Abstract
Scleractinian coral are experiencing global and regional stressors. Microplastics (<5 mm) are an additional stressor that may cause adverse effects on coral. Experiments were conducted to investigate ingestion size limits and retention times of microspheres in a two-day exposure as well as observing growth responses in a 12-week exposure in two Atlantic species, Pseudodiploria clivosa and Acropora cervicornis. In the two-day exposure, P. clivosa ingested a higher number of microspheres ranging in size from 425 μm-2.8 mm than A. cervicornis. Both species egested the majority of microspheres within 48 h of ingestion. In the long-term exposure, calcification and tissue surface area were negatively affected in the treatment group of both species. Exposure also negatively affected buoyant weight in A. cervicornis but not in P. clivosa. The results indicate that microplastics can affect growth responses, yet additional research is warranted to investigate potential synergistic impacts of microplastics and other stressors.
Keywords: Coral, Growth, Ingestion, Microplastic, Retention
Graphical Abstract
1. Introduction
Coral reefs have been heavily impacted by anthropogenic stressors. The condition of reef habitats have declined dramatically in the past few decades due to global stressors such as ocean acidification and increased temperatures (Lesser 1997; Hoegh-Guldberg et al., 2007; Anthony et al., 2008; Eakin et al., 2019) as well as by local stressors such as sedimentation and pollution (Pastorok and Bilyard 1985; Rogers 1990; Lamb et al., 2018). Specifically, plastic pollution may also have detrimental impacts on coral reefs, but potential effects have not been adequately investigated.
The presence of microplastics (MPs) has been documented in nearly every marine environment. Even remote habitats such as the Arctic Ocean and hadal trenches contain MPs (Jamieson et al., 2019; Kanhai et al., 2020; Tekman et al., 2020; Peng et al., 2020). Of the 265 million tons of plastic produced globally in 2010 (Plastic Europe, 2011), upwards of 12.7 million tons entered the world’s oceans (Jambeck et al., 2015). Assuming the same percentage, 4.8% of the 359 million tons produced globally in 2018 (Plastic Europe, 2019) potentially introduced 17.5 million tons into the oceans. Should plastic production and management continue at the “Business as Usual” pace, by 2040 nearly 300 million metric tons of plastic will enter the aquatic environment annually (Lau et al., 2020). The exponential production of plastic (Lau et al., 2020) and the growing pervasiveness of plastics in the marine environment has led to increased research efforts on the potential impacts that plastics may have on marine ecosystems.
Microplastics, small pieces of plastic <5 mm, can originate from various sources including degraded macroplastic, industrial processes, synthetic clothing, tire fibers, and personal care products (GESAMP 2015; Thompson 2015; Boucher and Friot 2017). Once in the marine environment, MPs tend to serve as a surface for microbial community growth as well as attracting pollutants (Teuten et al., 2007; Andrady 2011; Zettler et al., 2013). As a result, the buoyancy of particles decreases and they become more bioavailable to organisms in the environment (Ye and Andrady 1991; Teuten et al., 2007), including benthic organisms such as scleractinian coral.
Scleractinian corals are suspension feeders that ingest plankton from the water column. Whether actively or passively, corals have been shown to ingest microplastics (Hall et al., 2015a; Allen et al., 2017; Hankins et al., 2018; Reichert et al., 2018; Axworthy and Padilla-Gamiño 2019). Daily metabolism requirements rely heavily on photosynthesis from the corals’ symbiotic zooxanthellae (Muscatine and Porter 1977; Porter et al., 1989). Nonetheless, heterotrophic feeding becomes important when light is restricted or during bleaching events when zooxanthellae density is low or absent (Grottoli et al., 2006; Palardy et al., 2008; Anthony et al., 2009; Bessell-Browne et al., 2014). Ingested microplastics could potentially reduce the energy available for calcification, reproduction, and survival by inhibiting ingestion and digestion of food resources. There is also evidence that the physical interaction, that is, mere exposure to, microplastics impair feeding responses (Reichert et al., 2018, 2019; Savinelli et al., 2020) as well as diminish immune system and anti-stress responses (Tang et al., 2018).
Another emerging issue of microplastics is pollutant toxicity. In addition to the polymer composition and potential additives, plastic debris will also absorb contaminants, including metals from the water column (Mato et al., 2001; Ogata et al., 2009; Ashton et al., 2010; Holmes et al., 2012; Van et al., 2012; Rochman et al., 2013a). These contaminated microplastics have been shown to be bioavailable across many genera in the food web (Teuten et al., 2009; Fossi et al., 2012; Besseling et al., 2013; Rochman et al., 2013b; Chua et al., 2014); some plastic additives, such as phthalic acid esters, have been detected in at least 75% of corals sampled in the Maldives (Saliu et al., 2019; Montano et al., 2020). Contaminant uptake by coral has been shown to have adverse effects that include reduced fertilization, metabolism, and photosynthesis (Pait et al., 2007). Additionally, harmful pathogens, such as Vibrio spp., have been found on microplastics (Zettler et al., 2013; Kirstein et al., 2016), potentially making them vectors for infectious coral diseases, such as those caused by Vibrio coralliilyticus (Ben-Haim et al., 2003) and Vibrio shiloi (Kushmaro et al., 1996). Hallofoliculina, a ciliate known to cause skeletal eroding band syndrome/Caribbean ciliate infection (Antonius and Lispcomb, 2000), has also been detected on marine plastic (Goldstein et al., 2014). Whether through ingestion, exposure, or both, microplastics may be impacting coral feeding and health.
Acropora cervicornis and Pseudodiploria clivosa were chosen for study because of their different morphologies. Previous experiments demonstrated that the large-polyp coral, Montastraea cavernosa had a higher ingestion rate of a microplastics (850 μm-2.8 mm) compared to the smaller-polyp Orbicella faveolata (Hankins et al., 2018). Pseudodiploria clivosa is a large-polyp, mounding species commonly found in the Atlantic and Caribbean. Acropora cervicornis is a small-polyp, branching species that was historically a prominent reef-building coral in the Atlantic (Cramer et al., 2020). Over the past decades, A. cervicornis has experienced drastic declines (Aronson and Precht 2001; Jackson et al., 2014) and is a listed threatened species under the U.S. Endangered Species Act of 1973. There are concerted conservation efforts in the Atlantic and Caribbean to ensure the species’ survival (Herlan and Lirman 2008; Hollarsmith et al., 2012; Ware et al., 2020). Although P. clivosa is not listed as threatened, both species are susceptible to coral diseases plaguing the Florida Keys and the Caribbean (Aronson and Precht 2001; Precht et al., 2016; Kramer et al., 2019). The objectives of this study were to expand on previous research (Hankins et al., 2018) to (1) determine ingested size ranges (212 μm–2.8 mm) and retention times of microspheres with additional Atlantic/Caribbean species and (2) investigate long-term effects of exposure to microspheres on two species of scleractinian coral with differing morphologies. Knowledge gained from this study will provide a better understanding of the impacts microplastics may have on scleractinian coral growth and if such impacts are species-specific.
2. Materials and methods
Two laboratory experiments were conducted using the Caribbean, scleractinian coral species, Pseudodiploria clivosa and Acropora cervicornis. Coral were collected from Florida Keys National Marine Sanctuary Coral Nursery Program and Mote Marine Laboratory’s in-situ coral nursery located near Looe Key (Permit number FKNMS-2011–036). Coral were shipped to an indoor coral research facility at the U.S. Environmental Protection Agency’s Gulf Ecosystem Measurement and Modeling Division in Gulf Breeze, Florida. Coral arrived with signs of stress such as mucus production and polyp retraction but otherwise healthy; within 24 h mucus production ceased and polyps expanded. Corals were maintained for at least three months in recirculating culture systems (∼1000 L) prior to moving them to experimental systems for exposure. Both culture and experimental systems were kept at a temperature of 26.0 ± 1.0 °C and salinity of 35.0 ± 0.3 ppt. Other water quality parameters such as pH (YSI® Ecosence pH100), calcium (Salifert®), alkalinity (Salifert®), magnesium (Salifert®), ammonia (HACH®), and nitrate (HACH®) were all measured prior to experimental exposures and either weekly or bi-weekly thereafter to ensure accordance with culture condition parameters (Borneman, 2001; Delbeek and Sprung 2005; Holmes-Farley 2004). Lighting was provided by metal halide lights on a 10.5:13.5 light:dark cycle. Light intensity in culture and experimental systems ranged from 25.0 to 40.0 W m−2 depending on age of the bulbs and placement within the system. In each experiment, at least three parent colonies of P. clivosa were used and cut into 2.5–4 cm2 fragments using a Gryphon Corp. Aquasaw XL (Model C-40). A. cervicornis were cut with the Gryphon saw into ∼3 cm non-branching lengths and were from two genotypes (genotyped in 2008 by Illian Burns and later confirmed by Mote Marine Laboratory in 2017). Fragments were acclimated in the experimental system five days prior to the start of the experiment. During all experiments 100% cotton clothing was worn by laboratory personnel. Previous exposure to microplastics was assumed as corals originated from a natural environment and could have been exposed to microplastics in culture or in their natural environment. Therefore, easily identifiable microplastics were used in the experiments. Commercially available, fluorescent, high-density polyethylene microspheres/microplastics (MPs) were used in the smaller size classes (≤1000 μm) and white, polyethylene MP in the larger (>1.7 mm) size classes (Cospheric®). Polyethylene is a common polymer found in marine sediment and/or surface waters (Ng and Obbard 2006, Teuten et al., 2007; Erni-Cassola et al., 2017, 2019).
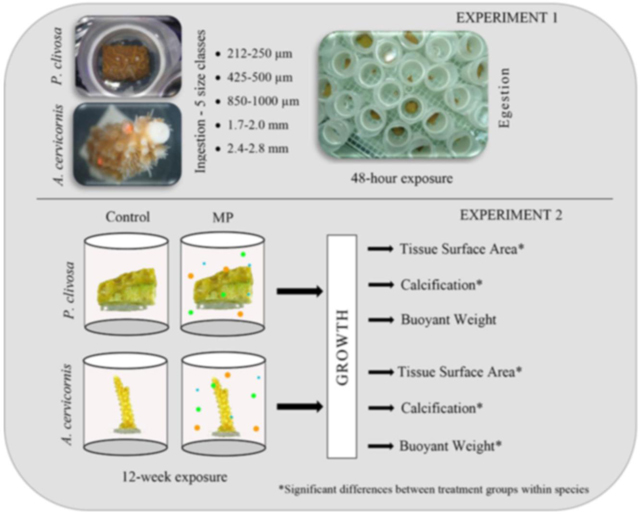
2.1. Experiment 1: ingestion size ranges (425 μm-2.8 mm) and retention times
In the first experiment, ingestion in coral polyps and retention after 48 h was determined for different size microspheres (MP). The experimental system (∼850 L) was identical to the system used by Hankins et al. (2018) whereby a 2” diameter polyvinyl chloride (PVC) pipe with 118 μm Nitex® was used as a chamber for individual fragments. Pseudodiploria clivosa fragments sat freely on egg crate (i.e. louvered ceiling panels) within the chamber; Acropora cervicornis fragments were glued onto a 2.5 cm diameter polycarbonate disc so that the coral fragments would be oriented vertically in the chamber. Experimental chambers (n = 10/species/treatment) contained one coral fragment each and were placed on egg crate. Water flowed continuously (4 L−1 min) in the recirculating system and two pumps were placed in the tank for additional water circulation.
Five size classes of uncured MP were applied to coral fragments (n = 10 per species for each of five size classes). The MP size classes used were: (1) 212–250 μm, (2) 425–500 μm, (3) 850–1000 μm, (4) 1.7–2.0 mm, (5) 2.4–2.8 mm, and (6) a control group not exposed to MPs. Microspheres were not cured in this experiment to ensure that they floated to the surface if not initially ingested or if later egested. To elicit a feeding response, food (5–50 μm Golden Pearls® Brine Shrimp Direct, Ogden, UT) was added to seawater at a concentration of 156 mg/100 mL. The seawater/food mixture was used to apply three MPs of the same size class to a coral fragment’s polyps within the PVC chamber using a 1 mL transfer pipette. Each fragment was visually monitored for 20 min to observe ingestion. Microspheres not ingested after 20 min were removed from the chamber. The control group was fed in the same manner with the Golden Pearls but without MP application. The number of MPs in the water at 48 h after ingestion were counted to determine if the coral polyp had egested the MPs. Data were recorded as the percentage of MPs egested per fragment. A one-way ANOVA was performed (Minitab 16, Inc.) along with Tukey’s post-hoc pairwise comparison between MP size ranges within a species; figures were generated in SigmaPlot 14®.
2.2. Experiment 2: growth effects of coral exposed to microspheres
The second experiment was designed to determine whether microplastic (MP) exposure, and possible ingestion, affected growth in the two coral species over a 12-week exposure period. Three size classes of fluorescent, polyethylene MPs were used in this experiment: 212–250, 425–500, and 850–1000 μm. The density of each of the three size classes was 1.002 ± 0.006 g cc−1. The approximate density of 26.0 °C and 35.0 ppt salinity sea water is 1.025 g cc−1, denser than the MPs.
For MPs to be bioavailable to the coral they must remain in the water column. To maintain neutral buoyancy, MPs can be cured to encourage biofouling which will increase their initial density. A study was conducted to determine if prior curing of MPs affected buoyancy. MPs of each size class were placed in separate 80 μm mesh containers and placed in a culture system to cure for six weeks. The containers consisted of clear polycarbonate tube cut into 2” lengths. Nitex mesh (80 μm) was adhered by silicone to either end of the tube that held the MPs. The number of MPs in suspension (10 mg−1 L per size class) was determined in 100 mL water samples for the following: (1) cured MPs immediately after surface agitation by use of a metal spatula, (2) cured MPs 15 min after agitation, and (3) non-cured MPs immediately after agitation. The water samples were vacuum filtered onto membrane filters (Millipore Isopore® 10.0 μm pore size) whereby the collected MPs were quantified visually with the naked-eye (425–500 μm and 850–1000 μm) or under an Olympus® SZ61TR stereo microscope (212–250 μm).
Prior to initiating the experiment, MPs were cured, enabling them to stay in the water column longer making them more bioavailable to coral. Sixteen 8 L plastic, circular chambers were used as experimental chambers (n = 4 chambers/species/treatment) and contained in a water bath to maintain temperature (26.0 ± 0.5 °C) (Fig. S1). Each chamber contained six fragments of one species (n = 24 fragments/species/treatment), either A. cervicornis or P. clivosa which were randomly assigned to species-specific chambers. All parent colonies in both species that were used for fragmentation were present in both the control and MP treatments. Fragments were glued to acrylic pegs to sit securely in egg crate. Each species consisted of a control treatment and MP exposed treatment resulting in four treatments. Each chamber of the exposed treatment contained all three MP size classes at a dose of 30 mg L−1 (10 mg L−1 per size class). The number of particles for each size class were counted for each size class at 30 mg L−1, either with the naked eye or under an Olympus® SZ61TR stereo microscope. The average number of particles for each size class was 11,213 for 212–250 μm, 215 for 425–500 μm, and 24 for the 850–1000 μm. To ensure consistent bioavailability of the microspheres throughout the experiment, chambers were drained, cleaned, and re-dosed every 3–4 weeks. The curing process of the MPs were staggered to ensure six weeks of curing for every dose.
The experimental setup was modeled after Hankins et al. (2018), however, a flow-through design had to be implemented as preliminary observations in static water showed that A. cervicornis polyp extension was reduced after approximately two weeks and tissue loss ensued after three weeks. For flow-through, each chamber was outfitted with 200 μm Nitex® mesh over the drain to prevent microspheres from escaping. A refugium, i.e. sump, supplied system water to one of four headboxes (Fig. S1). Each headbox had a corresponding manifold that contained six ports that supplied water to the chambers, each of which had a needle valve near the bottom to adjust water flow. Each chamber had a pump (Hydor® Koralia Nano 240) that sat in the center of the egg crate directing water downwards. Surface water within the chambers was agitated once a day to mimic wave action to re-suspend MPs that may have floated to the water surface.
Corals were fed two-three times a week whether or not calcification was measured (water flow was turned off for two days when measuring calcification, see section 2.2.2). Each chamber was fed 10 mL from a mixture of Golden Pearls® (Brine Shrimp Direct, Ogden, UT) (0.256 g 5–50 μm, 0.338 g 50–100 μm, and 0.296 g 100–200 μm) coral food in 260 mL seawater.
2.2.1. Tissue surface area
Prior to exposure, each coral fragment was photographed in fixed positions on a rotating camera stand (Fournie et al., 2012; Enzor et al., 2018; Vivian et al., 2019). Briefly, a mask of tissue area was generated in image processing software (Adobe® Photoshop® CS3 version 10.0, ©1990–2007 Adobe® Systems Inc.). The mask was then exported to ImageJ software (ImageJ 1.38x, Wayne Rasband, National Institutes of Health, USA; http://rsb.info.nih.gov/ij/; public domain) to determine surface area values. Tissue surface area (TSA) was measured for all fragments at week 0, 3, 6, 9, and 12 and converted to three-dimensional (3D) surface area (Vivian et al., 2019). The difference in the 3D surface area was determined for the overall change in the 12-week experiment. Data were tested for normality using Anderson -Darling test and difference in treatments per species was analyzed using a one-way ANOVA (Minitab 19, Inc.); figures generated in SigmaPlot 14®.
2.2.2. Calcification
Total alkalinity was measured during the acclimation period to ensure uniformity across all chambers. After acclimation, total alkalinity was measured every 2–3 weeks by using open celled titration (Metrohm Titrando 905) and was used to estimate/calculate calcification based off the alkalinity anomaly principle, which assumes that for every 1 mol of calcium carbonate precipitated, total alkalinity decreases by 2 mol (Kinsey 1978). This method is ideal as a non-destructive protocol to measure short term calcification (Smith and Kinsey 1978; Chisholm and Gattuso 1991). Water from each chamber was collected (time point 1) and water flow was immediately turned off. The chambers remained static for two days but with water within the chamber still circulating. Prior to turning the water flow back on, water samples were collected (time point 2). Alkalinity was measured at each time point to determine calcification by the following equation:
Where, AT is total alkalinity (μmol) and G is calcification, i.e. calcium carbonate precipitated (μmol). Calcification data were tested for normality using Anderson -Darling test and analyzed using a two-factor ANOVA (Minitab 19, Inc.); figures generated in SigmaPlot 14®.
2.3. Buoyant weight
The buoyant weight technique was used to determine the change in weight of coral over the course of the 12-week exposure (Jokiel et al., 1978). This technique was modified using recent advances in balance technology which allowed for a simplified and more time efficient procedure. Coral fragments were individually weighed using a Mettler Toledo® MS403S New Classic MF balance outfitted with a density determination kit (Mettler Toledo® MS-DNY-43 (Fig. S2). The salinity was measured from the experimental system. The temperature was measured by a precision thermometer in the balance’s glass beaker. Density of the sea water was calculated from these salinity and temperature measurements. Seawater density was then entered into the balance which has a central processing unit. By slightly altering the manufacturer’s operating procedure, the balance converted weight of coral in seawater to weight in air by using Archimedes Principle. Prior to measurements, the balance and procedure where verified by measuring validation weights. Buoyant weight was measured at week 0, 3, 6, 9, and 12. The overall change in weight was determined for the 12-week experiment. Data were tested for normality using Anderson -Darling test and analyzed using a one-factor analysis of variance (ANOVA) (Minitab 19, Inc.); figures generated in SigmaPlot 14®.
3. Results
3.1. Experiment 1: determination of ingestion size ranges (425 μm-2.8 mm) and retention times
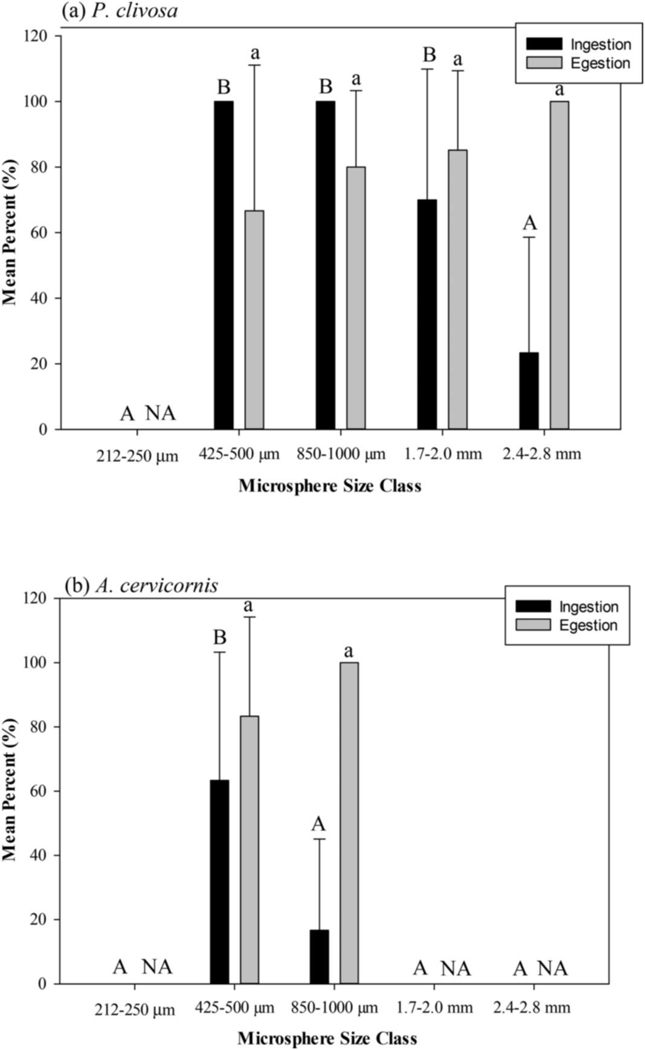
Pseudodiploria clivosa did not ingest any MPs in the 212–250 μm size class. The species did ingest 100% of MPs offered in the 425–500 and 850–1000 μm size classes and a mean of 70.0% (SD = 39.9) and 23.3% (SD = 35.3) for the 1.7–2.0 and 2.4–2.8 mm size classes, respectively, with a significant difference between size classes (ANOVA, df = 4, F = 36.25, p = 0.000). Ingestion of the 212–250 μm and 2.4–2.8 mm size classes were significantly different from the other size classes (Fig. 1; Tukey’s post-hoc p < 0.05). In addition to the 212–250 μm size class, Acropora cervicornis did not ingest either the 1.7–2.0 or the 2.4–2.8 mm size classes, however, it is important to note observations for lack of ingestion. In both P. clivosa and A. cervicornis, the addition of the 212–250 μm size MPs did not elicit a feeding response, i.e. tentacles did not actively grab onto MPs and bring to mouth. In A. cervicornis, a feeding response was observed in 1.7–2.0 and 2.4–2.8 mm size classes, although it appeared that the coral was not physically able to get MPs into the polyps’ mouths. A. cervicornis did ingest the 425–500 μm (mean 63.3%, SD = 39.9) and the 850–1000 μm (mean 16.7%, SD = 28.3) size classes, respectively. A significant difference was observed among the A. cervicornis treatments (ANOVA, df = 4, F = 15.70, p = 0.000), with only the 425–500 μm having a significantly higher ingestion than the 850–1000 μm size class (Fig. 1; Tukey’s post-hoc p < 0.05). P. clivosa and A. cervicornis egested at least 79% of all ingested MPs; P. clivosa 79.80% (SD = 31.11), A. cervicornis 87.88% (SD = 26.97). There was no significant difference in egestion rates among the size classes in either species (P. clivosa: ANOVA, df = 3, F = 1.28. p = 0.300, A. cervicornis, ANOVA, df = 1, F = 0.82, p = 0.389) (Fig. 1).
Fig. 1.
Mean percent with standard deviation of ingested and egested microspheres in (a) Pseudodiploria clivosa and (b) Acropora cervicornis. Capital letters indicate significant difference between ingested size classes (Tukey’s post-hoc). Lower case letters indicate significant difference between egested size classes (Tukey’s post-hoc). NA = not applicable. Note: mechanism for lack of ingestion for A. cervicornis not the same for all size classes, as described in results.
3.2. Experiment 2: growth effects of coral exposed to microspheres
In the MP curing study, the average number of particles of cured MPs in suspension immediately following agitation was nearly double (100.2 particles 100 mL−1) the number of non-cured MP (53.9 particles 100 mL−1). Fifteen minutes after agitation, the number of cured MPs in suspension (56.7 particles 100 mL1−) decreased to levels near the numbers of non-cured MPs immediately after suspension (Table S1). Those MPs not in suspension in all treatments floated to the water surface.
3.2.1. Tissue surface area
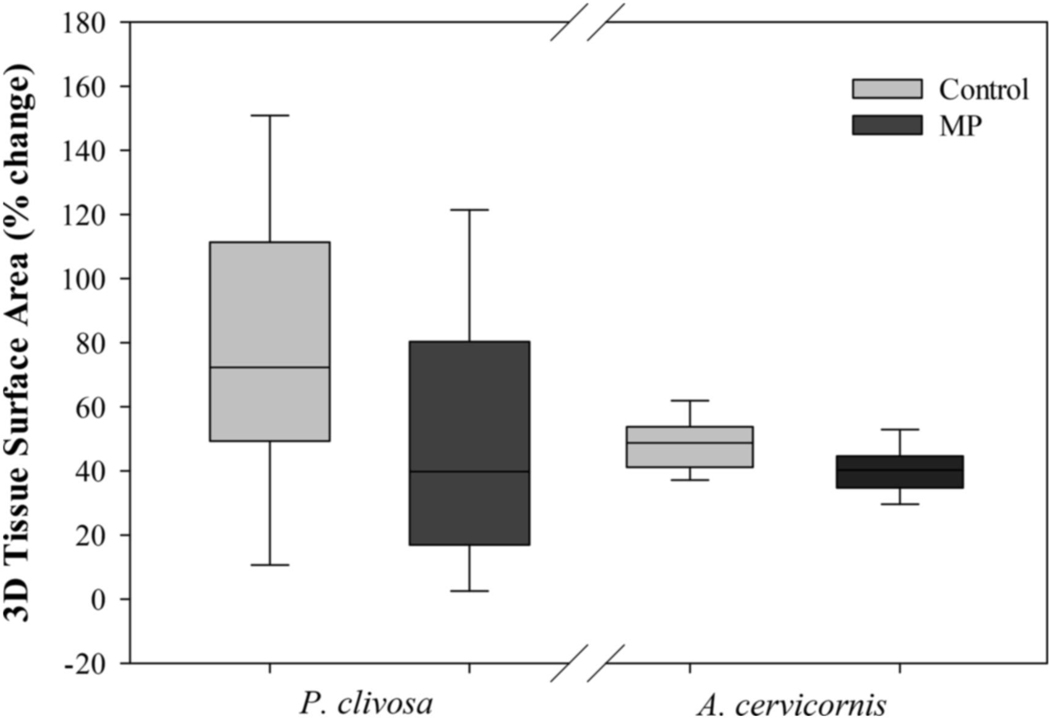
Measurements were observed at five time points to establish change of four time periods. Three-dimensional tissue surface area (TSA) measures over the four time periods were not normally distributed in P. clivosa. As there is no comparable non-parametric test for a two-factor ANOVA, the percent change in TSA over the 12-week exposure was used for statistical analysis for both P. clivosa and A. cervicornis. Mean TSA of P. clivosa increased 46.94 and 35.95% for the control and MP treatments, respectively, in the first three weeks of the experiment. Throughout the remainder of the experiment, TSA did not change more that 11% between time points (Fig. S3). Over the 12-week exposure, P. clivosa TSA increased in both control and MP treatments with the mean increasing 79.20% (SD = 45.48) and 48.07% (SD = 42.95), respectively (Fig. 2). There was a significant difference in TSA between the treatments measured at the end of the study (ANOVA, df = 1, F = 5.95, p = 0.019) with the MP treatment having less tissue growth than the control treatment. Likewise, the mean percent change of TSA for A. cervicornis was higher in the control treatment (48.83%, SD = 9.12)) than the MP treatment (40.32%, SD = 7.98) resulting in a significant difference (ANOVA, df = 1, F = 11.85, p = 0.001) (Fig. 2).
Fig. 2.
Mean percent change of tissue surface area in Pseudodiploria clivosa and Acropora cervicornis over the 12-week exposure.
3.2.2. Calcification
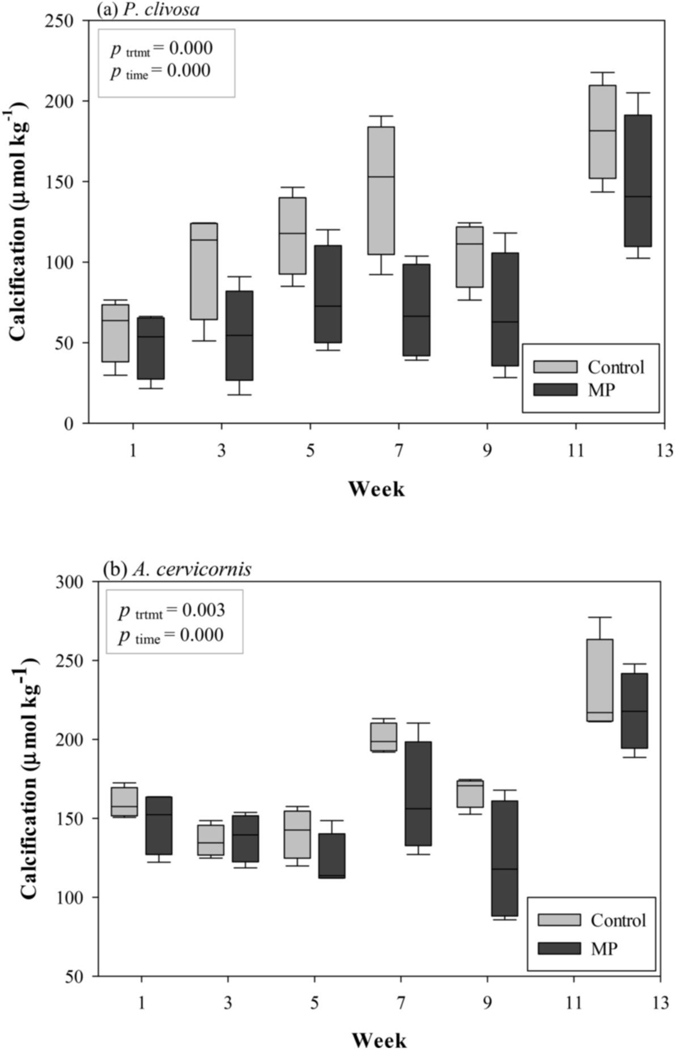
No significant differences in calcification were observed during acclimation (week 0) between the designated control and microplastic treatments in either species (ANOVA, P. clivosa, df = 1, F = 0.00, p > 0.05; A. cervicornis, df = 1, F = 0.15, p > 0.05). Mean calcification across both treatments over the 12-week exposure was 97.91 μmol kg−1 (SD = 49.74) and 162.04 μmol kg−1 (SD = 40.11) in P. clivosa and A. cervicornis, respectively. Mean calcification in the P. clivosa control treatment were higher than the MP treatment weeks 1–12. Mean calcification in the A. cervicornis control treatment was higher than the MP treatment in all weeks except week 3 (Fig. 3). The two-factor ANOVA revealed significant effects (p < 0.05) at both main levels, treatment and time, but no interaction effects for either P. clivosa or A. cervicornis (Table S2).
Fig. 3.
Mean calcification of treatment chambers for (a) Pseudodiploria clivosa and (b) Acropora cervircornis.
3.2.3. Buoyant weight
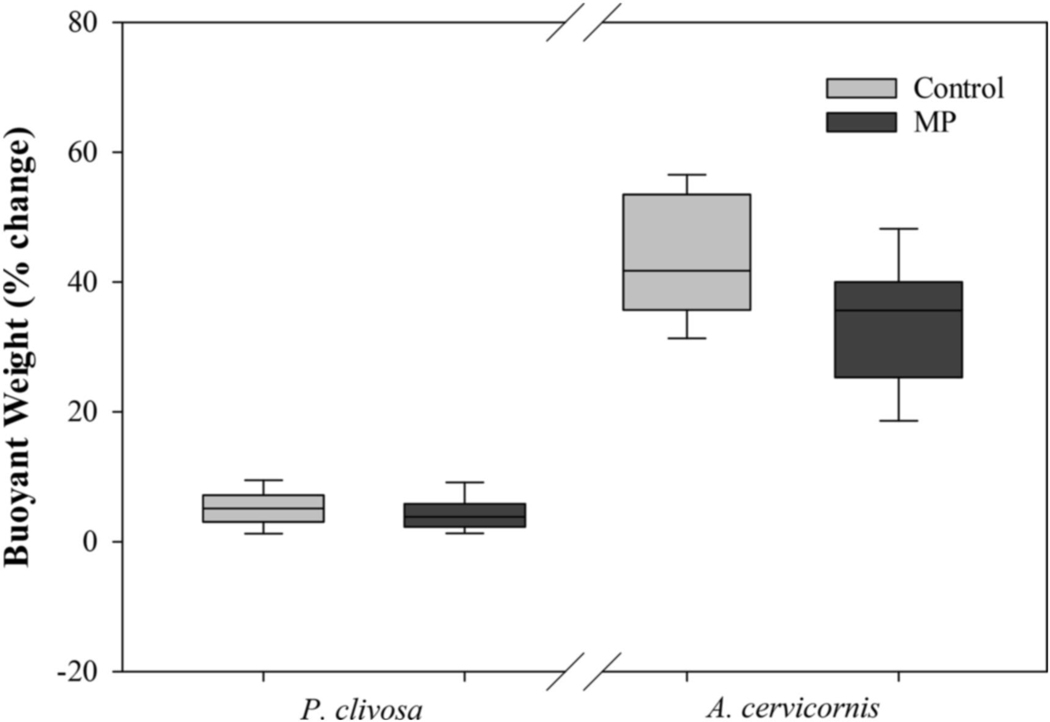
Buoyant weight was measured at five time points to establish change over four time periods. However, buoyant weight data from the four time periods was not normally distributed in P. clivosa or A. cervicornis. As there is no comparable non-parametric test for a two-factor ANOVA, the change in buoyant weight over the 12-week exposure was used for statistical analysis for both P. clivosa and A. cervicornis. Buoyant weight increased in both the control and MP treatments in both species. Mean percent change in buoyant weight of both P. clivosa and A. cervicornis was higher in the control treatments than the MP treatments (Fig. 4). However, there was no significance difference between treatments in P. clivosa (ANOVA, df = 1, F = 0.90, p = 0.348). Buoyant weight in A. cervicornis was significantly higher in the control treatment than the MP treatment (ANOVA, df = 1, F = 7074, p = 0.01).
Fig. 4.
Mean percent change of buoyant weight of Pseudodiploria clivosa and Acropora. cervicornis over the 12-week exposure.
4. Discussion
The results of this study contribute to the growing literature to characterize adverse effects of plastics on scleractinian coral (Richards and Beger 2011, Lamb et al., 2018; Reichert et al., 2018, 2019, Tang et al., 2018). Specifically, this study investigated the ingestion and retention rates in a two-day exposure to microplastics, as well as examining the 12-week growth responses in two Atlantic species of coral, the mounding species Pseudodiploria clivosa and the branching species Acropora cervicornis. Observations indicated that P. clivosa ingested a large size range of MPs, whereas A. cervicornis ingested a narrower range. As species in the laboratory have been acclimated to feeding during the day, these observations are expected to be similar to nighttime observations when reef corals normally feed. Both species egested greater than 79% of ingested MPs within 48 h. Furthermore, both P. clivosa and A. cervicornis exhibited reduced calcification and tissue surface area in the 12-week exposure to MPs. Buoyant weight was affected in A. cervicornis but not in P. clivosa.
In the two-day experiment, P. clivosa and A. cervicornis displayed differences in ingestion of microsphere size classes. Neither species exhibited a feeding response to the 212–250 μm size class. Feeding responses (e.g. active ingestion) were defined by tentacle capture of MP, delivery to mouth, and enveloping the particle. The same response was found in Montastraea cavernosa and Orbicella faveolata (Hankins et al., 2018), suggesting that the minimal size microplastic that triggers a feeding response by scleractinian coral is between 212 and 500 μm and is not species specific. Although not actively ingested, small or light MPs may inadvertently be consumed (Hankins et al., 2018; Axworthy and Padilla-Gamiño, 2019) with larger prey as coral are suspension feeders. P. clivosa ingested MPs from all remaining size classes ranging from 425 μm to 2.8 mm. Coral fragments ingested 100% of MPs administered at the 425–500 and the 850–1000 μm size classes after which ingestion decreased with increasing microsphere size. As P. clivosa ingested MPs at the largest size class (2.4–2.8 mm), a maximum MP capable of being ingested by P. clivosa was not established. However, A. cervicornis ingested the 425–500 and the 850–1000 μm size classes but not the 212–250 μm, 1.4–1.7 mm or the 2.4–2.8 mm size classes indicating that the maximum MP size A. cervicornis may ingest is between 850 μm and 1.4 mm. A. cervicornis did not ingest the 212–250 μm size class because those MPs did not induce a feeding response. The same is not true for the larger size classes, A. cervicornis brought the MPs to the mouth, however, within minutes the microplastic was released. Similar blockages have been seen in the cold-water coral Lophelia pertusa where macroplastic pieces acted as obstacles for zooplankton ingestion by the coral (Chapron et al., 2018). It is unknown if short-term blockage from MPs, as seen in A. cervicornis, would have detrimental impacts of zooplankton ingestion.
The results of experiment two demonstrated the adverse effects microplastics can have on coral growth, however, growth strategies must be taken into consideration when interpreting results. The mean percent change of tissue surface area in P. clivosa over the course of the experiment was influenced by the tissue growth in the first three weeks of the experiment. The cutting of the coral fragments may be the cause of variation in tissue growth between time points. Since the fragments were cut into squares exposing skeleton, each of those four sides created a clean, bare surface resulting in fast tissue generation in the first three weeks of exposure in both treatments. This process is similar to observations made whereby tissue regeneration dramatically declined over time in coral inflicted with experimental lesions (Meesters et al., 1997; Cróquer et al., 2002; Sabine et al., 2015). Such surfaces were not available for A. cervicornis as the cut surface was glued to a base, which is reflected in the narrow range of 7.50–12.46% tissue surface area change between time points. Environmental conditions have been shown to slow tissue regeneration (Hall et al., 2015b; Sabine et al., 2015) and, although not specifically addressed in the scope of this study, the reduced growth of P. clivosa tissue in the MP exposure may have been influenced in the first weeks after cutting suggesting that coral may be more susceptible to effects of microplastic exposure proceeding natural fragmentation events such as hurricanes or storms.
Additionally, the effects of calcification over time in this study must be approached with caution. Alkalinity is a measure of bicarbonate in the water that is available for uptake by coral to convert into calcium carbonate (Borneman 2001; Delbeek and Sprung 2005); higher alkalinity levels, within acceptable culture ranges, provide coral a greater potential for calcification. Time point 1 titrations for total alkalinity measurements ranged from 1958.87 to 3278.18 μmol kg−1 throughout the experiment which is close to the 2100–2700 μmol kg−1 range found in natural reef habitats (Watanabe et al., 2006; Yates et al., 2007; Lantz et al., 2013). The variances in calcification found across time for both P. clivosa and A. cervicornis, regardless of treatment were likely dependent on the available bicarbonate in the experimental system and why it is important that comparisons are made only between the differences of time points 1 and 2.
Though water chemistry may affect calcification over time, the effects of calcification across treatment groups were less confounding. There were calcification differences between the control and MP treatments in P. clivosa and A. cervicornis over the course of the study. At every week measured, the mean calcification was lower in the MP treatments than the controls; the only exception was at week 3 when the mean calcification in A. cervicornis was 2.27 μmol kg−1 higher than the control. These results are similar to other studies showing that chronic exposure to microplastics can affect coral growth. In the cold-water coral, Lophelia pertusa, calcification was reduced when exposed to 500 μm polyethylene microspheres at a concentration of 350 spheres L−1 (Chapron et al., 2018). These results were confirmed by Mouchi et al. (2019) who observed L. pertusa to have reduced septal growth. Reichert et al. (2019) showed that the hermatypic coral, Heliopora coerulea had reduced calcification rates when exposed to irregular shaped polyethylene microplastics ranging in size from 65 to 410 μm with a concentration of 2.5 mg L−1.
The significant difference of buoyant weight between control and MP treatments in A. cervicornis but not P. clivosa may be explained by the growth rate of each species. As indicated by the results of this study, mean A. cervicornis calcification over the course of the experiment was markedly higher than the mean calcification of P. clivosa. The lack of response in the P. clivosa in the MP treatment may be due to the sensitivities of the buoyant weight technique needing an extended observation period (Schoepf et al., 2017). It seems reasonable that the slower growing species may take longer to exhibit significant differences in weight between treatments.
Overall, the long-term exposure of coral to the microspheres displayed adverse effects on growth in both Pseudodiploria clivosa and Acropora cervicornis. Results of this study showed that P. clivosa has a higher rate of ingestion of microplastics than A. cervicornis. Conversely, the branching morphology of A. cervicornis had a higher probability of coral-microplastic interactions. Observations during experiment two indicated that after water agitation, numerous cured MPs adhered to the surface of A. cervicornis, whereas, P. clivosa had fewer particles on their surface These interactions result in adhesion of MP to the coral surface which can result in significant removal of MP from the water column compared to ingestion alone (Martin et al., 2019; Corona et al., 2020). While A. cervicornis may not ingest as many microplastics as P. clivosa, the branching species may use a substantial portion their daily energy budget to remove MPs from their surface. Not only is energy expenditure a concern during egestion and/or plastic removal, but microplastics are considered a contaminant of emerging concern due to the introduction of pollutants and their potential toxicity that can effect coral survival, reproduction, growth and development (Allmand et al., 1998; Negri and Heyward 2000; Negri et al., 2002; Markey et al., 2007; Bielmyer et al., 2010; Schwarz et al., 2013; Renegar et al., 2016; Flores et al., 2020).
5. Conclusion
Global stressors such as elevated temperature and increased atmospheric carbon dioxide are impacting coral reef habitats worldwide (Hughes et al., 2003; Hoegh-Guldberg et al., 2007). Additional studies for better understanding of the interactions between coral and microplastics can help inform local policy development and mitigation strategies. Future research should be conducted investigating effects of irregular shaped and/or various sizes of microplastic on coral. An additional focus should look at possible synergistic effects of global or regional stressors such as bleaching events and disease outbreaks. Increasing our knowledge will help guide coral protection on a local level in hopes of improving the resiliency of coral, making them less susceptible to global environmental threats. Whether through ingestion or mere exposure to microplastics, the results of this study provide evidence that faster growing coral species may be more heavily impacted than slower growing species.
Supplementary Material
Acknowledgements
We would like to thank Crystal Lilavios, Katherine Stanley-Lorson, and Lucas Doran for their laboratory assistance, Sandy Raimondo for statistical guidance, and William S. Fisher for manuscript review. We would also like to thank NOAA’s Florida Keys National Marine Sanctuary and Mote Marine Laboratory (Summerland Key, FL) for their assistance with coral collection. The views expressed in this article are those of the authors and do not necessarily reflect the views or policies of the U.S. EPA.
Footnotes
Author statement
Cheryl Hankins: Conceptualization, Formal analysis, Investigation, Resources, Writing – original draft, Visualization, Supervision, Project administration, Funding acquisition. Elizabeth Moso: Investigation, Data curation, Writing – review & editing. Danielle Lasseigne: Investigation, Data curation, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
References
- Allmand D, Tambutt_e _E, Girard JP, Jaubert J, 1998. Organic matrix synthesis in the scleractinian coral Stylophora pistillata: role in biomineralization and potential target of the organotin tributyltin. J. Exp. Biol. 201, 2001e2009. [DOI] [PubMed] [Google Scholar]
- Allen AS, Seymore A, Rittschof D, 2017. Chemoreception drives plastic consumption in hard coral. Mar. Pollut. Bull. 124, 198e205. [DOI] [PubMed] [Google Scholar]
- Andrady AL, 2011. Microplastics in the marine environment. Mar. Pollut. Bull. 62, 1596e1605. [DOI] [PubMed] [Google Scholar]
- Anthony KRN, Kline DI, Diaz-Pulido G, Hoegh-Guldberg O, 2008. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl. Acad. Sci. 105 (45), 17442e17446. 10.1073/pnas.0804478105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony KRN, Hoogenboom MO, Maynard JA, Grottoli AG, Middlebrook R, 2009. Energetics approach to predicting mortality risk from environmental stress: a case study of coral bleaching. Funct. Ecol. 23 (3), 539e550. [Google Scholar]
- Antonius AA, Lipscomb D, 2000. First protozoan coral-killer identified in the Indo-Pacific. Atoll Res. Bull. 481, 1e21. [Google Scholar]
- Ashton K, Holmes L, Turner A, 2010. Association of metals with plastic production pellets in the marine environment. Mar. Pollut. Bull. 60, 2050e2055. [DOI] [PubMed] [Google Scholar]
- Aronson RB, Precht WF, 2001. White-band disease and the changing face of Caribbean coral reefs. Hydrobiologia 460, 25e38. [Google Scholar]
- Axworthy JB, Padilla-Gamiño JL, 2019. Microplastic ingestion and heterotrophy in thermally stressed coral. Sci. Rep. 9 10.1038/s41598-019-54698-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Haim Y, Zicherman-Keren M, Rosenburg E, 2003. Temperature-regulated bleaching and lysis of the coral Pocillopora damicornis by the novel pathogen Vibrio coralliilyticus. J. Appl. Environ. Microbiol. 69 (7), 4326–4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besseling E, Wegner A, Foekema E, Van Den Heuvel-Greve M, Koelmans AA, 2013. Effects of microplastic on fitness and PCB bioaccumulation by the lugworm Arenicola marina (L.). Environ. Sci. Technol. 47, 593e600. [DOI] [PubMed] [Google Scholar]
- Bessell-Browne P, Stat M, Thomson D, Clode PL, 2014. Coscinaraea marshae coals that have survived prolonged bleaching exhibit signs of increased heterotrophic feeding. Coral Reefs 33, 795e804. [Google Scholar]
- Bielmyer GK, Grosell M, Bhagooli R, Baker AC, Langdon C, Gillette P, Capo TR, 2010. Differential effects of copper on three species of scleractinian corals and their algal symbionts. Aquat. Toxicol. (Amst.) 97 (2), 125e133. [DOI] [PubMed] [Google Scholar]
- Borneman EH, 2001. Water Chemistry: Parameters to Know and Maintain for Success with Coral. T.H.F. Publications, Inc., Aquarium corals: Selection, Husbandry, and Natural History, Neptune City, NJ, pp. 343e365. [Google Scholar]
- Boucher J, Friot D, 2017. Primary Microplastics in the Oceans: a Global Evaluation of Sources. IUCN, Gland, Switzerland, p. 43. [Google Scholar]
- Chua EM, Shimeta J, Nugegoda D, Morrison PD, Clarke BO, 2014. Assimilation of Polybrominated diphenyl ethers from microplastics by the marine amphipod, Allorchestes compressa. Environ. Sci. Technol. 48 (14), 8127e8134. [DOI] [PubMed] [Google Scholar]
- Chisholm JRM, Gattuso JP, 1991. Validation of the alkalinity anomaly technique for investigating calcification and photosynthesis in coral reef communities. Limnol. Oceanogr. 36 (6), 1232e1239. [Google Scholar]
- Chapron L, Peru E, Engler A, Ghiglione JF, Meistertzheim AL, Pruski AM, Purser A, V_etion G, Galand PE, Lartaud F, 2018. Macro- and microplastics affect cold-water corals growth, feeding and behavior. Sci. Rep. 8, 15299. 10.1038/s41598-018-33683-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona E, Martin C, Marasco R, Duarte CM, 2020. Passive and active removal of marine microplastics by a mushroom coral (Danafungia scruposa). Front. Mar. Sci. 7, 128. 10.3389/fmars.2020.00128. [DOI] [Google Scholar]
- Cramer KL, Jackson JBC, Donovan MK, Greenstein BJ, Korpanty CA, Cook GM, Pandolfi JM, 2020. Widespread loss of Caribbean acroporid corals was underway before coral bleaching and disease outbreaks. Sci. Adv. 6 (17), 10. 10.1126/sciadv.aax9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cr_oquer A, Villamizar E, Noriega N, 2002. Environmental factors affecting tissue regeneration of the reef-building coral Montastraea annularis (Faviidae) at Los Roques National Park, Venezuela. Rev. Biol. Trop. 50, 1055e1065. [PubMed] [Google Scholar]
- Delbeek CJ, Sprung J, 2005. Physical and chemical parameters of reef aquarium water. In: The Reef Aquarium: Science, Art, and Technology, ume 3. Two Little Fishies, Inc. d.b.a. Ricordea Publishing, Coconut Grove, FL, pp. 132e197. [Google Scholar]
- Eakin CM, Sweatman HPA, Brainard RE, 2019. The 2014–2017 global-scale coral bleaching event: insights and impacts. Coral Reefs 38, 539e545. [Google Scholar]
- Enzor LA, Hankins C, Vivian DN, Fisher WS, Barron MG, 2018. Calcification in Caribbean reef-building corals at high pCO2 levels. J. Exp. Mar. Biol. Ecol. 499, 9e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erni-Cassola G, Gibson MI, Thompson RC, Christie-Oleza JA, 2017. Lost, but found with Nile Red: a novel method for detecting and quantifying small microplastics (1 mm to 20 mm) in environmental samples. Environ. Sci. Technol. 51 (23), 13641e13648. [DOI] [PubMed] [Google Scholar]
- Erni-Cassola G, Zadjelovic V, Gibson MI, Christie-Oleza JA, 2019. Distribution of plastic polymer types in the marine environment; a meta-analysis. J. Hazard Mater. 369, 691e698. [DOI] [PubMed] [Google Scholar]
- Flores F, Kaserzon S, Elisei G, Ricardo G, Negri AP, 2020. Toxicity thresholds of three insecticides and two fungicides to larvae of the coral Acropora tenuis. PeerJ 8, e9615. 10.7717/peerj.9615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournie JW, Vivian DN, Yee SH, Courtney LA, Barron MG, 2012. Comparative sensitivity of six scleractinian corals to temperature and solar radiation. Dis. Aquat. Org. 99, 85e93. [DOI] [PubMed] [Google Scholar]
- Fossi MC, Panti C, Guerranti C, Coppola D, Giannetti M, Marsili L, Minutoli R, 2012. Are baleen whales exposed to the threat of microplastics? A case study of the Mediterranean fin whale (Balaenoptera physalus). Mar. Pollut. Bull. 64, 2374e2379. [DOI] [PubMed] [Google Scholar]
- GESAMP, 2015. Sources, fate and effects of microplastics in the marine environment: a global assessment. In: Kershaw PJ (Ed.), IMO/FAO/UNESCO-IOC/UNIDO/WMO/IAEA/UN/UNEP/UNDP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection. Rep. Stud. GESAMP No. 90, p. 96. [Google Scholar]
- Goldstein MC, Carson HS, Eriksen M, 2014. Relationship of diversity and habitat area in North Pacific plastic-associated rafting communities. Mar. Biol. 16, 1441e1453. 10.1007/s00227-014-2432-8. [DOI] [Google Scholar]
- Grottoli AG, Rogridues LJ, Palardy JE, 2006. Heterotrophic plasticity and resilience in bleached corals. Nature 440, 1186e1189. [DOI] [PubMed] [Google Scholar]
- Hall NM, Berry KLE, Rintoul L, Hoogenboom MO, 2015a. Microplastic ingestion by scleractinian corals. Mar. Biol. 162, 725e732. [Google Scholar]
- Hall ER, DeGroot BC, Fine M, 2015b. Lesion recovery of two scleractinian corals under low pH conditions: implications for restoration efforts. Mar. Pollut. Bull. 100 (1), 321e326. [DOI] [PubMed] [Google Scholar]
- Hankins C, Duffy A, Drisco K, 2018. Scleractinian coral microplastic ingestion: potential calcification effects, size limits, and retention. Mar. Pollut. Bull. 135, 587e593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlan J, Lirman D, 2008. Development of a coral nursery program for the threatened coral Acropora cervicornis in Florida. Proc. 11th Int. Coral Reef Symp. July, Ft. Lauderdale, Florida, pp. 7e11. [Google Scholar]
- Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E, Harvell CD, Sale PF, Edwards J, Caldeira K, Knowlton N, Eakin CM, Iglesias-Prieto R, Muthiga N, Bradbury RH, Dubi A, Hatziolos ME, 2007. Coral reefs under rapid climate change and ocean acidification. Science 318 (5857), 1737e1742. [DOI] [PubMed] [Google Scholar]
- Hollarsmith JA, Griffin SP, Moore TD, 2012. Success of outplanted Acropora cervicornis colonies in reef restoration. Proc 12th Int Coral Reef Symp. July, Cairns, Australia, pp. 9e13. [Google Scholar]
- Holmes L, Turner A, Thompson RC, 2012. Adsorption of trace metals to plastic resin pellets in the marine environment. Environ. Pollut. 160, 42e48. [DOI] [PubMed] [Google Scholar]
- Holmes-Farley R, 2004. Reef aquarium water parameters. Reefkeeping Online 3 (4). http://reefkeeping.com/issues/2004-05/rhf/index.php. (Accessed 9 September 2013). [Google Scholar]
- Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, Folke C, Grosberg R, Hoegh-Guldberg O, Jackson C, Kleypas J, Lough JM, Marshall P, Nystr€om M, Palumbi SR, Pandolfi JM, Rosen B, Roughgarden J, 2003. Climate change, human impacts, and the resilience of coral reefs. Science 301 (5635), 929e933. [DOI] [PubMed] [Google Scholar]
- Jackson JBC, Donovan MK, Cramer KL, Lam VYY, 2014. Status and Trends of Caribbean Coral Reefs. Global Coral Reef Monitoring Network. IUCN. [Google Scholar]
- Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryman M, Andrady A, Narayan R, Law KL, 2015. Plastic waste inputs from land into the ocean. Science 347 (6223), 768e771. [DOI] [PubMed] [Google Scholar]
- Jamieson AJ, Brooks LSR, Reid WDK, Piertney SB, Narayanaswamy BE, Linley TD, 2019. Microplastics and synthetic particles ingested by deep-sea amphipods in six of the deepest marine ecosystems on Earth. R. Soc. Open. Sci. 6, 180667. 10.1098/rsos.180667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokiel PL, Maragos JE, Franzisket L, 1978. Coral growth: buoyant weight technique. In: Stoddart DR, Johannes RE (Eds.), Coral Reefs: Research Methods. UNESCO; Paris, pp. 529e542. [Google Scholar]
- Kanhai LDK, Gardfeldt K, Krumpen T, Thompson RC, O’Connor I, 2020. Microplastics in sea ice and seawater beneath ice floes from the Arctic Ocean. Sci. Rep. 10, 5004. 10.1038/s41598-020-61948-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey DW, 1978. Alkalinity changes and coral reef calcification. Limnol. Oceanogr. 23, 989e991. [Google Scholar]
- Kirstein IV, Kirmizi S, Wichels A, Garin-Fernandez A, Erler R, L€oder M, Gerdts G, 2016. Dangerous hitchhikers? Evidence for potentially pathogenic Vibrio spp. on microplastic particles. Mar. Environ. Res. 120, 1e8. [DOI] [PubMed] [Google Scholar]
- Kramer PR, Roth L, Lang J, 2019. Map of Stony Coral Tissue Loss Disease Outbreak in the Caribbean. https://www.agrra.org/. (Accessed 10 June 2020).
- Kushmaro A, Loya Y, Fine M, Rosenberg E, 1996. Bacterial infection and coral bleaching. Nature 380, 396. [Google Scholar]
- Lamb JB, Willis BL, Fiorenza EA, Couch CS, Howard R, Rader DN, True JD, Kelly LA, Ahmad A, Jompa J, Harvell CD, 2018. Plastic waste associated with disease on coral reefs. Science 359, 460e462. [DOI] [PubMed] [Google Scholar]
- Lantz CA, Atkinson MJ, Winn CW, Kahng SE, 2013. Dissolved inorganic carbon and total alkalinity of a Hawaiian fringing reef: chemical techniques for monitoring the effects of ocean acidification on coral reefs. Coral Reefs 33, 105e115. [Google Scholar]
- Lau WWY, Shiran Y, Bailey RM, Cook E, Stuchtey MR, Koskella J, Velis CA, Godfrey L, Boucher J, Murphy MB, Thompson RC, Jankowska E, Castillo AC, Pilditch TD, Dixon B, Koerselman L, Kosior E, Favoino E, Gutberlet J, Baulch S, Atreya ME, Fischer D, He KK, Petit MM, Sumaila UR, Neil E, Bernhofen MV, Lawrence K, Palardy JE, 2020. Evaluating scenarios toward zero plastic pollution. Science 369 (6510), 1455e1461. [DOI] [PubMed] [Google Scholar]
- Lesser MP, 1997. Oxidative stress causes coral bleaching during exposure to elevated temperatures. Coral Reefs 16, 187e192. [Google Scholar]
- Markey K, Baird AH, Humphrey C, Negri AP, 2007. Insecticides and a fungicide affect multiple coral life stages. Mar. Ecol. Prog. Ser. 330, 127e137. [Google Scholar]
- Martin C, Corona E, Mahadik GA, Duarte CM, 2019. Adhesion to coral surface as a potential sink for marine microplastic. Environ. Pollut. 255, 113281 10.1016/j.envpol.2019.113281. [DOI] [PubMed] [Google Scholar]
- Mato Y, Isobe T, Takada H, Kanehiro H, Ohtake C, Kaminuma T, 2001. Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ. Sci. Technol. 35 (2), 318e324. [DOI] [PubMed] [Google Scholar]
- Meesters EH, Pauchli W, Bak RPM, 1997. Predicting regeneration of physical damage on reef-building coral by regeneration capacity and lesion shape. Mar. Ecol. Prog. Ser. 146, 91e99. [Google Scholar]
- Montano S, Seveso D, Maggioni D, Galli P, Corsarini S, 2020. Spatial variability of phthalates contamination in the reef-building corals Porites lutea, Pocillopora verrucosa, and Pavona varians. Mar. Pollut. Bull. 155, 111117. 10.1016/j.marpolbul.2019.03.043. [DOI] [PubMed] [Google Scholar]
- Mouchi V, Chapron L, Peru E, Pruski AM, Meistertzheim A, Vetion G, Galand PE, Lartaud, 2019. Long-term aquaria study suggests species-specific responses of two cold-water corals to macro- and microplastics exposure. Environ. Pollut. 253, 322e329. 10.1016/j.envpol.2019.07.024. [DOI] [PubMed] [Google Scholar]
- Muscatine L, Porter JW, 1977. Reef corals: mutualistic symbiosis adapted to nutrient-poor environments. Bioscience 27 (7), 454e460. [Google Scholar]
- Negri AP, Heyward AJ, 2000. Inhibition of fertilization and larval metamorphosis of the coral Acropora millepora (Ehrenberg, 1834) by petroleum products. Mar. Pollut. Bull. 41 (7e12), 420e427. [Google Scholar]
- Negri AP, Smith LD, Wester NS, Heyward AJ, 2002. Understanding ship grounding impacts on a coral reef: potential effects of anti-foulant paint contamination on coral recruitment. Mar. Pollut. Bull. 44, 111e117. [DOI] [PubMed] [Google Scholar]
- Ng KL, Obbard JP, 2006. Prevalence of microplastics in Singapore’s coastal marine environment. Mar. Pollut. Bull. 52 (7), 761e767. [DOI] [PubMed] [Google Scholar]
- Ogata Y, Takada H, Mizukawa K, Hirai H, Iwasa S, Endo S, Mato Y, Saha M, Okuda K, Nakashima A, Murakami M, Zurcher N, Booyatumanondo R, Zakaria MP, Dung LQ, Gordon M, Miguez C, Suzuki S, Moore C, Karapanagioti HK, Weerts S, McClurg T, Burres E, Smith W, Van Velkenburg M, Lang JS, Lang RC, Laursen D, Danner B, Stewardson N, Thompson RC, 2009. International pellet watch: global monitoring of persistent organic pollutants (POPs) in coastal water. 1. Initial phase data on PCBs, DDTs, and HCHs. Mar. Pollut. Bull. 58 (10), 1437e1446. [DOI] [PubMed] [Google Scholar]
- Pait AS, Whitall DR, Jeffrey CFG, Caldow C, Mason AL, Christensen JD, Monaco ME, Ramirez J, 2007. An Assessment of Chemical Contaminants in the Marine Sediments of Southwest Puerto Rico. Silver Spring, MD, NOAA/National Centers for Coastal Ocean Science, NOAA Technical Memorandum NOS NCCOS, p. 52. [Google Scholar]
- Palardy JE, Rodrigues LJ, Grottoli AG, 2008. The importance of zooplankton to the daily metabolic carbon requirements of health and bleached corals at two depths. J. Exp. Mar. Biol. Ecol. 367 (2), 180e188. [Google Scholar]
- Pastorok RA, Bilyard GR, 1985. Effects of sewage pollution on coral-reef communities. Mar. Ecol. Prog. Ser. 21, 175e189. [Google Scholar]
- Peng G, Bellerby R, Zhang F, Sun Xuerong, Li D, 2020. The ocean’s ultimate trashcan: hadal trenches as a major depositories for plastic pollution. Water Res. 168, 115121. 10.1016/j.watres.2019.115121. [DOI] [PubMed] [Google Scholar]
- Plastics Europe, 2011. Plastics - the Facts 2011. An Analysis of European Plastics Production, Demand and Recovery to 2010. [Google Scholar]
- Plastics Europe, 2019. Plastics - the Facts 2019. An Analysis of European Plastics, Production, Demand and Waste Data. [Google Scholar]
- Porter JW, Fitt W, Spero H, Rogers CS, White MW, 1989. Bleaching in reef corals: physiological and stable isotopic responses. Proc. Natl. Acad. Sci. 86, 9342e9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Precht WF, Gintert BE, Robbart ML, Fura R, van Woesik R, 2016. Unprecedented disease-related coral mortality in southeaster Florida. Sci. Rep. 6, 31374. 10.1038/srep31374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renegar DA, Turner NR, Riegl BM, Dodge RE, Knap AH, Schuler PA, 2016. Acute and subacute toxicity of the polycylic aromatic hydrocarbon 1-methylnapthalene to the shallow-water coral Porites divaricata: application of a novel exposure protocol. Environ. Toxicol. Chem. 10.1002/etc.3530. [DOI] [PubMed] [Google Scholar]
- Richards ZT, Beger M, 2011. A quantification of the standing stock of macro-debris in Majuro lagoon and its effect on hard coral communities. Mar. Pollut. Bull. 62, 1693e1701. [DOI] [PubMed] [Google Scholar]
- Riechert J, Schellenberg J, Schubert P, Wilke T, 2018. Responses of reef building corals to microplastic exposure. Environ. Pollut. 237, 955e960. 10.1016/j.envpol.2017.11.006. [DOI] [PubMed] [Google Scholar]
- Reichert J, Arnold AL, Hoogenboom MO, Schubert P, Wilke T, 2019. Impacts of microplastics on growth and health of hermatypic corals are species-specific. Environ. Pollut. 254, 113074. 10.1016/j.envpol.2019.113074. [DOI] [PubMed] [Google Scholar]
- Rochman CM, Hoh E, Hentschel BT, Kaye S, 2013a. Long-term field measurement of sorption of organic contaminants to five types of plastic pellets: implications for plastic marine debris. Environ. Sci. Technol. 47, 1646e1654. [DOI] [PubMed] [Google Scholar]
- Rochman CM, Hoh E, Kurobe T, Teh SJ, 2013b. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep. 3, 3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers C, 1990. Responses of coral reefs and reef organisms to sedimentation. Mar. Ecol. Prog. Ser. 62, 185e202. [Google Scholar]
- Sabine AM, Smith TB, Williams DE, Brandt ME, 2015. Environmental conditions influence tissue regeneration rates in scleractinian corals. Mar. Pollut. Bull. 95, 253e264. 10.1016/j.marpolbul.2015.04.006. [DOI] [PubMed] [Google Scholar]
- Saliu F, Montano S, Leoni B, Lasagni M, Galli P, 2019. Microplastics as a threat to coral reef environments: detection of phthalate esters in neuston and scleractinian corals from the Faafu Atoll. Maldives. Mar. Pollut. Bull. 142, 234e241. [DOI] [PubMed] [Google Scholar]
- Savinelli B, Fern_andez TV, Galasso NM, D’Anna G, Pipitone C, Prada F, Zenone A, Badalamenti F, Musco L, 2020. Microplastics impair feeding performance of a Mediterranean habitat-forming coral. Mar. Environ. Res. 155, 104887. 10.1016/j.marenvres.2020.104887. [DOI] [PubMed] [Google Scholar]
- Schoepf V, Hu X, Holcomb M, Cai W, Li Q, Wang Y, Xu H, Warner ME, Melman TF, Hoadley KD, Pettay DT, Matsui Y, Baumann JH, Grottoli AG, 2017. Coral calcification under environmental change: a direct comparison of the alkalinity anomaly and buoyant weight techniques. Coral Reefs 36, 13e25. 10.1007/s00338-016-1507-z. [DOI] [Google Scholar]
- Smith SV, Kinsey DW, 1978. Calcification and organic carbon metabolism as indicated by carbon dioxide. In: Stoddard DR, Johannes RE (Eds.), Coral Reefs: Research Methods. United Nations Educational, Scientific, and Cultural Organization, Paris, France, pp. 469e484. [Google Scholar]
- Schwarz JA, Mitchelmore CL, Jones R, O’Dea A, Seymore S, 2013. Exposure to copper induces oxidative stress responses and DNA damage in the coral Montasraea franksi. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 157 (3), 272e279. [DOI] [PubMed] [Google Scholar]
- Tang J, Ni X, Zhou Z, Wang L, Lin S, 2018. Acute microplastic exposure raises stress response and suppresses detoxification and immune capacities in the scleractinian coral Pocillopora damicornis. Environ. Pollut. 243 (A), 66e74. 10.1016/j.envpol.2018.08.045. [DOI] [PubMed] [Google Scholar]
- Tekman MB, Wekerle C, Lorenz C, Primpke S, Hasemann C, Gerdts G, Bergman, 2020. Tying up loose ends of microplastic pollution in the Arctic: distribution from the sea surface, through the water column to deep-sea sediments at the HAUSGARTEN observatory. Environ. Sci. Technol. 54 (7), 4049e4090. 10.1021/acs.est.9b06981. [DOI] [PubMed] [Google Scholar]
- Teuten EL, Rowland SJ, Galloway TS, Thompson RC, 2007. Potential for plastics to transport hydrophobic contaminants. Environ. Sci. Technol. 41, 7759e7764. [DOI] [PubMed] [Google Scholar]
- Teuten EL, Saquing JM, Knappe DRU, Barlaz MA, Jonsson S, Bj€orn A, Rowland SJ, Thompson RC, Galloway TS, Yamashita R, Ochi D, Watanuki Y, Moore C, Viet PH, Tana TS, Prudente M, Boonyatumanond R, Zakaria MP, Akkhavong K, Ogata Y, Hirai H, Iwasa S, Mizukawa K, Hagino Y, Imamura A, Saha M, Takada H, 2009. Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 2027e2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RC, 2015. Microplastics in the marine environment: sources, consequences and solutions. In: Bergmann M, Gutow L, Klages M. (Eds.), Marine Anthropogenic Litter. Springer, Berlin, Germany, pp. 185e200. [Google Scholar]
- Van A, Rochman CM, Flores EM, Hill KL, Vargas E, Vargas SA, Hoh E, 2012. Persistent organic pollutants in plastic marine debris found on beaches in San Diego, California. Chemosphere 86 (3), 258e263. [DOI] [PubMed] [Google Scholar]
- Vivian DN, Yee SH, Courtney LA, Fisher WS, 2019. Estimating 3-dimensional surface areas of small scleractinian corals. Caribb. J. Sci. 49 (2e3), 192e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware M, Garfield EN, Nedimyer K, Levy J, Kaufman L, Precht W, Winters RS, Miller SL, 2020. Survivorship and growth in staghorn coral (Acropora cervicornis) outplanting in the Florida Keys national marine sanctuary. PloS One 15 (5), e0231817. 10.1371/journal.pone.0231817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A, Kayenne H, Hata H, Kudo S, 2006. Analysis of the seawater CO2 system in the barrier reef-lagoon system of Palau using total alkalinity dissolved inorganic carbon diagrams. Limnol. Oceanogr. 51 (4), 1614e1628. [Google Scholar]
- Yates KK, Dufore C, Smiley N, Jackson C, Halley RB, 2007. Diurnal variation of oxygen and carbonate system parameters in Tampa Bay and Florida Bay. Mar. Chem. 104 (1e2), 110e124. [Google Scholar]
- Ye S, Andrady AL, 1991. Fouling of floating plastic debris under Biscayne Bay exposure conditions. Mar. Pollut. Bull. 22 (12), 608e613. [Google Scholar]
- Zettler ER, Mincer TJ, Amaral-Zettler LA, 2013. Life in the “plastisphere”: microbial communities on plastic marine debris. Environ. Sci. Technol. 47, 7137e7146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.