Abstract
Coronavirus disease 2019 (COVID-19), a novel etiology of end-stage lung disease, has resulted in major disruptions to the process of health care delivery worldwide. These disruptions have led to team-based innovations globally, resulting in a broad range of new processes in cardiopulmonary perioperative management. A key intersection of multidisciplinary teamwork and COVID-19 is found in lung transplantation, in which diverse teams collaborate throughout the perioperative period to achieve optimal outcomes. In this article, we describe the multidisciplinary approach taken by Mayo clinic in Florida to manage patients with COVID-19 presenting for lung transplantation.
Abbreviations and Acronyms: ARDS, acute respiratory distress syndrome; CAD, coronary artery disease; COVID-19, coronavirus disease 2019; ECMO, extracorporeal membrane oxygenation; ESLD, end-stage lung disease; ICU, intensive care unit; MCF, Mayo clinic in Florida; MDT, multidisciplinary team; OR, operating room; PCR, polymerase chain reaction; POD, postoperative day; PPE, personal protective equipment; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; VA, veno-arterial; VV, veno-venous
Coronavirus disease 2019 (COVID-19), a novel etiology of end-stage lung disease (ESLD), has resulted in major disruptions to the process of health care delivery worldwide.1 These disruptions have led to team-based innovations globally, resulting in a broad range of new processes impacting education, communication, and personal protective equipment (PPE) manufacturing.1,2 A key intersection of multidisciplinary teams (MDTs) and COVID-19 is found in lung transplantation, in which diverse teams collaborate throughout the perioperative period to achieve optimal outcomes in patients with ESLD.3,4 The first major case series describing early outcomes in lung transplantation focused on the surgical considerations for COVID-19 as well as preliminary proposed criteria for pursuing transplantation in patients with COVID-19–related ESLD.5 Since then, several other high-volume centers have published their institutional experience in lung transplantation for COVID-19.6,7 Although data on the total number of lung transplants for COVID-19 performed worldwide are lacking, a recent report noted that at least 60 lung transplantations had been performed throughout North America and European centers.8 Mayo clinic in Florida (MCF) has innovated a comprehensive multidisciplinary communication and clinical pathway for lung transplantation for COVID-19 from initial evaluation of patients to be transferred to MCF for lung transplantation evaluation and consideration for extracorporeal membrane oxygenation (ECMO) placement to lung transplantation and perioperative management. In this article, we describe our team’s process and its application in a series of 3 lung transplants performed for ESLD from COVID-19. Informed consent rules were followed per institutional policy, and this study was determined to be exempt from review by the institutional review board.
Pretransplant ECMO Implementation
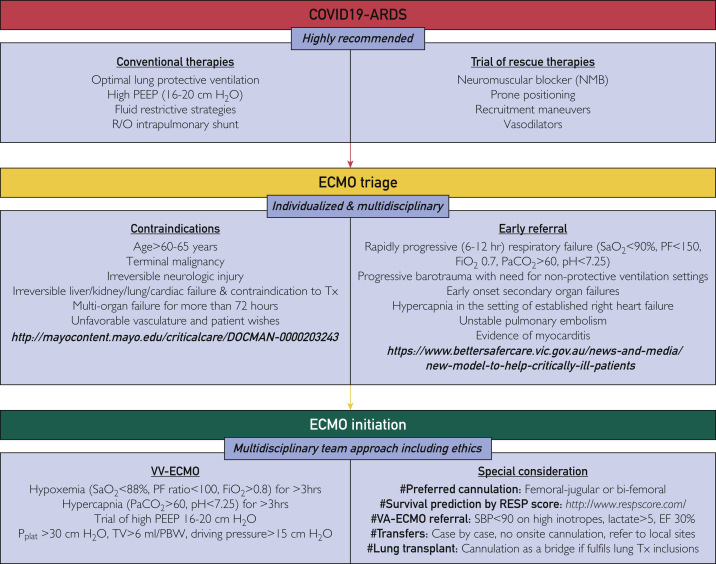
COVID-19 may lead to acute respiratory distress syndrome (ARDS) refractory to conventional therapy, requiring the use of ECMO to assist in decarboxylation and oxygenation.9 Current guidelines from the Extracorporeal Life Support Organization note that although conventional selection criteria should be used for COVID-19 ECMO implementation, a key difference between patients with COVID-19 and non–COVID-19 ECMO is the longer duration of ECMO required in patients with COVID-19–related ARDS.9 Although MCF follows standard Extracorporeal Life Support Organization criteria for ECMO selection, we developed early MDT communication and clinical pathways for both inpatient ECMO use and transfers from outside hospitals (Figures 1 and 2).
Figure 1.
Mayo criteria for extracorporeal membrane oxygenation initiation in coronavirus disease 2019. ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease 2019; ECMO, extracorporeal membrane oxygenation; EF, ejection fraction; FiO2, fraction of inspired oxygen; NMB, neuromuscular blocker; PaCO2, partial pressure of carbon dioxide within the blood; PBW, patient body weight; PEEP, positive end expiratory pressure; PF, PaO2/FiO2 ratio; R/O, rule out; RESP, respiratory; SaO2, oxygen saturation; SBP, systolic blood pressure; TV, tidal volume; Tx, transplantation; VV, veno-venous. ©2022 Mayo Foundation for Medical Education and Research. Materials and information are current as of February 24, 2022.
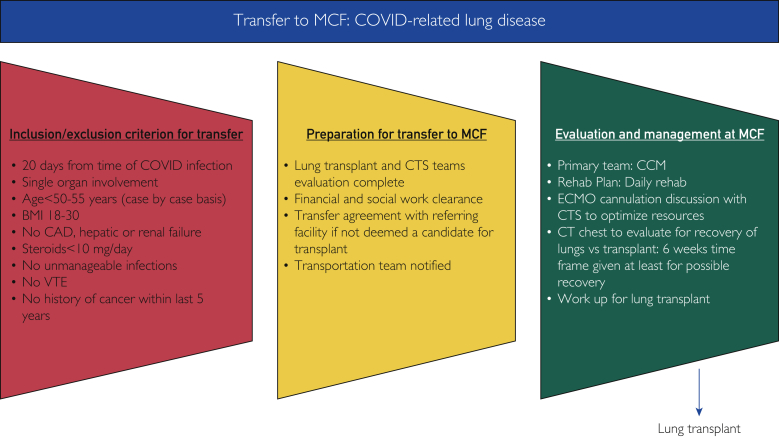
Figure 2.
Mayo clinic in Florida transfer coronavirus disease 2019–related lung disease criteria. BMI, body mass index; CAD, coronary artery disease; CCM, critical care medicine; COVID, coronavirus disease; CT, computed tomography; CTS, cardiothoracic surgery; ECMO, extracorporeal membrane oxygenation; MCF, Mayo clinic in Florida; VTE, venous thromboembolism. ©2022 Mayo Foundation for Medical Education and Research. Materials and information are current as of February 24, 2022.
Design and Implementation of MDT Pathway
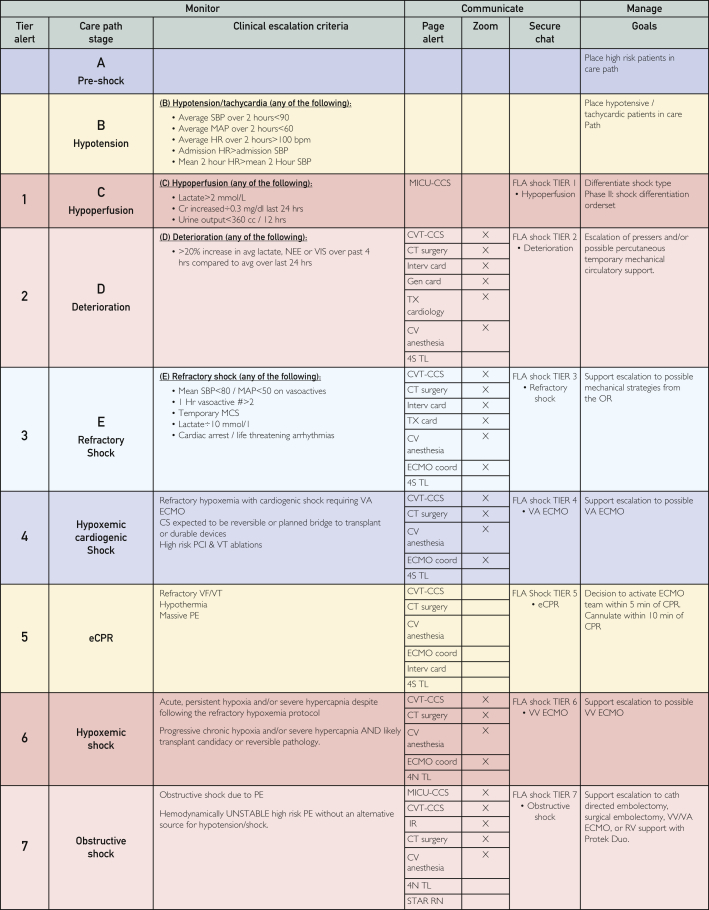
The MDT members represent medical and ancillary support specialties, including cardiothoracic surgeons, cardiac anesthesiologists, intensivists, transplant pulmonologists, intensive care unit (ICU) nursing leads, ethics committee representatives, perfusionists, ECMO specialists, and coordinators. Building upon a preexisting cardiogenic shock communication pathway (Figure 3), the MDT added tiers of communication that are activated when an outside hospital COVID-19 transfer or use of ECMO is being considered. Upon activation of the pathway, which is disseminated via the internal hospital communication system to individual pagers, the MDT members arrive at a virtual internet meeting room to discuss the situation and decide the next steps. In addition to the appropriate indication for ECMO, the type of ECMO support required—veno-venous (VV), veno-arterial (VA), or veno-arterial-venous—and the resource availability (ECMO units, staffing, and ICU bed) are also discussed. Unless cardiogenic shock is also present, VV ECMO is preferred in patients with isolated lung failure. If the patient is accepted for ECMO, each team member disseminates related information to their respective organizational downline in a hub-and-spoke communication model. These downline personnel include social workers, financial managers, physical therapists, perfusionists, and operating room (OR) staff.
Figure 3.
Mayo clinic in Florida cardiogenic shock communication pathway. CCS, critical care service; CS, cardiogenic shock; CT, computed tomography; CV, cardiovascular; CVT, cardiovascular and thoracic; ECMO, extracorporeal membrane oxygenation; eCPR, extracorporeal cardiopulmonary resuscitation; FLA, Florida; HR, heart rate; MAP, mean arterial pressure; MCS, mechanical circulatory support; MICU, medical intensive care unit; NEE, norepinephrine; PCI, percutaneous coronary intervention; PE, pulmonary embolism; RN, registered nurse; SBP, systolic blood pressure; STAR, stat team and response; VA, veno-arterial; VF, ventricular fibrillation; VIS, vasopressin; VT, ventricular tachycardia. ©2022 Mayo Foundation for Medical Education and Research. Materials and information are current as of February 24, 2022.
Transfer to OR for ECMO Cannulation
All ECMO cannulations in our institution are performed in OR. Before transferring the patient to OR, a preprocedural MDT huddle discusses the logistics of COVID-19 ECMO placement, including safety practices for ICU-to-OR transfer, individual staff PPE utilization, and ECMO cannulation imaging preferences, which mainly include transesophageal echocardiography, whereas fluoroscopy is used as needed.10,11 Each of these logistical components is reviewed for team fluency, and video resources are immediately available to provide additional training review for high-risk procedures such as correct PPE donning and doffing in patients with COVID-19.
Although VV ECMO cannulation can be performed in different configurations, our preferred approach is a combination of right femoral venous drainage with right internal jugular return cannulae. This configuration decreases the challenges of mobility with bifemoral cannulation and the potential for misdirection or malpositioning associated with the single double-lumen cannula via the right internal jugular approach. After ECMO is initiated, the patient is brought back to a dedicated cardiovascular and thoracic transplant ICU.
Care of Patients With COVID-19 on ECMO
Management of patients with COVID-19 remains a challenge, given the novelty of the disease. Despite having an established and well-functioning ECMO team for years, this novelty has necessitated institution of rapid adaptations to the management strategy in these patients. We employ a strategy of early percutaneous tracheostomy within the first week of cannulation to facilitate airway management and physiotherapy and decrease the need for sedation and analgesia. If the anatomy or body habitus is found to be not conducive for percutaneous tracheostomy in cardiovascular and thoracic transplant ICU, then we proceed with open tracheostomy in the OR. Ventilator settings are adjusted according to blood gas analysis with flexibility for both pressure- and volume-controlled mode with the aim of protecting the lungs. Our initial default settings are pressure control mode of ventilation with a rate of 10 breaths/min, positive end expiratory pressure of 10 centimeters of water (cm H2O) and inspiratory pressure of 10 cm H2O, and fraction of inspired oxygen of 40%-70% when the patients are heavily sedated. The mode of ventilator is switched to pressure support when the patient is awake.
Bivalirudin is the preferred anticoagulant used by our team for most of our patients, with the goal of achieving an activated partial thromboplastin time of 60-80 seconds. Multimodal analgesia and sedation pathways using opioids, propofol, benzodiazepines, and dexmedetomidine are initiated and transitioned from continuous intravenous to enteral route as the clinical status allows. Our published physiotherapy findings in patients on ECMO note the importance of aggressive early mobilization in patients with COVID-19 ECMO,12 and data also support the need for specific vigilance regarding hematologic complications in patients on ECMO secondary to COVID-19.13 If the patient is deemed to be a potential candidate for lung transplant, the pulmonary transplant team is consulted earlier in the course to initiate transplant work-up.
Transfer to Mayo Clinic for Lung Transplant and Listing Considerations for COVID-19
Before considering patients with COVID-19 for lung transplantation, guidelines for transfer to Mayo Clinic for lung transplant for COVID-19–related ESLD (Figure 2) and lung transplant listing considerations for COVID-19 were created. These guidelines have been approved by both the Lung Transplant Convergence Group on Mayo Clinic Enterprise Level and the Mayo Clinic Florida Hospital Practice Subcommittee. The criteria for consideration for transfer to Mayo Clinic for lung transplant evaluation for COVID-19–related lung disease are as follows:
Inclusion criteria:
-
○
Patient having completed more than 20 days from the initial diagnosis of COVID-19 (diagnosis is defined as severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2] polymerase chain reaction [PCR]–positive test result)
-
○
Single-organ involvement from COVID-19
-
○
Age of less than 50-55 years (older patients to be considered on a case-by-case basis)
-
○
Body mass index (calculated as the weight in kilograms divided by the height in meters squared) of 18-30 kg/m2
-
○
Steroids intake of less than 10 mg/d
-
○
Allocation of at least 6 weeks to evaluate for possible lung recovery on computed tomography scan of the chest (noncontrast) before being considered for lung transplant
-
○
Lung transplant evaluation per Lung Pre-Transplant Evaluation guideline
Exclusion criteria:
-
○
Coronary artery disease (CAD; case-by-case considerations)
-
○
Hepatic failure
-
○
Renal failure
-
○
Unmanageable infection
- ○ History of cancer in the past 5 years excluding the following:
-
•Prostate cancer
-
•Skin cancer
-
•Clear-cell cancer
-
•
-
○
Venous thromboembolism (patients are considered on a case-by-case basis, as patients with COVID-19 are at an increased risk of thromboembolism; patients with arterial clots are excluded, whereas those with venous thrombus are still considered for transplant)
Considerations for transfer to Mayo Clinic:
-
○
Evaluation by the lung transplant and cardiothoracic operation team
-
○
Financial evaluation for lung transplant work-up and listing
-
○
Social work assessment for transplant approval
-
○
Notification to the transport team to ensure that safe transportation is accomplished
Patient management at Mayo Clinic:
-
○
Critical care team serving as a primary care team
-
○
Daily rehabilitation
-
○
ECMO cannulation discussion with MDT to optimize resources
After the patient has completed lung transplant evaluation, the case is discussed in the lung transplant selection committee for consideration, and, if accepted, the patient is then listed for lung transplant.
Transplant Listing Considerations for COVID-19
Cypel and Keshavjee14 have summarized the salient points to consider when assessing a patient with COVID-19 for lung transplantation. We have added our experience-based points to those, which are listed below:
-
•
Basic guidelines for lung transplant listing are followed in all cases.
-
•
Age: patients aged less than 55 years are preferred; however, those aged less than 65 years can be considered on a case-by-case basis.
-
•
Single-organ dysfunction: ideally, patients with a single-organ dysfunction are considered for lung transplant listing. Exceptions are made on a case-by-case basis for patients with CAD or renal disease as our experience with this patient population increases. We have performed transplantations in patients with CAD successfully, and there have been case reports of lung-kidney transplants being performed for ESLD and renal failure after COVID-19.15
-
•
Patients who exhibit evidence of fibrocystic disease radiographically (post–COVID-19 fibrosis) and are not likely to recover are considered for transplant listing. Patients who have ground-glass changes or evidence of organizing pneumonia or consolidative pattern or ARDS related to COVID-19 are allowed time to see whether they would recover from their disease. King et al,16 in their recent publication, have described how to approach these patients in different clinical settings.
-
•
Time to recover from ARDS related to COVID-19 can be weeks to months in some cases. We have allowed months in certain cases with discharge of these patients to home or rehabilitation facilities. In other cases in which the risk of mortality is high, such as patients on high ECMO settings with progressive clinical decline, they can be considered for transplant listing sooner if they are not in the acute infectious phase of their disease.
-
•
Patients being considered for lung transplant listing need to be awake and able to participate in the discussion regarding transplant. Because most of these patients may be on narcotics, anxiolytics, and/or sedatives, especially those on mechanical ventilation and ECMO, it is important to also seek consent from caregivers in these cases. Patients whose neurologic status cannot be determined are not considered for transplant evaluation. It is important to recognize potential underlying depression, posttraumatic stress disorder, and anxiety in these patients, as these disorders can complicate their clinical progress and ability to participate in rehabilitation.17
-
•
Rehabilitation potential is the key when assessing these patients for transplantation. A trend toward improving rehabilitation potential over time is crucial for a good outcome after transplant. Most of these patients are assessed on a case-by-case basis depending on their baseline (preadmission) functional status and the functional trends shown during hospitalization.
-
•
It is important to have a negative SARS-CoV-2 PCR test before transplant listing to minimize the risk of infection of transplanted grafts. In the case of persistently positive SARS-CoV-2 PCR results from the upper or lower respiratory tract, it is important to determine the infectivity of the virus before listing.
Lung Transplantation Operative ProtocoL
Initial communication for lung transplantation acceptance is via the traditional email pathway, yet in the setting of limited ECMO resources, we have transitioned to a modification of the cardiogenic shock tier communication system to assess resources in real time from an MDT perspective. A pretransplantation huddle also occurs among the intraoperative team to cover aspects of transfer from ICU, intraoperative ECMO strategy, and postoperative ECMO considerations.
Bilateral clamshell thoracotomy with intraoperative VA ECMO is our preferred surgical approach for bilateral lung transplantation. For patients who are not already on ECMO before transplantation, central cannulation (aortic and right atrial) is performed. If already on VV ECMO, then the existing circuit is converted to VA by the addition of a central aortic cannula. Although we have not yet performed a COVID-19 lung transplant bridged preoperatively with VA ECMO, we have routinely used the existing configuration in other patients undergoing lung transplantation who were already on peripheral VA ECMO. Intraoperatively, we use a novel hybrid VA ECMO circuit that we have previously reported.18,19 To date, we have used this circuit on more than 125 lung transplantations, and all patients with COVID-19 have undergone transplantation by use of this novel ECMO circuit. After the lungs are implanted, we determine the need for postoperative ECMO prolongation on the basis of published criteria to assess the graft function.20 Postoperative plans are tailored to the ongoing use of ECMO for stabilization, and both early regional anesthetic techniques and early physiotherapy mobilization are foundational goals for our ICU recovery.
Our approach is grounded in early engagement of the lung transplant MDT to facilitate communication regarding the complexities of approaching transplantation in patients with COVID-19. To date, we have performed 3 bilateral lung transplantations in patients with COVID-19, which are discussed below.
Case 1
A man in his early 50s with a past medical history of hypertension and type 2 diabetes mellitus (A1c, 12%) presented to the emergency room in respiratory distress 1 week after the initial COVID-19 diagnosis. The imaging studies reported signs consistent with COVID-19 pneumonia as well as right upper lobe segmental pulmonary emboli. On hospital day 10, he required emergent VV ECMO support by a 25-French right femoral venous drainage and 19-French right internal jugular venous return cannula placement for worsening hypoxic respiratory failure. After the MDT meeting, the patient was listed for lung transplantation but continued aggressive rehabilitation on ECMO. By ECMO day 38, he was able to perform ambulatory exercises for 20 minutes a day. He underwent bilateral lung transplant on ECMO day 40, using the hybrid VA ECMO circuit via preexisting venous cannulae with the addition of an 18-French aortic cannula. Intraoperative course was complicated by bleeding, requiring transfusions; however, he did not require postoperative ECMO. He developed primary graft dysfunction grade 1 at 72 hours and cardiac tamponade 2 weeks after the operation, the latter requiring pericardial drainage and chest tube placement. He was discharged on postoperative day (POD) 34 and has excellent graft function at the 6-month follow-up.
Case 2
A man in his mid-50s with a past medical history of gout was transferred from an outside hospital for consideration of VV ECMO. His comorbidities included limited rehabilitation potential, preexisting pressure injuries, gastrointestinal bleeding, and right ventricular dysfunction with mild pulmonary hypertension. He underwent VV ECMO via right femoral and right internal jugular approaches, and a comprehensive ICU therapy was initiated for his comorbidities. During lung transplantation work-up, he was noted to have severe disease in his left anterior descending and circumflex arteries and was listed for combined lung transplant and 2-vessel coronary artery bypass grafting. The ECMO configuration was converted to VA ECMO for lung implantation. Once implantation was complete, we used the hybrid VA ECMO circuit to convert to cardiopulmonary bypass and performed a 2-vessel coronary artery bypass grafting with saphenous vein to the left anterior descending and obtuse marginal arteries. He was transitioned back to VV ECMO and transferred to the ICU where continuous renal replacement therapy had to be initiated for volume management. Both ECMO and continuous renal replacement therapy were discontinued on POD 8, and the patient was discharged on POD 143 because of marked physiotherapy-related requirements. He had excellent graft function on the last evaluation.
Case 3
A man in his early 50s with no past medical history underwent a course of VV ECMO for 79 days at an outside hospital. He was discharged to a long-term care facility on mechanical ventilation after ECMO was discontinued. During this initial hospitalization, he had multiple comorbidities, including deep venous thromboses, gastrointestinal bleeding, multidrug-resistant pneumonia, and 2 cardiac arrests. He was admitted to MCF for consideration of lung transplantation evaluation because of failure to wean off mechanical ventilation. Echocardiogram revealed a severely enlarged and dysfunctional right ventricle. Cardiac catheterization reported mild pulmonary hypertension and severe right CAD. We recommended and proceeded with right coronary artery percutaneous intervention, with subsequent lung transplantation listing 1 month after completion of dual antiplatelet therapy. Intraoperative support for bilateral lung transplantation was provided via hybrid VA ECMO, which was discontinued successfully at the end of the procedure. Although he developed primary graft dysfunction grade 1 at 48 hours, which resolved by 72 hours, he was discharged from the hospital and is doing well.
Conclusion
The intersection of COVID-19 and lung transplantation presents opportunities for MDT clinical care and communication. COVID-19 is a novel disease, and we, as clinicians, are still learning the clinical course of these patients. With an MDT approach, we have been able to learn when can we safely wait to allow the patients to recover from their COVID-19–related lung disease vs when a transplant is necessary. With time, our understanding of COVID-19–related lung disease progression has evolved, leading to multiple patients being delisted from the transplant waitlist for being too well who were initially evaluated and listed for lung transplant. Using a combination of pre–COVID-19 systems such as cardiogenic shock tier communication, transplantation selection committee, and MDT perioperative transplant care, our growing experience has allowed us to hone our novel MDT approach to communication and clinical care in patients with COVID-19 presenting for lung transplantation.
Footnotes
Potential Competing Interests: The authors report no competing interests.
References
- 1.Woolliscroft J.O. Innovation in response to the COVID-19 pandemic crisis. Acad Med. 2020;95(8):1140–1142. doi: 10.1097/ACM.0000000000003402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clifton W., Damon A., Martin A.K. Considerations and cautions for three-dimensional-printed personal protective equipment in the COVID-19 crisis. 3D Print Addit Manuf. 2020;7(3):97–99. doi: 10.1089/3dp.2020.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin AK, Fritz AV, Ramakrishna H. Multidisciplinary collaboration: the key to advancing lung transplantation outcomes. Indian J Thorac Card. Published online April 29, 2021. https://doi.org/10.1007/s12055-021-01182-5 [DOI] [PMC free article] [PubMed]
- 4.Fritz A.V., Martin A.K., Ramakrishna H. Practical considerations for developing a lung transplantation anesthesiology program. Indian J Thorac Cardiovasc Surg. 2021;37(suppl 3):445–453. doi: 10.1007/s12055-021-01217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bharat A., Machuca T.N., Querrey M., et al. Early outcomes after lung transplantation for severe COVID-19: a series of the first consecutive cases from four countries. Lancet Respir Med. 2021;9(5):487–497. doi: 10.1016/S2213-2600(21)00077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaheen L., Bremner R.M., Walia R., Smith M.A. Lung transplantation for coronavirus disease 2019 (COVID-19): the who, what, where, when, and why. J Thorac Cardiovasc Surg. 2022;163(3):865–868. doi: 10.1016/j.jtcvs.2021.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeung J.C., Cypel M., Chaparro C., Keshavjee S. Lung transplantation for acute COVID-19: the Toronto lung transplant program experience. CMAJ. 2021;193(38):E1494–E1497. doi: 10.1503/cmaj.211143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schweiger T., Hoetzenecker K. Commentary: post-COVID-19 acute respiratory distress syndrome and post-COVID-19 fibrosis-the new kids in town. J Thorac Cardiovasc Surg. 2022;163(3):869–870. doi: 10.1016/j.jtcvs.2021.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badulak J., Antonini M.V., Stead C.M., et al. Extracorporeal membrane oxygenation for COVID-19: updated 2021 guidelines from the extracorporeal life support organization. ASAIO J. 2021;67(5):485–495. doi: 10.1097/MAT.0000000000001422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ratzlaff R.A., Ripoll J.G., Cushenbery K.A., Lowman P.E., Diaz-Gomez J.L. Transthoracic echocardiogram-guided avalon catheter repositioning. Anesth Analg. 2017;125(1):48–51. doi: 10.1213/ANE.0000000000001909. [DOI] [PubMed] [Google Scholar]
- 11.Martin A.K., Allen W.L., Fritz A.V., Diaz-Gomez J.L. Successful rescue utilization of intraoperative tissue plasminogen activator in the setting of massive thrombosis of avalon catheter and patient in extremis with refractory hypoxemia. J Cardiothorac Vasc Anesth. 2018;32(5):2278–2281. doi: 10.1053/j.jvca.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Bohman J.K., Nei S.D., Mellon L.N., Ashmun R.S., Guru P.K. Physical therapy and sedation while on extracorporeal membrane oxygenation for COVID-19-associated acute respiratory distress syndrome. J Cardiothorac Vasc Anesth. 2022;36(2):524–528. doi: 10.1053/j.jvca.2021.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ripoll B., Rubino A., Besser M., et al. Observational study of thrombosis and bleeding in COVID-19 VV ECMO patients. Int J Artif Organs. 2022;45(2):239–242. doi: 10.1177/0391398821989065. [DOI] [PubMed] [Google Scholar]
- 14.Cypel M., Keshavjee S. When to consider lung transplantation for COVID-19. Lancet Respir Med. 2020;8(10):944–946. doi: 10.1016/S2213-2600(20)30393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guenthart B.A., Krishnan A., Alassar A., et al. First lung and kidney multi-organ transplant following COVID-19 Infection. J Heart Lung Transplant. 2021;40(8):856–859. doi: 10.1016/j.healun.2021.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King C.S., Mannem H., Kukreja J., et al. Lung transplantation for patients with COVID-19. Chest. 2022;161(1):169–178. doi: 10.1016/j.chest.2021.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmed H., Patel K., Greenwood D.C., et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis. J Rehabil Med. 2020;52(5) doi: 10.2340/16501977-2694. [DOI] [PubMed] [Google Scholar]
- 18.Thomas M., Martin A.K., Allen W.L., et al. Lung transplantation using a hybrid extracorporeal membrane oxygenation circuit. ASAIO J. 2020;66(10):e123–e125. doi: 10.1097/MAT.0000000000001157. [DOI] [PubMed] [Google Scholar]
- 19.Martin A.K., Harrison B.A., Fritz A.V., et al. Intraoperative management of a hybrid extracorporeal membrane oxygenation circuit for lung transplantation. J Card Surg. 2020;35(12):3560–3563. doi: 10.1111/jocs.15029. [DOI] [PubMed] [Google Scholar]
- 20.Hoetzenecker K., Schwarz S., Muckenhuber M., et al. Intraoperative extracorporeal membrane oxygenation and the possibility of postoperative prolongation improve survival in bilateral lung transplantation. J Thorac Cardiovasc Surg. 2018;155(5):2193–2206.e3. doi: 10.1016/j.jtcvs.2017.10.144. [DOI] [PubMed] [Google Scholar]