Highlights
-
•
Lymphopenia (<1500/mm3) was associated with 82% higher mortality in heart failure patients irrespective of ejection fraction.
-
•
Lymphopenia was a good predictor of all-cause readmission in heart failure patients with reduced ejection fraction.
-
•
Due to cost-effectiveness, easy availability, and ability to predict outcomes in the short-term and medium-term, lymphopenia can be a valuable tool in the mortality, readmission prediction model of heart failure.
Keywords: Heart failure, Lymphocyte, Absolute lymphocyte count, Mortality, Readmission
Abbreviations: ALC, absolute lymphocyte count; HR, hazard ratio; CI, confidence interval; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; LVEF, left ventricular ejection fraction; HFrEF, heart failure with reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; PSM, propensity-score matching
Abstract
Background
There is renewed interest in pursuing frugal and readily available laboratory markers to predict mortality and readmission in heart failure. We aim to determine the relationship between absolute lymphocyte count (ALC) and clinical outcomes in patients with heart failure hospitalization.
Methods
This was a retrospective cohort study of patients with heart failure. Patients were divided into two groups based on ALC, less than or equal to 1500 cells/mm3 and > 1500 cells/ mm3. The primary outcome was all-cause mortality. We did subgroup analysis based on ejection fraction and studied the association between ALC categories and clinical outcomes. Both ALC groups are matched by propensity score, outcomes were analyzed by Cox regression, and estimates are presented in hazard ratios (HR) and 95% confidence intervals (CI).
Results
We included 1029 patients in the pre-matched cohort and 766 patients in the propensity-score matched cohort. The median age was 64 years (IQR, 54–75), and 60.78% were male. In the matched cohort, ALC less than or equal to 1500 cells/mm3 had a higher risk of mortality compared with ALC > 1500 cells/mm3 (HR 1.51, 95% CI: 1.17–1.95; P = 0.002). These results were reproducible in subgroups of heart failure. When ALC was divided into four groups based on their levels, the lowest group of ALC had the highest risk of mortality.
Conclusions
In patients with heart failure and both subgroups, ALC less than or equal to 1500 cells/mm3 had a higher risk of mortality. Patients in lower groups of the ALC categories had a higher risk of mortality.
1. Introduction
Heart failure (HF) is a complex clinical syndrome associated with high morbidity and mortality. There is an increasing prevalence of HF due to the development of mortality-reducing therapies over the last 30 years. However, heart failure is still the most common cause of hospital readmissions in the United States [1], [2]. In patients hospitalized for acute heart failure, one-year mortality and readmission rate are 17.4% and 43.9%, respectively [1].
Various risk models from the trials, registries, and cohort studies have consistently demonstrated age, blood pressure, hemoglobin, sodium concentration, renal function, pro-brain natriuretic peptide (pro-BNP), left ventricular ejection fraction, no beta-blocker, and the dose of diuretic at discharge associated with mortality or readmission prediction [3]. In recent years, there is renewed interest in pursuing frugal and readily available laboratory markers to predict mortality and hospital readmission in HF. Lymphopenia in HF was first described in 1960 [4]. Subsequently, literature studied the role of neutrophil-to-lymphocyte ratio (NLR) [5], [6], [7], platelet-to-neutrophil ratio (PNR) [8], [9], [10], monocyte-lymphocyte ratio and lymphocyte percentage [11], [12] as potential independent predictors of mortality. However, these indices can be altered in conditions that affect neutrophils and platelets. Acute infection or stress can easily alter neutrophil count, while its effect on lymphocytes is minimal. Lymphocyte percentage is a proportional representation of total white blood cell count, and hence it can be affected with change in other cell lineages. Hence, we preferred absolute lymphocyte count (ALC) over other lymphocyte markers. Previous studies in heart failure patients have shown an association between lymphocyte count and mortality [13], [14], but there are very few studies that assessed an association between ALC grading and mortality, [14] and or association between ALC and readmission. We aimed to fill this void by assessing ALC's role in predicting mortality, all-cause readmission, cardiac readmission, and a composite of mortality or readmission at 30 days and 6 months in patients with acute heart failure.
1.1. Methods
1.1.1. Data source and study population
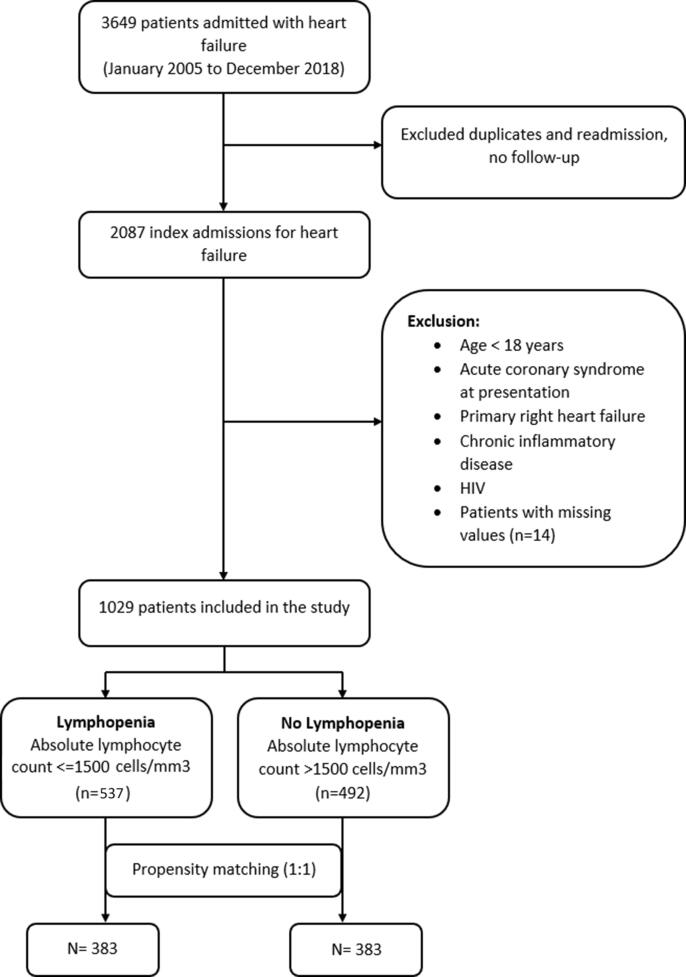
This is a single-center retrospective cohort study of acute heart failure hospitalization in the New York City. We collected information on consecutive patients hospitalized from January 2005 to December 2018, as per our inclusion and exclusion criteria mentioned below. The institutional review boards approved this study and permitted a waiver of informed consent from the study participants due to the retrospective nature of the study. Acute heart failure was defined according to the guideline criteria as acute onset (or worsening) of signs and symptoms of heart failure needing urgent hospitalization and intravenous treatment.[15] The exclusion criteria were: age < 18 years old, acute coronary syndrome at the time of presentation, primary right-sided heart failure, comorbidities that are known to affect the lymphocyte count, such as active infection, chronic inflammatory disease, chronic corticosteroid use in the past three months, and human immunodeficiency virus. The infection diagnosis was based on clinical information from the notes as documented by the admitting physician or laboratory criteria such as lactic acid and C-reactive protein suggestive of infection. For patients with previous heart failure, the first admission to the hospital was considered an index event. Fig. 1 summarizes patient selection.
Fig. 1.
Patient selection.
We reviewed and collected demographic, clinical, laboratory, medication information, and outcomes from the electronic medical records between June to October 2020. The definitions of all extracted data and outcomes were recorded separately and checked by two authors (M.M. and T.K.). All the data extraction was done manually, which was verified by a second physician. Any disparity was resolved by consulting the primary investigator. Patient confidentiality was protected by de-identification, and the data were stored in a locked, password-protected data storage device.
1.1.2. Primary predictor and outcomes
Our primary predictor was ALC, obtained at admission. ALC was measured by Sysmex automated hematology analyzer XN-10. We divided ALC into two groups, one with ALC ≤ 1500 cells/mm3 and the other > 1500 cells/mm3 (comparison group) based on median value obtained from our study as well as from a previous study.[16] We further divided ALC into 4 groups; Group A > 2000 cells/mm3 (comparison group), Group B 1501–2000 cells/mm3, Group C 1001–1500 cells/mm3, and Group D ≤ 1000 cells/mm3. This division was based on our IQR (interquartile range). Our primary outcome was all-cause mortality. Secondary outcomes were all-cause readmission, cardiac readmission, and a composite of readmission or mortality at 30 days and 6 months. All the outcomes were collected in ‘time to event’ format. They were defined as the time from discharge date to the date of occurrence of events. The clinical outcomes were determined by electronic medical records from all the hospitals of the organization. The occurrence of death was determined from the medical record, telephonic call, or social security death index master file by two-point identifier, name, and birth date. Cardiac readmission was defined as readmission due to heart failure, myocardial infarction, and/or arrhythmias as a primary discharge diagnosis. We performed a subgroup analysis by left ventricular ejection fraction (LVEF). Patients with LVEF ≤ 40% were grouped under heart failure with reduced ejection fraction (HFrEF), and with LVEF > 40% were grouped under heart failure with preserved ejection fraction (HFpEF).
1.1.3. Statistical analysis
Baseline characteristics of both groups of ALC were expressed using descriptive statistics. The continuous variables were demonstrated as median with interquartile range (IQR). Categorical variables were presented in frequency and proportion. The Mann-Whitney-Wilcoxon tests were applied to compare continuous variables. Fisher’s exact test or Pearson’s Chi2 tests were implemented to compare categorical variables. The Kaplan-Meier curves were used to demonstrate the rate of events in each group; the comparison between the two groups was calculated with the log-rank test. For death, censoring was applied at last objective evidence of survival available. For readmission, censoring was applied at six months. We built a multivariate cox-proportional hazard model to determine the hazard ratio (HR) and 95% confidence interval (CI) for the lymphopenia group compared with the group with normal ALC defined as ALC > 1500 cells/mm3. The final model was built by both the forward selection and backward elimination method. A p-value of 0.20 was set as a cut-off for the variable to include in the final multivariable model. The variables mentioned in the literature that can possibly be associated with the outcomes were also included in the final model. In the final model, we included age, gender, race, ejection fraction, coronary artery disease, stroke, cancer, hypertension, diabetes, atrial fibrillation, chronic lung disease, body mass index, pro-BNP, hemoglobin, sodium, glomerular filtration rate, albumin, and medications such as loop diuretic, beta blocker, ACE inhibitors or ARB, spironolactone, digoxin, aspirin/clopidogrel, warfarin/other anticoagulation, and statins. We also utilized a propensity-score matching methodology (PSM) to match patients with ALC ≤ 1500 cells/mm3 to those with > 1500 cells/mm3 at a 1:1 ratio. The nearest neighbor technique was adopted to match each patient in two groups by calculating propensity-score, with a caliper of 0.2. The variables used to calculate propensity-score are similar to those used in the multivariable model. After matching, we ran a univariate cox-proportional hazard analysis to determine the HR with 95% CI. We applied the test for proportionality assumption based on Schoenfeld Residuals. Missing data were not imputed. A p value < 0.05 was considered statistically significant. Statistical analysis was performed using STATA version 16.1 (StataCorp LLC).
1.2. Results
1.2.1. Baseline characteristics
We performed a retrospective cohort study of 1029 patients. The median follow-up period was 860 days (2.35 years). The median age was 64 years (IQR, 54–75), 61% (6 2 6) were males and 40.1% (4 1 3) were African American. Baseline demographic characteristics, laboratory parameters at admission, echocardiographic features, and discharge medications included in the study are in Table 1. As demonstrated in Table 1, patients with ALC ≤ 1500 cells/mm3 compared with patients with ALC > 1500 cells/mm3 were older (67 vs. 62 years; P < 0.001), had a lower median blood pressure (99.33 vs. 102.67 mmHg; P = 0.011), lower median body mass index (27.8 vs 30.3 kg/m2; P < 0.001), higher median pro-BNP levels (7924 vs. 3574 picograms/milliliter; P < 0.001), lower hemoglobin levels (11.6 vs. 12.3 g/dL; P < 0.001), and lower glomerular filtration rate (57.58 vs. 64.62 ml/min; P = 0.013). Other baseline characteristics were comparable in both groups. Baseline characteristics between the two groups after the PSM method are extrapolated in Table 2. The matched cohort had 766 patients based on 25 matched variables. The distribution of propensity-score between two groups and balance of covariates between two cohorts after matching are demonstrated in Supplemental Figure 1, Figure 2.
Table 1.
Baseline demographic and clinical characteristics of patients by absolute lymphocyte count before matching.
| All patients (N = 1029) | No Lymphopenia (ALC > 1500 cells/mm3) (N = 492) | Lymphopenia (ALC ≤ 1500 cells/mm3) (N = 537) | P-value | |
|---|---|---|---|---|
| Demographic | ||||
| Age (years), median (IQR) | 64 (54 – 75) | 62 (52.5 – 72) | 67 (56 – 78) | <0.001 |
| Male, n (%) | 626 (60.8) | 294 (59.8) | 332 (61.7) | 0.521 |
| White, n (%) | 86 (8.4) | 39 (7.9) | 47 (8.7) | 0.083 |
| Black, n (%) | 362 (35.2) | 190 (38.6) | 172 (32) | |
| Hispanic, n (%) | 582 (56.5) | 263 (53.5) | 319 (59.3) | |
| Comorbidities | ||||
| Hypertension, n (%) | 888 (86.2) | 426 (86.6) | 462 (85.9) | 0.741 |
| Diabetes, n (%) | 526 (51.1) | 260 (52.9) | 266 (49.4) | 0.275 |
| CAD, n (%) | 302 (29.4) | 136 (27.7) | 166 (31) | 0.25 |
| Stroke, n (%) | 93 (9) | 44 (8.9) | 49 (9.1) | 0.927 |
| Cancer, n (%) | 87 (8.5) | 44 (8.9) | 43 (8) | 0.584 |
| Atrial fibrillation, n (%) | 247 (24) | 115 (23.4) | 132 (24.5) | 0.663 |
| Chronic lung disease, n (%) | 193 (18.7) | 87 (17.7) | 106 (19.7) | 0.407 |
| Parameters on admission | ||||
| MAP (mm Hg), median (IQR) | 101 (89.7 – 115.3) | 102.67 (90.7 – 117.3) | 99.33 (89.3 – 113) | 0.011 |
| BMI, median (IQR) | 28.9 (24.8–35.4) | 30.3 (25.5–37) | 27.8 (24.3–33.7) | <0.001 |
| Pro-BNP (pg/ml), median (IQR) | 5179 (2157 – 12712) | 3574 (1553 – 8382) | 7924 (2614 – 17052) | <0.001 |
| Hb (g/dL), median (IQR) | 11.9 (10.5 – 13.4) | 12.3 (11–13.7) | 11.6 (10.1–13) | <0.001 |
| GFR (ml/min/m2), median (IQR) | 61.92 (37.9 – 85.5) | 64.62 (42.3–86.7) | 57.58 (35.3–85) | 0.013 |
| Sodium (mEq/L), median (IQR) | 139 (137 – 141) | 139 (137–142) | 139 (136–141) | 0.008 |
| Echocardiographic features | ||||
| EF > 40%, n (%) | 384 (37.3) | 193 (39.2) | 191 (35.5) | 0.217 |
| LAD (cm), median (IQR) | 4.3 (4 – 4.8) | 4.3 (4 – 4.8) | 4.4 (4 – 4.9) | 0.014 |
| LVIDD (cm), median (IQR) | 5.5 (4.9 – 6.1) | 5.5 (4.9 – 6) | 5.5 (5 – 6.1) | 0.99 |
| Discharge medications | ||||
| Loop diuretics, n (%) | 815 (79.2) | 382 (77.6) | 433 (80.6) | 0.238 |
| Beta blocker, n (%) | 821 (79.7) | 399 (81.1) | 422 (78.4) | 0.289 |
| ACE inhibitors/ARB, n (%) | 766 (74.4) | 372 (75.6) | 394 (73.2) | 0.383 |
| CCB, n (%) | 278 (27) | 124 (25.2) | 154 (28.6) | 0.217 |
| Spironolactone, n (%) | 188 (18.3) | 83 (16.9) | 105 (19.5) | 0.272 |
| Digoxin, n (%) | 282 (27.4) | 132 (26.8) | 150 (27.9) | 0.705 |
| Aspirin/clopidogrel, n (%) | 812 (78.8) | 404 (82.1) | 408 (75.8) | 0.014 |
| Anticoagulation, n (%) | 185 (18) | 79 (16.1) | 106 (19.7) | 0.128 |
| Statin, n (%) | 668 (64.9) | 341 (69.3) | 327 (60.8) | 0.004 |
| Outcomes | ||||
| Death, n (%) | 336 (32.6) | 137 (27.9) | 199 (37) | 0.002 |
| All-cause readmission, n (%) | 412 (40) | 164 (33.3) | 248 (46.1) | <0.001 |
| Cardiac readmission, n (%) | 301 (29.22) | 122 (24.80) | 179 (33.3) | 0.003 |
| Death or readmission, n (%) | 575 (55.8) | 239 (48.6) | 336 (62.5) | <0.001 |
Abbreviations: ACE – angiotensin converting enzyme, ALC – absolute lymphocyte count, ARB – angiotensin receptor blocker, BMI – body mass index, CAD – coronary artery disease, EF – ejection fraction, GFR – glomerular filtration rate, Hb – hemoglobin, IQR – interquartile range, LAD – left atrial diameter, LVIDD – left ventricular internal diastolic diameter, MAP – mean arterial pressure.
Table 2.
Baseline demographic and clinical characteristics of patients by absolute lymphocyte count after propensity-score matching.
| No Lymphopenia (ALC > 1500 cells/mm3) (N = 383) | Lymphopenia (ALC ≤ 1500 cells/mm3) (N = 383) | P-value | |
|---|---|---|---|
| Demographic | |||
| Age (years), median (IQR) | 64 (54 – 74) | 63 (53 – 74) | 0.67 |
| Male, n (%) | 154 (40.2) | 150 (39.2) | 0.768 |
| Black, n (%) | 157 (41) | 164 (42.8) | 0.608 |
| Comorbidities | |||
| Hypertension, n (%) | 335 (87.5) | 337 (88) | 0.826 |
| Diabetes, n (%) | 198 (51.7) | 210 (54.8) | 0.385 |
| CAD, n (%) | 116 (30.3) | 112 (29.2) | 0.752 |
| Stroke, n (%) | 36 (9.4) | 39 (10.2) | 0.715 |
| Cancer, n (%) | 35 (9.1) | 36 (9.4) | 0.901 |
| Atrial fibrillation, n (%) | 88 (22.98) | 88 (23) | 1.00 |
| Chronic lung disease, n (%) | 73 (19.1) | 70 (18.3) | 0.781 |
| Parameters on admission | |||
| MAP (mm Hg), median (IQR) | 102.67 (90.3 – 117.3) | 100 (89.3 – 113.7) | 0.17 |
| BMI, median (IQR) | 28.6 (24.9 – 35.3) | 29.4 (25 – 35.7) | 0.438 |
| Pro-BNP (pg/ml), median (IQR) | 5657 (1553–10317) | 6567 (2217–12941) | 0.33 |
| Hb (g/dL), median (IQR) | 12 (10.8 – 13.5) | 12 (10.4 – 13.3) | 0.18 |
| GFR (ml/min/m2), median (IQR) | 62.5 (39.3 – 86.3) | 61.2 (38.1 – 86.8) | 0.66 |
| Sodium (mEq/L), median (IQR) | 139 (137–142) | 139 (137 – 141) | 0.48 |
| Echocardiographic features | |||
| EF > 40%, n (%) | 146 (38.1) | 154 (40.2) | 0.554 |
| LAD, median (IQR) | 4.2 (4 – 4.8) | 4.3 (4 – 4.9) | 0.58 |
| LVIDD, median (IQR) | 5.5 (4.9 – 6) | 5.5 (5 – 6.1) | 0.80 |
| Discharge medications | |||
| Loop diuretics, n (%) | 308 (80.4) | 303 (79.1) | 0.653 |
| Beta blocker, n (%) | 305 (79.6) | 310 (80.9) | 0.65 |
| ACE inhibitors/ARB, n (%) | 290 (75.7) | 289 (75.5) | 0.933 |
| CCB, n (%) | 95 (24.8) | 118 (30.8) | 0.064 |
| Spironolactone, n (%) | 70 (18.3) | 64 (16.7) | 0.568 |
| Digoxin, n (%) | 101 (26.4) | 102 (26.6) | 0.935 |
| Aspirin/clopidogrel, n (%) | 307 (80.2) | 315 (82.3) | 0.459 |
| Anticoagulation, n (%) | 66 (17.2) | 63 (16.5) | 0.772 |
| Statin, n (%) | 251 (65.5) | 266 (69.5) | 0.247 |
| Outcomes | |||
| Death, n (%) | 112 (29.2) | 130 (34.7) | 0.104 |
| All-cause readmission, n (%) | 128 (33.4) | 176 (46) | <0.001 |
| Cardiac readmission, n (%) | 93 (24.3) | 129 (33.7) | 0.004 |
| Death or readmission, n (%) | 192 (50.1) | 238 (62.1) | 0.001 |
Abbreviations ACE – angiotensin converting enzyme, ALC – absolute lymphocyte count, ARB – angiotensin receptor blocker, BMI – body mass index, CAD – coronary artery disease, EF – ejection fraction, GFR – glomerular filtration rate, Hb – hemoglobin, LAD – left atrial diameter, LVIDD – left ventricular internal diastolic diameter, MAP – mean arterial pressure.
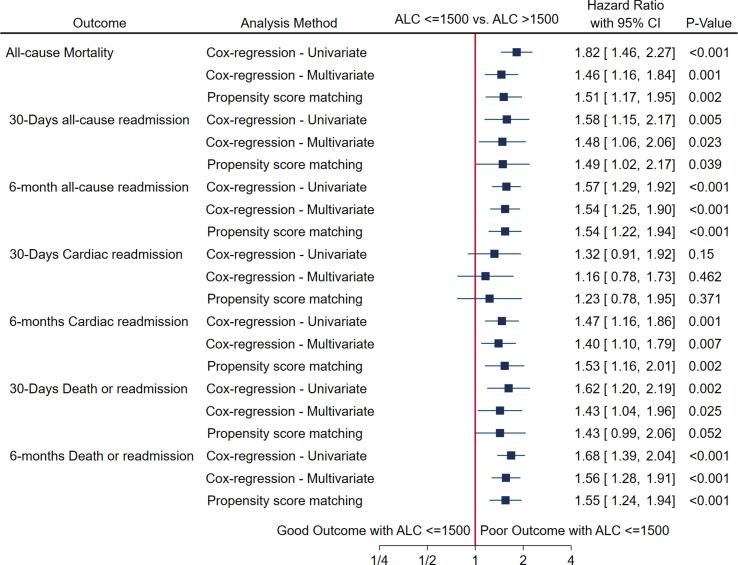
1.2.2. Primary outcome (Mortality) (Fig. 2)
Fig. 2.
Forest plot demonstrating primary and secondary outcomes in heart failure cohort by various methods.
Out of 1029 eligible patients, 137 (27.85%) patients died in cohort of ALC > 1500 cells/mm3 compared with 199 (36.99%) patients in cohort of ALC < 1500 cells/mm3 (P = 0.002) (Table 1). Using the PSM model, patients with ALC < 1500 were associated with increased hazard of mortality (HR 1.51, 95% CI: 1.17–1.95; P = 0.002). These results were similar to the results obtained with univariate and multivariate analysis (Supplemental Table 1).
1.2.3. Secondary outcomes (Fig. 2)
In the cohort of ALC ≤ 1500, 46.10% (2 4 8) patients were readmitted within 6 months compared with 33.33% (1 6 4) in the cohort of ALC > 1500 (P < 0.0001) (Table 1). In the PSM method, the analysis showed a higher hazard ratio for readmission in the cohort of ALC < 1500 cells/mm3 at 30 days and 6 months (30-day HR 1.49, 95% CI:1.02–2.17, P = 0.039; 6-month HR 1.54, 95% CI: 1.23–1.95, P < 0.001). The proportionality assumption was not violated (global test P = 0.47).
In the cohort of ALC ≤ 1500, 33.27% (1 7 9) patients had cardiac readmission within 6 months compared with 24.8% (1 2 2) in the cohort of ALC > 1500 (P = 0.003) (Table 1). Cox analysis and PSM method showed a similar rate of cardiac readmission at 30 days between two groups; however, at 6 months, ALC ≤ 1500 had a higher risk of cardiac readmission (adjusted HR 1.40, 95% CI: 1.10–1.79, P-0.007, by PSM HR 1.53, 95% CI: 1.16–2.01, P-0.002). The proportionality assumption was not violated (global test P = 0.90).
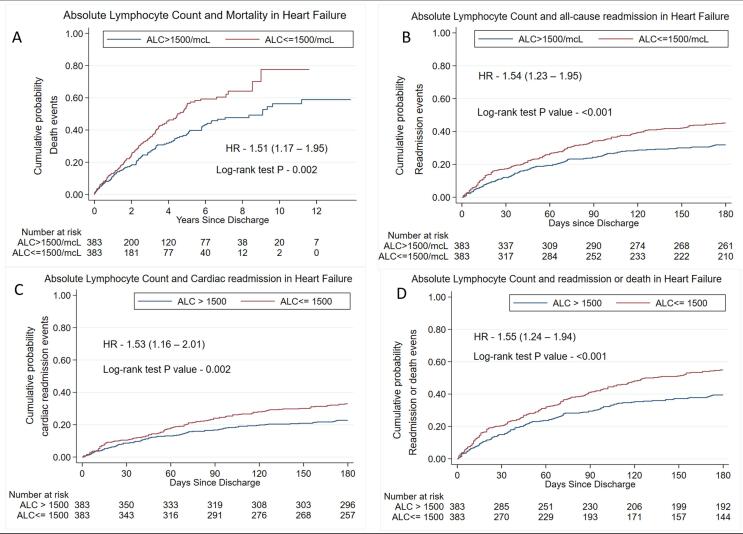
In the cohort of ALC ≤ 1500, 62.45% (3 3 6) patients died or were readmitted compared with 48.58% (2 3 9) in the cohort of ALC > 1500 (P < 0.001) (Table 1). By the PSM method, patients with ALC ≤ 1500 showed a higher hazard ratio for death or readmission at 30 days and 6 months (30-day HR 1.43, 95% CI:0.99–2.05, P-0.052; 6-month HR 1.55, 95% CI: 1.24–1.94, P < 0.001). The proportionality assumption was not violated (global test P = 0.60). Fig. 3 (Panel A-D) demonstrates Kaplan Meier graphs of all primary and secondary outcomes in propensity-score matched cohort. The log-rank test p-value for all the outcomes was statistically significant, as shown in the figures. Similar results were obtained using univariable and multivariable method for all secondary outcomes as shown in Supplemental Table 1.
Fig. 3.
Kaplan-Meier graphs for primary and secondary outcomes in propensity-score matched cohort, 3A. Kaplan-Meier graph of mortality; 3B. Kaplan-Meier graph of all-cause readmission, 3C. Kaplan-Meier graph of cardiac readmission; 3D. Kaplan-Meier graph of death or readmission.
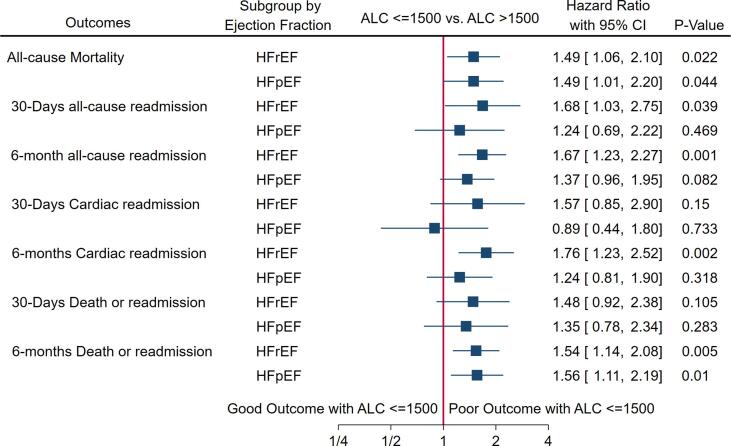
1.2.4. Subgroup analysis by Ejection Fraction (Fig. 4)
Fig. 4.
Forest plot of primary and secondary outcomes in subgroups of HFrEF and HFpEF in PSM analysis.
We performed a sub-group analysis in HFrEF and HFpEF to assess primary and secondary outcomes using the PSM method. There were 466 patients in HFrEF and 300 patients in HFpEF. We observed that ALC < 1500 cells/mm3 had a higher risk of mortality in both HFrEF (HR 1.49, 95% CI: 1.06–2.10, P-0.022) and HFpEF (HR 1.49, 95% CI: 1.01–2.20, P-0.044) subgroups. In the subgroup of HFrEF, patients with ALC < 1500 had a higher hazard of all-cause readmission at 30 days and 6 months. In the subgroup of HFpEF, patients with ALC < 1500 had a similar hazard of all-cause readmission at 30 days and 6 months. In the subgroup of HFrEF, patients with ALC < 1500 had a higher hazard of cardiac readmission at 30 days and 6 months, but at 30 days, the estimate was statistically insignificant. In the subgroup of HFpEF, patients with ALC < 1500 had a similar cardiac readmission hazard at 30 days and 6 months. In the subgroup of HFrEF, patients with ALC < 1500 had a higher hazard of a composite of death or readmission at 30 days and 6 months, but at 30 days, the estimate was statistically not significant. In the subgroup of HFpEF, patients with ALC < 1500 had a similar hazard of a composite of death or readmission at 30 days but had a higher hazard at 6 months. The p-value for interaction between type of heart failure and lymphopenia was not significant for all the outcomes (Supplemental Table S2).
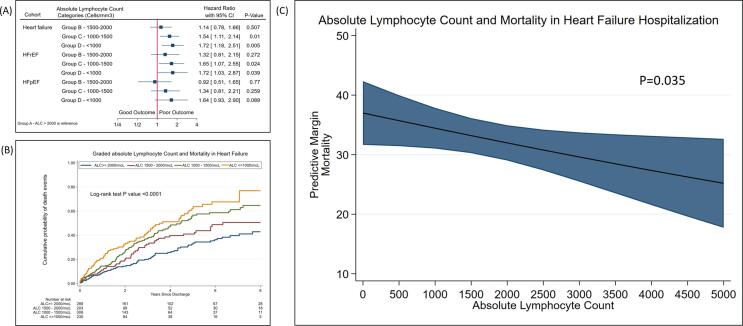
1.2.5. Degree of lymphopenia and mortality in full cohort and subgroups (Fig. 5. Panel A, B, C, Supplemental Table S3)
Fig. 5.
Panel (A) Forest plot of mortality and ALC categories in PSM Model; Panel (B) Kaplan-Meier graph of mortality by lymphopenia categories; Panel (C) Spline graph showing ALC and all-cause mortality, lymphocyte >= 2000 – reference group, lymphocyte 1500 – 2000 [HR (95% CI)] – 1.14 (0.78 – 1.65) P = 0.507, lymphocyte 1000 – 1500 – 1.54 (1.11 – 2.14) P = 0.010, lymphocyte <= 1000 – 1.72 (1.18 – 2.52) P = 0.005.
In the cohort of HF and subgroup of HFrEF, we observed a graded relationship between the degree of lymphopenia and mortality risk. The lower group of ALC was associated with a higher risk of mortality. It was consistent and statistically significant in groups C and D. In the HFpEF subgroup, we did not observe similar graded relationships likely due to insufficient power to detect the difference. We extrapolated the Kaplan-Meier graphs to demonstrate the association between ALC categories and mortality. We also showed linear association between ALC and all-cause mortality (p = 0.035).
1.3. Discussion
Significant findings of this study include the following: (1) ALC ≤ 1500 cells/mm3 is an independent predictor of mortality in heart failure and subgroups of HFrEF and HFpEF; (2) ALC ≤ 1500 cells/mm3 is an independent predictor of all-cause readmission, and a composite of death or readmission at 30 days and all-cause readmission, cardiac readmission, and composite of death or readmission at 6 months in heart failure; (3) in HFrEF, ALC ≤ 1500 cells/mm3 is an independent predictor of all-cause readmission at 30 days and 6 months, cardiac readmission and composite of readmission or death at 6 months; (4) in HFpEF, ALC ≤ 1500 cells/mm3 is an independent predictor of composite of readmission or death at 6 months; (5) four categories of ALC had a graded correlation with the risk of death in heart failure and subgroup of HFrEF, but in HFpEF it was not statistically significant, likely due to insufficient power.
One study has described the association between ALC and mortality at 1-year [16]. Few studies demonstrated other lymphocyte lineage methods such as NLR, PLR, or %L predicting mortality [5], [6], [7], [8], [9], [10]. The study by Boralkar et al. [5] described NLR as an independent predictor of all-cause mortality in patients hospitalized with HFpEF at 1, 2, or 3 years after admission. Huang et al. [17] showed that neutrophil count, NLR, reciprocal of lymphocyte, and PLR were independent predictors of mortality at 3 years. In a prospective longitudinal study, Charach et al. [18] found that ALC < 1600 cells/mm3 was an independent mortality predictor in patients with chronic HFrEF in the ambulatory setting. Among these multiple hematological indices that include lymphocytes, reciprocal of lymphocyte appears to be the best predictor of mortality in a head-to-head comparison to white blood cell count, neutrophil count, NLR, and PLR [17]. However, ALC is not well-studied in hospitalized heart failure patients for long-term mortality and readmission; additionally, no literature has shown association between grading of ALC and mortality. We used ALC as a predictor and demonstrated similar mortality results in heart failure patients and its subgroups of HFrEF and HFpEF. Uthamalingam et al. [19] divided NLR into tertiles and showed higher tertile had the highest mortality and 30-day readmission hazard. We found similar results in groups C and D of ALC for mortality when ALC was divided into four categories in heart failure and the subgroup HFrEF, which has not been described earlier. Minimal data are available on the association between ALC and other lymphopenia markers with readmission. One study [19] showed that a higher tertile of NLR is an independent predictor of 30-day readmission. In the current study, we assessed all-cause readmission, cardiac readmission, and a composite of readmission or death at short-term (30 days) and medium-term (60 days), which has not been studied prior for any lymphocyte marker.
The most accepted lymphopenia mechanism in heart failure is a state of chronic subclinical stress [20], inflammation [21] and sympathetic activation [22], where the cross-talk of this neuro-immuno-hormonal axis plays a critical role [23]. High cortisol levels [4], [24], stimulation of beta-adrenergic receptor in the lymphocytes due to increased sympathetic tone [22], and elevated cytokines [25] have all shown to cause lymphopenia. Systemic inflammation and the related cytokines in heart failure have been associated with the migration of monocytes to the myocardium, which leads to interstitial fibrosis and ventricular remodelling [26], leading to poor outcomes in HF. This further reflects our hypothesis that lymphopenia is associated with poor HF outcomes. It is important to stress that the cross-talk between immune, nervous and endocrinologic systems, and their effects on the heart is seen as a vicious cycle with no clear starting point [21]. Renin-angiotensin-aldosterone system inhibitors, mineralocorticoid antagonists, and beta-blockers prevent cardiac remodeling in HF that a chronic state of inflammation may partly drive. These therapies that have been proven to decrease mortality in patients with HF may affect the circulating lymphocytes [22]. It is tempting to speculate that ALC may serve as a measurement of response to a single or combination of these therapies [27], [28]. This opens the avenue for future research to explore lymphopenia as a marker of response to HF treatment.
Treatments targeting cytokines in HF have been a focus of interest given that cytokine production by lymphocytes and monocytes have been previously demonstrated [29]. TNF-α is a known driver of lymphopenia via increased susceptibility to apoptosis [30]. Agnoletti et al. hypothesized that TNF-α production by monocytes in patients with advanced HF could be due to the mesenteric vascular congestion, resulting in bacterial translocation and endotoxin release, leading to a feedback loop of chronic inflammation27. Targeting cytokines in HF, such as TNF-α [22], [31], and IL-1β [32], [33], have failed to show mortality benefit in randomized clinical trials. Hence, we assume that the underlying chronic inflammatory status present in HF patients appears to be a consequence rather than a cause [21]. This could explain why targeting the immune system in HF patients may be further downstream of the cascade that causes HF progression.
1.4. Study limitations
Our study was single-center and retrospective in nature, and fraught with inherent unmeasured biases that might exist despite robust adjustments. However, the size of the study population, and the consistency of results with prior studies reassure that the conclusions may be more generalizable. We analyzed by multivariate cox-regression and propensity-score matching methods, utilizing 25 variables for the robust adjustment and matching. We used ALC as the single estimate point to predict mortality. We did not evaluate the changes of ALC through time (to assess their predictive power through time). Moreover, we used ALC over lymphocyte percentage as ALC is a direct estimate while lymphocyte percentage is an indirect estimate of lymphocyte. However, superiority of one over the other is not established. It is essential to note that we have excluded patients with conditions, as mentioned in exclusion criteria, that affect ALC level, which removes potential biases and gives us a focused cohort to evaluate ALC's role. Since inflammatory parameters such as CRP, procalcitonin, or ESR were not commonly obtained in routine laboratories in our patients’ cohort, we could not correlate the severity of inflammation with the degree of lymphopenia. Though we obtained all the demographic, clinical, and laboratory characteristics from those hospitalized for the first time for HF in our medical center, we could not determine if prior admissions to other hospitals for HF exacerbation occurred. Also, patients might have had a readmission at a hospital outside our healthcare system, thereby not included in the study. However, given the large healthcare system and the specific patient population served by our hospital, it is very unlikely.
1.5. Conclusion
In conclusion, the present study showed that ALC could be a predictor of mortality in heart failure patients and its subgroups of HFrEF and HFpEF. Categories of ALC showed graded correlation with mortality from a higher group (>2000 cells/mm3, 1501–2000 cells/mm3 to lower group (≤1000 cells//mm3, and 1001–1500 cells/mm3). ALC is a good predictor of short-term (30-day) and medium-term (6-month) readmission-related outcomes in HF and HFrEF subgroups except for short-term cardiac readmission. ALC is not a good predictor of readmission-related outcomes in patients with HFpEF, and remains to be further investigated with sufficient power. This study supports the incorporation of a cost-effective, readily available ALC in the prognostication model of heart failure due to its ability to predict outcomes in the short-term and medium-term duration.
Authorship Contribution
All authors participated in the research and preparation of the manuscript as per the International Committee of Medical Journal Editors (ICMJE).
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: [Dr. Kalra is CEO of makeadent.org. The authors reported no potential conflict of interest, relationships with pharmaceutical companies, biomedical device manufacturers, or other corporations whose products or services are related to the subject matter of the article.]
Acknowledgment of Grant Support
makeadent.org Ram and Sanjita Kalra Aavishqaar Fund.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2022.100981.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Murphy S.P., Ibrahim N.E., Januzzi J.L. Heart Failure With Reduced Ejection Fraction: A Review. JAMA. 2020;324(5):488. doi: 10.1001/jama.2020.10262. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed A., Allman R.M., Fonarow G.C., Love T.E., Zannad F., Dell'Italia L.J., White M., Gheorghiade M. Incident heart failure hospitalization and subsequent mortality in chronic heart failure: a propensity-matched study. J. Card. Fail. 2008;14(3):211–218. doi: 10.1016/j.cardfail.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Passantino A., Monitillo F., Iacoviello M., Scrutinio D. Predicting mortality in patients with acute heart failure: Role of risk scores., World. J. Cardiol. 2015;7:902–911. doi: 10.4330/wjc.v7.i12.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hurdle A.D.F., Gyde O.H.B., Willoughby J.M.T. Occurrence of lymphopenia in heart failure. J. Clin. Pathol. 1966;19(1):60–64. doi: 10.1136/jcp.19.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boralkar K.A., Kobayashi Y., Amsallem M., Ataam J.A., Moneghetti K.J., Cauwenberghs N., Horne B.D., Knowlton K.U., Maecker H., Kuznetsova T., Heidenreich P.A., Haddad F. Value of Neutrophil to Lymphocyte Ratio and Its Trajectory in Patients Hospitalized With Acute Heart Failure and Preserved Ejection Fraction. Am. J. Cardiol. 2020;125(2):229–235. doi: 10.1016/j.amjcard.2019.10.020. [DOI] [PubMed] [Google Scholar]
- 6.Delcea C., Buzea C.A., Dan G.A. The neutrophil to lymphocyte ratio in heart failure: a comprehensive review. Rom. J. Intern. Med. 2019;57:296–314. doi: 10.2478/rjim-2019-0018. [DOI] [PubMed] [Google Scholar]
- 7.Afari M.E., Bhat T. Neutrophil to lymphocyte ratio (NLR) and cardiovascular diseases: an update. Expert Rev. Cardiovasc. Ther. 2016;14(5):573–577. doi: 10.1586/14779072.2016.1154788. [DOI] [PubMed] [Google Scholar]
- 8.Ye G.-L., Chen Q., Chen X., Liu Y.-Y., Yin T.-T., Meng Q.-H., Liu Y.-C., Wei H.-Q., Zhou Q.-H. The prognostic role of platelet-to-lymphocyte ratio in patients with acute heart failure: A cohort study. Sci. Rep. 2019;9:10639. doi: 10.1038/s41598-019-47143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durmus E., Kivrak T., Gerin F., Sunbul M., Sari I., Erdogan O. Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio are Predictors of Heart Failure. Brazilian Arch. Cardiol. 2015;105:606–613. doi: 10.5935/abc.20150126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pourafkari L., Wang C.K., Tajlil A., Afshar A.H., Schwartz M., Nader N.D. platelet-lymphocyte ratio in prediction of outcome of acute heart failure. Biomark. Med. 2018;12(1):63–70. doi: 10.2217/bmm-2017-0193. [DOI] [PubMed] [Google Scholar]
- 11.Ommen S.R., Hodge D.O., Rodeheffer R.J., McGregor C.G.A., Thomson S.P., Gibbons R.J. Predictive power of the relative lymphocyte concentration in patients with advanced heart failure. Circulation. 1998;97(1):19–22. doi: 10.1161/01.cir.97.1.19. [DOI] [PubMed] [Google Scholar]
- 12.Vaduganathan M., Ambrosy A.P., Greene S.J., Mentz R.J., Subacius H.P., Maggioni A.P., Swedberg K., Nodari S., Zannad F., Konstam M.A., Butler J., Gheorghiade M. Predictive value of low relative lymphocyte count in patients hospitalized for heart failure with reduced ejection fraction: insights from the EVEREST trial. Circ. Heart Fail. 2012;5(6):750–758. doi: 10.1161/CIRCHEARTFAILURE.112.970525. [DOI] [PubMed] [Google Scholar]
- 13.Acanfora D., Gheorghiade M., Trojano L., Furgi G., Pasini E., Picone C., Papa A., Iannuzzi G.L., Bonow R.O., Rengo F. Relative lymphocyte count: a prognostic indicator of mortality in elderly patients with congestive heart failure. Am. Heart J. 2001;142(1):167–173. doi: 10.1067/mhj.2001.115792. [DOI] [PubMed] [Google Scholar]
- 14.Marçula M., de Souza Buto M.F., Madaloso B.A., Nunes R.A.B., Cuoco M.A.R., de Paula R.S., Yamada A.T., Sandoval M.C., Botter D.A., Mansur A.J. Lymphocyte count and prognosis in patients with heart failure. Int. J. Cardiol. 2015;188:60–62. doi: 10.1016/j.ijcard.2015.04.043. [DOI] [PubMed] [Google Scholar]
- 15.P. Ponikowski, A.A. Voors, S.D. Anker, H. Bueno, J.G.F. Cleland, A.J.S. Coats, V. Falk, J.R. González-Juanatey, V.P. Harjola, E.A. Jankowska, M. Jessup, C. Linde, P. Nihoyannopoulos, J.T. Parissis, B. Pieske, J.P. Riley, G.M.C. Rosano, L.M. Ruilope, F. Ruschitzka, F.H. Rutten, P. van der Meer, G. Filippatos, J.J.V. McMurray, V. Aboyans, S. Achenbach, S. Agewall, N. Al-Attar, J.J. Atherton, J. Bauersachs, A.J. Camm, S. Carerj, C. Ceconi, A. Coca, P. Elliott, Ç. Erol, J. Ezekowitz, C. Fernández-Golfín, D. Fitzsimons, M. Guazzi, M. Guenoun, G. Hasenfuss, G. Hindricks, A.W. Hoes, B. Iung, T. Jaarsma, P. Kirchhof, J. Knuuti, P. Kolh, S. Konstantinides, M. Lainscak, P. Lancellotti, G.Y.H. Lip, F. Maisano, C. Mueller, M.C. Petrie, M.F. Piepoli, S.G. Priori, A. Torbicki, H. Tsutsui, D.J. van Veldhuisen, S. Windecker, C. Yancy, J.L. Zamorano, 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC, Eur. J. Heart Fail. 18 (2016) 891–975. https://doi.org/10.1002/ejhf.592. [DOI] [PubMed]
- 16.V. Carubelli, I. Bonadei, A.I. Castrini, E. Gorga, A. Ravera, C. Lombardi, M. Metra, Prognostic value of the absolute lymphocyte count in patients admitted for acute heart failure, J. Cardiovasc. Med. 18 (2017) 859–865. https://doi.org/10.2459/JCM.0000000000000428. [DOI] [PubMed]
- 17.Huang W.-M., Cheng H.-M., Huang C.-J., Guo C.-Y., Lu D.-Y., Lee C.-W., Hsu P.-F., Yu W.-C., Chen C.-H., Sung S.-H. Hemographic indices are associated with mortality in acute heart failure /692/4019/592/75/74 /692/4019/592/75/230 article. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-17754-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charach G., Grosskopf I., Roth A., Afek A., Wexler D., Sheps D., Weintraub M., Rabinovich A., Keren G., George J. Usefulness of total lymphocyte count as predictor of outcome in patients with chronic heart failure. Am. J. Cardiol. 2011;107(9):1353–1356. doi: 10.1016/j.amjcard.2010.12.049. [DOI] [PubMed] [Google Scholar]
- 19.Uthamalingam S., Patvardhan E.A., Subramanian S., Ahmed W., Martin W., Daley M., Capodilupo R. Utility of the neutrophil to lymphocyte ratio in predicting long-term outcomes in acute decompensated heart failure. Am. J. Cardiol. 2011;107(3):433–438. doi: 10.1016/j.amjcard.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 20.Pereg D., Chan J., Russell E., Berlin T., Mosseri M., Seabrook J.A., Koren G., Van Uum S. Cortisol and testosterone in hair as biological markers of systolic heart failure. Psychoneuroendocrinology. 2013;38(12):2875–2882. doi: 10.1016/j.psyneuen.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Van Linthout S., Tschöpe C. Inflammation - Cause or Consequence of Heart Failure or Both? Curr. Heart Fail. Rep. 2017;14(4):251–265. doi: 10.1007/s11897-017-0337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maisel A.S., Knowlton K.U., Fowler P., Rearden A., Ziegler M.G., Motulsky H.J., Insel P.A., Michel M.C. Adrenergic control of circulating lymphocyte subpopulations. Effects of congestive heart failure, dynamic exercise, and terbutaline treatment. J. Clin. Invest. 1990;85(2):462–467. doi: 10.1172/JCI114460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sivukhina E.V., Poskrebysheva A.S., Smurova I.V., Dolzhikov A.A., Morozov I.E., Jirikowski G.F., Grinevich V. Altered hypothalamic-pituitary-adrenal axis activity in patients with chronic heart failure. Horm. Metab. Res. = Horm. Und Stoffwechselforsch. = Horm. Metab. 2009;41(10):778–784. doi: 10.1055/s-0029-1224182. [DOI] [PubMed] [Google Scholar]
- 24.Yamaji M., Tsutamoto T., Kawahara C., Nishiyama K., Yamamoto T., Fujii M., Horie M. Serum cortisol as a useful predictor of cardiac events in patients with chronic heart failure: The impact of oxidative stress. Circ. Hear. Fail. 2009;2(6):608–615. doi: 10.1161/CIRCHEARTFAILURE.109.868513. [DOI] [PubMed] [Google Scholar]
- 25.Levine B., Kalman J., Mayer L., Fillit H.M., Packer M. Elevated Circulating Levels of Tumor Necrosis Factor in Severe Chronic Heart Failure. N. Engl. J. Med. 1990;323(4):236–241. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- 26.Wrigley B.J., Lip G.Y.H., Shantsila E. The role of monocytes and inflammation in the pathophysiology of heart failure. Eur. J. Heart Fail. 2011;13(11):1161–1171. doi: 10.1093/eurjhf/hfr122. [DOI] [PubMed] [Google Scholar]
- 27.Gullestad L., Ueland T., Brunsvig A., Kjekshus J., Simonsen S., Frøland S.S., Aukrust P. Effect of metoprolol on cytokine levels in chronic heart failure - A substudy in the metoprolol controlled-release randomised intervention trial in heart failure (MERIT-HF) Am. Heart J. 2001;141(3):418–421. doi: 10.1067/mhj.2001.112785. [DOI] [PubMed] [Google Scholar]
- 28.Topf A., Mirna M., Ohnewein B., Jirak P., Kopp K., Fejzic D., Haslinger M., Motloch L.J., Hoppe U.C., Berezin A., Lichtenauer M. The Diagnostic and Therapeutic Value of Multimarker Analysis in Heart Failure An Approach to Biomarker-Targeted Therapy. Front. Cardiovasc. Med. 2020;7 doi: 10.3389/fcvm.2020.579567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agnoletti L., Curello S., Malacarne F., Airò P., Cargnoni A., Valgimigli M., Ferrari R. Immune activation in severe heart failure: Does etiology play a role? Eur. Hear. J. Suppl. 2004;6:F22–F29. doi: 10.1016/j.ehjsup.2004.09.014. [DOI] [Google Scholar]
- 30.Gupta S., Gollapudi S. TNF-alpha-induced apoptosis in human naïve and memory CD8+ T cells in aged humans. Exp. Gerontol. 2006;41:69–77. doi: 10.1016/j.exger.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 31.E.S. Chung, M. Packer, K.H. Lo, A.A. Fasanmade, J.T. Willerson, Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (A, Circulation. 107 (2003) 3133–3140. https://doi.org/10.1161/01.CIR.0000077913.60364.D2. [DOI] [PubMed]
- 32.Van Tassell B.W., Trankle C.R., Canada J.M., Carbone S., Buckley L., Kadariya D., Del Buono M.G., Billingsley H., Wohlford G., Viscusi M., Oddi-Erdle C., Abouzaki N.A., Dixon D., Biondi-Zoccai G., Arena R., Abbate A. IL-1 Blockade in Patients With Heart Failure With Preserved Ejection Fraction. Circ. Heart Fail. 2018;11 doi: 10.1161/CIRCHEARTFAILURE.118.005036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ridker P.M., Everett B.M., Thuren T., MacFadyen J.G., Chang W.H., Ballantyne C., Fonseca F., Nicolau J., Koenig W., Anker S.D., Kastelein J.J.P., Cornel J.H., Pais P., Pella D., Genest J., Cifkova R., Lorenzatti A., Forster T., Kobalava Z., Vida-Simiti L., Flather M., Shimokawa H., Ogawa H., Dellborg M., Rossi P.R.F., Troquay R.P.T., Libby P., Glynn R.J. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017;377(12):1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.