Abstract
Background
With COVID-19 vaccine roll-out ongoing in many countries globally, monitoring of breakthrough infections is of great importance. Antibodies persist in the blood after a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Since COVID-19 vaccines induce immune response to the Spike protein of the virus, which is the main serosurveillance target to date, alternative targets should be explored to distinguish infection from vaccination.
Methods
Multiplex immunoassay data from 1,513 SARS-CoV-2 RT-qPCR-tested individuals (352 positive and 1,161 negative) without COVID-19 vaccination history were used to determine the accuracy of Nucleoprotein-specific immunoglobulin G (IgG) in detecting past SARS-CoV-2 infection. We also described Spike S1 and Nucleoprotein-specific IgG responses in 230 COVID-19 vaccinated individuals (Pfizer/BioNTech).
Results
The sensitivity of Nucleoprotein seropositivity was 85% (95% confidence interval: 80–90%) for mild COVID-19 in the first two months following symptom onset. Sensitivity was lower in asymptomatic individuals (67%, 50–81%). Participants who had experienced a SARS-CoV-2 infection up to 11 months preceding vaccination, as assessed by Spike S1 seropositivity or RT-qPCR, produced 2.7-fold higher median levels of IgG to Spike S1 ≥ 14 days after the first dose as compared to those unexposed to SARS-CoV-2 at ≥ 7 days after the second dose (p = 0.011). Nucleoprotein-specific IgG concentrations were not affected by vaccination in infection-naïve participants.
Conclusions
Serological responses to Nucleoprotein may prove helpful in identifying SARS-CoV-2 infections after vaccination. Furthermore, it can help interpret IgG to Spike S1 after COVID-19 vaccination as particularly high responses shortly after vaccination could be explained by prior exposure history.
Keywords: COVID-19, SARS-CoV-2, Nucleoprotein, Serosurveillance, immunoglobulin G, Multiplex immunoassay
1. Introduction
Since late 2020, multiple countries have initiated vaccine roll-out against COVID-19 which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. Breakthrough infections have been reported shortly after completion of the vaccination regimen [2]. Although COVID-19 vaccines were developed to prevent severe disease and mortality and not to provide sterile protection, it will remain important to monitor the frequency of breakthrough infections as well as their transmission potential, specifically as new SARS-CoV-2 variants emerge [3]. During the acute phase of an infection, molecular (RT-qPCR) and antigen tests are used to confirm symptomatic and asymptomatic breakthrough infections, i.e. after contact tracing or travel to a high-risk area. However, asymptomatic persons who do not seek testing will likely be missed. To ensure a complete picture of the frequency of breakthrough infections for surveillance purposes, frequent RT-qPCR testing would be needed which is time- and labor intensive as well as burdensome to individuals.
Serological assays can identify specific antibodies which indicate previous infection with SARS-CoV-2. SARS-CoV-2 serostatus can be determined high-throughput with multiplex immunoassays (MIA [4]), irrespective of the presence of clinical symptoms. Immunoglobulin G (IgG) antibodies persist for months after infection which widens the window of detection as compared to RT-qPCR and antigen tests [5]. This should provide a more accurate estimate of ongoing transmission in the general population. However, since the main serological marker used to date for SARS-CoV-2 is also the vaccine target, Spike S1 or RBD, alternative serological targets should be explored to distinguish past infection from vaccination. Nucleoprotein is one of the structural immunogenic SARS-CoV-2 proteins. Others have reported sensitivity estimates for seropositivity to Nucleoprotein ranging from 70% to 96% with specificity at ≥ 95%, depending on the assay and reference population used [6], [7], [8], [9]. Reference populations consisted of healthcare workers or hospitalized patients, which are not representative of the general population. Moreover, patients with severe symptoms produce higher antibody levels than those with mild or no symptoms which can lead to overestimation of sensitivity estimates [5], [10]. Hence, the reliability of Nucleoprotein to detect mild or asymptomatic infections, which represent the majority of COVID-19 cases [11], [12], is still unknown.
We previously described a bead-based detection method for simultaneous IgG detection to Spike S1 and Nucleocapsid [4]. In this study we aimed to determine the accuracy of seropositivity to Nucleoprotein and Spike S1 by time since primary RT-qPCR-confirmed infection with mild or asymptomatic SARS-CoV-2 using a prospective household survey as well as a nationwide population survey. Furthermore, we present initial findings of Spike S1 and Nucleoprotein-specific antibody responses in persons vaccinated against COVID-19 using the Pfizer/BioNTech vaccine.
2. Methods
2.1. Study design and population
2.1.1. Household cohort of infected and noninfected participants
A prospective cohort study was performed in households where one household member had tested positive for SARS-CoV-2 to determine within household transmission [13]. Patients with a RT-qPCR-confirmed SARS-CoV-2 infection (=index case) in the Municipal Health Service (GGD) Utrecht region, central Netherlands, were invited to participate with their household if they had at least one child under the age of 18 living at home. Households from 54 index cases were enrolled from March 24th to May 24th 2020 (with a total of 242 participants). Households were excluded if one or more of the household contacts did not want to participate in the study upfront. Furthermore, infants under the age of 1 were excluded. Most families were those of healthcare workers, for whom RT-qPCR testing was available at enrolment during the first pandemic wave (March/April 2020). Study nurses visited the families at their household within 24 h after inclusion and 2–3 weeks after inclusion to collect a naso- and oropharyngeal swab, oral fluid and a fecal sample. At 4–6 weeks after inclusion, a venous blood sample was collected for serological testing. In addition, a longer-term follow-up was done at 9–11 months after inclusion (February/March 2021) when the national vaccination campaign had started in the Netherlands. At this timepoint, participants who reported to have been vaccinated with Pfizer/BioNTech were selected to analyze serological responses following vaccination, Participants filled out a questionnaire at each sampling timepoint including data on demographic factors, symptoms and symptom onset, and vaccination data where applicable (brand product, number of vaccinations and their dates). The study was ethically approved by the Medical-Ethical Review Committee of the University Medical Center Utrecht (NL13529.041.06). All participants above the age of 12 gave written informed consent. Both parents or guardians of participating children below the age of 16 also gave written informed consent for participation of the child.
2.2. National cohort of noninfected, convalescent and vaccinated individuals
Serum samples were collected in an ongoing, nationwide longitudinal serosurveillance study; the PIENTER Corona (PICO) cohort study described by Vos et al. [14], [15]. Briefly, the PICO study emanated from a large-scale nationwide cross-sectional study performed in 2016–17 (PIENTER-3 [16]). Participants from the PIENTER-3 study who had consented to follow-up were invited to participate in the PICO study in April 2020 [14] and the cohort was extended with an additional nationwide random sample in June 2020 [15]. Two more rounds have been completed in October 2020 and February 2021. For this study, we used data from the February 2021 round. Participants were requested to return a self-collected finger-prick blood sample in a microtainer by mail and complete a questionnaire. Questions covered sociodemographic factors, clinical data (type and date of onset of symptoms), virological findings if applicable (SARS-CoV-2 RT-qPCR testing, and date and result of testing) and data on COVID-19 vaccination if applicable (brand product, number of vaccinations and their dates). We selected participants who had also participated in June and October 2020. This was done to determine SARS-CoV-2 exposure history in participants who had not undergone SARS-CoV-2 testing. Questions on clinical symptoms and SARS-CoV-2 confirmatory testing considered the time period between the prior study round (October 2020) and the current study round (February 2021). The study was ethically approved by the Medical Research Ethics Committees United MEC-U and registered under trial number NL8473. All participants above the age of 12 gave written informed consent. Both parents or legal guardians of participating children below the age of 16 years also gave written informed consent for participation of the child.
3. Laboratory methods
3.1. SARS-CoV-2 RT-qPCR testing in the household cohort
All available samples in the household cohort were tested for presence of SARS-CoV-2 as previously described [13], [17]. The results of the naso- and oropharyngeal swab, oral fluid and feces specimens were combined to one result: RT-qPCR negative (all negative) or RT-qPCR positive (any positive). Index cases were considered RT-qPCR positive even if they tested negative as they would have tested RT-qPCR positive with local health authorities prior to enrolment in the study.
3.2. Multiplex immunoassay for immunoglobulin G detection in the household and national cohorts
Serum was separated from blood clot and stored at −20 °C until analysis. Total IgG to Spike S1 and Nucleoprotein was measured with a MIA as previously described [4]. Median fluorescence intensity measurements were expressed as binding antibody units per milliliter (BAU/ml) using 5-parameter logistic interpolation of the International Standard for human anti-SARS-CoV-2 immunoglobulin (20/136 NIBSC standard) [18].
3.3. Statistical analyses
All statistical analyses were performed in R version 4.0.2 [19]. Calculation of seropositivity thresholds and associated assay performance is detailed in the Supplementary Methods. Sensitivity of seropositivity to Nucleoprotein and Spike S1 in detecting a past RT-qPCR-confirmed SARS-CoV-2 infection was determined in 1) hospitalized COVID-19 patients (Intensive Care Unit or ward), 2) mild COVID-19 patients (i.e., with COVID-19-related symptoms but not hospitalized), and 3) individuals with an asymptomatic infection. Specificity was determined in those who tested RT-qPCR negative. COVID-19-related symptoms were classified as fever, coughing, shortness of breath, loss of taste or smell, sore throat, headache, pain while breathing, runny nose, muscle ache, diarrhoea, (extreme) tiredness and/or nausea.
Data from the national and household cohort were analyzed jointly. Unvaccinated participants who underwent SARS-CoV-2 confirmatory testing between two weeks and 6 months prior to serological sampling were included in the study as follows. For the household cohort, all index cases were categorized as SARS-CoV-2 positive as well as any family members testing SARS-CoV-2 positive during study team visits at one day or 2–3 weeks after diagnosis of the index case (Supplementary Fig. 2A). Family members testing RT-qPCR negative at both of these timepoints were included in the negative group. Serological responses were measured 4–6 weeks after diagnosis of the index case. As this cohort commenced immediately following the start of the pandemic in the Netherlands (February/March 2020), these were all primary infections. For the national cohort, any participant who reported to have undergone SARS-CoV-2 confirmatory testing was included (Supplementary Fig. 2B). Those who reported a positive SARS-CoV-2 RT-qPCR test were included in the positive group. However, if they were Spike S1 seropositive in any prior study round, they were excluded, as it would not be a primary infection. Anyone testing SARS-CoV-2 negative was included in the negative group. Exclusion criteria were incomplete symptom data or other testing than RT-qPCR such as rapid antigen tests. The time since onset of symptoms was used to determine sensitivity over time since infection. For asymptomatic participants in the national cohort, the time since RT-qPCR testing date was used. In the household cohort, the time since onset of symptoms or diagnosis date for the index case was used if the time since onset of symptoms was unknown or in asymptomatic participants. Participants who reported to have been vaccinated against COVID-19 were analyzed separately (see below). For reference, sera from 27 hospitalized COVID-19 cases between 14 days and 2 months after onset of symptoms were analysed: 7 patients in the household cohort (Supplementary Fig. 2A), 10 patients from the Erasmus Medical Centre in Rotterdam (Medical Ethical Committee number METC 06/282) and 10 patients from the Dijklander hospital in Hoorn.
To describe IgG to Spike S1 and Nucleoprotein in a COVID-19 vaccinated study population, data from the two cohorts were also combined (Supplementary Fig. 2). Participants who reported to have completed one or two doses of COVID-19 vaccination were included. As nearly all participants had received Pfizer/BioNTech, participants with other vaccine brands were excluded. Furthermore, participants with incomplete vaccination information, such as vaccination dates, were excluded. Past infection with SARS-CoV-2 was based on RT-qPCR confirmation in the household cohort and Spike S1 seroconversion in a previous study round or self-reported RT-qPCR testing where available in the national cohort. Persons without a history of SARS-CoV-2 infection prior to vaccination are hereafter referred to as infection-naïve.
Sensitivity and specificity estimates, and their 95% confidence intervals (CIs) were calculated applying Receiver Operating Characteristic (ROC) curves using the pROC package in R (version 1.16.2 [20]). CIs were computed with 2,000 stratified bootstrap replicates. The Wilcoxon-Mann-Whitney test was used to compare IgG measurements between independent groups, the Wilcoxon signed ranks test for paired groups and the chi-squared test to test for differences in frequencies.
4. Results
4.1. SARS-CoV-2 RT-qPCR-tested study population
Overall, 1,513 individuals participated in this study. A total of 352 had tested RT-qPCR positive for SARS-CoV-2 (23%) and 1,161 negative (77%; Table 1 ). The majority was female (61% of the RT-qPCR positives and 61% of the RT-qPCR negatives) and in the age category 22–65 years old (71% of the RT-qPCR positives and 68% of the RT-qPCR negatives). Most of the RT-qPCR positives experienced mild COVID-19-related symptoms (90%) compared to 56% of the RT-qPCR negatives, while 10% of the RT-qPCR positives and 44% of the RT-qPCR negatives were asymptomatic.
Table 1.
General description of the RT-qPCR-confirmed SARS-CoV-2 study population.
| % (n) unless otherwise specified | SARS-CoV-2 positive | SARS-CoV-2 negative | p-value |
|---|---|---|---|
| N* | 352 | 1,161 | |
| Female | 61.1% (2 1 5) | 60.5% (7 0 2) | 0.885 |
| Age category (years) | |||
|
17.1% (60) | 16.0% (1 8 6) | |
|
71.0% (2 5 0) | 68.4% (7 9 4) | |
|
11.9% (42) | 15.6% (1 8 1) | 0.235 |
| COVID-19 related symptoms** | |||
|
10.2% (36) | 43.6% (5 0 6) | |
|
89.8% (3 1 6) | 56.4% (6 5 5) | <0.001 |
See Supplementary Fig. 2A and 2B for more information on sample availability and exclusion criteria.
Fever, coughing, shortness of breath, loss of taste or smell, sore throat, headache, pain while breathing, runny nose, muscle ache, diarrhoea, (extreme) tiredness and/or nausea.
4.2. Nucleoprotein and Spike S1 seropositivity to detect past SARS-CoV-2 infection
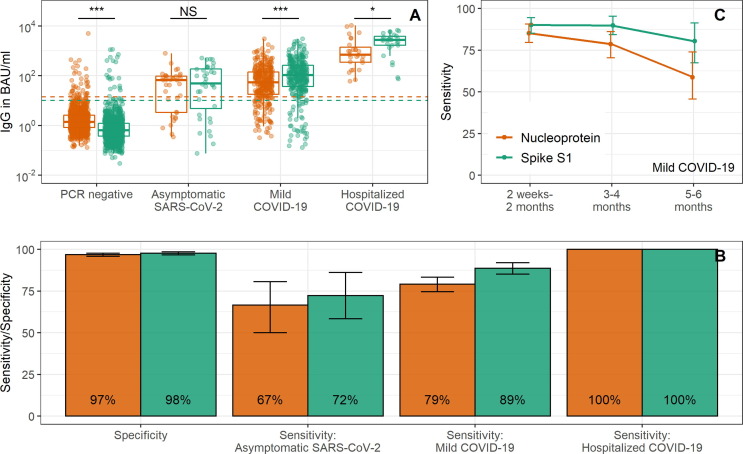
Nucleoprotein and Spike S1 IgG levels for four groups are shown in Fig. 1 A: RT-qPCR negative persons, RT-qPCR positive persons without symptoms, RT-qPCR positive persons with mild COVID-19 and RT-qPCR positive persons hospitalized with COVID-19. Sensitivity of Nucleoprotein was highest in hospitalized COVID-19 patients (100%) between two weeks and two months post onset of symptoms as compared to mild COVID-19 (79%, 95% CI: 75–84%) or asymptomatic SARS-CoV-2 (67%, 50–81%) between two weeks and six months following symptom onset/infection (Fig. 1B). This pattern was the same for seropositivity to Spike S1 (i.e., hospitalized: 100%, mild COVID-19: 89%, 85–92%, asymptomatic SARS-CoV-2: 72%, 56–86%). Sensitivity of Nucleoprotein for mild COVID-19 was highest shortly after infection: 85% (79–91%) at 2 weeks to 2 months following the onset of symptoms to 79% (70–86%) at 3–4 months and 59% (44–72%) at 5–6 months. This decline was faster than that seen for Spike S1 (from 90%, 85–94%, to 90%, 83–95% to 80%, 67–91%; Fig. 1C).
Fig. 1.
Nucleoprotein and Spike S1 IgG responses to detect SARS-CoV-2 infections. In (A) IgG concentrations to Nucleoprotein (orange) and Spike S1 (green) are shown for four groups: RT-qPCR negative persons, RT-qPCR positive persons without symptoms, RT-qPCR positive persons with mild COVID-19 and RT-qPCR positive persons hospitalized with COVID-19, along with the threshold for seropositivity (dashed horizontal line). Data for 2 weeks to 6 months since infection are shown. In (B) specificity and sensitivity estimates with 95% confidence intervals are shown for Nucleoprotein (orange) and Spike S1 (green) seropositivity. Data for 2 weeks to 6 months since infection are shown. In (C) sensitivity estimates with 95% confidence intervals of Nucleoprotein (orange) and Spike S1 (green) seropositivity are shown over time for RT-qPCR positive persons with mild COVID-19; this does not include repeated samples from the same individuals. S1: Spike S1, N: Nucleoprotein, IgG: immunoglobulin G, BAU/ml: binding antibody units; NS: not significant (p > 0.05); *: p < 0.05; **: p < 0.01; ***: p < 0.001.
Specificity in RT-qPCR negative tested persons was 97% (96–98%) for Nucleoprotein and 98% (97–99%) for Spike S1 (Fig. 1B). For persons who seroconverted to either Nucleoprotein or Spike S1 within the RT-qPCR-negative selection (n = 46), 19 had seroconverted to Nucleoprotein only (41%), 9 to Spike S1 only (20%) and 18 to both Nucleoprotein and Spike S1 (39%).
4.3. Spike S1 and Nucleoprotein IgG kinetics after COVID-19 vaccination with Pfizer/BioNTech
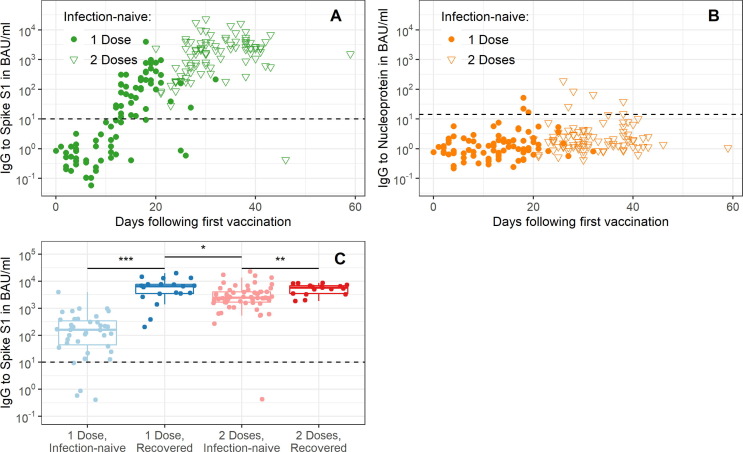
From the national cohort and the household cohort 24 and 19 vaccinated persons with a history of SARS-CoV-2 infection were available respectively, and from the national cohort an additional 187 infection-naïve vaccinated persons were available, totaling 230 persons (Supplementary Fig. 2). Of the 230 Pfizer/BioNTech vaccinated participants, 118 had received two doses at the time of sampling (51%), 172 were female (75%) and 177 were 18–65 years old (77%) vs. 53 who were > 65 years old (23%) (Table 2 ). In infection-naïve individuals (n = 179), IgG to Spike S1 showed a consistent response between individuals over time since vaccination (Fig. 2 A). After two weeks, 96% of the infection-naïve individuals who had received two doses of Pfizer/BioNTech had seroconverted to Spike S1 (126/131, Fig. 2A). The majority was seronegative for Nucleoprotein (93%, 122/131; Fig. 2B). Of the nine seropositive individuals, four were already seropositive for Nucleoprotein prior to vaccination but not for Spike S1. Participants who had experienced a SARS-CoV-2 infection preceding vaccination, produced 2.7-fold higher median levels of IgG to Spike S1 ≥ 14 days after the first dose as compared to those not infected with SARS-CoV-2 at ≥ 7 days after the second dose (6,480 vs. 2,438 BAU/ml, p = 0.011, Fig. 2C).
Table 2.
General description of the COVID-19 vaccinated study population.
| % (n) unless otherwise specified | |
|---|---|
| N* | 230 |
| Female | 75% (1 7 2) |
| Age category in years | |
|
77% (1 7 7) |
|
23% (53) |
| Healthcare worker | 75% (1 72) |
| Vaccination | |
|
51% (1 1 8) |
|
24, 0–59 (14–32) |
| Past SARS-CoV-2 infection | |
|
78% (1 7 9) |
|
14% (32) |
|
8% (19) |
IQR: interquartile range; IgG: immunoglobulin G.
See Supplementary Fig. 2A and 2C for more information on sample availability and exclusion criteria.
Fig. 2.
Nucleoprotein and Spike S1 IgG kinetics following COVID-19 vaccination. IgG measurements to Spike S1 (A) and Nucleoprotein (B) in infection-naïve individuals are shown over days since first vaccination. In (C) IgG measurements to Spike S1 are shown by prior exposure status and number of doses received, individuals were included if they were sampled ≥ 14 days after the first dose or ≥ 7 days after the second dose. In (A-C) the dashed horizontal line depicts the threshold for seropositivity. S1: Spike S1, N: Nucleoprotein, IgG: immunoglobulin G, BAU/ml: binding antibody units; NS: not significant (p > 0.05); *: p < 0.05; **: p < 0.01; ***: p < 0.001.
5. Discussion
As COVID-19 vaccines induce immune responses to the Spike protein, alternative serological targets need to be considered for serosurveillance of SARS-CoV-2 breakthrough infections. Here, we showed that seropositivity to Nucleoprotein can detect mild COVID-19 with a sensitivity of 85% between two weeks and two months following symptom onset compared to 90% for Spike S1. At 3–4 months post symptom onset, sensitivity declined to 79% for Nucleoprotein while it remained 90% for Spike S1.
Several publications have focused on the sensitivity of Nucleoprotein to detect past RT-qPCR-confirmed SARS-CoV-2 infections with estimates ranging from 70% to 96% [6], [7], [8], [9]. The wide range in the observed sensitivity estimates is likely due to differences in the used reference population, often consisting of hospitalized patients or healthcare workers with COVID-19, and differences related to the applied antibody detection platforms. Few have stratified results by symptomatic status of the reference populations or time since onset of symptoms [7], in spite of the fact that breakthrough infections are expected to be more frequently mild or asymptomatic than primary infections. In mildly symptomatic persons (healthcare workers), Mariën et al. reported a sensitivity of 70% within six weeks and 85% more than five months after symptom onset [7]. We also reported a sensitivity of 85% in mildly symptomatic patients between two weeks and two months after symptom onset though we saw a decline to 59% at 5–6 months after symptom onset. In persons with an asymptomatic SARS-CoV-2 infection in our study, sensitivity of Nucleoprotein was 67%; insufficient numbers were available to stratify this estimate further by time since infection. Our estimate for Spike S1 sensitivity in asymptomatic participants (72%; 26/36) was similar to that recently published by Vanshylla et al. combining IgG and IgA responses (77%; 34/44) [21]. Although Nucleoprotein sensitivity was lower in asymptomatic individuals, few to none of these individuals would have been identified by passive surveillance using RT-qPCR or antigen tests as the absence of symptoms would limit chances of seeking diagnosis.
Sensitivity decreased to 79% at 3–4 months following infection, a drop of 8% in 2 months. Choudhry et al. reported 31% seroreversion for Nucleoprotein IgG after three months in seroconverted healthcare workers in the United Kingdom using rapid IgG/IgM tests [22]. Others have likewise shown that Nucleoprotein IgG antibodies decline on average 1.5–2 times faster than those to Spike S1 [10], [23]. The higher rate of seroreversion for Nucleoprotein compared to Spike S1, means that regular serological measurements is recommended to ensure detection of breakthrough infections (e.g. 2–3 monthly intervals). The serological response to Nucleoprotein may vary more following COVID-19 vaccination as partial immunity might limit viral replication and thus exposure of the immune system to the viral Nucleoprotein. Asymptomatic and mild cases that might be missed by RT-qPCR or serological testing are unlikely to pose a risk of development of disease requiring hospitalization. However, they might still contribute to transmission of the virus.
Previous specificity estimates for bead-based assays were ≥ 97% based on pre-pandemic controls [7], [8], [9]. Here we likewise showed that 97% of the RT-qPCR-negative population was seronegative for Nucleoprotein. However, presence of SARS-CoV-2 could have been missed in our study due to no detectable SARS-CoV-2 RNA at the time of sampling or incorrect sampling thus lowering specificity. 18 out of 37 Nucleoprotein seropositive persons also seroconverted to Spike S1 which strengthens the hypothesis that these samples represent participants not being sampled optimally for RT-qPCR. The performance of an assay is a trade-off between sensitivity and specificity. As we expect the prevalence of breakthrough infections to be low, we focused on high specificity by setting a conservative seropositivity threshold.
Most of the COVID-19 vaccinated participants in the current study were healthcare workers who received Pfizer/BioNTech. IgG to Spike S1 after two doses showed comparable levels as those for healthcare workers from another study conducted in Rotterdam, the Netherlands [24], while a previously exposed population already produced robust IgG after one dose of Pfizer/BioNTech vaccine. Interestingly, the majority of the previously infected participants receiving one vaccination dose got infected approximately 11 months prior to vaccination (72%, 18/25). This suggests that infection up to a year prior to vaccination still enables robust boosting of IgG to Spike S1 as observed by others [25], [26], though numbers were small and nearly all were symptomatic. No Nucleoprotein responses should be seen following vaccination in an infection-naïve population as the Pfizer/BioNTech vaccine targets the Spike S1 antigen. Indeed, the majority of infection-naïve vaccinated persons was seronegative to Nucleoprotein (93%). It is possible that some of the Nucleoprotein-positives represent infections that were missed due to lack of symptoms and/or confirmatory testing by participants in the national cohort. However, since Nucleoprotein antibody concentrations were relatively low, these also may contain non-specific responses. No breakthrough infections were observed in our vaccinated population thus we were not able to determine the sensitivity of Nucleoprotein to detect such infections. Allen et al. showed that in 23 hospital workers with breakthrough infections after vaccination with Pfizer/BioNTech, 22% were symptomatic and 26% were seropositive to Nucleoprotein [27]. Bergwerk et al. studied 39 healthcare workers with breakthrough infections following Pfizer/BioNTech vaccination of which 67% were symptomatic [28]. Out of 22 with serological data available, 81% was seropositive to Nucleoprotein. Both studies used the Elecsys Roche anti-Nucleocapsid total antibodies assay. Although the number of participants is relatively small in both studies, the higher sensitivity estimate by Bergwerk et al. could be explained by the higher proportion of symptomatic infections. This would be in line with our findings of higher sensitivity in symptomatic compared to asymptomatic SARS-CoV-2 infections in unvaccinated persons. These estimates should be updated as more serological data become available in vaccinated populations.
There are strengths and weaknesses in the cohorts we used in this study. Study team nurses collecting samples and questionnaire data at pre-set sampling timepoints is the strength of the household cohort, but its weakness includes that results are likely to correlate within families (i.e., genetic relatedness and immune response). The national cohort is more representative of the general population, including more asymptomatic individuals, and the repeated cross-sectional design ensured that participants were included with different time frames since infection and/or vaccination. However, the weakness of this approach is that it relied on self-reported data. Several types of bias may arise from self-reported data including recall bias, e.g. those who tested SARS-CoV-2 positive might be more likely to remember the type of symptoms or test they received.
In conclusion, we showed that Nucleoprotein can detect prior SARS-CoV-2 infections with a sensitivity of 85% in a mildly symptomatic unvaccinated population between two weeks and two months after symptom onset. Serological responses to Nucleoprotein may thus prove helpful in identifying the frequency of SARS-CoV-2 infections in vaccinated persons, alongside molecular tests. Furthermore, it can help to interpret IgG to Spike S1 responses after COVID-19 vaccination as particularly high responses shortly after vaccination could be explained by prior exposure history.
Funding
This work was supported by the Dutch Ministry of Public Health, Welfare, and Sports (VWS).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to thank all study participants and the team at the laboratory conducting all RT-qPCR assays, represented by Bas van der Veer, Sharon van den Brink and Anne-Marie van den Brandt.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.03.009.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheikh A., McMenamin J., Taylor B., Robertson C. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397(10293):2461–2462. doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.den Hartog G., Schepp R.M., Kuijer M., GeurtsvanKessel C., van Beek J., Rots N., et al. SARS-CoV-2-Specific Antibody Detection for Seroepidemiology: A Multiplex Analysis Approach Accounting for Accurate Seroprevalence. J Infect Dis. 2020;222(9):1452–1461. doi: 10.1093/infdis/jiaa479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.den Hartog G., et al. Persistence of antibodies to SARS-CoV-2 in relation to symptoms in a nationwide prospective study. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fenwick C., Croxatto A., Coste A.T., Pojer F., André C., Pellaton C., et al. Changes in SARS-CoV-2 Spike versus Nucleoprotein Antibody Responses Impact the Estimates of Infections in Population-Based Seroprevalence Studies. J Virol. 2021;95(3) doi: 10.1128/JVI.01828-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marien J., et al. Evaluating SARS-CoV-2 spike and nucleocapsid proteins as targets for antibody detection in severe and mild COVID-19 cases using a Luminex bead-based assay. J Virol Methods. 2021;288 doi: 10.1016/j.jviromet.2020.114025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosado J., Pelleau S., Cockram C., Merkling S.H., Nekkab N., Demeret C., et al. Multiplex assays for the identification of serological signatures of SARS-CoV-2 infection: an antibody-based diagnostic and machine learning study. Lancet Microbe. 2021;2(2):e60–e69. doi: 10.1016/S2666-5247(20)30197-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fotis C., Meimetis N., Tsolakos N., Politou M., Akinosoglou K., Pliaka V., et al. Accurate SARS-CoV-2 seroprevalence surveys require robust multi-antigen assays. Sci Rep. 2021;11(1) doi: 10.1038/s41598-021-86035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dan J.M., Mateus J., Kato Y.u., Hastie K.M., Yu E.D., Faliti C.E., et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529) doi: 10.1126/science:abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Z., McGoogan J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 12.McDonald S.A., et al. Estimating the asymptomatic proportion of SARS-CoV-2 infection in the general population: Analysis of nationwide serosurvey data in the Netherlands. Eur J Epidemiol. 2021 doi: 10.1007/s10654-021-00768-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reukers D.F.M., et al. High infection secondary attack rates of SARS-CoV-2 in Dutch households revealed by dense sampling. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vos E.R.A., den Hartog G., Schepp R.M., Kaaijk P., van Vliet J., Helm K., et al. Nationwide seroprevalence of SARS-CoV-2 and identification of risk factors in the general population of the Netherlands during the first epidemic wave. J Epidemiol Community Health. 2021;75(6):489–495. doi: 10.1136/jech-2020-215678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vos E.R.A., et al. Associations between measures of social distancing and SARS-CoV-2 seropositivity: a nationwide population-based study in the Netherlands. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verberk J.D.M., Vos R.A., Mollema L., van Vliet J., van Weert J.W.M., de Melker H.E., et al. Third national biobank for population-based seroprevalence studies in the Netherlands, including the Caribbean Netherlands. BMC Infect Dis. 2019;19(1) doi: 10.1186/s12879-019-4019-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.KW., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. First WHO International Standard for anti-SARS-CoV-2 immunoglobulin (human). 2020 [cited 2021 7 June 2021]; Available from: https://www.nibsc.org/documents/ifu/20-136.pdf.
- 19.R Core Team, R: A language and environment for statistical computing. 2020, R Foundation for Statistical Computing: Vienna, Austria.
- 20.Robin X., et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinf. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanshylla K., Di Cristanziano V., Kleipass F., Dewald F., Schommers P., Gieselmann L., et al. Kinetics and correlates of the neutralizing antibody response to SARS-CoV-2 infection in humans. Cell Host Microbe. 2021;29(6):917–929.e4. doi: 10.1016/j.chom.2021.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choudhry N., et al. Disparities of SARS-CoV-2 Nucleoprotein-Specific IgG in Healthcare Workers in East London. UK. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.642723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wheatley A.K., Juno J.A., Wang J.J., Selva K.J., Reynaldi A., Tan H.-X., et al. Evolution of immune responses to SARS-CoV-2 in mild-moderate COVID-19. Nat Commun. 2021;12(1) doi: 10.1038/s41467-021-21444-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geers D., et al. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci Immunol. 2021;6(59) doi: 10.1126/sciimmunol.abj1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Favresse, J., et al., Early antibody response in health-care professionals after two doses of SARS-CoV-2 mRNA vaccine (BNT162b2). Clin Microbiol Infect, 2021 [DOI] [PMC free article] [PubMed]
- 26.Abu Jabal K., Ben-Amram H., Beiruti K., Batheesh Y., Sussan C., Zarka S., et al. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers, Israel, December 2020 to January 2021. 2021;26(6) doi: 10.2807/1560-7917.ES.2021.26.6.2100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allen N., Brady M., Carrion Martin A.I., Domegan L., Walsh C., Doherty L., et al. Serological markers of SARS-CoV-2 infection; anti-nucleocapsid antibody positivity may not be the ideal marker of natural infection in vaccinated individuals. J Infect. 2021;83(4):e9–e10. doi: 10.1016/j.jinf.2021.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bergwerk M., Gonen T., Lustig Y., Amit S., Lipsitch M., Cohen C., et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N Engl J Med. 2021;385(16):1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.