Abstract
Background
Collecting social determinants of health in electronic health records is time-consuming. Meanwhile, an Area Deprivation Index (ADI) aggregates sociodemographic information from census data. The objective of this study was to ascertain whether ADI is associated with stage of human papillomavirus (HPV)-related cancer at diagnosis.
Methods
We tested for the association between the stage of HPV-related cancer presentation and ADI as well as the association between stage and the value of each census-based measure using ordered logistic regression, adjusting for age, race and sex.
Results
Among 3247 cases of HPV-related cancers presenting to an urban academic medical center, the average age at diagnosis was 57. The average stage at diagnosis was Surveillance, Epidemiology and End Results Stage 3. In the study population, 43% of patients were female and 87% were white. In this study population, there was no association between stage of HPV-related cancer presentation and either aggregate or individual census variables.
Conclusions
These results may reflect insufficient sample size, a lack of socio-demographic diversity in our population, or suggest that simplifying social determinants of health into a single geocoded index is not a reliable surrogate for assessing a patient’s risk for HPV-related cancer.
Keywords: cancer, race, social determinants
Introduction
It is well established that increasing awareness on social and environmental factors play a critical role in determining health and health-related outcomes.1,2 The World Health Organization defines social determinants of health as ‘the conditions in which people are born, grow, live, work and age that shape health’.3 This includes socioeconomic status, education, employment and environment among others.3 The Institute of Medicine, the Department of Health and Human Services,4 the Association of American Medical Colleges,5 and numerous other organizations, institutions and independent healthcare providers have highlighted the importance of routinely collecting social and environmental determinants of health in electronic health records since these factors are large contributors to health inequity.6,7 In addition, the transition to accountable care organizations through the Patient Protection and Affordable Care Act has incentivized healthcare systems to track and manage these social and behavioral determinants of health to reduce utilization.7
At the same time, there are challenges to incorporating the collection of this type of information at the patient level and at the scale required to support population level health. A variety of tools exist including the PRAPARE, AHCS and IOM tools.8 Recently, electronic health records have become more complex and are now able to capture social determinants of health (SDOH) data, and the Office of the National Coordinator is leading efforts to standardize practices around how data are captured from a technical standpoint.8,9 Nevertheless, most clinics still rely on clinical staff implementing surveys of individual patients, which is both time-consuming and resource intensive.8 The benefits of collecting social and environmental data from the patient must be weighed against the increased burden placed upon patients and providers by collecting such data.10 Accessing these data at scale using related, collective measures such as those in the census could benefit population health studies now and facilitate implementation of these recommendations.
To address concerns about the scalability and sustainability of collecting SDOH,7 we test the theory that census is a reliable predictor of health outcomes in a novel population. The first outcome we studied was human papillomavirus (HPV). HPV is associated with high burden of oropharyngeal and anogenital cancers.11 These are some of the most preventable cancers and yet disparities exist due to a variety of factors including differences in access to screening, socioeconomic status, race/ethnicity and genetics.12 SDOH such as educational attainment,13 income14 and insurance status15 have been associated with worse outcomes. These outcomes depend on a complex relationship of individual and neighborhood-level social determinants. Teasing out the differences between these effects can be challenging, even with multilevel modeling techniques.16 Census data contain several neighborhood-level variables that have been linked to cancer outcomes including: socioeconomic status,12 educational attainment.13,17,18 The ability to geocode patient addresses and link them to relevant community-level social and environmental data can provide invaluable information for researchers as well as providers about the individual’s community without lengthening the clinical encounter.19
The American Community Survey (ACS) provides an opportunity to collect information about social, environmental and housing characteristics that can have an impact on health and health outcomes. The ACS is a nationwide, random sample survey conducted annually by the United States Census Bureau. Individual responses are aggregated into estimates for several geographic entities and made publicly available. According to the United States Census Bureau, a census tract is a relatively permanent geographical subdivision that contains approximately 4000 people. These were outlined in the early 1900s as permanent delineations large enough to make statistical comparisons across groups. A census block is a smaller unit with a minimum of 1500 people, but there are concerns about protecting the privacy of individuals by collecting data at this level.20 In addition, fewer variables are collected at the census block level.21 For these reasons, census tracts are utilized in this study. In addition to individual census variables, several indices have been created to encompass multiple socioeconomic components, which have been associated with health outcomes. An Area Deprivation Index (ADI) can be calculated to determine the overall socioeconomic status of the neighborhood of a given patient.
While neighborhood-level indicators are not a substitute for individually collected variables, they have shown utility in predicting health outcomes.22 Deprivation indices that have been studied include the Singh ADI,23 Townsend Index,24 Social Vulnerability Index,25 and a number of locally developed metrics. The majority of previous literature relies on a deprivation index composed of 17 different markers developed by Singh et al. through a factor analysis of 1990 American Community Survey. These studies demonstrated correlation with health outcomes such as readmissions,21 diabetes prevalence,26 and mortality.23 This study utilizes a different ADI with six of the markers. This six-variable ADI measure utilized in this study was selected because it can be calculated efficiently for a broad population using publicly available code.27 Furthermore, this institution’s research enterprise had successfully integrated this with existing electronic health record data. Finally, it was selected for its flexibility as there is potential to apply the measure to a variety of health outcomes in the future. So far, this ADI has shown correlation with hospital length of stay and hospital utilization in the first year of life as well as pediatric emergency medical services utilization. 28,29 Meanwhile, other indices have not been as useful predictors. The European Deprivation Index did not predict time to treatment or time to diagnosis in a population of cancer patients.28 In another study, the Neighborhood Disadvantage Index was not significantly correlated with birthweight of infants born to adolescent mothers after adjusting for patient-specific factors.29 The mixed evidence for the value of a quantitative index support the need for greater agreement in population-based studies looking at the utility of measuring SDOH.
The objective of this study is to ascertain whether ADI is associated with stage of HPV-related cancer at diagnosis. In the literature, cancer stage at disease presentation is commonly used as a surrogate for access to preventative measures as patients without access will present at higher stages.30–32 In one study of the Singh Index, women in the most-deprived group were more than 30% more likely to die from cervical cancer than the least-deprived group.33 A study in Sweden using a locally developed neighborhood deprivation index found that the risk of cervical cancer morbidity and mortality was significantly higher for patients with high neighborhood deprivation scores even after adjusting for individual patient factors.34 The same group also found similar results for lung cancer35 and prostate cancer.36
HPV was selected as the first diagnosis to study because the university data repository already contained staging information and ADI but had not previously studied the relationship between the two. The institution had plans to assess additional populations depending on the results of this study.
Methods
Study population
This retrospective case–control study was conducted in study population, which consisted of all patients presenting with an HPV-related cancer to Vanderbilt University Medical Center from 2010 to 2020. Vanderbilt University Medical Center is a tertiary referral center and has a large catchment area across the Southeastern United States. Cancer patients were identified at Vanderbilt University Medical Center (VUMC) through its Research Derivative using a retrospective study design. The Research Derivative (RD) is a database derived from the VUMC clinical systems and restructured for research.37 Ethical approval was obtained from the Vanderbilt University Institutional Review Board.
Subjects included in the analysis (1) carried at least two diagnostic codes (International Classification of Diseases-9 or International Classification of Diseases-10) for HPV-related cancers in the last 10 years, (2) had associated tumor registry information and (3) contained an address in the electronic health record, which could be geocoded to a census tract in Tennessee. Cancer diagnoses included in this study were as follows: anal, cervical, oropharyngeal, penile, vulvar and vaginal. There were 3706 patients in the RD with the requisite diagnostic codes in their electronic health record. From that starting set, 8 were excluded because of missing census information and 496 were excluded because they were missing the staging information from the National Tumor Registry. Age, race and gender were not predictive of missingness of cancer stage. The resulting population of 3247 individuals were included in the analysis (Fig. 1). Processed addresses were geocoded to census tracts using QGIS version 3.4.3 (Open Source Geospatial Foundation Project). Census variables were linked to clinical information derived from the RD and National Tumor Registry.
Fig. 1 .
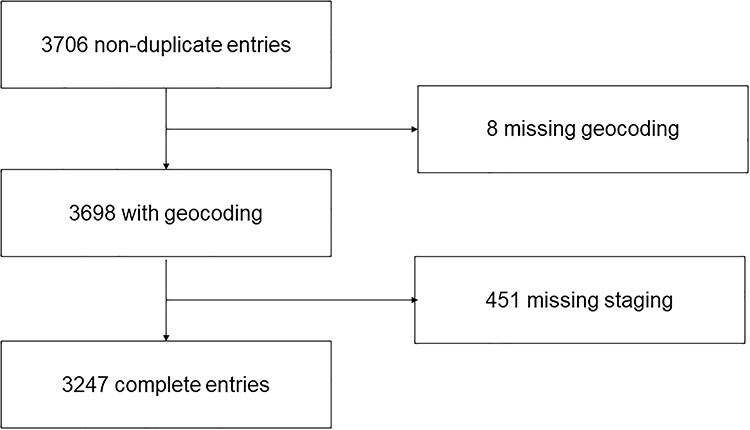
Schematic of patient inclusion criteria.
Outcome
The outcome of interest was stage at presentation for HPV-related cancers as derived from tumor registry information. Surveillance, Epidemiology and End Results (SEER) stage guidelines were utilized for this study.26 Stages 8 (Benign) and 9 (Unknown) were excluded from analysis. Stage 6 is undefined.
Covariates
Age-at-diagnosis was calculated from the date of birth and date of diagnosis as included in the cancer registry data. Sex was captured from cancer registry data and encoded as a binary variable with male as the reference. Race was captured from administratively assigned race in the electronic health record. Categories for race included White, Black, Indian or Alaska Native, Asian, Hispanic, other or unknown. Because of the low population of non-white participants, race was encoded as a binary variable (‘minority status’) for the purpose of analysis with White as the reference. Ethnicity (Hispanic, not Hispanic, unknown) could not be included in the model due to low prevalence of Hispanic participants (<1%).
Clinical information included SEER stage, age at diagnosis, race and other risk factors for HPV infection as indicated by diagnostic codes associated with patient encounters (prior sexually transmitted infections, presence of immunocompromising illness, HPV vaccination status, presence of drug or alcohol abuse). These clinical variables were selected a posteriori, based upon a review of relevant literature. These variables were selected as we believe they are associated with both ADI and HPV-related cancers. Number of sexual partners and tobacco use were not routinely collected in a standardized data format at the time of this study and therefore were not included in the model.
Because this was a retrospective study, individual variables such as individual income, educational qualification, employment status, family size, marital status and other variables were not available as these are not routinely collected in the electronic health record.
ADI
An ADI is calculated from the 2015 United States 5-year American Community Survey linking the address listed for the patient’s residence in the electronic health record with the census tract group with the same area. This method was developed and validated by Brokamp et al. and is available publically.38 ADI for each included census tract was calculated.38–40 The components included in this calculation are as follows: (1) fraction of households with incomes below the poverty level in the last 12 months, (2) median household income in the past 12 months in 2015 inflation-adjusted dollars, (3) fraction of population 25 and older with educational attainment of at least high school graduation (includes GED equivalency), (4) fraction of population with no health insurance coverage, (5) fraction of household receiving public assistance income or food stamps or SNAP in the last 12 months and (6) fraction of vacant housing. A principal component analysis was applied and the first component was selected, which explains 60% of the variation in census tract-level measurements. This is was reduced to a single ‘deprivation index’ that ranges from 0 to 1 with a higher value representing a census tract with increased deprivation.38 In addition to modeling the effect of the overall ADI, models were also created with each of the individual components.
Statistical analysis
Demographic characteristics and cancer stage were summarized using percentages, means and standard deviations. Ordinal logistic regression models were used to identify associations between a range of census tract variables with stage at diagnosis. This model was selected to predict the response of the ordinal variable to multiple inputs. The ordinal dependent variable was cancer stage at presentation (stage 0 to 7). A higher cancer stage increases the risk of a worse outcome. We constructed a separate model for each census variable. All models were adjusted for age at diagnosis, race and sex. Each model followed the following regression equation:
Cancer Stage at Diagnosis = Census Variable + Age + Minority Status + Sex
Analyses were summarized with odds ratios (OR) and 95% confidence intervals (CI). In addition, given previous studies showing the nonlinear relationship with ADI,34,41 we further examined this nonlinear relationship in this study population using ADI quartiles. Since observations of participants living in the same census tract may not be independent, we used robust estimate of variance to account for clustering within census tracts28,42 Secondary analyses tested for interactions between race and census track variables (ADI, median income and fraction with high school education) as well as sex and the same set of census tract variables using likelihood ratio tests. All analysis was conducted in STATA/SE version 15 (StataCorp LLC, College Station, Texas).
Results
Among 3247 cases of HPV-related cancers, the average age at diagnosis was 57 (standard deviation: 13.5). The majority of patients were male (57%) and white (87%) (Table 1). The average ADI for the cohort was 0.38 [interquartile range (IQR): 0.15]. The average fraction of census tract with high school education was 0.87 (IQR: 0.12). The average census median income was $46,875 (IQR: $24,825). The distributions of individual and aggregate census markers are displayed in Table 2 and Fig. 2. The average stage at diagnosis was SEER Stage 3. The most frequent cancer locations were the tonsils, rectum and tongue (Table 3).
Table 1.
Participant demographics
| Total (%) | Stage 0 | Stage 1 | Stage 2 | Stage 3 | Stage 4 | Stage 5 | Stage 7 | ||
|---|---|---|---|---|---|---|---|---|---|
| Total | 3247 (100%) | 370 (12%) | 923 (28%) | 293 (9%) | 602 (19%) | 388 (12%) | 89 (3%) | 582 (18%) | |
| Age | |||||||||
| Missing | 40 (1) | 2 | 8 | 3 | 9 | 4 | 1 | 13 | |
| <30 | 109 (3) | 23 | 31 | 4 | 11 | 7 | 1 | 32 | |
| 30–40 | 220 (7) | 45 | 74 | 18 | 27 | 15 | 3 | 38 | |
| 40–50 | 586 (18) | 81 | 151 | 50 | 105 | 78 | 20 | 101 | |
| 50–60 | 937 (29) | 108 | 232 | 86 | 216 | 130 | 19 | 146 | |
| 60–70 | 844 (26) | 81 | 241 | 72 | 164 | 110 | 27 | 149 | |
| 70–80 | 381 (12) | 22 | 137 | 43 | 62 | 36 | 11 | 70 | |
| >80 | 130 (4) | 8 | 49 | 17 | 8 | 8 | 7 | 33 | |
| Sex | |||||||||
| Male | 1836 (57) | 160 | 487 | 150 | 431 | 246 | 50 | 312 | |
| Female | 1411 (42) | 210 | 436 | 143 | 171 | 142 | 39 | 270 | |
| Race | |||||||||
| Asian | 33 (1) | 6 | 11 | 0 | 3 | 5 | 0 | 8 | |
| Black | 261 (8) | 40 | 67 | 30 | 34 | 28 | 8 | 54 | |
| Hispanic | 45 (1) | 5 | 18 | 2 | 6 | 6 | 2 | 6 | |
| Native American | 6 (0) | 3 | 0 | 0 | 0 | 1 | 2 | ||
| White | 2822 (87) | 312 | 813 | 256 | 544 | 336 | 75 | 486 | |
| Other | 9 (0) | 3 | 2 | 0 | 2 | 1 | 0 | 1 | |
| Missing | 71 (2) | 4 | 9 | 5 | 13 | 12 | 3 | 25 | |
| Ethnicity | |||||||||
| Hispanic | 45 (1) | 5 | 18 | 2 | 6 | 6 | 2 | 6 | |
| Not Hispanic | 3115 (96) | 361 | 890 | 284 | 583 | 372 | 84 | 541 | |
| Unknown | 87 (3) | 4 | 15 | 7 | 13 | 10 | 3 | 35 |
Table 2.
Census variables median (IQR)
| Total | Stage 0 | Stage 1 | Stage 2 | Stage 3 | Stage 4 | Stage 5 | Stage 7 | Missing | |
|---|---|---|---|---|---|---|---|---|---|
| Deprivation Index | 0.38 (0.15) | 0.38 (0.15) | 0.38 (0.15) | 0.39 (0.16) | 0.38 (0.15) | 0.39 (0.15) | 0.39 (0.14) | 0.38 (0.17) | 0.39 (0.15) |
| Fraction assisted income | 0.14 (0.13) | 0.13 (0.14) | 0.14 (0.13) | 0.15 (0.14) | 0.13 (0.14) | 0.14 (0.13) | 0.16 (0.12) | 0.14 (0.15) | 0.14 (0.13) |
| Fraction high school education | 0.87 (0.12) | 0.87 (0.11) | 0.87 (0.12) | 0.86 (0.11) | 0.87 (0.11) | 0.86 (0.12) | 0.86 (0.10) | 0.86 (0.13) | 0.86 (0.13) |
| Median income | 46875 (24825) | 48007 (25392.5) | 46944 (24475) | 44329 (23228.25) | 48250 (24967.25) | 47539 (22897.25) | 46819 (20581) | 46762 (26594.5) | 45589 (24552) |
| Fraction no health insurance | 0.11 (0.07) | 0.12 (0.07) | 0.11 (0.07) | 0.12 (0.07) | 0.12 (0.07) | 0.12 (0.07) | 0.11 (0.07) | 0.11 (0.07) | 0.11 (0.07) |
| Fraction poverty | 0.14 (0.14) | 0.13 (0.15) | 0.14 (0.14) | 0.15 (0.14) | 0.14 (0.14) | 0.14 (0.13) | 0.15 (0.16) | 0.14 (0.14) | 0.15 (0.13) |
| Fraction vacant housing | 0.09 (0.08) | 0.09 (0.07) | 0.10 (0.08) | 0.10 (0.07) | 0.09 (0.08) | 0.10 (0.07) | 0.09 (0.08) | 0.09 (0.08) | 0.10 (0.09) |
Fig. 2 .
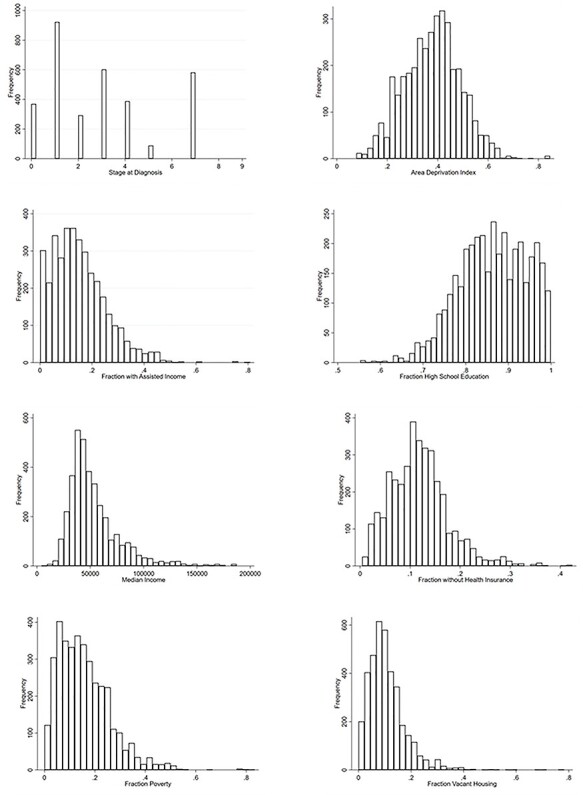
Frequency distribution of independent and dependent variables.
Table 3.
Most frequent cancer types
| Cancer type | Frequency |
|---|---|
| Tonsils | 348 |
| Rectum | 304 |
| Tongue | 282 |
| Cervix | 182 |
| Vulva | 90 |
| Penis | 61 |
| Anus | 55 |
| Rectosigmoid colon | 52 |
| Corpus uteri | 51 |
| Vagina | 49 |
The majority of patients (78%) in this cohort reside in the state of Tennessee. According to the American Community Survey Factfinder, 48.7% of the state’s population identifies as male. Meanwhile, 78% of the state population identifies as white. For the 1490 census tracts in TN, the average ADI was calculated to be 0.42 (standard deviation: 0.12). Thus, a 0.1-unit change in deprivation index is equivalent to an effect size of 0.80 (i.e. 0.1/0.12). The average ADI weighted by population was calculated to be 0.40, suggesting that the most deprived individuals live in the most populous census tracts.
Ordinal logistic regression results are in Table 4. ADI was not significantly associated with stage at diagnosis (P > 0.05) after controlling for minority status, age-at-diagnosis, and sex. Each of the six components of the area deprivation index were also not found to be significantly associated with stage at diagnosis (P > 0.05). Furthermore, no particular quartile was significantly different from the remaining. Interaction terms between race and ADI, income, and education were not significant and therefore not included in the models. Similarly, interaction terms between sex and ADI, income, and education were not significant and therefore not included in the models. In addition, the variable clustering estimator analysis shows that stage of HPV-related cancer stage at diagnosis does not vary by ADI even after accounting for clustering within census tracts. Additional analysis at the county level also showed similarly insignificant results.
Table 4.
Ordinal logistic regression results of relationship between patient characteristics and stage of presentation
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| Census variable description | OR | CI | P-value | OR | CI | P-value |
| Deprivation Index | 1.47 | (0.84, 2.55) | 0.18 | 1.40 | (0.79, 2.48) | 0.26 |
| Fraction assisted income | 1.59 | (0.87, 2.89) | 0.13 | 1.47 | (0.78, 2.75) | 0.23 |
| Fraction high school education | 0.56 | (0.26, 1.20) | 0.14 | 0.59 | (0.27, 1.28) | 0.18 |
| Median income | 1.00 | (1.00, 1.00) | 0.32 | 1.01 | (1.00, 1.01) | 0.36 |
| Fraction no health insurance | 1.37 | (0.45, 4.17) | 0.58 | 1.31 | (0.42, 4.05) | 0.64 |
| Fraction poverty | 1.38 | (0.75, 2.51) | 0.30 | 1.27 | (0.68, 2.38) | 0.44 |
| Fraction vacant housing | 1.70 | (0.74, 3.89) | 0.21 | 1.70 | (0.75, 3.87) | 0.20 |
The prevalence of risk factors (count of lifetime sexually transmitted infections, presence of immunocompromising illness, HPV vaccination status, presence of drug or alcohol abuse) in the dataset were too low to include in the analysis. We were not able to do additional sub-analysis by cancer type due to small numbers.
Discussion
Main finding
Based on previous studies of mortality in cervical cancer patients33,34, we predicted patients in neighborhoods with greater deprivation would present at higher stage-at-diagnosis. For this study population, ADI was not found to be significantly associated with stage at presentation in a cohort of HPV-related cancers.
What is already known on this topic
Incorporating social and environmental factors into electronic health records is an issue with a variety of potential solutions.4,7,8,19 Census-based deprivation indices have been utilized in a variety of settings from predicting individual health outcomes23,34–36 to hospital utilization.22,41 Many studies have shown strong correlations while others have not.24,29 Different deprivation indices contain different subsets of census markers, however, demonstrating the lack of consensus on which variables are important for measuring socioeconomic characteristics. Census-based deprivation indices have been utilized in a variety of settings from predicting individual health outcomes to hospital utilization. Clearly, there is a need for agreement on what should be measured before we can determine the utility of such indices. These findings also raise the concern for publication bias around ADI.
For HPV-related cancers specifically, both Singh et al.33 as well as Li et al.36 found correlations between degree of deprivation and cervical cancer mortality. We were not able to replicate these results in our study of the broader HPV-related cancer population.
Our results could also be explained by the fact that neighborhood estimates are not a perfect substitute for measuring individual characteristics. An analysis of a small area deprivation measure in New Zealand demonstrated that even in the most deprived areas, 10% of individuals have none of the characteristics of deprivation, thereby demonstrating the poor correlation between these individuals and their corresponding neighborhood deprivation level.42 In contrast, a study that surveyed people individually about financial insecurity and housing stability found statistically significant correlations with health outcomes, specifically blood pressure and cholesterol levels.43 This suggests the need for increased screening at the individual level rather than reliance on geocoded estimates. Individual variables such as individual income, educational qualification, employment status, family size and marital status likely play an important and complex role in health outcomes and according to the results of this study are not adequately reflected in the calculated index.
Our differing results also reflect the complexity of SDOH. Epidemiologic studies alone have not been sufficient to address the causes of inequality. Research does not exist outside of the political, economic and cultural contexts.44 We recognize that a single index can oversimplify the complex biologic and epigenetic mechanisms that underlie these disparities.45
What this study adds
Our findings are in contrast to previous studies, which have found correlations between other neighborhood deprivation indices and health outcomes.23,41,46 The inconsistency in our results compared to previous studies could be explained by variability in what is meant by socioeconomic deprivation, poor correlation between geocoded estimates and individual SDOH status, or insufficient statistical power. The results of this study question the use of ADI as a scalable and sustainable surrogate for collecting SDOH. According to our results, ADI did not show predictable and repeatable results in this population of patients at a tertiary care center. The results of our study suggest that geocoded indices may not be a reliable surrogate for capturing the complexity of underlying individual socio-demographic variables.
Strengths of this study are the fact that it takes advantage of routinely collected information and does not pose an additional burden to clinicians or patients. Another strength is this analysis incorporates disease-specific outcome rather than previous studies, which rely on hospital utilization data as a surrogate. This study model proposed here could be replicated in other disease-based cohorts. To eliminate variability in quality of treatment or hospital-based differences in care, this analysis was designed to utilize stage-at-diagnosis.
This method aims to support the conceptual framework provided by Hiatt and Breen, which suggests that cancer outcomes are a complex relationship between social determinants, biological factors and medical interventions. In this framework, social determinants refer to ‘physical and built environments that are part of or the result of human activity’. Examples included in this framework include occupation, income, education and health-insurance coverage. Outcomes are defined as those collected in cancer registries.47 By using publicly available census data, we hope to better understand the associations between these socioeconomic factors and HPV-related cancer outcomes.
Limitations
It is unclear if the results of this study are generalizable to the general population. This study was conducted in a tertiary referral center in the South, which may not reflect the socio-demographic diversity of all clinical practices. The ADI is lower than the general population of Tennessee. Moreover, the study was conducted in a population with 90% of persons identifying as white, higher than the state-wide average, which limits the ability to accurately account for race as a covariate.
The statistical methods utilized in this analysis were not complex enough to account for measurement error associated with the census. The census polls a subset of the population to make estimates for a given census tract. These estimates are less reliable for census tracts with smaller populations. Since Tennessee is a predominantly rural state, this could lead to measurement error in our population. In addition, one could argue that variable clustering estimation does not adequately account for similarities between persons in the same census tract and that geospatial techniques might better account for this geographic proximity. Of note, census subdivisions were created for the purpose of the US Census not calculating SDOH and therefore may group dissimilar households. For these reasons, correlations between census variables and health outcomes are inconsistent.
In order to draw stronger conclusions about the general population, this study ought to be replicated in larger datasets with greater racial diversity. Because this was conducted as a retrospective chart review, we are unfortunately limited in the types of populations and variables that can be studied. Future studies should collect individual-level variables to compare these to the neighborhood-level factors. Additional disease-specific variables, such as sexual practices or smoking status would have enriched the findings of this study. Future studies of larger cohorts would also allow for the study of subgroups of disease populations and narrower socioeconomic subgroups. For example, studying the outcomes of oropharyngeal squamous cell carcinoma may be more informative than comparison across all HPV-related cancers. Alternatively, future iterations of this model could incorporate genetic ancestry to better account for biological differences rather and socially constructed perceptions of race. In addition, alternate geospatial techniques, such as geographically weighted regression analysis, could be considered to better account for geographic similarities and measurement error.
In conclusion, ADI is not associated with stage of HPV-related cancer at presentation. These results suggest that simplifying SDOH into a single geocoded index is not a reliable surrogate for assessing a patient’s risk for HPV-related cancer. These findings should be confirmed by larger studies that are more reflective of the socioeconomic characteristics of the general population.
Acknowledgements
The authors would like to thank Drs. Marta Crispens, Ron Alvarez, Cody Chastain for their clinical input. Research reported in this publication was conducted under the auspices of the Precision Medicine and Health Disparities Collaborative (Vanderbilt-Meharry-Miami Center of Excellence in Precision Medicine and Population Health).
Rohini Chakravarthy, MD and MBA Candidate
Sarah Stallings, PhD, Research Assistant Professor
Digna R. Velez Edwards, PhD MS, Professor of Obstetrics and Gynecology, Director of Women's Health Research
Sifang Kathy Zhao, PhD Candidate
Douglas Conway, MS, Informatics Project Manager
J. Sunil Rao, PhD, Professor and Interim Chair of Public Health Services and Director
Melinda Aldrich, PhD MS, Associate Professor of Medicine and Thoracic Surgery and Bioinformatics
Erin Kobetz, PhD MPH, Associate Professor of Medicine, Public Health Sciences and Obstetrics/Gynecology, Director of Jay Weiss Institute for Health Equity at Sylvester Comprehensive Cancer Center, Senior Associate Dean for Health Disparities
Consuelo H. Wilkins, MD MSCI, Professor of Medicine and Vice President for Health Equity
Contributor Information
Rohini Chakravarthy, Vanderbilt University School of Medicine, Nashville, TN, USA.
Sarah C Stallings, Department of Medicine, Division of Geriatrics, Vanderbilt University Medical Center, Nashville, TN, USA.
Digna R Velez Edwards, Division of Quantitative Sciences, Department of Obstetrics and Gynecology, Vanderbilt University Medical Center, Nashville, TN, USA; Department of Biomedical Informatics, Vanderbilt University, Nashville, TN, USA; Institute for Medicine and Public Health, Vanderbilt Epidemiology Center, Vanderbilt University Medical Center, Nashville, TN, USA.
Sifang Kathy Zhao, Institute for Medicine and Public Health, Vanderbilt Epidemiology Center, Vanderbilt University Medical Center, Nashville, TN, USA.
Douglas Conway, Vanderbilt Institute for Clinical and Translational Research, Vanderbilt University Medical Center, Nashville, TN, USA.
J Sunil Rao, Department of Public Health Sciences, University of Miami School of Medicine, Miami, FL, USA; Division of Biostatistics, University of Miami School of Medicine, Miami, FL, USA.
Melinda C Aldrich, Department of Thoracic Surgery, Vanderbilt University Medical Center, Nashville, TN, USA; Division of Genetic Medicine, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN.
Erin Kobetz, Department of Public Health Sciences, University of Miami School of Medicine, Miami, FL, USA; Department of Medicine, University of Miami, Coral Gables, FL, USA.
Consuelo H Wilkins, Department of Medicine, Division of Geriatrics, Vanderbilt University Medical Center, Nashville, TN, USA; Meharry-Vanderbilt Alliance, Nashville, TN, USA; Office of Health Equity, Vanderbilt University Medical Center, Nashville, TN, USA.
Funding
This work is supported by National Institute on Minority Health and Health Disparities and National Human Genome Research, Institute of the National Institutes of Health (grant number U54MD010722). This work was also supported by the Vanderbilt Institute for Clinical and Translational Research (VICTR), Clinical and Translational Science Award from the National Center for Advancing Translational Science (grant number UL1TR00445), the Vanderbilt Medical Scholars Research Program, and the Meharry-Vanderbilt Alliance.
References
- 1. Marmot MG, Smith GD, Stansfeld S et al. Health inequalities among British civil servants: the Whitehall II study. Lancet 1991;337(8754):1387–93. [DOI] [PubMed] [Google Scholar]
- 2. WHO Commission on Social Determinants of Health, and World Health Organization . Closing the Gap in a Generation: Health Equity through Action on the Social Determinants of Health: Commission on Social Determinants of Health Final Report. World Health Organization, 2008. [Google Scholar]
- 3. WHO | About Social Determinants of Health. WHO. http://www.who.int/social_determinants/sdh_definition/en/ (24 June 2019, date last accessed). [Google Scholar]
- 4. Freiji M, Dullabh P, Hovey L et al. Incorporating Social Determinants of Health in Electronic Health Records: A Qualitative Study of Perspectives on Current Practices among Top Vendors. US Department of Health and Human Services, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alberti P. Achieving Health Equity: How Academic Medicine Is Addressing the Social Determinants of Health. Association of American Medical Colleges, 2016. [Google Scholar]
- 6. Schroeder SA. Shattuck lecture. We can do better--improving the health of the American people. N Engl J Med 2007;357(12):1221–8. [DOI] [PubMed] [Google Scholar]
- 7. Committee on the Recommended Social and Behavioral Domains and Measures for Electronic Health Records, Board on Population Health and Public Health Practice, Institute of Medicine . Capturing Social and Behavioral Domains and Measures in Electronic Health Records: Phase 2. National Academies Press (US), 2015. http://www.ncbi.nlm.nih.gov/books/NBK268995/ (15 May 2019, date last accessed). [PubMed] [Google Scholar]
- 8. Cantor MN, Thorpe L. Integrating data on social determinants of health into electronic health records. Health Aff (Millwood) 2018;37(4):585–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arons A, DeSilvey S, Fichtenberg C, Gottlieb L. Documenting social determinants of health-related clinical activities using standardized medical vocabularies. JAMIA Open 2019;2(1):81–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Phillips RL, Liaw W, Crampton P et al. How other countries use deprivation indices-and why the United States desperately needs one. Health Aff (Millwood) 2016;35(11):1991–8. [DOI] [PubMed] [Google Scholar]
- 11. de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer 2017;141(4):664–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Musselwhite LW, Oliveira CM, Kwaramba T et al. Racial/ethnic disparities in cervical cancer screening and outcomes. Acta Cytol 2016;60(6):518–26. [DOI] [PubMed] [Google Scholar]
- 13. Damiani G, Basso D, Acampora A et al. The impact of level of education on adherence to breast and cervical cancer screening: evidence from a systematic review and meta-analysis. Prev Med 2015;81:281–9. [DOI] [PubMed] [Google Scholar]
- 14. Chen H-Y, Kessler CL, Mori N, Chauhan SP. Cervical cancer screening in the United States, 1993-2010: characteristics of women who are never screened. J Womens Health (Larchmt) 2012;21(11):1132–8. [DOI] [PubMed] [Google Scholar]
- 15. Wookey VB, Appiah AK, Kallam A et al. HPV status and survival in non-Oropharyngeal squamous cell carcinoma of the head and neck. Anticancer Res 2019;39(4):1907–14. [DOI] [PubMed] [Google Scholar]
- 16. Diez Roux AV. Investigating neighborhood and area effects on health. Am J Public Health 2001;91(11):1783–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shack L, Jordan C, Thomson CS et al. Variation in incidence of breast, lung and cervical cancer and malignant melanoma of skin by socioeconomic group in England. BMC Cancer 2008;8:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spadea T, DʼErrico A, Demaria M, et al. Educational inequalities in cancer incidence in Turin, Italy. Eur J Cancer Prev. 2009;18(3):169–178. [DOI] [PubMed] [Google Scholar]
- 19. Bazemore AW, Cottrell EK, Gold R et al. Community vital signs: incorporating geocoded social determinants into electronic records to promote patient and population health. J Am Med Inform Assoc 2016;23(2):407–12. [DOI] [PubMed] [Google Scholar]
- 20. Census Tracts and Block Numbering Areas . Geographic Areas Reference Manual. Bureau of the Census, 1994. https://www.census.gov/programs-surveys/geography/guidance/geographic-areas-reference-manual.html. [Google Scholar]
- 21. Cantor MN, Chandras R, Pulgarin C. FACETS: using open data to measure community social determinants of health. J Am Med Inform Assoc 2018;25(4):419–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Taylor CB, Ahn D, Winkleby MA. Neighborhood and individual socioeconomic determinants of hospitalization. Am J Prev Med 2006;31(2):127–34. [DOI] [PubMed] [Google Scholar]
- 23. Singh GK. Area deprivation and widening inequalities in US mortality, 1969-1998. Am J Public Health 2003;93(7):1137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Putnam LR, Tsao K, Nguyen HT et al. The impact of socioeconomic status on Appendiceal perforation in Pediatric appendicitis. J Pediatr 2016;170:156, e1–60. [DOI] [PubMed] [Google Scholar]
- 25. Yee CW, Cunningham SD, Ickovics JR. Application of the social vulnerability index for identifying teen pregnancy intervention need in the United States. Matern Child Health J Published online June 21 2019. [DOI] [PubMed] [Google Scholar]
- 26. Summary Stage 2018 . Codes and Coding Instructions. National Cancer Institute, 2018. [Google Scholar]
- 27. Brokamp C. A Nationwide Community deprivation index. Github. https://github.com/geomarker-io/dep_index (29 April 2020, date last accessed). [Google Scholar]
- 28. Rogers W. Regression standard errors in clustered samples. Stata Tech Bull 1994;3(13). [Google Scholar]
- 29. Madkour AS, Harville EW, Xie Y. Neighborhood disadvantage, racial concentration and the birthweight of infants born to adolescent mothers. Matern Child Health J 2014;18(3):663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sankaranarayanan R, Nene BM, Shastri SS et al. HPV screening for cervical cancer in rural India. N Engl J Med 2009;360(14):1385–94. [DOI] [PubMed] [Google Scholar]
- 31. Robbins AS, Han X, Ward EM et al. Association between the affordable care act dependent coverage expansion and cervical cancer stage and treatment in young women. JAMA 2015;314(20):2189–91. [DOI] [PubMed] [Google Scholar]
- 32. Saghari S, Ghamsary M, Marie-Mitchell A et al. Sociodemographic predictors of delayed- versus early-stage cervical cancer in California. Ann Epidemiol 2015;25(4):250–5. [DOI] [PubMed] [Google Scholar]
- 33. Singh GK, Miller BA, Hankey BF, Edwards BK. Persistent area socioeconomic disparities in U.S. incidence of cervical cancer, mortality, stage, and survival, 1975-2000. Cancer 2004;101(5):1051–7. [DOI] [PubMed] [Google Scholar]
- 34. Li X, Sundquist J, Calling S et al. Neighborhood deprivation and risk of cervical cancer morbidity and mortality: a multilevel analysis from Sweden. Gynecol Oncol 2012;127(2):283–9. [DOI] [PubMed] [Google Scholar]
- 35. Li X, Sundquist J, Zöller B, Sundquist K. Neighborhood deprivation and lung cancer incidence and mortality: a multilevel analysis from Sweden. J Thorac Oncol 2015;10(2):256–63. [DOI] [PubMed] [Google Scholar]
- 36. Li X, Sundquist K, Sundquist J. Neighborhood deprivation and prostate cancer mortality: a multilevel analysis from Sweden. Prostate Cancer Prostatic Dis 2012;15(2):128–34. [DOI] [PubMed] [Google Scholar]
- 37. Danciu I, Cowan JD, Basford M et al. Secondary use of clinical data: the Vanderbilt approach. J Biomed Inform 2014;52:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brokamp C, Beck AF, Goyal NK et al. Material community deprivation and hospital utilization during the first year of life: an urban population-based cohort study. Ann Epidemiol 2019;30:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Riney LC, Brokamp C, Beck AF et al. Emergency medical services utilization is associated with community deprivation in children. Prehosp Emerg Care Published online August 17 2018;1–8. [DOI] [PubMed] [Google Scholar]
- 40. Brokamp C, LeMasters GK, Ryan PH. Residential mobility impacts exposure assessment and community socioeconomic characteristics in longitudinal epidemiology studies. J Expo Sci Environ Epidemiol 2016;26(4):428–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kind AJH, Jencks S, Brock J et al. Neighborhood socioeconomic disadvantage and 30-day rehospitalization: a retrospective cohort study. Ann Intern Med 2014;161(11):765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics 2000;56(2):645–6. [DOI] [PubMed] [Google Scholar]
- 43. Berkowitz SA, Hulberg AC, Standish S et al. Addressing unmet basic resource needs as part of chronic Cardiometabolic disease management. JAMA Intern Med 2017;177(2):244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rustin M. The causes of inequality: why social epidemiology is not enough. Soundings, London 2018;68:94–109. [Google Scholar]
- 45. Braveman P, Gottlieb L. The social determinants of health: it’s time to consider the causes of the causes. Public Health Rep 2014;129(Suppl 2):19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sheets L, Petroski GF, Jaddoo J et al. The effect of Neighborhood disadvantage on diabetes prevalence. AMIA Annu Symp Proc 2017;2017:1547–53. [PMC free article] [PubMed] [Google Scholar]
- 47. Hiatt RA, Breen N. The social determinants of cancer: a challenge for Transdisciplinary science. American J Prev Med 2008;35(2, Supplement):S141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]


