ABSTRACT
Subacute thyroiditis is the most common cause of painful thyroiditis, which usually occurs after an acute viral upper respiratory tract infection. Rare cases of subacute thyroiditis have been reported after administration of viral vaccines. Here, we report four cases of subacute thyroiditis after administration of the COVID-19 mRNA vaccine (Pfizer/BioNTech®). We describe the clinical, laboratory and imaging features of five cases of subacute thyroiditis after COVID-19 mRNA vaccine (Pfizer/BioNTech®). COVID-19 mRNA vaccine (Pfizer/BioNTech®)-associated subacute thyroiditis may present with clinical findings typical of classic subacute thyroiditis such as fever, neck pain, weakness, and tremor within a few days following vaccination. Subacute thyroiditis may be focal or may progress with diffuse bilateral involvement. Depending on the extent of subacute thyroiditis involvement, significant increases in acute-phase reactants can be observed. COVID-19 mRNA vaccine (Pfizer/BioNTech®) associated subacute thyroiditis responds quite well to non-steroidal anti-inflammatory therapy. Clinicians should be aware of the risk of developing subacute thyroiditis after vaccination.
KEYWORDS: COVID-19, SARS-CoV-2 vaccine, subacute thyroiditis
Introduction
COVID-19 causes a variety of clinical scenarios, from a flu-like syndrome to more serious conditions such as acute respiratory distress syndrome and death. Risk factors for critical illness include advanced age, chronic lung disease, serious cardiovascular diseases, immunodeficiencies, and chronic diseases such as diabetes mellitus.1 As a result of recent research, it is known that many complications related to the endocrine system develop in patients with COVID-19. However, its clinical significance and impact on prognosis are still not fully understood.2
COVID-19 infection causes thyroid dysfunction through destruction of the thyroid gland and immune-mediated mechanisms. Discovery of an effective vaccine against COVID-19 is an important step in the fight against the epidemic.3 Although the relationship between upper respiratory tract viruses and subacute thyroiditis is well known, rare cases have been reported with inactivated vaccines or live attenuated vaccines such as influenza.4,5 There are very limited data on subacute thyroiditis following COVID-19 vaccine.6,7 The emergence of an autoimmune response as a result of the viral protein concentration peaking within 24–48 hours after vaccine administration has been suggested as a possible mechanism.8 To date, only a few cases of subacute thyroiditis have been reported in the literature after mRNA COVID-19 vaccination.6,9,10 Here, we present five cases diagnosed with subacute thyroiditis following COVID-19 mRNA vaccine (Pfizer/BioNTech®) administration.
Material and methods
Case 1
A 41-year-old Caucasian male patient admitted to our endocrinology outpatient clinic with complaints of anterior neck pain, fatigue and palpitation. His past medical history was unremarkable for any disease such as thyroid disorders, upper respiratory system infection or COVID-19. The patient did not use any medication. He had no family history of autoimmune disease. The patient had a second dose of Pfizer/BioNTech® vaccine for COVID-19 3 days before his admission. The patient stated that he had mild neck pain and fatigue after the first dose of vaccine administered 30 days before the second dose. Thyroid-stimulating hormone (TSH) was suppressed to the lower limit of normal in the laboratory control performed after the first dose of vaccine. In the laboratory control performed after the first dose of vaccine, TSH was suppressed up to 0.38 mIU/L, and fT4 and fT3 were within the normal reference range.
Case 2
A 40-year-old Caucasian female patient was admitted to our outpatient clinic with anterior neck pain. Subclinical hyperthyroidism was detected in the laboratory examinations of the patient performed before admission to our clinic. The patient had complaints of neck pain, palpitation and sweating for about 1 month (Table 1). It was learned that the patient’s symptoms started 6 days after the second dose of COVID-19 mRNA vaccine (Pfizer/BioNTech®). A diagnosis of subacute thyroiditis was made due to acute-phase reactant elevation and a painful and sensitive thyroid gland on neck examination. Naproxen sodium 550 mg/8 h and propranolol 20 mg/12 h treatment were started. TSH suppression deepened and free thyroxine (fT4) and free triiodothyronine (fT3) increased above normal reference values at the first week follow-up under medical treatment. Minimal decreases in acute-phase reactants were observed. The patient’s treatment compliance was weak and symptomatic relief was not achieved in the second week of the treatment. Then the patient was admitted to our clinic. He had no known prior thyroid disorders and no recent history of upper respiratory tract infection or COVID-19 infection.
Table 1.
Laboratory test results of cases after vaccination
| First case | Second case | Third case | Fourth case | Fifth case | |
|---|---|---|---|---|---|
| Initial symptom | Neck pain, fatigue, palpitation | Neck pain, palpitation, sweating | Neck pain, nervousness, fatigue | Neck pain | Neck pain, headache, palpitation, sweating and tremor |
| Post-vaccination symptom onset time | 8th day | 6th day | 4th day | 6th day | 9th day |
| TSH (0.27–4.2 mIU/L) | 0.01 | 0.18 | 1.1 | 0.01 | 0.24 |
| fT4 (0.93–1.7 ng/dL) | 3.18 | 1.58 | 1.55 | 2.02 | 1.58 |
| fT3 (2–4.4 ng/dL) | 9.35 | 3.77 | 3.78 | 4.6 | 4.32 |
| ESR (<20 mm/h) | 32 | 80 | 28 | 34 | 44 |
| CRP (0–5 mg/L) | 124 | 34 | 15 | 27 | 18 |
TSH: Thyroid stimulating hormone, fT4: Free T4, fT3: Free T3, ESR: Erythrocyte sedimentation rate, CRP: C-reactive protein.
Case 3
A 40-year-old Caucasian male patient admitted to our outpatient clinic with complaints of left anterior neck pain, nervousness and fatigue that started 2 weeks ago. He did not have any disease or regular medication in his medical history. He did not have a recent upper respiratory infection or COVID-19 infection. He has been smoking for 15 years. He had no family history of autoimmune disease. He had received the first dose of COVID-19 mRNA vaccine (Pfizer/BioNTech®) on August 1, 2021. The patient’s symptoms started to occur 4 days after the vaccination and gradually increased.
Case 4
A 26-year-old Caucasian female patient was referred to our outpatient clinic with laboratory findings of hyperthyroidism. The patient had a complaint of pain on the left side of the neck for about 2 weeks. She had received two doses of inactive COVID-19 vaccine (CoronaVac®, Sinovac Life Sciences, Beijing, China) on January 14, 2021 and February 11, 2021. She did not have any complaints after these vaccinations. She had received the first dose of COVID-19 mRNA vaccine (Pfizer/BioNTech®) on July 1, 2021. Twenty days after the COVID-19 mRNA vaccine (Pfizer/BioNTech®) routine tests revealed TSH: 0.01 mIU/mL, fT3: 4.43 ng/L, fT4: 1.95 ng/dL. Her medical history did not indicate any disease or upper respiratory system infection and COVID-19. She had no family history of autoimmune disease.
Case 5
A 44-year-old Caucasian female patient was admitted to our outpatient clinic with complaints of anterior neck pain, headache, palpitation, sweating and tremor for about 2 months. She did not use any medication regularly, but her pain was relieved when she used non-steroidal anti-inflammatory drugs during periods of increased pain. She had received two doses of COVID-19 mRNA vaccine (Pfizer/BioNTech®) on April 19, 2021 and May 20, 2021. She stated that her symptoms started after the first dose of vaccine and gradually increased after the second dose of vaccine. The patient was using levothyroxine 50 mcg/day for Hashimoto’s thyroiditis. She did not have a recent upper respiratory infection or COVID-19 infection.
Results
Case 1
On his physical examination, his heart rate was 97/min; body temperature was 37.3°C; blood pressure was 135/80 mmHg; respiratory rate was 18/min. Oropharyngeal and ontologic examinations were normal, and thyroid gland was sensitive, painful, and enlarged. His nasopharyngeal swab test for SARS-CoV-2 was negative. Laboratory examination revealed elevated fT4 and fT3 levels, as well as suppressed TSH (Table 1). While anti-thyroglobulin (Anti-Tg), anti-thyroid peroxidase (Anti-TPO), and thyrotropin receptor antibodies (TRAb) were negative; erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels were high (Table 1). Thyroid ultrasonography (USG) revealed a decrease in bilateral focal parenchymal echogenicity and heterogeneous tissue with pseudonodular areas. Decreased blood flow was observed in Doppler USG (Figure 1a,b). The patient was diagnosed with subacute thyroiditis possibly associated with the COVID-19 mRNA vaccine (Pfizer/BioNTech®). Symptomatic relief was achieved within a few days of initiation of acetylsalicylic acid 500 mg/6 h and propranolol 20 mg/12 h. Neck pain continued for an intermittent period, but no additional medication was needed in the follow-up. Within the next few weeks, the symptoms resolved completely and medication was stopped. In his biochemical follow-up, non-symptomatic overt hypothyroidism developed (Figure 2). He was followed up without levothyroxine replacement, and hypothyroidism resolved in the following months. At the last visit, TSH, fT3 and fT4 were in the normal range and acute-phase reactants were in normal limits. Therefore, the patient was evaluated as euthyroid and in complete remission.
Figure 1.
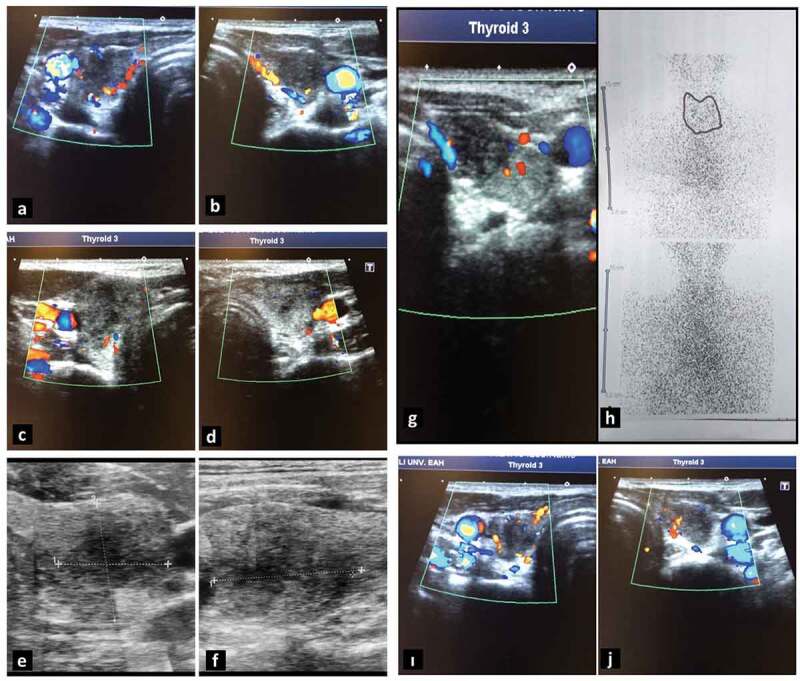
Thyroid ultrasonography of the cases. (a, b) First case. (a) Right lobe and (b) left lobe. (c, d) Second case. (a) Right lobe and (b) left lobe. (e, f) Third case. (e) Axial resolution and (f) longitudinal resolution of the left lobe. (g, h) Fourth case: (g) thyroid ultrasonography of the left lobe and (h) thyroid scintigraphy imaging. (i, j) Fifth case. (i) Right lobe and (j) left lobe.
Figure 2.
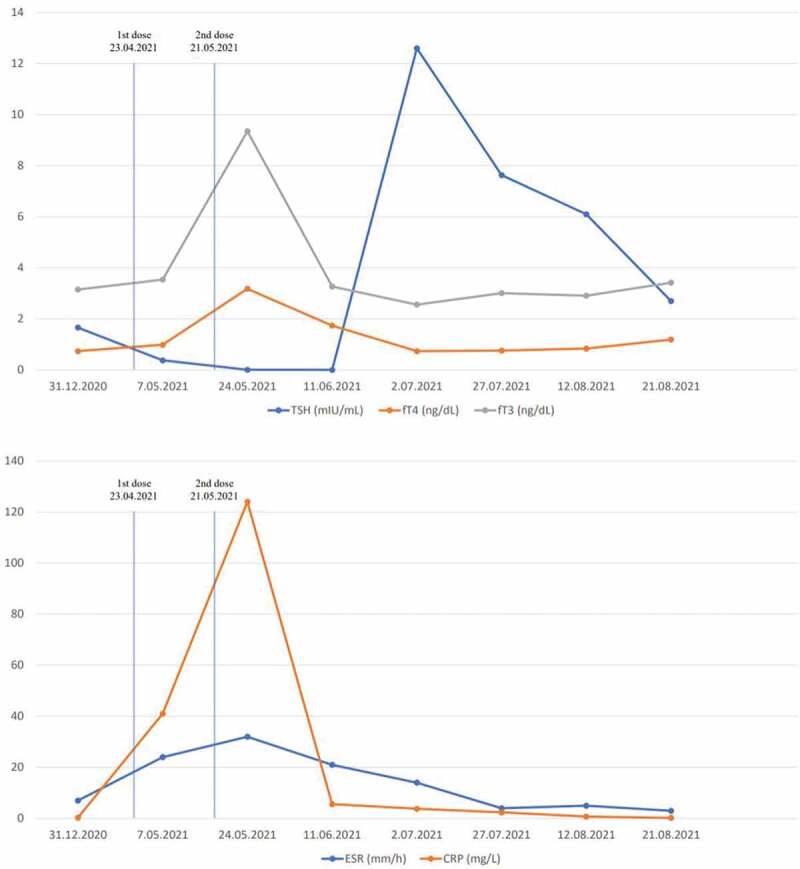
Follow-up laboratory results of the first case.
Case 2
On her physical examination, her heart rate was 83/min; body temperature was 36.3°C; blood pressure was 120/75 mmHg; respiratory rate was 15/min. Thyroid gland was sensitive and painful. Thyroid USG revealed heterogeneity in the thyroid parenchyma, bilateral multiple hypoechoic areas, and decreased blood flow on Doppler USG (Figure 1c,d). Laboratory examination revealed elevated fT4 and fT3 levels, as well as suppressed TSH (Figure 3). Anti-Tg was positive at low titer (160 IU/mL, reference range: 0–115 IU/mL), Anti-TPO and TRAb were negative. The patient was considered for subacute thyroiditis possibly associated with the COVID-19 mRNA vaccine (Pfizer/BioNTech®). Acetylsalicylic acid 500 mg/6 h and propranolol 20 mg/12 h treatment were initiated. The symptoms of the patient were decreased on the second week of control. She advised to be controlled on the first month.
Figure 3.
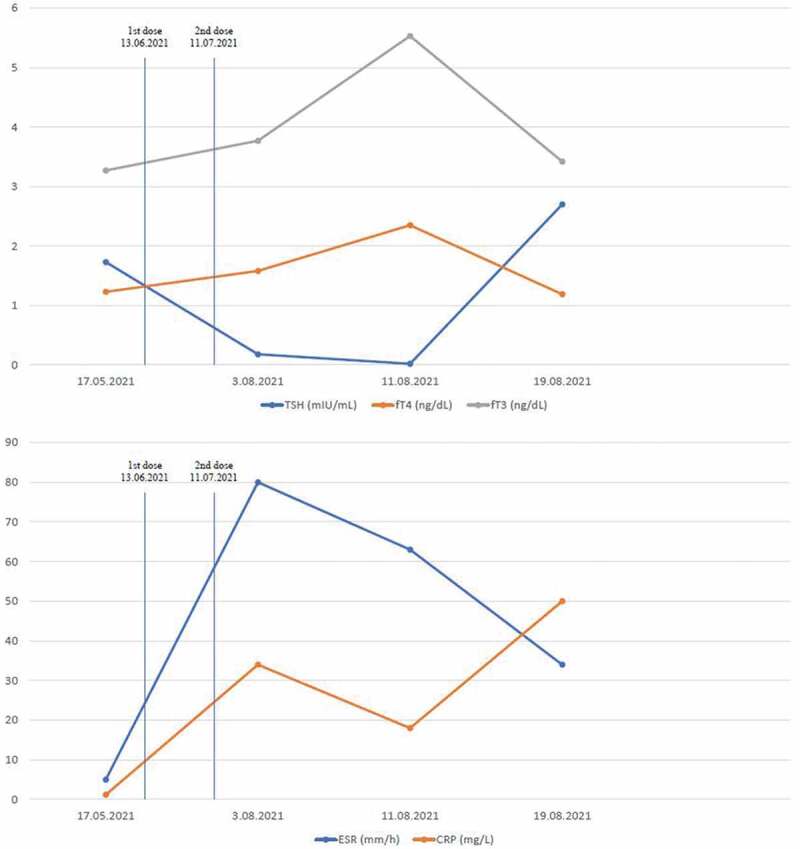
Follow-up laboratory results of the second case.
Case 3
On his physical examination, his heart rate was 78/min; body temperature was 36.6°C; blood pressure was 130/85 mmHg; respiratory rate was 15/min. Thyroid gland was sensitive, painful, and enlarged. Laboratory examination revealed fT4, fT3 and TSH levels were normal. Anti-Tg and Anti-TPO were negative. ESR levels and CRP levels were high (Table 1). On the thyroid USG, a 1.5–2 cm subcapsular heterogeneous hypoechoic thyroiditis area was observed in the left lobe (Figure 1e,f). The patient was considered for subacute thyroiditis associated with the COVID-19 mRNA vaccine (Pfizer/BioNTech®). Ibuprofen 600 mg/8 h treatment was started. The patient was called for outpatient control two weeks later.
Case 4
On her physical examination, her heart rate was 81/min; body temperature was 36.7°C; blood pressure was 120/70 mmHg; respiratory rate was 14/min. Thyroid gland examination revealed tenderness and warmth in the left thyroid lobe. Laboratory examination revealed elevated fT4 and fT3 levels, as well as suppressed TSH (Table 1). Increased Anti-Tg: 562 IU/mL (Reference range: 0–115 IU/mL) and Anti-TPO: 424 IU/mL (0–34 IU/mL) were detected. TRAb was negative. ESR and CRP levels were high (Table 1). In thyroid USG, approximately 2 cm thyroiditis area with irregular border, heterogeneous, hypoechoic and decreased blood flow on Doppler USG was observed in the left lobe (Figure 1g). A suppressed thyroid gland was detected in 99mTc pertechnetate thyroid scintigraphy imaging (Figure 1h). 99mTc uptake at the 20th minute was 1.13% (reference value range: 0.3–3%). The patient was considered for subacute thyroiditis associated with the COVID-19 mRNA vaccine (Pfizer/BioNTech®). Acetylsalicylic acid 500 mg/6 h and propranolol 20 mg/12 h treatment were initiated. The symptoms of the patient were resolved on the second week of control. At the follow-up 1 month later, TSH, fT3, and fT4 were in the normal range and acute-phase reactants were normal. Treatment was stopped. The patient was called for outpatient follow-up 1 month later in terms of the risk of developing hypothyroidism after subacute thyroiditis.
Case 5
On her physical examination, her heart rate was 88/min; body temperature was 36.5°C; blood pressure was 110/70 mmHg; respiratory rate was 14/min. Thyroid gland was sensitive, painful, and enlarged. Laboratory examination revealed elevated fT4 and fT3 levels, as well as suppressed TSH. Anti-TPO: 362 IU/mL (Reference range: 0–75 IU/mL) was detected. Anti-Tg and TRAb were negative. ESR and CRP levels were high (Table 1). Diffuse heterogeneity and hypoechoic areas were observed in thyroid USG. Thyroid blood flow was decreased in Doppler USG (Figure 1i,j). The patient was considered for subacute thyroiditis associated with the COVID-19 mRNA vaccine (Pfizer/BioNTech®). Ibuprofen 600 mg/8 h treatment was started. Within the next weeks, the symptoms resolved completely. At the first month control, TSH, fT3 and fT4 were in the normal range and acute-phase reactants were normal. Thus, ibuprofen treatment was terminated. The patient was called for outpatient follow-up 1 month later in terms of the risk of developing hypothyroidism after subacute thyroiditis.
Discussion
Subacute thyroiditis, which usually develops after upper respiratory tract infection, is the most common cause of painful thyroiditis. However, there have been rare cases of subacute thyroiditis reported with inactivated viral vaccines or live attenuated vaccines. Here, we present cases of subacute thyroiditis after administration of COVID-19 mRNA vaccine (Pfizer/BioNTech®) in five patients without a history of COVID-19 and upper respiratory tract infection. In these days of increasing vaccination, which plays a key role in the fight against the COVID-19 pandemic, we think that these cases should be reported in order to pay attention for the vaccine associated complications.
The first case of subacute thyroiditis associated with the SARS-CoV-2 infection was reported in an 18-year-old Italian woman. Due to the rapid spread of the COVID-19 pandemic, several cases of subclinical and atypical thyroiditis have been reported.11,12 Complaints such as neck pain, fever, malaise and odynophagia have been observed in the vast majority of COVID-19-related subacute thyroiditis cases, similar to classical subacute thyroiditis. In a review of 21 cases, 76% of the patients developed subacute thyroiditis after COVID-19 and 24% during COVID-19 infection.12 Entry of SARS-CoV-2 into host cells is mediated by angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine 2 (TMPRSS2). Although it primarily affects the respiratory system, SARS-CoV-2 can affect many systems by showing a wide organotropism. The pathophysiological characterization of COVID-19 on the endocrine system is still not fully understood.2 Increased expression of ACE2 and TMPRSS2 in the hypothalamo-pituitary system, thyroid follicular cells and adreno-gonadal endocrine organs has been suggested as a possible pathophysiological mechanism.13 The lack of a history of COVID-19 in our cases ruled out the possibility of SARS-CoV-2 associated subacute thyroiditis.
Although subacute thyroiditis usually occurs after viral infections, a few cases of subacute thyroiditis have been reported following vaccinations such as influenza.14 Vaccination is an important tool in combating epidemics, and many vaccines, including mRNA-based vaccines, have been proven to prevent serious infection and mortality with COVID-19. Data on the relationship between vaccines and autoimmune conditions are limited. Although broad homology between viral elements and the human proteome may exert protective effects by increasing immune tolerance, it may also facilitate pathological autoimmune processes. An immune reaction against foreign pathogenic elements resembling human proteins can turn into an autoimmune process targeting homologous self-proteins.15
Cross-reaction between the SARS-CoV-2 spike protein and nucleoprotein targeted by the COVID-19 mRNA vaccine and thyroid cell antigens stands out as a possible mechanism.6 In a recent preclinical study, SARS-CoV-2 spike protein and nucleoprotein; it has been found that it reacts strongly with tissue proteins such as thyroid peroxidase (TPO), transglutaminase 3, ENA, myelin basic protein, mitochondria, nuclear antigen, α-myosin, collagen, claudin.16 In the THYRCOV study conducted in patients hospitalized for COVID-19, thyrotoxicosis was detected in 58 patients (20.2%) and hypothyroidism was detected in 15 patients (5.2%).17 In a study conducted in the intensive care unit, the prevalence of destructive thyroiditis was found to be 10% in patients with COVID-19 and 0.5% in patients without COVID-19.18 Recently, Graves’ disease developed after Pfizer/BioNTech® mRNA vaccine was reported in two health-care workers who were previously known to be healthy.19
Conclusion
Vaccination rates against COVID-19 infection are increasing rapidly all over the world. Considering the vaccine recommendations against COVID-19 infection, we think that we will see more cases as a result of increased immunological reactions. Therefore, clinicians should be aware of the possible side effects of the vaccine.
Funding Statement
The authors reported that there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the authors.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Data availability statement
The datasets used are available from the corresponding author on reasonable request.
Abbreviations
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| fT4 | Free thyroxine |
| fT3 | Free triiodothyronine |
| TSH | Thyroid stimulating hormone |
| ACE2 | Angiotensin converting enzyme 2 |
| TMPRSS2 | Transmembrane protease serine 2 |
References
- 1.Certain medical conditions and risk for severe COVID-19 illness | CDC [Internet]. [accessed 2020. Sep 7]. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fneed-extra-precautions%2Fgroups-at-higher-risk.html.
- 2.Lisco G, De Tullio A, Stragapede A, Solimando AG, Albanese F, Capobianco M, Giagulli VA, Guastamacchia E, De Pergola G, Vacca A, et al. COVID-19 and the endocrine system: a comprehensive review on the theme. J Clin Med [Internet]. 2021. [accessed 2021 Aug 17];10:2920. doi: 10.3390/jcm10132920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ku CR, Jung KY, Ahn CH, Moon JS, Lee JH, Kim EH, Kwon H, Kim HK, Suh S, Hong S, et al. COVID-19 vaccination for endocrine patients: a position statement from the Korean Endocrine Society. Endocrinol Metab [Internet]. 2021. [accessed 2021 Aug 17];36:757–65. doi: 10.3803/EnM.2021.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altay FA, Güz G, Altay M.. Subacute thyroiditis following seasonal influenza vaccination. Hum Vaccin Immunother [Internet] 2016. [accessed 2021 Aug 17]; 12:1033–34. doi: 10.1080/21645515.2015.1117716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Girgis CM, Russo RR, Benson K.. Subacute thyroiditis following the H1N1 vaccine. J Endocrinol Invest [Internet]. 2010. [accessed 2021 Aug 17];33:506. doi: 10.1007/BF03346633. [DOI] [PubMed] [Google Scholar]
- 6.Franquemont S, Galvez J. Subacute thyroiditis after mRNA vaccine for COVID-19. J Endocr Soc [Internet]. 2021. [accessed 2021 Aug 17];5:A956–7. doi: 10.1210/jendso/bvab048.1954. [DOI] [Google Scholar]
- 7.İremli BG, Şendur SN, Ünlütürk U. Three cases of subacute thyroiditis following SARS-CoV-2 vaccine: postvaccination Asia syndrome. J Clin Endocrinol Metab [Internet]. 2021. [accessed 2021 Aug 17];XX:1–6. https://academic.oup.com/jcem/advance-article/doi/10.1210/clinem/dgab373/6287003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dotan A, Muller S, Kanduc D, David P, Halpert G, Shoenfeld Y. The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun Rev. 2021;20:102792. doi: 10.1016/j.autrev.2021.102792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kyriacou A, Ioakim S, Syed AA. COVID-19 vaccination and a severe pain in the neck. Eur J Intern Med [Internet]. 2021. [accessed 2021 Nov 13]. doi: 10.1016/j.ejim.2021.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siolos A, Gartzonika K, Tigas S. Thyroiditis following vaccination against COVID-19: report of two cases and review of the literature. Metab Open [Internet]. 2021. [accessed 2021 Nov 13];12:100136. doi: 10.1016/j.metop.2021.100136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brancatella A, Ricci D, Viola N, Sgrò D, Santini F, Latrofa F. Subacute thyroiditis after Sars-COV-2 infection. J Clin Endocrinol Metab [Internet]. 2020. [accessed 2021 Aug 18];105:2367–70. doi: 10.1210/clinem/dgaa276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rehman MAU, Farooq H, Ali MM, Rehman MEU, Dar QA, Hussain A. The association of subacute thyroiditis with COVID-19: a systematic review. SN Compr Clin Med [Internet]. 2021. accessed 2021 Aug 18;3:1. https://pmc/articles/PMC8082479/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lazartigues E, Qadir MMF, Mauvais-Jarvis F. Endocrine significance of SARS-CoV-2’s reliance on ACE2. Endocrinology [Internet]. 2020. [accessed 2021 Aug 18];161:1–7. doi: 10.1210/endocr/bqaa108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Momani MS, Zayed AA, Bakri FG. Subacute thyroiditis following influenza vaccine: a case report and literature review. Ital J Med. 2015;9:384–86. doi: 10.4081/itjm.2015.542. [DOI] [Google Scholar]
- 15.Segal Y, Shoenfeld Y. Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell Mol Immunol [Internet]. 2018. [accessed 2021 Aug 21];15:586–94. 2017 156. doi: 10.1038/cmi.2017.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol [Internet]. 2020. [accessed 2021 Aug 21];217:108480. doi: 10.1016/j.clim.2020.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lania A, Sandri MT, Cellini M, Mirani M, Lavezzi E, Mazziotti G. Thyrotoxicosis in patients with COVID-19: the THYRCOV study. Eur J Endocrinol [Internet]. 2020. [accessed 2021 Aug 21];183:381–87. doi: 10.1530/EJE-20-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller I, Cannavaro D, Dazzi D, Covelli D, Mantovani G, Muscatello A, Ferrante E, Orsi E, Resi V, Longari V, et al. SARS-CoV-2-related atypical thyroiditis. Lancet Diabetes Endocrinol [Internet]. 2020. [accessed 2021 Aug 21];8:739–41. doi: 10.1016/S2213-8587(20)30266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vera-Lastra O, Navarro AO, Domiguez MPC, Medina G, Valadez TIS, Jara LJ. Two cases of graves’ disease following SARS-CoV-2 vaccination: an autoimmune/inflammatory syndrome induced by adjuvants [Internet]. 2021. [accessed 2021 Aug 18]. https://www.liebertpub.com/doi/abs/10.1089/thy.2021.0142.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used are available from the corresponding author on reasonable request.


