ABSTRACT
Immunotherapy for cancer has attracted considerable attention. As one of the immunotherapeutics, tumor vaccines exert great potential for cancer immunotherapy. The most important components in tumor vaccines are antigens and adjuvants, which determine the therapeutic safety and efficacy, respectively. After decades of research, many types of adjuvants have been developed. Although these adjuvants can induce strong and long-lasting immune responses in tumor immunity, they also cause more severe toxic side effects and are therefore not suitable for use in humans. With the development of innate immunity research, pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) are receiving more attention in vaccine design. However, whether they have the potential to become new adjuvants remains to be elucidated. The purpose of this review is to provide newideas for the research and development of new adjuvants by discussing the mechanisms and related functions of PAMPs and DAMPs.
KEYWORDS: Cancer, immunotherapy, tumor vaccine, adjuvant, DAMPs, PAMPs
Introduction
Classical methodologies for the treatment of malignant tumors are surgery, radiotherapy and chemotherapy. Although these methodologies are widely applied, such methodologies sometimes lose their effect for some tumors and produce obvious toxic and side effects to the human body1. With tumor immunotherapy as a hot spot of current research, tumor vaccines have attracted more attention and are achieving much success.
Cancer vaccine generally consists of two parts: antigen and adjuvant. Adjuvant can improve the problems such as weak immunogenicity of vaccine and weak immune response.2 Therefore, they play an important role in the development of vaccines. Aluminum is the first adjuvant approved for use in human vaccines in the 1920s.3 Although adjuvant research has been going on for almost a century, few have been approved certified for use in humans. Because of its safety, aluminum is still widely used, such as adsorbed diphtheria-tetanus combination vaccine (Shanghai Institute of Biological Products Co., Ltd) and recombinant hepatitis B vaccine (Merck & Co., Inc.), especially in children. MF59, a proprietary adjuvant containing squalene, is included in a seasonal subunit influenza vaccine licensed by the Italian Regulatory Authority in 1997 and subsequently by several other countries, for example, Fluad (Seqirus). ASO4, formulated with MPL and aluminum salt, came into the market in 2005, which is an improvement of the traditional aluminum salt adjuvant. It’s currently used for hepatitis B vaccine (MPL with aluminum phosphate), such as Fendrix (GSK), and bivalent cervical cancer vaccine (MPL with aluminum hydroxide) such as Cervarix (GSK). More than 100 adjuvants are explored today, but the most of them are still in the stage of preclinical animal experiments or used by veterinarians. Safer and more effective human adjuvants are urgently needed. In this study, the mechanism and research progress of pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) in triggering immune responses were reviewed, which might help to provide more possibilities for the development of new adjuvants.
Classification of tumor vaccines
Multiple therapeutic cancer vaccine platforms have been developed, including peptide-based, protein-based, viral-based, recombinant vector including yeast-based and bacterial-based, whole tumor cell and pulsed dendritic cells (DCs).4 There are three therapeutic cancer vaccines have been approved by the U.S. Food and Drug Administration (FDA): (1) Bacillus Calmette-Guerin (BCG, TheraCys®), a live attenuated strain of Mycobacterium bovis for non-muscle invasive bladder carcinoma; (2) Sipuleucel-T (Provenge®), a DC vaccine for metastatic castration-resistant prostate cancer (mCRPC); and (3) talimogene laherparepvec (T-VEC or Imlygic®), an oncolytic viral-based vaccine for advanced melanoma.
Peptide/protein-based vaccine
The most common vaccine platform is peptide/protein-based vaccine. These vaccines are relatively simple to develop. However, the simplicity of the platform may be in question if its short amino acid sequence does not encode enough antigenic material to induce an immune response. Therefore, peptide vaccines usually require an immune adjuvant. Protein vaccines are more expensive to produce than peptide vaccines due to their larger amino acid sequence, but they may cause a stronger response. In addition, peptide and protein vaccines or protein vaccines are limited by HLA.
Whole tumor cell vaccines
Whole tumor cell vaccines, divided into autologous or allogeneic vaccines, could present a variety of tumor antigens. The lack of specificity has a possibility of diluting the immune response. However, additional stimulation is required by granulocyte-macrophage colony-stimulating factor (GM-CSF) or calmette-gue bacilli ´ rin (BCG). Autologous vaccines require harvesting the tumor from the patient, which is not always possible. GM-CSF-transduced autologous tumor cell vaccines have been extensively studied, but have not yet been approved by the FDA. Allogeneic whole tumor cell vaccines contain several established malignant cell lines and provide an unlimited supply of tumor antigens at a low cost. However, promising phase II studies have been followed by negative phase III trials, and this is the reason why there is currently no FDA approval for an allogeneic whole tumor cell vaccine.5
Recombinant poxvirus vaccines
Recombinant poxvirus vaccines are reported to be safe and can express large amounts of exogenous DNA. Several ongoing trials are investigating recombinant vectors, with most using modified poxviruses such as vaccinia or fowlpox viruses.6 The immunogenic potential of vaccinia is self-limiting, but hosts usually neutralize the virus after one or two vaccinations leading to the significant decline in the effectiveness of the vaccine.6
DCs vaccines
DCs are specialized antigen-presenting cells (APCs) that activate T lymphocytes via major histocompatibility complex (MHC) signals.7 DCs vaccines can be infected with peptides or protein pulses, or with viral vectors.8 They require complex preparation, but the platform has proven successful.8 The FDA approved Sipuleucel-T (Provenge) for metastatic castration-resistant prostate cancer (mCRPC) in 2010. However, the phase 3 study also found certain cerebrovascular risks with the vaccine.9
Possible problems with cancer vaccines
Many kinds of cancer vaccines exist, but few of them are actually used in the clinic due to some problems which vaccines may exist.10 First, in the selection of vaccine antigen, whether the selected antigen is the highly expressed antigen in the tumor tissue remains unknown. Second, in the process of vaccine delivery, antigen epitopes are lost, and appropriate delivery vectors are selected. Finally, even if the vaccine works, the tumor’s immune escape mechanism can make the vaccine less effective. These reasons that make cancer vaccine research difficult.
Factors influencing the efficacy of tumor vaccines
Tumor vaccines are mainly composed of antigens and adjuvants, which amplify the anti-tumor immune responses in tumor patients through active immunization.11
Classification and introduction of tumor antigens
The recognition of tumor antigens is of great significance for the development of tumor vaccines.12 On the basis of the expression and localization of tumor vaccines, tumor-associated antigens (TAAs) and tumor-specific antigens (TSAs) can be generally classified. TAAs were selected as antigen for some vaccines in many tumors and can cause specific T-cell and humoral responses.13 Unfortunately, TAAs are also expressed in normal cells, leaving the risk of leading to tolerance. TSAs are more attractive therapeutic target than TAAs because they are rarely expressed in normal cells and are directly regarded as foreign antigens by the immune system.14 TSAs-targeted vaccines have the advantages of being safer, more specific, and more effective.15 However, TSAs are not perfect targets. Vaccines based on TSAs can only kill part of the tumor cells if the targeted neoantigens come from mutated subclones. On the other hand, they are also limited by the vast diversity and individual specificity of somatic mutations in different tumor types.16
Different kinds of TAAs exist, for example, carcinoembryonic antigen (CEA) for gastrointestinal cancer.17 The HPV-16 E7 antigen is a tumor-associated antigen (TAA) that is commonly expressed in HPV-induced tumors, but has low immunogenicity.18 An oncolytic adenovirus encoding an SA-4-1BBL adjuvant, when fused with the HPV-16 E7 antigen, produces a specific antitumor effect in a mouse model of cervical cancer.19,20 MUC4 is an attractive TAA that functionally contributes to the pathogenesis of PC; however, it is over-expressed in mouse and human pancreatic tumors.18 The recombination of the MUC4 domain, along with predicted immunogenic T-cell epitopes, elicited cellular and humoral anti-MUC4 responses, indicating its potential as a vaccine candidate for PC therapy.21 TSAs are theoretically more attractive therapeutic targets because the specific immune response induced by TSAs is not affected by central or peripheral tolerance. Moreover, targeting TSAs is unlikely to induce autoimmunity. As a result, it appears to be an ideal target for therapeutic cancer vaccines and T-cell-based cancer immunotherapy. Several types of TSAs have been identified in different types of cancer, including melanoma, lung, liver, and kidney cancers.22 PSA and MART-1 have been studied deeply as two TSAs.8
Classification and introduction of adjuvants
Adjuvant is also one of the important components of tumor vaccines, which enhances the efficacy of vaccines by enhancing the immunogenicity of antigens. Adjuvant can also act as a delivery system to deliver antigens to antigen presenting cells (APCs) to promote induction of antigen-specific immune responses.23 In addition, adjuvant have the advantages of making the vaccine more stable, protecting the antigen from being broken down by enzymes in vivo, and reducing the dose of expected efficacy.24 Aluminum salt adjuvant was the first vaccine adjuvant approved by the FDA for use in human vaccines, and it is also one of the most widely used adjuvants in vaccine production. With the deepening of research, in addition to traditional aluminum salt adjuvants, pattern recognition receptors (PRRs) agonists (Toll-like receptor [TLR] agonists), polymer materials (PEI), polypeptides and other novel adjuvants have been found to exert immune activation effects.25
Definitions of PAMPs and DAMPs and their main functions
Charles Janeway proposed that the development of immune system is aimed at protecting the body against infectious pathogens rather than harmless foreign antigen, and then came to the concept of pathogen-associated molecular patterns PAMPs.26
The definition of PAMPs
PAMPs are some conserved non-self and highly expressed molecular motifs. Most of them are fundamentally functional components, such as LPS, which is an elementary component of Gram-negative bacterial cell membrane and flagellin, nucleic acid of viruses such as double-stranded RNA (dsRNA), single-stranded RNA (ssRNA) and DNA.27 The mechanism of the body in detecting pathogen is that the PRRs recognize the conserved molecular motifs of the pathogen, which are absent in humans.28 The combination of PAMPs to PRRs then gives a sign of exogenous dangers that warns the immunity system and stimulates a series of pro-inflammatory and antimicrobic responses to fight against foreign invaders.26
The receptors and function of PAMPs
The receptors that recognize PAMPs are called PRRs, which are expressed by native immune cells. Most of them are located on the surface of cell membrane such as TLR4 and TLR5, which recognizes LPS and flagellin,29respectively. Some of them are located in the endosome such as TLR3, which is combined with dsRNA. TLR7/8 and TLR10 are expressed on the endosome as well.30 RLRs and NLRs are another kind of receptors that are located in the cytoplasm. The activation of PRRs will stimulate a series of intracellular signaling pathways such as adapter molecules, kinases and transcription factors that results in a range of gene expression and molecules synthesis.31 It also directs adaptive immune responses also.
However, the “stranger theory” cannot explain the damage in the sterile inflammation conditions such as trauma, ischemia and tumor, which can unexpectedly lead to strong immune responses. On this basis, the “danger theory” was proposed by Polly Matzinger.32 The danger theory came from a clinical trial about kidney transplantation33 and was rarely identified until HMGB1 and acid crystals were recognized as DAMPs.34,35
The definition of DMAPs
DAMPs are a range of intracellular molecules that are not only released passively by dead cells but can also be expressed actively by live cells facing with life-threatening stress.36 They include ATP, HMGB1, HSPs, and uric acid. DAMPs have physiological function in the cell and can fulfill extra functions when exposed to the external environment.37
The location and function of DMAPs
Location is the key factor that determines the functions of DAMPs. Histones, genomic DNA and HMGB1 are located in the nucleus physically, which usually work as DNA chaperones that are combined with DNA and regulate the transcription and translation actions.38 However, when they come to the extracellular environment, the DAMPs will exert strong pro-inflammatory activity. Time is another critical point as well. Initially, people considered DAMPs as a signal of cellular death because they thought DAMPs could only be released by death cells.39 However, it has turned out that DAMPs can be passively released and active secreted as well. In addition, they can only be released by necrotic cells rather than apoptotic cells.35
The function of DAMPs varies. They have physiological function such as regulating the transcription, adjusting the calcium homeostasis40 and the proliferation and differentiation of cells.41 When pathogen causes damage in the body, they trigger inflammation and innate immunity to eliminate damage. DAMPs can combine with PRRs that participate in the host defense by stimulating signal transduction and activating downstream signal pathway.31 They also regulate the immune responses and promote adaptive immunity by recruiting DCs42 and APCs, triggering inflammation and tissues healing.43
The majority of DAMPs participate in the maturation of DCs, Some of them recruit and activate immune cells such as macrophages (S100, ATP) and neutrophils (HMGB1, histones, mtDNA)44–46 They can promote the secretion of pro-inflammatory factors (tumor necrosis factor-alpha (TNF-α), IL-1, IL-6, etc)47 and cytokines (IL-4, IL-10, IL-12, and IL-18)48 as well. Some of DAMPs are involved in anti-tumor immunity, such as ATP and calreticulin. The calreticulin presents a signal of “eat me” that triggers the phagocytosis.49 Additionally, DAMPs play important roles in tissue repair and promote the migration and proliferation of stem cells. They produce pro-angiogenic mediators and promote mucosal epithelial cells and myoblaster proliferation.50 The extracellular matrix is a crucial environment that cells depend on. Some DAMPs are involved in extracellular matrix production and collagen synthetization in the skin.44 However, the excessive repair causes immunological diseases as well. The robust pro-inflammatory response may go beyond the tolerance of patients. Some diseases such as colitis, SLE, vitiligo and cirrhosis are closely related to DAMPs.51
HMGB1
HMGB1 is a chromatin protein and works similar to a DNA chaperone that participates in transcription replication and DNA repair.52 When relocated into the external environment, it interacts with various receptors and activates immune responses to estimate pathogens and promote the repairs. Thus, that it can lead to immune diseases as well when it overreacts.53 A recent study indicated that hepatocyte injury in hepatitis B would release HMGB1 which leads to exacerbate the pathological injury.54
RAGE and TLRs are classically defined receptors. RAGE is a multifunctional transmembrane protein of the immunoglobulin superfamily. It is lowly expressed by most normal tissues but highly expressed in the lungs in physiological conditions.55 Only in the pathological condition can it be highly expressed by different tissues, especially on the surface of endothelial cells and leukocytes.56
When HMGB1 is combined with RAGE, the signal will be delivered by NF-κB and ERK/MAP kinase to enhance the expression of adhesion molecule such as VCAM-1 and ICAM-1. It also promotes the secretion of cytokines such as CXCL12 to take part in the recruitment and migration indirectly on account of the complex of HMGB1 and CXCL12 will activate CXCR4, which promotes the migration.50 TLRs are type-1 transmembrane proteins. TLR9 can recognize the CpG-ODNs and HMGB1 complex and enhance plasmacytoid DCs to produce cytokine.57 TLR2 is located in the nucleosome and can activate DCs and microphages when combined with HMGB1, while TLR4 participates in inflammation and immune regulation.58
ATP
ATP is a universal energy source, which also has vascular activation function.59 The receptors of ATP are P2 receptors, which can be further subdivided into P2YRs and P2XRs.45P2YR is also involved in chronic diseases such as atherosclerosis, transplant rejection, and asthma and is related to chronic inflammation conditions such as allergic airway diseases and asthma.60 P2Y2R plays a role in mucociliary clearance and wound healing.45 Apoptotic cells release ATP that combines with P2Y2R, which sets off a signal of “find me” that triggers the phagocytic activity and the clearance of apoptotic cells. Among P2XRs, P2X7R is highly expressed on mast cells, macrophages, microglia and DCs.61 It promotes the inflammatory defense against the pathogens and cancers. ATP can be passively released by dead tumor cells. When combined with P2X7R on DCs, it can promote cytotoxic CD8+ T cells to kill cancer cells.62
In general, PAMP and DAMP play a role in the body’s anti-tumor immunity by activating DC cells and enhancing antigen delivery, thus killing tumor cells through the antibody and Fas-FasL pathways (Figure 1).
Figure 1.
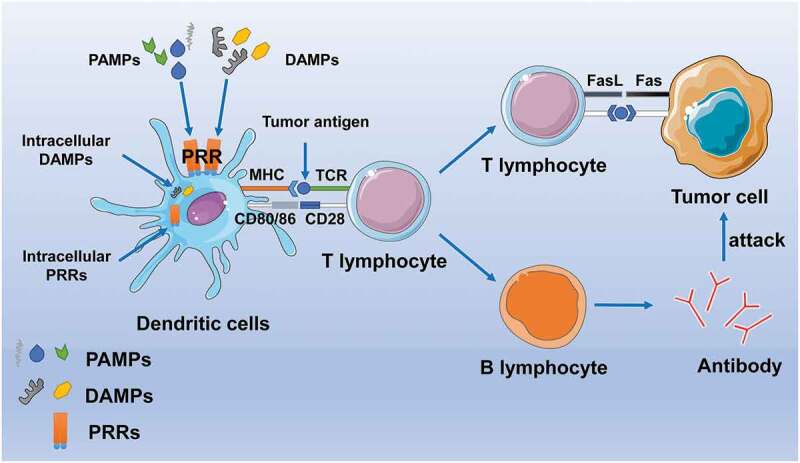
Schematic expression of several PAMP and DAMP signals. TLR and IL-1 R usually share a common signaling event. PAMP and DAMP bind to corresponding ligands, TLR dimer forms a homodimer or heterodimer, and conformational changes occur. MyD88 was the first adaptor molecule to be identified and is involved in all TLR (except TLR3)-induced signal transduction.MyD88 relies on a pathway to initiate a series of events that lead to the activation of IRAK kinase, which leads to the activation of NF-κB transcription factor and the expression of the pro-validation factor gene. In response to TLR stimulation, the MYD88-dependent pathway also activates the interferon regulatory factor (IRF) family of transcription factors IRF1, IRF5, or IRF7 phosphorylation mecha-intensity, which lead to formation of their translocated nuclei and bind to target interferon stimulation-responsive elements (ISRES) with the transcription co-activators association. IRF1 and IRF7 are involved in transcriptional control of type I interferon, while IRF5 is involved in the transcription of all pro-inflammatory cytokines tested to date by TLRs.
Application of PAMPs in vaccine
Two main types of (PAMPs) exist. One is similar to a kind of bacterial cell wall components mainly composed of sugars and lipids, including lipopolysaccharides, peptidoglycan for the adjuvants in RNA vaccines, lipoteichoic acid, mannose, lipoproteins, flagellins for the adjuvants in DNA vaccines, lipids for the adjuvants in RNA and DNA vaccines.44 The other includes viral products and bacterial cell nucleus components, such as non-methylated oligonucleotide CpG DNA for the adjuvants in DNA and RNA vaccines, ssRNA and dsRNA for the adjuvants in DNA vaccines.48 PAMPs are potential vaccine adjuvants that activate macrophages and DCs. The main research applications at present are as follows.
Application of flagellin in tumor vaccine
Flagellin is found to possess both satisfactory immunogenicity and surprising adjuvant activity.63 TLR is a natural immune PRR that can recognize pathogens and activate innate immunity.64 As shown in studies utilizing mouse models, flagellin, a TLR5 ligand, decreases immune tolerance for autoantigens and promotes immune responses to tumor antigens.65 TLR5 functions as a bacterial flagellin receptor that mobilizes nuclear factor NF-κB and stimulates TNF-α production via a MyD88-dependent pathway.66 Flagellin can also be recognized by the other members of the NLR family, including NLR family CARD domain-containing protein 4 (NLRC4, which triggers inflammasome formation)67,68 and neuronal apoptosis inhibitory protein 5 (NAIP5, the inflammasome receptor for bacteria) .69For example, an enhanced tumor-specific CD8+ T-cell immune response was obtained via TLR5 stimulation in a therapeutic cancer vaccine model.70 As a result, flagellin has been used in the development of tumor vaccines and cancer immunotherapy.71,72
Application of LPS in tumor vaccine
LPS has been used as an adjuvant for a long time, but it is dangerous due to its endotoxin essence.73 As a modifier of LPS, MPLA has lower toxicity and retains immune stimulation activity. MPLA combines with basic cell growth factor, which increases IgG and IFN-γ levels in mice, leading to DCs-induced cytokine activation of CTLs to demonstrate nonspecific anti-tumor effects.73
Application of CPG-ODN in tumor vaccine
Studies have shown that k3-CPG-ODN (a TLR9-targeted PAMP and a common pharmaceutical additive) can be added to the seasonal influenza cleaved vaccine to enhance the induction of protective type 1 cell immune response, and it can also inhibit the induction of specific IgE production, thereby greatly reducing the body’s allergic reaction.74
A CpG-oligodeoxynucleotides (CpG-OND) crosslinked aminated β-glucan-ovalbumin dual targeting nanoparticle (CpG-OND-AG-OVA) plays dual roles as ionic crosslinker and immunopotentiator,75 which enhances the ability of antigen uptake and the process of protein hydrolysis, resulting in APCs maturation, inducing robust Th1 and Th2-type immune responses comparable to Freund’s adjuvant without obvious toxicity.76–78
Hence, PAMPs can be used as potential as adjuvants. Studies have shown that PAMPs are immobilized with apatite within the pores and on the surface of mesoporous silica (MS) in the preparation of novel MS-Ap-PAMP adjuvants.79 Particle size is important for its activity.79–82 An appropriate size of particles can facilitate its absorption by immune cells, and the antigen can be loaded into the biological barrier as a result.83–85 The size of the granular adjuvant is related to the intensity and type of immune response induced.79 In a previous study, cell-mediated anti-tumor immunity that was markedly improved compared with to commercial alum adjuvant in vitro and in vivo.58
Application of DAMPs in vaccine
DAMPs, a type of endogenous adjuvants, are recognized by receptors expressed on APCS. They function by activating the NF-κB pathway, which induces cell maturation and production of inflammatory cytokines.86 DAMPs are composed of the following 4 types: 1) intracellular protein molecules, HMGB1 and HSP for the adjuvants in DNA and RNA vaccines, S100 for the adjuvants in DNA vaccines; 2) non-protein purine molecules and their degradation products, ATP, ADP, uric acid, adenosine for the adjuvants in DNA vaccines; 3) extracellular matrix degradation products (hyaluronic acid, heparin sulfate for the adjuvants in DNA vaccines; 4) a group of cytokines secreted by leukocytes (IL-1, IL-33 for the adjuvants in DNA and RNA vaccines).
Application of HMGB1 in tumor vaccine
As an endogenous adjuvant, HMGB1 and heat shock protein (HSP) are most commonly used among DAMPs.87 HMGB1 is expressed in all eukaryotic cells and is a highly conserved nuclear protein consisting of two DNA-binding regions (box A and box B) and a negatively charged C-terminal tail. HMGB1 can stimulate innate immune response by combining TIL2/L and RAGE, thus promoting T-cell activation and regulating adaptive immune responses.88,89 Studies have shown that HMGB1 can enhance anti-influenza immunity when injected with DNA vaccine.90 Moreover, HMGB1 can effectively enhance the protective efficacy and cellular immune responses of tuberculosis subunit vaccine.91 HMGB1 has also been shown to play an inflammatory role when used in combination with the human immunodeficiency virus-1-Ag-encoded DNA vaccine by enhancing the antibody responses and the CD8+T-cell IFN-γ response.90,92 In addition, extracellular HMGB1, as an adjuvant, has been shown to enhance the immunogenicity of apoptotic lymphoma cells and induce antibody responses to soluble ovalbumin.93 It has also been shown to delay tumor growth and increase tumor-free survival in mice as an adjuvant.93
Application of HSP in tumor vaccine
(HSP), first discovered in Drosophila and expressed in bacteria and mammals, is a highly conserved molecule that induces anti-tumor and anti-infective immunity as an adjuvant.94 Studies have also shown that vaccines administered in combination with HSP peptide complexes increase the responses of CTL, NK, and NKT cells to tumor and viral antigens.49 Furthermore, the prophylactic inoculation of microbial HSP produces significant protective immunity against bacterial, fungal, and mycobacterial infections.95 HSP70, a widely used adjuvant, has been shown to help newborn mice fight herpes simplex virus.96 Studies have shown that HSP70L1, a novel HSP derived from DCs, can promote DC maturation and stimulate the secretion of TNF, MIP-1 and chemokine IP-10 by combining with the receptors on DCs. The ability of HSP70L1 to activate DCs has demonstrated that it may provide new possibilities for adjuvants used in peptide immunity.97 We summarized some signal events of PAMPs and DAMPs (Figure 2). In Table 1, we listed the common DAMP receptors, downstream, and their roles in immunity. In Table 2, the vaccines with DAMPs and PAMPs that have advanced to clinical studies and also a subsection for those in preclinical studies.
Figure 2.

Schematic expression of how PAMPs and DAMPs enhance the body’s anti-tumor immunity. PAMPs and DAMPs bind to PRR receptors in the APC membrane or cytoplasm and enhance APC activation and uptake of tumor antigens, presenting them to T cells to activate them. On the one hand, activated T cells induced apoptosis of tumor cells through the Fas/FasL pathway. On the other hand, they can also assist B cell activation to produce specific antibodies to destroy the tumor.
Table 1.
Common DAMP receptors, downstream, and their roles in immunity
| DAMPS | Receptor | Downstream | Role in immunity |
|---|---|---|---|
| HMGB1 | TLR2, TLR4, TLR9 | MyD88-TRAF6-NF-κB MAPK/ERK |
Induce cytokine release and recruit leucocytes |
| Histones | TLR2, TLR4 | MyD88-TRAF6-NF-κB MAPK/ERK |
Activate human polymorphonuclear neutrophils |
| HSP | TLR2, TLR4 | MyD88-TRAF6-NF-κB MAPK/ERK |
Promote maturation and activity of DCs |
| IL-1 | IL-1 R | MyD88-TRAF6-NF-κB MAPK/ERK |
Promote tumor cell proliferation and neo-vascularization |
| Mitochondrial N-formyl peptides | FPR1 | ERK, Akt, and STAT3 | Enhance vascular permeability |
| CIRBP | TLR4 | MyD88-TRAF6-NF-κB MAPK/ERK |
Promote cell migration |
| S100 calcium-binding proteins |
RAGE | NOX2, NF-κB | Activate endothelial cells, macrophages, and lymphocytes |
| ATP | P2XR | (Na+, Ca2+, K+) p38 MAPK, PLA2 |
Recruit macrophages and promote clearance of apoptotic cells |
| HDGF | NUCLEOLIN | NCL-PI3K-AKT | Stimulate the growth and proliferation of fibroblasts, endothelial cells, vascular smooth and some hepatoma cells |
| fHA | TLR4 | TIRAP, MyD88 and Trif | Induces DCs to produce the proinflammatory cytokine and upregulate costimulatory molecules |
| mtDNA | TLR9, FPR1 | cGAS-STING-IRF3 | Activate circulating neutrophils to mediate tissue injury |
Table 2.
Summary of vaccines with DAMPs and PAMPs and on going clinical trials results
| Registration | Patient population | Drug | Result |
|---|---|---|---|
| NCT00524277 | Breast cancer, node-positive or high-risk node- negative | GP2 peptide 1 GM-CSF vaccine | No difference in DFS between study arm and control arm |
| Dnr151:785/2001 | Metastatic breast cancer, HER2 positive |
HER2- plasmid DNA vaccine 1 GM-CSF and IL-2 | No toxic effects, immune response identified |
| NCT00121173 | Cervical Cancer | HSP70+ pNGVL4a-Sig/E7(detox) | The incidence was reduced effectively after long-term observation, but the relationship between components was not clear |
| NCT00971737 | Metastatic breast cancer, HER2 negative |
MCF-7 vaccine 1 GM-CSF and IL-2 | Significant improvement in DSS in vaccinated patients with immunity loss |
| NCT01079741 | Melanoma | NY-ESO-1 protein, Poly-ICLC and Montanide | Significantly improved the CD4+ T-cell response of patients compared with Poly-ICLC alone |
| NCT02254772 | Extranodal Marginal Zone B-cell Lymphoma of Mucosa-associated Lymphoid Tissue Nodal Marginal Zone B-cell Lymphoma Recurrent Grade 1 Follicular Lymphoma Recurrent Grade 2 Follicular Lymphoma Recurrent Marginal Zone Lymphoma Recurrent Small Lymphocytic Lymphoma Splenic Marginal Zone Lymphoma |
Ipilimumab and SD-101 | No significant therapeutic effect has been observed yet |
| NCT02501473 | Follicular Low Grade Non-Hodgkin’s Lymphoma | G100, Pembrolizumab and Rituximab | Possibly reduced the mortality of application of Pembrolizumab with dose dependence leaving alone the small sample capacity |
| NCT02041429 | Recurrent Breast Cancer Metastatic Breast Cancer |
Ruxolitinib and Paclitaxel | The efficacy of Paclitaxel had been improved to some extent, but the sample size needed to be enlarged |
| NCT02151448 | Malignant Neoplasm of Pancreas Metastatic to Peritoneal Surface Malignant Peritoneal Mesothelioma Peritoneal Carcinomatosis |
IFN-α-2b, Celecoxib and rintatolimod | Phase 1/2 trial proved that the vaccine was effective, and the specific mechanism needs to be followed up |
Data from References115–120
GP2, Glycoprotein 2; GM-CSF, Granulocyte-macrophage Colony Stimulating Factor; HER2,human epidermal growth factor receptor-2; HSP70, heat shock proteins 70; MCF-7, Michigan Cancer Foundation-7; IL-2, Interleukin-2; NY-ESO-1, New York esophageal squamous cell carcinoma 1; Poly-ICLC, poly-L-lysine and carboxymethylcellulose; IFN, Interferon.
Advantages of nucleic acid vaccine
Future nucleic acid vaccines will have the following advantages: 1) direct DNA inoculation: avoiding the tedious process of preparing traditional vaccines. 2) After nucleic acid vaccine inoculation, protein antigens can directly combine with MHC-I and MHC-II molecules to form immune complexes, which can cause CTL reaction as well as live attenuated vaccine or carrier vaccine, but there is no risk of the latter’s virulence rebound. 3) The process of presentation of immune antigenic peptides produced by genes is very similar to that produced by natural infection, which is particularly important for protective immunity caused by conformational epitopes. In vitro synthesis of protein epitopes by current recombination techniques often results in the alteration or deletion of epitopes. 4) Nucleic acid vaccines have common physical and chemical properties, which provide the possibility of combined immunization. 5) As a recombinant plasmid, the nucleic acid vaccine can proliferate rapidly in the engineered coliform bacteria, and it is simple to extract and purify, which can greatly reduce the cost and save time and effort. 6) Nucleic acid vaccines have little influence on the host’s preexisting immunity, which is one of their incomparable advantages of nucleic acid vaccines. 7) Nucleic acid vaccines can be used not only for prevention, but also for treatment.
Future development direction of nucleic acid vaccine
Although nucleic acid vaccines have considerable advantages, there is still a lot of room for improvement. In the next five years, the following research directions may be achieved: 1) Vaccines can be combined with immune checkpoint, antibody, oncolytic virus, and other therapies to improve their therapeutic efficacy. 2) With the further development of related studies, the search for new TSAs as targets can more accurately stimulate the immune system to kill tumor cells. 3) Individualized treatment for different patients will also become a new trend in the development of nucleic acid vaccines. 4) Given that adjuvants play an important role in enhancing immune induction and immune response in cancer vaccines, the discovery and research of new adjuvants have become increasingly important. 5) In addition, delivery vectors for cancer vaccines are also an important component. A safe and efficient delivery vector can improve the expression efficiency of antigen epitopes in the body and induce specific immunity. At the same time, it is also an important breakthrough in the clinical development of cancer vaccines.
The deficiency of the innate immune system activation signal is one of the reasons for the reduced immune response of the vaccine. In most cases, adjuvants need to be added to the preparation to achieve protective response, and DAMPs and PAMRs can make up for this deficiency by activating the innate immunity of the human body. As the function of PRRs, such as TLRs, has become apparent over the past few decades, various PRR ligands have been developed for use as adjuvants of PAMPs.
Conclusion
The answer to the question of whether PAMPs and DAMPS can be used as adjuvants for cancer vaccines is yes, and after more than a decade of research on PAMPs and DAMPS, the understanding of their roles in innate and adaptive immunity is becoming clearer. However, the current variety of PAMPs and DAMPs is not very rich. The discovery of new PAMPs and DAMPs and their specific effects, as well as their interaction with TAA and TSA as adjuvants, has become increasingly important.
Finally, as adjuvants, PAMPs/DAMPs have the following advantages over traditional adjuvants. First, PAMPs/DAMPs enhance specific immune responses. Secondly, PAMPs/DAMPs induce less autoimmune response, which also results in less toxic side effects. PAMPs/DAMPs can then be expressed intracellular to produce immune factors and enhance the immune response. However, traditional adjuvants are easily engulfed and cleared by the body’s innate immune system, so that they are not conducive to the release of specific cytokines. In addition, the preparation process of PAMPs/DAMPs is simple and economical.
However, there are problems with PAMPS/DAMPS. The first is its reaction mechanism in the immune response process, which needs to be further clarified. Secondly, over-expression of PAMPs/DAMPs may lead to the development of other diseases, such as tumors. Although this is less likely in the short term, the long-term effects need to be observed further.
Funding Statement
This project is supported by grants from the National Natural Science Foundation of China (Nos. 81871869, 81803080 and 82072814), the Natural Science Foundation of Jiangsu Province (No. BK20180990), Jiangsu Province Social Development Key Projects (Nos. BE2020641, BE2020640), Key Research Development project of Xuzhou (No. KC19082), the Natural Science Key Project of Jiangsu Provincial Education Department (No. 19KJA470001), Youth Technology Innovation Team of Xuzhou Medical University (No. TD202003), Jiangsu Provincial Key Medical Discipline, The Project of Invigorating Health Care through Science, Technology and Education (No. ZDXKA2016014) and the Qing Lan Project of Jiangsu Province.
Disclosure statement
The authors declare that they have no competing interests.
References
- 1.Wang JJ, Lei KF, Han F.. Tumor microenvironment: recent advances in various cancer treatments. Eur Rev Med Pharmacol Sci. 2018;22:3855–64. [DOI] [PubMed] [Google Scholar]
- 2.Garcon N, Chomez P, Van Mechelen M.. GlaxoSmithKline adjuvant systems in vaccines: concepts, achievements and perspectives. Expert Rev Vaccines. 2007;6:723–39. doi: 10.1586/14760584.6.5.723. [DOI] [PubMed] [Google Scholar]
- 3.Golos A, Lutynska A. Aluminium-adjuvanted vaccines–a review of the current state of knowledge. Przegl Epidemiol. 2015;69:871–74. [PubMed] [Google Scholar]
- 4.Gatti-Mays ME, Redman JM, Collins JM, Bilusic M. Cancer vaccines: enhanced immunogenic modulation through therapeutic combinations. Hum Vaccin Immunother. 2017;13:2561–74. doi: 10.1080/21645515.2017.1364322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aldous AR, Dong JZ. Personalized neoantigen vaccines: a new approach to cancer immunotherapy. Bioorg Med Chem. 2018;26:2842–49. doi: 10.1016/j.bmc.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 6.Schlom J, Hodge JW, Palena C, Tsang KY, Jochems C, Greiner JW, Farsaci B, Madan RA, Heery CR, Gulley JL. Therapeutic cancer vaccines. Adv Cancer Res. 2014;121:67–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 8.DeMaria PJ, Cancer Vaccines BM. Cancer vaccines. Hematol Oncol Clin North Am. 2019;33(2):199–214. doi: 10.1016/j.hoc.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Handy CE, Antonarakis ES. Sipuleucel-T for the treatment of prostate cancer: novel insights and future directions. Future Oncol. 2018;14:907–17. doi: 10.2217/fon-2017-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burke EE, Kodumudi K, Ramamoorthi G, Czerniecki BJ. Vaccine therapies for Breast Cancer. Surg Oncol Clin N Am. 2019;28:353–67. doi: 10.1016/j.soc.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Tiptiri-Kourpeti A, Spyridopoulou K, Pappa A, Chlichlia K. DNA vaccines to attack cancer: strategies for improving immunogenicity and efficacy. Pharmacol Ther. 2016;165:32–49. [DOI] [PubMed] [Google Scholar]
- 12.Pilla L, Ferrone S, Maccalli C. Methods for improving the immunogenicity and efficacy of cancer vaccines. Expert Opin Biol Ther. 2018;18:765–84. doi: 10.1080/14712598.2018.1485649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banerjee K, Kumar S, Ross KA, Gautam S, Poelaert B, Nasser MW, Aithal A, Bhatia R, Wannemuehler MJ, Narasimhan B, et al. Emerging trends in the immunotherapy of pancreatic cancer. Cancer Lett. 2018;417:35–46. doi: 10.1016/j.canlet.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner S, Mullins CS, Linnebacher M. Colorectal cancer vaccines: tumor-associated antigens vs neoantigens. World J Gastroenterol. 2018;24:5418–32. doi: 10.3748/wjg.v24.i48.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tran T, Blanc C, Granier C, Saldmann A, Tanchot C, Tartour E. Therapeutic cancer vaccine: building the future from lessons of the past. Semin Immunopathol. 2019;41:69–85. doi: 10.1007/s00281-018-0691-z. [DOI] [PubMed] [Google Scholar]
- 16.Peng M, Mo Y, Wang Y, Wu P, Zhang Y, Xiong F, Guo C, Wu X, Li Y, Li X, et al. Neoantigen vaccine: an emerging tumor immunotherapy. Mol Cancer. 2019;18:128. doi: 10.1186/s12943-019-1055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veisi Malekshahi Z, Hashemi Goradel N, Shakouri Khomartash M, Maleksabet A, Kadkhodazadeh M, Kardar GA, Negahdari B. CEA plasmid as therapeutic DNA vaccination against colorectal Cancer. Iran J Immunol. 2019;16:235–45. [DOI] [PubMed] [Google Scholar]
- 18.Li YL, Qiu XH, Shen C, Liu JN, Zhang J. Vaccination of full-length HPV16 E6 or E7 protein inhibits the growth of HPV16 associated tumors. Oncol Rep. 2010;24:1323–29. [DOI] [PubMed] [Google Scholar]
- 19.Martinez-Perez AG, Perez-Trujillo JJ, Garza-Morales R, Ramirez-Avila NE, Loera-Arias MJ, Gomez-Gutierrez JG, Saucedo-Cardenas O, Garcia-Garcia A, Rodriguez-Rocha H, Montes-de-Oca-Luna R, et al. An oncolytic adenovirus encoding SA-4-1BBL adjuvant fused to HPV-16 E7 antigen produces a specific antitumor effect in a Cancer Mouse model. Vaccines (Basel). 2021;9:149. doi: 10.3390/vaccines9020149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garza-Morales R, Perez-Trujillo JJ, Martinez-Jaramillo E, Saucedo-Cardenas O, Loera-Arias MJ, Garcia-Garcia A, Rodriguez-Rocha H, Yolcu E, Shirwan H, Gomez-Gutierrez J, et al. A DNA vaccine encoding SA-4-1BBL fused to HPV-16 E7 antigen has prophylactic and therapeutic efficacy in a cervical Cancer Mouse model. Cancers (Basel). 2019;11:96. doi: 10.3390/cancers11010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gautam SK, Kumar S, Dam V, Ghersi D, Jain M, Batra SK. MUCIN-4 (MUC4) is a novel tumor antigen in pancreatic cancer immunotherapy. Semin Immunol. 2020;47:101391. doi: 10.1016/j.smim.2020.101391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan RY, Chung WH, Chu MT, Chen SJ, Chen HC, Zheng L, Hung S-I. Recent development and clinical application of cancer vaccine: targeting neoantigens. J Immunol Res. 2018;2018:4325874. doi: 10.1155/2018/4325874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Temizoz B, Kuroda E, Ishii KJ. Vaccine adjuvants as potential cancer immunotherapeutics. Int Immunol. 2016;28:329–38. doi: 10.1093/intimm/dxw015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pellegrino P, Clementi E, Radice S. On vaccine’s adjuvants and autoimmunity: current evidence and future perspectives. Autoimmun Rev. 2015;14:880–88. doi: 10.1016/j.autrev.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 25.Shah RR, Hassett KJ, Brito LA. Overview of vaccine adjuvants: introduction, history, and current status. Methods Mol Biol. 2017;1494:1–13. [DOI] [PubMed] [Google Scholar]
- 26.Janeway CA Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/SQB.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Satoh T, Akira S, Gordon S. Toll-Like Receptor Signaling and Its Inducible Proteins. Microbiol Spectr. 2016;4:1–7. doi: 10.1128/microbiolspec.MCHD-0040-2016. [DOI] [PubMed] [Google Scholar]
- 28.Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85:85–95. doi: 10.1016/S0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 29.Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev. 2012;249:158–75. doi: 10.1111/j.1600-065X.2012.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delgado MA, Elmaoued RA, Davis AS, Kyei G, Deretic V. Toll-like receptors control autophagy. EMBO J. 2008;27:1110–21. doi: 10.1038/emboj.2008.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cui J, Chen Y, Wang HY, Wang RF. Mechanisms and pathways of innate immune activation and regulation in health and cancer. Hum Vaccin Immunother. 2014;10:3270–85. doi: 10.4161/21645515.2014.979640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 33.Land W, Schneeberger H, Schleibner S, Illner WD, Abendroth D, Rutili G, Arfors KE, Messmer K. The beneficial effect of human recombinant superoxide dismutase on acute and chronic rejection events in recipients of cadaveric renal transplants. Transplantation. 1994;57:211–17. doi: 10.1097/00007890-199401001-00010. [DOI] [PubMed] [Google Scholar]
- 34.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–21. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 35.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–95. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 36.Fleshner M, Crane CR. Exosomes, DAMPs and miRNA: features of stress physiology and immune homeostasis. Trends Immunol. 2017;38:768–76. doi: 10.1016/j.it.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel S. Danger-Associated Molecular Patterns (DAMPs): the derivatives and triggers of inflammation. Curr Allergy Asthma Rep. 2018;18:63. doi: 10.1007/s11882-018-0817-3. [DOI] [PubMed] [Google Scholar]
- 38.Kang R, Chen R, Zhang Q, Hou W, Wu S, Cao L, Huang J, Yu Y, Fan XG, Yan Z, et al. HMGB1 in health and disease. Mol Aspects Med. 2014;40:1–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garg AD, Dudek AM, Agostinis P. Cancer immunogenicity, danger signals, and DAMPs: what, when, and how? Biofactors. 2013;39:355–67. doi: 10.1002/biof.1125. [DOI] [PubMed] [Google Scholar]
- 40.Raghavan M, Wijeyesakere SJ, Peters LR, Del Cid N. Calreticulin in the immune system: ins and outs. Trends Immunol. 2013;34:13–21. doi: 10.1016/j.it.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Donato R, Cannon BR, Sorci G, Riuzzi F, Hsu K, Weber DJ, Geczy CL. Functions of S100 proteins. Curr Mol Med. 2013;13:24–57. doi: 10.2174/156652413804486214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nace G, Evankovich J, Eid R, Tsung A. Dendritic cells and damage-associated molecular patterns: endogenous danger signals linking innate and adaptive immunity. J Innate Immun. 2012;4:6–15. doi: 10.1159/000334245. [DOI] [PubMed] [Google Scholar]
- 43.Wilgus TA. Alerting the body to tissue injury: the role of alarmins and DAMPs in cutaneous wound healing. Curr Pathobiol Rep. 2018;6:55–60. doi: 10.1007/s40139-018-0162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. [DOI] [PubMed] [Google Scholar]
- 45.Idzko M, Ferrari D, Eltzschig HK. Nucleotide signalling during inflammation. Nature. 2014;509:310–17. doi: 10.1038/nature13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Venereau E, Ceriotti C, Bianchi ME. DAMPs from cell death to new life. Front Immunol. 2015;6:422. doi: 10.3389/fimmu.2015.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krentz T, Allen S. Bacterial translocation in critical illness. J Small Anim Pract. 2017;58:191–98. doi: 10.1111/jsap.12626. [DOI] [PubMed] [Google Scholar]
- 48.Zindel J, Kubes P. DAMPs, PAMPs, and LAMPs in immunity and sterile inflammation. Annu Rev Pathol. 2020;15:493–518. doi: 10.1146/annurev-pathmechdis-012419-032847. [DOI] [PubMed] [Google Scholar]
- 49.Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12:860–75. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- 50.Schiraldi M, Raucci A, Munoz LM, Livoti E, Celona B, Venereau E, Apuzzo T, De Marchis F, Pedotti M, Bachi A, et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med. 2012;209:551–63. doi: 10.1084/jem.20111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ellson CD, Dunmore R, Hogaboam CM, Sleeman MA, Murray LA. Danger-associated molecular patterns and danger signals in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2014;51:163–68. [DOI] [PubMed] [Google Scholar]
- 52.Mandke P, Vasquez KM. Interactions of high mobility group box protein 1 (HMGB1) with nucleic acids: implications in DNA repair and immune responses. DNA Repair (Amst). 2019;83:102701. doi: 10.1016/j.dnarep.2019.102701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harris HE, Andersson U, Pisetsky DS. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat Rev Rheumatol. 2012;8:195–202. doi: 10.1038/nrrheum.2011.222. [DOI] [PubMed] [Google Scholar]
- 54.Yang Q, Shi Y, Yang Y, Lou G, Chen Z. The sterile inflammation in the exacerbation of HBV-associated liver injury. Mediators Inflamm. 2015;2015:508681. doi: 10.1155/2015/508681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oczypok EA, Perkins TN, Oury TD. All the “RAGE” in lung disease: the receptor for advanced glycation endproducts (RAGE) is a major mediator of pulmonary inflammatory responses. Paediatr Respir Rev. 2017;23:40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kierdorf K, Fritz G. RAGE regulation and signaling in inflammation and beyond. J Leukoc Biol. 2013;94:55–68. doi: 10.1189/jlb.1012519. [DOI] [PubMed] [Google Scholar]
- 57.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–96. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 58.Wang X, Sun R, Wei H, Tian Z. High-mobility group box 1 (HMGB1)-Toll-like receptor (TLR)4-interleukin (IL)-23-IL-17A axis in drug-induced damage-associated lethal hepatitis: interaction of gammadelta T cells with macrophages. Hepatology. 2013;57:373–84. doi: 10.1002/hep.25982. [DOI] [PubMed] [Google Scholar]
- 59.Szeto V, Chen NH, Sun HS, Feng ZP. The role of KATP channels in cerebral ischemic stroke and diabetes. Acta Pharmacol Sin. 2018;39:683–94. doi: 10.1038/aps.2018.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kouzaki H, Iijima K, Kobayashi T, O’Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol. 2011;186:4375–87. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol. 2011;11:201–12. doi: 10.1038/nri2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–78. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 63.Cui B, Liu X, Fang Y, Zhou P, Zhang Y, Wang Y. Flagellin as a vaccine adjuvant. Expert Rev Vaccines. 2018;17:335–49. doi: 10.1080/14760584.2018.1457443. [DOI] [PubMed] [Google Scholar]
- 64.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 65.Smith KD, Andersen-Nissen E, Hayashi F, Strobe K, Bergman MA, Barrett SL, Cookson BT, Aderem A. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat Immunol. 2003;4:1247–53. doi: 10.1038/ni1011. [DOI] [PubMed] [Google Scholar]
- 66.Miao EA, Andersen-Nissen E, Warren SE, Aderem A. TLR5 and Ipaf: dual sensors of bacterial flagellin in the innate immune system. Semin Immunopathol. 2007;29:275–88. doi: 10.1007/s00281-007-0078-z. [DOI] [PubMed] [Google Scholar]
- 67.Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006;7:569–75. [DOI] [PubMed] [Google Scholar]
- 68.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–18. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 69.Lightfield KL, Persson J, Brubaker SW, Witte CE, von Moltke J, Dunipace EA, Henry T, Sun Y-H, Cado D, Dietrich WF, et al. Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat Immunol. 2008;9:1171–78. doi: 10.1038/ni.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Faham A, Altin JG. Antigen-containing liposomes engrafted with flagellin-related peptides are effective vaccines that can induce potent antitumor immunity and immunotherapeutic effect. J Immunol. 2010;185:1744–54. doi: 10.4049/jimmunol.1000027. [DOI] [PubMed] [Google Scholar]
- 71.Hayashi T, Momota M, Kuroda E, Kusakabe T, Kobari S, Makisaka K, Ohno Y, Suzuki Y, Nakagawa F, Lee MSJ, et al. DAMP-inducing adjuvant and PAMP adjuvants parallelly enhance protective Type-2 and Type-1 immune responses to influenza split vaccination. Front Immunol. 2018;9:2619. doi: 10.3389/fimmu.2018.02619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Panda AK. Induction of anti-tumor immunity and T-cell responses using nanodelivery systems engrafting TLR-5 ligand. Expert Rev Vaccines. 2011;10:155–57. doi: 10.1586/erv.10.164. [DOI] [PubMed] [Google Scholar]
- 73.Rossol M, Heine H, Meusch U, Quandt D, Klein C, Sweet MJ, Hauschildt S. LPS-induced cytokine production in human monocytes and macrophages. Crit Rev Immunol. 2011;31:379–446. doi: 10.1615/CritRevImmunol.v31.i5.20. [DOI] [PubMed] [Google Scholar]
- 74.Mochizuki S, Morishita H, Kobiyama K, Aoshi T, Ishii KJ, Sakurai K. Immunization with antigenic peptides complexed with beta-glucan induces potent cytotoxic T-lymphocyte activity in combination with CpG-ODNs. J Control Release. 2015;220:495–502. doi: 10.1016/j.jconrel.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 75.Jin JW, Tang SQ, Rong MZ, Zhang MQ. Synergistic effect of dual targeting vaccine adjuvant with aminated beta-glucan and CpG-oligodeoxynucleotides for both humoral and cellular immune responses. Acta Biomater. 2018;78:211–23. doi: 10.1016/j.actbio.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 76.Doita M, Rasmussen LT, Seljelid R, Lipsky PE. Effect of soluble aminated beta-1,3-D-polyglucose on human monocytes: stimulation of cytokine and prostaglandin E2 production but not antigen-presenting function. J Leukoc Biol. 1991;49:342–51. doi: 10.1002/jlb.49.4.342. [DOI] [PubMed] [Google Scholar]
- 77.Tincer G, Yerlikaya S, Yagci FC, Kahraman T, Atanur OM, Erbatur O, Gursel I. Immunostimulatory activity of polysaccharide-poly(I:C) nanoparticles. Biomaterials. 2011;32:4275–82. doi: 10.1016/j.biomaterials.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 78.Han J, Cai J, Borjihan W, Ganbold T, Rana TM, Baigude H. Preparation of novel curdlan nanoparticles for intracellular siRNA delivery. Carbohydr Polym. 2015;117:324–30. doi: 10.1016/j.carbpol.2014.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oyewumi MO, Kumar A, Cui Z. Nano-microparticles as immune adjuvants: correlating particle sizes and the resultant immune responses. Expert Rev Vaccines. 2010;9:1095–107. doi: 10.1586/erv.10.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li X, Sloat BR, Yanasarn N, Cui Z. Relationship between the size of nanoparticles and their adjuvant activity: data from a study with an improved experimental design. Eur J Pharm Biopharm. 2011;78:107–16. doi: 10.1016/j.ejpb.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gutierro I, Hernandez RM, Igartua M, Gascon AR, Pedraz JL. Size dependent immune response after subcutaneous, oral and intranasal administration of BSA loaded nanospheres. Vaccine. 2002;21:67–77. doi: 10.1016/S0264-410X(02)00435-8. [DOI] [PubMed] [Google Scholar]
- 82.Fifis T, Gamvrellis A, Crimeen-Irwin B, Pietersz GA, Li J, Mottram PL, McKenzie IFC, Plebanski M. Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. J Immunol. 2004;173:3148–54. doi: 10.4049/jimmunol.173.5.3148. [DOI] [PubMed] [Google Scholar]
- 83.Xiang SD, Scholzen A, Minigo G, David C, Apostolopoulos V, Mottram PL, Plebanski M. Pathogen recognition and development of particulate vaccines: does size matter? Methods. 2006;40:1–9. doi: 10.1016/j.ymeth.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 84.Russell J, Mumper, Z, Cui, Moses, et al. Nanotemplate engineering of cell specific nanoparticles. Journal of Dispersion Science and Technology. 2003;24:569–88. [Google Scholar]
- 85.Panyam J, Labhasetwar V. Dynamics of endocytosis and exocytosis of poly(D,L-lactide-co-glycolide) nanoparticles in vascular smooth muscle cells. Pharm Res. 2003;20:212–20. doi: 10.1023/A:1022219003551. [DOI] [PubMed] [Google Scholar]
- 86.Miyake Y, Yamasaki S. Sensing necrotic cells. Adv Exp Med Biol. 2012;738:144–52. [DOI] [PubMed] [Google Scholar]
- 87.Ciocca DR, Cayado-Gutierrez N, Maccioni M, Cuello-Carrion FD. Heat shock proteins (HSPs) based anti-cancer vaccines. Curr Mol Med. 2012;12:1183–97. doi: 10.2174/156652412803306684. [DOI] [PubMed] [Google Scholar]
- 88.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–88. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 89.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 90.Fagone P, Shedlock DJ, Bao H, Kawalekar OU, Yan J, Gupta D, Morrow MP, Patel A, Kobinger GP, Muthumani K, et al. Molecular adjuvant HMGB1 enhances anti-influenza immunity during DNA vaccination. Gene Ther. 2011;18:1070–77. doi: 10.1038/gt.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grover A, Troudt J, Foster C, Basaraba R, Izzo A. High mobility group box 1 acts as an adjuvant for tuberculosis subunit vaccines. Immunology. 2014;142:111–23. doi: 10.1111/imm.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Muthumani G, Laddy DJ, Sundaram SG, Fagone P, Shedlock DJ, Kannan S, Wu L, Chung CW, Lankaraman KM, Burns J, et al. Co-immunization with an optimized plasmid-encoded immune stimulatory interleukin, high-mobility group box 1 protein, results in enhanced interferon-gamma secretion by antigen-specific CD8 T cells. Immunology. 2009;128:e612–20. doi: 10.1111/j.1365-2567.2009.03044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rovere-Querini P, Capobianco A, Scaffidi P, Valentinis B, Catalanotti F, Giazzon M, Dumitriu IE, Müller S, Iannacone M, Traversari C, et al. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep. 2004;5:825–30. doi: 10.1038/sj.embor.7400205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dhakal J, Brah GS, Agrawal RK, Pawar HN, Kaur D, Verma R. Over-expression of gene encoding heat shock protein 70 from Mycobacterium tuberculosis and its evaluation as vaccine adjuvant. Indian J Med Microbiol. 2013;31:123–29. [DOI] [PubMed] [Google Scholar]
- 95.Brenner BG, Wainberg MA. Heat shock protein-based therapeutic strategies against human immunodeficiency virus type 1 infection. Infect Dis Obstet Gynecol. 1999;7:80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pack CD, Kumaraguru U, Suvas S, Rouse BT. Heat-shock protein 70 acts as an effective adjuvant in neonatal mice and confers protection against challenge with herpes simplex virus. Vaccine. 2005;23:3526–34. doi: 10.1016/j.vaccine.2005.01.152. [DOI] [PubMed] [Google Scholar]
- 97.Wan T, Zhou X, Chen G, An H, Chen T, Zhang W, Liu S, Jiang Y, Yang F, Wu Y, et al. Novel heat shock protein Hsp70L1 activates dendritic cells and acts as a Th1 polarizing adjuvant. Blood. 2004;103:1747–54. doi: 10.1182/blood-2003-08-2828. [DOI] [PubMed] [Google Scholar]
- 98.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–62. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chandrashekaran V, Seth RK, Dattaroy D, Alhasson F, Ziolenka J, Carson J, Berger FG, Kalyanaraman B, Diehl AM, Chatterjee S, et al. HMGB1-RAGE pathway drives peroxynitrite signaling-induced IBD-like inflammation in murine nonalcoholic fatty liver disease. Redox Biol. 2017;13:8–19. doi: 10.1016/j.redox.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.de Paulis A, Prevete N, Rossi FW, Rivellese F, Salerno F, Delfino G, Liccardo B, Avilla E, Montuori N, Mascolo M, et al. Helicobacter pylori Hp(2-20) promotes migration and proliferation of gastric epithelial cells by interacting with formyl peptide receptors in vitro and accelerates gastric mucosal healing in vivo. J Immunol. 2009;183:3761–69. doi: 10.4049/jimmunol.0900863. [DOI] [PubMed] [Google Scholar]
- 101.Feng H, Zeng Y, Graner MW, Likhacheva A, Katsanis E. Exogenous stress proteins enhance the immunogenicity of apoptotic tumor cells and stimulate antitumor immunity. Blood. 2003;101:245–52. doi: 10.1182/blood-2002-05-1580. [DOI] [PubMed] [Google Scholar]
- 102.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/S0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 103.Holen I, Lefley DV, Francis SE, Rennicks S, Bradbury S, Coleman RE, Ottewell P. IL-1 drives breast cancer growth and bone metastasis in vivo. Oncotarget. 2016;7:75571–84. doi: 10.18632/oncotarget.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee WL, Slutsky AS. Sepsis and endothelial permeability. N Engl J Med. 2010;363:689–91. doi: 10.1056/NEJMcibr1007320. [DOI] [PubMed] [Google Scholar]
- 105.Lu M, Ge Q, Wang G, Luo Y, Wang X, Jiang W, Liu X, Wu C-L, Xiao Y, Wang X, et al. CIRBP is a novel oncogene in human bladder cancer inducing expression of HIF-1alpha. Cell Death Dis. 2018;9:1046. doi: 10.1038/s41419-018-1109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qiang X, Yang WL, Wu R, Zhou M, Jacob A, Dong W, Kuncewitch M, Ji Y, Yang H, Wang H, et al. Cold-inducible RNA-binding protein (CIRP) triggers inflammatory responses in hemorrhagic shock and sepsis. Nat Med. 2013;19:1489–95. doi: 10.1038/nm.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tesar BM, Jiang D, Liang J, Palmer SM, Noble PW, Goldstein DR. The role of hyaluronan degradation products as innate alloimmune agonists. Am J Transplant. 2006;6:2622–35. doi: 10.1111/j.1600-6143.2006.01537.x. [DOI] [PubMed] [Google Scholar]
- 108.Venereau E, De Leo F, Mezzapelle R, Careccia G, Musco G, Bianchi ME. HMGB1 as biomarker and drug target. Pharmacol Res. 2016;111:534–44. doi: 10.1016/j.phrs.2016.06.031. [DOI] [PubMed] [Google Scholar]
- 109.Wei Y, Chen X, Liang C, Ling Y, Yang X, Ye X, Zhang H, Yang P, Cui X, Ren Y, et al. A noncoding regulatory RNAs network driven by Circ-CDYL acts specifically in the early stages hepatocellular carcinoma. Hepatology. 2020;71:130–47. doi: 10.1002/hep.30795. [DOI] [PubMed] [Google Scholar]
- 110.West AP, Khoury-Hanold W, Staron M, Tal MC, Pineda CM, Lang SM, Bestwick M, Duguay BA, Raimundo N, MacDuff DA, et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature. 2015;520:553–57. doi: 10.1038/nature14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu J, Zhang X, Monestier M, Esmon NL, Esmon CT. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J Immunol. 2011;187:2626–31. doi: 10.4049/jimmunol.1003930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yan X, Chen S, Huang H, Peng T, Lan M, Yang X, Dong M, Chen S, Xu A, Huang S, et al. Functional variation of IL-1R-associated kinases in the conserved MyD88-TRAF6 pathway during evolution. J Immunol. 2020;204:832–43. doi: 10.4049/jimmunol.1900222. [DOI] [PubMed] [Google Scholar]
- 113.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–07. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhou Z, Yamamoto Y, Sugai F, Yoshida K, Kishima Y, Sumi H, Nakamura H, Sakoda S. Hepatoma-derived growth factor is a neurotrophic factor harbored in the nucleus. J Biol Chem. 2004;279(26):27320–26. doi: 10.1074/jbc.M308650200. [DOI] [PubMed] [Google Scholar]
- 115.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-López A, Hagenbeek A, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 116.Faivre S, Santoro A, Kelley RK, Gane E, Costentin CE, Gueorguieva I, Smith C, Cleverly A, Lahn MM, Raymond E, et al. Novel transforming growth factor beta receptor I kinase inhibitor galunisertib (LY2157299) in advanced hepatocellular carcinoma. Liver Int. 2019;39:1468–77. doi: 10.1111/liv.14113. [DOI] [PubMed] [Google Scholar]
- 117.Sathianathen NJ, Philippou YA, Kuntz GM, Konety BR, Gupta S, Lamb AD, et al. Taxane-based chemohormonal therapy for metastatic hormone-sensitive prostate cancer. Cochrane Database Syst Rev. 2018(10):CD012816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Giannelli G, Santoro A, Kelley RK, Gane E, Paradis V, Cleverly A, Smith C, Estrem ST, Man M, Wang S, et al . Correction: biomarkers and overall survival in patients with advanced hepatocellular carcinoma treated with TGF-betaRI inhibitor galunisertib. PLoS One. 2020;15:e0235580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Takeoka T, Nagase H, Kurose K, Ohue Y, Yamasaki M, Takiguchi S, Sato E, Isobe M, Kanazawa T, Matsumoto M, et al. NY-ESO-1 protein Cancer vaccine with poly-ICLC and OK-432: rapid and strong induction of NY-ESO-1-specific immune responses by Poly-ICLC. J Immunother. 2017;40:140–47. doi: 10.1097/CJI.0000000000000162. [DOI] [PubMed] [Google Scholar]
- 120.Trimble CL, Peng S, Kos F, Gravitt P, Viscidi R, Sugar E, Pardoll D, Wu TC. A phase I trial of a human papillomavirus DNA vaccine for HPV16+ cervical intraepithelial neoplasia 2/3. Clin Cancer Res. 2009;15:361–67. doi: 10.1158/1078-0432.CCR-08-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]


