Abstract
Background
Aim of the present report was to investigate the repercussions of COVID-19 pandemic on the procedural volumes and on the main indications of pediatric digestive endoscopy in Italy.
Methods
An online survey was distributed at the beginning of December 2020 to Italian digestive endoscopy centers. Data were collected comparing two selected time intervals: the first from 1st of February 2019 to 30th June 2019 and the second from 1st February 2020 to 30th June 2020.
Results
Responses to the survey came from 24 pediatric endoscopy Units. Globally, a reduction of 37.2% was observed between 2019 and 2020 periods with a significant decrease in median number of procedures (111 vs 57, p < 0.001). Both the median number of procedures performed for new diagnoses and those for follow-up purposes significantly decreased in 2020 (63 vs 36, p < 0.001 and 42 vs 21, p< 0.001, respectively). We reported a drastic reduction of procedures performed for suspected Celiac Disease and Functional Gastrointestinal Disorders (55.1% and 58.0%, respectively). Diagnostic endoscopies for suspected IBD decreased of 15.5%, whereas procedures for Mucosal Healing (MH) assessment reduced of 48.3%.
Conclusions
Our study provides real-world data outlining the meaningful impact of COVID-19 on pediatric endoscopy practice in Italy.
Keywords: Endoscopy, COVID-19, Pediatric Endoscopy
1. Introduction
A novel, deadly, coronavirus (SARS-CoV-2) was identified in Wuhan city (China) in 2019 and found to be the causative agent of a severe acute respiratory syndrome (COVID-19) [1]. After the initial outbreak in China, COVID-19 exponentially affected countries all around the world and rapidly acquired the size of a pandemic. In Italy, on February 20th 2020, the first report of a severe case of COVID-19 in a young male from Codogno, a town just south of Milan, was rapidly followed by the identification of clusters of infected patients in the densely populated Lombardy region and in nearby regions of Northern Italy [2]. Starting from March 9th to May 4th, the Italian government, the first in Europe, responded to such unexpected challenge with unprecedented measures and established a strict lock-down [3]. Public healthcare was reorganized in order to receive the upcoming inflow of COVID-19 patients and to minimize COVID-19 transmission [4]. Although the Pediatric Gastroenterology Units were not primarily involved in the care of COVID-19 patients, many of them underwent drastic modifications [5]. Both in adult and in children, non-urgent diagnostic procedures were canceled or rescheduled, particularly digestive endoscopy procedures [6].
Healthcare providers in Endoscopy Units are at increased risk of infection by COVID-19 from inhalation of airborne droplets, conjunctival contact [7] and potential fecal-oral transmission of the virus [8,9]. Moreover, aerosolized infections have been reported during upper GI endoscopy, making it a high-risk procedure [6]. In addition, the presence of live virus has been demonstrated in patients’ stools [10].
As a result, a widespread reduction of procedural volumes was observed, both in Italy and in several other countries [11], [12], [13]. Most of the data regarding changing patterns of digestive endoscopy during COVID-19 pandemic arise from adult literature, whereas pediatric data are limited [14].
Indeed, digestive endoscopy in pediatrics has some peculiar features. Firstly, diagnostic indications are different from adult patients. As an example, cancer surveillance, which represents a relevant indication for colonoscopy in adult populations, is rarely performed in children and adolescents, resulting in far lower volumes of pediatric colonoscopies. Additionally, digestive endoscopy in children is generally performed under deep sedation and/or general anesthesia in order to ensure patient safety, comfort and cooperation [15]. Lastly, in children, there is a higher proportion of patients with mild or asymptomatic COVID-19 disease and gastrointestinal (GI) symptoms are generally more frequent at the time of endoscopy [14]. On the other hand, diagnostic endoscopic procedures are essential to the assessment, treatment, and care of infants and children with a vast number of GI conditions [16]. Diagnosis of chronic GI disorders, such as Inflammatory Bowel Diseases (IBD) and Eosinophilic Gastroenteritis (EGE) whose incidence rate is increasing in pediatric population, relies on well-established endoscopic and histologic criteria [17,18]. Similarly, despite the latest European Society of Gastroenterology Hepatology and Nutrition (ESPGHAN) guidelines consider the possibility of a biopsy-sparing approach for the diagnosis of celiac disease in a selected group of children, a relevant proportion of patients still require a duodenal biopsy for the diagnosis of such condition [19].
Aim of the present report performed by the Endoscopy Working Group of the Italian Society of Gastroenterology and Nutrition (SIGENP) was to conduct a survey among pediatric endoscopy Units in Italy to investigate how COVID-19 has influenced the procedural volumes, indications, health care provider's safety perceptions and waiting time of pediatric endoscopic procedures.
2. Methods
2.1. Survey design
An online survey (Supplementary file 1) was developed by the Endoscopy Working Group of the Italian Society of Pediatric Gastroenterology, Hepatology and Nutrition.
The survey was completely anonymized and it was conducted using a Google form and was structured into the following domains:
-
I)
Region and Center of endoscopy practicing
-
II)
Monthly procedural volumes before and during COVID-19 in two selected periods: from 1 st of February 2019 to 30th June 2019 and from 1st February 2020- 30 th June 2020
-
III)
Indications for endoscopy and diagnosis
-
IV)
Impact of COVID-19 outbreak on scheduling/planning endoscopy
-
V)
Impact of COVID-19 outbreak on the delay of procedures
-
VI)
Impact of COVID-19 outbreak on personal safety.
At the beginning of December 2020, the survey was distributed to Italian GI endoscopy centers via email by the Italian Society of Pediatric Gastroenterology, Hepatology and Nutrition. The survey was open for 10 weeks from 15th December 2020 to 20th April 2021 and was sent to 30 centers practicing pediatric endoscopy in Italy.
2.2. Statistical analysis
Data were collected, analyzed and extracted with graphs and analysis performed using SPSS (IBM SPSS Inc., Chicago, Illinois). Percentages were calculated based on the total number of survey participants and the number of responses to each individual question. Data were collected and analyzed by means of descriptive statistics as a mean and standard deviation or medians and interquartile ranges, where indicated. Categorical variables were compared using the χ2 or the Fisher test, where appropriate. Pairwise comparisons of procedural numbers were performed between pre-COVID-19 and COVID-19 periods using Wilcoxon signed-rank tests. All differences were considered significant at two-sided P-value <0.05.
3. Results
3.1. Impact of COVID-19 on procedural volumes
Overall, 24 out of 30 (80%) pediatric GI endoscopy Units coming from 13 different regions in Italy were included in the survey (Supplementary Appendix 1). A total of 4138 GI endoscopic procedures were performed from the 1st February 2019 to the 30th of June 2019 across the 24 centers included the survey. In contrast, during the period ranging from the 1st February 2020 and the 30th of June 2020, 2599 endoscopies were reported with a percentage decrease of 37.2%. The median number of procedures performed in 2019 period was 111 (IQR: 156), whereas the median number of endoscopic examinations performed in 2020 period was 57 (IQR: 93) (p<0.001). At a single center level, all those participating to the survey (100%) experienced a reduction in endoscopic procedures in 2020 when compared to 2019.
Table 1 shows the monthly distribution of endoscopic procedures in 2019 and 2020 periods. A decrease of the absolute number of endoscopies from 2019 to 2020 has been observed for each month of the analyzed period. Nevertheless, when comparing individually each single month of the study period, median number of monthly endoscopic procedures significantly decreased in March, April, May and June (p<0.001, <0.001, <0.001 and 0.004, respectively) but not in February (p = 0.053) (Table 1).
Table 1.
Monthly distribution of endoscopic procedures in 2019 and 2020.
| Month | 2019 | 2020 | p-value | Percentage decrease |
|---|---|---|---|---|
| February | ||||
| Total | 797 | 725 | −9.0% | |
| Median (IQR) | 24 (24) | 18 (23) | 0.053 | |
| March | ||||
| Total | 828 | 383 | −53.7% | |
| Median (IQR) | 23 (26) | 8 (19) | < 0.001* | |
| April | ||||
| Total | 823 | 295 | −64.1% | |
| Median (IQR) | 19 (25) | 7 (14) | < 0.001* | |
| May | ||||
| Total | 927 | 520 | −43.9% | |
| Median (IQR) | 26 (31) | 12 (22) | < 0.001* | |
| June | ||||
| Total | 760 | 674 | −11.3% | |
| Median (IQR) | 20 (31) | 14 (24) | 0.003* |
*Below threshold of statistical significance (p = 0.05).
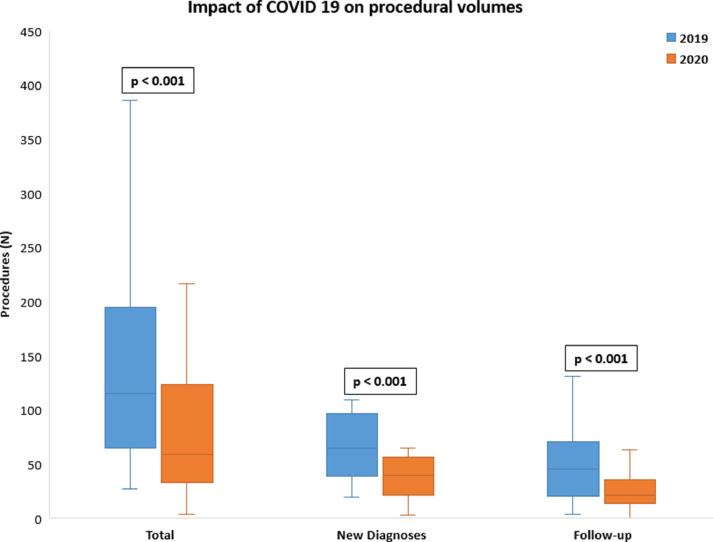
The reported total number of endoscopic procedures performed for new diagnoses was 2369 in the period from the 1st February 2019 to the 30th of June 2019 and 1321 during the period from the 1st February 2020 to the 30th of June 2020 (percentage decrease: −44.2%). The median number of endoscopic procedures performed per center for new diagnoses was significantly lower in 2020 period, when compared to 2019 one (p<0.001). The percentage decrease in total number of endoscopies performed for follow-up purposes was −30.3% (1074 vs 1542, respectively). Again, the median number of procedures performed for follow-up purposes was significantly lower in 2020 compared to 2019 (p<0.001) (Fig. 1).
Fig. 1.
Mean endoscopic procedures performed in 2019 and 2020 period.
3.2. Impact of COVID-19 on indication for endoscopic procedures
Main presenting symptoms leading to diagnostic endoscopic procedures are summarized in Table 2 . A significant reduction in the total number of endoscopies performed for recurrent abdominal pain, anemia, vomiting, weight loss and dyspepsia was observed (Table 2). In contrast, a slighter reduction in the total number of endoscopies performed for hematochezia and melena was reported. Table 3 summarizes the main presumptive diagnosis at endoscopy procedure in 2019 and 2020 periods. A consistent reduction in the number of diagnostic endoscopic procedures for new diagnoses of celiac disease, EGE and Eosinophilic Esophagitis (EoE), gastroesophageal reflux disease (GERD), Helicobacter pylori infection (Hp) and functional gastrointestinal disorders (FGID) was detected. The total number of endoscopies performed for suspected Celiac Disease across the 24 participating centers decreased from 621 to 279 from 2019 to 2020 period (percentage decrease: −55.1%). Median number of endoscopic procedures performed per center for suspected Celiac Disease was drastically lower in 2019 compared to 2020 period (p<0.001). Similarly, a significant reduction in the median number of procedures performed for suspected GERD, eosinophilic gastroenteropathy/EoE, Hp and to rule out FGID was observed from 2019 to 2020 period. In contrast, the number of endoscopic procedures performed for suspected IBD did not decrease significantly with a reduction of only 15.5%.
Table 2.
Main presenting symptoms at endoscopic procedure.
| Main presenting symptom at endoscopic procedure performed for diagnostic purpose | 2019 | 2020 | Percentage decrease | p-value |
|---|---|---|---|---|
| Hematochezia | ||||
| Total | 271 | 229 | −15.5% | 0.107 |
| Median (IQR) | 7 (10) | 7 (7) | ||
| Melena | ||||
| Total | 78 | 54 | −26.9% | 0.286 |
| Median (IQR) | 2 (3) | 2 (3) | ||
| Recurrent abdominal pain | ||||
| Total | 742 | 336 | −54.7% | < 0.001* |
| Median (IQR) | 21 (19) | 10 (14) | ||
| Weight loss | ||||
| Total | 265 | 164 | −38.1% | 0.002* |
| Median (IQR) | 6 (12) | 5 (6) | ||
| Anemia | ||||
| Total | 213 | 129 | −39.4% | 0.001* |
| Median (IQR) | 6 (10) | 4 (5) | ||
| Swallowing difficulties | ||||
| Total | 246 | 222 | −9.7% | 0.732 |
| Median (IQR) | 5 (10) | 5 (9) | ||
| Dyspepsia | ||||
| Total | 299 | 150 | −49.8% | < 0.001* |
| Median (IQR) | 9 (10) | 3 (5) | ||
| Vomiting | ||||
| Total | 229 | 128 | −44.1% | 0.007* |
| Median (IQR) | 5 (15) | 2 (7) |
*Below the threshold of statistical significance (p = 0.05).
Table 3.
Main presumptive diagnoses at endoscopic procedure and main indications for follow-up procedures.
| Presumptive diagnosis at endoscopy | 2019 | 2020 | Percentage decrease | p-value |
|---|---|---|---|---|
| IBD | ||||
| Total | 258 | 218 | −15.5% | 0.210 |
| Median (IQR) | 7 (11) | 7 (10) | ||
| HP Gastritis | ||||
| Total | 207 | 122 | −41.1% | 0.001* |
| Median (IQR) | 3 (9) | 2 (4) | ||
| Eosinophilic Gastroenteropathy | ||||
| Total | 129 | 63 | −51.2% | 0.001* |
| Median (IQR) | 2 (5) | 1 (3) | ||
| GERD | ||||
| Total | 291 | 133 | −54.3% | <0.001* |
| Median (IQR) | 7 (13) | 2 (7) | ||
| Celiac Disease | ||||
| Total | 621 | 279 | −55.1% | <0.001* |
| Median (IQR) | 27 (27) | 8 (9) | ||
| FGID | ||||
| Total | 417 | 175 | −58.0% | 0.003* |
| Median (IQR) | 9 (19) | 3(9) | ||
| Main indication for follow-up endoscopy | ||||
| IBD, disease flare | 0.223 | |||
| Total | 292 | 244 | −16.4% | |
| Median (IQR) | 9 (14) | 6 (7) | ||
| IBD, mucosal healing assessment | 0.001* | |||
| Total | 211 | 109 | −48.3% | |
| Median (IQR) | 5 (13) | 1 (7) | ||
| Eosinophilic gastroentheropaty | 0.002* | |||
| Total | 194 | 90 | −53.6% | |
| Median (IQR) | 5 (12) | 2 (5) | ||
| GERD | 0.005* | |||
| Total | 139 | 50 | −64.0% | |
| Median (IQR) | 2 (7) | 0 (3) | ||
*Below the threshold of statistical significance (p = 0.05).
In addition, we observed a considerable reduction in the number of endoscopies performed for the assessment of Mucosal Healing (MH) in patients with IBD, for the follow up of both eosinophilic gastroenteropathies and GERD and for the confirmation of Hp eradication (p = 0.01, =0.001, <0.001, =0.01). In contrast, the median number of procedures performed for IBD recrudescence did not decrease significantly (Table 3).
3.3. Impact of COVID-19 on physician perception of endoscopy delay and personal safety
Participants were asked to rate their level of perceived priority for the execution of endoscopic procedures during the 2019 and 2020 periods. Priority was expressed as a number ranging from 1 (minimum priority) to 5 (maximum priority) Table 4 . When looking at perceived priority for new diagnoses of IBD, 100% of responders reported either maximum (5) or high (4) priority in both 2019 and 2020. Conversely, when considering IBD endoscopic re-assessment, the proportion of responders reporting high or maximum priority decreased significantly from 2019 to 2020 (62.5% vs 25%, respectively, p = 0.008).
Table 4.
Perceived priority for the execution of endoscopic procedures during the 2019 and 2020 periods.
| Perceived priority | 2019 | 2020 |
|---|---|---|
| IBD: new diagnosis, n (%) | ||
| 1 | 0 (0) | 0 (0) |
| 2 | 0 (0) | 0 (0) |
| 3 | 0 (0) | 0 (0) |
| 4 | 0 (0) | 3 (12.5) |
| 5 | 23 (95.8) | 20 (83.3) |
| Missing | (4.2) | 1 (4.2) |
| IBD: endoscopic re-assessment, n (%) | ||
| 1 | 0 (0) | 2 (8.3) |
| 2 | 2 (8.3) | 7 (29.2) |
| 3 | 7 (29.2) | 9 (37.5) |
| 4 | 11 (45.8) | 4 (16.7) |
| 5 | 4 (16.7) | 2 (8.3) |
| Celiac Disease, n (%) | ||
| 1 | 0 (0) | 0 (0) |
| 2 | 1 (4.2) | 6 (25.0) |
| 3 | 8 (33.3) | 6 (25.0) |
| 4 | 11 (45.8) | 9 (37.5) |
| 5 | 4 (16.7) | 2 (8.3) |
| Missing | 1 (4.2) | 1 (4.2) |
| EoE: new diagnosis, n (%) | ||
| 1 | 0 (0) | 0 (0) |
| 2 | 0 (0) | 0 (0) |
| 3 | 1 (4.2) | 3 (12.5) |
| 4 | 6 (25.0) | 10 (41.7) |
| 5 | 16 (66.7) | 10 (41.7) |
| Missing | 1 (4.2) | 1 (4.2) |
| EoE: endoscopic re-assessment, n (%) | ||
| 1 | 0 (0) | 9 (37.5) |
| 2 | 3 (12.5) | 5 (20.8) |
| 3 | 11 (45.8) | 3 (12.5) |
| 4 | 7 (29.2) | 6 (25) |
| 5 | 2 (8.3) | 0 (0) |
| Missing | 1 (4.2) | 1 (4.2) |
| GERD: new diagnosis, n (%) | ||
| 1 | 1 (4.2) | 4 (16.7) |
| 2 | 2 (8.3) | 7 (29.2) |
| 3 | 12 (50) | 8 (33.3) |
| 4 | 9 (37.5) | 4 (16.7) |
| 5 | 0 (0) | 0 (0) |
| Missing | 0 (0) | 1 (4.2) |
| GERD: re-assessment, n (%) | ||
| 1 | 6 (25.0) | 16 (66.7) |
| 2 | 9 (37.5) | 3 (12.5) |
| 3 | 5 (20.8) | 3 (12.5) |
| 4 | 4 (16.7) | 1 (4.2) |
| 5 | 0 (0) | 0 (0) |
| Missing | 0 (0) | 1 (4.2) |
| FGID, n (%) | ||
| 1 | 1 (4.2) | 16 (66.7) |
| 2 | 6 (25) | 6 (25.0) |
| 3 | 6 (25) | 1 (4.2) |
| 4 | 9 (37.5) | 0 (0) |
| 5 | 1 (4.2) | 0 (0) |
| Missing | 1 (4.2) | 1 (4.2) |
Concerns on personal safety and delay of endoscopic procedures
Participants to survey were further asked to rate the personal perception of safety and their perception on the increase of waiting lists for endoscopic procedures, expressing their perception with a number ranging from 1 (minimum) to 5 (maximum). When asked about institutional screening protocols for patients undergoing endoscopic procedures, 16/24 (66.7%) of the pediatric endoscopists who participated to the survey rated 5 (maximum safety), 7/24 (29.2%) rated 4 (very high safety) and only one out of 24 (4.2%) rated 3 (moderate safety). When asked about appropriateness of personal protective equipment (PPE) for the execution of endoscopic procedures, 17/24 (70.8%) of the participants to the survey rated 5, 6/24 (25%) rated 4 and only one out of 24 (4.2%) rated 3. Further, 23/24 (95.8%) of the participants experienced delay in endoscopic procedures due to the willingness of the families to postpone the exam owing to the ongoing COVID-19 pandemic.
Moreover, when asked to rate the personal perception regarding elongation of waiting lists for endoscopic examinations, 17/24 (70.8%) participants reported consistent increase of waiting lists whereas only 7/24 (29.2%) reported only a slight to moderate increase. Table 5 . shows survey responders’ perception of waiting lists elongation according to the different indications for the endoscopic procedures.
Table 5.
Perceived waiting lists elongation according to the different indications for the endoscopic procedures in 2020.
| Waiting list elongation | |
|---|---|
| CD: new diagnosis, n (%) | |
| 1 | 11 (45.8) |
| 2 | 10 (41.7) |
| 3 | 3 (12.5) |
| 4 | 0 (0) |
| 5 | 0 (0) |
| Missing | (4.2) |
| UC: new diagnosis, n (%) | |
| 1 | 13 (54.2) |
| 2 | 8 (33.3) |
| 3 | 3 (12.5) |
| 4 | 0 (0) |
| 5 | 0(0) |
| Celiac Disease, n (%) | |
| 1 | 1 (4.2) |
| 2 | 4 (16.7) |
| 3 | 6 (25.0) |
| 4 | 7 (29.2) |
| 5 | 6 (25.0) |
| EoE: new diagnosis, n (%) | |
| 1 | 5 (20.8) |
| 2 | 8 (33.3) |
| 3 | 8 (33.3) |
| 4 | 2 (8.4) |
| 5 | 0 (0) |
| Missing | 1 (4.2) |
| GERD: new diagnosis, n (%) | |
| 1 | 0 (0) |
| 2 | 5 (20.8) |
| 3 | 8 (33.3) |
| 4 | 4 (16.7) |
| 5 | 7 (29.2) |
| Missing | 1 (4.2) |
| FGID, n (%) | |
| 1 | 0 (0) |
| 2 | 1 (4.2) |
| 3 | 3 (12.5) |
| 4 | 7 (29.2) |
| 5 | 12 (50.0) |
| Missing | 1 (4.2) |
| CD: treatment escalation, n (%) | |
| 1 | 1 (4.2) |
| 2 | 12 (50.0) |
| 3 | 5 (20.8) |
| 4 | 4 (16.7) |
| 5 | 0 (0) |
| Missing | 1 (4.2) |
| CD: MH assessment, n (%) | |
| 1 | 1 (4.2) |
| 2 | 6 (25.0) |
| 3 | 2 (8.3) |
| 4 | 8 (33.3) |
| 5 | 6 (25.0) |
| Missing | 1 (4.2) |
| UC: treatment escalation, n (%) | |
| 1 | 2 (8.3) |
| 2 | 12 (50.0) |
| 3 | 6 (25.0) |
| 4 | 3 (12.5) |
| 5 | 0 (0) |
| Missing | 1 (4.2) |
| UC: MH assessment, n (%) | |
| 1 | 1 (4.2) |
| 2 | 6 (25.0) |
| 3 | 2 (8.3) |
| 4 | 8 (33.3) |
| 5 | 6 (25.0) |
| Missing | 1 (4.2) |
Lastly, participants were asked to rate from 1 (minimum) to 5 (maximum) the personal safety perception regarding pre-endoscopy screening protocols and the adequacy of PPE supplied in the endoscopy theater. Sixteen (66.7%) participants reported maximum perceived safety, 7 (29.2%) reported high perceived safety and only one responder (4.2%) reported moderate safety regarding pre-endoscopy COVID-19 testing. Seventeen (70.8%) responders to survey reported maximum safety, 6 (25%) of them high safety and only one (4.2%) reported only moderate safety regarding PPE provision.
4. Discussion
We conducted a web-based survey among Italian pediatric gastroenterology centers practicing digestive endoscopy, aiming to assess the modifications of digestive endoscopy practice in a period of intense viral circulation. The results of our survey, covering the vast majority of Italian centers performing pediatric GI endoscopy, likely offer a reliable picture of the Italian pediatric GI endoscopy practice during COVID-19 outbreak. Our data outline the meaningful impact of the pandemic on the execution of pediatric digestive endoscopy in Italy. Indeed, all the centers responding to the interview experienced a reduction in procedural volumes with and an overall reduction of 37.2% comparing the 2020 and the 2019 period. These findings are consistent with a recent survey conducted among 12 centers across Europe conducted in April 2020, shortly after the publication of ESPGHAN recommendations. Although not reporting the absolute number of endoscopic procedures and their relative reduction between 2020 and 2019, all the centers participating to the survey (12/12, 100%) had to cancel or postpone elective endoscopies due to COVID-19 outbreak, mainly beginning in mid-March 2020 [20].
Interestingly, besides the absolute reduction of all digestive endoscopic procedures, we have learnt from our report that in a setting of endoscopic constraint a modulation of the indications for digestive endoscopy procedures is possible. Indeed, a significant reduction of endoscopies performed for new diagnoses of celiac disease, FGID, suspected HP infection, and eosinophilic gastroenteropathy was reported, whereas only a smaller reduction was observed for IBD new diagnosis. Again, our findings are comparable to those published by Ruan and colleagues [14]. The authors performed an international survey aiming to assess changes in pediatric endoscopic practice during the first months of pandemic outbreak, reporting that more than 80% of centers participating to the survey experienced a reduction of more than 90% of normal procedural volumes. Moreover, when addressing for the use of pediatric endoscopy to guide treatment decisions, the authors reported that only little more than 15% of participants responded positively to the possibility to treat new-onset IBD without endoscopy during COVID-19 pandemic.
The modifications we observed in IBD endoscopy are in line with the recommendations published by International adult and Pediatric IBD Societies [21,22]. Indeed, endoscopic volumes did not decrease significantly for neither new diagnoses of suspected new-onset IBD nor disease flares. Conversely, median number of elective endoscopic procedures scheduled for MH re-assessment reduced significantly. Treatment goal in IBD has remarkably evolved during the last years moving from symptoms alleviation to MH and deep remission. This concept, delineated as “treat to target” strategy has now become the mainstay of IBD therapeutic strategies, both in adults and in children [23]. The cancelation of elective endoscopic procedure for MH assessment in IBD patients with clinically silent disease has led to a “reactive” use of endoscopy leading to potentially missing of subclinical recrudescence of the disease. The results of our survey are consistent with those published by those recently on behalf of SIGENP IBD working group [24]. Indeed, in this multicenter retrospective study performed in 21 Italian centers managing pediatric IBD, Arrigo et al. reported a significant reduction in hospital admissions performed for endoscopic re-evaluation during COVID-19 outbreak, whereas the number of hospitalizations for disease flares remained unchanged [24]. Regarding the monitoring of IBD patients, our results offer insights on possible surrogate markers of endoscopic re-assessment. A tight combined clinical and biochemical monitoring [25] may be considered as a reliable endpoint of the “treat to target” strategy in the follow-up of pediatric IBD patients.
As regards to the dramatic reduction of endoscopies performed for suspected Celiac Disease, some consideration have to be taken into account. In January 2020, ESPGHAN released an update of Celiac Disease guidelines, recommending a biopsy-sparing approach in all children with IgA antitransglutaminase (TGA-IgA) autoantibodies ≥ 10 ULN and positive endomysial antibodies (EMA-IgA) regardless of symptoms and the assessment of genetic predisposition (HLA DQ2-DQ8) [19]. Therefore, application of such new recommendations may have contributed to the decreased use of endoscopy for the diagnosis of Celiac Disease. In addition, because of the challenge to access endoscopy and in order to tackle SARS-CoV-2 spreading, the Italian Society of Gastroenterology, Hepatology and Nutrition (SIGENP) released practical recommendations for the management and care of Celiac Disease patients during COVID-19 outbreak, proposing the extension of the “biopsy-free” approach also for patients with TGA-IgA > 7.5 ULN and positive EMA-IgA. Overall, the reduction of procedural volumes for non-urgent endoscopies offers the opportunity to reconsider the role of biopsy-sparing approach for the diagnosis of celiac disease. Indeed, such practice is widely adopted in pediatric setting and the latest guidelines [19] have somehow extended the possibility of performing non- diagnosis of celiac disease without using endoscopy and histology. In addition, there have been some reports suggesting that lower threshold of TGA-IgA threshold in EMA-positive children may accurately predict celiac disease [26]. Similarly, a biopsy-sparing approach has been proposed also in adult practice [27], has been implemented during COVID-19 outbreak (https://www.bsg.org.uk/covid-19-advice/covid-19-specific-non-biopsy-protocol-guidancefor-those-with-suspected-coeliacdisease/) and proven to be safe in adult cohorts [28].
The recent update of joint ESPGHAN/NASPGHAN guidelines for the management of HP in children and adolescents recommend against a “test and treat” strategy in pediatric populations [29]. However, such management approach in may gain significance and be re-evaluated in a setting of endoscopic constraint, especially in pediatric age, where the risk of HP-related complications is extremely low.
Similarly, in a setting of shortage of endoscopic procedures, empiric proton pump inhibitors (PPI) therapy (or so-called PPI trial) may reveal a reasonable approach to confirm GERD when it is suspected also in pediatric populations with typical symptoms [30].
A further lesson may be provided from the dramatic reduction of endoscopic procedures performed for FGID as primary indication. On one hand, such reduction does not cause much concern because of the limited clinical value of gastrointestinal endoscopy in children with symptoms indicative of FGID and because of the lack of clear clinical relevance of the minor macroscopic and histopathological changes frequently encountered on endoscopy and/or histology. On the other hand, our findings draw the attention to the significant number of potentially unnecessary endoscopic procedures performed for FGID and suggest to implement Rome IV criteria to achieve a positive clinical diagnosis for FGID [31].
When looking at the distribution of the answers in prioritizing clinical indications to endoscopy in 2020, responders to survey assigned highest priority rates to IBD and EoE new diagnoses, an intermediate priority to IBD and EoE endoscopic re-assessment whereas celiac disease, GERD and FGID received among all the lowest priority rates. Moreover, as was to be expected, priority rates did not change for IBD new diagnosis but varied consistently for MH assessment, as for EoE, Celiac Disease, GERD and FGID. Accordingly, when asked to rate the elongation of waiting lists for access to endoscopic procedures, CD and UC new diagnosis showed only little elongation of waiting lists with only little more than 10% of responders reporting moderate. Conversely, participants to survey reported a moderate delay in the access to endoscopy to guide CD and UC treatment escalation and new-onset suspected EoE. Again, the longest waiting lists were reported for new diagnosis of Celiac Disease, new-onset and re-assessment of GERD and FGID.
Overall, a high-perceived safety regarding both pre-endoscopy screening protocols and PPE provision was reported. These data reflect a prompt and uniform adherence to national and international recommendations along with the fact that Italy was one of the first countries to face COVID-19 outbreak and thus required early efforts to counteract virus spreading and have been reported also in previously published multicenter national survey conducted among adult endoscopists [32].
The changes in pediatric digestive endoscopy management are in line with the indications of both the Italian National Health System and the recommendations published by International Societies [6,33] as most of non-urgent activities were postponed. However, despite the tight adherence to such recommendations, the effects of these changes are uncertain and should be closely monitored. Moreover, a further challenge will be represented by the necessity of rescheduling canceled procedures, a path that should be guided by giving clinical judgment on the basis of priority [34]. Our study certainly has some limitations. We provided data from 24 out of 30 pediatric centers performing endoscopy in Italy. However, they cover most of the procedural volumes in Italy and likely represent a reliable snapshot of pediatric digestive endoscopy management during the lockdown in one of the countries more severely hit by COVID-19 pandemic. Moreover, our study does not assess directly the impact of the COVID-19 pandemic on the consequences of missed diagnoses, as we did not provide the exact number of diagnoses performed during the two periods of interest. Nevertheless, it can be reasonably assumed that the significant proportions of missed diagnoses will have a negative impact on several GI affections’ course. Undoubtedly, more studies will be needed in the near future to assess such aspect. However, it is reasonable to assert that the negative effects of pandemic will extend beyond the spreading and the complications of the infections. Despite the abovementioned limitations, our data provided adds relevant information on how COVID-19 pandemic affected pediatric digestive endoscopy practice in Italy. These data should support appropriate and timely decision making and planning of support for Endoscopy Units in the months and years to come.
Conflict of interest
None declared.
Footnotes
Funding: No specific funding was received for this work.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.dld.2022.02.010.
Contributor Information
SIGENP Endoscopy Working Group:
Emanuele Dabizzi, Marco Deganello Saccomani, Michele Di Toma, Simona Gatti, Maria Teresa Illiceto, Sara Isoldi, Marta Maino, Maristella Pellegrino, and Caterina Strisciuglio
Appendix A. Collaborators equally contributed to the work:
SIGENP Endoscopy Working Group:
-
-
Emanuele Dabizzi, MD; Gastrointestinal and Interventional Endoscopy Unit, Surgical Department, AUSL Bologna, Bologna, Italy
-
-
Marco Deganello Saccomani, MD; Department of Pediatrics, Woman's and Child's University Hospital of Verona, Italy
-
-
Michele Di Toma, MD; Department of Medical and Surgical Sciences, University of Foggia, Foggia, Italy
-
-
Simona Gatti, MD; Department of Pediatrics, Università Politecnica delle Marche, Ancona, Italy
-
-
Maria Teresa Illiceto, MD; Pediatric Gastroenterology and Digestive Endoscopic Unit, Department of Pediatrics, “Santo Spirito” Hospital of Pescara, Italy
-
-
Sara Isoldi, MD; Maternal and Child Health Department, Sapienza - University of Rome, Santa Maria Goretti Hospital, Polo Pontino, Latina, Italy
-
-
Marta Maino, MD; Interventional Endoscopic Unit, ASST Hospital “San Gerardo”, Monza, Italy.
-
-
Maristella Pellegrino, MD; Pediatric Surgery Unit, Maternal and Child Department, ASST GOM of Niguarda, Milan, Italy
-
-
Caterina Strisciuglio, MD; Department of Woman, Child and General and Specialistic Surgery, University of Campania “Luigi Vanvitelli”, Naples, Italy
Appendix B. Supplementary materials
References
- 1.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Distante C., Piscitelli P., Miani A. Covid-19 outbreak progression in Italian regions: approaching the peak by the end of march in Northern Italy and first week of april in Southern Italy. IJERPH. 2020;17:3025. doi: 10.3390/ijerph17093025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenbaum L. Facing Covid-19 in Italy — ethics, logistics, and therapeutics on the epidemic's front line. N Engl J Med. 2020;382:1873–1875. doi: 10.1056/NEJMp2005492. [DOI] [PubMed] [Google Scholar]
- 4.Romani G., Dal Mas F., Massaro M., Cobianchi L., Modenese M., Barcellini A., et al. Population health strategies to support hospital and intensive care unit resiliency during the COVID-19 pandemic: the Italian experience. Popul Health Manag. 2021;24:174–181. doi: 10.1089/pop.2020.0255. [DOI] [PubMed] [Google Scholar]
- 5.Maida M., Sferrazza S., Savarino E., Ricciardiello L., Repici A., Morisco F., et al. Impact of the COVID-19 pandemic on gastroenterology divisions in Italy: a national survey. Digest Liver Dis. 2020;52:808–815. doi: 10.1016/j.dld.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gralnek I.M., Hassan C., Beilenhoff U., Antonelli G., Ebigbo A., Pellisè M., et al. ESGE and ESGENA Position Statement on gastrointestinal endoscopy and the COVID-19 pandemic. Endoscopy. 2020;52:483–490. doi: 10.1055/a-1155-6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson N.M., Norton A., Young F.P., Collins D.W. Airborne transmission of severe acute respiratory syndrome coronavirus-2 to healthcare workers: a narrative review. Anaesthesia. 2020;75:1086–1095. doi: 10.1111/anae.15093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parasa S., Desai M., Thoguluva Chandrasekar V., Patel H.K., Kennedy K.F., Roesch T., et al. Prevalence of gastrointestinal symptoms and fecal viral shedding in patients with coronavirus disease 2019: a systematic review and meta-analysis. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu J., Han B., Wang J. COVID-19: gastrointestinal manifestations and potential fecal–oral transmission. Gastroenterology. 2020;158:1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158 doi: 10.1053/j.gastro.2020.02.055. 1831–1833.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belle A., Barret M., Bernardini D., Tarrerias A.-.L., Bories E., Costil V., et al. Impact of the COVID-19 pandemic on gastrointestinal endoscopy activity in France. Endoscopy. 2020;52:1111–1115. doi: 10.1055/a-1201-9618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lantinga M.A., Theunissen F., ter Borg P.C.J., Bruno M.J., Ouwendijk R.J.T., Siersema P.D., et al. Impact of the COVID-19 pandemic on gastrointestinal endoscopy in the Netherlands: analysis of a prospective endoscopy database. Endoscopy. 2021;53:166–170. doi: 10.1055/a-1272-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parasa S., Reddy N., Faigel D.O., Repici A., Emura F., Sharma P. Global impact of the COVID-19 pandemic on endoscopy: an international survey of 252 centers from 55 countries. Gastroenterology. 2020;159 doi: 10.1053/j.gastro.2020.06.009. 1579–1581.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruan W., Fishman D.S., Lerner D.G., Engevik M.A., Elmunzer B.J., Walsh C.M., et al. Changes in pediatric endoscopic practice during the coronavirus disease 2019 pandemic: results from an international survey. Gastroenterology. 2020;159:1547–1550. doi: 10.1053/j.gastro.2020.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tringali A., Thomson M., Dumonceau J.-.M., Tavares M., Tabbers M., Furlano R., et al. Pediatric gastrointestinal endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) and European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) Guideline Executive summary. Endoscopy. 2016;49:83–91. doi: 10.1055/s-0042-111002. [DOI] [PubMed] [Google Scholar]
- 16.Lightdale J.R., Acosta R., Shergill A.K., Chandrasekhara V., Chathadi K., Early D., et al. Modifications in endoscopic practice for pediatric patients. Gastrointest. Endosc. 2014;79:699–710. doi: 10.1016/j.gie.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Levine A., Koletzko S., Turner D., Escher J.C., Cucchiara S., de Ridder L., et al. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. 2014;58:795–806. doi: 10.1097/MPG.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 18.Dellon E.S., Liacouras C.A., Molina-Infante J., Furuta G.T., Spergel J.M., Zevit N., et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: proceedings of the AGREE Conference. Gastroenterology. 2018;155 doi: 10.1053/j.gastro.2018.07.009. 1022–1033.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Husby S., Koletzko S., Korponay-Szabó I., Kurppa K., Mearin M.L., Ribes-Koninckx C., et al. European society paediatric gastroenterology, hepatology and nutrition guidelines for diagnosing coeliac disease 2020. J Pediatr Gastroenterol Nutr. 2020;70:141–156. doi: 10.1097/MPG.0000000000002497. [DOI] [PubMed] [Google Scholar]
- 20.Athiana I., Légeret C., Bontems P., Dall'Oglio L., De Angelis P., Dias J.A., et al. Significant variations across European centres in implementing recommended guidelines for the paediatric gastroenterology endoscopy suite during the COVID-19 pandemic. JPGN Reports. 2021;2:e061. doi: 10.1097/PG9.0000000000000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Amico F., Danese S., Peyrin-Biroulet L., Ailsa H., Kucharzik T., Magro F., et al. Inflammatory bowel disease management during the coronavirus-19 outbreak: a survey from the European Crohn’s and colitis organization. Gastroenterology. 2020;159 doi: 10.1053/j.gastro.2020.04.059. 14–19.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubin D.T., Feuerstein J.D., Wang A.Y., Cohen R.D. AGA clinical practice update on management of inflammatory bowel disease during the COVID-19 pandemic: expert commentary. Gastroenterology. 2020;159:350–357. doi: 10.1053/j.gastro.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turner D., Ricciuto A., Lewis A., D’Amico F., Dhaliwal J., Griffiths A.M., et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160:1570–1583. doi: 10.1053/j.gastro.2020.12.031. [DOI] [PubMed] [Google Scholar]
- 24.Arrigo S., Alvisi P., Banzato C., Bramuzzo M., Celano R., Civitelli F., et al. Impact of COVID-19 pandemic on the management of paediatric inflammatory bowel disease: an Italian multicentre study on behalf of the SIGENP IBD Group. Digest Liver Dis. 2021;53:283–288. doi: 10.1016/j.dld.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colombel J.-.F., Panaccione R., Bossuyt P., Lukas M., Baert F., Vaňásek T., et al. Effect of tight control management on Crohn's disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2017;390:2779–2789. doi: 10.1016/S0140-6736(17)32641-7. [DOI] [PubMed] [Google Scholar]
- 26.Trovato C.M., Montuori M., Cucchiara S., Oliva S. ESPGHAN ‘biopsy-sparing’ guidelines for celiac disease in children with low antitransglutaminase during COVID-19. Eur J Gastroenterol Hepatol. 2020;32:1523–1526. doi: 10.1097/MEG.0000000000001924. [DOI] [PubMed] [Google Scholar]
- 27.Fuchs V., Kurppa K., Huhtala H., Laurila K., Mäki M., Collin P., et al. Serology-based criteria for adult coeliac disease have excellent accuracy across the range of pre-test probabilities. Aliment Pharmacol Ther. 2019;49:277–284. doi: 10.1111/apt.15109. [DOI] [PubMed] [Google Scholar]
- 28.Tashtoush L.B., Bosanko N.C., Broad S.R., Chan Y.J., Singhal N., Saji S., et al. Letter: the BSG COVID-19 interim coeliac disease guidance no-biopsy approach is safe in adults. Aliment Pharmacol Ther. 2021;54:1090–1092. doi: 10.1111/apt.16553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones N.L., Koletzko S., Goodman K., Bontems P., Cadranel S., Casswall T., et al. Joint ESPGHAN/NASPGHAN guidelines for the management of helicobacter pylori in children and adolescents (Update 2016) J Pediatr Gastroenterol Nutr. 2017;64:991–1003. doi: 10.1097/MPG.0000000000001594. [DOI] [PubMed] [Google Scholar]
- 30.Katz P.O., Gerson L.B., Vela M.F. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308–328. doi: 10.1038/ajg.2012.444. [DOI] [PubMed] [Google Scholar]
- 31.Hyams J.S., Di Lorenzo C., Saps M., Shulman R.J., Staiano A., van Tilburg M. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2016;150 doi: 10.1053/j.gastro.2016.02.015. 1456–1468.e2. [DOI] [PubMed] [Google Scholar]
- 32.Vassallo R., Venezia L., Zullo A., Stasi E., Milazzo G., Soncini M., et al. Safety and protection in endoscopic services during phase II of COVID-19 pandemic: a national survey. Eur J Gastroenterol Hepatol. 2021;33:974–976. doi: 10.1097/MEG.0000000000002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Repici A., Maselli R., Colombo M., Gabbiadini R., Spadaccini M., Anderloni A., et al. Coronavirus (COVID-19) outbreak: what the department of endoscopy should know. Gastrointest Endosc. 2020;92:192–197. doi: 10.1016/j.gie.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Danese S., Sands B., Ng S.C., Peyrin-Biroulet L. The day after COVID-19 in IBD: how to go back to ‘normal.’. Nat Rev Gastroenterol Hepatol. 2020;17:441–443. doi: 10.1038/s41575-020-0322-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.