ABSTRACT
Heat activation at a sublethal temperature is widely applied to promote Bacillus species spore germination. This treatment also has the potential to be employed in food processing to eliminate undesired bacterial spores by enhancing their germination and then inactivating the less-heat-resistant germinated spores at a milder temperature. However, incorrect heat treatment could also generate heat damage in spores and lead to more heterogeneous spore germination. Here, the heat activation and heat damage profile of Bacillus subtilis spores was determined by testing spore germination and outgrowth at both population and single-spore levels. The heat treatments used were 40 to 80°C and for 0 to 300 min. The results were as follows. (i) Heat activation at 40 to 70°C promoted l-valine- and l-asparagine-glucose-fructose-potassium (AGFK)-induced germination in a time-dependent manner. (ii) The optimal heat activation temperatures for AGFK and l-valine germination via the GerB plus GerK or GerA germinant receptors were 65°C and 50 to 65°C, respectively. (iii) Heat inactivation of dormant spores appeared at 70°C, and the heat damage of molecules essential for germination and growth began at 70 and 65°C, respectively. (iv) Heat treatment at 75°C resulted in both activation of germination and damage to the germination apparatus, and 80°C treatment caused more pronounced heat damage. (v) For the spores that should withstand adverse environmental temperatures in nature, heat activation seemed functional for a subsequent optimal germination process, while heat damage affected both germination and outgrowth.
IMPORTANCE Bacterial spores are thermal-stress-resistant structures that can thus survive food preservation strategies and revive through the process of spore germination. The more heat resistant spores are, the more heterogeneous their germination upon the addition of germinants. Upon germination, spores can cause food spoilage and food intoxication. Here, we provide new information on both heat activation and inactivation regimes and their effects on the (heterogeneity of) spore germination.
KEYWORDS: Bacillus subtilis, bacterial spores, heat activation, spore germination, germination heterogeneity
INTRODUCTION
Applying mild heat for a short to moderate time (e.g., pasteurization treatment at 72°C for at least 15 s) is one of the conventional food processing procedures to extend shelf life in the food industry (1, 2). In this process, vegetative pathogens or spoilage organisms lose their viability, although bacterial spores survive. Not only that, heat treatment at sublethal temperatures (e.g., 60 to 75°C) can increase and synchronize the spore germination of Bacillales and Clostridiales (2–5). Bacillus subtilis spores have higher heat resistance than their vegetative forms due to the following factors (2–4). (i) The spore core has a low water content (25 to 45% wet weight) and a high level of dipicolinic acid (DPA) chelated to divalent cations, predominantly Ca2+ (Ca2+-DPA) (25% of core dry weight). (ii) Spore DNA is saturated with α/β-type small acid-soluble protein (SASP). (iii) Spore cortex peptidoglycan, characterized by the muramic-δ-lactam moiety and low level of peptide cross-linking, also contributes to spore wet heat resistance. However, upon increasing the temperature above 80°C, spore damage and inactivation occur (6). Damage accumulated in spore molecules then results in more heterogeneous germination and slowed spore outgrowth (6). To analyze spore thermal physiology such that heat activation can be optimally applied for the stimulation of spore germination, it is necessary to have a detailed analysis of the effects of heat treatment, even at relatively mild temperatures.
Heat activation studies have mainly focused on spores of members of the order Bacillales (5, 7–11). These studies have indicated that a sublethal heat treatment can reversibly activate spores’ germinant receptor (GR)-dependent germination, which is induced by physiological small-molecule nutrients or hydrostatic pressures of ∼150 MPa. In contrast, sublethal thermal treatment has no effect on GR-independent germination triggered by exogenous Ca2+-DPA, dodecylamine, or hydrostatic pressures of ∼550 MPa (4, 9, 12). In the heat activation process, temperature is not the only variable, as treatment time also affects outcomes. Luu et al. indicated that the best activation of B. subtilis spores was reached after 15 min at 75°C with l-valine-triggered germination via the GerA GR, whereas 4 h at 75°C was needed for optimal germination in l-asparagine, glucose, fructose, and potassium (AGFK)-triggered germination via the GerB and GerK GRs (9). When both temperature and time duration of heat treatment are considered, the following questions need to be addressed. (i) What is the best time-temperature combination for heat activation? (ii) When does heat activation shift to heat inactivation during long treatment times?
In the current paper, we employed B. subtilis strain PS832 spores to measure the time-temperature-activation/inactivation profile at sublethal temperatures. Spores were treated at 40 to 80°C for 15 to 300 min, followed by inducing germination by adding l-valine or AGFK. The spore viability after a variety of treatments was also tested to probe for heat inactivation. Spore germination and subsequent outgrowth and cell growth were monitored at the population level by microplate spectrophotometric analysis and at the single-cell level by phase-contrast microscopy.
RESULTS
Heat treatments at 40 to 70°C promote B. subtilis spore germination in a time-dependent manner.
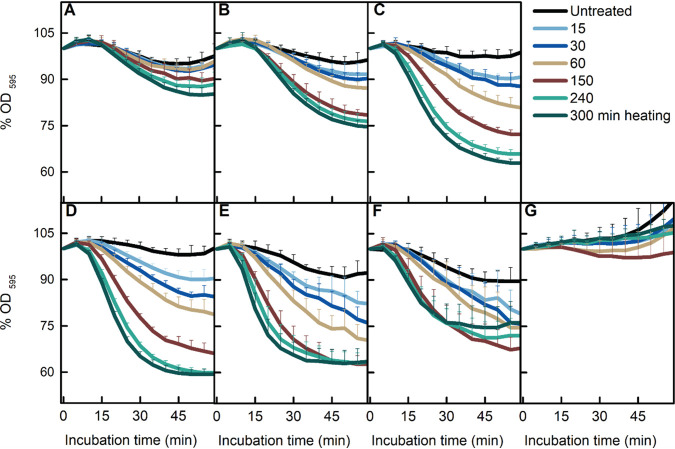
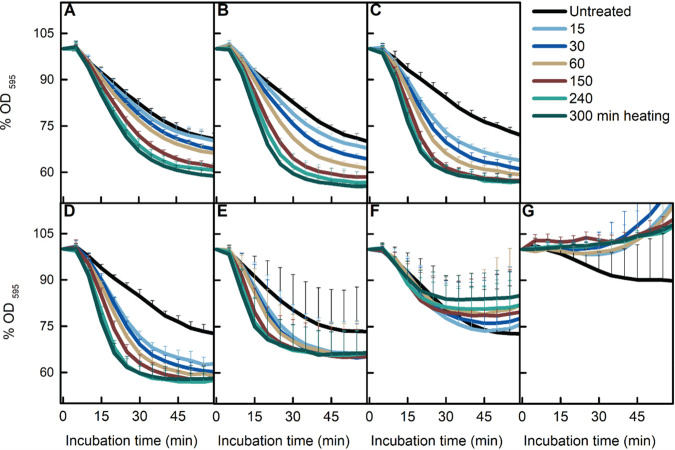
Heat activation at sublethal temperatures is a common procedure to promote homogeneous spore germination in the laboratory. However, the temperatures applied differ among different laboratories, with 65, 70, 75, and 80°C used for B. subtilis spore heat activation (9, 13–15). In addition, as mentioned above, heat treatments at 75°C enhance AGFK-induced spore germination in a time-dependent manner (9). However, it is not clear whether different temperatures promote germination in a similar pattern. First, we focused on the four commonly used heat activation temperatures mentioned above. As shown by the results in Fig. 1D to G, with the exception of 80°C, the commonly used temperatures promoted AGFK-induced germination in a time-dependent positive manner. However, the 65°C treatment resulted in the largest decrease in the optical density at 595 nm (OD595), which represents the most complete germination. To test the germination promotion efficiency at lower temperatures, the same measurements were taken on spores treated at 40, 50, and 60°C. As shown by the results in Fig. 1A to C, the time-dependent positive correlation was observed again. According to the data presented here, the optimum condition of heat activation for AGFK-induced germination when completion of germination was monitored was 300 min at 65°C. Notably, the magnitude of the drop in the OD595 gradually decreased as the temperature was increased and was completely abolished at 80°C (Fig. 1G). These results suggested that the activation of germination by heat might have vanished at 80°C or been subsumed by damage at this temperature. Similar results, albeit with subtle differences, were observed for l-valine-induced germination (Fig. 2A to G). First, the optimal heat activation temperature for l-valine-induced germination was 50 to 65°C. Second, treatment at 75°C, while clearly promoting l-valine-induced germination, also decreased the magnitude of the maximum OD595 decrease. These results suggested there was a transition from only heat activation to heat activation plus damage to spores affecting germination at around 75°C.
FIG 1.
Effect of heat treatment on AGFK-induced spore germination. B. subtilis PS832 spores were germinated at 37°C with (10 mM each) AGFK in HEPES buffer after heat treatments for various times (0, 15, 30, 60, 150, 240, and 300 min) at 40 (A), 50 (B), 60 (C), 65 (D), 70 (E), 75 (F), and 80°C (G). Spore germination was measured by the drop in the optical density due to the release of Ca2+-DPA and water uptake. Values shown are the mean values and standard deviations of duplicate or triplicate measurements on at least two experiments.
FIG 2.
Effect of heat treatment on l-valine-induced spore germination. B. subtilis PS832 spores were germinated at 37°C with 10 mM l-valine in HEPES buffer after heat treatment for various times (0, 15, 30, 60, 150, 240, and 300 min) at 40 (A), 50 (B), 60 (C), 65 (D), 70 (E), 75 (F), or 80°C (G). Spore germination was measured by the drop in optical density due to the release of Ca2+-DPA and water uptake. Values shown are the mean values and standard deviations of duplicate or triplicate measurements in two or three experiments.
Heat damage accumulates at 70°C.
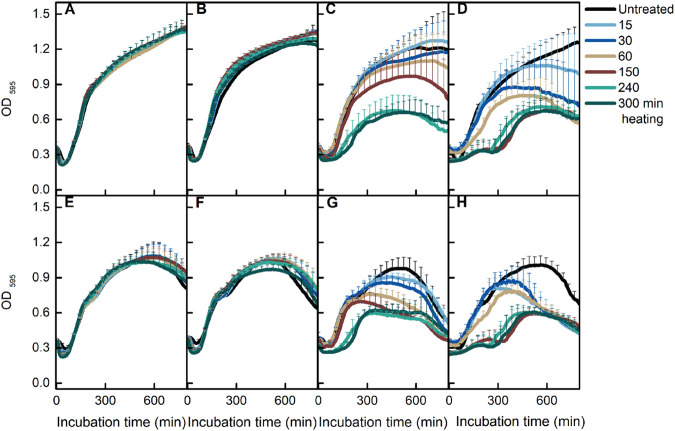
To determine the temperature at which heat damage occurs, we tested the viability of spores treated at 65 to 80°C for various times. Clearly, 80°C treatment led to significant killing of spores (Fig. 3). Careful analysis of the results of the other three temperatures also showed a small but significant decrease in spore viability for the spores treated for 300 min at 75°C (Fig. 3B). To test the effect of heat treatments on cell growth, we tracked the change in spore OD595 in LB medium supplemented with AGFK or l-valine. Thermal treatments of 60 and 70°C did not affect B. subtilis growth, whereas exposure to 75 and 80°C at longer heat treatment times (150, 240, and 300 min) decreased the yield of growing cells when LB medium was supplemented with either l-valine or AGFK (Fig. 4A to H). These results suggested that both damage and inactivation of spores take place at 70 to 75°C.
FIG 3.
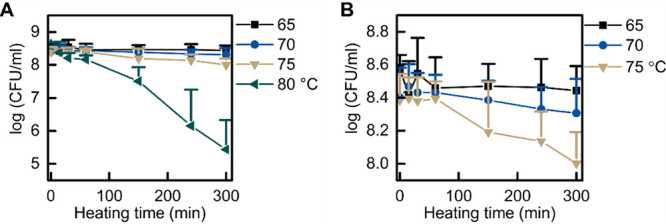
Effect of heat treatment on spore viability. B. subtilis PS832 spores were incubated at 37°C on LB agar plates supplemented with (10 mM each) AGFK after heat treatment at 65, 70, 75, or 80°C for various times (0, 15, 30, 60, 150, 240, and 300 min). (A) Viability of spores pretreated at 65, 70, 75, and 80°C. (B) An enlarged portion of panel A (the x axis remains the same, but the y axis zooms in on the range between log 8 and log 8.8). Values shown are the mean values and standard deviations of triplicate measurements in two or three experiments.
FIG 4.
Effect of heat treatment on spore germination and cell growth. B. subtilis PS832 spores were heated for various times (0, 15, 30, 60, 150, 240, and 300 min) at 65 (A and E), 70 (B and F), 75 (C and G), and 80°C (D and H). Spore germination and cell growth were monitored by the change of optical density in LB medium supplemented with (10 mM each) AGFK (A to D) or 10 mM l-valine (E to H). Values shown are the mean values and standard deviations of triplicate measurements in two or three experiments.
Effects of heat treatment at the single-spore level.
Our population data indicated that treatments at 65 to 75°C resulted in a time-dependent positive enhancement of AGFK-induced spore germination at the population level, and detectable heat damage/inactivation were observed in spores treated at 70 to 75°C for long times. Considering that the population level data cannot provide a detailed view of what sublethal heat does to individual B. subtilis spores, we employed phase-contrast microscopy to track AGFK-induced spore germination, outgrowth, and cell growth after treatments at 65, 70, 75, and 80°C. By analyzing the time-lapse images, germination plots and growth plots of individual spores were created by SporeTrackerX (ImageJ macro) (Fig. 5). Based on the changes in plot profiles, germination, burst (the cell’s escape from the spore coat), and first cell division events were detected and marked for further analysis. The heat treatment durations at 65 to 70°C were 30, 150, and 300 min, while treatments for 15 and 60 min at 80°C were added because of a lack of sufficient germination events for quantitative analysis in the groups with prolonged heat treatment (Table 1).
FIG 5.
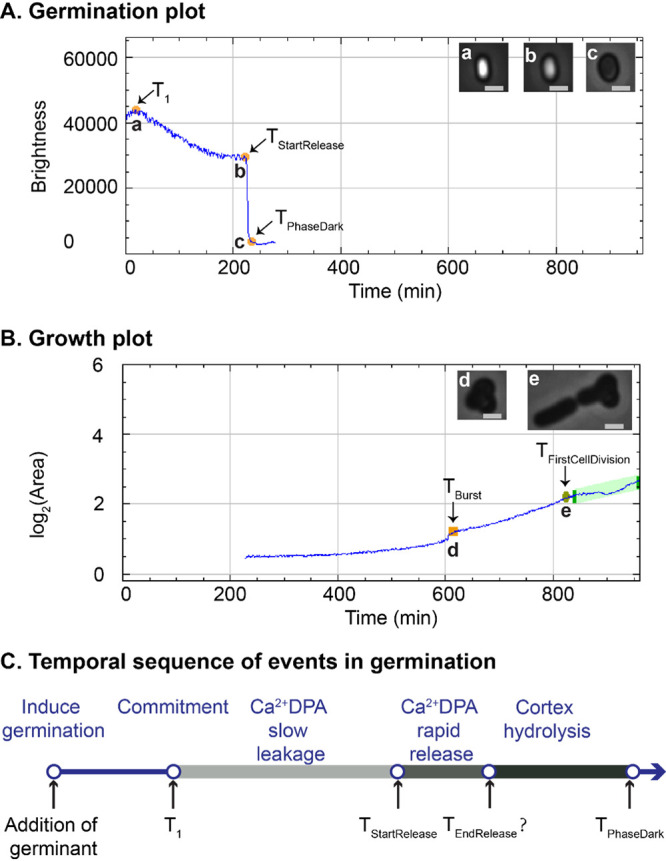
Germination and growth plots of a single B. subtilis spore (treated at 80°C for 150 min), created by SporeTrackerX. (A) In the germination plot, two phases of brightness drop at different speeds were observed. The slow drop was considered the slow leakage of Ca2+-DPA. The fast drop was likely due to both the rapid Ca2+-DPA release and the subsequent cortex hydrolysis induced by the released Ca2+-DPA. The time of the start of rapid Ca2+-DPA release (TStartRelease), time of complete phase darkening (TPhaseDark), and time of the start of the slow drop in brightness (T1) were assessed during spore germination by SporeTrackerX. (B) The time of burst (TBurst) and time of first cell division (TFirstCellDivision) were determined, and the various events are marked in the growth plot. Micrographs of spores and cells at each time point are shown at the top right of panels A and B. The brightness display ranges for images a and b and images c to e are 0 to 40,000 and 0 to 25,000, respectively. Scale bar, 1 μm. (C). The temporal sequence of events in germination, adapted from the work of Wang et al. (19). The top shows the temporal sequence of germination events, and the bottom indicates the time points detected in the current work as shown in panel A. For the rapid Ca2+-DPA release, we only estimated its initiation from the germination plot.
TABLE 1.
Germination parameters of individual heat-treated B. subtilis PS832 spores in time-lapse imagesa
| Nutrient(s), treatment |
Time (min) of indicated eventb |
No. of germination events (% germination) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
T
1
|
P value | ΔTSlowLeakage |
P value | ΔTPhaseDarkening |
P value | ||||||
| Temp (°C) | Time (min) | Median | IQR | Median | IQR | Median | IQR | ||||
| AGFK | |||||||||||
| Untreated | 12.0 | 4.7 | 40.0 | 65.1 | 12.1 | 3.9 | 184 (100) | ||||
| 65 | 30 | 9.0 | 3.2 | < 0.01 | 15.0 | 29.1 | < 0.01 | 11.0 | 3.0 | < 0.01 | 216 (100) |
| 150 | 5.2 | 2.1 | < 0.01 | 5.0 | 12.9 | < 0.01 | 10.2 | 3.0 | < 0.01 | 263 (100) | |
| 300 | 4.0 | 0.0 | < 0.01 | 3.2 | 10.4 | < 0.01 | 11.1 | 4.0 | < 0.01 | 231 (98.7) | |
| 70 | 30 | 10.3 | 7.0 | < 0.01 | 19.1 | 36.4 | < 0.01 | 11.1 | 2.4 | < 0.01 | 204 (99.5) |
| 150 | 5.5 | 1.0 | < 0.01 | 3.9 | 9.9 | < 0.01 | 12.2 | 3.9 | 0.13 | 225 (99.6) | |
| 300 | 4.0 | 2.3 | < 0.01 | 3.0 | 6.9 | < 0.01 | 14.1 | 4.3 | < 0.01 | 213 (100) | |
| 75 | 30 | 8.0 | 2.0 | < 0.01 | 23.1 | 40.0 | < 0.01 | 13.1 | 3.8 | < 0.01 | 211 (100) |
| 150 | 6.0 | 2.1 | < 0.01 | 11.0 | 25.3 | < 0.01 | 17.1 | 5.9 | < 0.01 | 176 (90.7) | |
| 300 | 3.0 | 1.0 | < 0.01 | 4.0 | 10.0 | < 0.01 | 19.2 | 9.4 | < 0.01 | 126 (88.7) | |
| 80 | 15 | 7.0 | 4.1 | < 0.01 | 29.1 | 56.0 | 0.02 | 14.2 | 5.4 | < 0.01 | 231 (92) |
| 30 | 6.0 | 2.0 | < 0.01 | 59.5 | 99.4 | 0.04 | 15.2 | 6.9 | < 0.01 | 122 (77.2) | |
| 60 | 8.0 | 1.7 | < 0.01 | 66.0 | 304.1 | < 0.01 | 16.2 | 9.0 | < 0.01 | 153 (59.8) | |
| 150 | 10.0 | 1.0 | 0.19 | 48.0 | 140.0 | 0.53 | 16.1 | 18.1 | 0.42 | 11 (12.4) | |
| 300 | 0 (0) | ||||||||||
| l-Valine | |||||||||||
| Untreated | 7.5 | 1.0 | 11.1 | 22.0 | 13.3 | 5.1 | 112 (100) | ||||
| 75 | 30 | 7.0 | 1.0 | < 0.01 | 10.1 | 15.8 | 0.84 | 12.1 | 3.5 | < 0.01 | 99 (98) |
| 150 | 3.0 | 0.5 | < 0.01 | 7.0 | 37.5 | 0.32 | 15.1 | 10.4 | 0.40 | 75 (87.2) | |
| 300 | 3.0 | 1.0 | < 0.01 | 0.1 | 17.0 | 0.02 | 19.3 | 10.0 | < 0.01 | 61 (77.2) | |
As described in the legend to Fig. 5, B. subtilis PS832 spores were treated with various temperature-time conditions, followed by being germinated and grown on a MOPS-agarose pad with (10 mM each) AGFK and 10 mM l-valine. Data were collected by SporeTrackerX (ImageJ Macro) as described in Materials and Methods. The significance of differences between heat-treated groups and the untreated group was measured by the Mann-Whitney test.
T1, time when spore commitment occurred following exposure to germinant; ΔTSlowLeakage, time between T1 and TStartRelease (start of rapid Ca2+-DPA release); ΔTPhaseDarkening, time between TStartRelease and TPhaseDark (time of the appearance of the phase-dark spore); IQR, interquartile range.
Damage to the germination apparatus occurs at 70°C.
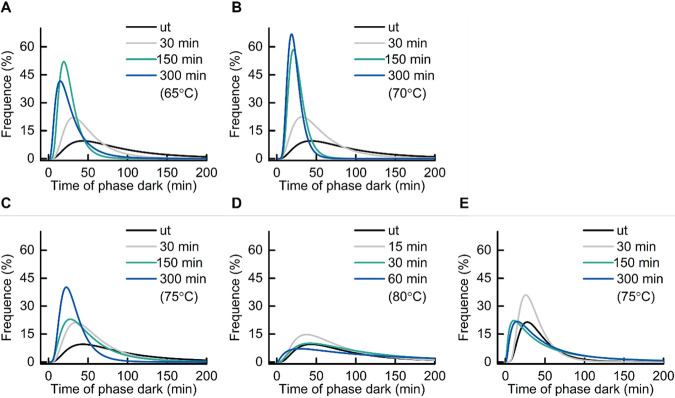
As shown by the results in Fig. 5A, the spore germination plot can be divided into three segments, the first before the drop in brightness, followed by a slow drop in brightness and then a fast drop in brightness. Wang et al. observed slow leakage of Ca2+-DPA in B. subtilis spore germination, along with a slow decrease in differential interference contrast image intensity, followed by rapid release of the remaining Ca2+-DPA and cortex hydrolysis during spore germination, and they further suggested that spores become committed to germinating at the starting point of the slow Ca2+-DPA leakage (16). We speculated that the slow decrease of spore brightness in the germination plot was due to the slow leakage of Ca2+-DPA, and time 1 (T1) was set as the time point when spore commitment occurred (Fig. 5C). The fast drop in brightness in our germination plot was attributed to the rapid release of Ca2+-DPA and cortex hydrolysis triggered by the release of Ca2+-DPA. We refer to the following parameters: ΔTSlowLeakage is the time between T1 and TStartRelease and ΔTPhaseDarkening is the time between TStartRelease and TPhaseDark. Our live-imaging data showed that AGFK-induced germination was shortened by heat treatments at 65 to 75°C in a time-dependent positive manner (Fig. 6A to D). The TPhaseDark values for untreated spores and those treated at 65 to 75°C for 300 min were 66.6, 21.1, 22.1, and 30.2 min, respectively. In addition, 65 to 75°C treatments shortened T1 and ΔTSlowLeakage in a time-dependent positive manner, and 70 to 75°C treatments prolonged the ΔTPhaseDarkening in a time-dependent positive manner (Table 1). Given the fact that the release of Ca2+-DPA is via the SpoVA channel in the spore inner membrane and is slowed markedly when the cortex lytic enzyme CwlJ is absent, SpoVA proteins and cortex lytic enzymes are potential heat damage targets when the thermal treatment temperatures rise above 70°C (17).
FIG 6.
Frequency distribution of the times of appearance of phase dark spores (TPhaseDark). B. subtilis PS832 spores were preheated at 65, 70, 75, or 80°C for various times or not heated (ut, untreated). Subsequently, spores were immobilized on a MOPS-agarose pad at 37°C with (10 mM each) AGFK (A to D) or 10 mM l-valine (E) for 16 h of time-lapse imaging. The line profile is the log-normal distribution fit of the time to phase darkening. The numbers of germinated spores examined by microscopy are given in Table 1.
Treatment at 65°C can be sufficient to damage essential cell growth molecules stored in spores.
The accumulated heat damage, potentially in the germination apparatus, is likely responsible for the broad TPhaseDark distribution of spores treated at 75 and 80°C, differing from the narrow distribution seen in longer treatment times at 70°C, indicative for heat-induced germination activation. Instead, at 80°C, the distribution at TPhaseDark approached the TPhaseDark distribution of untreated spores at all treatment times applied (Fig. 6C and D). In addition, decreased germination efficiency was observed in spores treated at 75 and 80°C, and this decline had a positive correlation with time/temperature (Table 1, Fig. 7). The subsequent outgrowth and cell growth of heat-treated spores was also monitored in our time-lapse imaging. We noticed a remarkable decrease (∼41%) in the cell division events of spores treated at 70°C for 300 min compared to those of untreated spores, although 100% of spores of both groups germinated (Tables 1 and 2, Fig. 7). The decline of cell division events occurred in spores treated at 65°C for 150 min and increased when the treatment temperature and time increased (Tables 1 and 2, Fig. 7). These data suggest that 65°C was sufficient to damage molecules essential for growth that were stored in spores, although spores themselves have high heat resistance. We did not find time-/temperature-dependent effects of heat on outgrowth, except that an inverse correlation between the treatment and the burst time was observed in the 80°C groups.
FIG 7.
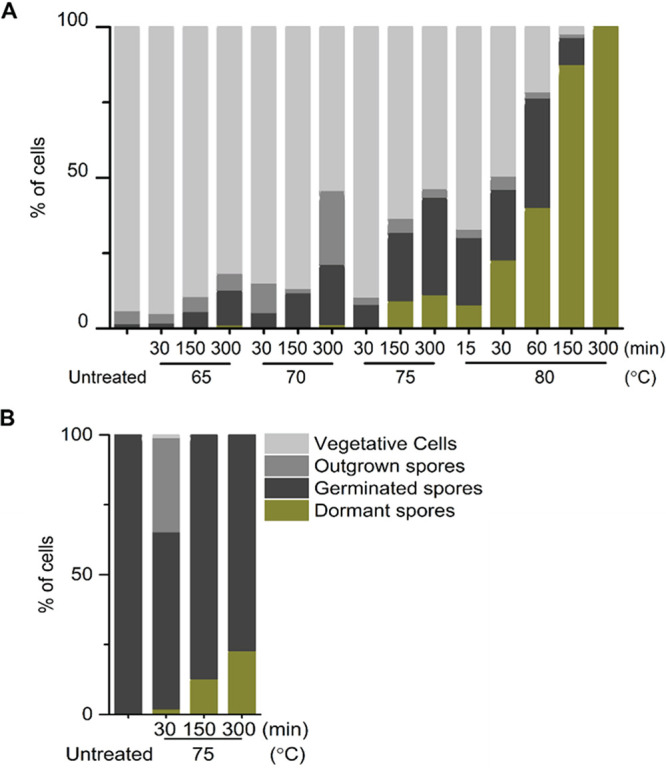
Quantification of spores with different fates after 16 h of time-lapse tracking. B. subtilis PS832 spores in water were heated at 65, 70, 75, or 80°C for different times. Subsequently, spores were immobilized on a MOPS-agarose pad at 37°C with (10 mM each) AGFK (A) or 10 mM l-valine (B) for 16 h of time-lapse imaging. The numbers of individual spores examined by microscopy are given in Tables 1 and 2.
TABLE 2.
Outgrowth and growth parameters of individual heat-treated B. subtilis PS832 spores in time-lapse imagesa
| Nutrient(s), treatment |
Time (min) of indicated eventb |
No. (%) of indicated events |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ΔTburst |
P value | ΔTFirstCellDivision |
P value |
T
Generation
|
P value | |||||||
| Temp (°C) | Time (min) | Median | IQR | Median | IQR | Median | IQR | Burst | First cell division | |||
| AGFK | ||||||||||||
| Untreated | 219.8 | 106.5 | 192.0 | 81.0 | 250.6 | 91.4 | 159 (86.4) | 172 (93.5) | ||||
| 65 | 30 | 204.8 | 89.5 | 0.13 | 163.0 | 58.5 | < 0.01 | 210.5 | 94.1 | < 0.01 | 175 (81.0) | 205 (94.9) |
| 150 | 205.7 | 62.1 | < 0.01 | 179.5 | 58.0 | 0.14 | 288.1 | 230.9 | < 0.01 | 235 (89.4) | 231 (87.8) | |
| 300 | 239.3 | 118.7 | 0.14 | 167.0 | 62.0 | < 0.01 | 218.8 | 88.5 | < 0.01 | 196 (83.8) | 189 (80.8) | |
| 70 | 30 | 229.8 | 145.5 | 0.23 | 179.0 | 85.3 | 0.64 | 241.8 | 66.7 | 0.67 | 183 (89.3) | 173 (84.4) |
| 150 | 222.3 | 93.6 | 0.61 | 150.0 | 44.8 | < 0.01 | 189.4 | 57.3 | < 0.01 | 188 (83.2) | 190 (84.1) | |
| 300 | 287.8 | 145.4 | < 0.01 | 242.5 | 87.5 | < 0.01 | 371.6 | 149.2 | < 0.01 | 163 (76.5) | 117 (54.9) | |
| 75 | 30 | 197.9 | 118.0 | 0.02 | 186.0 | 55.0 | 0.37 | 206.9 | 58.9 | < 0.01 | 186 (88.2) | 185 (87.7) |
| 150 | 284.9 | 124.7 | < 0.01 | 201.0 | 72.8 | 0.03 | 232.7 | 103.8 | 0.12 | 130 (67.0) | 120 (61.9) | |
| 300 | 269.8 | 102.6 | < 0.01 | 177.5 | 54.0 | 0.29 | 183.0 | 60.6 | < 0.01 | 74 (52.1) | 75 (52.8) | |
| 80 | 15 | 234.8 | 93.9 | 0.05 | 211.0 | 73.8 | < 0.01 | 293.2 | 216.2 | < 0.01 | 162 (64.5) | 162 (64.5) |
| 30 | 271.9 | 125.4 | < 0.01 | 179.0 | 80.0 | 0.53 | 246.9 | 114.2 | 0.94 | 83 (52.5) | 77 (48.7) | |
| 60 | 307.0 | 196.3 | < 0.01 | 206.0 | 96.5 | 0.03 | 204.1 | 61.0 | < 0.01 | 54 (21.1) | 53 (20.7) | |
| 150 | 499.8 | 34.0 | < 0.01 | 331.0 | 49.0 | 0.03 | 222.4 | 0.0 | 0.70 | 3 (3.4) | 2 (2.2) | |
| 300 | 1 (0.7) | 0 (0) | ||||||||||
| l-Valine | ||||||||||||
| Untreated | 0 (0) | 0 (0) | ||||||||||
| 75 | 30 | 584.8 | 261.0 | 519.9 | 35 (34.7) | 1 (1.0) | ||||||
| 150 | 0 (0) | 0 (0) | ||||||||||
| 300 | 0 (0) | 0 (0) | ||||||||||
As described in the legend to Fig. 5, B. subtilis PS832 spores were treated with various temperature-time conditions, followed by being germinated and grown on a MOPS-agarose pad with (10 mM each) AGFK and 10 mM l-valine. Data were collected by SporeTrackerX (ImageJ Macro) as described in Materials and Methods. The significance of differences between heat-treated groups and the untreated group was measured by the Mann-Whitney test.
ΔTburst, time between TPhaseDark (time of appearance of phase-dark spore) and TBurst (time of escape of cell from spore coat); ΔTFirstCellDivision, time between TBurst and TFirstCellDivision (time of first cell division); TGeneration, time between the first division and the end of the linear part of the log2(area)-versus-time plot; IQR, interquartile range.
Treatment at 75°C affects l-valine- and AGFK-induced germination similarly.
At the population level, 75°C treatment promoted AGFK-induced germination in a time-dependent manner, which was different from the results with l-valine-triggered germination (Fig. 1F and 2F). To better understand this difference, l-valine-induced germination was tracked by phase-contrast microscopy after spore heating at 75°C. Unlike spores supplemented with AGFK and MOPS (morpholinepropanesulfonic acid) medium, no cell division events occurred under nutrient conditions with l-valine and MOPS. Similar to the cell growth in LB medium (Fig. 4), AGFK acted as nutritional assistance for cell growth. In both l-valine- and AGFK-induced germination, the germination efficiency decreased with increased heat treatment time. In addition, T1 and ΔTSlowLeakage were shortened in a time-dependent positive manner, and ΔTPhaseDarkening was prolonged in a time-dependent positive manner (Table 1). In other words, the 75°C heat treatment affected AGFK- and l-valine-induced germination in the same way. The detectable time-dependent positive correlation in AGFK-induced germination at the population level was due to the bigger differences in the germination parameters of different groups. However, it was not clear why AGFK-induced germination was more sensitive to the change in heat activation time.
DISCUSSION
Heterogeneous spore germination in processed food is a major concern for food spoilage and foodborne disease. Promoting synchronous germination and then inactivating germinated spores and vegetative cells would extend food shelf life at minimal cost in the decontamination process (10). In the laboratory, spore germination heterogeneity can be stimulated by a heat activation procedure consisting of an exposure for a given time period to sublethal temperatures. However, with elevated temperatures, heat treatment could result in the accumulation of damage and further increases in the germination and outgrowth heterogeneity (18, 19). Thus, thermal treatment can in fact create increased difficulties in food processing. In the current work, we measured the spore germination and growth profiles after spore heat treatment at sublethal temperatures for a variety of treatment times. We hoped to determine the optimal heating time/temperature combination for optimal B. subtilis spore germination and outgrowth.
Our study showed that 50 to 65°C is the optimal heat activation temperature for B. subtilis PS832 spores obtained from cells sporulated in 2× SG medium (37) at 37°C. The 50 to 65°C treatment resulted in the largest decrease in OD595 without any germination apparatus damage. Such damage, as well as damage to molecules essential for growth stored in the dormant spore, which is linked to increased levels of spore inactivation, starts accumulating after treatment at 70 to 75°C. Published differential scanning calorimetry (DSC) thermogram profiles of B. subtilis spores present a reversible endothermic peak at around 60°C, which is followed by a second, irreversible endothermic peak initiated at around 70°C (20). It is suggested that the reversible endothermic peak is the outcome of heat activation, and the following irreversible peak is referred to as an inactivation peak. Our measured results fit the prediction from the B. subtilis thermogram profile. While we did not measure the heat activation/inactivation temperatures for other spore formers, it is likely that spores of other Bacillus species require similar heat activation/inactivation temperatures, based on their typical thermogram profiles. Indeed, the activation endothermic peaks are at 56°C for Bacillus megaterium spores and ∼55°C for Bacillus cereus spores (21, 22). Notably, the heat activation temperature has a positive correlation with heat resistance. For instance, superdormant spores or very-heat-resistant spores require higher heat activation temperatures (5, 23, 24). It would be useful to investigate the optimal heat activation conditions for heat-resistant spores that are major troublemakers in the food industry (2).
Sublethal heat activation is reversible in a temperature-dependent manner (25). Various phenomena have been observed in heat-activated spores, including but not limited to some release of Ca2+-DPA, a loss of coat proteins and small acid-soluble proteins, a change in the ultrastructure of the spore coat, including the development of comblike striations on the coat, and reversible protein denaturation (13, 26–30). However, how heat activation works and why spores require 50 to 65°C to gain the optimal heat activation is still unclear. Studies have proposed that heat activation might promote germination by directly increasing the level of functional GRs and affecting the state of the spore inner membrane, which surrounds the GRs (9, 31). Our data support the notion that GRs’ response to nutrient triggers has an important role in heat activation, considering the different germination behaviors in l-valine- and AGFK-induced germination processes seen previously and in the current work.
The exact mechanism of heat activation of spores remains unclear, as does the full mechanism of heat damage to spores. One study showed that the inner membrane of the spore was damaged in the spore heat inactivation process (32). Other studies also suggested that moist heat (e.g., 89°C for 2 h) killed spores by protein denaturation in B. subtilis, B. cereus, B. megaterium, and Clostridium perfringens spores (6, 19, 33). The current work shows that the effects of thermal treatment on spores are different in different growth stages, including germination, outgrowth, and subsequent vegetative cell growth. Further study should focus on the relation between the thermal properties of bacterial spores and their proteome-wide analysis. Such work might identify one or more macromolecules and/or processes key to the various thermal stress effects described in the current study.
MATERIALS AND METHODS
Strain used and spore preparation.
B. subtilis strain PS832, a prototrophic derivative of B. subtilis strain 168, was obtained from the laboratory of Peter Setlow. Spores were obtained from cells cultured at 37°C in 2× SG medium in Erlenmeyer flasks under continuous rotation at 200 rpm for 2 days, using the procedure detailed by Abhyankar et al. (34). Two-day sporulation cultures were harvested, followed by extensive washing with MilliQ water and further purification of phase-bright spores using HistoDenz density gradient centrifugation (35). Small volumes of aqueous spore suspensions (OD600 of ∼60, in MilliQ water) were dispensed, frozen, and stored at −80°C until use, after confirming the phase brightness of the spores (≥98%) by phase-contrast microscopy, as well as the absence of any visible debris.
Heat treatment.
The heat treatment procedure was modified based on Luu’s methods, in which spores were prepared at 37°C on 2× SG medium agar plates (9). Here, 200-μL amounts of aqueous spore suspensions (OD600 of ∼2, ∼3 × 108/ml) were incubated at 45, 50, 55, 60, 65, 70, 75, or 80°C for 0, 15, 30, 60, 150, 240, and 300 min, followed by cooling in a water-ice bath (≥15 min).
Monitoring germination and growth at the population level.
The exchange of spore core Ca2+-DPA for water during germination results in a drop in phase brightness, such that spore germination can be monitored by following spores’ optical density at 595 nm (OD595). Heat-treated aqueous spore suspensions (final OD595 of ∼1, 150 μL) were dispensed into 96-well microtiter plates and germinated (and grown) in either HEPES buffer (25 mM) or LB medium supplemented with either l-valine (10 mM) or AGFK (l-asparagine, glucose, fructose, and potassium chloride at 10 mM each). The OD595 was measured every 5 min at 37°C from cultures that underwent continuous shaking in a Multiskan FC microplate photometer (Thermo Fisher Scientific). Data were collected from at least two independent experiments with at least two replicates for each individual experimental condition.
Spore viability after heat treatment.
Heat-treated aqueous spore suspensions (OD595 of ∼2) were diluted 1/10 in MilliQ water at room temperature. Aliquots of further dilutions were plated on LB agar plates that were incubated at 37°C overnight, followed by incubation at room temperature until no further colonies appeared. Colonies were counted to determine spore viability in least two independent biological experiments, with at least two replicates for each heat treatment condition.
Monitoring germination, outgrowth, and cell growth of individual spores.
Heat-treated PS832 spores (0.5 μl, OD595 of ∼2) were immobilized on 1% agarose pads supplemented with MOPS minimal medium and additional nutrient germinants (10 mM valine or 10 mM each AGFK) in an air-containing chamber, as described elsewhere (36). Time-lapse phase-contrast images were captured by a phase-contrast microscope, which was coupled with a Nikon Ti microscope, a numeric aperture (NA) 1.45 Plan Apo λ 100× oil Ph3 DM objective, a C11440‐22CU Hamamatsu ORCA flash 4.0 camera, and NIS Elements software. Spores from each heat treatment condition were imaged twice at 37°C to track spore germination, outgrowth, and cell growth in detail. For each imaging process, 6 to 9 fields of view were recorded in parallel once every 1 min for 16 h.
Images were analyzed by using the modified ImageJ macro SporeTrackerX, which runs with the assistance of the ImageJ plugin ObjectJ (13). SporeTrackerX is capable of assessing multiple germination, outgrowth, and cell growth events based on the drop in spore brightness, “jump-like” surface area increase, and cell surface area increase in phase-contrast time-lapse images (13, 36). Briefly, the spore for analysis was labeled in the first frame of the time-lapse images, and subsequently, the changes in brightness and the log2(area) of the spore over time were assessed, stored, and plotted by SporeTrackerX, as shown by the results in Fig. 1. The conspicuous fast drop is almost certainly due to the rapid Ca2+-DPA release and the subsequent cortex hydrolysis induced by the released Ca2+-DPA (Fig. 1A and C) (4). Both the time of the start of rapid Ca2+-DPA release (TStartRelease) and the time of the appearance of the phase-dark spore (TPhaseDark) were marked and stored for quantitative analysis. In addition, a slow decline in brightness occurred before the rapid drop, and the start time of the slow drop of brightness was stored as T1 (Fig. 5A and C). The slow drop is most likely due to the slow leakage of Ca2+-DPA (4). In the growth plot, TBurst was defined as the time of escape of the cell from the spore coat, TFirstCellDivision was the time of the first cell division, and the generation time (TGeneration) was the time between the first division and the end of the linear part of the log2(area)-versus-time plot. (Fig. 5B). Based on the measurements described above, SporeTrackerX also calculated the following parameters: ΔTSlowLeakage is the time between T1 and TStartRelease, ΔTPhaseDarkening is the time between TStartRelease and TPhaseDark, ΔTburst is the time between TPhaseDark and TBurst, and ΔTFirstCellDivision is the time between TBurst and TFirstCellDivision.
ACKNOWLEDGMENTS
We thank the Van Leeuwenhoek Centre for Advanced Microscopy at the University of Amsterdam for the use of the microscope.
J.W. acknowledges the China Scholarship Council for a Ph.D. fellowship.
Juan Wen acquired funding. Juan Wen and Arend L. de Vos prepared the spore samples for investigation and formal analysis. Jan P. P. M. Smelt performed the modeling. Norbert O. E. Vischer created the image analysis software SporeTrackerX and assisted in the analyses. Juan Wen, Jan P. P. M. Smelt, and Arend L. De Vos wrote the original draft. Peter Setlow supervised the work and revised and edited the manuscript. Stanley Brul conceptualized the work, acquired funding, and supervised the work and wrote, reviewed, and edited the manuscript.
Contributor Information
Stanley Brul, Email: s.brul@uva.nl.
Danilo Ercolini, University of Naples Federico II.
REFERENCES
- 1.Smelt JPPM, Brul S. 2014. Thermal inactivation of microorganisms. Crit Rev Food Sci Nutr 54:1371–1385. 10.1080/10408398.2011.637645. [DOI] [PubMed] [Google Scholar]
- 2.Wells-Bennik MHJ, Eijlander RT, den Besten HMW, Berendsen EM, Warda AK, Krawczyk AO, Nierop Groot MN, Xiao Y, Zwietering MH, Kuipers OP, Abee T. 2016. Bacterial spores in food: survival, emergence, and outgrowth. Annu Rev Food Sci Technol 7:457–482. 10.1146/annurev-food-041715-033144. [DOI] [PubMed] [Google Scholar]
- 3.Abhyankar W, Pandey R, Ter Beek A, Brul S, de Koning LJ, de Koster CG. 2015. Reinforcement of Bacillus subtilis spores by cross-linking of outer coat proteins during maturation. Food Microbiol 45:54–62. 10.1016/j.fm.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Setlow P, Wang S, Li Y-Q. 2017. Germination of spores of the orders Bacillales and Clostridiales. Annu Rev Microbiol 71:459–477. 10.1146/annurev-micro-090816-093558. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Palacios A, Lejeune JT. 2011. Moist-heat resistance, spore aging, and superdormancy in Clostridium difficile. Appl Environ Microbiol 77:3085–3091. 10.1128/AEM.01589-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smelt JPPM, Bos AP, Kort R, Brul S. 2008. Modelling the effect of sub(lethal) heat treatment of Bacillus subtilis spores on germination rate and outgrowth to exponentially growing vegetative cells. Int J Food Microbiol 128:34–40. 10.1016/j.ijfoodmicro.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Levinson HS, Hyatt MT. 1970. Effects of temperature on activation, germination, and outgrowth of Bacillus megaterium spores. J Bacteriol 101:58–64. 10.1128/jb.101.1.58-64.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keynan A, Evanchik Z, Halvorson HO, Hastings JW. 1964. Activation of bacterial endospores. J Bacteriol 88:313–318. 10.1128/jb.88.2.313-318.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luu S, Cruz-Mora J, Setlow B, Feeherry FE, Doona CJ, Setlow P. 2015. The effects of heat activation on Bacillus spore germination, with nutrients or under high pressure, with or without various germination proteins. Appl Environ Microbiol 81:2927–2938. 10.1128/AEM.00193-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Løvdal IS, Hovda MB, Granum PE, Rosnes JT. 2011. Promoting Bacillus cereus spore germination for subsequent inactivation by mild heat treatment. J Food Prot 74:2079–2089. 10.4315/0362-028X.JFP-11-292. [DOI] [PubMed] [Google Scholar]
- 11.Paidhungat M, Setlow P. 2000. Role of ger proteins in nutrient and nonnutrient triggering of spore germination in Bacillus subtilis. J Bacteriol 182:2513–2519. 10.1128/JB.182.9.2513-2519.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Black EP, Koziol-Dube K, Guan D, Wei J, Setlow B, Cortezzo DE, Hoover DG, Setlow P. 2005. Factors influencing germination of Bacillus subtilis spores via activation of nutrient receptors by high pressure. Appl Environ Microbiol 71:5879–5887. 10.1128/AEM.71.10.5879-5887.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Omardien S, Ter Beek A, Vischer N, Montijn R, Schuren F, Brul S. 2018. Evaluating novel synthetic compounds active against Bacillus subtilis and Bacillus cereus spores using Live imaging with SporeTrackerX. Sci Rep 8:1–13. 10.1038/s41598-018-27529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leuschner RGK, Lillford PJ. 1999. Effects of temperature and heat activation on germination of individual spores of Bacillus subtilis. Lett Appl Microbiol 29:228–232. 10.1046/j.1365-2672.1999.00604.x. [DOI] [PubMed] [Google Scholar]
- 15.Sinai L, Rosenberg A, Smith Y, Segev E, Ben-Yehuda S. 2015. The molecular timeline of a reviving bacterial spore. Mol Cell 57:695–707. 10.1016/j.molcel.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang S, Setlow P, Li Y-Q. 2015. Slow leakage of Ca-dipicolinic acid from individual Bacillus spores during initiation of spore germination. J Bacteriol 197:1095–1103. 10.1128/JB.02490-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang P, Thomas S, Li Y-Q, Setlow P. 2012. Effects of cortex peptidoglycan structure and cortex hydrolysis on the kinetics of Ca2-dipicolinic acid release during Bacillus subtilis spore germination. J Bacteriol 194:646–652. 10.1128/JB.06452-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warda AK, den Besten HMW, Sha N, Abee T, Nierop Groot MN. 2015. Influence of food matrix on outgrowth heterogeneity of heat damaged Bacillus cereus spores. Int J Food Microbiol 201:27–34. 10.1016/j.ijfoodmicro.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 19.He L, Chen Z, Wang S, Wu M, Setlow P, Li Y-Q. 2018. Germination, outgrowth, and vegetative growth kinetics of dry heat treated individual spores of Bacillus species. Appl Environ Microbiol 84:e02618-17. 10.1128/AEM.02618-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ablett S, Darke AH, Lillford PJ, Martin DR. 1999. Glass formation and dormancy in bacterial spores. Int J Food Sci 34:59–69. 10.1046/j.1365-2621.1999.00240.x. [DOI] [Google Scholar]
- 21.Leuschner RGK, Lillford PJ. 2003. Thermal properties of bacterial spores and biopolymers. Int J Food Microbiol 80:131–143. 10.1016/S0168-1605(02)00139-3. [DOI] [PubMed] [Google Scholar]
- 22.Belliveau BH, Beaman TC, Pankratz HS, Gerhardt P. 1992. Heat killing of bacterial spores analyzed by differential scanning calorimetry. J Bacteriol 174:4463–4474. 10.1128/jb.174.13.4463-4474.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berendsen EM, Zwietering MH, Kuipers OP, Wells-Bennik MHJ. 2015. Two distinct groups within the Bacillus subtilis group display significantly different spore heat resistance properties. Food Microbiol 45:18–25. 10.1016/j.fm.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh S, Zhang P, Li Y-Q, Setlow P. 2009. Superdormant spores of Bacillus species have elevated wet-heat resistance and temperature requirements for heat activation. J Bacteriol 191:5584–5591. 10.1128/JB.00736-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Busta FF, John Ordal Z. 1964. Heat-activation kinetics of endospores of Bacillus subtilis. J Food Sci 29:345–353. 10.1111/j.1365-2621.1964.tb01742.x. [DOI] [Google Scholar]
- 26.Alimova A, Katz A, Gottlieb P, Alfano RR. 2006. Proteins and dipicolinic acid released during heat shock activation of Bacillus subtilis spores probed by optical spectroscopy. Appl Opt 45:445–450. 10.1364/ao.45.000445. [DOI] [PubMed] [Google Scholar]
- 27.Zhang P, Setlow P, Li Y. 2009. Characterization of single heat-activated Bacillus spores using laser tweezers Raman spectroscopy. Opt Express 17:16480–16491. 10.1364/OE.17.016480. [DOI] [PubMed] [Google Scholar]
- 28.Wang G, Zhang P, Paredes-Sabja D, Green C, Setlow P, Sarker MR, Li Y-Q. 2011. Analysis of the germination of individual Clostridium perfringens spores and its heterogeneity. J Appl Microbiol 111:1212–1223. 10.1111/j.1365-2672.2011.05135.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhang P, Kong L, Setlow P, Li Y-Q. 2010. Characterization of wet-heat inactivation of single spores of bacillus species by dual-trap Raman spectroscopy and elastic light scattering. Appl Environ Microbiol 76:1796–1805. 10.1128/AEM.02851-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luu S, Setlow P. 2014. Analysis of the loss in heat and acid resistance during germination of spores of Bacillus species. J Bacteriol 196:1733–1740. 10.1128/JB.01555-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang P, Garner W, Yi X, Yu J, Li Y-Q, Setlow P. 2010. Factors affecting variability in time between addition of nutrient germinants and rapid dipicolinic acid release during germination of spores of Bacillus species. J Bacteriol 192:3608–3619. 10.1128/JB.00345-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan L, Ismail BB, Hou F, Muhammad AI, Zou M, Ding T, Liu D. 2019. Thermosonication damages the inner membrane of Bacillus subtilis spores and impels their inactivation. Food Res Int 125:108514. 10.1016/j.foodres.2019.108514. [DOI] [PubMed] [Google Scholar]
- 33.Wang G, Paredes-Sabja D, Sarker MR, Green C, Setlow P, Li Y-Q. 2012. Effects of wet heat treatment on the germination of individual spores of Clostridium perfringens. J Appl Microbiol 113:824–836. 10.1111/j.1365-2672.2012.05387.x. [DOI] [PubMed] [Google Scholar]
- 34.Abhyankar WR, Kamphorst K, Swarge BN, van Veen H, van der Wel NN, Brul S, de Koster CG, de Koning LJ. 2016. The influence of sporulation conditions on the spore coat protein composition of Bacillus subtilis spores. Front Microbiol 7:1636. 10.3389/fmicb.2016.01636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wen J, Pasman R, Manders EMM, Setlow P, Brul S. 2019. Visualization of germinosomes and the inner membrane in Bacillus subtilis spores. J Vis Exp 2019:e59388. 10.3791/59388. [DOI] [PubMed] [Google Scholar]
- 36.Pandey R, Ter Beek A, Vischer NOE, Jan PP, Brul S, Manders EMM. 2013. Live cell imaging of germination and outgrowth of individual Bacillus subtilis spores; the effect of heat stress quantitatively analyzed with SporeTracker. PLoS One 8:e58972. 10.1371/journal.pone.0058972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leighton TJ, Doi RH. 1971. The stability of messenger ribonucleic acid during sporulation in Bacillus subtilis. J Biol Chem 246:3189–3195. [PubMed] [Google Scholar]