case series approaches: correction for violation of assumption and evaluation of bias. Pharmacoepidemiol Drug Saf. 2013;22:819–25. doi: 10.1002/pds.3451. [DOI] [PubMed] [Google Scholar]
- 37.Petersen I, Douglas I, Whitaker H. Self controlled case series methods: an alternative to standard epidemiological study designs. BMJ. 2016;354:i4515. doi: 10.1136/bmj.i4515. [DOI] [PubMed] [Google Scholar]
- 38.Baker MA, Lieu TA, Li L, Hua W, Qiang Y, Kawai AT, Fireman BH, Martin DB, Nguyen MD. A vaccine study design selection framework for the postlicensure rapid immunization safety monitoring program. Am J Epidemiol. 2015;181:608–18. doi: 10.1093/aje/kwu322. [DOI] [PubMed] [Google Scholar]
- 39.Li R, Stewart B, Weintraub E. Evaluating efficiency and statistical power of self-controlled case series and self-controlled risk interval designs in vaccine safety. J Biopharm Stat. 2016;26:686–93. doi: 10.1080/10543406.2015.1052819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Webb AJS, Brain SAE, Wood R, Rinaldi S, Turner MR. Seasonal variation in Guillain-Barré syndrome: a systematic review, meta-analysis and Oxfordshire cohort study. J Neurol Neurosurg Psychiatry. 2015;86:1196–201. doi: 10.1136/jnnp-2014-309056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


