ABSTRACT
The biosynthesis of prodigiosin in the model prodigiosin-producing strain, Serratia sp. ATCC 39006, is significantly influenced by environmental and cellular signals. However, a comprehensive regulatory mechanism for this process has not been well established. In the present study, we demonstrate that organic hydroperoxide activates prodigiosin biosynthesis in an OhrR-dependent manner. Specifically, the MarR-family transcriptional repressor OhrR (Ser39006_RS05455) binds to its operator located far upstream of the promoter region of the prodigiosin biosynthesis operon (319 to 286 nucleotides [nt] upstream of the transcription start site) and negatively regulates the expression of prodigiosin biosynthesis genes. Organic hydroperoxide disassociates the binding between OhrR and its operator, thereby promoting the prodigiosin production. Moreover, OhrR modulates the resistance of Serratia sp. ATCC 39006 to organic hydroperoxide by regulating the transcription of its own gene and the downstream cotranscribed ohr gene. These results demonstrate that OhrR is a pleiotropic repressor that modulates the prodigiosin production and the resistance of Serratia sp. ATCC 39006 to organic hydroperoxide stress.
IMPORTANCE Bacteria naturally encounter various environmental and cellular stresses. Organic hydroperoxides generated from the oxidation of polyunsaturated fatty acids are widely distributed and usually cause lethal oxidative stress by damaging cellular components. OhrR is known as a regulator that modulates the resistance of bacteria to organic hydroperoxide stress. In the current study, organic hydroperoxide disassociates OhrR from the promoter of prodigiosin biosynthesis gene cluster, thus promoting transcription of pigA to -O genes. In this model, organic hydroperoxide acts as an inducer of prodigiosin synthesis in Serratia sp. ATCC 39006. These results improve our understanding of the regulatory network of prodigiosin synthesis and serve as an example for identifying the cross talk between the stress responses and the regulation of secondary metabolism.
KEYWORDS: Serratia sp. ATCC 39006, prodigiosin synthesis, organic hydroperoxide, OhrR, transcriptional regulation
INTRODUCTION
Bacterial secondary metabolites exhibit biological and ecological activities that confer benefits to their producers, including antibacterial activities, antifungal activities, quorum sensing, and communication between cells (1). The expression of secondary metabolites biosynthetic genes is highly regulated by multiple transcription factors that respond to various intra- and extracellular signals (1). Studying environmental and cellular signals in association with their regulators will enhance the yield of secondary metabolite in industrial processes.
Organic hydroperoxides resulting from the oxidation of polyunsaturated fatty acids lead to bacterial cell death by inducing oxidative stress that damages cellular components (2–4). Organic hydroperoxide resistance regulator (OhrR) belongs to the MarR-family transcriptional regulator and acts as a response regulator of organic hydroperoxide stress signals. Under normal conditions, OhrR binds to its operator and represses the transcription of its target gene, ohr, which encodes a thiol-dependent peroxidase and plays an essential role in detoxifying organic hydroperoxides (5, 6). When organic hydroperoxide stress occurs, OhrR dissociates from its binding site, which leads to the expression of ohr to neutralize organic hydroperoxide (7–15). In addition to the oxidative stress response, OhrR is also involved in regulating virulence or secondary metabolism (16–20).
Serratia species are well-known for their ability to produce the red tripyrrole pigment prodigiosin that is a promising microbial secondary metabolite with multiple biological activities (21–24). In Serratia spp., prodigiosin biosynthesis genes are arranged as a polycistron and form the pig operon (23, 25). In the model strain Serratia sp. ATCC 39006, the pig operon comprises 15 structural genes, including pigA to -O (pigA-O) (26, 27). Multiple transcription factors bind to or release from the promoter region of the pig operon to regulate the expression of prodigiosin biosynthesis genes in response to various environmental signals, such as two-component systems PhoB/PhoR (response to phosphate limitation signal) and RssA/RssB (response to iron ion signal) (28–30) and transcriptional regulators PigT (response to gluconate signal) (31), HexS (response to acidification signal) (32), Crp (response to cyclic AMP) (33), and SmaR/SpnR (response to the quorum-sensing signal molecule N-acyl-homoserine-lactone) (34, 35). In addition, numerous environmental signals have been observed to affect prodigiosin production (22, 23), but their regulatory mechanisms remain unknown. Revealing these regulatory mechanisms can facilitate the optimization of industrial fermentation via genetic engineering to improve prodigiosin yield.
In the present study, we show that OhrR modulates the resistance of Serratia sp. ATCC 39006 to organic hydroperoxide by regulating the transcription of ohr. In addition, OhrR acts as a transcriptional repressor of prodigiosin biosynthesis genes in Serratia sp. ATCC 39006. Specifically, organic hydroperoxide induces prodigiosin production by disassociating OhrR from its binding site, thereby leading to the derepression of pigA-O genes and the enhancement of prodigiosin yield. In summary, this study represents the induction of organic hydroperoxide to prodigiosin biosynthesis in Serratia sp. ATCC 39006 in an OhrR-dependent manner.
RESULTS
Identification of Serratia sp. ATCC 39006 OhrR.
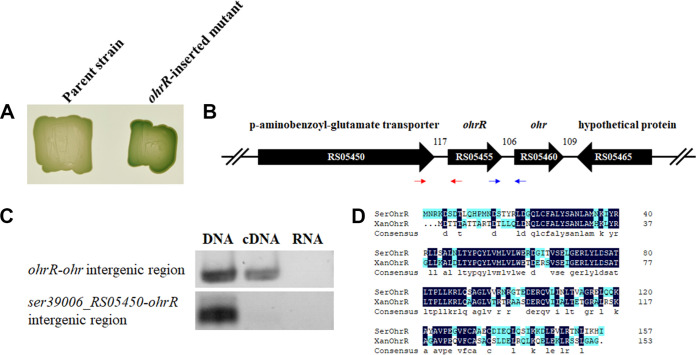
To identify the regulator affecting the transcription of the prodigiosin biosynthetic operon, we performed transposon mutagenesis analysis with the ΔpigA/pBBR1MCS5-Ppig-lacZ strain that harbors a pig promoter-lacZ reporter plasmid in the prodigiosin-negative strain as we described previously (36). Among 5,000 mutants, 7 bluer transformants growing on LB agar plate with X-gal were selected to identify the insertion site by sequencing (Table S1), and 1 of the 7 mutants harbored the insertion in the ser39006_RS05455 gene that was annotated as a MarR-family transcriptional regulator (Fig. 1A). The ser39006_RS05455 gene is located between ser39006_RS05450 and ser39006_RS05460, and these three genes are transcribed in the same direction (Fig. 1B). Reverse transcriptase PCR (RT-PCR) indicated that ser39006_RS05455 is not cotranscribed with ser39006_RS05450 but rather forms a polycistron with ser39006_RS05460 that encodes the organic hydroperoxide resistance protein, Ohr (Fig. 1C). The ser39006_RS05455 is located upstream of the ohr gene (Fig. 1B), and the amino acid sequence of Ser39006_RS05455 exhibits 65.10% identity to Xanthomonas phaseoli pv. phaseoli OhrR (GenBank accession AAK62673.1) (Fig. 1D), leading to the designation of this gene as ohrR.
FIG 1.
Identification of ohrR in Serratia sp. ATCC 39006. (A) Colonies of the parent strain (ΔpigA/pBBR1MCS5-Ppig-lacZ) and the ohrR-inserted strain (mini-Tn5 insertional mutant) grown on LB agar supplemented with kanamycin, gentamicin, and X-gal. (B) Schematic diagram of ohrR and its neighboring genes. Numbers indicate the distance (bp) between genes. The prefix (Ser39006_) is omitted for each gene name, while the suffix ID is shown inside the thick arrows. Red and blue thin arrows indicate primer binding sites for ohrR-RTF/ohrR-RTR and ohr-RTF/ohr-RTR, respectively. (C) Amplification of the ohrR-ohr and ser39006_RS05450-ohrR intergenic regions using genomic DNA, cDNA, and RNA as the template. (D) Amino acid sequence alignment of Serratia sp. ATCC 39006 OhrR (SerOhrR) and Xanthomonas phaseoli pv. phaseoli OhrR (XanOhrR).
OhrR regulates the expression of ohrR-ohr operon in response to organic hydroperoxide.
OhrR has been reported to modulate the resistance of some bacteria to organic hydroperoxide (i.e., tert-butyl hydroperoxide [tBHP]) stress by controlling the transcription of its own gene and ohr (6, 14, 37, 38). To analyze the regulatory pattern of OhrR in Serratia sp. ATCC 39006, we constructed an ohrR in-frame deletion mutant ΔohrR and its complemented strain CohrR. The quantitative real-time PCR (qRT-PCR) result showed that the transcription levels of ohrR and ohr in ΔohrR were significantly increased compared to those in the wild type (WT) and CohrR (Fig. 2A), indicating that OhrR negatively regulates the transcription of its own operon.
FIG 2.
Ohr represses the transcription of its own operon. (A) Transcription levels of ohrR and ohr in the WT, ΔohrR, and CohrR. Transcription levels of evaluated genes in different strains were normalized to 16S rRNA gene (internal control). The relative transcription values of evaluated genes in the WT strain are set as 1, while those in the ΔohrR and CohrR strains are presented as fold changes. (B) EMSA verification of OhrR binding to the PohrR probe that harbors the promoter region of the ohrR-ohr operon. Labeled probe (0.2 nM) was added to each reaction mixture, and the OhrR concentration in each reaction mixture is shown above the figure. Lane S: 100-fold unlabeled specific competitive probe. Lane N: 100-fold unlabeled nonspecific competitive probe. Arrow indicates free probes, and bracket indicates the protein-DNA complexes. (C) Prodigiosin yield per cell unit (A534/OD600) for the WT and WT/ohrR-FLAG. (D) ChIP-qPCR analysis of the ohrR promoter region enrichment by C-terminal 3×FLAG-labeled OhrR in WT/ohrR-FLAG, and the WT was used as a negative control. The relative enrichment value of the ohrR promoter by OhrR is shown as %INPUT. Untreated (–tBHP): Serratia sp. ATCC 39006 cells were cross-linked without the tBHP treatment. Treated (+tBHP): Serratia sp. ATCC 39006 cells were treated with 5 mM tBHP for 30 min before cross-linking. (E) DNase I footprinting assay of the OhrR and ohrR promoter. Each reaction mixture contains 300 ng of FAM-labeled ohrR promoter probe, and the OhrR concentration is shown to the left of the figure. Control: without addition of OhrR protein. The nucleotide sequences of the protected region (gray background) and the ohrR promoter are shown below the figure. The predicted TSS is shown by the arrow and bolding, while the start codon is also bolded. Numbers located on both sides of the nucleotide sequence indicate the distance (nt) from the predicted TSS. Experimental data are shown as means ± standard deviation (SD) (n = 3), and statistical significance was evaluated using Student’s t tests. NS, no statistical significance; ***, P < 0.001.
To identify whether OhrR directly repressed the transcription of ohrR-ohr operon, N-terminal His6-tagged OhrR protein was expressed and purified to perform in vitro electrophoretic mobility shift assay (EMSA). The result revealed that OhrR binds specifically to the promoter of its own operon (probe PohrR) (Fig. 2B). To confirm this result, a chromosomal C-terminal 3×FLAG tag was attached to OhrR in the WT to generate WT/ohrR-FLAG for chromatin immunoprecipitation (ChIP)-quantitative PCR (qPCR) assay. The WT and WT/ohrR-FLAG exhibited similar cell growth (Fig. S2A) and prodigiosin production (Fig. 2C), indicating that the C-terminal 3×FLAG tag did not affect the function of OhrR. The ChIP-qPCR result indicated that the enrichment of the promoter of ohrR-ohr operon in WT/ohrR-FLAG was significantly higher (about 46-fold) than that in the WT (untreated bars in Fig. 2D). Thus, OhrR represses the transcription of ohrR-ohr operon by binding directly to its own promoter. Then, the DNase I footprinting assay revealed that the protective region of OhrR on its own promoter is up to 56 nucleotides (nt) in length, extending from 84 to 29 nt upstream of the ohrR start codon (Fig. 2E). The transcription start site (TSS, +1) of ohrR was predicted to be 10 nt upstream of the ohrR start codon based on the analysis with Berkeley Drosophila Genome Project (BDGP) promoter prediction tool (https://www.fruitfly.org/seq_tools/promoter.html) (Fig. 2E) (39). Altogether, these results suggest that OhrR directly represses the transcription of its own operon by blocking the binding of RNA polymerase to the promoter region.
To determine whether OhrR senses organic hydroperoxide stress in Serratia sp. ATCC 39006, we performed a drop plate assay to compare the resistance of WT, ΔohrR, Δohr, and ΔohrR-ohr to tBHP. The cell growth of Δohr and ΔohrR-ohr was totally inhibited in the tBHP-containing medium (Fig. 3A), indicating that Ohr is essential for withstanding organic hydroperoxide stress in Serratia sp. ATCC 39006. In addition, the ΔohrR was able to grow at all the cell concentration gradients, while the WT growth was significantly decreased (Fig. 3A), indicating that OhrR modulates the resistance of Serratia sp. ATCC 39006 to organic hydroperoxide stress.
FIG 3.
OhrR responds to organic hydroperoxide stress. (A) Sensitivity analysis of the WT, ΔohrR, Δohr, and ΔohrR-ohr strains to the organic hydroperoxide exposure. Cells were serially diluted and spotted onto LB agar (control) or LB agar containing 0.1 mM tBHP, followed by incubation at 30°C for 48 h. (B) Transcriptional analysis of ohr induced by different concentrations of tBHP. Transcriptional levels of ohr in the WT treated with different concentrations of tBHP (0, 1, 2, and 5 mM) for 30 min were determined and normalized to 16S rRNA gene (internal control). The relative transcription value of ohr in the WT without tBHP treatment is set as 1, while the levels in tBHP-treated cells are shown as fold changes. (C) Transcriptional levels of ohr in the WT induced by 5 mM tBHP over different times (0, 5, 10, 20, and 30 min) were determined and normalized to 16S rRNA gene (internal control). The relative transcription value of ohr in the WT without tBHP treatment is set as 1, while those in tBHP-treated cells are shown as fold changes. (D) EMSA of the tBHP-based effects on the binding of OhrR to the PohrR probe. The concentrations of tBHP and OhrR in each reaction mixture are shown above the figure. Labeled probe (0.2 nM) was added to each reaction mixture. Experimental data are shown as means ± SD (n = 3).
We next sought whether OhrR regulates its own operon in response to tBHP. The qRT-PCR result indicated that the transcription of ohr in the WT was induced by tBHP in a dose-dependent manner (Fig. 3B) and increased with the extension of tBHP treatment time (Fig. 3C), suggesting that the tBHP stress induces the transcription of ohrR-ohr operon. Then, the ChIP-qPCR and EMSAs were performed to further analyze whether the induction of tBHP to the transcription of ohrR-ohr operon is OhrR-dependent. The ChIP-qPCR result showed that the tBHP treatment led to the disappearance of the ohrR promoter enrichment (Fig. 2D), while the EMSA indicated that the binding of OhrR to the probe PohrR was weakened by tBHP in a dose-dependent manner (Fig. 3D). Thus, OhrR regulates the expression of the ohrR-ohr operon in response to organic hydroperoxide stress in Serratia sp. ATCC 39006.
OhrR negatively regulates the prodigiosin production in Serratia sp. ATCC 39006.
Transposon mutagenesis experiment suggested that OhrR influenced prodigiosin production (Fig. 1A). Shake flask fermentation experiment demonstrated that the prodigiosin yield per cell unit (A534/optical density at 600 nm [OD600]) of ΔohrR was significantly higher than that of the WT and CohrR (Fig. 4A). This result indicated that OhrR negatively regulates prodigiosin production. Furthermore, the WT, ΔohrR, and CohrR exhibited similar cell growths (Fig. S2B), indicating that the increased prodigiosin production in ΔohrR was not caused by the change in cell growth. In addition, the cell growth and prodigiosin production of Δohr were similar to those of the WT (Fig. 4A, Fig. S2C), suggesting that OhrR represses the prodigiosin production independently of its regulation of ohr transcription.
FIG 4.
OhrR directly represses the transcription of pigA-O genes. (A) Prodigiosin yield per cell unit (A534/OD600). (B) Expression analysis of C-terminal 3×FLAG-labeled PigA in WT/pigA-FLAG and ΔohrR/pigA-FLAG strains. Untreated (–tBHP): cells were grown in LB medium. Treated (+tBHP): cells were grown in LB medium supplemented with 0.05 mM tBHP. (C) ChIP-qPCR analysis of the pig operon promoter region enrichment by FLAG-labeled OhrR in WT/ohrR-FLAG. The WT was used as negative control. The relative enrichment of the pig operon promoter by OhrR is shown as %INPUT. Untreated (–tBHP): cells without the tBHP treatment were cross-linked. Treated (+tBHP): cells were treated with 5 mM tBHP for 30 min before cross-linking. (D) EMSA verification of OhrR binding to Ppig probe that harbors the promoter region of the pig operon. Labeled probe (0.2 nM) was added to each reaction mixture. OhrR concentrations in each reaction mixture are shown above the figure. Lane S, 100-fold unlabeled specific competitive probe. Lane N, 100-fold unlabeled nonspecific competitive probe. The arrow indicates free probes, and the bracket indicates protein-DNA complexes. (E) DNase I footprinting assay of OhrR and the pig operon promoter. Each reaction mixture contained 300 ng of FAM-labeled pig promoter probe, and the OhrR concentration is shown to the left of the figure (control: without addition of OhrR protein). The nucleotide sequences of the protected region (bolded residues) and the pig promoter are shown below the figure. Arrows indicate inverted repeats. The TSS of pigA is shown by an arrow and bolding. Numbers located on both sides of the nucleotide sequence indicate the distance (nt) from the TSS. The −35 and −10 regions are shown within a box. Shaded aera: ROP1 box. (F) Identification of the OhrR binding site in the pig promoter. EMSA of the association between OhrR and Ppig probe, in addition to the mutated Ppig probe (Ppig-mut). OhrR concentrations in each reaction mixture and nucleotide sequences of the predicted binding site and the mutated binding site are shown above the figure. The arrow indicates free probes, and the bracket indicates protein-DNA complexes. (G) Prodigiosin yield and pigA transcription levels in WT/PaacC1-pig and ΔohrR/PaacC1-pig strains. Transcription levels of genes in different strains were determined and normalized to 16S rRNA gene (internal control). The transcription value of pigA in WT/PaacC1-pig is set as 1 and the value of ΔohrR/PaacC1-pig is shown as fold change. Experimental data are shown as means ± SD (n = 3), and significant differences between data sets were evaluated with Student’s t tests. NS, no statistical significance; ***, P < 0.001.
OhrR directly represses the transcription of prodigiosin biosynthesis genes.
We next considered whether OhrR negatively regulates prodigiosin production by repressing the transcription of pigA-O genes. The qRT-PCR was carried out to compare the transcription of 15 pig genes in the WT and ΔohrR. The results showed that the transcription levels of 15 pig genes in ΔohrR were significantly higher than those in the WT (Table 1). Moreover, Western blotting was performed to estimate the effect of OhrR on the expression of PigA. The result demonstrated that the expression of PigA was higher in ΔohrR/pigA-FLAG than in WT/pigA-FLAG (untreated bands in Fig. 4B and Fig. S3). Thus, OhrR negatively regulates prodigiosin production by repressing the expression of prodigiosin synthesis genes.
TABLE 1.
Transcriptional changes of pigA-O genes in ΔohrR relative to those in the WT
| Genes | Fold changea | P valueb |
|---|---|---|
| pigA | 2.05 | 3.69E−04 |
| pigB | 2.16 | 2.66E−04 |
| pigC | 1.84 | 1.67E−04 |
| pigD | 1.75 | 7.50E−03 |
| pigE | 1.89 | 4.53E−06 |
| pigF | 1.87 | 2.31E−05 |
| pigG | 2.05 | 5.15E−04 |
| pigH | 2.09 | 6.26E−05 |
| pigI | 1.95 | 1.19E−04 |
| pigJ | 1.80 | 2.08E−03 |
| pigK | 2.00 | 1.30E−05 |
| pigL | 1.97 | 1.84E−03 |
| pigM | 1.77 | 4.70E−05 |
| pigN | 1.80 | 7.60E−04 |
| pigO | 1.86 | 2.10E−04 |
Fold change data represent the ΔohrR/WT ratios for each pig gene.
The P value represents the statistical significance level of the differential transcription.
We next evaluated whether OhrR represses the transcription of pigA-O genes by binding directly to the promoter of the pig operon. The ChIP-qPCR result showed that the enrichment of the promoter of pig operon in WT/ohrR-FLAG was considerably higher (about 56-fold) than that in the WT (untreated bars in Fig. 4C), indicating that OhrR binds directly to the promoter of the pig operon in vivo. The EMSA result indicated that His6-OhrR bound specifically to the promoter region of the pig operon (probe Ppig) in vitro (Fig. 4D). The binding site of OhrR on the pig promoter was determined using the DNase I footprinting assay. In the presence of OhrR, a 34-nt protected region was identified from −319 to −286 nt upstream of the TSS (+1) of the pig operon (Fig. 4E). The binding sequences of OhrR in many bacteria comprise AT-rich inverted repeats (12, 14, 15, 20, 40). Accordingly, the nucleotide sequence analysis for this protected region revealed an imperfect palindromic sequence of TTTACTTG|CAATAAAA. To verify whether this palindromic sequence is the OhrR binding site, the sequence was mutated and labeled for EMSA. The result showed that OhrR did not bind to the mutant probe (Ppig-mut) (Fig. 4F), indicating that this 8-nt imperfect inverted repeat is essential for OhrR binding. Based on the above results, OhrR binds directly to the pig operon promoter to repress the transcription of prodigiosin biosynthesis genes.
To further confirm that OhrR negatively regulates prodigiosin production by repressing the transcription of pigA-O genes, we used a constitutive promoter, PaacC1, to replace the native pig promoter in ΔohrR, resulting in the ΔohrR/PaacC1-pig strain. The cell growth, prodigiosin yield, and pigA transcription level in ΔohrR/PaacC1-pig did not differ significantly from those in the previously constructed WT/PaacC1-pig strain (36) (Fig. 4G, Fig. S2D). Thus, OhrR negatively regulates prodigiosin production by repressing the transcription of pig genes.
Organic hydroperoxide induces prodigiosin production in an OhrR-dependent manner.
Organic hydroperoxide tBHP diminished the binding between OhrR and the promoter of its own operon, leading us to next evaluate whether the tBHP treatment could weaken the binding of OhrR to the pig promoter. The effect of 0.05 mM tBHP on the cell growth and prodigiosin production was analyzed. As shown in Fig. S2E, the addition of 0.05 mM tBHP exerted no effect on the cell growth of all tested strains, but the prodigiosin yield of tBHP-treated WT cells was improved to a level similar to that of ΔohrR, which was significantly higher than that of untreated WT cells (Fig. 5A). In addition, the tBHP treatment had no effect on the prodigiosin production of ΔohrR (Fig. 5A). Thus, low concentration of organic hydroperoxide improves the prodigiosin production of Serratia sp. ATCC 39006 in an OhrR-dependent manner. The qRT-PCR and Western blotting indicated that the transcription level (Fig. 5B) and protein expression level (Fig. 4B) of pigA were greatly elevated in the tBHP-treated WT cells, but these levels did not change in ΔohrR. The EMSA result showed that in vitro binding of OhrR to probe Ppig was weakened by tBHP in a dose-dependent manner (Fig. 5C), while the ChIP-qPCR revealed that in vivo enrichment of the promoter region of pig operon by OhrR was eliminated by the tBHP addition to fermentation broth (treated bars in Fig. 4C). Thus, the tBHP stress relieves the transcriptional repression of OhrR to the pig operon, thereby increasing the prodigiosin production. Consequently, these results indicate that organic hydroperoxide induces the prodigiosin production of Serratia sp. ATCC 39006 in an OhrR-dependent manner.
FIG 5.

Organic hydroperoxide induces prodigiosin production. (A) Prodigiosin yield per cell unit (A534/OD600) and (B) transcriptional analysis of pigA in the WT and ΔohrR strains grown in LB (untreated [–tBHP]) or grown in LB supplemented with 0.05 mM tBHP (treated [+tBHP]). Transcription levels of pigA in different strains were determined and normalized to 16S rRNA gene (internal control). The relative transcription value of pigA in the WT grown in LB medium is set as 1, while those in other strains are shown as fold changes. (C) EMSA of the effects of tBHP on the binding of OhrR to probe Ppig. The concentrations of tBHP and OhrR in each reaction mixture are shown above the figure. Labeled probe (0.2 nM) was added to each reaction mixture. Experimental data are shown as means ± SD (n = 3), and significant differences between data sets were evaluated with Student’s t tests. NS, no statistical significance; ***, P < 0.001.
DISCUSSION
Bacteria are naturally exposed to various stresses due to constantly changing environments, including the changes in temperature, osmolarity, and pH, leading to the presence of complicated stress response systems that facilitate bacterial survival under various environmental stresses. OhrR modulates the resistance of some bacteria to organic hydroperoxide stress by controlling the expression of thiol-dependent peroxidase gene, ohr (11, 14, 17). The locus of ohr in genomes is usually close to that of ohrR, with the gene arrangement typically organized in one of the following two patterns. First, in Pseudomonas aeruginosa and Xanthomonas phaseoli pv. phaseoli, ohrR is located upstream of ohr, and both genes are cotranscribed (41, 42). In this arrangement, the OhrR binding site is located in the promoter region of its own operon. Second, in Streptomyces coelicolor (15), Streptomyces avermitilis (20), Bacillus subtilis (13), and Agrobacterium tumefaciens (12), ohrR and ohr are transcribed in different directions and share an intergenic promoter region that harbors the OhrR binding site. In both models, OhrR acts as a transcriptional repressor to repress the expression of both genes (7, 8, 37). In this study, we present the physiological function of OhrR (Ser39006_RS05455) in Serratia sp. ATCC 39006 (Fig. 6). The ohrR and ohr are cotranscribed, and OhrR represses the transcription of its own operon. Organic hydroperoxide exposure decreases the DNA-binding ability of OhrR and derepresses the transcription of the ohrR-ohr operon, thereby modulating the resistance of Serratia sp. ATCC 39006 to organic hydroperoxide stress.
FIG 6.
Proposed model of the OhrR-dependent modulation of prodigiosin production and the resistance to organic hydroperoxide stress in Serratia sp. ATCC 39006. Thin arrows indicate activation, gene expression, or production activities. Blunt arrows indicate repression activities.
In addition to regulating organic hydroperoxide resistance, OhrR also acts as a pleiotropic regulator to modulate multiple physiological processes. For example, OhrR acts as a global transcriptional regulator that participates in the enhancement of virulence in many pathogenic microorganisms (16–19). Furthermore, OhrR is involved in regulating the secondary metabolism of Streptomyces (20). In the present study, OhrR is found to regulate prodigiosin biosynthesis. In this model, OhrR acts as a transcriptional repressor of prodigiosin biosynthesis genes by binding directly to the promoter region of the pig operon (Table 1, Fig. 4C and D), resulting in the negative regulation of prodigiosin production (Fig. 4A). Organic hydroperoxide disassociates OhrR from its binding site (Fig. 4C, Fig. 5C), leading to the improvement of the transcription of pig operon (Fig. 5B), thereby significantly elevating prodigiosin yield (Fig. 5A). These results illustrate that organic hydroperoxide induces prodigiosin biosynthesis in Serratia sp. ATCC 39006 in an OhrR-dependent manner. Prodigiosin production is affected by various environmental signals, including temperature, dissolved oxygen, carbon sources, nitrogen sources, pH, metal ions, secondary messengers, and quorum-sensing molecules (22, 23). This report presents the underlying mechanism of prodigiosin biosynthesis induced by organic hydroperoxide, further improving our knowledge of the regulatory network of prodigiosin production. We identified an OhrR binding site upstream of pig operon, harboring an AT-rich imperfect palindromic sequence of TTTACTTG|CAATAAAA (Fig. 4E). Mutation of this sequence resulted in the abolishment of OhrR binding (Fig. 4F), indicating that this inverted repeat is critical for OhrR recognition of its target. This is coincident with previous research in which the binding sequences of OhrR in many bacteria comprise AT-rich inverted repeats (12, 14, 15, 20, 40) (Fig. S4A). Similar AT-rich sequence is also found in the OhrR protected region upstream of ohrR-ohr operon (Fig. S4B). In future study, the conserved binding motif of OhrR could be employed to scan other OhrR regulons in the genome of Serratia sp. ATCC 39006, thereby revealing more physiological functions of OhrR.
The transcriptional regulation of prodigiosin synthesis genes can be classified into two groups in bacteria according to the presence or absence of pathway-specific regulators. In the first, as exemplified by the model undecylprodigiosin-producing strain Streptomyces coelicolor, undecylprodigiosin biosynthesis genes are under the control of two pathway-specific regulators, RedD and RedZ (23). Multiple global regulators and pleiotropic regulators typically modulate undecylprodigiosin biosynthesis by regulating the transcription of redD and redZ. In the second model, as exemplified by the prodigiosin-producing strain Serratia sp. ATCC 39006, prodigiosin synthesis genes are consecutively arranged and transcribed as a polycistron, while no pathway-specific regulator is involved in modulating the transcription of the prodigiosin biosynthesis genes (23). An intergenic region of 922 bp between the pig operon and its upstream gene harbors the promoter region of pig operon and abundant operators (25). Previous research has shown that multiple transcription factors, including Fnr (36), PhoR (28), and PigT (31), regulate the pig operon by binding to the region close to the RNA polymerase binding site that covers the −10 and −35 regions. An element located −320 to −200 nt upstream of the TSS was identified previously, and a mutation in this element resulted in the enhanced transcription of pigA-O genes and the hyperproduction of prodigiosin (shaded areas in Fig. 4E) (43). This element was consequently designated repressor of pigment 1 (ROP1). Slater et al. speculated that the ROP1 box might be important for binding of the transcription repressors of pig operon (43). In the present study, we found an OhrR operator within the ROP1 box (Fig. 4E). Given that the OhrR operator is relatively far upstream of the TSS, this study proposed three possible regulatory patterns of OhrR: (i) OhrR represses the transcription of the pig operon by modulating DNA conformation, (ii) OhrR prevents some unknown transcriptional activator from promoting the transcription of the pig operon by direct interaction or steric hindrance, or (iii) OhrR cooperates with some other transcriptional repressors to repress the pig operon.
Prodigiosin is of great interest due to its inherent biological activities, including anticancer, antibacterial, antioxidant, antialgal, and immunosuppressive characteristics (21–23). Thus, enhancing prodigiosin yield would promote the widespread application of prodigiosin. In this study, we found that the addition of low concentration of organic hydroperoxide is able to significantly enhance prodigiosin yield, thereby providing a new method to improve prodigiosin production.
MATERIALS AND METHODS
Bacterial strains, plasmids, primers, oligonucleotides, and culture conditions.
Serratia sp. ATCC 39006 and Escherichia coli strains used in this study are listed in Table 2. The primers and oligonucleotides are presented in Table 3. The constructed strains and plasmids were verified by PCR and DNA sequencing. Serratia sp. ATCC 39006 and E. coli were grown at 30 and 37°C, respectively. For liquid culture, the strains were cultured in 100-mL flasks containing 25 mL Luria-Bertani (LB) medium with 200 rpm shaking. For solid culture, 1.8% (wt/vol) agar was added into LB medium. The solid M9 medium, solid LCS medium, and LC liquid were used in the conjugation experiment (36). Antibiotics used in this study were purchased from Beijing Solarbio Science & Technology Co., Ltd. The final concentrations of kanamycin (Km) and gentamicin (Gm) were 50 μg/mL and 15 μg/mL, respectively.
TABLE 2.
Strains and plasmids used in this study
| Strains or plasmids | Usage or description | Source |
|---|---|---|
| Strains | ||
| E. coli | ||
| S17-1(λpir) | Routine transconjugation host and cloning host for pMMB1 plasmid variants; recA pro hsdR recA::RP4-2-Tc::Mu λpir TmpR SpR SmR | Laboratory stock |
| DH5α | Routine cloning host; fhuA2 lacΔU169 phoA glnV44 Φ80' lacZΔM15 gyrA96 recA1 relA1 endA1 thi-1 hsdR17 | Laboratory stock |
| BL21(DE3) | Protein expression host; dcm ompT hsdS (rB−mB−) gal | Laboratory stock |
| BL21(DE3)/pET28-ohrR | Heterologous expression of N-terminal His6-tagged Serratia sp. ATCC 39006 OhrR protein | This study |
| UQ3022 | Donor strain for the mini-Tn5 transposon containing plasmid, pRL27 | 45 |
| Serratia sp. ATCC 39006 | ||
| Wild type (WT) | Serratia sp. ATCC 39006 (car+ pig+ lac−) | 26 |
| ΔohrR | ohrR (Ser39006_RS05455) deletion mutant | This study |
| CohrR | ΔohrR complemented by pBBR1MCS2-CohrR | This study |
| Δohr | ohr (Ser39006_RS05460) deletion mutant | This study |
| ΔohrR-ohr | ohrR-ohr double-deletion mutant | This study |
| Δpig | pig operon deletion mutant | This study |
| WT/ohrR-FLAG | C-terminal 3×FLAG-tagged ohrR transformant | This study |
| WT/pigA-FLAG | C-terminal 3×FLAG-tagged pigA transformant | 36 |
| ΔohrR/pigA-FLAG | C-terminal 3×FLAG-tagged pigA in ΔohrR | This study |
| WT/PaacC1-pig | Replace the promoter region of pig operon with aacC1 promoter | 36 |
| ΔohrR/PaacC1-pig | Replace the promoter region of pig operon with aacC1 promoter in ΔohrR | This study |
| CohrR/PaacC1-pig | Replace the promoter region of pig operon with aacC1 promoter in CohrR | This study |
| ΔpigA/pBBR1MCS5-Ppig-lacZ | ΔpigA harboring a lacZ reporter plasmid with a pigA promoter | 36 |
| Plasmids | ||
| pET-28a(+) | Vector for heterologous protein expression in E. coli; Kmr | Novagen |
| pET28-OhrR | Vector for heterologous expression of N-terminal His6-tagged Serratia sp. ATCC 39006 OhrR | This study |
| pMV-PigA-M | Vector containing the nucleotide sequence of EMSA probe pigA, with its predicted OhrR binding sites mutated. | Synthesized by BGI; genomics in this study. |
| pRL27 | Plasmid containing Mini-Tn5 transposon and a Pir protein-dependent DNA replication origin (oriR6K); Kmr | 45 |
| pBBR1MCS2 | Broad-host-range vector; Kmr | 46 |
| pBBR1MCS2-PaacC1 | Vector for complementary; Kmr | Laboratory stock |
| pBBR1MCS5-Ppig-lacZ | Reporter vector for identifying the transcriptional regulators binding to the pig promoter | 36 |
| pBBR1MCS2-CohrR | Vector for complement ohrR in ΔohrR mutant | This study |
| pMMB1 | Suicide plasmid for Serratia sp. ATCC 39006, replacing the DNA replication origin of pK19mobsacB with a Pir protein-dependent oriR6K from pKNG101; sacB (modified from Bacillus subtilis) lacZ Kmr | 36 |
| pMMB1-DohrR | Plasmid used for in-frame deletion of ohrR gene | This study |
| pMMB1-Dohr | Plasmid used for in-frame deletion of ohr gene | This study |
| pMMB1-DohrR-ohr | Plasmid used for in-frame double deletion of ohrR-ohr operon | This study |
| pMMB1-ohrR-FlagKin | Plasmid used to knock-in 3×FLAG tag to the C terminus of ohrR gene | This study |
| pMMB1-pigA-FlagKin | Plasmid used to knock-in 3×FLAG tag to the C terminus of pigA gene | 36 |
| pMMB1-PaacC1-pig | Plasmid used to replace Ppig with PaacC1 | 36 |
TABLE 3.
Primers and oligonucleotide used in this study
| Primer or oligonucleotide | Nucleotide sequence | Usage |
|---|---|---|
| Primers | ||
| Tn5-F | CAGCAACACCTTCTTCACGA | Mapping the transposon insertion site |
| Tn5-R | AACAAGCCAGGGATGTAACG | Mapping the transposon insertion site |
| ohrR-5F | TTGGATCCTAAAAGCCAGTGGTATTGC | ohrR upstream homologous arm |
| ohrR-5R | CCGGAATTCATAGGTCAAATTCAGCGC | ohrR upstream homologous arm |
| ohrR-3F | CCGGAATTCGAAGTGTTACGTACCAACCTG | ohrR downstream homologous arm |
| ohrR-3R | CCTACTAGTATGGCGGAAAACGATAGT | ohrR downstream homologous arm |
| ohrR-UF | GGTATTTATCCGCATTTGCA | Verification of Δfnr deletion mutant |
| ohrR-DR | TTCTTGCCCTCCTGTCAG | Verification of Δfnr deletion mutant |
| ohrR-OF | CGAAAGTCTGCACCAACT | Verification of Δfnr deletion mutant |
| ohrR-OR | CCTGGAGTGAAGCTTATGAT | Verification of Δfnr deletion mutant |
| ohrR-InF | CACAATATCTGGTGATGCTAGTG | Verification of Δfnr deletion mutant |
| ohrR-InR | CCTTCAGGCACAGCGTAG | Verification of Δfnr deletion mutant |
| ohr-5F | AACTGGGGATCCGGACCTACTATGCATTGATACTTC | ohr upstream homologous arm |
| ohr-5R | TACTTTGAATTCTTCAATAGACATAATAGACTCCAGATA | ohr upstream homologous arm |
| ohr-3F | GGCAACGAATTCATCGATGTAACGCTTAGTATCATTTAA | ohr downstream homologous arm |
| ohr-3R | AGAAGCCTGCAGCAATAAATCGCTCAATCAGATAAC | ohr downstream homologous arm |
| ohr-UF | AGAGTGTGATATTGAGCAGTTACA | Verification of Δohr deletion mutant |
| ohr-DR | AATGATGATTGCAACCATGGAAGG | Verification of Δohr deletion mutant |
| ohr-OF | CAGTCATTCCGTTAGCACCGGTAT | Verification of Δohr deletion mutant |
| ohr-OR | CCATCAGTTAAATGAGGCATCAGT | Verification of Δohr deletion mutant |
| ohr-InF | AAAGTATTGTACGTTGCTCATGCC | Verification of Δohr deletion mutant |
| ohr-InR | AGTGAAATCTTCAGTTCTACTTCA | Verification of Δohr deletion mutant |
| ohrR-CF | TAATTTGGATCCTAAACGATGTAAATTTGCG | Complementation of ohrR |
| ohrR-CR | CTTGCCGAATTCCTCCTGTCAGATATGCTTG | Complementation of ohrR |
| ohrR-RTF | GGTATTTATCCGCATTTGCA | Verification of cotranscription of ohrR and Ser39006_RS05450 |
| ohrR-RTR | AACAGACGGCGATAGATT | Verification of cotranscription of ohrR and Ser39006_RS05450 |
| ohr-RTF | AGAGTGTGATATTGAGCAGTTACA | Verification of cotranscription of ohrR and ohr |
| ohr-RTR | TTCAATAGACATAATAGACTCCAGATA | Verification of cotranscription of ohrR and ohr |
| ohrR-FlagConF | TTACCGGGATCCACTGGATGGTCAACTGTGTTTTGC | Construction of pMMB1-ohrR-FlagKin plasmid |
| ohrR-FlagConR | AAATCTCTGCAGTCAGTTCTACTTCAATACCGAACC | Construction of pMMB1-ohrR-FlagKin plasmid |
| ohrR-FlagKinFa | GATATGCTTGATCAGGTTGGTACGTAACACT | Construction of pMMB1-ohrR-FlagKin plasmid |
| ohrR-FlagKinR | CATATCACTAGTTGACAGGAGGGCAAGAAGGTTTTTTTTCG | Construction of pMMB1-ohrR-FlagKin plasmid |
| Flag-Fa | GGAGGTGGCGATTACAAGGATGAC | Construction of pMMB1-ohrR-FlagKin plasmid |
| Flag-R | GGACTAGTTTTATCGTCATCATCTTTGTAGTC | Construction of pMMB1-ohrR-FlagKin plasmid |
| 16S-QF | CAACTTAACAGACCGCCTGCGTGC | qRT-PCR primer for 16S rRNA |
| 16S-QR | GCAGAAGAAGCACCGGCTAACTCC | qRT-PCR primer for 16S rRNA |
| ohrR-QF | ATGAACAGAAAAGATAGCGA | qRT-PCR primer for ohrR |
| ohrR-QR | AACAGACGGCGATAGATT | qRT-PCR primer for ohrR |
| pigA-QF | TGGAGCAGTGTCGGTTGATGCTCT | qRT-PCR primer for pigA |
| pigA-QR | GAACCGCGTACTCGGAAATCAGCA | qRT-PCR primer for pigA |
| pigB-QF | TGCTGGGTCATTCGTTTGGTGGCT | qRT-PCR primer for pigB |
| pigB-QR | ATGATCGTCCATCCAGTTGGCCAC | qRT-PCR primer for pigB |
| pigC-QF | CAGTGCCATTGCCGTGATTCTACA | qRT-PCR primer for pigC |
| pigC-QR | CTTATCGGTGCTCCCTGACAGAGG | qRT-PCR primer for pigC |
| pigD-QF | ACTGCCCACCGATAAGCGACAGTT | qRT-PCR primer for pigD |
| pigD-QR | ACTCATCGTCACTGACCGGTCCTA | qRT-PCR primer for pigD |
| pigE-QF | GATCAAACCTTCGCTGGTGCCGTA | qRT-PCR primer for pigE |
| pigE-QR | GGAATTTCCATGTCGTGTGCCAGT | qRT-PCR primer for pigE |
| pigF-QF | GGCGGTATCGAAAGCAGTCCTCAG | qRT-PCR primer for pigF |
| pigF-QR | ACTATCAGTGTACGAGGACCGGAT | qRT-PCR primer for pigF |
| pigG-QF | GAGCAAGGTCTCGATTGGCATGCA | qRT-PCR primer for pigG |
| pigG-QR | AGCCGTTGCACCAGCGCAACCATA | qRT-PCR primer for pigG |
| pigH-QF | CGCTGTCAGCGTTGAAGCAGTGTT | qRT-PCR primer for pigH |
| pigH-QR | GCATCATGGTCAATGGCGGAGTCG | qRT-PCR primer for pigH |
| pigI-QF | ATGTGCTGTCCAATCACGCCAGTT | qRT-PCR primer for pigI |
| pigI-QR | CGCGGCGCAATCTTTCTGTTCGCT | qRT-PCR primer for pigI |
| pigJ-QF | GACGCAATTGTATGCGCTGAATAG | qRT-PCR primer for pigJ |
| pigJ-QR | TACTGGCAATGATGATCGCCACAC | qRT-PCR primer for pigJ |
| pigK-QF | GGATTATTGCGCTTGCGAGAGCCT | qRT-PCR primer for pigK |
| pigK-QR | GGCGTCCATCGAGCTTATGTGAAT | qRT-PCR primer for pigK |
| pigL-QF | CAACGGATGGTGAAACAGCGGGCA | qRT-PCR primer for pigL |
| pigL-QR | CACTCGGCCGATGAGTCAGTGTAA | qRT-PCR primer for pigL |
| pigM-QF | TGTCGCTGATCTGGTGGCTTATCA | qRT-PCR primer for pigM |
| pigM-QR | AATAACGCGTATAGTTGTGCCTGT | qRT-PCR primer for pigM |
| pigN-QF | CAATGGGTAGCCATCGTGGTTTAT | qRT-PCR primer for pigN |
| pigN-QR | CCGGAGCAGAGGATCTGGCCTTTA | qRT-PCR primer for pigN |
| pigO-QF | TTGCTACTCGCTCACCAAGACGGC | qRT-PCR primer for pigO |
| pigO-QR | TTCAGCAGATCCAGACCTCGAACA | qRT-PCR primer for pigO |
| ohrR-EF | TGCGACGGATCCATGAACAGAAAAGATAGCGATACC | Construction of expression vector for His6-OhrR |
| ohrR-ER | CTCCTGAAGCTTTCAGATATGCTTGATCAGGTTGGT | Construction of expression vector for His6-OhrR |
| ohrR-PF | GTTGGTGGTGTGGTATCTG | Amplification of EMSA probe PohrR |
| ohrR-PR | TCGGTAAGTACTGTCGTTCAT | Amplification of EMSA probe PohrR |
| pigA-PF | CATGTGTTAATTGTGGGTATG | Amplification of EMSA probe Ppig or Ppig-mut |
| pigA-PR | TATACGCTGACTCATAAATATCTG | Amplification of EMSA probe Ppig or Ppig-mut |
| ohrR-FootFb | TGTAAAACGACGGCCAGTGTTGGTGGTGTGGTATCTG | Amplification of DNase I footprinting probe for ohrR |
| ohrR-FootR | CAGGAAACAGCTATGACCTCGGTAAGTACTGTCGTTCAT | Amplification of DNase I footprinting probe for ohrR |
| pigA-FootFb | TGTAAAACGACGGCCAGTGTCATCAGTGTCGTTTTAGC | Amplification of DNase I footprinting probe for pigA |
| pigA-FootR | CAGGAAACAGCTATGACCTATACGCTGACTCATAAATATCTG | Amplification of DNase I footprinting probe for pigA |
| ohrR-ChIP-F | GCTGTGGTGTTTCGGAAT | ChIP-qPCR primer for ohrR |
| ohrR-ChIP-R | TCGGTAAGTACTGTCGTTCATG | ChIP-qPCR primer for ohrR |
| pigA-ChIP-F | GTTACTGGTAACTGGAAAGCTATT | ChIP-qPCR primer for pigA |
| pigA-ChIP-R | GACAGGTTAAAATCCATAAAACAC | ChIP-qPCR primer for pigA |
| Oligonucleotide | ||
| 3×FLAG tag | GGAGGTGGCGATTACAAGGATGACGACGATAAGGACTATAAGGACGATGATGACAAGGACTACAAAGATGATGACGATAAA | Template for cloning 3×FLAG tag sequence |
5′-Phosphorylated primer.
5′-FAM-labeled primer.
Transposon mutagenesis and mapping the transposon insertion sites.
The transposon mutagenesis and the identification of transposon insertion sites in Serratia sp. ATCC 39006 were performed as described previously (36).
Gene manipulation and complementation.
To perform in-frame deletion of ohrR, upstream homologous arm (positions −859 to +150 relative to the start codon) and downstream homologous arm (positions +439 to +1390 relative to the start codon) were amplified by primers ohrR-5F/ohrR-5R and ohrR-3F/ohrR-3R, respectively. The homologous arms were then digested and ligated to pMMB1 vector (36), resulting in the ohrR in-frame deletion vector, pMMB1-DohrR. The ohr in-frame deletion vector, pMMB1-Dohr, and the ohrR-ohr operon in-frame deletion vector, pMMB1-DohrR-ohr, were constructed using the same method. After verification by sequencing, the deletion vectors were transferred from E. coli S17-1(λpir) to Serratia sp. ATCC 39006 by conjugation. The procedures for plasmid construction and mutant screening were performed as described previously (36) and are shown in Fig. S1.
To complement ohrR, the ribosome-binding-site-containing open reading frame (ORF) of the ohrR gene was amplified using primers ohrR-CF/ohrR-CR. The resulting DNA fragment was digested and ligated to the complementary vector pBBR1MCS2-PaacC1 to obtain the ohrR-complementary vector pBBR1MCS2-CohrR. The vector was then conjugated into ΔohrR, resulting in the complementation strain, CohrR.
To construct the C-terminal 3×FLAG-tagged OhrR strain WT/ohrR-FLAG, an 827-bp fragment containing the homologous arms flanking the 3′-terminal region of ohrR gene was amplified using primers ohrR-FlagConF and ohrR-FlagConR. The yielding fragment was then ligated to the plasmid pMMB1, and the resulting plasmid was used as a template to amplify the fragment F1 using the primers ohrR-FlagKinF and ohrR-FlagKinR. The oligonucleotide encoding 3×FLAG tag (DYKDDDDKGDYKDDDDKIDYKDDDDK) (shown in Table 3) was amplified using the primers Flag-F/Flag-R, resulting in fragment F2. Then, the fragments F1 and F2 were digested with restriction enzyme SpeI and ligated to acquire the 3×FLAG tag knock-in vector, pMMB1-ohrR-FlagKin. The plasmid was conjugated into WT, resulting in the C-terminal 3×FLAG-tagged OhrR strain WT/ohrR-FLAG.
To replace the native promoter (Ppig) of pig operon with the constitutive promoter PaacC1, we conjugated the previously constructed Ppig-replacement plasmid (36), pMMB1-PaacC1-pig, into ΔohrR to obtain the ΔohrR/PaacC1-pig strain.
Prodigiosin yield analysis.
The shake flask fermentation and prodigiosin production analysis were slightly modified from previous studies (36, 43). LB broth was used as seed medium and fermentation medium. Fermentation was performed in a 100-mL flask containing 25 mL LB medium at 30°C, 200 rpm for 12 h. The Serratia sp. ATCC 39006 cells were collected from 3.6 mL fermentation broth and added with 1 mL acidified methanol (pH 3.0). After centrifugation at 12,000 rpm for 2 min, the prodigiosin yield was determined by measuring the absorbance at 534 nm of the supernatant.
Drop plate assay.
The drop plate assay was performed to analyze the resistance of Serratia sp. ATCC 39006 to organic hydroperoxide. Overnight cultured LB seed broth of Serratia sp. ATCC 39006 was adjusted to an OD600 of ∼0.8. After gradient dilution, the cell suspension was dropped onto LB agar plates with or without tert-butyl hydroperoxide (tBHP) and cultured at 30°C for 48 h. The cell growth was observed to analyze the resistance to tBHP.
qRT-PCR.
The overnight cultured seed broth was inoculated into the fermentation medium with an inoculum size of 1%. After culture at 30°C, 200 rpm for 12 h, the cells were collected by centrifugation at 8,000 rpm and 4°C for RNA extraction. To analyze the effect of tBHP on the transcription of ohr gene, we treated the cells cultured in fermentation medium for 12 h with 5 mM tBHP for 5, 10, 20, and 30 min or treated them with different concentration of tBHP (1, 2, and 5 mM) for 30 min before RNA extraction. The RNA isolation, reverse transcription, and qRT-PCR experiments were performed as described previously (36). The 16S rRNA was used an internal control gene.
Purification of His6-tagged OhrR protein.
The ohrR ORF amplified with the primers ohrR-EF/ohrR-ER was inserted into pET-28a(+), resulting in the expression vector pET28-ohrR. The vector was then transformed into E. coli BL21(DE3) to obtain the N-terminal His6-tagged OhrR expressing strain, BL21(DE3)/pET28-ohrR. The expressing strain was cultured at 37°C, 220 rpm until OD600 of 0.6 to 0.8, and the induction was initiated by adding 0.4 mM IPTG (isopropyl-β-d-thiogalactopyranoside). Then, the cells were cultured overnight at 16°C, 180 rpm before harvest. The collected cells were resuspended in phosphate-buffered saline (PBS) containing 1× protease inhibitor (catalog no. CW2200, CoWin Biosciences, China). After ultrasonication treatment with a sonicator (JY88-IIN, Ningbo Scientz Biotechnology Co., Ltd., China), the supernatant containing His6-tagged OhrR was collected and loaded into Ni-agarose resin (catalog no. CW0010, CoWin Biosciences) for affinity chromatography purification. The purified protein was dialyzed at 4°C in the dialysis buffer, which contains 20 mM HEPES (pH 7.6), 1 mM EDTA, 10 mM (NH4)2SO4, 1 mM dithiothreitol (DTT), 30 mM KCl, 0.2% Tween 20, and 20% glycerol. The protein concentration was measured using Quick Start Bradford protein 1× dye reagent (Bio-Rad).
EMSA.
EMSA was performed as described in a previous study using a 2nd generation DIG gel shift kit (catalog no. 03353591910, Roche) (36). DNA probes were amplified and digoxigenin (DIG) labeled according to the manufacturer’s protocol. The binding reaction mixture containing DNA probe, OhrR protein, binding buffer, and poly d(I-C) was incubated at 30°C for 40 min. When necessary, unlabeled specific/nonspecific competitor and tBHP were added. Then, the mixtures were loaded onto 5% native polyacrylamide gels, and the electrophoresis was performed at a constant 100 V for 120 min. The DNA bands were transferred to Roche positive charged nylon membrane (catalog no. 11417240001) at a constant 400 mA for 30 min. Anti-DIG antibody was used to interact with the DIG-labeled DNA probe, and the signal was recorded using the chemiluminescence method. The site-mutated Ppig-mut probe was amplified using the primers pigA-PF/pigA-PR with plasmid pMV-PigA-M as the template.
ChIP-qPCR.
ChIP-qPCR was performed as described previously with minor modifications (36). Anti-FLAG gel (Bimake) was balanced with the buffer containing HEPES 50 mM, NaCl 137 mM, EDTA 1 mM, Tris-HCl 10 mM, bovine serum albumin (BSA) 0.5 mg/mL, and salmon sperm DNA 0.1 mg/mL (pH 8.0). Overnight cultured seed broth was inoculated into the fermentation medium with an inoculum size of 1%. After 12 h of fermentation, formaldehyde was added to a final concentration of 1% to perform cross-linking reaction. After shaking at 30°C, 150 rpm for 30 min, we terminated the cross-linking by adding 0.1 M glycine and gently shaking for 5 min. The cells were collected and washed twice with ice-cold PBS, and 0.5 g washed cells were resuspended in 3 mL ice-cold lysis buffer (HEPES 50 mM, NaCl 137 mM, EDTA 1 mM, Tris-HCl 10 mM [pH 8.0]; protease inhibitor cocktail was added before sonication). Sonication was performed to break the genomic DNA into 250- to 500-bp fragments. After centrifugation at 4°C, the supernatant was collected and the protein concentration was adjusted to 4 mg/mL. A 10-μL aliquot of supernatant was used as an input sample, 1 mL supernatant was added with 40 μL balanced anti-FLAG gel, and the sample was rotated gently at 4°C for 3 h. After collection by centrifugation at 4°C, 3,000 rpm for 1 min, the gel was washed by buffer I (HEPES 50 mM, NaCl 500 mM, EDTA 1 mM, Tris-HCl 10 mM [pH 8.0]), buffer II (LiCl 250 mM, NP-40 1%, deoxycholate 1%, EDTA 1 mM, Tris-HCl 10 mM [pH 8.0]), and buffer III (EDTA 1 mM, Tris-HCl 50 mM [pH 8.0]). The immune complexes were then eluted from the gel by fresh elution buffer (SDS 1%, NaHCO3 0.1 M). Then, 1 mL eluate was supplied by 40 μL 5 M NaCl and incubated at 65°C for 3 h to acquire ChIP sample. The DNA in the input sample and ChIP sample were precipitated using ethanol and resuspended in 200 μL double-distilled water (ddH2O) to acquire the template for qPCR analysis. To analyze the effect of tBHP on the enrichment of target DNA by OhrR, the cells cultured in fermentation medium for 12 h were treated with 5 mM tBHP for 30 min before formaldehyde cross-linking.
DNase I footprinting assay.
DNase I footprinting was performed as described in a previous study with minor modifications (44). 6-Carboxyfluorescein (FAM)-labeled DNA fragments containing putative OhrR binding sites were amplified and purified to acquire the DNase I footprinting probes. The reaction mixture containing 300 ng probe, binding buffer (the 2nd generation DIG gel shift kit, Roche), and various concentrations of protein was incubated at 30°C for 30 min. After DNase I treatment, the digested probe was purified and sequenced. Data analysis was performed using GeneMarker software program (vision 2.2.0).
Western blotting.
The protein samples were prepared as described previously (36). The samples containing equal amount of total protein were loaded to SDS-PAGE and separated at 150 V for 70 min. The separated proteins were electroblotted to polyvinylidene difluoride (PVDF) membrane (Roche) at 400 mA for 90 min. After blocking with skim milk (5% in Tris-buffered saline with Tween 20 [TBST]), the membrane was incubated with primary antibody (anti-FLAG tag mouse monoclonal antibody, catalog no. CW0287, CoWin Biosciences, dilution: 1:10,000), followed by incubation with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (catalog no. CW0102, CoWin Biosciences, dilution: 1:10,000). The target protein was then detected using eECL Western blotting kit (catalog no. CW0049, CoWin Biosciences).
ACKNOWLEDGMENTS
This research was funded by National Natural Science Foundation of China (31800020, 31970036, 31900401), Natural Science Foundation of Jiangsu Province (BK20181009, BK20171163, BK20210920), Natural Science Foundation of Xuzhou City (KC19196), Six Talent Peaks Project of Jiangsu Province (JNHB-103), Natural Science Foundation of the Jiangsu Higher Education Institutions of China (20KJB180001, 20KJA180007), and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Footnotes
Supplemental material is available online only.
Contributor Information
Cong Liu, Email: liucong0426@126.com.
Arpita Bose, Washington University in St. Louis.
REFERENCES
- 1.Daniel-Ivad M, Pimentel-Elardo S, Nodwell JR. 2018. Control of specialized metabolism by signaling and transcriptional regulation: opportunities for new platforms for drug discovery? Annu Rev Microbiol 72:25–48. 10.1146/annurev-micro-022618-042458. [DOI] [PubMed] [Google Scholar]
- 2.Fang FC. 2011. Antimicrobial actions of reactive oxygen species. mBio 2:e00141-11. 10.1128/mBio.00141-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayala A, Munoz MF, Arguelles S. 2014. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 2014:360438. 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gęgotek A, Skrzydlewska E. 2019. Biological effect of protein modifications by lipid peroxidation products. Chem Phys Lipids 221:46–52. 10.1016/j.chemphyslip.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Cussiol JR, Alves SV, de Oliveira MA, Netto LE. 2003. Organic hydroperoxide resistance gene encodes a thiol-dependent peroxidase. J Biol Chem 278:11570–11578. 10.1074/jbc.M300252200. [DOI] [PubMed] [Google Scholar]
- 6.Mongkolsuk S, Praituan W, Loprasert S, Fuangthong M, Chamnongpol S. 1998. Identification and characterization of a new organic hydroperoxide resistance (ohr) gene with a novel pattern of oxidative stress regulation from Xanthomonas campestris pv. phaseoli. J Bacteriol 180:2636–2643. 10.1128/JB.180.10.2636-2643.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deochand DK, Grove A. 2017. MarR family transcription factors: dynamic variations on a common scaffold. Crit Rev Biochem Mol Biol 52:595–613. 10.1080/10409238.2017.1344612. [DOI] [PubMed] [Google Scholar]
- 8.Dubbs JM, Mongkolsuk S. 2012. Peroxide-sensing transcriptional regulators in bacteria. J Bacteriol 194:5495–5503. 10.1128/JB.00304-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caswell CC, Baumgartner JE, Martin DW, Roop RM, II.. 2012. Characterization of the organic hydroperoxide resistance system of Brucella abortus 2308. J Bacteriol 194:5065–5072. 10.1128/JB.00873-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen NX, Chu YJ, Ni B, Hsu P, Wong HC. 2021. Organic hydroperoxide resistance gene ohr (VPA1681) confers protection against organic peroxides in the presence of alkyl hydroperoxide reductase genes in Vibrio parahaemolyticus. Appl Environ Microbiol 87:e00861-21. 10.1128/AEM.00861-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen SJ, Shu HY, Lin GH. 2021. Regulation of tert-butyl hydroperoxide resistance by chromosomal OhrR in A. baumannii ATCC 19606. Microorganisms 9:629. 10.3390/microorganisms9030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chuchue T, Tanboon W, Prapagdee B, Dubbs JM, Vattanaviboon P, Mongkolsuk S. 2006. ohrR and ohr are the primary sensor/regulator and protective genes against organic hydroperoxide stress in Agrobacterium tumefaciens. J Bacteriol 188:842–851. 10.1128/JB.188.3.842-851.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuangthong M, Atichartpongkul S, Mongkolsuk S, Helmann JD. 2001. OhrR is a repressor of ohrA, a key organic hydroperoxide resistance determinant in Bacillus subtilis. J Bacteriol 183:4134–4141. 10.1128/JB.183.14.4134-4141.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mongkolsuk S, Panmanee W, Atichartpongkul S, Vattanaviboon P, Whangsuk W, Fuangthong M, Eiamphungporn W, Sukchawalit R, Utamapongchai S. 2002. The repressor for an organic peroxide-inducible operon is uniquely regulated at multiple levels. Mol Microbiol 44:793–802. 10.1046/j.1365-2958.2002.02919.x. [DOI] [PubMed] [Google Scholar]
- 15.Oh SY, Shin JH, Roe JH. 2007. Dual role of OhrR as a repressor and an activator in response to organic hydroperoxides in Streptomyces coelicolor. J Bacteriol 189:6284–6292. 10.1128/JB.00632-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clair G, Lorphelin A, Armengaud J, Duport C. 2013. OhrRA functions as a redox-responsive system controlling toxinogenesis in Bacillus cereus. J Proteomics 94:527–539. 10.1016/j.jprot.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 17.Gupta A, Pande A, Sabrin A, Thapa SS, Gioe BW, Grove A. 2019. MarR family transcription factors from Burkholderia species: hidden clues to control of virulence-associated genes. Microbiol Mol Biol Rev 83:e00039-18. 10.1128/MMBR.00039-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klomsiri C, Panmanee W, Dharmsthiti S, Vattanaviboon P, Mongkolsuk S. 2005. Novel roles of ohrR-ohr in Xanthomonas sensing, metabolism, and physiological adaptive response to lipid hydroperoxide. J Bacteriol 187:3277–3281. 10.1128/JB.187.9.3277-3281.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Previato-Mello M, Meireles DA, Netto LES, da Silva Neto JF. 2017. Global transcriptional response to organic hydroperoxide and the role of OhrR in the control of virulence traits in Chromobacterium violaceum. Infect Immun 85:e00017-17. 10.1128/IAI.00017-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun M, Lyu M, Wen Y, Song Y, Li J, Chen Z. 2018. Organic peroxide-sensing repressor OhrR regulates organic hydroperoxide stress resistance and avermectin production in Streptomyces avermitilis. Front Microbiol 9:1398. 10.3389/fmicb.2018.01398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.You Z, Zhang S, Liu X, Zhang J, Wang Y, Peng Y, Wu W. 2019. Insights into the anti-infective properties of prodiginines. Appl Microbiol Biotechnol 103:2873–2887. 10.1007/s00253-019-09641-1. [DOI] [PubMed] [Google Scholar]
- 22.Yip CH, Yarkoni O, Ajioka J, Wan KL, Nathan S. 2019. Recent advancements in high-level synthesis of the promising clinical drug, prodigiosin. Appl Microbiol Biotechnol 103:1667–1680. 10.1007/s00253-018-09611-z. [DOI] [PubMed] [Google Scholar]
- 23.Williamson NR, Fineran PC, Leeper FJ, Salmond GP. 2006. The biosynthesis and regulation of bacterial prodiginines. Nat Rev Microbiol 4:887–899. 10.1038/nrmicro1531. [DOI] [PubMed] [Google Scholar]
- 24.Hu DX, Withall DM, Challis GL, Thomson RJ. 2016. Structure, chemical synthesis, and biosynthesis of prodiginine natural products. Chem Rev 116:7818–7853. 10.1021/acs.chemrev.6b00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris AKP, Williamson NR, Slater H, Cox A, Abbasi S, Foulds I, Simonsen HT, Leeper FJ, Salmond GPC. 2004. The Serratia gene cluster encoding biosynthesis of the red antibiotic, prodigiosin, shows species- and strain-dependent genome context variation. Microbiology (Reading) 150:3547–3560. 10.1099/mic.0.27222-0. [DOI] [PubMed] [Google Scholar]
- 26.Thomson NR, Crow MA, McGowan SJ, Cox A, Salmond GP. 2000. Biosynthesis of carbapenem antibiotic and prodigiosin pigment in Serratia is under quorum sensing control. Mol Microbiol 36:539–556. 10.1046/j.1365-2958.2000.01872.x. [DOI] [PubMed] [Google Scholar]
- 27.Williamson NR, Simonsen HT, Ahmed RA, Goldet G, Slater H, Woodley L, Leeper FJ, Salmond GP. 2005. Biosynthesis of the red antibiotic, prodigiosin, in Serratia: identification of a novel 2-methyl-3-n-amyl-pyrrole (MAP) assembly pathway, definition of the terminal condensing enzyme, and implications for undecylprodigiosin biosynthesis in Streptomyces. Mol Microbiol 56:971–989. 10.1111/j.1365-2958.2005.04602.x. [DOI] [PubMed] [Google Scholar]
- 28.Gristwood T, Fineran PC, Everson L, Williamson NR, Salmond GP. 2009. The PhoBR two-component system regulates antibiotic biosynthesis in Serratia in response to phosphate. BMC Microbiol 9:112. 10.1186/1471-2180-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horng YT, Chang KC, Liu YN, Lai HC, Soo PC. 2010. The RssB/RssA two-component system regulates biosynthesis of the tripyrrole antibiotic, prodigiosin, in Serratia marcescens. Int J Med Microbiol 300:304–312. 10.1016/j.ijmm.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Lin CS, Tsai YH, Chang CJ, Tseng SF, Wu TR, Lu CC, Wu TS, Lu JJ, Horng JT, Martel J, Ojcius DM, Lai HC, Young JD. 2016. An iron detection system determines bacterial swarming initiation and biofilm formation. Sci Rep 6:36747. 10.1038/srep36747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fineran PC, Everson L, Slater H, Salmond GPC. 2005. A GntR family transcriptional regulator (PigT) controls gluconate-mediated repression and defines a new, independent pathway for regulation of the tripyrrole antibiotic, prodigiosin, in Serratia. Microbiology (Reading) 151:3833–3845. 10.1099/mic.0.28251-0. [DOI] [PubMed] [Google Scholar]
- 32.Stella NA, Fender JE, Lahr RM, Kalivoda EJ, Shanks RM. 2012. The LysR transcription factor, HexS, is required for glucose inhibition of prodigiosin production by Serratia marcescens. Adv Microbiol 2. 10.4236/aim.2012.24065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalivoda EJ, Stella NA, Aston MA, Fender JE, Thompson PP, Kowalski RP, Shanks RM. 2010. Cyclic AMP negatively regulates prodigiosin production by Serratia marcescens. Res Microbiol 161:158–167. 10.1016/j.resmic.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fineran PC, Slater H, Everson L, Hughes K, Salmond GP. 2005. Biosynthesis of tripyrrole and beta-lactam secondary metabolites in Serratia: integration of quorum sensing with multiple new regulatory components in the control of prodigiosin and carbapenem antibiotic production. Mol Microbiol 56:1495–1517. 10.1111/j.1365-2958.2005.04660.x. [DOI] [PubMed] [Google Scholar]
- 35.Takayama Y, Kato N. 2016. Switch of SpnR function from activating to inhibiting quorum sensing by its exogenous addition. Biochem Biophys Res Commun 477:993–997. 10.1016/j.bbrc.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 36.Sun D, Zhou X, Liu C, Zhu J, Ru Y, Liu W, Liu J. 2021. Fnr negatively regulates prodigiosin synthesis in Serratia sp. ATCC 39006 during aerobic fermentation. Front Microbiol 12:734854. 10.3389/fmicb.2021.734854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panmanee W, Vattanaviboon P, Poole LB, Mongkolsuk S. 2006. Novel organic hydroperoxide-sensing and responding mechanisms for OhrR, a major bacterial sensor and regulator of organic hydroperoxide stress. J Bacteriol 188:1389–1395. 10.1128/JB.188.4.1389-1395.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panmanee W, Vattanaviboon P, Eiamphungporn W, Whangsuk W, Sallabhan R, Mongkolsuk S. 2002. OhrR, a transcription repressor that senses and responds to changes in organic peroxide levels in Xanthomonas campestris pv. phaseoli. Mol Microbiol 45:1647–1654. 10.1046/j.1365-2958.2002.03116.x. [DOI] [PubMed] [Google Scholar]
- 39.Reese MG. 2001. Application of a time-delay neural network to promoter annotation in the Drosophila melanogaster genome. Comput Chem 26:51–56. 10.1016/S0097-8485(01)00099-7. [DOI] [PubMed] [Google Scholar]
- 40.Fuangthong M, Helmann JD. 2002. The OhrR repressor senses organic hydroperoxides by reversible formation of a cysteine-sulfenic acid derivative. Proc Natl Acad Sci USA 99:6690–6695. 10.1073/pnas.102483199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sukchawalit R, Loprasert S, Atichartpongkul S, Mongkolsuk S. 2001. Complex regulation of the organic hydroperoxide resistance gene (ohr) from Xanthomonas involves OhrR, a novel organic peroxide-inducible negative regulator, and posttranscriptional modifications. J Bacteriol 183:4405–4412. 10.1128/JB.183.15.4405-4412.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Atichartpongkul S, Fuangthong M, Vattanaviboon P, Mongkolsuk S. 2010. Analyses of the regulatory mechanism and physiological roles of Pseudomonas aeruginosa OhrR, a transcription regulator and a sensor of organic hydroperoxides. J Bacteriol 192:2093–2101. 10.1128/JB.01510-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slater H, Crow M, Everson L, Salmond GP. 2003. Phosphate availability regulates biosynthesis of two antibiotics, prodigiosin and carbapenem, in Serratia via both quorum-sensing-dependent and -independent pathways. Mol Microbiol 47:303–320. 10.1046/j.1365-2958.2003.03295.x. [DOI] [PubMed] [Google Scholar]
- 44.Liu W, Chen Y, Zhou X, Liu J, Zhu J, Wang S, Liu C, Sun D. 2020. The cyclic AMP receptor protein, Crp, is required for the decolorization of acid yellow 36 in Shewanella putrefaciens CN32. Front Microbiol 11:596372. 10.3389/fmicb.2020.596372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larsen RA, Wilson MM, Guss AM, Metcalf WW. 2002. Genetic analysis of pigment biosynthesis in Xanthobacter autotrophicus Py2 using a new, highly efficient transposon mutagenesis system that is functional in a wide variety of bacteria. Arch Microbiol 178:193–201. 10.1007/s00203-002-0442-2. [DOI] [PubMed] [Google Scholar]
- 46.Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM, II, Peterson KM. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176. 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 and Fig. S1 to S4. Download aem.02041-21-s0001.pdf, PDF file, 3.9 MB (3.9MB, pdf)