Abstract
Importance
The actual risk of thrombotic events after Covid-19 vaccination is unknown.
Objective
To evaluate the risk of thrombotic events after Covid-19 vaccination.
Design
Retrospective cohort study which included consecutive adult patients vaccinated with the first dose of Covid-19 vaccine between January 1 and May 30, 2021, and a historic control group, defined as consecutive patients vaccinated with influenza vaccine between March 1 and July 30, 2019.
Setting
Hospital Italiano de Buenos Aires, a tertiary hospital in Argentina.
Participants
Non-Hospitalized Adults vaccinated with the first dose of a Covid-19 vaccine.
Exposure
Vaccination with Covid-19 vaccines available during the study period: Gam-COVID-Vac (Sputnik), ChAdOx1 nCoV-19 (AstraZeneca/Oxford or Covishield), BBIBP-CorV (Beijing Institute of Biological Products) (Sinopharm). Active comparator group exposure was Influenza vaccine.
Main outcome
Primary endpoint was cumulative incidence of any symptomatic thrombotic event at 30 days, defined as the occurrence of at least one of the following: symptomatic acute deep venous thrombosis (DVT); symptomatic acute pulmonary embolism (PE); acute ischemic stroke (AIS); acute coronary syndrome (ACS) or arterial thrombosis.
Results
From a total of 29,985 adult patients who received at least a first dose of Covid-19 vaccine during study period and 24,777 who received Influenza vaccine in 2019, we excluded those who were vaccinated during hospitalization. We finally included 29,918 and 24,753 patients respectively. Median age was 73 years old (IQR 75–81) and 67% were females in both groups. Thirty six subjects in the Covid-19 vaccination group (36/29,918) and 15 patients in the Influenza vaccination group (15/24,753) presented at least one thrombotic event. The cumulative incidence of any thrombotic event at 30 days was 12 per 10,000 (95%CI 9–17) for Covid-19 group and 6 per 10,000 (95%CI 4–10) for Influenza group (p-value=0.022).
Conclusions and relevance
This study shows a significant increase in thrombotic events in subjects vaccinated with Covid-19 vaccines in comparison to a control group. The clinical implication of these findings should be interpreted with caution, in light of the high effectiveness of vaccination and the inherent risk of thrombosis from Covid-19 infection itself.
Keywords: Covid-19, Thrombotic events, Vaccines
1. Introduction
The development of new vaccines and the implementation of vaccination programmes in less than a year after the discovery of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) was a milestone of scientific progress [1]. In countries that achieved rapid vaccination coverage of a large percentage of their population, the pandemic was initially brought under control [2]. However, this rapid development and implementation of vaccination programmes has led to the occurrence of novel adverse events that were not described in the clinical trials on which the emergency approval of these vaccines was based [3,4].
Vaccine-Induced Immune Thrombocytopenia and Thrombosis (VITT) is a syndrome with a similar mechanism to that described for heparin-induced thrombocytopenia. Pathogenic pathways involve the production of high titers of antibodies to platelet factor 4 [5], [6], [7]. Patients first described with thrombotic events (TEs) after vaccination were young and healthy with thrombosis at unusual sites, thrombocytopenia, and elevated d-dimer [8]. The occurrence of these rare adverse events has led to temporary interruption of vaccination campaigns in some countries [9]. The incidence of these thrombotic complications varies depending on the report, type of vaccine and registration of these adverse effects [10].
Argentina started the Covid-19 vaccination campaign using a staggered priority scheme: population aged at least 80 years and health personnel were targeted first [11]. By June 2021, more than 19 million vaccines had been administered from an estimated population of 45,605,826, and approximately 2600 adverse events had been reported [12,13]. Although 99.2% of these events were classified as mild or moderate, serious adverse effects have been reported. However, this surveillance depends on active reporting by healthcare systems and most likely suffers from under-reporting.
Data regarding vaccines administered outside North America and Europe, such as BBIBP-CorV (Sinopharm) and Gam-COVID-Vac (Sputnik V) is scarce. These vaccines have been widely administered in Latin America and Asia and information regarding their safety profile is lacking [14].
Therefore, we designed the present study in order to evaluate the occurrence of TEs outside clinical trials. This study aims to estimate the incidence of TEs in a closed cohort of patients vaccinated with different Covid-19 vaccines at 30 days and to compare it with Influenza vaccine as an active comparator [15,16].
2. Materials and methods
2.1. Study design
Retrospective cohort study with patients vaccinated with a first dose of Gam-COVID-Vac (Sputnik), ChAdOx1 nCoV-19 (AstraZeneca/Oxford or Covishield) or BBIBP-CorV (Beijing Institute of Biological Products; Sinopharm) from January 1 to May 30, 2021, and a historic control group with patients vaccinated with influenza vaccine from March 1 to July 30, 2019.
Follow-up began with vaccination (Covid-19 or Influenza) until the occurrence of any of the following events: a thrombotic event, a positive Covid-19 polymerase chain reaction (PCR) test, death, or censored data (30 days of follow-up).
2.2. Participants and setting
The study took place at Hospital Italiano de Buenos Aires (HIBA), a tertiary hospital in Argentina. It has its own private insurance plan (PS-HIBA) with approximately 180,907 affiliated patients and 10,200 health workers in March 2021.
The vaccination campaign started in January 2021, using a staggered priority scheme. The available vaccines were: Gam-COVID-Vac, ChAdOx1 nCoV-19, BBIBP-CorV. We included subjects older than 17 years of age, affiliated to PS-HIBA or health workers, who were vaccinated as outpatients with a first dose.
Ethical approval was obtained from Institutional Review Board (CEPI#6062) and all data was treated with confidentiality.
2.3. Data collection
All patient health information is stored in a single clinical data repository (CDR) fed by the hospital electronic health record (EHR), evaluated and accredited by Healthcare Information and Management Systems Society as Level 7+.
The CDR comprehensive stores clinical documents for each patient, from different sources such as test results, images, clinical notes, outpatient visits, emergency department visits, in-hospital care, among others. We used these high quality secondary databases.
For data capture of potential thrombotic events we screened all key studies ordered and active problems registered during 30 days after vaccine application. For this purpose, order sets included were: computed tomography pulmonary angiograms (CTPA), arterial and venous doppler ultrasound, ventilation/perfusion (V/Q) scans, central nervous system computed tomography (CT) and magnetic resonance image (MRI), coronary and pulmonary angiographies.
Additionally, based on International Classification of Diseases version 10 [ICD-10] we included clinical related problems (eAppendix 1).
2.4. Event definition
The primary endpoint was any symptomatic thrombotic event, defined as the occurrence of at least one of the following: symptomatic acute deep venous thrombosis (DVT); symptomatic acute pulmonary embolism (PE); acute ischemic stroke (AIS); acute coronary syndrome (ACS); arterial thrombosis. Results were also reported for each thrombotic event end point separately.
DVT was defined as a positive eco doppler ultrasound with compatible symptoms; PE was defined as a positive chest angiotomography or ventilation/perfusion scan; AIS was defined as pathological acute finding of the central nervous system in the CT or MRI showing acute infarction with compatible symptoms, classified by etiology using Stop Stroke Study TOAST system [17]; ACS was defined as clinical interpretation according to physician criteria, classified as with ST elevation or without ST elevation based on electrocardiographic findings. Meanwhile, arterial thrombosis was defined as peripheral arterial thrombosis of the limbs with a pathological finding in a vascular study such as arterial doppler ultrasounds or angiography. All diagnoses were validated by physicians.
2.4.1. Sample size and statistical analysis
We did not use sample size estimation due to the design: closed cohort, in a local context with a shortage of sufficient vaccines. All subjects vaccinated during the study period were included. Data were presented as mean and standard deviation (SD) for quantitative variables, or median and interquartile range (IQR). Categorical variables were reported as absolute and relative frequencies. Comparisons between groups were assessed using Chi-square or Fisher's exact test for categorical variables and the Mann-Whitney U test or Student's t-test for continuous variables as appropriate.
Time to event analysis was performed in order to estimate the rates of thrombotic events, reported as cumulative incidences at 30 days per 10,000 person-years, with their corresponding 95% confidence intervals (95%CI). Preespecified subgroup analyses were performed according to sex, age, vaccine type, and vaccine platform, reporting p-value from log-rank test. A Kaplan–Meier analysis with a log-rank test was used for the univariate analysis.
In order to compare TE in the Covid-19 vaccination group with the Influenza vaccination group, we performed a Cox proportional-hazards regression model. Comparison of the survival curves and Schoenfeld's global test were used to test the proportional-hazards assumption for multivariate model. Results were presented as hazard ratios (HRs) and 95%CI. Covariates significantly associated in the univariable and those with clinical relevance were included in the multivariable model.
Statistical analyses were performed using STATA 17.0 (Stata Corporation, College Station, TX), and differences were considered statistically significant at p-values<0.05.
3. Results
3.1. Participants
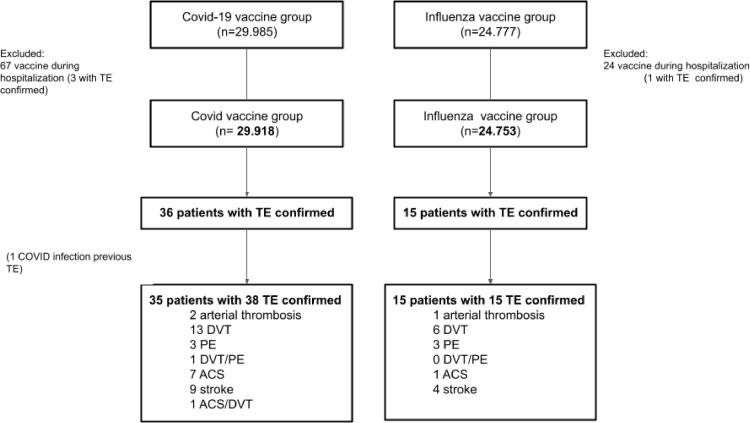
From a total of 29,985 adult patients who received at least the first dose of a Covid-19 vaccine (Covid-19 group) in our institution and 24,777 who received influenza vaccine in 2019 (Influenza group), we excluded those who were vaccinated during hospitalization. We finally included 29,918 and 24,753 patients respectively for the analysis (Fig. 1 ). One patient became infected with the SARS-CoV-2 coronavirus after Covid-19 vaccination and prior to a TE. A total of 9272 patients were included in both cohorts, in the Influenza Group in 2019 and in the Covid-19 group in 2021. Regarding baseline characteristics, median age was 73 years old (IQR 75–81) and roughly 67% were females in both groups (Table 1 ). Both groups showed a bimodal distribution regarding age, with younger population representing health workers, and older population representing patients with an indication for influenza vaccine or prioritized for early Covid-19 vaccine (Fig. 1).
Fig. 1.
Flowchart. TE, thrombotic event; DVT, deep venous thrombosis; PE, pulmonary embolism; ACS: acute coronary syndrome.
Table 1.
Baseline characteristics of patients by vaccine group.
| Covid-19 vaccine | Influenza vaccine | p value | |
| n: 29.918 | n: 24.753 | ||
| Demographic | |||
| Age (years, median IQR) | 73 (65–81) | 73 (65–81) | 0.083 |
| Female (n,%) | 20,066 (67.1) | 66.90% 16,559 (66.9) | 0.668 |
| Health worker (n,%) | 4510 (15.1) | 1430 (5.8) | 0.001 |
| Medical history | |||
| Previous thromboembolic event (n,%) | 1308 (4.4) | 1058 (4.3) | 0.576 |
| Hypertension (n,%) | 16,874 (56.4) | 14,812 (59.8) | 0.001 |
| Obesity (n,%)a | 7551(25.2) | 6491 (26.2) | 0.009 |
| BMI (median, IQR) | 26.93 (24.06–30.32) | 27.03 (24.09–30.41) | 0.066 |
| COPD (n,%) | 1262 (4.2) | 1295 (5.2) | 0.001 |
| Renal Failure (n,%) | 810 (2.7) | 958 (3.9) | 0.001 |
| Diabetes (n,%) | 3180 (11) | 2906 (12) | 0.001 |
| Atrial Fibrillation (n,%) | 1621 (5.4) | 1556 (6.3) | 0.001 |
| Heart Failure (n,%) | 1026 (3.4) | 1053 (4.3) | 0.001 |
| Coronary Artery Disease (n,%) | 1535 (5.1) | 1486 (6.0) | 0.001 |
| Stroke (n,%) | 1171 (3.9) | 1058 (4.3) | 0.034 |
| Cancer (solid tumor)b (n,%) | 1865 (6.2) | 1550 (6.3) | 0.892 |
| Onco Hematological disease (n,%) | 189 (0.6) | 255 (1.0) | 0.001 |
| Cirrhosis (n,%) | 204 (0.7) | 224 (0.9) | 0.003 |
| Smoker (n,%) | 5941 (19.9) | 4522 (18.3) | 0.001 |
| Interstitial lung disease (n,%) | 117 (0.4) | 118 (0.5) | 0.128 |
| Transplant (n,%) | 28 (0.1) | 95 (0.4) | 0.001 |
| Lung (n) | 1 | 3 | |
| Liver (n) | 6 | 12 | |
| Kidney (n) | 11 | 32 | |
| Heart Failure (n) | 3 | 11 | |
| Bone Marrow (n) | 7 | 37 | |
| Thrombophilia (n,%) | 19 (0.1) | 33 (0.1) | 0.008 |
| Major surgery last 30 days c (n,%) | 231 (0.8) | 240 (1.0) | 0.013 |
| Rheumatic disorder d (n,%) | 292 (1.0) | 327 (1.3) | 0.001 |
| Previous Covid-19 (n,%) | 1506 (5.0) | N/A | N/A |
| Influenza Vaccine (previous year) (n,%) | 7406 (24.8) | N/A | N/A |
| Previous medication (n,%) | |||
| Antiaggregants | 2360 (7.9) | 3075 (12.4) | 0.001 |
| Aspirin | 2247 (7.5) | 2936 (11.9) | 0.001 |
| Clopidogrel | 216 (0.7) | 293 (1.2) | 0.001 |
| Other antiaggregant | 11 (0.04) | 17 (0.07) | 0.101 |
| Oral anticoagulation | 1043 (3.5) | 1134 (4.6) | 0.001 |
| Dicumarinics | 657 (2.2) | 990 (4.0) | 0.001 |
| Direct oral anticoagulant drugs e | 389 (1.3) | 148 (0.6) | 0.001 |
| Low weight heparins | 620 (2.1) | 620 (2.5) | 0.001 |
| Antipsychotic | 987 (3.3) | 936 (3.8) | 0.002 |
| Antidepressant | 3224 (10.8) | 2948 (11.9) | 0.001 |
| Systemic steroids | 2000 (6.7) | 2192 (8.9) | 0.001 |
| Immunotherapy f | 22 (0.07) | 23 (0.09) | 0.431 |
| Only women subgroup (n,%) | |||
| Hormone Therapy | 4 (0.01) | 28 (0.11) | 0.001 |
| Contraceptive pills | 87 (0.43) | 96 (0.43) | 0.048 |
| Pregnancy | 48 (0.24) | 824 (0.24) | 0.001 |
| Puerperium | 11 (0.05) | 30 (0.18) | 0.001 |
Obesity define as body mass index ≥ 30.
Solid tumor excluding skin cancer other than melanoma diagnosed in the last 5 years.
Major surgery define as a surgery of more than 90 min.
Rheumatic disorders including rheumatoid arthritis, lupus, mixed connective tissue disease, dermatomyositis, polymyositis scleroderma and Sjogren.
Include: rivaroxaban, apixaban, dabigatrán etexilato.
Include: ipilimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab and avelumab
Abbreviations: BMI,body mass index; COPD, chronic obstructive pulmonary diseases; N/A, not applicable.
Hypertension, obesity, smoking, and diabetes were the most frequent comorbidities in both groups, with a significantly higher prevalence in the Influenza group. There was also a higher use of antiplatelets drugs, anticoagulation, antipsychotics and systemic steroids in the Influenza group.
There was a higher proportion of health workers in the Covid-19 vaccinated group (15.07% vs 5.78%; p < 0.001), while there were more transplant patients in the Influenza vaccinated group (0.09% vs. 0.38%; p < 0.001).
3.2. Studies performed
In the Covid-19 vaccine group 384 subjects from 29,918 (1.28%) had at least one study performed during 30 days of follow-up, compared to 531 from 24,753 (2.14%) in the Influenza group (p < 0.001). In the Covid-19 vaccine group 180 subjects underwent studies to rule out venous thrombotic events (24 CTPA, 3 V/Q scans and 159 venous doppler ultrasounds) while 233 subjects were studied in de Influenza group (17CTPA, 3 V/Q scans and 228 venous doppler ultrasounds). In regard to neurologic studies related to arterial events, 119 subjects (107 brain MRI and 16 head CT scans) and 198 (121 brain MRI and 82 head CT scans) were performed in the Covid-19 vaccine group and in the Influenza group, respectively.
3.3. Primary outcome
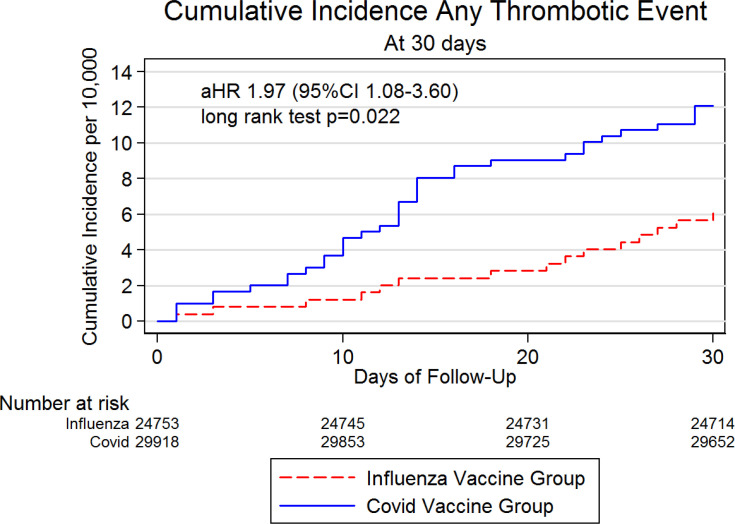
Thirty six subjects in the Covid-19 group (36/29,918) and 15 patients in the Influenza group (15/24,753) developed at least one TE. The cumulative incidence of any TE during the 30 days of follow-up was 12 per 10,000 (95%CI 9–17) for Covid-19 vaccinated group and 6 per 10,000 (95%CI 4–10) for Influenza vaccinated group (log-rank p-value=0.022) (Table 2 ). Cumulative incidence curves are presented in Fig. 2 . The adjusted HR (by sex, age, previous TE, major surgery) for TEs was 1.97 (95%CI 1.08–3.60; p < 0.001) in the Covid-19 vaccine group, compared with Influenza vaccine group.
Table 2.
Thrombotic event incidence.
| Covid-19 Vaccine | Influenza vaccine | |||||
|---|---|---|---|---|---|---|
| Any covid vaccine | Gam-COVID-Vac (Sputnik) | ChAdOx1 nCoV-19 (AstraZeneca/ Oxford) | BBIBP-CorV (Sinopharm) | Any influenza vaccine | p-value (a) | |
| 29,918 | 18,291 | 7057 | 4570 | 24,753 | ||
| Primary outcome (combined) | 0.022 | |||||
| Any thrombotic events 7 days (per 10,000) | 3 (95%CI 1–5) | 2 (95%CI 1–6) | 1 (95%CI 0–10) | 7 (95%CI 2–20) | 1 (95%CI 0–3) | |
| Any thrombotic events 14 days (per 10,000) | 8 (95%CI 5–12) | 10 (95%CI 5–13) | 7 (95%CI 3–17) | 11 (95%CI 5–26) | 2 (95%CI 1–5) | |
| Any thrombotic events 21 days (per 10,000) | 9 (95%CI 6–13) | 9 (95%CI 5–14) | 9 (95%CI 4–19) | 11 (95%CI 5–26) | 3 (95%CI 2–6) | |
| ANY thrombotic events 30 days (per 10,000) | 12 (95%CI 9–17) | 12 (95%CI 8–18) | 11 (95%CI 6–23) | 15 (95%CI 7–32) | 6 (95%CI 4–10) | |
| Primary outcomes at 30 days (per 10,000) (independent) | ||||||
| Venous thrombotic event (DVT/PE) | 5 (95%CI 3–8) | 5 (95%CI 3–9) | 6 (95%CI 2–15) | 4 (95%CI 1–17) | 2 (95%CI 1–5) | 0.212 |
| Deep venous thrombosis (DVT) | 5 (95%CI 3–8) | 5 (95%CI 3–9) | 6 (95%CI 2–15) | 4 (95%CI 1–17) | 2 (95%CI 1–5) | 0.124 |
| Pulmonary embolism (PE) | 1 (95%CI 0–4) | 2 (95%CI 1–5) | 1 (95%CI 0–10) | – | 1 (95%CI 1–4) | 0.897 |
| Stroke | 3 (95%CI 1–5) | 2 (95%CI 1–6) | 4 (95%CI 1–13) | 2 (95%CI 0–16) | 2 (95%CI 1–4) | 0.405 |
| Acute Coronary Syndrome | 3 (95%CI 1–5) | 3 (95%CI 1–7) | 1 (95%CI 1–10) | 4 (95%CI 1–17) | 0 (95%CI 0–3) | 0.039 |
| Arterial Thrombotic Event | 1 (95%CI 0–3) | 1 (95%CI 0–4) | – | 2 (95%CI 0–16) | 0 (95%CI 0–3) | 0.677 |
aComparison between any covid vaccine and Influenza vaccine.
Abbreviations: DVT, deep venous thrombosis; PE, pulmonary embolism.
Fig. 2.
Cumulative incidence of thrombotic events by vaccine group
* aHR = adjusted HR (by sex, age, previous TE, major surgery).
Regarding independent outcomes, no significant differences were found in TEs categories (DVT, PE, Stroke or arterial TE), except cumulative incidence of ACS, higher in Covid-19 vaccine group (p = 0.039) (Table 2).
3.3.1. Subgroup analysis in Covid-19 vaccine group
No difference was detected in the prespecified subgroup analysis by sex (p = 0.682) with cumulative incidence in male 13 per 10,000 (95%CI 8–23) versus 11 per 10,000 in women (95%CI 8–17).
On the other hand, TEs cumulative incidence was significatively higher in subjects older than 65 years than younger (p = 0.0099) with cumulative incidence 15 per 10,000 (95%CI 11–21) versus 3 per 10,000 (95%CI 1–11) respectively.
No differences were detected in the prespecified subgroup analysis by Covid-19 vaccine type: 12 per 10,000 (95%CI 8–18) in Gam-COVID-Vac; 11 per 10,000 (95%CI 6–23) in ChAdOx1 nCoV-19; and 15 per 10,000 (95%CI 7–32) in BBIBP-CorV (p = 0.784).
The comparison between adenovirus vector platform based vaccine (ChAdOx1 nCoV-19 and Gam-COVID-Vac 15 per 10,000; 95%CI 7–32) and inactivated virus vaccine (BBIBP-CorV 11 per 10,000; 95%CI 8–17) did not demonstrate significant differences (e Table 1).
3.3.2. Associated factors to thrombotic events in Covid-19 vaccine group
Patients with TEs were older (p < 0.001), with more frequent previous TE (11.11% vs. 4.36%; p = 0.048), renal failure (p = 0.002) and history of major surgery in the last 30 days (p < 0.001), in comparison to patients without TEs (Tables 3 and e Table 2).
Table 3.
Factors associated with thrombotic events in Covid-19 vaccine group.
| Covid-19 vaccine group (n: 29,918) | |||
| Any thrombotic event(n: 36) | No event(n: 29,882) | p-value | |
|---|---|---|---|
| Demographic | |||
| Age (years, median IQR) | 80.05 (75.44–87.09) | 73.08 (65.37–80.72) | 0.001 |
| Male (n,%) | 13 (36.11%) | 9839 (32.93%) | 0.684 |
| Previous thromboembolic events (n,%) | 4 (11.11%) | 1304 (4.36%) | 0.048 |
| HIBA health worker | 1 (2.78%) | 4509 (15.09%) | 0.039 |
| Medical history | |||
| BMI (median, IQR) | 26.22 (23.08–29.21) | 26.94 (24.06–30.32) | 0.249 |
| Renal Failure (n,%) | 4 (11.11%) | 806 (2.70%) | 0.002 |
| Oncohematologic disease (n,%) | 1 (2.78%) | 188 (0.63%) | 0.057 |
| Transplant (n,%) | – | 28 (0.09%) | 0.854 |
| Thrombophilia (n,%) | – | 19 (0.06%) | 0.880 |
| Major surgery last 30 days (n,%) | 4 (11.11%) | 227 (0.76%) | 0.001 |
| Rheumatic disorder (n,%) | – | 292 (0.98%) | 0.551 |
| Previous Covid-19 (n,%) | 3 (8.33%) | 1503 (5.03%) | 0.365 |
| Influenza Vaccine (last year) | 5 (13.89%) | 7401 (24.77%) | 0.131 |
| Previous medication (n,%) | |||
| Antiaggregants | 5 (13.89%) | 2355 (7.88%) | 0.181 |
| Aspirin | 5 (13.89%) | 2242 (7.50%) | 0.146 |
| Clopidogrel | 2 (5.56%) | 214 (0.72%) | 0.001 |
| Other | – | 11 (0.04%) | 0.908 |
| Oral anticoagulation | 3 (8.33%) | 1040 (3.48%) | 0.113 |
| Dicoumarinics | 3 (8.33%) | 654 (2.19%) | 0.012 |
| Direct oral anticoagulant drugs | – | 389 (1.30%) | 0.491 |
| Low weight heparins | 2 (5.56%) | 618 (2.07%) | 0.142 |
| Antipsychotic | 2 (5.56%) | 985 (3.30%) | 0.448 |
| Antidepressant | 4 (11.11%) | 3220 (10.78%) | 0.948 |
| Systemic steroids | 4 1(1.11%) | 1996 (6.68%) | 0.287 |
| Immunotherapy (n,%) | – | 22 (0.07%) | 0.871 |
| Hormone Therapy | – | 4 (0.01%) | 0.945 |
| Only women subgroup (n,%) | n: 23 | n: 20,043 | |
| Contraceptive pills | – | 87 (0.43%) | 0.752 |
| Pregnancy (n,%) | – | 48 (0.24%) | 0.814 |
| Puerperium (n,%) | – | 11 (0.05%) | 0.911 |
Abbreviations: BM, body mass index; HIBA, Hospital Italiano de Buenos Aires; IQR, interquartile range.
3.4. Secondary outcomes
The cumulative incidence of death at 30 days was significantly higher in the Influenza vaccine group with 11 per 10,000 (95%CI 0.07–0.15), compared to Covid-19 vaccine group with 1 per 10,000 in (95%CI 0.00–0.03)(p < 0.001) (e Table 3 ).
Regarding Covid-19 breakthrough infection after 30 days of follow up, it was 84 per 10,000 vaccinated subjects (95%CI 74–95) with no difference between vaccine types.
3.5. Characteristics of thrombotic events in Covid-19 vaccine group
Out of a total of 53 TEs in 50 patients from both groups, 38 events occurred in the 36 patients in the Covid-19 vaccinated group.
In the Covid-19 vaccine group, the median time from vaccination to the TEs was 13 days. Most of the patients needed hospitalization (24/36; 67%). The thirty day mortality found in patients with TEs was 2.8% (1/36) in the Covid-19 vaccinated group. Most of the patients with thrombotic events had classical risk factors for TE (cancer, thrombophilia, recent surgery or previous VTE for venous TEs and hypertension, diabetes, coronary artery disease for arterial TEs) (eTable 4).
Mild thrombocytopenia was present in only 3 patients with TE, with no patients presenting with less than 90,000 platelets. Anti-Platelet Factor 4 was not measured in any patient. There were no patients with a diagnosis of cerebral vein thrombosis (CVST) or VITT.
4. Discussion
This study shows a statistically significant higher cumulative incidence of thrombotic events at 30 days in subjects vaccinated with Covid-19 vaccines in comparison to a control group of subjects vaccinated with Influenza vaccine.
4.1. Key results in context
The comparison of the TE incidence found in this study with previous reports leads us to interesting findings. For instance, Smajda et al., describe the results of a report of the anti-SARS-CoV-2 vaccination thrombotic risk by the World Health Organization Global Database for Individual Case Safety Reports (VigiBase), world's largest pharmacovigilance database [18]. They found a cumulative incidence 0.059 TEs per 10,000 at 90 days. The cumulative incidence of TEs found in our cohort was much higher, 12 TEs per 10,000 vaccinated subjects at 30 days. This difference could be partially explained by the nature of the reports. While our cohort actively searched for symptomatic thrombotic events, VigBase report is a pharmacovigilance database, showing really reporting incidence instead of event incidence, as the authors acknowledge in their statement.
In a retrospective cohort study, Pottegård et al., found an excess of 11 venous thromboembolic events per 100,000 vaccinations with ChAdOx1 nCoV-19 vaccine, in comparison to no vaccinated historical control population [19]. Cumulative incidence of combined arterial and venous thrombotic events, arterial events and venous events at day 28 was 5.04, 2.95 and 2.09 per 10,000 vaccinated subjects, respectively. Once again, this cumulative incidence is lower than the one found in the present study. Differently to our study, Pottegård et al., included cases only on the basis of clinical diagnosis recorded by physicians, leading to possible under-reporting. Another significant difference is that this large cohort only included patients between 18 and 65 years old. Older age is a well-known risk factor for thrombotic events, so this lower incidence is also expected. More recent reports found no increased incidence of stroke, acute myocardial infarct or pulmonary embolism in a population-based French study of subjects older than 75 years vaccinated with BNT162b2 mRNA [20]. The European database of suspected adverse drugs reaction reports (EudraVigilance), reports only on vaccines approved by the European Union, not having reports on Gam-COVID-Vac or BBIBP-CorV vaccines [21]. In this database, on the bases of 69 million of ChAdOx1 nCoV-19 vaccine doses applied, they report an incidence of pulmonary embolism and deep venous thrombosis of 1.43 per 10,000, ACS of 0.085 per 10,000 and AIS of 0.16 per 10,000. These incidences are much lower than found in our report. Meanwhile, Vaccine Adverse Event Reporting System (VAERS), a national early warning system to detect possible safety problems in the United States-licensed vaccines (co-managed by the Centers for Disease Control and Prevention and the United States Food and Drug Administration) does not include information on either of the vaccines reports in the present study [22].
Our findings are in line with Hippisley-Cox et al. [23]. In this self-controlled case series involving 29 million people, they found an increased relative incidence of venous thromboembolism admissions in the 8–14 days after ChAdOx1 nCoV-19 vaccination and an increased relative incidence of arterial events after BNT162b2 mRNA vaccination.
Finally, it is important to take into account that Covid-19 is associated with increased risk of thrombotic events, and vaccination could reduce the incidence of TE by reducing the risk of infection [24].
4.2. Subgroup analysis
First descriptions of thrombotic events after Covid-19 vaccination and some possible physiopathological mechanisms were related to adenoviral vector-based technology [25,26]. In spite of this, we found no difference between thrombotic events according to the vaccine platform comparing adenoviral vector-based (Gam-COVID-Vac and ChAdOx1 nCoV-19) versus inactivated virus platform (BBIBP-CorV). It should be noted that the low number of events preclude definitive conclusions.
4.3. Risk factors
With regard to factors associated with increased risk of TE in the Covid-19 vaccine group, we found age as an important risk factor, adding a 5% increase risk for each year of age older, and we found no difference in risk between sexes. These findings are contrary to previous observations that found increased risk of a specific type of thrombosis (CVST) in younger women [27]. Most patients with TE in our series had classical risk factors (cancer, previous VTE, thrombophilia or recent surgery) and no manifestation of VITT, suggesting that events found have a different mechanism than the one described for VITT or no relation at all with the vaccines. Interestingly, Liu et al. reported laboratory findings consistent with an alteration in coagulation profile and an inflammatory pattern, not immune mediated, in healthy volunteers who received BBIBP-CorV inactivated vaccine [28].
4.4. Limitations and strengths
Several limitations of this study must be acknowledged. First, this is a retrospective monocentric study, subject to confounding factors, for instance, influenza vaccinated subjects used antithrombotic medication more often than Covid-19 vaccine group. To account for known confounders, we adjusted the analysis for factors known to be associated with TE (age, previous thrombotic events, cancer, major surgery, pregnancy, obesity, medications and thrombophilia). There could also be a lower threshold to order studies in the Covid-19 vaccine group due to early publications of thrombotic risk. Nevertheless, a higher proportion of subjects in the Influenza group underwent relevant diagnostic studies, probably due to restrictions to patient's mobility and difficulties to access to health care during pandemic. The combination of lower studies performed with a higher incidence of thrombotic events, reinforces the finding of a higher risk of thrombotic events in Covid-19 group and raises the possibility of underdiagnoses in this group.
We also addressed the limitation related to the possible loss of events. EHR completeness and complete follow up allowed certainty about symptomatic events detection. Second, even though this is a big cohort, it may be insufficient to detect events with very low incidence but high clinical impact (such as CVST or VITT). This study might not have enough power to detect differences between vaccine types (unbalanced groups to real world data).
Third, this cohort was limited to patients vaccinated with the first dose, with vaccination restricted to health care workers and patients over 65 years of age during the study period. Also, due to vaccine shortages during this period, the Health Ministry of Argentina favored a policy of prioritizing the first doses in a larger population and delaying the second doses. The occurrence of TE in patients who received the second dose was not addressed in the present study.
Finally, the study was limited to vaccines approved in Argentina at the studied period, which did not include RNA vaccines such as mRNA-1273 SARS-CoV-2 Vaccine (Moderna) or BNT162b2 (Pfizer). So these results should not be extrapolated to different vaccines.
Conversely, the construction of a control group allowed us to evaluate the excess of risk attributable to Covid-19 vaccine. Another strength of the present study is the inclusion of vaccines not present in other studies such as Gam-COVID-Vac and BBIBP-CorV, but widely used worldwide. Finally it has the strength of systematically detecting TEs thanks to the systematic search.
5. Conclusions
In the present study, we found evidence of statistically significant excess of thrombotic events associated with different Covid-19 vaccines in comparison with an active control group. Nevertheless, this finding should be considered in the context of an overwhelming evidence of Covid-19 vaccines efficacy, and the well documented thrombotic risk associated to Covid-19 infection itself.
Key points
Question: Is Covid-19 vaccination associated with thrombotic events?
Findings: This retrospective cohort study of 29,918 Covid-19 vaccinated subjects with Gam-COVID-Vac, ChAdOx1 nCoV-19, BBIBP-CorV vaccines, showed a statistically significant increase risk of arterial and venous thrombotic events compared to a control group of 24,753 subjects vaccinated with Influenza vaccine.
Meaning: The clinical relevance of this finding should be interpreted in the context of an overwhelming evidence of Covid-19 vaccines efficacy, and the well documented thrombotic risk associated to Covid-19 infection itself.
Declaration of Competing Interest
None of the above authors claim to have any conflict of interest to declare.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ejim.2022.03.002.
Appendix. Supplementary materials
Reference
- 1.Pischel L., Yildirim I., Omer S.B. Fast development of high-quality vaccines in a pandemic. Chest. 2021 doi: 10.1016/j.chest.2021.03.063. Published online April 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dagan N., Barda N., Kepten E., et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. New Engl J Med. 2021;384(15):1412–1423. doi: 10.1056/nejmoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swissmedic ©C. Side effects of COVID-19 vaccines in Switzerland – update. Accessed June 11, 2021. https://www.swissmedic.ch/swissmedic/en/home/news/coronavirus-covid-19/nebenwirkungen-covid-19-impfungen-update.html.
- 4.CDC . CDC; 2021. Myocarditis and pericarditis following mRNA COVID-19 vaccination.https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/myocarditis.html Published June 11Accessed June 11, 2021. [Google Scholar]
- 5.Scully M., Singh D., Lown R., et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(23):2202–2211. doi: 10.1056/NEJMoa2105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schultz N.H., Sørvoll I.H., Michelsen A.E., et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(22):2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muir K.L., Kallam A., Koepsell S.A., Gundabolu K. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N Engl J Med. 2021;384(20):1964–1965. doi: 10.1056/NEJMc2105869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pavord S., Scully M., Hunt B.J., et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med. 2021 doi: 10.1056/NEJMoa2109908. Published online August 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.A.C. Pinho EMA's safety committee continues investigation of COVID-19 vaccine astrazeneca and thromboembolic events – further update. Published March 15, 2021. Accessed June 11, 2021. https://www.ema.europa.eu/en/news/emas-safety-committee-continues-investigation-covid-19-vaccine-astrazeneca-thromboembolic-events.
- 10.Chan B, Odutayo A, Jüni P, et al. Risk of Vaccine-Induced Thrombotic Thrombocytopenia (VITT) following the AstraZeneca/COVISHIELD Adenovirus Vector COVID-19 Vaccines. Science Briefs of the Ontario COVID-19 Science Advisory Table. 2021;2(28) doi: 10.47326/ocsat.2021.02.28.1.0. [DOI] [Google Scholar]
- 11.Macchia A., Ferrante D., Angeleri P., et al. Evaluation of a COVID-19 vaccine campaign and SARS-CoV-2 infection and mortality among adults aged 60 years and older in a middle-income country. JAMA Netw Open. 2021;4(10) doi: 10.1001/jamanetworkopen.2021.30800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monitor público de vacunación. Published February 23, 2021. Accessed June 27, 2021. https://www.argentina.gob.ar/coronavirus/vacuna/aplicadas.
- 13.Banco de recursos de comunicación del ministerio de salud de la nación. Accessed June 27, 2021. https://bancos.salud.gob.ar/recurso/10deg-informe-de-seguridad-en-vacunas.
- 14.Ritchie H., Mathieu E., Rodés-Guirao L., et al. Coronavirus Pandemic (COVID-19) 2020 https://ourworldindata.org/coronavirus Published online March 5Accessed September 9, 2021. [Google Scholar]
- 15.Huitfeldt A., Hernan M.A., Kalager M., Robins J.M. Comparative effectiveness research using observational data: active comparators to emulate target trials with inactive comparators. EGEMS. 2016;4(1):1234. doi: 10.13063/2327-9214.1234. Wash DC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vickers E.R., McClure D.L., Naleway A.L., et al. Risk of venous thromboembolism following influenza vaccination in adults aged 50years and older in the vaccine safety datalink. Vaccine. 2017;35(43):5872–5877. doi: 10.1016/j.vaccine.2017.08.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ay H., Furie K.L., Singhal A., Smith W.S., Sorensen A.G., Koroshetz W.J. An evidence-based causative classification system for acute ischemic stroke. Ann Neurol. 2005;58(5):688–697. doi: 10.1002/ana.20617. [DOI] [PubMed] [Google Scholar]
- 18.Smadja D.M., Yue Q.Y., Chocron R., Sanchez O., Lillo-Le Louet A. Vaccination against COVID-19: insight from arterial and venous thrombosis occurrence using data from VigiBase. Eur Respir J. 2021;58(1) doi: 10.1183/13993003.00956-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pottegård A., Lund L.C., Karlstad Ø., et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ. 2021;373:n1114. doi: 10.1136/bmj.n1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jabagi M.J., Botton J., Bertrand M., et al. Myocardial infarction, stroke, and pulmonary embolism after BNT162b2 mRNA COVID-19 vaccine in people aged 75 years or older. JAMA. 2021 doi: 10.1001/jama.2021.21699. Published online November 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oracle BI interactive dashboards-DAP. Accessed February 7, 2022. https://dap.ema.europa.eu/analytics/saw.dll?PortalPages.
- 22.VAERS - About Us. Accessed February 7, 2022. https://vaers.hhs.gov/about.html.
- 23.Hippisley-Cox J., Patone M., Mei X.W., et al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ. 2021;374:n1931. doi: 10.1136/bmj.n1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lodigiani C., Iapichino G., Carenzo L., et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haimei M.A. Concern about the adverse effects of thrombocytopenia and thrombosis after adenovirus-vectored COVID-19 vaccination. Clin Appl Thromb Hemost. 2021;27 doi: 10.1177/10760296211040110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashrani A.A., Crusan D.J., Petterson T., Bailey K., Heit J.A. Age- and sex-specific incidence of cerebral venous sinus thrombosis associated with Ad26.COV2.S COVID-19 vaccination. JAMA Intern Med. 2021 doi: 10.1001/jamainternmed.2021.6352. Published online November 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J., Wang J., Xu J., et al. Comprehensive investigations revealed consistent pathophysiological alterations after vaccination with COVID-19 vaccines. Cell Discov. 2021;7(1):99. doi: 10.1038/s41421-021-00329-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.