ABSTRACT
Prophylactic vaccines are crucial in modern healthcare and have been used successfully to combat bacterial and viral infectious diseases. Infections like polio and smallpox, which were dreaded historically, and which devastated the human race over many centuries, are now rare. Smallpox has been eradicated completely and polio is nearly eradicated because of vaccines. Vaccines differ fundamentally from other classes of medicines in that they are usually administered as a preventive measure to a healthy individual rather than to a sick person already with an infection, although exceptions to this practice exist. Most currently used prophylactic vaccines are based on established platforms, but many vaccine candidates, in late development stages, including several COVID-19 vaccines, use highly novel vaccine platforms not available historically. History of infectious diseases and prophylactic vaccines are filled with important scientific lessons, and thus provide valuable insights for the future. With hindsight, historically there were some ethically questionable approaches to testing vaccines and the germ warfare against native populations in the Americas and other regions. In this review, we examine key historical lessons learned with prophylactic vaccines with reflections on current healthcare dilemmas and controversies with respect to influenza and COVID-19 vaccines.
KEYWORDS: Vaccines, virology, infectious diseases, prophylactic vaccines, preventive medicines, history of vaccines, vaccination
Introduction
Vaccination has been relatively successful in preventing many childhood-related infections and saving millions of lives. Well in excess of 10 million lives were saved between mid-1960s and 2015 with viral vaccines1, 2 such as measles, mumps, rubella, chickenpox and hepatitis A, produced from acceptable cell culture substrates. However, approximately 1.5 million children under 5 years still die annually from vaccine-preventable diseases, mainly due to the lack of access to essential childhood vaccines.3,4 By contrast, major pathogens, including smallpox, polio, rabies that killed hundreds of millions of individuals in the last few centuries, are largely under control because of availability of safe and effective prophylactic vaccines. The WHO estimates that currently available vaccines prevent 2–3 million deaths worldwide annually.3 Smallpox was eradicated formally about 40 years ago and animal borne virus, rinderpest, was the second pathogen to be eradicated, in 2011. Remarkable progress has been made toward eradicating Polio due to global vaccination efforts since 1960s and relatively recent more effective single antigen vaccine or various combination vaccines1 that contain inactivated polio virus. Polio is set to become the second human infectious virus that will be fully eradicated. Other vaccine preventable diseases are largely under control with more than 90% to 100% decrease in morbidity compared to 20th Century annual cases because of protection that vaccines offer in conferring herd immunity1.
Early developments in inoculation (precursor to vaccination) were due to the efforts in Asia Minor, Africa and East Asian societies, in particular Chinese and Muslim countries. Later vaccine developments, particularly by British, French and German scientists contributed significantly to vaccine development between the 17th–20th Centuries. Spain was the first country to undertake a general public health vaccination program by transporting vaccines from Europe to the Americas to vaccinate millions of people against smallpox.5
History provides many lessons in dealing with infectious diseases. It is also obvious that current efforts to combat COVID-19 as well as other potential future pandemics requires us to work in close collaborations globally since “no one is safe until everyone is safe.” This review critically examines key past successes with vaccine developments, as well as key mistakes made in the past in vaccine discovery and vaccination. The focus of this review is on active immunization using commercially developed vaccines rather than on passive immunization where an individual receives antibodies from another infected individual (or animal). We also highlight how certain human actions with respect to vaccines and vaccinations have affected indigenous populations in the colonized world with the goal that current vaccination efforts do not lead to similar catastrophic outcomes for marginalized populations.
Inoculation, Edward Jenner and Lady Montague – eradication of “Angel of Death”
Start of modern vaccination refers to the study by English physician, Edward Jenner, in the 18th century. Jenner recognized that milkmaids infected with cowpox virus were immune to the smallpox virus. He injected pus from a pustule of a cowpox patient to his gardener’s son and subsequently challenged him with smallpox virus and observed that the child survived. His 1798 published findings is one of the most influential studies in medical history.6 He named this practice Variolae vaccinae denoting cowpox; the term vaccine originates from the Latin word vacca for cow.
By today’s norms, Jenner’s experiment would be considered unethical, and his experiment had many flaws. However, this study was a precursor to the development of smallpox vaccine and paved the way for the discovery and development of many vaccines used currently. Smallpox was eventually eradicated in 1979 and children are no longer vaccinated against smallpox. However, smallpox vaccines are still stockpiled by various countries for threats against bioterrorism.
Prior to vaccines and vaccination, inoculation (also referred to as variolation or engrafting) was common globally; cultures in Asia Minor, Middle East, Africa, India and China, were already using inoculation to immunize children.7,8 This practice firmly established the concept that an individual who recovered from a disease generally did not catch the same disease again.
Well before Jenner’s time, inoculation was widely used in Britain, New England, and Russia due to the efforts of Lady Montague (1689–1762), who learnt about inoculation in Istanbul. Lady Montague was herself disfigured by smallpox and her children were inoculated in Istanbul; she mentioned in her letters home how old women would inoculate children with attenuated/ inactivated smallpox viruses from prior mild cases. The practice also became widespread in Russia after Catherine the Great was inoculated, well before Jenner’s milkmaid and cowpox observation.
Similar developments were evolving in other regions; in Boston, an African slave, Onesimus (circa 1600s–1700s), detailed an inoculation that he had undergone which he claimed protected him from smallpox for life. This led to inoculation of many Bostonians once smallpox emerged as a major cause of death (1,000 deaths of the estimated population of 10,000 in the 1700s).9,10
An alternative inoculation process, involving nasal insufflation of cotton buds that contained a small amount of powdered substance from smallpox, very likely from smallpox scabs, was also common in China and India.11–13
Aftermath of the first vaccine
Inoculation, however, worked partially and occasionally caused serious complications or even death. Jenner’s success, therefore, was a major achievement and his results and related later experiments by Pasteur, Toussaint2 and others, in animals and subsequently in humans for other infectious diseases, by transferring an artificially weakened (attenuated) pathogen (e.g., anthrax, rabies) from the same species (i.e., from animal to animal or human to human), were pioneering projects in modern vaccine discovery and development. These successes collectively led to vaccination as the major public health initiative to combat infectious diseases globally.
Colonization – consequences of infectious diseases in the colonies
Colonization by Spanish, British, French, Dutch and other European nations with the consequent spread of new European diseases like smallpox in the new colonies, drastically changed human history. This had a cataclysmic effect on many indigenous populations, causing precipitous declines in their populations due to lack of natural immunity. It is estimated that more than 20 million natives died in the 16th Century not long after the Spanish established a new colony in Mexico because of previously unknown infections that Europeans brought to the continent.14–17 This rapid decline in the native population had a subsequent effect on much needed workforce as new settlements required expanded workforce. In turn, the colonizers needed to identify new cheap workforce sources which resulted in massive slavery of Africans by European nations.
Similar outcomes were noted in other parts of the world where native populations suffered similar consequences of colonialism. The First Australians suffered 80–96% population loss during the first 10 years of European settlement in the 18th Century; smallpox, measles and other diseases played a significant role.18
There was also intentional spread of infections in the new colonies with similar catastrophic consequences, as a crude and primary form of biological warfare. One example is the deliberate attempt to spread smallpox in Massachusetts to native Americans via infected blankets and other material; mentioned in the letters of Sir Jeffery Amherst (chief of British forces in North America in the mid-18th Century).19,20 Deliberate spread of smallpox and perhaps other infectious diseases amongst native Australians was also suggested during European settlement in the late 18th Century.21
Vaccination in the Americas by the Spanish
The first mass vaccination campaign was probably the vaccination of millions of individuals in the Americas against smallpox by the Spanish in the early 19th Century. Transporting vaccines to long destinations over many months, especially by sea was however, extremely difficult in the 19th Century. The Spanish found a solution; creating a human chain, akin to cold chain that is used currently, to carry the vaccine to the New World. For this highly unethical and immoral chain, 22 orphans were taken on the voyage and one after another were infected until the expedition reached the colonies in the Americas. Several trips were then made to major settlements in North and South America, and subsequently millions were vaccinated against smallpox.22–25
Eradication of “Angel of Death”
Variola viruses causing smallpox are of two major types: variola minor and variola major. Variola minor was a strain of smallpox that was less common and only caused mild infection. Variola major, however, was commonly known as the smallpox strain that originated approximately 3000 years ago either in Egypt or India and caused global havoc over many centuries. It bore many names like the Angel of Death and speckled monster. In the 20th Century alone, smallpox caused 100–300 million deaths.26,27
Victor Zhdanov (1914–1987), a highly influential Russian epidemiologist and deputy health minister of the Soviet Union advocated for the total eradication of the smallpox virus. Many were skeptical since previous eradication programs for malaria, yellow fever, hookworm and other infections had not been feasible. In 1959, the World Health Organization (WHO) undertook this challenge; progress was very slow until 1966, when an annual US$2.4 million funding was secured the appointment of well-known epidemiologist, Dr Donald A. Henderson (1928–2016), as the head of WHO’s Smallpox Eradication Unit.27 Other relatively simple and novel approaches, such as the introduction of the bifurcated needle for vaccine administration, expedited the eradication program and it was easy to teach vaccinators who were not medically trained, the bifurcated needle also required much less vaccine so the production capacity was no longer the critical issue.
In 1975, “Operation Smallpox Zero” program was launched by WHO which was treated with skepticism by many experts. In 1980, the WHO officially declared that smallpox was eradicated due largely to a globally successful vaccination campaign that lasted many decades. It required international collaborative public health measures, adopting rigorous scientific principles and pragmatically adopting other methods as required, providing appropriate monetary support, and public involvement at many levels of society. Smallpox eradication is probably the greatest public health achievements in history. A similar eradication of polio is close to being achieved.
Summary of lessons from history
The preceding discussion on the history of vaccines and vaccination has provided useful lessons for the future of vaccines. These include:
Colonization and its effects on emergence of infectious diseases especially in Indigenous populations and how to protect vulnerable native peoples.
Importance of critical observations to document ways of protecting individuals as well as the general population against infectious diseases.
Use of ethical testing practices for testing vaccine efficacy and safety.
Importance of childhood and adult mass-vaccination programs to confer herd immunity in the population (e.g., lessons recently learned from outbreaks of mumps and pertussis) and to eradicate specific infections linked to specific contagions.
Collaborative targeted international efforts, and injection of appropriate funds to bring about stepwise and rapid changes in vaccine development.
The importance of political will, leadership and role models to promote greater acceptance of vaccines quickly especially in an era of vaccine hesitancy.
Keeping these lessons from the history of vaccines and vaccination in mind this review now moves to more contemporary considerations facing society in the era of recurrent influenza outbreaks and the current pandemic.
Types and classes of vaccines
Many vaccine types have been developed since Jenner’s historic breakthrough and majority of these vaccines are summarized in Table 1 and depicted in Figure 1. Most of these vaccines, however, use established discovery/development platforms, where the pathogen is grown using cell culture. Globally, most of these vaccines are provided as pediatric vaccines. However, since Jenner’s time, and as a result of the many lessons learnt, novel methods and vaccine discovery strategies have evolved to arrive at safe and effective vaccines.
Table 1.
Vaccine immunization programs*
| Age | Disease | Vaccine brand/Manufacturer** | Vaccine type** | |
|---|---|---|---|---|
| Childhood | Birth | Hepatitis B | H-B-Vax® II Paediatric/or Engerix B® Paediatric/GlaxoSmithKline | Recombinant vaccines, single dose vial or prefilled syringe, aluminum hydroxide adjuvant |
| 2 Months |
|
|
|
|
| 4 Months |
|
|
|
|
| 6 Months | Diphtheria, tetanus, pertussis, hepatitis B, polio, Haemophilus influenzae type b |
|
As above | |
| 12 Months |
|
|
|
|
| 18 Months |
|
|
|
|
| 4 Years | Diphtheria, tetanus, pertussis, polio |
|
|
|
| Adolescent | 7 Years |
|
|
|
| 12–13 Years |
|
|
|
|
| 14–16 Years |
|
|
As above | |
| Adult | 15–49 Years | Pneumococcal | Pneumovax® 23/Merck Sharp & Dohme | Pneumococcal conjugated polysaccharide vaccine |
| 50 Years and over |
|
|
As above | |
| 65 Years and over | Pneumococcal | Pneumovax® 23/Merck Sharp & Dohme | As above | |
| 70–79 Years | Shingles (herpes zoster) | Zostavax®/Merck Sharp & Dohme or Shingrix® | Zoster live/attenuated virus vaccine, lyophilized | |
| Pregnant women |
|
(2)Fluvax® CSL (5 years)/CSL Ltd (2)Agrippal®/Seqirus Vaccines Ltd (2)Fluarix®/GlaxoSmithKline (2)Influvac®/Mylan Health (2)Vaxigrip®/Sanofi-Pasteur |
|
|
| Other Vaccines | 6 Months and over | Influenza | Fluvax® CSL (5 years)/CSL Ltd Agrippal®/Seqirus Vaccines Ltd Fluarix®/GlaxoSmithKline Influvac®/Mylan Health Vaxigrip®/Sanofi-Pasteur |
All of them as above |
| No age limit | Rabies |
|
|
|
| 2 Months and over | Japanese encephalitis |
|
|
|
| 9 Months and over | Yellow fever | Stamaril®/Sanofi-Aventis | Live attenuated virus, lyophilized |
*This is not an exhaustive list. Adapted from the National Immunization Program Schedule of Australia, NWS Immunization Schedule and WHO.28–32 Many other countries (like the US, UK) have specific lists to suit the vaccination programs in their individual countries.
Figure 1.
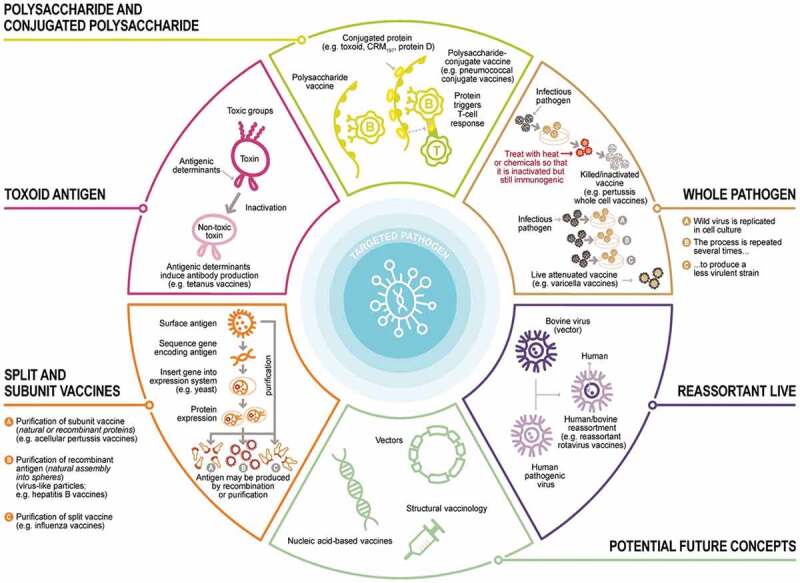
Different types/classes of vaccines. Vaccines are produced using different processes. Vaccines may contain live attenuated pathogens (usually viruses), inactivated whole pathogens, toxoids (an inactivated form of the toxin produced by bacteria that causes the disease), or parts of pathogens (e.g., natural or recombinant proteins, polysaccharides, conjugated polysaccharide or virus-like particles). Adapted from.35
Conjugate vaccines, where a specific bacterial surface sugar group (i.e., polysaccharide) that is conjugated covalently to a toxoid (a chemically modified pathogen toxin, which is no longer toxic but is still antigenic and can be used as a vaccine), usually diphtheria or tetanus protein, have emerged. Conjugation is necessary since polysaccharides are weakly immunogenic, but toxoids elicit good immunogenicity while displaying fewer side effects. Combined conjugate toxoid vaccines have been developed against Haemophilus influenzae type b (Hib) and pneumococcus which are used as combination vaccines for childhood immunization (Table 1). Recently new conjugated pneumococcal vaccines have been developed with additional pneumococcal capsular polysaccharide from different serotypes to provide broader coverage for different regions of the world.
Other childhood vaccines including diphtheria, tetanus, pertussis, polio, hepatitis B plus HibCV are constituents of the hexavalent pediatric vaccine (Table 1).
Attenuated or inactivated viral vaccines are another vaccine class that use established platforms to prepare vaccines. In the attenuated vaccine, a pathogen is passed many times through cells until it is not infectious/ virulent in humans. Some of the influenza and polio vaccines are examples of attenuated vaccines. In such vaccines, the virus is inactivated chemically or using elevated temperature so that it cannot reproduce anymore. Chemical inactivation uses either formalin, β-mercapto-ethanol or other similar chemicals, Rabies vaccine is an example.36 Occasionally, further processing is needed to split the virus to prepare a subunit vaccine with fewer side effects. Some influenza vaccines are examples of this kind of vaccine. Usually, a surfactant, like Triton-100, is used to split the virus. Exposing the virus to a surfactant induces splitting by dissolving the viral membrane and exposing inner regions of the virus. This process is used for most annual seasonal influenza vaccines. Formulation stability is a major issue with this process since splitting generates a non-homogeneous mixture.
Novel vaccine platforms have emerged in recent years including nucleic acid-based (e.g., mRNA), nanoparticle complex formation or viral vector based-vaccines that have been used to develop COVID-19 vaccines and other emerging vaccines. In the nucleic acid type vaccines, DNA or RNA representing a small section of a virus, for example, surface protein, is used as the antigen. In the case of mRNA, an encapsulating delivery system needs to be used since RNA is highly unstable and is short-lived in the body. Pfizer/BioNTech, Moderna and other COVID-19 vaccines are of this class. Research on nucleic acid (both RNA and DNA)-based vaccines have been ongoing for many decades. However, only recently RNA vaccine efforts have come to fruition and generated multiple, hugely successful mRNA vaccines for COVID-19, while no successful human DNA vaccine exists currently.
With the viral vector system, a part of the target virus is incorporated into a different virus that is harmless and nonreplicating. As a vector or carrier, adenoviruses (of human or animal origin) that cause common cold, or measles, vaccinia or other viruses may be used. Examples of this type of vaccines include Merck’s Ebola vaccine Ervebo®, which is an attenuated recombinant vesicular stomatitis viral vaccine, AstraZeneca’s and Johnson & Johnson’s COVID-19 vaccines which both use chimpanzee adenovirus viral vector while Sputnik V COVID-19 vaccine uses a human adenovirus vector, and Bavarian Nordic’s smallpox vaccine, Imvanex®, that uses a modified Vaccinia Ankara vector among other vectors.
Current vaccines and immunization programs
Recent step-wise refinements in vaccine discovery/development have led to a paradigm shift in the management of infectious diseases and vaccines are now an integral part of modern healthcare and are first-line defense to prevent human infections. In addition to inactivated or attenuated vaccine production methods, subunit, recombinant vector using short DNA/RNA fragments and polysaccharide vaccines have been developed. Sixteen childhood and adolescent vaccines37 are now routinely administered from birth to age 4 years (Table 1); most of these vaccines require multiple doses to elicit long-lasting immunity. Some of these vaccines are combination vaccines such as DTaP-HepB-IPV (diphtheria, tetanus, and acellular pertussis, hepatitis B, and inactivated poliovirus vaccine).
While many childhood vaccines provide life-long immunity, the protective effects of some vaccines decline with age, requiring additional booster shots (Table 1). For example, a single dose of measles vaccine provides over 90% protection that lasts a lifetime, but booster shots are required to bring the protection to higher levels. Influenza is an exception to this; instead of booster doses, seasonal influenza vaccines are administered each year because of influenza viral shift and drift. At present, trivalent or quadrivalent seasonal influenza vaccines are available for children 6 months and older as well as adults.38 Hunt for a universal influenza vaccine is vigorously being pursued by both vaccine industry and academia. Such efforts focus primarily on the constant regions of the virus such as the M2 protein or the stem of HA both of which have not shown mutations over the years. However, these viral domains are not very immunogenic and hence a successful universal influenza vaccine candidate is yet to be identified.
Other vaccines such as for travel vaccinations also exist and are administrated as required against many different types of pathogens (Table 1). These include for example, seasonal influenza, yellow fever, rabies and zoster vaccines. WHO provides recommendations for routine immunization schedules for these39 and each individual country adopts a schedule of vaccination from birth to adolescence; most country schedules are highly similar. As a result of vaccine hesitancy and regional conflicts (like the Yemen and Syrian wars), highly infectious vaccine-preventable diseases such as measles are on the rise in many countries including some developed countries and various public health measures have been implemented to increase mass vaccination programs (for example, mandating vaccination prior to school admission/entry).
Pandemic readiness – emerging issues with current influenza vaccines
Pandemic preparedness schemes were adopted by some countries following the 2005 bird influenza outbreak, the 2009 swine influenza pandemic and the 2014–16 Ebola outbreak. Many nations, including Australia, launched their pandemic (and biosecurity) procedures at the start of the COVID-19 pandemic. However, according to the Global Preparedness Monitoring Board (GPMB) and the WHO, no country was fully prepared or could mitigate global health emergencies like the current coronavirus outbreak.40,41 The existing pandemic readiness frameworks were insufficient for the global COVID-19 pandemic caused by SARS-CoV-2; partly because most preparedness schemes were established to deal with only an influenza pandemic.
Influenza avoids our immune system due to extensive viral drift (small number of mutations in the viral gene leading to changes in key surface glycoproteins [hemagglutinin, HA, and neuraminidase, NA]) and shift (a major structural change in virus, giving rise to a new HA and/or NA, which may be categorized as a new subtype),42 requiring vaccination annually.43,44 The WHO reports that over 500,000 individuals die annually from influenza,43–46 including high-risk groups (children, the elderly and pregnant women). The USA Centers for Disease Control and Prevention (CDC) estimated that the 2009 influenza pandemic, caused by Influenza A (H1N1pdm09 – the swine influenza), killed approximately 600,000 individuals.47,48 The COVID-19 pandemic has revealed that the world was not prepared for a more virulent pandemic virus that can spread and kill rapidly and extensively.
There have been many other influenza pandemics in history such as the Russian influenza in 1889 (which killed ~1 million), Asian influenza in 1957 (~1.1 million deaths), Hong Kong influenza in 1968 (1 million casualty); with the 1918 Spanish influenza being the most deadly (50 million deaths, ~5% of the world’s population at that time).49 Another influenza pandemic is expected by many experts, thus understanding historical and emerging influenza outbreaks might assist in dealing swiftly when another pandemic emerges.
Current influenza vaccines: element of ‘guessing’ and hit-and-miss efficacy
Current influenza vaccines consist of one influenza A strain (H1N1) and (H3N2), together with one or two influenza B virus strains that are used in either the trivalent or quadrivalent vaccines respectively.50 There are 18 different subtypes of hemagglutinins (HA) and 11 subtypes of neuraminidases (NA) in influenza viruses.50 Only a small number of influenza A viruses, mainly H1N1 and H3N2 subtypes, have humans as hosts.
The critical public health issue is the selection of the viral strain (subtype) for seasonal influenza vaccines as this directly affects the vaccine’s efficacy. The WHO reviews worldwide viral surveillance data to determine which strain(s) to recommend annually for different regions. Mismatch between ‘guessed’ or ‘predicted’ strains (vaccine components) and actual circulating viral strains at a particular time in a specific region results in the vaccine’s relatively poor performance.51 While artificial intelligence (AI) and machine learning (ML) may provide better predictions of the viral strains for annual vaccines, these techniques are in their infancy.52 Each viral strain is produced separately and mixed with the other strain(s) to yield either a trivalent or quadrivalent influenza vaccine.
Influenza vaccines are 60–85% effective in children and 30–60% in adults <65 years old.53 In the last several decades, however, the effectiveness has fluctuated considerably and sometimes it is as low as 10%. Over the last several years, seven influenza variant strains have been observed; this rapid emergence of different strains is very concerning.
Moreover, different surfactant amounts are required to split different influenza viral strains, reflecting different intrinsic viral structural stabilities (unpublished observations from the primary author). This may result in the proportions of unsplit virus being for different strains viruses. Additionally, influenza viruses have a diverse range of HA and NA sequences and somewhat dissimilar glycosylation profiles,54 which would affect their intrinsic stabilities and the types/amounts of protein aggregates formed. In general, however, each strain undergoes an identical manufacturing process and these critical quality attribute differences are not routinely considered during manufacturing, due to lack of sensitive methods to reliably detect virion differences. Such issues need to be understood fully to yield vaccines with high formulation stabilities.55
Lack of sensitive in-situ methods in vaccine manufacturing
Many of the R&D issues with vaccines are known, however, regulatory agencies are slow to implement new developments. An example is the widely used Single Radial Immunodiffusion (SRID) assay,56 developed in the 1960s, and still used to determine HA content and vaccine stability/potency. More sensitive techniques have been developed as alternatives but these are not commonly accepted either by the vaccine industry or regulatory agencies. Relying on SRID to estimate vaccine formulation stability/potency may be misleading especially for those vaccines that contain inadequately split virus; other techniques are thus urgently needed.
Lack of manufacturing controls and insufficient product characterization
Many parameters influence influenza vaccine stability including for example, flock age for egg-based vaccines, initial titer concentration, type of surfactant, initial and residual surfactant concentrations, number of filtration steps and final HA concentration. Several unanswered questions with respect to influenza vaccine development/manufacturing include:
What other process-parameters directly affect virus yield?
What is the relationship between viral yield and virus strain?
Does immunosuppression have a considerable impact on yield?
What is the temperature sensitivity of the virus yield?
What is the effect of inoculate concentration on virus yield?
What (and how much) growth factors and/or nutrients enhance virus growth?
How does glycosylation (type/extent) of the virus influence yield?
How do different surfactants (and their concentrations) interact with the virus?
How does intrinsic virus stability influence final (finished) formulation stability?
What type of particles are formed during manufacturing?
Can we elucidate aggregation of HA in detail and how this affects formulation stability?
Can we develop rapid and sensitive potency ranking/screening methods?
How do different additives affect stability?
Understanding these vaccine formulation issues would lead to more precise control of each vaccine production step and a consistent viral yield in each vaccine batch.
Vaccine supply and demand gaps
From the current global influenza vaccine manufacturing capacity, there would be a vaccine shortage if another influenza pandemic emerged. Although cell-based influenza vaccine manufacturing platforms exist, majority of the current influenza vaccines are egg-based. The current annual capacity for producing a pandemic influenza vaccine is estimated primarily on the capacity to produce the current seasonal vaccine. The world’s annual capacity for the production of egg-based influenza vaccine is approximately 1.5 billion doses for seasonal vaccine and about 8 billion doses for a potential influenza pandemic.57 As a result, only ~60% of the world’s population would have enough vaccine in one year if another influenza pandemic was to occur. Two billion more doses would be required to vaccinate the desired 70% of the population to confer herd immunity.57 Limitations to pandemic influenza vaccine availability are: (i) need to plan at least 6 months ahead, (ii) availability of sufficient chicken eggs of the right quality, (iii) unpredictable viral yields, and (iv) very low viral yield per embryonated chicken egg for some viral strains.58,59 These limitations also result in higher manufacturing costs, limited production volumes and unequitable vaccine distribution globally. New influenza vaccine manufacturing/formulation technologies are therefore urgently needed.
COVID-19 vaccines – a paradigm shift in vaccine development
SARS-CoV-2 generated COVID-19 pandemic
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is the novel coronavirus (Figure 2) that emerged in China in late 2019 and caused Coronavirus Disease 2019 (COVID-19) which is now a pandemic. So far (July 2021), the confirmed cases have exceeded hundred millions of cases globally and 4 millions of deaths.60 A recent estimate found that its basic reproductive number (R0) is between 3.6 and 6.1 depending on the country61 with an incubation time of 2–14 days. More critically, asymptomatic individuals spread the virus rapidly, making SARS-CoV-2 one of the most transmissible viruses.62–64 This was very concerning initially since there was no therapy and vaccines have only now become available. However, many countries implemented their pandemic readiness frameworks or biosecurity action procedures well before WHO’s COVID-19 pandemic declaration. However, no country was fully prepared for this pandemic,40,41 especially as more virulent (delta) strains emerge.
Figure 2.
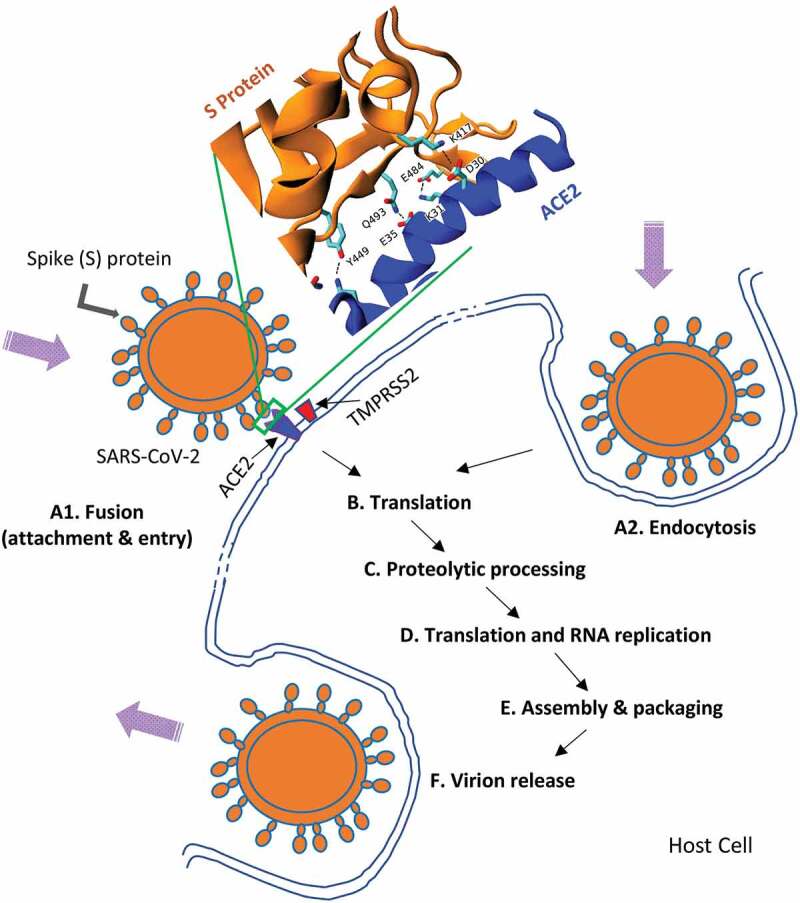
A highly simplified representation of the SARS-CoV-2 cell cycle. (A1) Virus entry generally occurs via surface glycoprotein (Spike protein, S-protein) binding to ACE2 receptor, TMPRSS2 also has a critical role to play in this process. Detailed interactions between S protein–ACE2 are also shown (courtesy of Dr. Serdar Kuyucak, USyd (A2). The virus can also enter by endocytosis (B&C). Subsequently, uncoating of viral proteins, primary translation, polyprotein processing and transcription take place. (D). Translation and viral RNA synthesis then occur (E). Other key steps such as accessory protein-host interactions take place prior to assembly and viral maturation. (F). Finally, release of mature viruses occurs via endocytosis.
Coronaviruses on the rise
Multiple coronavirus outbreaks have occurred since the early 2000s; original SARS-CoV-1 in 2002–3, several swine coronaviruses cases, Middle East Respiratory Syndrome (MERS), and finally SARS-CoV-2 in 2019; clearly coronavirus infections are on the rise. While all these outbreaks originated from bats, intermediate hosts include civets for SARS-CoV-1, camels for MERS while the intermediate host for SARS-CoV-2 is yet to be firmly established. The virus has surface glycoproteins called Spike protein (S-protein) that play a key role in cellular attachment and entry into the host (Figure 2). It is also the main target for immunity and hence many vaccines use S-protein as the antigen. SARS coronavirus is one of the largest single strand RNA viruses but unusually it has a proof-reading mechanism, so for example, compared to Influenza, it mutates more slowly. Nevertheless, it is an RNA virus, so it still mutates and different variants of SARS-CoV-2 including the UK, South Africa, India, California and Brazil variants, have appeared within a year or so. Some of these variants (like the delta variant) are more virulent and spread more rapidly but their full characteristics are yet to be fully determined. These variants are a major concern in case the immunity afforded by current vaccines do not protect individuals fully against emerging variants.
Role of regulatory agencies – approval of COVID-19 vaccines
One of the lessons learnt from history with respect to small molecule drugs and vaccines is a robust but timely approval and drug registration framework. Role of regulatory agencies in each country is generally clear and transparent; in general, the public has confidence in the drug (vaccine) approval process. Regulatory reviews of new vaccines to ensure quality, safety and efficacy has traditionally been a lengthy and slow process. Every R&D and manufacturing step, safety, efficacy and stability data must be examined prior to granting approval. Several years ago, new approval pathways were introduced in different jurisdictions to expedite approval; for example, Investigational New Drug (IND) Application, Priority Review, Breakthrough Therapy, Accelerated Approval, and Fast Track pathways by the USA’s FDA,65–67 and PRIME (Priority Medicines) of the European Medicines Agency (EMA).68 These pathways are generally more rapid than the traditional pathways; however, there are strict conditions for a medicine to be considered via these expediated pathways, like an unmet medical need. During this COVID-19 pandemic, however, there has been criticism of regulatory agencies for taking too long to approve a particular vaccine, especially if the same vaccine was approved in another jurisdiction. In contrast, regulatory agencies also faced criticisms about by some for approval of some vaccines too rapidly so that quality, safety, and efficacy attributes might have been compromised. What this pandemic has demonstrated though is the ability of regulatory agencies to be responsive to public demands for timely approval of vaccines in an environment where there are very few other mechanisms for controlling the pandemic.
Specific COVID-19 vaccines
As of July 2021, 21 vaccines have been authorized for emergency use in different jurisdictions and over 300 are in various stages of development.69,70 Major approved vaccines, listed in Table 2, fall into two categories: those based on established platforms, e.g., inactivated virus, and those based on novel vaccine platforms, e.g., mRNA-based vaccines. The latter can be further categorized as nucleic acid-based or viral vector-based vaccines, which are the two major classes.
Table 2.
Major approved COVID-19 vaccines for emergency use†
| Type | Company | Country | Price/dose (US$)* | Efficacy (%)* | Dosage recommendation | Approval# | Storage conditions | Common/Rare side effects** |
|---|---|---|---|---|---|---|---|---|
| mRNA | BioNtech Pfizer |
Germany USA |
19.5 | 95 | 2 doses, 3 weeks apart for 12 years and older (as of May 2021) | US, EU, UK, Turkey, Australia, Japan |
|
Common: Chills, headache, tiredness, and injection site adverse events (swelling, pain, redness) Rare: Can trigger anaphylaxis |
| Moderna | USA | 25–37 | 95 | 2 doses, 4 weeks apart for 18 years and older (other ages are being considered as of May 2021) | US, EU |
|
Common: As above Rare: As above |
|
| Viral Vector-based | AstraZeneca/ Oxford University |
UK | 2.15–5.25 | ~70 | 2 doses, 4–12 weeks apart for 18 years and older | EU, UK, Australia, India | Up to 6 months at 2–8°C |
Common: As above Rare: Blood clots |
| Johnson & Johnson | USA Belgium |
10 | 66–72 | 1 dose for 18 years and older (under study for <18) | USA | Up to 3 months at 2–8°C |
Common: As above (but less severe) Rare: Blood clots |
|
| Sputnik V (Gamalaya Institute) | Russia | 10 | 91.4 | 2 | Russia, India, Turkey, Iran, Argentina (>60 countries) |
|
Common: As above and flu-like symptoms, fatigue Rare: Unknown |
|
| Inactivated Virus | CoronaVacTM (Sinovac) | China | 40–60 | 50–91 | 2 | China, UAE, Indonesia, Turkey, Brazil | 2–8°C |
Common: As above, allergic reaction, cough, fever Rare: Unknown |
| Sinopharm | China | 18–40 | 2 | China, Hungary, Venezuela, Sri Lanka (>40 countries) | 2–8°C |
Common: As above, fatigue Rare: Nausea and acute disseminated encephalomyelitis |
||
| Covaxin® (Bharat Biotech) | India | 2 | India | 2–8°C |
Common: As above, fatigue, fever Rare: Unknown |
|||
| Protein-based | Novavax | USA | 16 | 89.3 | 2 doses, 3 weeks apart, being tested for adults 18–84 ages | Undergoing evaluation (Australia, EU, US, and others) | Up to 3 months at 2–8°C |
Common: As above, fatigue, myalgia Rare: Unknown |
Vaccines using novel platforms include mRNA-based vaccines (Pfizer/BioNtech and Moderna), adenovirus vector-based vaccines (AstraZeneca/Oxford University, Johnson & Johnson and Sputnik V), and protein-based (Novavax). Vaccines that have been prepared using established platforms use inactivated virus (CoronaVacTM by Sinovac, Sinopharm vaccine, and Covaxin® by Bharat Biotech). Prior to COVID-19, no vaccine on the market had utilized these novel platforms; therefore, every aspect of this vaccine development paradigm has required detailed regulatory scrutiny. The various clinical trial phases (I to IV) are traditionally designed to facilitate intense scrutiny but the design of these vaccine trials were very diverse. For example, many differences between trial protocol end-points might have existed although Pfizer, Moderna, AstraZeneca and Johnson & Johnson trials apparently had similar primary endpoints.76 Other parameters may have affected efficacy assessments, for example, during the clinical trials of the AstraZeneca vaccine, results from different countries varied, presumably due to dosing errors.
The various phases of vaccine trials are designed to examine quality, safety, and efficacy of the product; if these attributes are sub-optimal, theoretically these would be picked up during one/more of the trial phases. This is what was observed for the University of Queensland (UQ) vaccine candidate; trials were discontinued due to an intrinsic design problem in the foundation technology. A ‘molecular clamp’ was employed to maintain the trimeric S-protein conformation, as in the actual virus, via a peptide from HIV as ‘a clamp.’ However, during phase I trials, some volunteers displayed false positive results in HIV tests and hence the trial was discontinued to prevent a potential public health distrust in the vaccine.77
Rapid approval and use of Sputnik V vaccine in Russia, and Sinovac and Sinopharm vaccines in China, before human phase III trials were completed, have raised concerns about possible political interference in the approval processes in these countries. These controversies, however, did not hinder the early roll-out of these vaccines in many countries.78 For example, Sinovac vaccine was used in Turkey, UAE, Indonesia, and Brazil before any peer-reviewed publications became available on clinical trial evidence. Turkey traditionally requires EMA approval for new vaccines and medications, but mass vaccination started in the country without such approval. Some of these vaccines have subsequently been shown to be safe and effective.79
Most vaccines against SARS-CoV-2, the virus causing the COVID-19 pandemic, herald a new scientific era in vaccine discovery, development and manufacture. It is unprecedented historically that several COVID-19 vaccines have become available so rapidly for a pathogen that has initiated a pandemic within a year of its genetic sequence being determined. Vaccine development processes are generally slow, taking on average up to 10 years to develop a vaccine. In addition, many diseases including HIV AIDS do not have a vaccine, even after several decades of intense research. There are several reasons for this rapid development of COVID-19 vaccines:
Most of the vaccine platforms (like mRNA or viral vector delivery) were already established.
Large-scale vaccine clinical trials and large-scale manufacturing are two very expensive and risky steps in vaccine development. Significant funding from agencies like The Gates Foundation, many governments and philanthropists from around the globe added significant momentum to the discovery process.
Some clinical trial phases were run in parallel or combined, e.g., phase I & II trials were combined or phase II trial was conducted with a large group of volunteers and overlapped with phase III trials.
There was no shortage of volunteers for these trials in many countries.
Rapid peer-reviewed publications (sometimes 1 week after submission) and availability of manuscripts in open access format facilitated scientific scrutiny of key findings.
Recent prior history with human coronaviruses.
Rapid identification of virus protein sequence including neutralization epitopes of S protein as the major antigen.
Ability to grow SARS-CoV-2 rapidly in different cell lines and to assay the virus (and its variants) accurately and rapidly.
Some concerns have emerged about several COVID-19 vaccines related to serious but rare adverse reactions such as blood clots with low platelet numbers, thrombocytopenia syndrome80 from AstraZeneca vaccine, resulting in public health recommendations for this vaccine in individuals over 50 years in Australia and over 30 years in the UK. With new technologies, rare but serious side- effects may only be observed once mass vaccination begins as is the case with the Astra Zeneca vaccine. Johnson & Johnson vaccine, being a vector-based vaccine is also under scrutiny for blood clots. Other ongoing concerns include lack of full efficacy against newly emerging viral variants. Another adverse event is anaphylaxis observed with Pfizer/BioNtech mRNA-based COVID-19 vaccine in individuals with prior history of severe allergies.81,82
To ease global travel restrictions amid this pandemic, fresh discussions on vaccine passports have started. Some countries see such passports necessary for safe international travel and opening of borders while in some jurisdictions it is argued that mandatory vaccinations and other forced restrictions would diminish legal rights.83
The current COVID-19 pandemic has highlighted the gap between global supply and demand for vaccines. Several European Union (EU) countries blocked export of COVID-19 vaccines that were manufactured in mainland EU84,85 including a shipment to Australia.86 Similarly, India temporarily blocked shipments of COVID-19 vaccines manufactured by Serum Institute of India because of rapidly rising COVID-19 cases in India.87 To ensure a globally equitable and responsible access to COVID-19 vaccines, a collaboration between WHO, Gavi, the Vaccine Alliance, The Coalition for Epidemic Preparedness, CEPI and United Nations Children’s Fund, UNICEF have established COVAX, whose aim is to accelerate the development and manufacture of COVID-19 vaccines, and to guarantee fair and equitable access for every country.88 COVAX has already delivered some vaccines to needy regions of the world; however, underdeveloped countries (who often have the greatest need) have experienced severe vaccine shortages during this COVID-19 pandemic.
What happens if annual COVID-19 vaccinations are necessary?
Vaccines against SARS-CoV-2 may stop individuals getting sick or reduce the severity of disease; however, the virus might still be prevalent in the environment. In addition, because of relatively slow mass vaccination rates, global herd immunity may not be achieved in a timely manner and more virulent variants with more extensive mutations may emerge which may lead to new strains. To make matters worse, the virus might be able to jump to another animal host. In such a scenario, annual COVID-19 vaccinations might be necessary, as with seasonal influenza vaccination. A more elegant approach might be to develop a combination influenza–COVID-19 vaccine. This is being contemplated but such a combination vaccine would require either similar or complementary platforms (either SARS-CoV-2 needs to be grown in eggs or influenza virus needs to be cultured in the same cell line as SARS-CoV-2) and would need to be in acceptable final vaccine preparations (like fully liquid and thermostable). A combination vaccine might also overcome current issues with COVID-19 vaccines (inadequate manufacturing capacity, storage, transport and delivery). A combination vaccine might also be “self-adjuvanting,” where the relatively low effectiveness of current influenza vaccines might be enhanced.
Conclusions
Vaccine development has traditionally been a slow and arduous process, but significant collaborative/targeted international research efforts and injection of substantial amounts of money have resulted in successful vaccine developments within a much shorter timespan especially during this pandemic. WHO has been listing ongoing prophylactic vaccine clinical trials since 2007;89 trials in progress include vaccines for HIV, malaria, TB, Zika, Ebola and of course SARS-CoV-2.
Despite these remarkable vaccine discovery advancements, unwavering collaborative research is urgently needed to maintain such stepwise discoveries in vaccine development and timely commercial availability. Further advancements in vaccine discovery, development and formulation should pave the way for availability of much safer and more efficacious vaccines and restore much needed public confidence in vaccines and mass vaccination programs, as a key public health strategy, during pandemics.
Notes
A combination vaccine consists of several different vaccines in the same dosage form. Many childhood vaccines are combination vaccines such as VAXELIS™ (Diphtheria and Tetanus Toxoids and Acellular Pertussis, Inactivated Poliovirus, Haemophilus b Conjugate and Hepatitis B Vaccine).
Jean Joseph Henri Toussaint (1847–1890) was a French bacteriologist contemporary of Pasteur and worked on various infectious diseases like chicken cholera and tuberculosis. He is credited with the development of the first anthrax vaccine via chemical inactivation but during his lifetime only Pasteur received full recognition.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the author(s).
References
- 1.Orenstein WA, Ahmed R.. Simply put: vaccination saves lives. Proc Natl Acad Sci. 2017;114(16):4031. doi: 10.1073/pnas.1704507114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olshansky SJ, Hayflick L.. The role of the WI-38 cell strain in saving lives and reducing morbidity. AIMS Public Health. 2017;4(2):127. doi: 10.3934/publichealth.2017.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okwo-Bele J-M. Together we can close the immunization gap [accessed Aug 9]. https://apps.who.int/mediacentre/commentaries/vaccine-preventable-diseases/en/index.html.
- 4.WHO Immunization. [accessed Aug 9]. https://www.who.int/news-room/facts-in-pictures/detail/immunization.
- 5.The history of vaccines. [accessed May 3]. https://www.historyofvaccines.org/timeline/all.
- 6.Jenner E. An inquiry into the causes and effects of the variolae vaccinae: a disease discovered in some of the western counties of England, particularly Gloucestershire, and known by the name of the cow pox. England, London: Printed for the author, by S. Low and sold by Law [etc.]; 1800. [Google Scholar]
- 7.Plotkin S, Bazin H. Vaccination: a history from Lady Montagu to genetic engineering. Montrouge, France: John Libbey Eurotext; 2011. [Google Scholar]
- 8.Snowden F. HIST 234: epidemics in western society since 1600 [accessed Oct 23]. https://oyc.yale.edu/history/hist-234/lecture-1.
- 9.Mather C. New World Encyclopedia. [accessed Aug 3]. https://www.newworldencyclopedia.org/entry/Cotton_Mather.
- 10.How an enslaved African man in Boston helped save generations from smallpox [accessed Apr 24]. https://www.history.com/news/smallpox-vaccine-onesimus-slave-cotton-mather.
- 11.Williams G. Angel of death: the story of smallpox. Basingstoke (UK): Palgrave Macmillan; 2010. [Google Scholar]
- 12.Silverstein AM. A history of immunology. San Diego (USA): Academic Press; 1989. [Google Scholar]
- 13.Henderson D. Smallpox: the death of a disease. New York (USA): Prometheus Books; 2009. [Google Scholar]
- 14.Hamnett BR. A concise history of Mexico. 3. Cambridge: Cambridge University Press; 2019. [Google Scholar]
- 15.Gibson C. The Aztecs under Spanish rule: a history of the Indians of the Valley of Mexico 1519-1810. Stanford: Stanford University Press; 1964. [Google Scholar]
- 16.Lockhart J. The Nahuas after the conquest a social and cultural history of the Indians of central Mexico, sixteenth through eighteenth centuries. Stanford (CA): Stanford University Press; 1992. [Google Scholar]
- 17.History of Mexico. [accessed Aug 3]. https://www.history.com/topics/mexico/history-of-mexico.
- 18.Harris J. Hiding the bodies: the myth of the humane colonisation of Australia. J Aborig Hist. 2003;27:79–104. [Google Scholar]
- 19.Fenn EA. Biological warfare in eighteenth-century North America: beyond Jeffery Amherst. J Am Hist. 2000;86(4):1552–80. doi: 10.2307/2567577. [DOI] [PubMed] [Google Scholar]
- 20.Kiger PJ. Did colonists give infected blankets to native Americans as biological warfare? [accessed Aug 3]. https://www.history.com/news/colonists-native-americans-smallpox-blankets.
- 21.Dowling PJ. “A great deal of sickness” introduced diseases among the aboriginal people of colonial Southeast Australia 1788-1900. Australian National University; 1997 [accessed Aug 3].. https://openresearch-repository.anu.edu.au/bitstream/1885/7529/1/02Whole_Dowling.pdf.
- 22.Franco-Paredes C, Lammoglia L, Santos-Preciado JI. The Spanish Royal Philanthropic expedition to bring smallpox vaccination to the new world and Asia in the 19th century. Clin Infect Dis. 2005;41(9):1285–89. doi: 10.1086/496930. [DOI] [PubMed] [Google Scholar]
- 23.Kean S. 22 Orphans gave up everything to distribute the world’s first vaccine. The Atlantic; 2021.
- 24.Romeo J. How children took the smallpox vaccine around the world. JSTOR Daily; 2020.
- 25.Mark C, Rigau-Pérez JG. The world’s first immunization campaign: the Spanish smallpox vaccine expedition, 1803–1813. Bull Hist Med. 2009;83(1):63–94. doi: 10.1353/bhm.0.0173. [DOI] [PubMed] [Google Scholar]
- 26.Flight C. Smallpox: eradicating the scourge. BBC; 2011.
- 27.Chapter 1: smallpox—eradicating and ancient scourge. [accessed May 3]. https://www.who.int/about/history/publications/public_health_stories/en/.
- 28.Health, A. G. D. o. National immunisation program schedule. [accessed Mar 24]. https://www.health.gov.au/health-topics/immunisation/immunisation-throughout-life/national-immunisation-program-schedule.
- 29.Government, N . NSW immunisation schedule; 2020. July 1 [accessed Mar 24]. https://www.health.nsw.gov.au/immunisation/Pages/schedule-changes.aspx.
- 30.Slonim M, Starr M, Blashki G. Are we there yet? Travel vaccinations for Australian children. Aust Fam Physician. 2014;43:378–81. [PubMed] [Google Scholar]
- 31.WHO Table 1: summary of WHO position papers - recommendations for routine immunization. [accessed 2019]. https://www.who.int/immunization/policy/Immunization_routine_table1.pdf.
- 32.WHO Table 2: summary of WHO position papers - recommended routine immunizations for children. [accessed Nov 15]. https://www.who.int/immunization/policy/Immunization_routine_table2.pdf.
- 33.Australian immunisation handbook. [accessed Aug 3]. https://immunisationhandbook.health.gov.au/vaccines.
- 34.Medicine finder. [accessed Aug 3]. https://www.nps.org.au/medicine-finder/.
- 35.Vetter V, Denizer G, Friedland LR, Krishnan J, Shapiro M. Understanding modern-day vaccines: what you need to know. Ann Med. 2018;50(2):110–20. doi: 10.1080/07853890.2017.1407035. [DOI] [PubMed] [Google Scholar]
- 36.Kayser V, Françon A, Pinton H, Saluzzo J-F, Trout BL. Rational design of rabies vaccine formulation for enhanced stability. Turk J Med Sci. 2017;47:987–95. doi: 10.3906/sag-1610-82. [DOI] [PubMed] [Google Scholar]
- 37.CDC Table 3. Recommended child and adolescent immunization schedule by medical indication, United States; 2021. [accessed 2021]. https://www.cdc.gov/vaccines/schedules/hcp/imz/child-indications.html.
- 38.Kayser V, Reslan M. Pivotal biology, chemistry, biochemistry, and biophysical concepts of biologics and biosimilars. In: Ramzan I editor. Biologics, biosimilars, and biobetters: an introduction for pharmacists, physicians, and other health practitioners. USA: John Wiley & Sons, Inc.; 2020. p. 89–107. [Google Scholar]
- 39.WHO WHO recommendations for routine immunization - summary tables. [accessed May 3]. https://www.who.int/immunization/policy/immunization_tables/en/.
- 40.A WORLD AT RISK - Annual report on global preparedness for health emergencies. [accessed May 3]. https://apps.who.int/gpmb/assets/annual_report/GPMB_annualreport_2019.pdf.
- 41.WHO WHO Director-General’s opening remarks at the media briefing on COVID-19-5 March 2020. [accessed Mar 5]. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—5-march-2020.
- 42.How the Flu virus can change: “Drift” and “Shift.” [accessed May 2]. https://www.cdc.gov/flu/about/viruses/change.htm.
- 43.Influenza. [accessed Mar 5].https://www.ncirs.org.au/sites/default/files/2020-04/Influenza-fact-sheet_March%202020_Final.pdf.
- 44.Influenza (Seasonal). [accessed May 3]. http://www.who.int/mediacentre/factsheets/fs211/en/.
- 45.Soema PC, van Riet E, Kersten G, Amorij J-P. Development of cross-protective influenza A vaccines based on cellular responses. Front Immunol. 2015;6:1–9. doi: 10.3389/fimmu.2015.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.WHO influenza: burden of disease. [accessed Dec 5]. https://www.who.int/influenza/surveillance_monitoring/bod/en/.
- 47.Dawood FS, Iuliano AD, Reed C, Meltzer MI, Shay DK, Cheng P-Y, Bandaranayake D, Breiman RF, Brooks WA, Buchy P, et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis. 2012;12(9):687–95. doi: 10.1016/S1473-3099(12)70121-4. [DOI] [PubMed] [Google Scholar]
- 48.CDC 2009 H1N1 pandemic (H1N1pdm09 virus). [accessed Mar 24]. https://www.cdc.gov/flu/pandemic-resources/2009-h1n1-pandemic.html.
- 49.Johnson NPAS, Mueller JJ. Updating the accounts: global mortality of the 1918-1920 “Spanish” influenza pandemic. Bull Hist Med. 2002;76(1):1086–3176. doi: 10.1353/bhm.2002.0022. [DOI] [PubMed] [Google Scholar]
- 50.Types of influenza viruses. [accessed May 3]. https://www.cdc.gov/flu/about/viruses/types.htm.
- 51.Selecting viruses for the seasonal influenza vaccine. [accessed May 2]. https://www.cdc.gov/flu/prevent/vaccine-selection.htm.
- 52.Murayama T, Shimizu N, Fujita S, Wakamiya S, Aramaki E. Predicting regional influenza epidemics with uncertainty estimation using commuting data in Japan. PLOS ONE. 2021;16(4):e0250417. doi: 10.1371/journal.pone.0250417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Influenza vaccines for Australians. [accessed May 3]. https://www.ncirs.org.au/sites/default/files/2018-12/Influenza_FAQs.pdf.
- 54.Cruz E, Cain J, Crossett B, Kayser V. Site-specific glycosylation profile of influenza A (H1N1) hemagglutinin through tandem mass spectrometry. Hum Vaccin Immunother. 2018;14(3):508–17. doi: 10.1080/21645515.2017.1377871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sahin Z, Akkoc S, Neeleman R, Haines J, Kayser V. Nile Red fluorescence spectrum decomposition enables rapid screening of large protein aggregates in complex biopharmaceutical formulations like influenza vaccines. Vaccine. 2017;35(23):3026–32. doi: 10.1016/j.vaccine.2017.04.066. [DOI] [PubMed] [Google Scholar]
- 56.Fahey JL, McKelvey EM. Quantitative determination of serum immunoglobulins in antibody-agar plates. J Immunol. 1965;94:84. [PubMed] [Google Scholar]
- 57.Sparrow E, Wood JG, Chadwick C, Newall AT, Torvaldsen S, Moen A, Torelli G. Global production capacity of seasonal and pandemic influenza vaccines in 2019. Vaccine. 2021;39(3):512–20. doi: 10.1016/j.vaccine.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hampson AW. Vaccines for pandemic influenza. The history of our current vaccines, their limitations and the requirements to deal with a pandemic threat. Ann Acad Med Singap. 2008;37:510. [PubMed] [Google Scholar]
- 59.Tree JA, Richardson C, Fooks AR, Clegg JC, Looby D. Comparison of large-scale mammalian cell culture systems with egg culture for the production of influenza virus A vaccine strains. Vaccine. 2001;19(25):3444–50. doi: 10.1016/S0264-410X(01)00053-6. [DOI] [PubMed] [Google Scholar]
- 60.CSSE JH. Coronavirus COVID-19 global cases by Johns Hopkins CSSE. [accessed May 3]. https://www.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6.
- 61.Ke R, Romero-Severson E, Sanche S, Hengartner N. Estimating the reproductive number R0 of SARS-CoV-2 in the United States and eight European countries and implications for vaccination. J Theor Biol. 2021;517:110621. doi: 10.1016/j.jtbi.2021.110621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382(13):1199–207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Riou J, Althaus CL. Pattern of early human-to-human transmission of Wuhan 2019 novel coronavirus (2019-nCoV), December 2019 to January 2020. Eurosurveillance. 2020;25(4):2000058. doi: 10.2807/1560-7917.ES.2020.25.4.2000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.WHO report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19). [accessed Mar 5]. https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf.
- 65.Investigational New Drug (IND) application. [accessed May 3]. https://www.fda.gov/drugs/types-applications/investigational-new-drug-ind-application.
- 66.Breakthrough therapy designation requests. [accessed May 3]. https://www.fda.gov/drugs/ind-activity/breakthrough-therapy-designation-requests.
- 67.Fast track designation requests. [accessed May 3]. https://www.fda.gov/drugs/ind-activity/fast-track-designation-requests.
- 68.PRIME – PRIORITY MEDICINES: paving the way for promising medicines for patients. [accessed May 3]. https://www.ema.europa.eu/en/documents/leaflet/prime-paving-way-promising-medicines-patients-factsheet_en.pdf.
- 69.Craven J. COVID-19 vaccine tracker. [accessed Apr 22]. https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-vaccine-tracker.
- 70.COVID-19 vaccine tracker. [accessed Apr 22]. https://vac-lshtm.shinyapps.io/ncov_vaccine_landscape/.
- 71.Terry M. UPDATED comparing COVID-19 vaccines: timelines, types and prices. [accessed May 3]. https://www.biospace.com/article/comparing-covid-19-vaccines-pfizer-biontech-moderna-astrazeneca-oxford-j-and-j-russia-s-sputnik-v/.
- 72.Weedon A, Walden M. China’s COVID-19 vaccines are being given the green light without the data being revealed. Why? https://www.abc.net.au/news/2021-01-22/china-coronavirus-covid-19-vaccine-equity-sinovac-sinopharm/13066856.
- 73.Katella K. Comparing the COVID-19 vaccines: how are they different? [accessed May 3]. https://www.yalemedicine.org/news/covid-19-vaccine-comparison.
- 74.Global COVID-19 vaccine summary: side effects. [accessed May 29]. https://www.medicalnewstoday.com/articles/global-covid-19-vaccine-summary-side-effects#Currently-authorized-COVID-19-vaccines.
- 75.Coronavirus disease (COVID-19): vaccines safety. [accessed May 29]. https://www.who.int/news-room/q-a-detail/coronavirus-disease-(covid-19)-vaccines-safety.
- 76.Doshi P. Covid-19 vaccine trial protocols released. BMJ. 2020;371:m4058. doi: 10.1136/bmj.m4058. [DOI] [PubMed] [Google Scholar]
- 77.Queensland, T. U. o. Molecular clamp vaccines: lessons from a setback; 2020. [accessed May 29]. https://www.nature.com/articles/d42473-020-00504-2?source=globalbiodefense.
- 78.Schiffling S, Breen L. Russian COVID vaccine: why more and more countries are turning to Sputnik V. [accessed May 29]. https://theconversation.com/russian-covid-vaccine-why-more-and-more-countries-are-turning-to-sputnik-v-159158.
- 79.Jones I, Roy P. Sputnik V COVID-19 vaccine candidate appears safe and effective. The Lancet. 2021;397(10275):642–43. doi: 10.1016/S0140-6736(21)00191-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.WHO report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19). [accessed Aug 2]. https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf.
- 81.CDC Pfizer-BioNTech COVID-19 vaccine overview and safety. [accessed May 29]. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/Pfizer-BioNTech.html.
- 82.Department of Health, A. G. ATAGI statement on AstraZeneca vaccine in response to new vaccine safety concerns. [accessed May 3]. https://www.health.gov.au/news/atagi-statement-on-astrazeneca-vaccine-in-response-to-new-vaccine-safety-concerns.
- 83.Stupp C. U.K., EU face privacy criticism for Covid-19 passport plans. The Wall Street J. 2021. https://www.wsj.com/articles/u-k-eu-face-privacy-criticism-for-covid-19-passport-plans-11617960601 [Google Scholar]
- 84.EU threatens to hold back coronavirus vaccine exports to safeguard its own supply. ABC; 2021.
- 85.Coronavirus: EU stops short of vaccine export ban. BBC; 2021.
- 86.Italy, EU refuse AstraZeneca request to ship 250,000 doses of vaccine to Australia. ABC; 2021.
- 87.Findlay S, Peel M, Mancini DP. India blocks vaccine exports in blow to dozens of nations. Financial Times; 2021.
- 88.WHO COVAX — working for global equitable access to COVID-19 vaccines. [accessed May 3]. https://www.who.int/initiatives/act-accelerator/covax.
- 89.WHO WHO vaccine pipeline tracker. [accessed May 3]. https://docs.google.com/spreadsheets/d/19otvINcayJURCMg76xWO4KvuyedYbMZDcXqbyJGdcZM/pubhtml#.


