ABSTRACT
Host-associated microbial biofilms can provide protection against pathogen establishment. In many host-microbe symbioses (including, but not limited to humans, plants, insects, and amphibians), there is a correlation between host-associated microbial diversity and pathogen infection risk. Diversity may prevent infection by pathogens through sampling effects and niche complementarity, but an alternative hypothesis may be that microbial biomass is confounded with diversity and that host-associated biofilms are deterring pathogen establishment through space preemption. In this study, we use the amphibian system as a model for host-microbe-pathogen interactions to ask two questions: (i) is bacterial richness confounded with biofilm thickness or cell density, and (ii) to what extent do biofilm thickness, cell density, and bacterial richness each deter the establishment of the amphibian fungal pathogen Batrachochytrium dendrobatidis? To answer these questions, we built a custom biofilm microcosm that mimics the host-environment interface by allowing nutrients to diffuse out of a fine-pore biofilm scaffolding. This created a competitive environment in which bacteria and the fungal pathogen compete for colonization space. We then challenged bacterial biofilms ranging in community richness, biofilm thickness, bacterial cell density, and B. dendrobatidis (also known as Bd)-inhibitory metabolite production with live B. dendrobatidis zoospores to determine how B. dendrobatidis establishment success on membranes varies. We found that biofilm thickness and B. dendrobatidis-inhibitory isolate richness work in complement to reduce B. dendrobatidis establishment success. This work underscores that physical aspects of biofilm communities can play a large role in pathogen inhibition, and in many studies, these traits are not studied.
IMPORTANCE Our finding highlights the fact that diversity, as measured through 16S rRNA gene sequencing, may obscure the true mechanisms behind microbe-mediated pathogen defense and that physical space occupation by biofilm-forming symbionts may significantly contribute to pathogen protection. These findings have implications across a wide range of host-microbe systems since 16S rRNA gene sequencing is a standard tool used across many microbial systems. Further, our results are potentially relevant to many host-pathogen systems since host-associated bacterial biofilms are ubiquitous.
KEYWORDS: biofilms, fungal-bacterial interactions, pathogens, microbial ecology
INTRODUCTION
Host-associated microbiota are widely acknowledged to play a crucial role in host health (1), such as by providing protection against pathogen establishment (2–5). In particular, there is a recurring observation that low microbial diversity of microbiota is associated with higher probabilities of infection by microbial pathogens. This relationship is found across many different systems, including humans (6), insects (7), plants (8), and animals (9, 10). However, the mechanism by which diversity deters pathogen establishment is not well understood, and diversity alone provides an incomplete explanation of how microbe-mediated pathogen protection is achieved (11).
In the amphibian system, the emerging fungal pathogen Batrachochytrium dendrobatidis (also known as Bd) has been linked to widespread declines in amphibian populations (12). The severity of infection is influenced by a variety of both intrinsic (host) and extrinsic (environmental) factors (13–16). Among these factors is the host-associated microbiota (17–19), which may influence pathogen establishment by deterring or outcompeting B. dendrobatidis seeking to colonize host tissue. Like in many other systems, low richness and diversity of the symbiotic microbiota correlate with increased susceptibility to pathogen infection (9, 10, 17), but it is not clear why diversity is consistently correlated with lower infection risk, both in amphibian systems and beyond.
Microbial diversity is hypothesized to prevent pathogen success through multiple possible mechanisms. Greater diversity may result in sampling effects (20, 21), whereby having a greater number of symbionts increases the probability of having at least one microbe capable of inhibiting pathogen growth. Diverse communities may also use resources more efficiently due to increased niche complementarity (20), which results in less pathogen success because of decreased nutrient availability (22–25). High overall diversity may also correlate with high diversity of bacteria that produce antimicrobial (or antipathogen) metabolites. There are many different types of secondary metabolites that are capable of inhibiting pathogens such as B. dendrobatidis in vitro (26, 27), and these metabolites are potentially produced by many different types of bacteria (26, 28). Coculture of microbes may also produce novel metabolites not previously observed in monococulture (28), which may have complementary effects against pathogens (29, 30). Thus, increasing microbial diversity may reduce B. dendrobatidis establishment through a variety of ecological mechanisms, including, but not limited to, sampling effects, complementary contest competition, and synergistic interference competition.
It is also possible, however, that diversity itself does not influence pathogen success but is instead an indicator of high microbial biomass (31). More diverse biofilms are generally more uniform (32) and also thicker (33) than nondiverse biofilms, and the extracellular matrix of biofilms is known to reduce invasion success of microbes (34). Therefore, highly diverse microbial communities may be correlated with biofilm thickness or greater cell density, which, in turn, deters B. dendrobatidis settlement through space preemption. The effect of microbial biomass on pathogen establishment success in vivo is generally poorly studied because most microbial studies use amplicon sequencing, which generates proportional, not absolute, abundances (35). Further, there are few economically affordable in vitro systems that can measure the effect of microbial biomass or biofilm thickness on pathogen success. Therefore, most previous work correlating bacterial richness or diversity (9, 10) with pathogen success does not account for the effects of biofilm biomass on pathogen establishment, even though it has been demonstrated that biofilm thickness does affect microbial invasion success (33, 34).
In this study, we investigated the currently unexplored relationship between biofilm thickness and cell density on B. dendrobatidis establishment and discuss their importance as possible mechanisms through which microbial diversity may mitigate pathogen success. We exposed 62 bacterial biofilm communities of various diversity to active B. dendrobatidis zoospores and ask two questions: (i) is microbial richness confounded by biofilm thickness or microbial cell density in bacterial communities, and (ii) to what extent does bacterial richness deter B. dendrobatidis establishment after accounting for effects of microbial biomass? We controlled for possible confounding effects of B. dendrobatidis inhibitory capacity by explicitly measuring the B. dendrobatidis inhibitory capacity of each biofilm member and asked how the presence of B. dendrobatidis inhibitory bacteria alters B. dendrobatidis establishment success in diverse and nondiverse biofilms. We also describe the relationship between microbial richness and biofilm biomass. Finally, we discussed the various ways in which symbiotic microbes may deter pathogen establishment and whether there was support for those mechanisms in our results.
RESULTS
Our experiment measured the establishment success of B. dendrobatidis across 62 unique bacterial biofilm communities (and 12 no-bacteria controls) through both zoosporangia counts (microscopy) and B. dendrobatidis internal transcribed spacer 3 (ITS-3) copy abundance (quantitative PCR [qPCR]) (Table S1 in the supplemental material). The final data set included bacterial biofilms ranging from 1 to 8 in richness (mean, 2.7), bacterial cell densities (as measured by qPCR copies) ranging from 3 to 1,339,922 reads, and biofilm thickness (as measured by crystal violet intensity) ranging from OD at 590 nm (OD590) from 0 to 1.28 (Fig. S1a). Of the known 40 introduced isolates, 23 unique isolates were observed in the pre-B. dendrobatidis-exposure biofilms (Fig. 1). B. dendrobatidis establishment success ranged from 0 to 1,922 zoosporangia per mm2 (mean, 163), or 0 to 58,581 qPCR copies of the B. dendrobatidis ITS1-3/5.8Chytr gene region (Fig. S1b).
FIG 1.
Isolate identity and characteristics. (Left) All isolates introduced into the experiment. Isolate names are presented as genus-species combinations with isolate ID in parenthesis. Appearance in experiment after introduction (first column) is indicated by a solid circle; absence in the experiment despite being introduced at least once is indicated by an X. The inverse growth rate of each isolate is shown as time to reach half of the maximum cell density (in hours, at OD600). Biofilm thickness of each isolate in monoculture was determined in 96-well plates by measuring the intensity of crystal violet staining as OD590. The relative diameter of the zone of inhibition on a plate with B. dendrobatidis zoospores is shown, where the maximum zone diameter (isolate with the largest circle) was 10 mm, and the minimum zone diameter of zero is denoted with no circle. Note that isolate 24D was detected in one post-B. dendrobatidis-exposure jar but not in pre-B. dendrobatidis-exposure jars (which is what we use to calculated observed richness); therefore, it is marked as “not observed.”
Correlations between variables.
To summarize the basic trends and relationships within our data, we assessed the degree of correlation and multicollinearity between our variables using a principal-component analysis (Fig. 2). We verified that our two response metrics (B. dendrobatidis qPCR copies and B. dendrobatidis microscopy counts) were strongly, but not perfectly, correlated (correlation, 0.65; t72, [i.e., t with df = 72] 7.288; P = 3.251e−10) (Fig. 3b). Therefore, we ran all subsequent models with either B. dendrobatidis qPCR copies or B. dendrobatidis microscopy counts as response variables independently. All predictor variables were also significantly correlated with each other (Table S2). Biofilm thickness and bacterial cell density, which both measure different aspects of “biofilm biomass,” shared a significant amount of variation (correlation, 0.669; t72, 7.63; P = 7.51e−11) (Fig. 2 and Fig. 3b). Community richness correlated with both biofilm thickness and cell density (Fig. 2 and 3; Table S2). Additionally, there was a correlation between biofilm thickness and inhibitory designation: biofilm communities comprised of B. dendrobatidis-inhibitory bacteria were generally thicker than biofilms comprised of non-B. dendrobatidis-inhibitory bacteria (slope, 0.540; 95% credible interval [CI], 0.131 to 0.966; probability of direction [PD], 0.0056) (Fig. S2).
FIG 2.
Principal-component analysis of all predictor and response variables. B. dendrobatidis qPCR copy and B. dendrobatidis microscopy counts are tightly correlated (R2 = 0.66, t55 = 6.56, P < 0.001). Response metrics are also correlated (see Fig. 3b), but variance inflation factors for individual predictors are less than 6, including interactions. Dots represent samples and are colored by B. dendrobatidis microscopy counts.
FIG 3.
Bacterial community richness, cell density, and biofilm thickness are correlated. (a) Bacterial community richness (x axis) is correlated with both bacterial cell density (y axis) and biofilm thickness (crystal violet intensity; colored dots). Points are jittered horizontally but not vertically. Bacterial community richness was calculated by summing the number of observed ASVs after data filtering. (b) The two metrics to measure biofilm biomass (thickness and cell density) are strongly correlated (R2 = 0.669, t72 = 7.63, P = 7.51e−11).
Community composition predicts biofilm thickness.
Biofilm thickness (as measured by crystal violet density) generally increased with community richness (Fig. 3), but thickness did not always increase with additional isolates (Fig. S3). Some communities with additional members (i.e., 3 compared to 1 or 10 compared to 3) decreased in biofilm thickness, even if overall trends showed thickness increasing with richness. In contrast, cell density behaved as a saturating function of community richness (Fig. 3). Thus, competitive exclusion of biofilm-forming bacteria by other less prolific biofilm-forming bacteria may lead to reduced biofilm thickness, even if total gene copy number increases.
We asked whether biofilm thickness could be predicted by community composition with the following three models: (i) additive (predicted by sum of individual isolate contributions), (ii) complementary (predicted by the thickest biofilm-forming individual isolate), or (iii) proportional (predicted by weighted average of thickness based on community composition). The complementary model (mean squared error [MSE], 0.174) predicted biofilm thickness of mixed communities better than the additive (MSE, 0.326) and proportional (MSE, 0.325) models (Fig. S4). The complementary model also had the least bias in its estimates (mean biased estimate [MBE], −0.0371). A few biofilms were poorly predicted by the complementary model (Fig. S4), but we also found a low relative abundance of the isolate with the best biofilm-forming ability in those communities (Fig. S4). The additive model overpredicted biofilm thickness on average (MBE, 0.312) and generally modeled the upper limit of biofilm thickness in mixed communities. In contrast, the proportional model underpredicted biofilm thickness (MBE, −0.388). Therefore, biofilm thickness was most accurately predicted by a community’s most prolific biofilm-forming member unless that member was not the dominant member in the community.
Biofilm traits that predict B. dendrobatidis success.
First, we removed variation in B. dendrobatidis inhibitory capacity and asked whether B. dendrobatidis establishment success correlated with biofilm attributes in biofilms composed of non-B. dendrobatidis-inhibitory bacteria only (Fig. 4). We found that B. dendrobatidis microscopy counts were negatively affected by biofilm thickness (slope, −0.727; 95% CI, −1.18 to −0.268; PD, 0.0011) and bacterial cell density (slope, −0.222; 95% CI, −0.441 to 0.00135; PD, 0.0237), but not by bacterial richness (slope, −0.0946; 95% CI, −0.233 to 0.531; PD, 0.09). These trends were mirrored in B. dendrobatidis qPCR copy abundance: B. dendrobatidis qPCR copies decreased strongly with biofilm thickness (slope, −0.579; 95% CI, −1.01 to −0.122; PD, 0.0063) and cell density (slope, −0.246; 95% CI, −0.458 to −0.0256; PD, 0.0136), but not with biofilm richness (slope, 0.0547; 95% CI, −0.0836 to 0.193; PD, 0.216).
FIG 4.
Biofilm cell density and thickness predict B. dendrobatidis establishment success for biofilms composed of non-B. dendrobatidis-inhibitory bacteria, while community richness predicts B. dendrobatidis establishment success for biofilms composed of B. dendrobatidis-inhibitory bacteria. The y axis is the residual variation in B. dendrobatidis counts or copies after subtracting effects of all other predictors (as estimated by Bayesian linear models). Lines indicate Bayesian-estimated best-fit slopes (with 95% simulated credible intervals as transparent ribbons) for biofilms where fraction inhibitory is exactly zero (gray) and where fraction inhibitory is 1 (salmon). Individual data points are colored by the fraction of isolates that are B. dendrobatidis inhibitory or not.
Next, we asked which biofilm traits predicted B. dendrobatidis establishment success in biofilms composed of empirically verified B. dendrobatidis-inhibitory bacteria. B. dendrobatidis microscopy counts were not predicted by any biofilm attributes: biofilm thickness, cell density, and richness were all poor predictors of microscopy counts (PD for all > 0.288) (Fig. 4). B. dendrobatidis qPCR copies were predicted by richness (slope, −0.556; 95% CI, −0.936 to −0.187; PD, 0.0025), but not biofilm thickness (slope, −0.0171; 95% CI, −1.26 to 1.14; PD, 0.489) or cell density (slope, 0.302; 95% CI, −0.27 to 0.865; PD, 0.148).
In both B. dendrobatidis microscopy and B. dendrobatidis qPCR copy models, the intercepts for biofilms composed of B. dendrobatidis-inhibitory bacteria were lower than for biofilms composed of non-B. dendrobatidis-inhibitory bacteria (differences in intercept, coefficient estimate, −0.782 and −0.691; PD, 0.0029 and 0.0053, respectively, for microscopy and qPCR copies). This suggests that B. dendrobatidis establishment success was lower when biofilm members were inhibitory against B. dendrobatidis, regardless of biofilm thickness, cell density, or richness. Thus, the lack of effect of biofilm thickness and cell density in biofilms composed of B. dendrobatidis-inhibitory bacteria was not because thicker biofilms did not prevent B. dendrobatidis establishment but, rather, because thin biofilms prevented B. dendrobatidis establishment similarly to thick ones if isolates were B. dendrobatidis inhibitory.
To further verify that effects of biofilm thickness and cell density were not confounded by richness (with which they are intrinsically tied), we analyzed a subset of microcosms with single-isolate biofilms to assess whether biofilm thickness and cell density predict B. dendrobatidis microscopy counts and B. dendrobatidis qPCR copies in single-isolate biofilms. For non-B. dendrobatidis-inhibitory isolates, there were strong effects of biofilm thickness and cell density on B. dendrobatidis microscopy counts and qPCR copies (PD < 0.02 for all) (Fig. S5). In contrast, biofilms composed of single B. dendrobatidis-inhibitory bacteria show there were no meaningful correlations between biofilm thickness or cell density on B. dendrobatidis success. This mirrors patterns found above, which suggests that effects of biofilm thickness on B. dendrobatidis microscopy counts and qPCR copies (respectively) are possibly masked by dominating effects of B. dendrobatidis-inhibitory metabolites.
We further probed the effect of B. dendrobatidis inhibitory capacity on B. dendrobatidis establishment success by asking whether the zone of inhibition produced by individual isolates in plate assays (Fig. 1) predicted the degree to which B. dendrobatidis success was prohibited in microcosm biofilms. We restricted this analysis to only biofilms with B. dendrobatidis-inhibitory members. We considered three alternative B. dendrobatidis-inhibition models, (i) additive (B. dendrobatidis success is predicted by sum of inhibitory zones of individual isolates), (ii) complementary (B. dendrobatidis success is predicted by the inhibitory zone of the most effective B. dendrobatidis-inhibitory isolate), and (iii) average (B. dendrobatidis is predicted by the average inhibitory zone of all isolates). None of these models predicted either B. dendrobatidis microscopy counts or qPCR copies (all PD > 0.025) (Fig. S6). Thus, while B. dendrobatidis inhibitory richness reduces B. dendrobatidis qPCR copies in the full data set (see above), the size of the zone of inhibition produced by individual bacterial isolates in plate assays did not predict B. dendrobatidis success in mixed-biofilm settings.
Isolate effects.
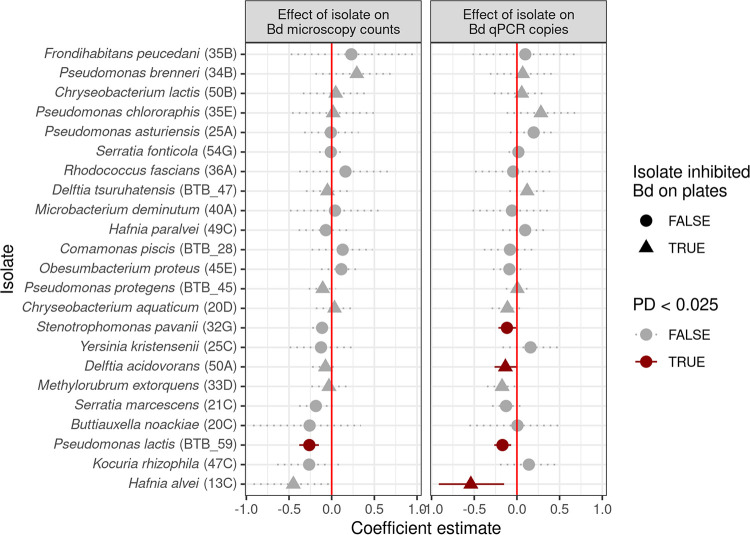
We asked whether any individual isolates were correlated with reductions in B. dendrobatidis establishment success. We found that four isolates had strong negative effects on B. dendrobatidis microscopy counts as follows: Pseudomonas lactis (BTB_59), Stenotrophomonas pavanii (32G), Hafnia alvei (13C), and Delftia acidovorans (50A) (Fig. 5). Only P. lactis was associated with reductions in both B. dendrobatidis microscopy counts and B. dendrobatidis qPCR copies; the other three isolates were correlated with fewer B. dendrobatidis qPCR copies but not fewer B. dendrobatidis microscopy counts. P. lactis and S. pavanii both do not inhibit B. dendrobatidis in preliminary B. dendrobatidis-inhibitory assays (Fig. 1). P. lactis was an avid biofilm former (Fig. 1; Fig. S7), while S. pavanii was a strong competitor among other isolates (Fig. S7). In contrast, H. alvei and D. acidovorans both inhibit B. dendrobatidis in plate assays, although neither of them generated above-average zones of inhibitions on B. dendrobatidis lawns.
FIG 5.
Effects of isolate abundance on B. dendrobatidis microscopy counts (left) and B. dendrobatidis qPCR copies (right). Each horizontal line represents the 95% credible interval for each isolate predictor in a Bayesian linear mixed model. Solid lines indicate predictors where the one-sided posterior probability (PD) is less than 0.025. Isolates’ genus and species (and isolate ID) are listed in the y axis.
DISCUSSION
Biofilm formation is key to microbial colonization of hosts by both beneficial and pathogenic microbes (36–38). The ecology and molecular signaling involved in biofilm formation, maintenance, and persistence have been studied extensively in both biological (39–41) and nonbiological systems (42, 43). However, microbial interactions within a biofilm are complex, and the ecological forces that drive interactions can vary. Elucidating biofilm community dynamics is therefore relevant to host systems for a variety of reasons (44), including having a better understanding of the role microbiota play in deterring pathogen establishment in host systems. Here, we investigate the relative contributions of biofilm thickness, bacterial cell density, and bacterial richness on establishment of the amphibian pathogen B. dendrobatidis. Although our experiment is based on an amphibian-focused system, our findings are potentially applicable across many similar ecological settings given the ubiquity of host-associated biofilms.
Microbe-mediated protection against pathogens in our system versus others.
Microbe-mediated pathogen protection is found in a wide range of host systems. It has long been recognized that the presence of healthy gut bacteria in animals (including humans) prevents infection by certain gastrointestinal pathogens (2, 45–47) and that plant pathogens are suppressed by the microbiota living on and within plant tissue (48–50). Evidence for a protective role of host-associated microbiota has also been observed in seaweeds (51), insects (7, 52, 53), and amphibians (17–19, 54). Thus, microbe-mediated pathogen suppression appears to be a widespread ecological phenomenon across many different hosts and biological systems.
In our model system, we are interested in how the skin-associated microbiota of a locally endangered amphibian, Anaxyrus boreas boreas (the Boreal toad), are able to prevent establishment of the fungal pathogen B. dendrobatidis. There are a variety of host and environmental attributes that affect B. dendrobatidis success, including host identity (55), differences in immunological responses (16), and environmental stressors (56, 57). Thus, while there are numerous approaches used to reduce the risk of amphibian populations to this devastating disease, microbe-mediated protection against pathogens has gained traction as a flexible and effective way to reduce B. dendrobatidis infection risk. There is strong evidence that the skin-associated microbiota on amphibians play a protective role against infection by B. dendrobatidis (17, 18). Specifically, bioaugmentation of beneficial microbes has been shown to increase protection against B. dendrobatidis (19). Therefore, while there are many host and environmental factors that potentially influence B. dendrobatidis success, the host-associated microbiota represent an aspect of host health that can be directly manipulated to affect infection outcome.
The primary mechanisms by which amphibian-associated microbiota are hypothesized to reduce B. dendrobatidis infection is through the production of B. dendrobatidis-inhibitory metabolites (26, 58). The presence and effectiveness of antimicrobial metabolite production against pathogen establishment have also been observed in many other systems, including seaweeds (51), insects (52, 59, 60), and humans (61, 62). However, we can also draw from other host systems to ask whether there are other ecological mechanisms that may reduce pathogen success. For example, links between antibiotic use and higher rates of secondary gastrointestinal infections in humans suggest that microbial knockdown of natural gut microbiota may increase host susceptibility to pathogen establishment (2). Additionally, it has been shown in the mouse system that commensal bacteria that are closely related to invading pathogens can protect against pathogen establishment, which suggests that niche overlap between resident and invader bacteria can predict pathogen establishment (24, 63). Therefore, while there is abundant literature on the diversity and role of microbially derived antimicrobial compounds in the context of pathogen establishment, there is also evidence that pathogens may be deterred through contest competition (space or resource preemption).
In our study, we explore the impact that biofilm thickness and cell density have on B. dendrobatidis establishment success. Biofilm thickness, which is measured through crystal violet staining, estimates the total amount of extracellular polymeric substances (EPS) in the biofilm matrix. The biofilm matrix allows cells to adhere to a surface and can also capture nutrients (39). Therefore, biofilm thickness can be considered a rough metric of space preemption by limiting access to the nutrient source by invaders. Conversely, quantitative PCR of the 16S rRNA gene provides an estimate of biofilm cell density, which correlates with total living microbial biomass. When biofilm community members use complementary niche space, the total amount of supported biomass should theoretically increase (64). Cell density can therefore be considered a rough metric of nutrient use efficiency, which may impact invasions through niche occupation (25). Although both biofilm thickness and cell density may reduce pathogen success through space and nutrient preemption, they are also possibly correlated with community richness (see next section). Here, we measure all three biofilm traits in custom microbial biofilms to assess whether either thickness, cell density, or richness affects pathogen establishment success of B. dendrobatidis.
Richness is confounded with bacterial biomass in biofilms.
It has previously been observed that community richness (9, 10) and richness of B. dendrobatidis-inhibitory bacteria (30) correlate with decreased B. dendrobatidis success in the field and in the lab. However, most experiments use 16S rRNA gene sequencing, which provides only proportional abundance estimates of bacteria (35). This means absolute microbial biomass is not taken into account. Correlations between amphibian skin microbial diversity and pathogen success may therefore be conflated with effects of microbial biomass in general.
We demonstrate in our experiment that increasing microbial richness significantly increases both biofilm thickness and cell density (Fig. 3; Fig. S3). This is corroborated by work done in nonamphibian biofilm systems, where increasing biofilm richness results in increased thickness and evenness in the biofilm matrix (32, 33). We find in our system that biofilm thickness was best predicted by the maximum biofilm thickness of any single-isolate member, which suggests that sampling effects (wherein higher richness leads to a higher likelihood of having at least one prolific biofilm former) are largely responsible for the correlation between richness and thickness (Fig. S4). In contrast, cell density was observed to be a saturating function of community richness (Fig. 3), where additional members to a biofilm did not typically reduce qPCR copy number density in the sample. This implies that resource efficiency increases with biofilm diversity since more bacterial cells (estimated via 16S gene copies) are being supported by an equivalent number of resources. Richness of a community therefore impacts both biofilm thickness and cell density, likely through sampling effects and niche complementarity, respectively.
Our finding that bacterial richness is conflated with biofilm thickness and cell density in biofilm communities has implications for the way diversity trends in microbial ecology should be interpreted. Most data are derived from 16S rRNA gene sequences, which are proportional in nature (35). Nonquantitative amplicon sequencing results may therefore mask signals of microbial biomass in host-associated studies. Although there are advanced statistical techniques that can partially account for false signals in proportional data (e.g., ANCOM [65]), the interpretive limitations of 16S sequencing approaches should be regarded more carefully in the future.
Biofilm thickness prevents establishment of B. dendrobatidis.
Previous work has shown that preexisting biofilms can exclude other bacteria from establishing by creating an extracellular matrix that excludes nonsister cells (33, 34, 66). Bacteria that attempt invasion are only able to grow on top of the matrix and are dislodged easily by shear forces (34). It was unknown whether a similar interaction occurs between resident bacteria and invading chytrid fungi since fungi are both physiologically and biochemically different from bacteria.
For example, the size difference between chytrid zoospores (3 to 5 μm) or zoosporangia (10 to 15 μm) and bacteria (0.1 to 1 μm) may result in different invasion dynamics than bacteria-bacteria invasions. Additionally, it is unknown the extent to which bacteria are able to communicate with chytrid fungi through molecular signals (or signal blockers). Some bacteria produce molecules that quench quorum-sensing molecules of other bacterial pathogens, which can prevent establishment on hosts (67, 68). B. dendrobatidis growth is also partially limited by the presence of a quorum-sensing molecule, tryptophol (69), which has also been found in bacterial cultures isolated from amphibians (28). This suggests bacteria may be able to halt B. dendrobatidis growth by saturating tryptophol signals. However, the concentrations of tryptophol found on amphibian skin in vivo were many times smaller than the required dose to suppress B. dendrobatidis growth in vitro (69), so it is unknown whether the concentration of quorum-sensing molecules produced by skin-associated bacteria is sufficient to affect B. dendrobatidis establishment.
We provide evidence that suggests establishment success of the chytrid fungus B. dendrobatidis is deterred by the thickness and cell density of bacterial biofilms, even after accounting for community richness (Fig. 4). The correlation between non-B. dendrobatidis-inhibitory biofilm thickness and B. dendrobatidis establishment success persists across single-strain biofilm comparisons (Fig. S5). We also find that presence of the prolific biofilm-forming isolate BTB_59 (Pseudomonas lactis) is strongly associated with a decrease in both B. dendrobatidis microscopy counts and qPCR copies. Pseudomonas lactis does not inhibit B. dendrobatidis in plate assays (Fig. 1) and is not a dominant competitor in mixed biofilms (Fig. S7) (Fig. 1). In summary, our data suggest that physical space preemption by resident biofilms may play an important role in pathogen establishment, even against fungal pathogens like B. dendrobatidis.
While microbial biofilm biomass (thickness and cell density) is strongly associated with decreased B. dendrobatidis establishment success in both multi- and single-isolate biofilms, this effect is masked in the presence of isolates that produce B. dendrobatidis-inhibitory metabolites (Fig. 4). The intercept for B. dendrobatidis establishment is lower in B. dendrobatidis-inhibitory biofilms than non-B. dendrobatidis-inhibitory biofilms, which suggests that even “thin” biofilms composed of B. dendrobatidis-inhibitory isolates are capable of reducing B. dendrobatidis establishment success. Thus, our data suggest that protection against B. dendrobatidis occurs through both contest competition (through space or nutrient preemption) and interference competition (by producing B. dendrobatidis-inhibitory metabolites), which operate simultaneously and in complement to each other within a biofilm environment.
Richness of B. dendrobatidis-inhibitory bacteria reduces B. dendrobatidis success.
While previous amphibian work finds correlations between whole-community diversity and B. dendrobatidis infection severity (9), we did not find any relationship between richness of non-B. dendrobatidis-inhibitory bacteria and B. dendrobatidis establishment success in any models (Fig. 4). Rather, we found that richness of B. dendrobatidis-inhibitory bacteria specifically strongly predicted marginal B. dendrobatidis qPCR copies (but not B. dendrobatidis microscopy counts). These correlations persisted even after accounting for variation in B. dendrobatidis microscopy counts (Fig. 4), suggesting that the amount of B. dendrobatidis genetic material per zoosporangia is reduced by richness of B. dendrobatidis-inhibitory bacteria. We speculate that some B. dendrobatidis-inhibitory metabolites may affect zoospore production (rather than zoosporangia development) in B. dendrobatidis, resulting in different amounts of genetic content despite similar zoosporangia counts. Previous B. dendrobatidis-inhibitory assays have observed that certain compounds cause general cell lysis, while others seem to specifically affect zoospore development within the zoosporangia (29). Therefore, it is possible that richer communities may reduce B. dendrobatidis infection intensity through having a diverse suite of B. dendrobatidis-inhibitory compounds that target different life stages.
We considered whether correlations with B. dendrobatidis-inhibitory richness were due to sampling effects, where richer communities are simply more likely to have powerful inhibitors. If sampling effects were responsible for the negative correlation between B. dendrobatidis-inhibitory richness and B. dendrobatidis establishment success, one might expect that isolates with large inhibition zones in plate assays (Fig. 1) would also be strong individual predictors of B. dendrobatidis establishment success (Fig. 5). However, this is not the case. The two B. dendrobatidis-inhibitory isolates that were most strongly associated with decreased B. dendrobatidis establishment success were Delftia acidovorans (50A) and Hafnia alvei (13C), neither of which had above-average inhibition zone diameters in plate assays (Fig. 1). We acknowledge that plate assays generate imperfect metrics of B. dendrobatidis-inhibitory ability since the size of the zone of inhibition is also dependent on factors that affect diffusion rate through agar, such as molecule size, solubility, and chemistry (70). However, given the data we have available, there is little evidence that sampling effects are driving correlations between B. dendrobatidis inhibitory richness and decreased B. dendrobatidis establishment success.
Discussion of methodology and assumptions.
Our novel biofilm microcosm system was a powerful tool to ask our questions because it allowed for explicit competition between resident biofilm bacteria and B. dendrobatidis zoospores. The membrane scaffolding allowed nutrients to diffuse out of the scaffolding itself, which created a competitive environment where both resident biofilm bacteria and possible invading pathogens must compete for adherence and access to a nutrient-rich source. This differs from previous systems because (i) the nutrient source diffuses from the surface itself (unlike 96-well plate biofilm systems, or microfluidic systems), and (ii) invading pathogens are not forced to settle on the surface because our system is suspended in aquatic settings (unlike other membrane systems, such as the one found in Piovia-Scott et al. [30]). Thus, B. dendrobatidis zoosporangia that are not attached to the membrane (but are simply sitting on top of the bacteria biofilm) are easily washed away through shear forces, just as they could be when amphibians move through water in the wild. This provides a more accurate depiction of how B. dendrobatidis-bacteria interactions may occur on host membrane surfaces.
Although our biofilm microcosms allow us to probe interactions between bacteria and B. dendrobatidis on a host-like surface, they do not simulate the intracellular infection process that occurs in real amphibians. B. dendrobatidis infects amphibians by invading the hosts’ epidermis and forming zoosporangia intracellularly. Our system is limited in that we cannot explore how bacterial biofilm and B. dendrobatidis interactions change when B. dendrobatidis is already embedded in host tissue. Our findings are relevant to interactions that occur between B. dendrobatidis and host-associated skin microbiota prior to successful invasion of amphibian skin cells, but may not accurately model interactions between intracellular B. dendrobatidis and extracellular skin microbiota.
We also made the assumption that biofilm densities in our artificial system were comparable to bacterial densities on amphibian skin in vivo. We did not measure bacterial density on wild-caught A. boreas (the species from which isolates were collected), but previous studies have estimated 16S rRNA gene read densities on other amphibian species like Salamandra salamandra (71) to be approximately 5.7 × 103 rRNA genes copies per mm2. Comparatively, if we assume our area sampled is 1/4 in. squared (the approximate area of the tip of our swabs), our biofilms had an average density of 1.3 × 103 and a maximum density of 8.3 × 103 16S rRNA genes reads/mm2. Therefore, our estimated bacterial densities are within the range of previously quantified bacterial densities found on amphibian skin.
Bacterial densities were measured using qPCR of swabs, so we must also acknowledge that extracellular DNA may impact the number of qPCR reads detected (72). Since biofilms were expected to differ in thickness, removal of extracellular DNA (eDNA) (for example, through DNases) would be logistically challenging to accomplish in an unbiased way across treatments. Biofilm swabs were also frozen between sampling and DNA extraction, which may cause living cells to lyse and become sensitive to digestion by DNases. Therefore, instead of possibly introducing variability in DNA content through uneven eDNA removal, we chose to extract all DNA found in biofilms while acknowledging that this will likely include eDNA or DNA from nonliving cells.
The method by which we characterized isolates’ B. dendrobatidis inhibitory ability also has some limitations. Individual isolate colonies were dotted on B. dendrobatidis lawns to assess whether bacteria produced a zone of inhibition. This assay is considered a standard microbiological method by which to test for inhibitory capacity (73), but the method also comes with assumptions. First, as mentioned above, B. dendrobatidis-inhibitory metabolites are assumed to be water soluble so that they can diffuse through the liquid-agar matrix. There may be some isolates that produce no zone of inhibition despite producing B. dendrobatidis-inhibitory compounds because the compounds may not be water soluble. Additionally, the size of the zone of inhibition may not necessarily reflect the potency of an antimicrobial agent since molecule size and chemistry will influence the speed at which it travels through the agar (70). Slow-diffusing molecules may yield a false-negative inhibitory identification. Isolates are also typically tested in isolation (i.e., in monoculture). Some studies have shown that coculture of microbes may trigger production of previously absent metabolites (28), which means that mixed biofilm communities may produce B. dendrobatidis-inhibitory compounds even if all members tested non-B. dendrobatidis inhibitory during our isolate screening. However, given the strong and persistent effect of biofilm thickness on B. dendrobatidis establishment across both multispecies and monospecies biofilms, we do not expect the possible emergent production of B. dendrobatidis-inhibitory metabolites in mixed biofilms to compromise our finding that biofilm thickness can limit B. dendrobatidis establishment success in non-B. dendrobatidis-inhibitory biofilms.
Finally, it was not possible to simultaneously sample biofilms for thickness and cell density and expose biofilms to B. dendrobatidis, so we used duplicate microcosms inoculated with identical communities to correlate biofilm traits with B. dendrobatidis establishment success. To verify that communities between duplicates were similar, we sampled microbial communities from both pre-B. dendrobatidis-exposure and post-B. dendrobatidis-exposure duplicate sets and compared their composition to verify that richness and community structure were similar. While there were small deviations in isolate relative abundance between pre- and postexposure membranes as a result of B. dendrobatidis exposure and treatment itself, membership was largely consistent between replicates (see supplemental methods). All microcosms with large community differences in pre-B. dendrobatidis-exposure and post-B. dendrobatidis-exposure duplicates were removed from analysis.
Conclusion.
Across many host systems, there is a recurrent observation that microbial diversity of the host-associated biofilm community is correlated with decreased pathogen success. Here, we investigate a few potential mechanisms underlying this diversity-invasion trend, including the possibility that diversity is confounded with biofilm biomass, which may deter pathogen establishment through space or nutrient preemption. We find that biofilm thickness and fungal-inhibitory capacity of biofilm members work together to deter establishment of the fungal pathogen Batrachochytrium dendrobatidis. In the absence of B. dendrobatidis-inhibitory bacteria, biofilm thickness strongly reduced B. dendrobatidis establishment success. However, the presence of B. dendrobatidis-inhibitory bacteria in biofilms strongly reduced B. dendrobatidis zoosporangia establishment (as determined through microscopy) regardless of biofilm thickness. Lastly, increasing richness of B. dendrobatidis-inhibitory bacteria further decreased B. dendrobatidis qPCR gene copies per zoosporangia. These findings support the idea that biofilm thickness and B. dendrobatidis-inhibitory isolate diversity are complementary traits through which host-associated microbiota can protect hosts against colonization by pathogens. Future approaches in microbiota-mediated pathogen prevention techniques could therefore consider both interference and contest competition as mechanisms in preventing pathogen establishment.
MATERIALS AND METHODS
Standardized terms.
To standardize language used in the manuscript, we define B. dendrobatidis establishment success to mean either B. dendrobatidis microscopy counts (number of zoosporangia per mm2) or B. dendrobatidis qPCR copies (number of estimated gene copies of the ITS1-3/5.8S region). Additionally, we define bacterial biofilm biomass as a general term that was quantified by two metrics, (i) biofilm thickness, as measured by crystal violet (CV) stain intensity, and (ii) biofilm cell density, as measured by qPCR copies of the 515F/806R region. The term “richness” describes the number of unique isolates found in a mixed bacterial biofilm community. Any reference to the “biofilm” refers to the bacterial community. The phrase “fraction inhibitory” refers to what fraction of bacteria in the biofilm community was considered B. dendrobatidis inhibitory or non-B. dendrobatidis inhibitory, given the single-isolate B. dendrobatidis inhibition assay results (see supplemental methods in the supplemental material).
Custom biofilm microcosm.
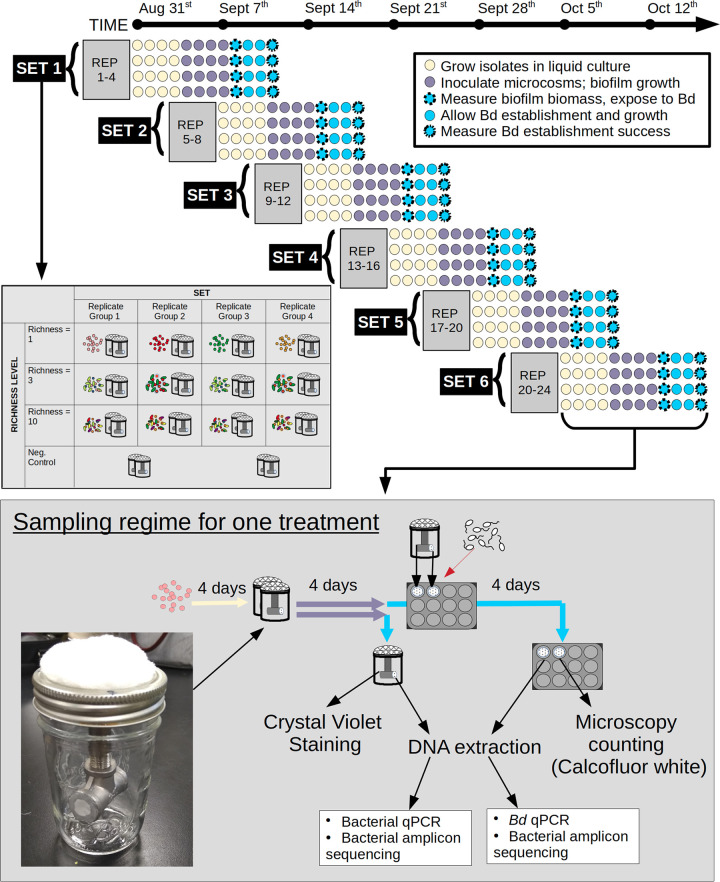
We designed and built custom biofilm microcosms that allowed us to explicitly measure biofilm richness, thickness, and cell density of a bacterial community and correlate these variables with subsequent B. dendrobatidis establishment success (inset image in Fig. 6). This system is unique from others because it allows the gradual diffusion of nutrient media out of a biofilm scaffolding suspended in an aquatic setting, thereby creating a natural competitive environment upon which microbes compete for establishment space. This mimics amphibian skin, which itself secretes mucus in an aquatic environment that attracts microbial settlement. The goal in designing these microcosms was to create an environment that better mimicked the competitive habitats found on host tissue, which may include competition for space.
FIG 6.
Diagram of full experimental design, including nested structure of treatments within replicates, and replicates within sets. (Top) From 31 August to 17 October, 5 sets of samples were conducted. Sets were this size because of space limitations in incubators. (Middle) Within each set were two replicates, which included 7 treatments (bacterial richnesses 1, 3, and 10 for inhibitory and noninhibitory biofilms, plus 1 control). Replicates were nested such that the treatment with 10 isolates were inclusive of the isolates found in richness 3, which were, in turn, inclusive of the isolate found in richness 1. (Bottom) The sampling regime for each treatment occurred across the span of 12 days. In short, isolates were introduced to biofilm microcosms in duplicate and allowed to form biofilms for 4 days. One microcosm was destructively sampled to measure biofilm thickness (CV), bacterial cell density (qPCR), and bacterial community composition (amplicon sequencing). Biofilms from the other duplicate microcosm were exposed to 1 × 106 active B. dendrobatidis zoospores in 12-well plates and incubated for 4 days. To sample B. dendrobatidis establishment success, one duplicate membrane from each jar was visualized with a fluorescent fungal stain (CFW), and B. dendrobatidis zoosporangia were counted. The DNA from the other duplicate membrane was extracted, amplified using quantitative PCR for B. dendrobatidis copy abundance, and amplicon sequenced for bacterial community composition post-B. dendrobatidis exposure.
The biofilm microcosms were built from 4-inch-diameter glass mason jars with hand-sewn cotton-and-cheesecloth caps, which allowed oxygen exchange in the jar while preventing contamination. Stainless steel nipple and tee pipe fittings were suspended in sterilized spring water. On either side of the tee were 0.22-μm-pore-size track-etch membranes (Whatman; catalog no. 05-715-115), attached using silicone caulking. Nutrients (1% tryptone broth) were added into the stainless steel nutrient tube, which diffused out of the 0.22-μm membrane pores and fed biofilm growth. Given the small pore size, bacteria cannot migrate into the nutrient tube. Thus, growth is encouraged on the membrane itself from which nutrients are sourced. Since the membrane scaffolding is suspended in water, biofilm recruitment is passive and mimics host tissue systems such as amphibian skin in an aquatic environment. Details about the system, including pilot experiments, are described in the “Pilots” section of the supplemental methods.
Summary of experimental design.
We used a collection of 40 known isolates in our experiment (Fig. 1), which varied in biofilm-forming ability and were either B. dendrobatidis inhibitory (20 isolates) or not B. dendrobatidis inhibitory (20 isolates) (see supplementary methods of isolate characterization methods). Random mixed bacterial biofilm communities (ranging in introduced richness from 1 to 10 and with various B. dendrobatidis-inhibitory capacities) were assembled by introducing bacteria into biofilm microcosms 4 days before B. dendrobatidis exposure. Four-day-old biofilms were then exposed to active B. dendrobatidis zoospores. Biofilm composition (16S rRNA gene sequencing), thickness (crystal violet staining), and cell density (bacterial qPCR) were measured directly prior to B. dendrobatidis exposure. B. dendrobatidis establishment success was measured 4 days after B. dendrobatidis exposure by both microscopy counts and fungal qPCR. Details on all steps are elaborated below.
Design of biofilm community composition.
Biofilm communities were grouped in “replicate groups” that included 3 “richness levels” of 1, 3, or 10 bacterial strains. Within each replicate group, each higher-richness-level community was inclusive of lower-level members. For example, the 10-richness community would include all isolates of the 3-richness community, and the 3-richness community would include the 1-richness isolate. Note that these compositions is in reference to introduced communities only: we could not control which isolates became extinct in microcosms during biofilm formation, so these “nested” community designs are not necessarily preserved in the final results. Biofilms were designed to be composed of either entirely B. dendrobatidis-inhibitory or non-B. dendrobatidis-inhibitory isolates (see “Isolate screening” in the supplemental methods), but there were a few exceptions (see “Community assembly notes” in the supplemental methods). Multiple replicate groups were combined into a temporal “set.” Due to incubator size capacity, four replicate groups were tested at once and are referred to collectively as a set. In total, we had 24 replicate groups (24× 10-richness communities, 24× 3-richness communities, and 24× 1-richness communities; richnesses are introduced richnesses) plus 12 no-bacterial controls. Each unique community was inoculated into two jars at once (“duplicate” jars) so that one duplicate could be destructively sampled for bacterial biofilm biomass and community composition, while the other could be exposed to B. dendrobatidis zoospores and sampled for B. dendrobatidis qPCR copy numbers and microscopy counts. Each temporal set included 14 treatments (28 jars) total (4× 10-richness communities, 4× 3-richness communities, 4× 1-richness communities, and 2× no-bacterial controls) A visual depiction of our experiment can be seen in Fig. 6.
Bacterial biofilm inoculation and growth.
Isolates for each set were grown in 8 mL of 1% tryptone broth at 21°C for 4 days in a shaker at 130 rpm. After 4 days, isolate cultures were standardized to an OD600 of 0.05 and combined in equal proportions for each mixed culture. Microcosms were inoculated in duplicate with 1 mL of mixed OD600 of 0.05 communities and incubated at 21°C in a shaker at 130 rpm. Every 24 h, microcosms were fed 500 μL of 1% tryptone broth through the nutrient tube. Biofilms were allowed to develop for 4 days, which was found to be an adequate amount of time to reach maximum biofilm thickness (see “Pilots” in the supplemental material). One of each duplicate pair of microcosms was sampled for biofilm thickness and bacterial community composition (“Bacterial biofilm community characterization before B. dendrobatidis exposure”), while the other was exposed to B. dendrobatidis zoospores (“B. dendrobatidis exposure” below).
(i) Bacterial biofilm community characterization before B. dendrobatidis exposure.
To sample biofilm thickness, membranes with 4-day-old biofilms were fixed in 1 mL of ice-cold methanol on ice for 15 min. Following fixation, membranes were soaked in 1 mL of deionized water (diH2O) for 5 min to remove any trace methanol. Membranes were then placed biofilm side down into 500 μL of 0.1% crystal violet for 10 min and then gently dipped in 1 mL of diH2O twice to remove excess crystal violet stain before setting out to dry. A total of 84 quarter-inch punchouts of dried membranes were placed in a 96-well plate for extraction of crystal violet (74, 75).
To extract bound crystal violet on membranes, 200 μL of 30% acetic acid was added to each well containing a quarter-inch membrane punchout. The membranes were incubated in acid for 20 min. Each well was pipetted up and down 5 times, and 100 μL from each well was transferred into a new 96-well plate and read for absorbance at OD590 three times. OD measurements per well were averaged. Since membranes naturally bind some crystal violet even with no bacterial membrane, the mean control membrane values were subtracted from treatment membrane values.
To determine biofilm community composition, membranes were rinsed with 1 mL of sterile deionized water, peeled off the stainless steel hardware, and put directly into a cryotube and frozen at −20°C. Members were later extracted for DNA (see below). DNA extract was used for both qPCR quantification of biofilm biomass and also 16S rRNA gene sequencing to determine community composition.
(ii) B. dendrobatidis exposure.
The second of each duplicate microcosm pair was exposed to B. dendrobatidis. First, all membranes were peeled from their hardware using flame and ethanol-sterilized forceps and placed in non-tissue culture-treated 12-well plates containing 1 mL 0.1% tryptone agar per well. This concentration mimics the concentration of tryptone in the nutrient tubes. Flame and ethanol-sterilized stainless steel washers were placed in each well to keep the membranes flush to the agar, and 1 × 106 B. dendrobatidis zoospores (in 1 mL of sterile spring water) was added to each well. Active zoospore solutions had been obtained by flooding B. dendrobatidis plates with sterile spring water, which were diluted to 1 × 106 zoospores per mL using hemocytometer counts. B. dendrobatidis plates had previously been prepared by spreading 750 μL of liquid B. dendrobatidis stock culture onto 1% tryptone plates.
Every 24 h, B. dendrobatidis-exposed membranes were transferred to a new well with fresh 0.1% tryptone agar and fresh spring water. On the fourth day, membranes were sampled for B. dendrobatidis settlement. One of the membranes from each microcosm was rinsed with 1 mL sterile deionized water and swabbed with a cotton swab. Swabbing was done by placing membranes on an ethanol-and-bleach-sterilized surface and twisting the tip of the swab back and forth 10 times to sample an approximately equal surface area each time. The tips of the cotton swabs were then broken off into cryotubes and frozen at −20°C for later DNA extraction, qPCR for B. dendrobatidis copy number, and sequencing for bacterial community composition. The second membrane was fixed with 1 mL of 100% ice-cold methanol for 15 min on ice and then soaked in 1 mL diH2O for 5 min to remove remaining methanol. Membranes were placed biofilm side down into 250 μL of 50 μM calcofluor white (CFW) for 15 min. Membranes were gently dipped in 1 mL diH2O to remove remaining stain and then visualized using a fluorescence microscope to count and measure B. dendrobatidis.
Microscopy counting methods.
Whole membranes were placed under a fluorescence microscope using a DAPI (4′,6-diamidino-2-phenylindole) filter. A target of 15 fields of view at ×20 magnification was captured for each membrane in the pattern shown in Fig. S8a. From each field of view, the total number of zoosporangia were counted using ImageJ.
Additionally, at least 5 fields of view (one from each quadrant of the membrane plus one in the center; see Fig. S8b) were captured at ×40 magnification to estimate average B. dendrobatidis zoosporangia size. The microscope lens was placed in each of the quadrants at random, and then the field of view was adjusted until the first cluster of zoosporangia (over 2 zoosporangia, if possible) was observed. On membranes where there were less clusters than quadrants, we scanned the entire membrane and took pictures of all possible clusters. On membranes where clusters were extremely abundant, we also took extra photos at random points between five primary target sections to better capture the variation in sporangia size.
Source of bacterial isolates.
Bacterial isolates were obtained from Anaxyrus boreas in summer 2019 by swabbing toad skin with sterile rayon swabs and smearing samples on 1% tryptone agar plates. Isolates were identified using Sanger sequencing and BLAST (76) (see supplemental methods for details). Bacteria were isolated and stored frozen in a 15% glycerol solution at −80°C and revived in 1% tryptone broth. All isolates were screened for growth rate, biofilm-forming capabilities, and B. dendrobatidis inhibitory capacity. Only isolates with reasonable growth rates (amount of time it takes to reach half the carrying capacity [1/2kmax] < 20 h) and who were biofilm formers (crystal violet intensities significantly greater than controls) were included in the experiment. Methods for each of these screening steps are found in the supplemental methods, and a summary of isolate characteristics is shown in Fig. 1.
B. dendrobatidis culturing and maintenance.
B. dendrobatidis was grown in in 50-mL tissue culture flasks in 1.6% tryptone broth. Three stocks were kept at all times, which were all derived from the same master stock (passaged a total of 5 times). Medium was refreshed every week by replacing approximately half of the B. dendrobatidis culture with fresh medium. The experiment lasted a total of 2 months.
DNA extraction.
Genomic DNA was extracted from pre- and post-B. dendrobatidis-exposure membranes and swabs using the Qiagen blood and tissue kit (catalog no. 69506) in benchtop single-tube extractions, according to manufacturer protocol, including the initial recommended lysozyme digestion for 30 min at 37°C. Centrifuge speeds for AW2 buffer washes were changed to 17,000 × g for 5 min and 2 min, respectively, to accommodate our centrifuge limits. Extraction controls were included in each batch of extractions. Benches were cleaned with 10% bleach and 70% ethanol before and after all extractions.
Bacterial PCR.
All pre- and post-B. dendrobatidis-exposure DNA extracts were amplified for the 16S rRNA gene V4 region and barcoded using 515F (5′-GTG CCA GCM GCC GCG GTA A-3′) and 806R (5′-GGA CTA CHV GGG TWT CTA AT-3′) primers (77). Sequencing was done on the Illumina MiSeq platform using V2 2 × 150-bp chemistry at the University of Colorado Boulder.
Bacterial and B. dendrobatidis qPCR.
We used the ABsolute qPCR SYBR Green mix with no ROX (Thermo; catalog no. AB-1159/A) and Agilent qPCR plates (part no. 410088) in the Agilent Stratagene Mx3000P machine to quantify copy number for bacteria (pre-B. dendrobatidis exposure) and B. dendrobatidis (post-B. dendrobatidis exposure) in duplicate. For duplicates whose difference in estimated copy number was in the 95th percentile or above of variation in copy number between all qPCRs, a third qPCR was done. The 515F/806R universal bacterial primers (same as in the PCR section) were used for bacterial qPCR, whereas the ITS1-3 (5′-CCTTGATATAATACAGTGTGCCATATGTC-3′) and 5.8SChytr (5′-AGCCAAGAGATCCGTTGTCAAA-3′) primers were used for B. dendrobatidis qPCR (78). The final primer concentration was 0.5 μM for each primer. The PCR cycle for bacterial qPCR was as follows: 95°C for 15 min, 40 cycles of 94°C for 45 s, 50°C for 1 min, and 72°C for 90 s, followed by 72°C for 10 min. The PCR cycle for B. dendrobatidis qPCR was 95°C for 15 min followed by 50 cycles of 95°C for 15 s and 60°C for 1 min (78). Bacterial qPCRs amplified poorly in test reactions, which we hypothesized was because bacterial DNA concentrations were too high. Therefore, we diluted bacterial DNA extract 1:5 with molecular-grade water before use in qPCRs. For both bacterial and B. dendrobatidis qPCRs, 5 μL of diluted and undiluted DNA, respectively, was added to each master mix reaction.
DNA copy number standards were created by diluting a custom G-block fragment (IDT Technologies) according to the manufacturer’s instructions into 10× serial dilutions. The G-block sequence was created by combining the 16S rRNA gene V4 sequence from E. coli (79) and ITS1-3/5.8S sequence for B. dendrobatidis (80) with additional random sequences to pad either end of the fragment (see supplemental methods for sequence). Final copy number per microliter of the original G-block solution was 1.95 × 1010; dilutions between 10−2 and 10−10 were further diluted 1:5, and 5 μL of standard was added to each reaction. Standards were heated to 50°C for 5 min, vortexed for 5 s, and centrifuged for 1 min at 6,000 × g before every use to produce consistent standards.
Copy number estimates were calculated in R by comparing automatically generated Gt (fluorescence intensity) thresholds from the Agilent Mx3000P program against our qPCR template standard curves. Our “no-template controls” (NTCs) yielded increased SYBR fluorescence starting around 34 cycles for B. dendrobatidis reactions and 32 cycles for bacterial reactions, but both these cycle numbers were significantly beyond the threshold cycle number (CT) required to reach the Gt threshold for all real samples, which suggests either that NTC DNA template content was very low or that nontarget amplification (like primer-dimers) occurred after excessive cycles.
Bioinformatic pipeline for sequencing results.
16S rRNA genes sequences were demultiplexed with QIIME2’s (81, 82) demux plugin (83, 84) using the emp-paired (paired-end) function. Resulting paired-ends were trimmed to 150 bp each and denoised with the DADA2 plugin (85) in QIIME2. The resulting amplicon sequence variant (ASV) table (5,939,614 reads) was assigned taxonomy using QIIME2’s q2-feature-classifier plugin (86) using a pretrained Bayes classifier for SILVA 138 on the 515F/806R region (86, 87). All taxonomic strings including the string “mitochondria,” “chloroplast, ” and “archaea” were removed, which resulted in the loss of 9 features (ASVs) and 664 reads (0.01% of reads). All sequences were successfully inserted into the 16S tree.
Sequence identification.
After exporting the ASV table from the QIIME2 pipeline into R 4.1.0 (88), we observed that both ASV abundance (determined by multiplying proportional abundances by qPCR copy number) and ASV relative abundance were strongly bimodal. We filtered the ASV table to remove the low-abundance reads by using a custom threshold and kmeans clustering approach. In brief, we used R’s kmeans clustering function (stats package [88]) to sort ASVs into high- and low-abundance clusters. We compare these clusters with a thresholding approach and manually removed samples for which ASV patterns did not appear strongly bimodal. Fifteen jars out of 168 were removed from analysis. Only 1.06% of reads were lost but reduced the number of ASVs in the data set from 897 to 32. At this point, we also removed treatments in which pre- and post-B. dendrobatidis-exposure biofilm communities (which were measured from duplicate jars) differed drastically in community membership (ASVs present) and composition (relative ranks of ASV abundances).
After removing low-abundance reads and jars that appeared contaminated based on comparisons between duplicates, there were 32 remaining ASVs that were observed in the experiment (across all jars). The remaining observed 32 ASVs were mapped to our 40 known reference isolate sequences. We created a de novo phylogenetic tree of our 515F/806R sequences and full-length reference isolate 27F/1492R sequences by aligning and masking sequences with MAFFT (89) in the alignment qiime2 plugin and generating a rapid bootstrap RAxML tree using the GTRCAT substitution model (90, 91) (Fig. S9). Each ASV was manually matched to a reference isolate sequence by comparing its taxonomy, phylogenetic distance, and ASV occurrence pattern in the experiment relative to which samples each isolate was introduced. We successfully mapped all 32 ASVs to 24 reference isolates (Table S3). One of the mapped isolates was only observed in post-B. dendrobatidis-exposure jars (isolate 24D) and is therefore not included in subsequent analyses (see paragraph below for explanation). Some reference isolates mapped to multiple ASVs, which were all Pseudomonas and extremely similar in phylogenetic distance and occurrence pattern in the data set. A full description of the thresholding and matching process is described in the supplemental methods (“Identifying contaminants using kmeans clustering and thresholding”). Additionally, a full description of reference isolate sequencing and identification can be found in the supplemental methods under “Creation of reference databases.”
The observed richness (number of observed ASVs) for each pre-B. dendrobatidis-exposure jar was recorded and used for subsequent analyses. This included 23 (of 24) observed ASVs. We used pre-B. dendrobatidis-exposure (as opposed to post-B. dendrobatidis-exposure) richness levels as our “observed richness” variable since this would have been the richness of the community when B. dendrobatidis was introduced. Post-B. dendrobatidis-exposure communities may be affected by exposure to B. dendrobatidis, so these data were only used to verify that pre- and post-B. dendrobatidis-exposure communities were similar and were not used in richness analyses.
Statistical analysis.
All subsequent analyses were conducted in R 4.1.0 (88) using the package tidyverse (92) for data manipulation.
Since several measured biofilm qualities were expected to be correlated, we first assessed the degree of correlation between various predictor and response metrics by conducting a principal-component analysis (PCA) (prcomp in base R; plotted using the ggbiplots package [93]). Then, we tested for correlations (Pearson’s product-moment correlation using cor.test, base R) between predictor and between response variables. The relationship between the fraction of inhibitory ASVs and biofilm thickness (CV) was also tested using a basic linear model (lm). Predictor variables were significantly correlated, so we calculated the variance inflation factor (vif from the car package [94]) of mock linear mixed-effects models using lmer from the lme4 package (95) to test for multicollinearity. Variance inflation factors were successfully reduced to <4.1 after centering predictors around zero (Table S4). VIFs over 10 are generally indicative of strong multicollinearity, and a threshold of 5 is often used to determine whether predictors should be removed (96, 97). We decided to include all predictors in our model since we did not encounter any model convergence issues and found strong consistency in estimated coefficients across different trial models. In subsequent models, we center all predictors except for “fraction inhibitory” so that the effect of “no B. dendrobatidis inhibitory isolates present” will remain the baseline within the model.
To investigate how community structure influenced biofilm thickness, we assessed whether we could predict biofilm thickness (CV) of microcosm communities based on community composition and individual isolate biofilm-forming abilities as measured through previous 96-well plate assays (See “Isolate screening” in supplemental methods). We considered three biofilm thickness prediction models, (i) additive (predicted microcosm-biofilm thickness was calculated by summing biofilm thickness of individual members), (ii) complementary (predicted microcosm-biofilm thickness was equal to the maximum biofilm thickness of any individual isolate), and (iii) proportional (predicted microcosm-biofilm thickness was a weighted mean of all isolate members). Since biofilm thickness of individual isolates was measured in 96-well plates and not in microcosms, we could not compare biofilm thickness measurements between individual isolates and mixed biofilm communities directly. Instead, we used a subset of the data where “microcosm” biofilm communities contained only one isolate to generate a Bayesian mixed-effects linear model (stan_lmer from the package rstanarm [98, 99]) that estimated the relationship between single-isolate microcosm biofilm thickness measurement and the corresponding single-isolate 96-well plate biofilm thickness measurement. Set was included as a random effect. This model was used to assess whether biofilm thickness prediction models over- or underestimated biofilm thickness compared to observed mixed-community biofilms. To quantify the quality of each biofilm thickness model, we calculated the mean square error (MSE) and mean bias error (MBE) of each model.
To test for effects of biofilm richness, thickness, and cell density on B. dendrobatidis establishment success, we built Bayesian linear regression models. We tested two biofilm models, one with B. dendrobatidis microscopy counts as the response metric and the other with B. dendrobatidis qPCR copies as the response metric. Both models include richness, cell density, thickness, and “fraction of isolates that were inhibitory” (i.e., “fraction inhibitory”) as fixed effects and “set” as a random effect. We allowed fraction inhibitory to interact with richness, cell density, and thickness. “Non-B. dendrobatidis-inhibitory” biofilms were used as the baseline in the model (FractionInhibitory = 0), so posterior distributions for “B. dendrobatidis-inhibitory” biofilm coefficients (when FractionInhibitory = 1) were calculated by adding the appropriate interaction (:FractionInhibitory) sampling distribution to baseline non-B. dendrobatidis-inhibitory sampling distributions. To test whether B. dendrobatidis genetic content within sporangia differed across treatments, we built a third biofilm model with B. dendrobatidis qPCR copies as a response metric but included B. dendrobatidis microscopy counts as an additional predictor (along with all other predictors listed above).
Since richness and biofilm thickness/cell density are inherently confounded, we analyzed a subset of our data that included only biofilms with single-isolate communities to remove confounding effects of richness. Here, we built linear mixed models using stan_lmer to ask whether B. dendrobatidis microscopy counts or qPCR copies decreased when single-isolate biofilms were thicker (higher CV) or denser (more bacterial qPCR copies) (four models total). Set was included as a random effect.
To test whether plate assay results from individual B. dendrobatidis-inhibitory microbes could predict B. dendrobatidis establishment success, we built the following three linear mixed models for each of the two B. dendrobatidis establishment success metrics: (i) additive model where B. dendrobatidis success is predicted by summing the diameters of zones of inhibition of each isolate, (ii) complementary model (where B. dendrobatidis success is predicted by the diameter of the zone of inhibition of the most effective single isolate), and (iii) average model (where B. dendrobatidis success is predicted by the mean diameter of zones of inhibition of all isolates in biofilm).
Lastly, we tested whether individual isolates could predict either B. dendrobatidis microscopy or B. dendrobatidis qPCR copies. The log10 of isolate reads (as estimated by qPCR) was used to predict either B. dendrobatidis microscopy counts (log10) or B. dendrobatidis qPCR copies (log10) using stan_lmer.
For each set of models, the one-sided probability of direction (PD) was determined by calculating the fraction of posterior draws that were greater than zero. Statistical significance was determined by asking whether the 95% credible interval for posterior samples overlapped zero (or, equivalently, whether the PD was less than 0.025). Credible intervals (CIs) were calculated with the “highest-density interval” method using “ci” from the package bayestestR (100). In all Bayesian models described above, weakly informative default priors were used, and adapt_delta and the number of iterations were increased to 0.99 and 5,000, respectively, to improve convergence and credible interval estimates.
Data availability.
All sequences can be found at EBI/ENA under accession no. PRJEB46498 (secondary accession no. in SRA, ERP130685; https://www.ebi.ac.uk/ena/browser/view/PRJEB46498?show=reads). Codes for data processing, analysis, and figure generation can be found on our GitHub page at https://github.com/mech3132/BiofilmMicrocosm.
ACKNOWLEDGMENTS
We thank T. Korpita for his B. dendrobatidis collection and assistance in maintaining B. dendrobatidis cultures and A. Weier for his assistance in laboratory maintenance, molecular work, and image processing for B. dendrobatidis zoosporangia counts.
Funding support for this work was provided by Morris Animal Foundation (MAF; grant D19ZO-044) to V.J.M. and the Natural Science and Engineering Search Council of Canada (PGSD3), Colorado Mountain Club Foundation, and the Department of Ecology and Evolutionary Biology (CU Boulder) to M.Y.C.
Footnotes
Supplemental material is available online only.
Contributor Information
Valerie J. McKenzie, Email: valerie.mckenzie@colorado.edu.
Irina S. Druzhinina, Nanjing Agricultural University
REFERENCES
- 1.Gilbert SF, Sapp J, Tauber AI. 2012. A symbiotic view of life: we have never been individuals. Q Rev Biol 87:325–341. 10.1086/668166. [DOI] [PubMed] [Google Scholar]
- 2.Buffie CG, Pamer EG. 2013. Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol 13:790–801. 10.1038/nri3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fraune S, Anton-Erxleben F, Augustin R, Franzenburg S, Knop M, Schröder K, Willoweit-Ohl D, Bosch TC. 2015. Bacteria–bacteria interactions within the microbiota of the ancestral metazoan Hydra contribute to fungal resistance. ISME J 9:1543–1556. 10.1038/ismej.2014.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rebollar EA, Martínez-Ugalde E, Orta AH. 2020. The amphibian skin microbiome and its protective role against chytridiomycosis. Herpetologica 76:167–177. 10.1655/0018-0831-76.2.167. [DOI] [Google Scholar]
- 5.Ubeda C, Djukovic A, Isaac S. 2017. Roles of the intestinal microbiota in pathogen protection. Clin Transl Immunology 6:e128. 10.1038/cti.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravi A, Halstead FD, Bamford A, Casey A, Thomson NM, van Schaik W, Snelson C, Goulden R, Foster-Nyarko E, Savva GM, Whitehouse T, Pallen MJ, Oppenheim BA. 2019. Loss of microbial diversity and pathogen domination of the gut microbiota in critically ill patients. Microb Genom 5:e000293. 10.1099/mgen.0.000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dillon R, Vennard C, Buckling A, Charnley A. 2005. Diversity of locust gut bacteria protects against pathogen invasion. Ecol Lett 8:1291–1298. 10.1111/j.1461-0248.2005.00828.x. [DOI] [Google Scholar]
- 8.Matos A, Kerkhof L, Garland J. 2005. Effects of microbial community diversity on the survival of Pseudomonas aeruginosa in the wheat rhizosphere. Microb Ecol 49:257–264. 10.1007/s00248-004-0179-3. [DOI] [PubMed] [Google Scholar]
- 9.Longo AV, Savage AE, Hewson I, Zamudio KR. 2015. Seasonal and ontogenetic variation of skin microbial communities and relationships to natural disease dynamics in declining amphibians. R Soc Open Sci 2:140377. 10.1098/rsos.140377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rebollar EA, Hughey MC, Medina D, Harris RN, Ibáñez R, Belden LK. 2016. Skin bacterial diversity of Panamanian frogs is associated with host susceptibility and presence of Batrachochytrium dendrobatidis. ISME J 10:1682–1695. 10.1038/ismej.2015.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shade A. 2017. Diversity is the question, not the answer. ISME J 11:1–6. 10.1038/ismej.2016.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheele BC, Pasmans F, Skerratt LF, Berger L, Martel A, Beukema W, Acevedo AA, Burrowes PA, Carvalho T, Catenazzi A, De la Riva I, Fisher MC, Flechas SV, Foster CN, Frías-Álvarez P, Garner TWJ, Gratwicke B, Guayasamin JM, Hirschfeld M, Kolby JE, Kosch TA, La Marca E, Lindenmayer DB, Lips KR, Longo AV, Maneyro R, McDonald CA, Mendelson J, Palacios-Rodriguez P, Parra-Olea G, Richards-Zawacki CL, Rödel M-O, Rovito SM, Soto-Azat C, Toledo LF, Voyles J, Weldon C, Whitfield SM, Wilkinson M, Zamudio KR, Canessa S. 2019. Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science 363:1459–1463. 10.1126/science.aav0379. [DOI] [PubMed] [Google Scholar]
- 13.Andre SE, Parker J, Briggs CJ. 2008. Effect of temperature on host response to Batrachochytrium dendrobatidis infection in the mountain yellow-legged frog (Rana muscosa). J Wildl Dis 44:716–720. 10.7589/0090-3558-44.3.716. [DOI] [PubMed] [Google Scholar]
- 14.Chatfield MW, Richards-Zawacki CL. 2011. Elevated temperature as a treatment for Batrachochytrium dendrobatidis infection in captive frogs. Dis Aquat Organ 94:235–238. 10.3354/dao02337. [DOI] [PubMed] [Google Scholar]
- 15.Piotrowski JS, Annis SL, Longcore JE. 2004. Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia 96:9–15. 10.2307/3761981. [DOI] [PubMed] [Google Scholar]
- 16.Savage AE, Zamudio KR. 2016. Adaptive tolerance to a pathogenic fungus drives major histocompatibility complex evolution in natural amphibian populations. Proc Biol Sci 283:20153115. 10.1098/rspb.2015.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becker MH, Harris RN. 2010. Cutaneous bacteria of the redback salamander prevent morbidity associated with a lethal disease. PLoS One 5:e10957. 10.1371/journal.pone.0010957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris RN, Brucker RM, Walke JB, Becker MH, Schwantes CR, Flaherty DC, Lam BA, Woodhams DC, Briggs CJ, Vredenburg VT, Minbiole KPC. 2009. Skin microbes on frogs prevent morbidity and mortality caused by a lethal skin fungus. ISME J 3:818–824. 10.1038/ismej.2009.27. [DOI] [PubMed] [Google Scholar]
- 19.Kueneman JG, Woodhams DC, Harris R, Archer HM, Knight R, McKenzie VJ. 2016. Probiotic treatment restores protection against lethal fungal infection lost during amphibian captivity. Proc R Soc B 283:20161553. 10.1098/rspb.2016.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fargione JE, Tilman D. 2005. Diversity decreases invasion via both sampling and complementarity effects. Ecol Lett 8:604–611. 10.1111/j.1461-0248.2005.00753.x. [DOI] [Google Scholar]
- 21.Vila JC, Jones ML, Patel M, Bell T, Rosindell J. 2019. Uncovering the rules of microbial community invasions. Nat Ecol Evol 3:1162–1171. 10.1038/s41559-019-0952-9. [DOI] [PubMed] [Google Scholar]
- 22.Jousset A, Schulz W, Scheu S, Eisenhauer N. 2011. Intraspecific genotypic richness and relatedness predict the invasibility of microbial communities. ISME J 5:1108–1114. 10.1038/ismej.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mallon CA, Van Elsas JD, Salles JF. 2015. Microbial invasions: the process, patterns, and mechanisms. Trends Microbiol 23:719–729. 10.1016/j.tim.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Maltby R, Leatham-Jensen MP, Gibson T, Cohen PS, Conway T. 2013. Nutritional basis for colonization resistance by human commensal Escherichia coli strains HS and Nissle 1917 against E. coli O157: H7 in the mouse intestine. PLoS One 8:e53957. 10.1371/journal.pone.0053957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Elsas JD, Chiurazzi M, Mallon CA, Elhottova D, Kristufek V, Salles JF. 2012. Microbial diversity determines the invasion of soil by a bacterial pathogen. Proc Natl Acad Sci USA 109:1159–1164. 10.1073/pnas.1109326109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woodhams DC, Alford RA, Antwis RE, Archer H, Becker MH, Belden LK, Bell SC, Bletz M, Daskin JH, Davis LR, Flechas SV, Lauer A, Gonzalez A, Harris RN, Holden WM, Hughey MC, Ibáñez R, Knight R, Kueneman J, Rabemananjara F, Reinert LK, Rollins-Smith LA, Roman-Rodriguez F, Shaw SD, Walke JB, McKenzie V. 2015. Antifungal isolates database of amphibian skin-associated bacteria and function against emerging fungal pathogens: ecological Archives E096-059. Ecology 96:595–595. 10.1890/14-1837.1. [DOI] [Google Scholar]
- 27.Yasumiba K, Bell S, Alford R. 2016. Cell density effects of frog skin bacteria on their capacity to inhibit growth of the chytrid fungus, Batrachochytrium dendrobatidis. Microb Ecol 71:124–130. 10.1007/s00248-015-0701-9. [DOI] [PubMed] [Google Scholar]
- 28.Loudon AH, Holland JA, Umile TP, Burzynski EA, Minbiole KP, Harris RN. 2014. Interactions between amphibians’ symbiotic bacteria cause the production of emergent anti-fungal metabolites. Front Microbiol 5:441. 10.3389/fmicb.2014.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bell SC, Alford RA, Garland S, Padilla G, Thomas AD. 2013. Screening bacterial metabolites for inhibitory effects against Batrachochytrium dendrobatidis using a spectrophotometric assay. Dis Aquat Organ 103:77–85. 10.3354/dao02560. [DOI] [PubMed] [Google Scholar]
- 30.Piovia-Scott J, Rejmanek D, Woodhams DC, Worth SJ, Kenny H, McKenzie V, Lawler SP, Foley JE. 2017. Greater species richness of bacterial skin symbionts better suppresses the amphibian fungal pathogen Batrachochytrium dendrobatidis. Microb Ecol 74:217–226. 10.1007/s00248-016-0916-4. [DOI] [PubMed] [Google Scholar]
- 31.Wang C, Liu D, Bai E. 2018. Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition. Soil Biol Biochem 120:126–133. 10.1016/j.soilbio.2018.02.003. [DOI] [Google Scholar]
- 32.Murga R, Stewart PS, Daly D. 1995. Quantitative analysis of biofilm thickness variability. Biotechnol Bioeng 45:503–510. 10.1002/bit.260450607. [DOI] [PubMed] [Google Scholar]
- 33.Burmølle M, Webb JS, Rao D, Hansen LH, Sørensen SJ, Kjelleberg S. 2006. Enhanced biofilm formation and increased resistance to antimicrobial agents and bacterial invasion are caused by synergistic interactions in multispecies biofilms. Appl Environ Microbiol 72:3916–3923. 10.1128/AEM.03022-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nadell CD, Drescher K, Wingreen NS, Bassler BL. 2015. Extracellular matrix structure governs invasion resistance in bacterial biofilms. ISME J 9:1700–1709. 10.1038/ismej.2014.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gloor GB, Macklaim JM, Pawlowsky-Glahn V, Egozcue JJ. 2017. Microbiome datasets are compositional: and this is not optional. Front Microbiol 8:2224. 10.3389/fmicb.2017.02224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Egan S, Harder T, Burke C, Steinberg P, Kjelleberg S, Thomas T. 2013. The seaweed holobiont: understanding seaweed–bacteria interactions. FEMS Microbiol Rev 37:462–476. 10.1111/1574-6976.12011. [DOI] [PubMed] [Google Scholar]
- 37.Latasa C, Roux A, Toledo-Arana A, Ghigo J-M, Gamazo C, Penadés JR, Lasa I. 2005. BapA, a large secreted protein required for biofilm formation and host colonization of Salmonella enterica serovar Enteritidis. Mol Microbiol 58:1322–1339. 10.1111/j.1365-2958.2005.04907.x. [DOI] [PubMed] [Google Scholar]
- 38.Liu X, Zhang K, Liu Y, Zou D, Wang D, Xie Z. 2020. Effects of calcium and signal sensing systems on Azorhizobium caulinodans biofilm formation and host colonization. Front Microbiol 11:563367. 10.3389/fmicb.2020.563367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flemming H-C, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S. 2016. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol 14:563–575. 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- 40.Marsh PD. 2005. Dental plaque: biological significance of a biofilm and community life-style. J Clin Periodontol 32:7–15. 10.1111/j.1600-051X.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- 41.Nett J, Andes D. 2006. Candida albicans biofilm development, modeling a host–pathogen interaction. Curr Opin Microbiol 9:340–345. 10.1016/j.mib.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 42.Feazel LM, Baumgartner LK, Peterson KL, Frank DN, Harris JK, Pace NR. 2009. Opportunistic pathogens enriched in showerhead biofilms. Proc Natl Acad Sci USA 106:16393–16399. 10.1073/pnas.0908446106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Percival S, Walker J. 1999. Potable water and biofilms: a review of the public health implications. Biofouling 14:99–115. 10.1080/08927019909378402. [DOI] [Google Scholar]
- 44.Moser C, Pedersen HT, Lerche CJ, Kolpen M, Line L, Thomsen K, Høiby N, Jensen PØ. 2017. Biofilms and host response–helpful or harmful. APMIS 125:320–338. 10.1111/apm.12674. [DOI] [PubMed] [Google Scholar]
- 45.Bohnhoff M, Drake BL, Miller CP. 1954. Effect of streptomycin on susceptibility of intestinal tract to experimental Salmonella infection. Proc Soc Exp Biol Med 86:132–137. 10.3181/00379727-86-21030. [DOI] [PubMed] [Google Scholar]
- 46.Freter R. 1955. The fatal enteric cholera infection in the guinea pig, achieved by inhibition of normal enteric flora. J Infect Dis 97:57–65. 10.1093/infdis/97.1.57. [DOI] [PubMed] [Google Scholar]
- 47.Kamada N, Chen GY, Inohara N, Núñez G. 2013. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol 14:685–690. 10.1038/ni.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bulgarelli D, Schlaeppi K, Spaepen S, Van Themaat EVL, Schulze-Lefert P. 2013. Structure and functions of the bacterial microbiota of plants. Annu Rev Plant Biol 64:807–838. 10.1146/annurev-arplant-050312-120106. [DOI] [PubMed] [Google Scholar]
- 49.Song Y, Wilson AJ, Zhang X-C, Thoms D, Sohrabi R, Song S, Geissmann Q, Liu Y, Walgren L, He SY, Haney CH. 2021. FERONIA restricts Pseudomonas in the rhizosphere microbiome via regulation of reactive oxygen species. Nat Plants 7:644–654. 10.1038/s41477-021-00914-0. [DOI] [PubMed] [Google Scholar]
- 50.Teixeira PJ, Colaianni NR, Law TF, Conway JM, Gilbert S, Li H, Salas-González I, Panda D, Del Risco NM, Finkel OM. 2021. Specific modulation of the root immune system by a community of commensal bacteria. Proc Natl Acad Sci USA 118:e2100678118. 10.1073/pnas.2100678118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saha M, Weinberger F. 2019. Microbial “gardening” by a seaweed holobiont: surface metabolites attract protective and deter pathogenic epibacterial settlement. J Ecol 107:2255–2265. 10.1111/1365-2745.13193. [DOI] [Google Scholar]
- 52.Forsgren E, Olofsson TC, Vásquez A, Fries I. 2010. Novel lactic acid bacteria inhibiting Paenibacillus larvae in honey bee larvae. Apidologie 41:99–108. 10.1051/apido/2009065. [DOI] [Google Scholar]
- 53.Oliver KM, Moran NA, Hunter MS. 2005. Variation in resistance to parasitism in aphids is due to symbionts not host genotype. Proc Natl Acad Sci USA 102:12795–12800. 10.1073/pnas.0506131102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kueneman JG, Woodhams DC, Van Treuren W, Archer HM, Knight R, McKenzie VJ. 2016. Inhibitory bacteria reduce fungi on early life stages of endangered Colorado boreal toads (Anaxyrus boreas). ISME J 10:934–944. 10.1038/ismej.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gervasi S, Gondhalekar C, Olson DH, Blaustein AR. 2013. Host identity matters in the amphibian-Batrachochytrium dendrobatidis system: fine-scale patterns of variation in responses to a multi-host pathogen. PLoS One 8:e54490-11. 10.1371/journal.pone.0054490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson PT, McKenzie VJ, Peterson AC, Kerby JL, Brown J, Blaustein AR, Jackson T. 2011. Regional decline of an iconic amphibian associated with elevation, land-use change, and invasive species. Conserv Biol 25:556–566. 10.1111/j.1523-1739.2010.01645.x. [DOI] [PubMed] [Google Scholar]
- 57.Rohr JR, Raffel TR. 2010. Linking global climate and temperature variability to widespread amphibian declines putatively caused by disease. Proc Natl Acad Sci USA 107:8269–8274. 10.1073/pnas.0912883107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bletz MC, Loudon AH, Becker MH, Bell SC, Woodhams DC, Minbiole KP, Harris RN. 2013. Mitigating amphibian chytridiomycosis with bioaugmentation: characteristics of effective probiotics and strategies for their selection and use. Ecol Lett 16:807–820. 10.1111/ele.12099. [DOI] [PubMed] [Google Scholar]
- 59.Alippi AM, Reynaldi FJ. 2006. Inhibition of the growth of Paenibacillus larvae, the causal agent of American foulbrood of honeybees, by selected strains of aerobic spore-forming bacteria isolated from apiarian sources. J Invertebr Pathol 91:141–146. 10.1016/j.jip.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 60.Sabaté DC, Carrillo L, Audisio MC. 2009. Inhibition of Paenibacillus larvae and Ascosphaera apis by Bacillus subtilis isolated from honeybee gut and honey samples. Res Microbiol 160:193–199. 10.1016/j.resmic.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 61.Abt MC, Pamer EG. 2014. Commensal bacteria mediated defenses against pathogens. Curr Opin Immunol 29:16–22. 10.1016/j.coi.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hammami R, Fernandez B, Lacroix C, Fliss I. 2013. Anti-infective properties of bacteriocins: an update. Cell Mol Life Sci 70:2947–2967. 10.1007/s00018-012-1202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stecher B, Chaffron S, Käppeli R, Hapfelmeier S, Freedrich S, Weber TC, Kirundi J, Suar M, McCoy KD, von Mering C, Macpherson AJ, Hardt W-D. 2010. Like will to like: abundances of closely related species can predict susceptibility to intestinal colonization by pathogenic and commensal bacteria. PLoS Pathog 6:e1000711. 10.1371/journal.ppat.1000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mensah S, Du Toit B, Seifert T. 2018. Diversity–biomass relationship across forest layers: implications for niche complementarity and selection effects. Oecologia 187:783–795. 10.1007/s00442-018-4144-0. [DOI] [PubMed] [Google Scholar]
- 65.Mandal S, Van Treuren W, White RA, Eggesbø M, Knight R, Peddada SD. 2015. Analysis of composition of microbiomes: a novel method for studying microbial composition. Microb Ecol Health Dis 26:27663. 10.3402/mehd.v26.27663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xavier JB, Foster KR. 2007. Cooperation and conflict in microbial biofilms. Proc Natl Acad Sci USA 104:876–881. 10.1073/pnas.0607651104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kusari P, Kusari S, Spiteller M, Kayser O. 2015. Implications of endophyte-plant crosstalk in light of quorum responses for plant biotechnology. Appl Microbiol Biotechnol 99:5383–5390. 10.1007/s00253-015-6660-8. [DOI] [PubMed] [Google Scholar]
- 68.Vinoj G, Vaseeharan B, Thomas S, Spiers AJ, Shanthi S. 2014. Quorum-quenching activity of the AHL-lactonase from Bacillus licheniformis DAHB1 inhibits Vibrio biofilm formation in vitro and reduces shrimp intestinal colonisation and mortality. Mar Biotechnol (NY) 16:707–715. 10.1007/s10126-014-9585-9. [DOI] [PubMed] [Google Scholar]
- 69.Verbrugghe E, Adriaensen C, Martel A, Vanhaecke L, Pasmans F. 2018. Growth regulation in amphibian pathogenic chytrid fungi by the quorum sensing metabolite tryptophol. Front Microbiol 9:3277. 10.3389/fmicb.2018.03277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kunin CM, Edmondson WP. 1968. Inhibitor of antibiotics in bacteriologic agar. Proc Soc Exp Biol Med 129:118–122. 10.3181/00379727-129-33264. [DOI] [PubMed] [Google Scholar]
- 71.Bletz MC, Kelly M, Sabino-Pinto J, Bales E, Van Praet S, Bert W, Boyen F, Vences M, Steinfartz S, Pasmans F, Martel A. 2018. Disruption of skin microbiota contributes to salamander disease. Proc R Soc B 285:20180758. 10.1098/rspb.2018.0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tang L, Schramm A, Neu TR, Revsbech NP, Meyer RL. 2013. Extracellular DNA in adhesion and biofilm formation of four environmental isolates: a quantitative study. FEMS Microbiol Ecol 86:394–403. 10.1111/1574-6941.12168. [DOI] [PubMed] [Google Scholar]
- 73.Barry AL. 1986. Procedure for testing antimicrobial agents in agar media: theoretical considerations, p 1–26. In Lorian V (ed), Antibiotics in laboratory medicine. Williams & Wilkins, Baltimore, MD. [Google Scholar]
- 74.Borlee BR, Goldman AD, Murakami K, Samudrala R, Wozniak DJ, Parsek MR. 2010. Pseudomonas aeruginosa uses a cyclic-di-GMP-regulated adhesin to reinforce the biofilm extracellular matrix. Mol Microbiol 75:827–842. 10.1111/j.1365-2958.2009.06991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.O'Toole GA. 2011. Microtiter dish biofilm formation assay. JoVE 10.3791/2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.National Center for Biotechnology Information, Camacho C. 2008. BLAST (r) command line applications user manual. National Center for Biotechnology Information, Bethesda, MD. [Google Scholar]
- 77.Carini P, Marsden PJ, Leff JW, Morgan EE, Strickland MS, Fierer N. 2017. Relic DNA is abundant in soil and obscures estimates of soil microbial diversity. Nat Microbiol 2:16242. 10.1038/nmicrobiol.2016.242. [DOI] [PubMed] [Google Scholar]
- 78.Hyatt AD, Boyle DG, Olsen V, Boyle DB, Berger L, Obendorf D, Dalton A, Kriger K, Heros M, Hines H, Phillott R, Campbell R, Marantelli G, Gleason F, Coiling A. 2007. Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis. Dis Aquat Organ 73:175–192. 10.3354/dao073175. [DOI] [PubMed] [Google Scholar]
- 79.Fellner P, Ehresmann C, Stiegler P, Ebel J. 1972. Partial nucleotide sequence of 16S ribosomal RNA from E. coli. Nature New Biol 239:1–5. [PubMed] [Google Scholar]
- 80.Schloegel LM, Toledo LF, Longcore JE, Greenspan SE, Vieira CA, Lee M, Zhao S, Wangen C, Ferreira CM, Hipolito M, Davies AJ, Cuomo CA, Daszak P, James TY. 2012. Novel, panzootic and hybrid genotypes of amphibian chytridiomycosis associated with the bullfrog trade. Mol Ecol 21:5162–5177. 10.1111/j.1365-294X.2012.05710.x. [DOI] [PubMed] [Google Scholar]
- 81.Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Caraballo-Rodríguez AM, Chase J, Cope EK, Da Silva R, Diener C, Dorrestein PC, Douglas GM, Durall DM, Duvallet C, Edwardson CF, Ernst M, Estaki M, Fouquier J, Gauglitz JM, Gibbons SM, Gibson DL, Gonzalez A, Gorlick K, Guo J, Hillmann B, Holmes S, Holste H, Huttenhower C, Huttley GA, Janssen S, Jarmusch AK, Jiang L, Kaehler BD, Kang KB, Keefe CR, Keim P, Kelley ST, Knights D, et al. 2019. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857., 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high throughput community sequencing data. Nat Methods 7:335–336. 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hamady M, Walker JJ, Harris JK, Gold NJ, Knight R. 2008. Error-correcting barcoded primers for pyrosequencing hundreds of samples in multiplex. Nat Methods 5:235–237. 10.1038/nmeth.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hamady M, Knight R. 2009. Microbial community profiling for human microbiome projects: tools, techniques, and challenges. Genome Res 19:1141–1152. 10.1101/gr.085464.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. 2016. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bokulich NA, Kaehler BD, Rideout JR, Dillon M, Bolyen E, Knight R, Huttley GA, Caporaso JG. 2018. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 6:90. 10.1186/s40168-018-0470-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Robeson MS, II, O’Rourke DR, Kaehler BD, Ziemski M, Dillon MR, Foster JT, Bokulich NA. 2020. RESCRIPt: reproducible sequence taxonomy reference database management for the masses. PLoS Comput Bio 17:e1009581. 10.1371/journal.pcbi.1009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.R Core Team. 2021. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 89.Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780. 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stamatakis A, Hoover P, Rougemont J. 2008. A rapid bootstrap algorithm for the RAxML web servers. Syst Biol 57:758–771. 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- 92.Wickham H. 2017. The tidyverse. R Package version 11.1. https://cran.r-project.org/web/packages/tidyverse/index.html.
- 93.Vu VQ. 2011. ggbiplot: a ggplot2 based biplot. https://rdrr.io/github/vqv/ggbiplot/.
- 94.Fox J, Weisberg S, Adler D, Bates D, Baud-Bovy G, Ellison S, Firth D, Friendly M, Gorjanc G, Graves S. 2012. Package ‘car’. https://cran.r-project.org/web/packages/car/index.html.
- 95.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 96.Gareth J, Daniela W, Trevor H, Robert T. 2013. An introduction to statistical learning: with applications in R. Spinger, New York, NY. [Google Scholar]
- 97.Menard S. 2002. Applied logistic regression analysis. Sage Publications, Thousand Oaks, CA. [Google Scholar]
- 98.Stan Development Team. 2016. rstanarm: Bayesian applied regression modeling via Stan. https://mc-stan.org/rstanarm.
- 99.Stan Development Team. 2020. RStan: the R interface to Stan. https://cran.r-project.org/web/packages/rstan/vignettes/rstan.html.
- 100.Makowski D, Ben-Shachar MS, Lüdecke D. 2019. bayestestR: describing effects and their uncertainty, existence and significance within the Bayesian framework. JOSS 4:1541. 10.21105/joss.01541. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental methods and pilot descriptions, Fig. S1 to S9, and Tables S1 to S4. Download aem.01604-21-s0001.pdf, PDF file, 1.5 MB (3MB, pdf)
Data Availability Statement
All sequences can be found at EBI/ENA under accession no. PRJEB46498 (secondary accession no. in SRA, ERP130685; https://www.ebi.ac.uk/ena/browser/view/PRJEB46498?show=reads). Codes for data processing, analysis, and figure generation can be found on our GitHub page at https://github.com/mech3132/BiofilmMicrocosm.