ABSTRACT
Bacteria adapt to the constantly changing environment by regulating their metabolism. The global transcriptional regulator CodY is known to regulate metabolism in low-G+C Gram-positive bacteria. Systems-level identification of its direct targets by proteome and chromatin immunoprecipitation followed by sequencing (ChIP-seq) assays have rarely been reported. Here, we identified that CodY serves as an activator or a repressor of hundreds of genes involved in nitrogen metabolism, carbohydrate metabolism, and transcription through iTRAQ proteome and ChIP-seq. Combined with the electrophoretic mobility shift assay (EMSA), apart from the genes associated with amino acid biosynthesis (ilvD, leuA, optS, ybbD, dtpT, and pepN), genes involved in cell wall synthesis (murD and ftsW) and nisin immunity (nisI) were identified as being regulated by CodY. Moreover, it was demonstrated by nisin resistance assay that CodY activated the transcription of nisI and contributed to nisin immunity. Intriguingly, CodY showed a self-regulation through binding to the motif AAAGGTGTGACAACT in the coding sequence (CDS) region of codY, as verified by DNase I footprinting assay and MEME analysis. In addition, a novel conserved AT-rich motif, AATWTTCTGACAATT, was obtained in L. lactis F44. This study provides new insights into the comprehensive CodY regulation in L. lactis by controlling metabolism, nisin immunity, and self-expression.
IMPORTANCE Lactococcus lactis, a species of lactic acid bacteria (LAB) widely used in food fermentation, has been the model strain in genetic engineering, and its application has extended from food to microbial cell factories. CodY is a global regulator in low-G+C Gram-positive bacteria. Its function and direct target genes at the genome-level are little known in L. lactis. In this study, we describe the comprehensive regulation mechanism of CodY. It widely modulated the metabolism of nitrogen and carbohydrate, cell wall synthesis, and nisin immunity in L. lactis F44, and its expression level was regulated by feedback control.
KEYWORDS: Lactococcus lactis, transcriptional regulator, CodY, metabolism, nisin
INTRODUCTION
Lactococcus lactis has been widely used in food fermentation for centuries, and it has generally recognized as safe (GRAS) status (1). Over the last couple of decades, its application has been enlarged from food fermentation to microbial cell factories (2), and it has been used as a vehicle for delivering therapeutics and live vaccines (3–5). Nisin, a lanthionine-containing antimicrobial peptide generated from L. lactis, serves as an effective inhibitor of Clostridioides difficile (6). Hence, nisin and L. lactis show potential for treatment of C. difficile-associated diarrhea (7).
CodY, a global transcriptional regulator, is associated with starvation, stringent response, and virulence in many low-G+C Gram-positive bacteria (8–10). It is activated by GTP and branched-chain amino acids (BCAAs)—leucine, isoleucine, and valine. However, GTP seems to be ineffective in CodY activation in L. lactis (11). Recently, a cis-acting attenuator was identified to coordinate with trans-acting CodY in the regulation of BCAA biosynthesis in both Staphylococcus aureus and Listeria monocytogenes (12, 13). CodY can serve as an activator by binding to the promoter region to increase the transcription level and as a repressor by binding within the coding sequence to repress (14). CodY binding requires a 15-nucleotide (nt) palindromic AT-rich sequence, 5′-AATTTTCWGAAAATT-3′, of the target gene in L. lactis, Bacillus subtilis, and L. monocytogenes (11, 14, 15). Another potential CodY motif, 5′-CTGTCAG-3′, was identified by using the transcriptome in L. lactis KF147 at near-zero growth rates, but the direct binding was not verified (16). Furthermore, the full-length crystal structure of B. subtilis CodY in the absence of its ligands was determined. It was dimeric under the concentrations similar to those found in bacterial cells, while could form tetramers at the concentration of crystallization (17). According to the crystal structure and motif, we previously investigated the interaction mechanism between CodY protein and DNA in L. lactis by molecular simulation, revealing the identities of nine key residues in CodY-DNA binding, among which Ser186, Arg218, and Lys242 play a vital role (18).
CodY regulation has been widely investigated in several Gram-positive bacteria, but the genome-scale identification of the direct target genes of CodY has been revealed only in L. monocytogenes, S. aureus, and B. subtilis (11, 14, 19). In L. monocytogenes, a range of genes involved in metabolism, virulence, and motility were identified to be under the direct control of CodY in vivo by using chromatin immunoprecipitation followed by sequencing (ChIP-seq) (11), and the virulence was controlled by CodY via directly activating the master virulence activator gene prfA (20). In B. subtilis, DNA affinity purification sequencing (IDAP-seq) was used to identify a subset of the strongest CodY binding sites, and a 17-nt motif, ATTTTCWGAAWWTTCWG, was obtained according to MEME analysis (10). In L. lactis, key pathways for nitrogen metabolism were identified as being modulated by CodY, such as amino acid biosynthesis and the assimilation of peptides, through transcriptome analysis (21), but the genome-wide analysis of its direct targets in vivo has not been revealed.
In this study, we investigated CodY function and its direct target genes in L. lactis through ChIP-seq and iTRAQ quantitative proteome analysis. The results showed that CodY can function as a repressor and/or an activator involved in amino acid and carbohydrate metabolism, cell wall synthesis, nisin immunity, and self-regulation; such direct regulations were verified by electrophoretic mobility shift assay (EMSA) and real-time quantitative reverse transcription-PCR (qRT-PCR), and the binding sequences of CodY were identified by DNase I footprinting assay. In summary, we give an insight into the comprehensive CodY regulation in L. lactis.
RESULTS
Investigation of the molecular mechanism of CodY by proteomic analysis.
Since CodY is highly conserved in low-G+C Gram-positive bacteria, the nucleotide sequence of L. lactis F44 CodY was conserved in 42 L. lactis strains (>90% identity) (see Fig. S1 in the supplemental material). To assess the regulation function of CodY in L. lactis, the quantitative iTRAQ liquid chromatography-tandem mass spectrometry (LC-MS/MS) proteomic analysis was performed on wild-type (WT) and codY knockout (ΔcodY) strains. Samples were harvested at the log, each with two biological replicates. After filtration, the qualified spectra matched 1,393 proteins, about 62.6% of the 2,223 predicted proteins in the L. lactis F44 genome (see Fig. S2A in the supplemental material). Among them, 357 proteins were tested to have over 30% of their sequences covered (Fig. S2B). The reproducibility of the two replicates evaluated by error distribution analysis demonstrated that the level of biological noise was relatively low (see Fig. S3 in the supplemental material).
To investigate CodY regulation, comparative proteomic analysis was conducted between the F44 WT and ΔcodY mutant strains. A total of 129 proteins were affected by CodY, among which 111 proteins were upregulated and 18 proteins were downregulated, respectively, in the ΔcodY strain compared with those in the WT, using a cutoff of >1.5-fold change (P < 0.05). Then 10 of the proteins were selected to be verified for their expression levels by the multiple-reaction monitoring (MRM) assay (see Table S1 in the supplemental material), indicated a positive correlation with the proteome result. Moreover, the significantly changed proteins were widely distributed in amino acid transport and metabolism, carbohydrate transport and metabolism, and transcription according to cluster of orthologous group (COG) functional category analysis (Fig. 1A). According to KEGG enrichment analysis, the proteins involved in the biosynthesis of amino acids, biosynthesis of antibiotics, nitrogen metabolism, and galactose metabolism were enriched (P < 0.0005) (Fig. 1B).
FIG 1.
Functional analysis of CodY regulation. (A) Cluster of orthologous group (COG) functional category analysis of differentially expressed proteins (with a cutoff of >1.5-fold change, P < 0.05). (B) Pathway classification according to the KEGG enrichment analysis of differentially expressed proteins. The ratio of the number of differentially expressed proteins to the number of total proteins in this pathway is shown (P < 0.005).
Based on the comparative proteomic analysis, amino acid transport and metabolism were widely affected by CodY. Notably, almost all proteins associated with BCAA biosynthesis (IlvA, IlvB, IlvC, IlvD, IlvH, LeuA, and LeuD) were significantly repressed by CodY (directly or indirectly [>3.0-fold]) (see Table S2 in the supplemental material), which is consistent with previous studies that the regulator CodY represses BCAA biosynthesis (11, 12). Besides that, expression (shown for each protein as fold change) of the amino acid transporter and permease (LysP, 0.64; DtpT, 1.66; YddD, 2.46; BcaT, 2.69; YdgC, 2.7; OptS, 5.2) and biosynthesis pathways of aspartate (AsnB, 2.54), arginine (ArgF, 3.91), glutamate (GltB, 4.49; GltD, 4.51), lysine (LysA, 2.42), and serine (SerB,1.68; SerC, 1.57) was found to be under the control of CodY.
Besides nitrogen metabolism, the results showed that carbohydrate intake and metabolism were also mediated by CodY. Cody repressed the essential component of the sugar-transporting phosphotransferase system (PTS)—phosphocarrier protein PtsH—which is involved in the metabolism of maltose and galactose (MalE, MalP, GalK, and LacZ). On the contrary, CodY activated 6-phosphogluconate dehydrogenase GntZ (0.61), which is the main generator of NADPH in the oxidative branch of the pentose phosphate pathway and 6-phospho-β-glucosidase BglA (0.66) associated with the β-glucoside metabolism. Moreover, fatty acid biosynthesis protein FabI was downregulated (0.67) in the ΔcodY strain, but the acyl carrier protein AcpP was inhibited by CodY (1.5).
Also, CodY, as a global regulator, could hierarchically mediate a series of transcriptional regulators: MarR family transcriptional regulator NapB (0.67) and Rrf2 family transcriptional regulator YffB (0.4) were activated by CodY. On the contrary, four cold shock proteins (CspC1, CspE, ATY87682.1, and CspB1), XRE family transcriptional regulator YhgC, and LysR family transcriptional regulator MleR (5.47) were repressed by CodY. Overall, the proteomic analysis reveals the potential function of CodY, while its directly regulated genes/pathways need further identification.
Genome-scale identification of genes regulated directly by CodY in vivo via ChIP-seq analysis.
To illuminate genes that are directly controlled by CodY, we performed the genome-scale chromatin immunoprecipitation combined with DNA sequence analysis (ChIP-seq). A 3×Flag-tagged CodY fusion protein expression gene was constructed with plasmid pLEB124 and was then transformed into the L. lactis F44 WT strain. The recombinant strain, FCodY-Flag, was grown in the seed medium to the log phase and cross-linked by formaldehyde. Subsequently, ChIP-seq was performed as described in Materials and Methods.
Through the ChIP-seq analysis, we found 91 loci harboring CodY binding peaks were enriched (>1.35-fold; P = e−5) compared with that of the input DNA (Fig. 2A; see Table S3 in the supplemental material). Among these 91 loci, 50 were situated in the intergenic region (55%) and 41 in the coding regions (45%) (Fig. 2B). Besides, 26 loci were mapped to the same transcriptional units that were significantly changed in the proteomic analysis (Fig. 2C; Table S3). It is noteworthy that the genes involved in the cell wall biosynthesis (murA, murD, ftsW, and ftsW1) and l-rhamnose synthesis related to cell wall formation (rmlA) were bound by CodY. Furthermore, the results demonstrated that the nisin immunity gene, nisI, was regulated by CodY. Additionally, we discovered that CodY had the potential to regulate itself by binding to its coding sequence (CDS) region.
FIG 2.
ChIP-seq analysis of CodY direct binding sites. (A) CodY binding sites in L. lactis F44 genome. The two outer circles represent the + and – strands of L. lactis F44 genome; each line represents a gene. The third and fourth circles represent the + and – strands of the proteome result. The orange lines represent the upregulated proteins (>1.5- and <2.5-fold), and the red lines represent the upregulated proteins (>2.5-fold). The dark green lines represent the downregulated proteins (<0.67). The gray lines represent proteins not significantly changed (>0.67- and <1.5-fold). The fifth and sixth circles represent the + and – strands of the ChIP-seq result. The orange lines represent the peaks with the fold enrichment of >1.4-fold and <2-fold. The red lines represent the peaks with the fold enrichment of >2-fold and <3-fold. The dull red lines represent the peaks with the fold enrichment of >3-fold. (B) Pie chart representing the distribution of binding sites in the genome based on the ChIP-seq result. (C) Venn diagram representing the numbers of significantly changed proteins identified in proteome results and the CodY binding genes found in the ChIP-seq result.
Combining the data of the proteome and ChIP-seq analysis, we obtained the candidates of targets directly regulated by CodY. The genes involved in amino acid transport (ybbD, pepN, optS, dtpT, and ydgC) and BCAA synthesis (leuA and ilvD) were found to be directly controlled by CodY, and their expression levels were more abundant in the codY null mutant than those in the WT. These results were consistent with the previous studies that nitrogen metabolism was the main pathway inhibited by CodY (22). Three genes related to nitrogen uptake, pepO, oppA, and oppC, were also identified to be directly controlled by CodY in the ChIP-seq results. Besides, uridine phosphorylase (Udp) was found to be directly repressed by CodY. Udp, a key enzyme in the pyrimidine salvage pathway, plays an important role in cell survivability by catalyzing the reversible phosphorolysis of converting uridine into uracil and ribose-1-phosphate (23).
Several genes without significant changes in the proteome were obtained to be directly bound by CodY in ChIP-seq results: for instance, the transcriptional regulator genes glnR, gntR, ydaF, and busR. This result was similar to those from previous studies that found GntR and GlnR were regulated directly by CodY in L. monocytogenes (11), and the cell wall physiology of pneumococci was impacted by the co-inactivation of CodY and nitrogen metabolism regulator GlnR (24). In addition, pfk (encoding ATP-dependent 6-phosphofructokinase) and pgl (encoding 6-phosphogluconolactonase), which are involved in the pentose phosphate pathway, could be directly regulated by CodY.
Verification of the direct regulatory genes of CodY.
We verified the direct bindings between CodY and the DNA regions of 17 genes identified from ChIP-seq in vitro by using electrophoretic mobility band shift assays (EMSAs). In order to obtain the His6-tagged CodY protein, the expression vector was constructed in Escherichia coli BL21(DE3), and then the protein was purified (>95% pure as observed by SDS-PAGE). The DNA probes (the DNA binding regions of genes) were amplified with PCR by using Cy5-labeled primers. The upstream region of the gene nox, which could not bind to CodY, was used as a negative control. In the result, in contrast to no direct binding in the negative control, 16 out of 17 candidates were verified with a clear gel shift (Fig. 3). These genes are involved in amino acid transport and metabolism (optS, ybbD, dtpT, pepN, ilvD, leuA, yncA/gltB, and ydgC/ydgD), are cell wall related (murA, murD, rmlA, ftsW, and ftsW1), encode transcriptional regulators (busR and codY), and are involved in nisin immunity (nisI). Notably, the genes associated with amino acid transport and metabolism, like ilvD and leuA (5.10-fold and 2.64-fold enriched in ChIP-seq, respectively), showed a relatively higher level of binding than the others.
FIG 3.
EMSA results of direct binding of CodY to the target genes. (A) EMSA analysis of CodY binding to the genes involved in amino acid synthesis and transport. (B) EMSA analysis of CodY binding to the genes involved in cell wall synthesis. (C) EMSA analysis of CodY binding to transcriptional regulators. (D) EMSA analysis of CodY binding to the nisin immunity gene and the negative control. The results were independently repeated at least three times. EMSA was performed with 10 nM BCAAs.
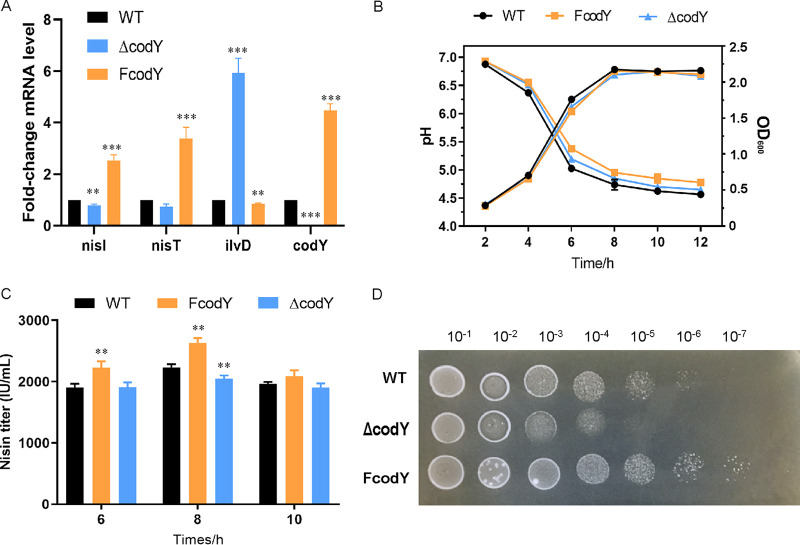
CodY contributes to nisin immunity.
The nisin immunity gene, nisI, was obtained to be directly bound by CodY in ChIP-seq, and the result was verified by EMSA (Fig. 3D). To further investigate the regulation mechanism, the transcription levels of nisI were determined by qRT-PCR assay. As shown in Fig. 4A, the expression levels of these genes were significantly increased in FcodY. NisI is a specific nisin immunity protein that can prevent nisin from reaching targets by binding to it (25). Thus, the nisin resistance assay was carried out to detect the CodY regulation of NisI. Growth characteristics (by optical density at 600 nm [OD600] and pH) and nisin yield during fermentation were determined (Fig. 4B and C). The results showed overexpression of codY could increase nisin yield by 17.8% and had no significant effect on cell growth. As shown in Fig. 4D, FcodY showed more resistance to nisin than the WT strain, whereas the ΔcodY mutant strain was deceased. The maximum valid dilutions of the strains on the plate with 8,000 IU/mL nisin were as follows: 1:106 for FcodY, 1:105 for F44, and 1:104 for the ΔcodY mutant. These results suggested that CodY could enhance the nisin immunity by directly activating the transcription of nisI.
FIG 4.
CodY contributes to nisin synthesis and immunity. (A) Transcriptional levels of nisI and nisT by qRT-PCR analysis. ilvD is the positive control. (B) Growth characteristics of the FcodY, ΔcodY mutant, and WT strains. (C) Nisin yields of the FcodY, ΔcodY, and WT strains. (D) The nisin resistance of the FcodY, ΔcodY, and WT strains. The ability was detected by the serial dilutions plated on the 8,000-IU/mL nisin plates. Error bars indicate the standard deviation (SD) from three independent experiments. **, P < 0.01, and ***, P < 0.001, by t test.
Identification of the CodY DNA binding region using DNase I footprinting.
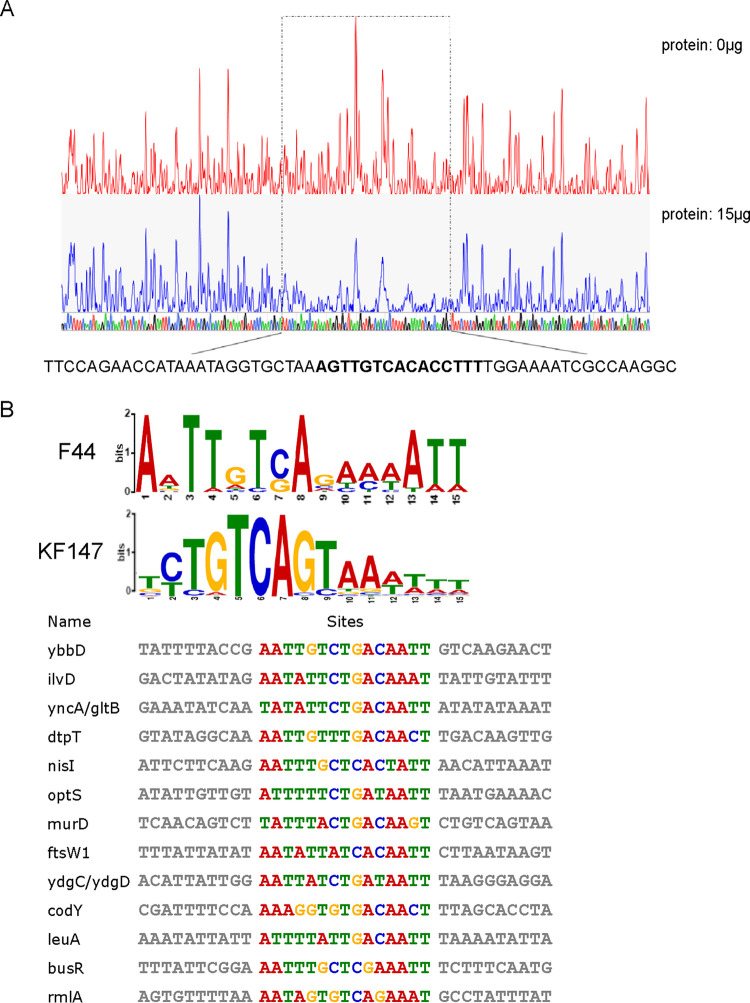
The ChIP-seq results demonstrated that CodY could bind to the CDS region of codY, and this was verified by in vitro EMSA (Fig. 3). To further identify the DNA binding motif, the DNase I footprinting assay was carried out. A 57-bp binding region (GCCTTGGCGATTTTCCAAAAGGTGTGACAACTTTAGCACCTATTTATGGTTCTGGAA [positions +317 to +373]) in the CDS region of codY (Fig. 5A) was protected by CodY from DNase I digestion. Moreover, the summit was in the middle of the 57-bp binding region obtained in ChIP-seq, indicating that CodY binds to the CDS region of the codY gene, which was suggested to be a self-regulatory mechanism.
FIG 5.
DNase I footprinting assay and MEME analysis. (A) DNase I footprinting result of CodY binding to codY. The DNA fragment from the CDS region of codY is labeled with FAM dye, incubated without CodY (red line) or with CodY (blue line). The peak represents the number of DNA fragments after DNase I digestion detected by DNA analyzer. DNA sequencing is shown at the bottom, and the sequence in boldface is the motif identified by using MEME analysis. The region protected by CodY from DNase I cleavage is indicated with dashed black boxes. (B) MEME analysis based on DNA-binding sequences obtained by DNase I footprinting and EMSA results.
Characterization of DNA sequences bound with CodY.
The ChIP-seq data showed the direct binding of CodY to regulatory genes involved in different metabolic pathways, such as nitrogen metabolism, transcriptional regulation, nisin immunity, and cell wall synthesis. According to the DNase I footprinting and EMSA results, the 15 binding regions were selected for analysis (see Table S4 in the supplemental material). After the MEME analysis (http://meme-suite.org/) (26), a conserved AT-rich motif was obtained (Fig. 5B). AATWTTCTGACAATT was identified in 13 regions, which was consistent with the conserved 15-nt palindromic AT-rich sequence 5′-AATTTTCWGAAAATT-3′ in L. lactis MG1363, B. subtilis, and L. monocytogenes (11, 14, 15) and which was similar to the motif 5′-TCTGTCAGTAAATTT-3′ identified in L. lactis KF147 (16). Moreover, the motif was in the 57-bp region identified by the DNase I footprinting assay. Coincidentally, the summit of the codY peak found in ChIP-seq was in this motif. These results suggest that CodY regulates self-transcription by binding to the region AAAGGTGTGACAACT.
DISCUSSION
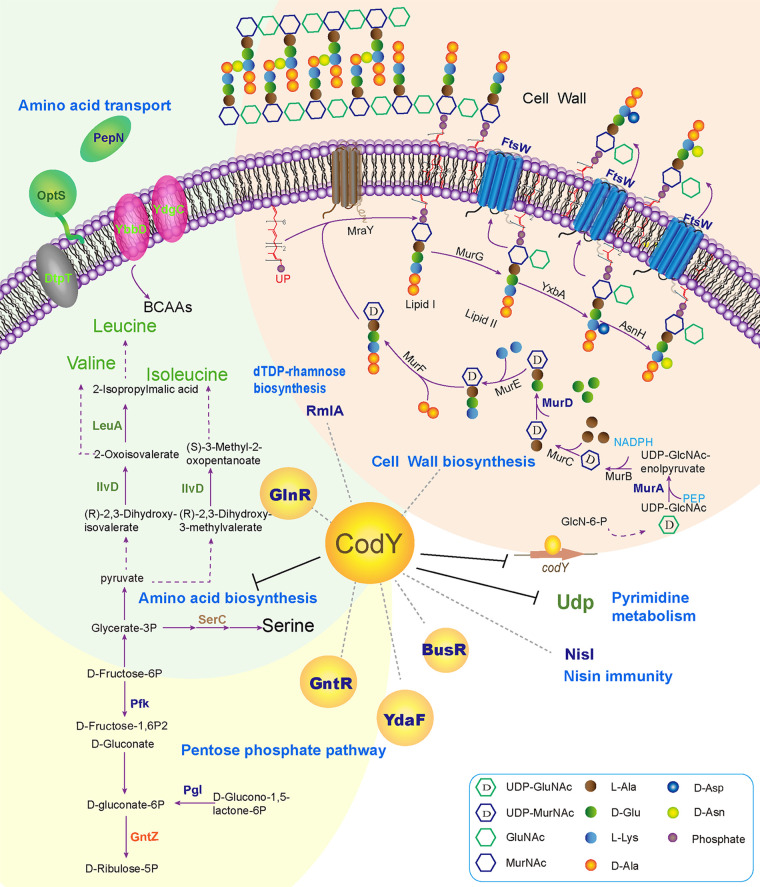
In this study, to further reveal the regulation mechanism of the global regulator CodY in L. lactis F44, a genome-wide analysis was carried out (Fig. 6), and several novel direct target genes of CodY and DNA binding motifs were discovered. Thus, this study throws light on the hierarchical control of CodY.
FIG 6.
The proposed model of CodY regulation mechanism. Green fonts represent genes downregulated by CodY based on the proteome and ChIP-seq results. Blue fonts represent genes regulated by CodY only obtained in ChIP-seq analysis. Orange fonts represent genes upregulated by CodY only obtained in proteome analysis. Brown fonts represent genes downregulated by CodY only obtained in proteome analysis.
To determine the complicated roles of CodY in L. lactis in vivo, we employed iTRAQ LC-MS/MS proteomic analysis and ChIP-seq: the proteomic analysis showed that CodY affected the expression of hundreds of genes, proving the global regulation role of CodY in L. lactis, and the ChIP-seq revealed that CodY controlled 92 regions directly in the log phase. However, there was some inconsistency between proteome and ChIP-seq, due to the influence of posttranscriptional modification and translation. In total, CodY mainly served as the repressor of the metabolic genes, especially nitrogen metabolism, but it could activate several genes, such as nisin immunity gene nisI.
CodY, a highly conserved global transcriptional regulator, exists in most species of low-G+C Gram-positive bacteria, including Bacillus, Streptococcus, Listeria, and Lactococcus (27), and it participates in a large number of metabolisms, including the uptake and metabolism of amino acid (7, 28), carbon metabolism (29), motility (15, 30), virulence (20, 31), cell division, and competence development (32) in Gram-positive bacteria. In these metabolisms, the transcriptional responses of CodY vary from target to target, which gives rise to the hierarchical control of gene expression: this is to some extent related to the activity change of CodY induced by the nutrient limitation (33). Similar to the previous studies, we found CodY repressed the genes involved in amino acid transport and metabolism—mainly BCAAs and glutamate. Especially, the expressions of leuA, ilvD, gltB, optS, ybbD, and ydgC/ydgD were repressed with strong affinity. Consistent with our results, the previous study revealed that ilvD, serC, gltB, pepC, pepO, and pepN were controlled directly by CodY in L. lactis (11, 22). In carbohydrate metabolism, we found that pfk was regulated by CodY. The previous study found pfk was less abundant under isoleucine starvation, which seemed to be under CodY and under stringent control in L. lactis IL-1403 according to transcriptomic and proteomic analyses (34).
Besides the above metabolic regulation, this study identified a novel role of CodY—regulating nisin immunity. We demonstrated that CodY directly bound to the region of nisI identified by ChIP-seq and EMSAs. Also, the transcriptional level of nisI was increased, the nisin-resistant ability was enhanced in FcodY (Fig. 4), and the summit of the binding region identified by ChIP-seq was found to be located at the promoter region of nisI (35). Altogether, it is suggested that nisin immunity is directly activated by CodY.
According to the EMSA and DNase footprinting assays, CodY could bind to the CDS region of its own gene, which implied a self-regulation mechanism (Fig. 3 and 6). A lot of transcriptional regulators have been identified to possess the feedback control. For instance, transcriptional regulator LrpA was identified to be bound to the transcriptional start site of its own promoter by DNase I footprinting (36), and in E. coli and Salmonella enterica serovar Typhimurium, the two-component system phoPQ was transcribed from a constitutive promoter controlled by a positive regulator which is regulated by PhoPQ (37). In the present work, we found CodY protein could bind to its own CDS region, which might serve as a negative self-regulator with the roadblock mechanism causing premature termination of transcription, demonstrating that CodY possesses a feedback control.
MATERIALS AND METHODS
Strains and growth conditions.
The strains and plasmids used in this study are listed in Tables 1 and 2. L. lactis subsp. lactis F44 (accession no. PRJNA419050) and its derivatives were cultivated in seed medium (1.5% yeast extract, 1.5% peptone, 2% KH2PO4, 1.5% sucrose, 0.15% NaCl, 0.015% MgSO4·7H2O [pH 7.2]) at 30°C. The growth characteristics were determined from three independent growth experiments. Cells for proteomic analysis were prepared at 30°C for 6 h (log phase) in seed medium and were collected by centrifugation at 8,228 × g for 5 min at 4°C. E. coli DH5α and BL21 were cultivated in Luria-Bertani (LB) broth at 37°C with shaking at 180 rpm.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Source |
|---|---|---|
| Strains | ||
| L. lactis | ||
| F44 | Genome shuffling of L. lactis YF11 (accession no. CGMCC7.52) | 46 |
| FcodY | L. lactis F44 strain with codY gene overexpression | This study |
| ΔcodY mutant | L. lactis F44 deletion mutant for codY gene | This study |
| FCodY-Flag | L. lactis F44 with plasmid 3×Flag-tagged CodY overexpression used for ChIP-seq assay | This study |
| E. coli | ||
| DH5α | Host for plasmid construction | Lab collection |
| BL21 | Host for expressing protein | Lab collection |
| His6-CodY | E. coli BL21 with plasmid pET28a-codY used for CodY protein purification | This study |
| Plasmids | This study | |
| pLEB124 | Vector containing p45 promoter with repA; Emr | 47 |
| pLEB124-codY | pLEB124 carrying the codY DNA region; Emr | This study |
| pET28a | Kanr, His tag | Lab collection |
| pET28a-codY | Pet28a carrying the codY DNA region; Kanr | This study |
| pNZ5319 | Cmr Emr | 48 |
| pNZ5319-ΔcodY | Plasmid for codY deletion; Cmr Emr | This study |
| pNZTS-Cre | cre gene cloned at the EcoRI and HindIII sites; Emr | Lab collection |
| pLEB124-codY-3×flag | pLEB124 carrying CodY and 3×Flag tag fusion protein; Emr | This study |
TABLE 2.
Primers used in this study
| Primer | 5′→3′ | Product length (bp) | Function |
|---|---|---|---|
| FcodY-F | CCCAAGCTTGGGAAAAATAGGAGAACAAAGTGGCT | 809 | To construct codY overexpression strain FcodY |
| FcodY-R | CGCGGATCCGCGGTGGTGATGGTGATGATGCTTTTAGAAATTACGTCCTGCAA | ||
| codY-up-F | CCGCTCGAGTTTCTGAATTGATTGTTATGGC | 1,117 | To construct knockout codY strain |
| codY-up-R | AGCTTTGTTTAAACGGTCACTCCATCTTGCAAAA | ||
| codY-down-F | TCCCCCGGGAAAGCCAGGGTCTAAGCG | 1,128 | ΔcodY |
| codY-down-R | CGAGCTCAACCACCCATCGCAAACT | ||
| nisI-F | AAAGTATCCCGAGGTCTGAATG | 132 | qRT-PCR |
| nisI-R | CAACGGCAAATGCTTCAGTA | ||
| nisT-F | TTTCAAGAAGGGCGTCAACT | 243 | |
| nisT-R | CTGTCCATCTTTCATAACCACG | ||
| ilvD-F | GTCTATGGTGGAACGATTGAACA | 236 | |
| ilvD-R | GGATTGGAAGAGGAATAAGGTAAAC | ||
| codY-F | ATACGAAGGGCGAGTTGCT | 171 | |
| codY-R | CTGGGAAGATTGCTAATGGAC | ||
| EMSA-optS-F | CCAAGTGTTATTCAAAGGTTCA | 311 | EMSA |
| EMSA-optS-R | TGTTTTTGTGCCACAGGC | ||
| EMSA-ybbD-F | GCACAAGCAGCAGGTTTG | 318 | |
| EMSA-ybbD-R | ACCTAGTGCAAGAAAATCACG | ||
| EMSA-leuA-F | TTATGTCTACCTAAAGCAACAAAAT | 478 | |
| EMSA-leuA-R | TTCCAGTTGTTTAGCAATCGT | ||
| EMSA-ilvD-F | TAACTGAGAAAGGAAAGCAAAA | 305 | |
| EMSA-ilvD-R | ATTTCCGTCCCAATCCAT | ||
| EMSA-yncA/gltB-F | AAAATCAGGCTGCCAAAGT | 317 | |
| EMSA-yncA/gltB-R | AAAAGGGCATGGTTACGATA | ||
| EMSA-dtpT-F | TTTTCAAAGAATCTGTATAGGCA | 300 | |
| EMSA-dtpT-R | TTGAAAGATAGACAAGTGCACC | ||
| EMSA-codY-F | ATGCATGTGTGATTAATACGAAG | 510 | |
| EMSA-codY-R | CAGCAATGACAGAAGCGATA | ||
| EMSA-ydgC/D-F | CATTTGAATGGTTTTCCCAG | 304 | |
| EMSA-ydgC/D-R | ATTTCTAATAGATTTTGGTCGATT | ||
| EMSA-busR-F | TTTTTTGTCCAACTTTAAGTTCAT | 455 | |
| EMSA-busR-R | TGACTATCGTTCTCCAATTACAG | ||
| EMSA-pepN-F | ATAAATGACAAAATAGGGGATAAT | 181 | |
| EMSA-pepN-R | TTTACAGCCATGTTTTCTCCTA | ||
| EMSA-rmlA-F | TGGTTGCTCAACCAAGAAGA | 209 | |
| EMSA-rmlA-R | GACCTCCATAATCATAATTAATCAA | ||
| EMSA-ftsW1-F | ATTATCTTGCTGTTTTTTTATTTAT | 336 | |
| EMSA-ftsW1-R | TTCATTATCTTTTTTTCCTCAT | ||
| EMSA-ftsW-F | AAGAAGGCGTGAAATAGTGC | 397 | |
| EMSA-ftsW-R | TAGTTGCAAATCAATGAATAACTT | ||
| EMSA-murA-F | ATGGAAGGTACTTTTGTGTTATAAT | 526 | |
| EMSA-murA-R | AGCCTTAGCTTCAATATAACCAG | ||
| EMSA-murD-F | TCACTGACGACTTTATCTAATCTA | 310 | |
| EMSA-murD-R | TATTACGCTCTAAGTCTCATACTTT | ||
| EMSA-noxE-F | GAAACAATGTGGCAAGCA | 242 | |
| EMSA-noxE-R | ACCGATAACTACGATTTTCAT | ||
Real-time qRT-PCR assay.
Total RNAs were extracted with ZR RNA MiniPrep (Zymo Research, USA). RNAs were reversed transcribed into first-strand cDNA with the RevertAid first strand cDNA synthesis kit (Thermo Scientific, USA). The transcriptions of genes were detected by the LightCycler 480 real-time PCR system (Roche Diagnostics, Ltd., Mannheim, Germany). The qPCRs were performed in 20-μL volumes consisting of 5 μL cDNA (100 ng), 10 μL LightCycler 480 SYBR green I master mix (Roche), 1 μL each primer (final concentration, 0.5 μM), and 3 μL RNase-free water. The PCR program was run as follows: preincubation at 95°C for 5 min, followed by 45 cycles of 95°C for 10 s, 55°C for 10 s, and 72°C for 20 s, with a melting curve cycle of 95°C for 5 s and 65°C for 1 min, and then 97°C for acquisition mode. The 16S rRNA gene was used as the endogenous control gene to normalize the expression of the target gene. Triplicate technical replicates were performed. The difference in the relative expression levels was calculated by the threshold cycle (2−ΔΔCT) method. The primers used for qRT-PCR are shown in Table 2.
Construction of the codY overexpression and knockout strains.
The primers of the genes used in the study are listed in Table 2. The gene codY was amplified with the addition of BamHI/SmaI restriction sites, and the PCR fragment was cloned into the plasmid pLEB124, which contained the multiple-cloning sites preceded by the P450 promoter. The resulting recombination plasmid, pLEB124-codY, was transformed into E. coli DH5α. After erythromycin (100 μg/mL) selection, the plasmid was extracted with the TIANprep Mini Plasmid kit (Tiangen, China) and transformed into L. lactis F44 by electroporation transformation to generate FcodY.
The homologous double-crossover recombination method was applied to construct an L. lactis codY knockout (ΔcodY) strain. The detailed method was done as described previously by Zhu et al. (38). The upstream and downstream fragments of codY were amplified with the L. lactis F44 chromosome as a template, and PCR fragments were ligated into the XhoI/PmeI and SmaI/SacI restriction sites of plasmid pNZ5319, respectively. Then the vector was transformed into E. coli DH5α. After that, the positive vector pNZ5319-ΔcodY was transformed into F44 competent cells by electroporation. After Cmr and Emr selection, the knocked-out strain with Cmr was obtained. Then pNZTS-Cre was transformed into the strain to remove the Cmr, and temperature-sensitive pNZTS-Cre was removed by cultivation with the temperature shift method (cultured at 28°C and then shifted to 42°C). After that, we obtained the ΔcodY knockout strain without Emr or Cmr, and the sequence was confirmed by DNA sequencing (Genewiz, Inc.).
iTRAQ quantitative proteomics.
Protein extraction and iTRAQ labeling were performed as in our previous study (39). In brief, samples were ground into powder in liquid nitrogen after centrifugation (8,228 × g, 5 min, 4°C). Protein extraction, protein quantitation, SDS-PAGE, and protein digestion were then conducted. The protein solution with 8 M urea was diluted with 100 mM TEAB (triethylammonium bicarbonate) 4 times. Trypsin Gold (Promega, USA) was applied to digest the proteins (protein/trypsin ratio of 40:1, 37°C, overnight). Afterward, a Strata X C18 column (Phenomenex, USA) was used to desalinate the peptides. The peptides were iTRAQ labeled by the ITRAQ reagent 8-plex kit (Applied Biosystems, CA). Then the labeled peptides were desalted with the Strata X C18 column again.
The peptides were separated by the Shimadzu LC-20AB high-performance liquid chromatography (HPLC) pump system with a high-pH reverse-phase (RP) column. They were resuspended with 2 mL buffer A (5% acetonitrile [ACN] and 95% H2O [pH 9.8]) and then loaded onto a column with 5-μm particles (Phenomenex, USA). The peptides were separated with the following parameters: 1-mL/min flow rate and a gradient of 5% buffer B (95% ACN and 5% H2O [pH 9.8]) for 10 min, from 5% to 35% buffer B for 40 min, from 35% to 95% buffer B for 1 min, 95% buffer B for 3 min, from 95% to 5% buffer B within 1 min, and finally 5% buffer B for 10 min. The absorbance of elution was monitored at 214 nm, and fractions were collected every 1 min. The eluted peptides were condensed into 20 fractions and vacuum dried.
After resuspension of each fraction in buffer C (0.1% formic acid [FA] in water and 2% ACN), the samples were centrifuged (20,000 × g) for 10 min and loaded onto a C18 trap column by an LC-20AD nano-HPLC instrument (Shimadzu, Japan). The flow rate was 5 μL/min for 8 min. After elution, the peptides were separated by an analytical C18 column with the following parameters: 300-nL/min flow rate and 8% to 35% buffer D (0.1% FA in ACN and 2% H2O) for 35 min, going up to 60% buffer D for 5 min, then 80% buffer D for 5 min, and then returning to 5% buffer D within 0.1 min, followed by 5% buffer D for 10 min.
Then the separated peptides were subjected to tandem mass spectrometry with Q Exactive (Thermo Fisher Scientific, CA) for data-dependent acquisition (DDA) detection by nano-electrospray ionization. Proteomic data analysis was performed as previously described (37).
MRM assay.
Multiple-reaction monitoring (MRM) analyses were carried out by a QTRAP 6500 mass spectrometer (SCIEX, Framingham, USA) with an LC-20AD nano-HPLC system (Shimadzu, Japan). The mobile phase consisted of buffer A (0.1% aqueous formic acid) and buffer B (98% acetonitrile with 0.1% formic acid). Peptides were separated by a C18 column (0.075- by 150-mm column, 3.6 μm) with a 300-nL/min flow rate, and the parameters were set up as follows: from 5% to 30% buffer B for 38 min, from 30% to 80% buffer B for 4 min, and then 80% buffer B for 8 min. A 2,400-V spray voltage, 23 lb/in2 nebulizer gas, and a 10-ms dwell time were used for the QTRAP 6500 mass spectrometer. To maximize specificity, the unit resolutions in both Q1 and Q3 quadrupoles were used to monitor multiple MRM transitions.
Skyline software was used to analyze the raw file obtained by QTRAP 6500. An indexed retention time (iRT) strategy was applied to define the chromatography of peptide. Unless interference was observed, all transitions for each peptide were used for quantitation. Samples were spiked with β-galactosidase for label-free data normalization. MSstats with the linear mixed-effects model was used. The P value was adjusted to control the false-discovery rate (FDR), with a cutoff of 0.05. All proteins with a fold change larger than 1.5 with a P value of <0.05 are considered significant proteins.
ChIP.
Chromatin immunoprecipitation (ChIP) was performed as previously described (40). L. lactis F44 was cross-linked by adding 1% formaldehyde in seed medium at 30°C for 10 min. Glycine at 125 mM was added to stop cross-linking. Pellets were washed twice in buffer A (150 mM NaCl, 20 mM Tris-HCl [pH 7.5]) with proteinase inhibitor cocktail (Roche) and resuspended in 400 μL lysis buffer (10 mM EDTA, 1% Triton X-100, 1% SDS, 50 mM Tris-HCl [pH 8.0], protease inhibitor cocktail) for 30 min. The chromatin was sonicated to 200 to 500 bp. After centrifugation (14,000 × g, 4°C, 10 min), 100 μL supernatant was incubated with 10 μg anti-Flag for 16 h at 4°C, and 2% of the supernatant was saved as the input. Then 30 μL protein G magnetic beads (Thermo Scientific, USA) was added to each immunoprecipitation (IP) reaction mixture, and the mixture was incubated for 2 h at 4°C with rotation. Immunoprecipitations were performed with a series of washes: wash buffer B (1% Triton X-100, 0.1% SDS, 150 mM NaCl, 20 mM Tris-HCl [pH 8.1], 2 mM EDTA), wash buffer C (1% Triton X-100, 0.1% SDS, 500 mM NaCl, 20 mM Tris-HCl [pH 8.1], 2 mM EDTA), wash buffer D (1% sodium deoxycholate, 1% NP-40, 0.25 M LiCl, 1 mM EDTA, 10 mM Tris-HCl [pH 8.1]), and TE buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA). The immune complexes were eluted with 400 μL elution buffer (0.1 M NaHCO3, 1% SDS) and incubated for 20 min at 65°C, 20 μL 5 M NaCl was added to reverse the cross-linking, and samples were incubated at 65°C overnight. To digest the proteins, 20 mg/mL proteinase K, 30 mM Tris-HCl (pH 6.5), and 10 mM EDTA were added to the samples, and then they were incubated for 1 h at 45°C. Then the DNA was purified by phenol-chloroform/isoamyl extraction.
ChIP-seq library construction, sequencing and analysis.
The DNA fragments (250 to 350 bp) were selected using SPRI beads and amplified by PCR for 15 cycles following repair and adaptor ligation steps. A Qubit fluorometer (Invitrogen, USA) and Bioanalyzer 2100 (Agilent, USA) were used to validate libraries. ChIP-seq libraries were sequenced (150-nt paired-end sequencing) using the HiSeq X Ten system (Illumina).
After filtering out low-quality reads by Trimmomatic (version 0.38), clean reads were mapped to the L. lactis genome by Bwa (version 0.7.15), allowing up to two mismatches. Samtools (version 1.3.1) was performed to eliminate PCR duplicates. Peak calling was carried out using MACS2 software (version 2.1.1.20160309) with default settings for a cutoff q value of <0.05. Data visualization was performed by IGV (version 2.3.91) using Wig files. KEGG (Kyoto Encyclopedia of Genes and Genomes [http://www.genome.jp/kegg/]) enrichment analysis (41) was performed by clusterProfiler (http://www.bioconductor.org/packages/release/bioc/html/clusterProfiler.html) in the R package (42).
Expression and purification of CodY.
The coding regions of codY were cloned into pET28a+, resulting in the addition of a C-terminal histidine tag. Isopropyl-1-thio-β-d-galactopyranoside (IPTG) was added at 0.5 mM to a 2-L culture (OD600 of ∼0.6) to induce the expression of CodY, with incubation at 18°C overnight. Cells were harvested, washed by binding buffer (50 mM Tris-HCl, 500 mM NaCl, 10 mM imidazole, 10% glycerol [pH 8.0]) containing 100 mg/mL lysozyme and 0.1 M phenylmethylsulfonyl fluoride (PMSF), and incubated on ice for 30 h, followed by sonication, adding 10 μg/mL RNase A and 5 μg/mL DNase I. After centrifugation (12,000 × g, 4°C, 10 min), protein extracts were purified by nickel-nitrilotriacetic acid agarose chromatography (GE Healthcare, Sweden). After being washed with wash buffer (10% glycerol, 20 mM Tris-Cl [pH 7.9], 500 mM NaCl), accompanied by addition of different concentrations of imidazole (20, 50, and 70 mM), proteins were eluted by elution buffer (500 mM NaCl, 20 mM Tris-Cl [pH 7.9], 10% glycerol, 250 mM imidazole). The eluted proteins were desalinated by a PD-10 desalination column (GE Healthcare, Sweden). The purified His6-CodY proteins were stored in buffer supplemented with 10% glycerol and kept at −20°C after being checked by SDS-PAGE.
EMSAs.
The promoter regions of genes were amplified by the L. lactis F44 genome using primers listed in Table 2. The DNA probes were labeled with Cy5 (Genewiz, China). The electrophoretic mobility shift assay (EMSA) was performed by the protocol previously described (43). For each reaction, about 10 ng of DNA probes was mixed with different amounts of His6-CodY proteins using binding buffer (20 mM Tris-base [pH 7.9], 1 mM dithiothreitol (DTT), 10 mM MgCl2, and 5% glycerol) containing 0.2 mg/mL nonspecific protein competitor calf bovine serum albumin (BSA) and 1 mg/mL nonspecific DNA competitor salmon sperm DNA and incubated for 20 min at 25°C. After incubation, protein binding and DNA binding were detected by 6% nondenaturing polyacrylamide gels in 0.5× TBE (Tris-borate-EDTA) running buffer at 4°C. The gels were scanned with an Azure c300 imager (Azure Biosystems, USA).
Nisin yield and resistance assays.
Nisin yield and resistance assays were performed as previously described (44). L. lactis strain F44, the ΔcodY mutant, and FcodY were cultivated to logarithmic phase. The broth was 10-fold serially diluted in normal saline and was plated on agar plates containing 8,000 IU/mL nisin, with incubation for 24 h at 30°C. All experiments were carried out in triplicate.
DNase I footprinting assay.
The DNase I footprinting experiment was performed by the protocol previously described by Wang et al. (45). The main contents are outlined as follows. The binding regions identified by ChIP-seq of murA and codY were PCR amplified by employing the primers listed in Table 2. The probes with 6-carboxyfluorescein (FAM) were labeled through the second round of PCR and were purified by the Wizard SV gel and PCR clean-up system (Promega). A 350-ng amount of murA/codY probe was used for binding reactions with different amounts of proteins (25°C, 30 min). Then 10 μL of 0.015 U DNase I (Promega) solution was added for digestion (37°C, 60 s). The phenol-chloroform extraction was performed to remove protein, and ethanol was used to precipitate DNA. Pellets were dissolved in MiniQ ultrapure water and examined with a 3500 DNA analyzer using Peak Scanner software (Applied Biosystems).
MEME motif prediction.
DNA sequence motifs were predicted using the motif sequence analysis tool MEME, which is available online (https://meme-suite.org/meme/) (26). Binding site searches were carried out using the summit regions (both the upstream and downstream 100 bp of the summit) obtained by ChIP-seq. Both strands were searched, and any number of sites per sequence was allowed.
Statistical analysis.
To evaluate the statistical significance of the gene expression level by qRT-PCR and nisin yield during fermentation, Student's t test was performed.
Data availability.
The ChIP-seq data files have been deposited in the Gene Expression Omnibus (GEO) and are accessible through accession no. GSE139902.
ACKNOWLEDGMENTS
This study was supported by the Funds for the National Key R&D Program of China (2019YFA0905600 and 2020YFA0907900), the National Natural Science Foundation of China (31770076), and Creative Research Groups of China (21621004). Jianjun Qiao was supported by “131” Innovative Personnel Training Project of Tianjin (China) and “MingShiZhiXiang” Meritocrat Project of Shaoxing (China).
We declare no conflict of interest.
Footnotes
Supplemental material is available online only.
Contributor Information
Jianjun Qiao, Email: jianjunq@tju.edu.cn.
Nicole R. Buan, University of Nebraska—Lincoln
REFERENCES
- 1.Wessels S, Axelsson L, Bech Hansen E, De Vuyst L, Laulund S, Lähteenmäki L, Lindgren S, Mollet B, Salminen S, Wright AV. 2004. The lactic acid bacteria, the food chain, and their regulation. Trends Food Sci Technol 15:498–505. 10.1016/j.tifs.2004.03.003. [DOI] [Google Scholar]
- 2.Song AA, In LLA, Lim SHE, Rahim RA. 2017. A review on Lactococcus lactis: from food to factory. Microb Cell Fact 16:55. 10.1186/s12934-017-0669-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao JY, Yuan XM, Xu Y, Yin WL, Lin LY, Pan XY, Yang GL, Wang CF, Shen JY. 2016. Live recombinant Lactococcus lactis vaccine expressing immobilization antigen (i-Ag) for protection against Ichthyophthirius multifiliis in goldfish. Fish Shellfish Immunol 58:302–308. 10.1016/j.fsi.2016.09.037. [DOI] [PubMed] [Google Scholar]
- 4.Guo S, Yan W, McDonough SP, Lin N, Wu KJ, He H, Xiang H, Yang M, Moreira MA, Chang YF. 2015. The recombinant Lactococcus lactis oral vaccine induces protection against C. difficile spore challenge in a mouse model. Vaccine 33:1586–1595. 10.1016/j.vaccine.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Lei H, Peng X, Jiao H, Zhao D, Ouyang J. 2015. Broadly protective immunity against divergent influenza viruses by oral co-administration of Lactococcus lactis expressing nucleoprotein adjuvanted with cholera toxin B subunit in mice. Microb Cell Fact 14:111. 10.1186/s12934-015-0287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lay CL, Dridi L, Bergeron MG, Ouellette M, Fliss IL. 2016. Nisin is an effective inhibitor of Clostridium difficile vegetative cells and spore germination. J Med Microbiol 65:169–175. 10.1099/jmm.0.000202. [DOI] [PubMed] [Google Scholar]
- 7.Dowdell P, Chankhamhaengdecha S, Panbangred W, Janvilisri T, Aroonnual A. 2020. Probiotic activity of Enterococcus faecium and Lactococcus lactis isolated from Thai fermented sausages and their protective effect against Clostridium difficile. Probiotics Antimicrob Proteins 12:641–648. 10.1007/s12602-019-09536-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stenz L, Francois P, Whiteson K, Wolz C, Linder P, Schrenzel J. 2011. The CodY pleiotropic repressor controls virulence in Gram-positive pathogens. FEMS Immunol Med Microbiol 62:123–139. 10.1111/j.1574-695X.2011.00812.x. [DOI] [PubMed] [Google Scholar]
- 9.Geiger T, Wolz C. 2014. Intersection of the stringent response and the CodY regulon in low GC Gram-positive bacteria. Int J Med Microbiol 304:150–155. 10.1016/j.ijmm.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Brinsmade SR. 2017. CodY, a master integrator of metabolism and virulence in Gram-positive bacteria. Curr Genet 63:417–425. 10.1007/s00294-016-0656-5. [DOI] [PubMed] [Google Scholar]
- 11.den Hengst CD, van Hijum SA, Geurts JM, Nauta A, Kok J, Kuipers OP. 2005. The Lactococcus lactis CodY regulon: identification of a conserved cis-regulatory element. J Biol Chem 280:34332–34342. 10.1074/jbc.M502349200. [DOI] [PubMed] [Google Scholar]
- 12.Kaiser JC, King AN, Grigg JC, Sheldon JR, Edgell DR, Murphy MEP, Brinsmade SR, Heinrichs DE. 2018. Repression of branched-chain amino acid synthesis in Staphylococcus aureus is mediated by isoleucine via CodY, and by a leucine-rich attenuator peptide. PLoS Genet 14:e1007159. 10.1371/journal.pgen.1007159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brenner M, Lobel L, Borovok I, Sigal N, Herskovits AA. 2018. Controlled branched-chain amino acids auxotrophy in Listeria monocytogenes allows isoleucine to serve as a host signal and virulence effector. PLoS Genet 14:e1007283. 10.1371/journal.pgen.1007283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belitsky BR, Sonenshein AL. 2013. Genome-wide identification of Bacillus subtilis CodY-binding sites at single-nucleotide resolution. Proc Natl Acad Sci USA 110:7026–7031. 10.1073/pnas.1300428110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lobel L, Herskovits AA. 2016. Systems level analyses reveal multiple regulatory activities of CodY controlling metabolism, motility and virulence in Listeria monocytogenes. PLoS Genet 12:e1005870. 10.1371/journal.pgen.1005870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ercan O, Bisschops MMM, Overkamp W, Jørgensen TR, Ram AF, Smid EJ, Pronk JT, Kuipers OP, Daran-Lapujade P, Kleerebezem M. 2015. Physiological and transcriptional responses of different industrial microbes at near-zero specific growth rates. Appl Environ Microbiol 81:5662–5670. 10.1128/AEM.00944-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levdikov VM, Blagova E, Young VL, Belitsky BR, Lebedev A, Sonenshein AL, Wilkinson AJ. 2017. Structure of the branched-chain amino acid and GTP-sensing global regulator, CodY, from Bacillus subtilis. J Biol Chem 292:2714–2728. 10.1074/jbc.M116.754309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan LC, Wu H, Zhao Y, Qin XY, Li YN. 2019. Molecular simulation of the interaction mechanism between CodY protein and DNA in Lactococcus lactis. Front Chem Sci Eng 13:133–139. 10.1007/s11705-018-1737-4. [DOI] [Google Scholar]
- 19.Majerczyk CD, Dunman PM, Luong TT, Lee CY, Sadykov MR, Somerville GA, Bodi K, Sonenshein AL. 2010. Direct targets of CodY in Staphylococcus aureus. J Bacteriol 192:2861–2877. 10.1128/JB.00220-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lobel L, Sigal N, Borovok I, Belitsky BR, Sonenshein AL, Herskovits AA. 2015. The metabolic regulator CodY links Listeria monocytogenes metabolism to virulence by directly activating the virulence regulatory gene prfA. Mol Microbiol 95:624–644. 10.1111/mmi.12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guédon E, Sperandio B, Pons N, Ehrlich SD, Renault P. 2005. Overall control of nitrogen metabolism in Lactococcus lactis by CodY, and possible models for CodY regulation in Firmicutes. Microbiology (Reading) 151:3895–3909. 10.1099/mic.0.28186-0. [DOI] [PubMed] [Google Scholar]
- 22.Guédon E, Serror P, Ehrlich SD, Renault P, Delorme C. 2001. Pleiotropic transcriptional repressor CodY senses the intracellular pool of branched-chain amino acids in Lactococcus lactis. Mol Microbiol 40:1227–1239. 10.1046/j.1365-2958.2001.02470.x. [DOI] [PubMed] [Google Scholar]
- 23.Liu YK, Lin TH, Liu PF. 2017. ATP alters protein folding and function of Escherichia coli uridine phosphorylase. Arch Biochem Biophys 634:11–20. 10.1016/j.abb.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Johnston C, Bootsma HJ, Aldridge C, Manuse S, Gisch N, Schwudke D, Hermans PW, Grangeasse C, Polard P, Vollmer W, Claverys JP. 2015. Co-inactivation of GlnR and CodY regulators impacts pneumococcal cell wall physiology. PLoS One 10:e0123702. 10.1371/journal.pone.0123702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeong JH, Ha SC. 2018. Crystal structure of NisI in a lipid-free form, the nisin immunity protein, from Lactococcus lactis. Antimicrob Agents Chemother 62:e01966-17. 10.1128/AAC.01966-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. 2009. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res 37:W202–W208. 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brinsmade SR, Kleijn RJ, Sauer U, Sonenshein AL. 2010. Regulation of CodY activity through modulation of intracellular branched-chain amino acid pools. J Bacteriol 192:6357–6368. 10.1128/JB.00937-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belitsky BR. 2015. Role of branched-chain amino acid transport in Bacillus subtilis CodY activity. J Bacteriol 197:1330–1338. 10.1128/JB.02563-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu WW, Wang Y, Wang T, Kong J. 2015. The global regulator CodY in Streptococcus thermophilus controls the metabolic network for escalating growth in the milk environment. Appl Environ Microbiol 81:2349–2358. 10.1128/AEM.03361-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindbäck T, Mols M, Basset C, Granum PE, Kuipers OP, Kovács ÁT. 2012. CodY, a pleiotropic regulator, influences multicellular behaviour and efficient production of virulence factors in Bacillus cereus. Environ Microbiol 14:2233–2246. 10.1111/j.1462-2920.2012.02766.x. [DOI] [PubMed] [Google Scholar]
- 31.Waters NR, Samuels DJ, Behera RK, Livny J, Rhee KY, Sadykov MR, Brinsmade SR. 2016. A spectrum of CodY activities drives metabolic reorganization and virulence gene expression in Staphylococcus aureus. Mol Microbiol 101:495–514. 10.1111/mmi.13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ercan O, Wels M, Smid EJ, Kleerebezem M. 2015. Molecular and metabolic adaptations of Lactococcus lactis at near-zero growth rates. Appl Environ Microbiol 81:320–331. 10.1128/AEM.02484-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brinsmade SR, Alexander EL, Livny J, Stettner AI, Segrè D, Rhee KY, Sonenshein AL. 2014. Hierarchical expression of genes controlled by the Bacillus subtilis global regulatory protein CodY. Proc Natl Acad Sci USA 111:8227–8232. 10.1073/pnas.1321308111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dressaire C, Redon E, Gitton C, Loubière P, Monnet V, Cocaign-Bousquet M. 2011. Investigation of the adaptation of Lactococcus lactis to isoleucine starvation integrating dynamic transcriptome and proteome information. Microb Cell Fact 10:S18. 10.1186/1475-2859-10-S1-S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li H, O'Sullivan DJ. 2006. Identification of a nisI promoter within the nisABCTIP operon that may enable establishment of nisin immunity prior to induction of the operon via signal transduction. J Bacteriol 188:8496–8503. 10.1128/JB.00946-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brinkman AB, Dahlke I, Tuininga JE, Lammers T, Dumay V, de Heus E, Lebbink JH, Thomm M, de Vos WM, van Der Oost J. 2000. An Lrp-like transcriptional regulator from the archaeon Pyrococcus furiosus is negatively autoregulated. J Biol Chem 275:38160–38169. 10.1074/jbc.M005916200. [DOI] [PubMed] [Google Scholar]
- 37.Groisman EA. 2016. Feedback control of two-component regulatory systems. Annu Rev Microbiol 70:103–124. 10.1146/annurev-micro-102215-095331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu D, Zhao K, Xu H, Zhang X, Bai Y, Saris PE, Qiao M. 2015. Construction of thyA deficient Lactococcus lactis using the Cre-loxP recombination system. Ann Microbiol 65:1659–1665. 10.1007/s13213-014-1005-x. [DOI] [Google Scholar]
- 39.Wu H, Zhao Y, Du Y, Miao S, Liu J, Li Y, Caiyin Q, Qiao J. 2018. Quantitative proteomics of Lactococcus lactis F44 under cross-stress of low pH and lactate. J Dairy Sci 101:6872–6884. 10.3168/jds.2018-14594. [DOI] [PubMed] [Google Scholar]
- 40.Li N, Wei S, Chen J, Yang F, Kong L, Chen C, Ding X, Chu Z. 2018. OsASR2 regulates the expression of a defence-related gene, Os2H16, by targeting the GT-1 cis-element. Plant Biotechnol J 16:771–783. 10.1111/pbi.12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Altermann E, Klaenhammer TR. 2005. PathwayVoyager: pathway mapping using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. BMC Genomics 6:60. 10.1186/1471-2164-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu G, Wang LG, Han Y, He QY. 2012. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16:284–287. 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song Z, Chen L, Wang J, Lu Y, Jiang W, Zhang W. 2014. A transcriptional regulator Sll0794 regulates tolerance to biofuel ethanol in photosynthetic Synechocystis sp. PCC 6803. Mol Cell Proteomics 13:3519–3532. 10.1074/mcp.M113.035675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu H, Liu J, Miao S, Zhao Y, Zhu H, Qiao M, Saris PEJ, Qiao J. 2018. Contribution of YthA, a PspC family transcriptional regulator of Lactococcus lactis F44 acid tolerance and nisin yield: a transcriptomic approach. Appl Environ Microbiol 84:e02483-17. 10.1128/AEM.02483-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Cen XF, Zhao GP, Wang J. 2012. Characterization of a new GlnR binding box in the promoter of amtB in Streptomyces coelicolor inferred a PhoP/GlnR competitive binding mechanism for transcriptional regulation of amtB. J Bacteriol 194:5237–5244. 10.1128/JB.00989-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang YF, Liu SY, Du YH, Feng WJ, Liu JH, Qiao JJ. 2014. Genome shuffling of Lactococcus lactis subspecies lactis YF11 for improving nisin Z production and comparative analysis. J Dairy Sci 97:2528–2541. 10.3168/jds.2013-7238. [DOI] [PubMed] [Google Scholar]
- 47.Zhang J, Caiyin Q, Feng W, Zhao X, Qiao B, Zhao G, Qiao J. 2016. Enhance nisin yield via improving acid-tolerant capability of Lactococcus lactis F44. Sci Rep 6:27973. 10.1038/srep27973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lambert JM, Bongers RS, Kleerebezem M. 2007. Cre-lox-based system for multiple gene deletions and selectable-marker removal in Lactobacillus plantarum. Appl Environ Microbiol 73:1126–1135. 10.1128/AEM.01473-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S4 and Tables S1 and S4. Download aem.01847-21-s0001.pdf, PDF file, 2.2 MB (2.2MB, pdf)
Table S2. Download aem.01847-21-s0002.xlsx, XLSX file, 0.3 MB (274.5KB, xlsx)
Table S3. Download aem.01847-21-s0003.xlsx, XLSX file, 0.02 MB (20.6KB, xlsx)
Data Availability Statement
The ChIP-seq data files have been deposited in the Gene Expression Omnibus (GEO) and are accessible through accession no. GSE139902.