Summary
Background
Although dysfunction of large-scale brain networks has been frequently demonstrated in patients with α-Synucleinopathy (α-Syn, i.e., Parkinson's disease, dementia with Lewy bodies, and multiple system atrophy), a consistent pattern of dysfunction remains unclear. We aim to investigate network dysfunction in patients with α-Syn through a meta-analysis.
Methods
Whole-brain seed-based resting-state functional connectivity studies (published before September 1st, 2020 in English) comparing α-Syn patients with healthy controls (HC) were retrieved from electronic databases (PubMed, Web of Science, and EMBASE). Seeds from each study were categorized into networks by their location within a priori functional networks. Seed-based effect size mapping with Permutation of Subject Images analysis of between-group effects identified the network systems in which α-Syn was associated with hyperconnectivity (increased connectivity in α-Syn vs. HC) or hypoconnectivity (decreased connectivity in α-Syn vs. HC) within and between each seed-network. This study was registered on PROSPERO (CRD42020210133).
Findings
In total, 136 seed-based voxel-wise resting-state functional connectivity datasets from 72 publications (3093 α-Syn patients and 3331 HC) were included in the meta-analysis. We found that α-Syn patients demonstrated imbalanced connectivity among subcortical network, cerebellum, and frontal parietal networks that involved in motor functioning and executive control. The patient group was associated with hypoconnectivity in default mode network and ventral attention network that involved in cognition and attention. Additionally, the patient group exhibited hyperconnectivity between neural systems involved in top-down emotion regulation and hypoconnectivity between networks involved in bottom-up emotion processing.
Interpretation
These findings supported neurocognitive models in which network dysfunction is tightly linked to motor, cognitive and psychiatric symptoms observed in α-Syn patients.
Keywords: α-Synucleinopathy, Resting-state functional connectivity, Meta-analysis, Brain networks, Parkinson's disease, Dementia with lewy body
Research in context.
Background of previous research
α-Synucleinopathy (α-Syn) refer to a series of neurodegenerative diseases, including Parkinson's disease, dementia with Lewy bodies, and multiple system atrophy. Previous studies referring dysfunctions in large-scale functional networks in α-Syn patients have been implicated in motor, cognitive and emotional dysregulation, which may contribute to the clinical symptoms of α-Syn.
Added value of this study
Through a meta-analytic overview of 72 resting state functional connectivity studies, our study clarified the dysfunction of brain networks in α-Syn patients, which implicated widespread abnormal crosstalk across brain networks involved in motor, cognition, attention, and emotion.
Implications of the available evidence
These findings provide an empirical foundation for neurodegenerative models in which network dysfunctions underlie motor, cognitive and psychiatric symptoms in α-Synucleinopathy patients, which also suggests development of subsequent precision intervention (such as neuromodulation) targeting specific brain networks to ameliorate corresponding symptoms.
Alt-text: Unlabelled box
Introduction
α-Synucleinopathy (α-Syn) refer to a series of neurodegenerative diseases, including Parkinson's disease (PD), dementia with Lewy bodies (DLB), and multiple system atrophy (MSA).1 The neuropathology of α-Syn is complicated as α-synuclein aggregation widely distributes across the brain from the brainstem to subcortical and cortical regions, which is aptly reflected by their protean clinical manifestations including motor, mood, cognitive, behavioral, and autonomic disturbances. However, it remains unclear on how the neuropathology would correlate to the various symptoms complex.2
During recent years, studies detecting functional brain networks using resting-state functional magnetic resonance imaging (rs-fMRI) by analyzing the temporal correlations in blood oxygen level dependent (BOLD) signal between pairs of brain regions are well established in neuroscience and neurodegenerative diseases.3 The most prominent example is the default mode network (DMN)-involved in self-reference, spontaneous cognition and aspects of consciousness,4 the frontoparietal network (FPN) – associated with numerous aspects of cognitive control,5 the somatomotor network (SMN) – relevant for motor execution and somatosensory components,3,5 and some other networks such as dorsal and ventral attention networks (DAN and VAN) – dynamic control of attention,6 and visual network (VIS).6 Dysfunctions within and between these networks give rise to the symptoms that characterize neuropsychiatric disorders.
In evaluating disturbances of functional networks in the three α-Syn diseases, seed-based resting-state functional connectivity (rsFC) is the most commonly used method. However, substantial spatial variance of seeds selection and varying seeds definition in different studies limit the establishment of coherent framework of network functioning in these diseases. A strategy raised by Kaiser and colleagues is categorizing seeds and related findings into a prior functional network parcellation6 based on their spatial locations, which is helpful to organize the various findings across studies to allow for direct replication and comparison.7 Apart from inconsistency of network selection, there is also a large variability across studies including sample size, medication, and clinical heterogeneity.8 Meta-analysis is an effective way to overcome the diversity and inconsistency of previously published work. To our knowledge, there was only one existing meta-analysis of seed-based rsFC studies for PD, which only focused on the corticobasal ganglia – thalamocortical network and motor dysfunction9 but no similar work for the less common α-Syn disorders, DLB and MSA.
Because of the large overlapping in pathology and clinical symptoms such as motor, cognitive, behavioural, and autonomic domains across the three α-Syn disorders, clinical differentiation among them remains a problematic diagnostic dilemma.10 It is increasingly recognized that existing clinical diagnostic categories may hinder the search for biomarkers in neuropsychiatric diseases because they are not clearly associated with distinct neurobiological abnormalities.11 Therefore, we proposed that from the functional networks point of view to build trans-diagnostic network dysfunction patterns across α-Syn to understand clinical perspectives, instead of the rigid diagnostic categories of α-Syn, is one way to address this issue. This also emphasizes the need to examine the full spectrum of functional brain networks to understand their functional consequences, rather than isolated regions or networks. Nonetheless, to our knowledge, there was no available study to discuss the converging network involvement in three α-Syn in a quantitative manner. Thus, we conducted this meta-analysis of seed-based rsFC studies to investigate the functional network disturbances of α-Syn. In addition, we also performed subgroup analyses and meta-regression analyses to detect the clinical relevance for network functioning in α-Syn.
Methods
Search strategy
This study was registered on PROSPERO (CRD42020210133) and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.12 Online search was conducted in PubMed, Web of Science, and EMBASE databases for literature published before September 1st, 2020. The keywords used in the search were (“Parkinson disease” OR “PD” OR “parkinsonism” OR “Dementia with Lewy Bodies” OR “Lewy body dementia” OR “DLB” OR “LBD” OR “Multiple system atrophy” OR “MSA”) AND (“functional magnetic resonance imaging” OR “fMRI” OR “resting state” OR “functional connectivity”). We then manually searched the references of the included literature and pertinent review articles.
Study selection criteria
The inclusion criteria were as follows:
-
(1)
Original peer-viewed fMRI study written in English;
-
(2)
Patients included in the study were diagnosed with PD (based on well-established criteria for PD13,14), DLB (on the basis of the McKeith criteria15,16) or MSA (on the basis of Second consensus criteria17);
-
(3)
Directly compared PD/DLB/MSA patients with healthy controls (HC);
-
(4)
Performed whole-brain seed-based rsFC analysis;
-
(5)
Reported peak coordinates in standard stereotaxic spaces (e.g., Montreal Neurological Institute (MNI) or Talairach).
Studies were excluded if (1) using other whole-brain rsFC methods, such as independent components analysis, which could cause methodology bias18; (2) the coordinates of between-group effects could not be retrieved even by contacting the author; (3) the sample and seeds completely overlapping reported in another publication. Studies on the same samples while using different seeds were considered as separate datasets. Meanwhile, different group of patients vs. a single HC group in a study were coded as distinct datasets.
Data extraction
To identify alterations in rsFC in patients with α-Syn, we extracted the locations of the seeds (e.g., the coordinates, a prior template, or a standard atlas) as well as the information of effect regions. For seeds in cortex, we categorized each into one of seven seed networks by its location within the prior networks parcellation,19 which included the DMN, limbic network (LN), FPN, DAN, VAN, SMN and VIS. Seeds that located in subcortical regions were classified as subcortical network, while seeds that located in cerebellum were classified as cerebellar network (CN). Then, the peak coordinates and t statistic of clusters with significant between-group differences were extracted. If only the Z score of the cluster was reported, it was converted to t statistic. The effects of rsFC were also categorized into two groups according to their direction: hyperconnectivity (α-Syn patients > HC) and hypoconnectivity (α-Syn patients < HC). Meanwhile, studies with null findings (i.e. those that reported no between-group differences in rsFC or whose effects did not survive statistical correction) were also included to reduce the publication bias.
Data extractions were conducted by two investigators independently (S.T. and YL.W.) and all information were double-checked by two investigators (S.T. and YL.W.). Any inconsistent results were discussed and resolved by consensus.
Statistical analysis
Meta-analysis
The meta-analysis was conducted by using the Seed-based d Mapping with Permutation of Subject Images (SDM-PSI) software, version 6.21 (https://www.sdmproject.com/), which is based on a novel algorithm for coordinate-based meta-analysis that conducts a standard subject-based permutation test to control the family wise error (FWE) rate.20 Firstly, effect size map was built for each individual experiment, by (a) converting the t value of each peak coordinate into an estimate of effect size (Hedge's g)21 and (b) convolving these peaks with a fully anisotropic unnormalized Gaussian kernel (α = 1, Full width at half maximum (FWHM) = 20 mm) within the boundaries of the default gray matter template as provided by SDM-PSI (voxel size = 2 × 2 × 2 mm3). The study conducted standard random-effects meta-analysis by imputing the brain maps of statistical effects for each study, which assumes the observed estimates of effect can vary across studies. We have not selected one variance covariance matrix during analysis as this is not specified in the software. Next, imputation was conducted separately for groups of experiments with different statistical thresholds as follows: (a) for each experiment, impute several study images that show realistic local spatial covariance, each set of images was named “imputed dataset”, one per experiment; (b) for each experiment, impute subject images that show realistic local spatial covariance. Then adapt them to the different imputed study images, so that the group analysis of the subject images of an imputed dataset returns the study images of that imputed dataset.22 Then, for each network, a subject-based permutation test was performed as follows: (a) creating a random permutation of the subjects and apply it to the subject images of the different imputed datasets; (b) conducting a group analysis of the permuted subject images for each imputed dataset separately to obtain a meta-analysis image; (c) obtaining a combined meta-analysis image from different imputed datasets by using Rubin's rules23; (d) saving the largest z-value from the combined meta-analysis image and (e) iterating steps (a) to (d) 1000 times and using the distribution of the largest z-values to threshold the combined meta-analysis image obtained from unpermuted data.20 Finally, the meta-analytic maps were thresholded using the FWE correction for multiple comparisons with a threshold of p < 0.05, and a cluster-wise extent threshold of k > 50 voxels to improve the reliability of the results.
Subgroup meta-analysis and meta-regression analysis
To estimate potential medication effects on the main meta-analysis findings, we performed subgroup-analyses of medication-naïve/OFF and medication-ON patients. Furthermore, to detect potential diverging patterns in each subtype of α-Syn, we also conducted subgroup-analyses of PD, DLB and MSA for sufficient datasets in seed networks of each disorder.
In addition, to investigate the potential effects of relevant clinical variables to the main findings, we further performed meta-regression analyses in α-Syn patients with the Unified Parkinson's Disease Rating Scale Part III (UPDRS III), Hoehn & Yahr stage, duration of illness and the Mini–Mental State Examination as regressors. For studies that used Movement Disorders Society-UPDRS III, we converted it to UPDRS III based on published formulas.24 The threshold was the same as the main analysis.
Sensitivity analysis, heterogeneity analysis and publication bias
Jackknife sensitivity analysis was conducted to evaluate the robustness and reliability of the results. This analysis excludes one study each time and repeats the main statistical analysis. If a previous significant main result remains significant in all or most of the combinations of studies, it can be regarded as replicable and stable.25 For each cluster in the main results, τ2 is used to account for the between-study heterogeneity. Other statistics for assessing heterogeneity include H2, and I2 statistic. A value of τ2 ≈ 0, H2 ≈ 1, I2 < 50% indicates good homogeneity among the studies. The study heterogeneity was assessed through conversion of the Q statistic into Z scores, clusters that showed significant heterogeneity and overlapped with the main results were considered heterogeneous between studies.26,27 Funnel plots and Egger's test in SDM-PSI were used to test the possibility of any publication bias. Funnel plots are generated to visualize any possible publication bias, while the Egger's test is a quantitative method of assessing asymmetry in the funnel plots and can therefore be used as an indicator for the presence of publication bias. Results showing p < 0.05 on Egger's test were considered to have significant publication bias.28
Quality assessment
To increase the quality of the current meta-analysis, we followed the 20-point checklist used in previous meta-analyses of rs-fMRI studies9,29 (Table S6 in Supplementary materials) to qualify each study. This checklist included quality of diagnostic procedures, assessments and reporting of critical clinical and demographic data, rs-fMRI acquisition parameters and quality of reported results and the use of statistical correction.
Role of funding source
The funders had no role in study design, data collection, analysis, interpretation, or writing of the report.
Results
Included studies and sample characteristics
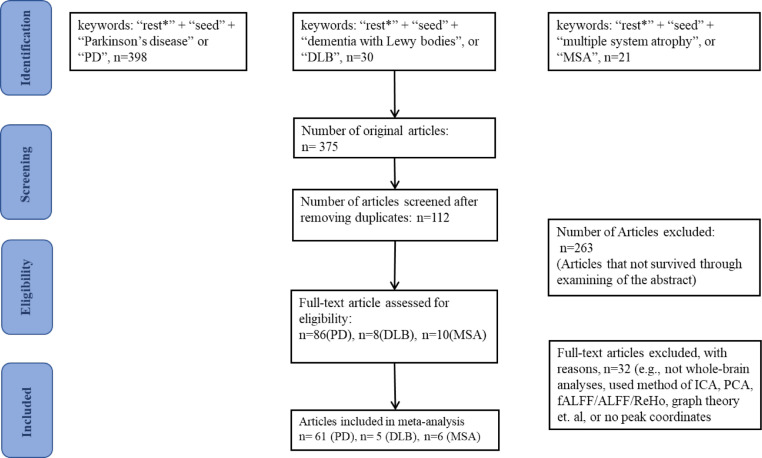
This study included 72 publications,30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101 with a total of 3093 non-overlapping patients (2636 PD patients, 142 DLB patients and 315 MSA patients) and 3331 HC. The flow chart of the search strategy and study selection is shown in Figure 1.
Figure 1.
Flowchart of the research strategy and literature selection. Abbreviations: ICA, independent component analysis; PCA, principal component analysis; (f)ALFF, (fractional) amplitude of low frequency fluctuation; ReHo, regional homogeneity.
Summarized sample characteristics are shown in Table S1 in supplementary materials. Detailed demographic and clinical characteristics are shown in Tables S2–4 in supplementary materials. After the seeds from each study were categorized into networks, 16 datasets with FPN seeds, 29 datasets with DMN seeds, 16 datasets with CN seeds, 60 datasets with subcortical network seeds, 6 datasets with VAN seeds, and 9 datasets with LN seeds were identified, and these networks were delivered to subsequent meta-analyses. Results of relatively small datasets (5 < n < 10) would need to be interpreted with caution, while the VIS and DAN seeds were not subjected to a quantitative meta-analysis because of their small datasets (both n = 3), which may not provide adequate statistical power.
Altered connectivity of motor control related networks
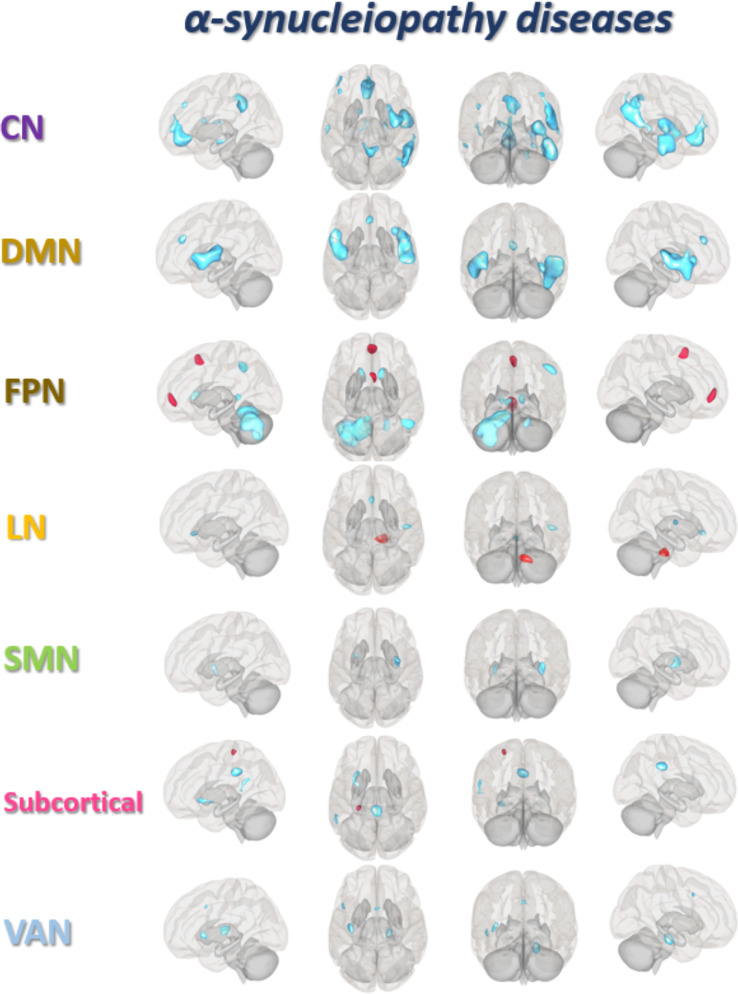
Connectivity alterations in patients with α-Syn were associated with motor and motor control related regions including the subcortical network, SMN, CN, FPN, and VAN. Comparing to HC, α-Syn patients demonstrated both increased connectivity between subcortical network seeds and SMN (the left post central gyrus, postCG) and decreased connectivity between SMN seeds and subcortical network (bilateral putamen). Decreased connectivity was also observed in α-Syn patients, between CN seeds and subcortical network (the right striatum), FPN (the right dorsal lateral prefrontal cortex), and from both CN and subcortical network seeds to VAN (the right insula/operculum). Further, α-Syn patients also demonstrated hyperconnectivity between FPN seeds and SMN (the right supplementary motor area) and hypoconnectivity between FPN seeds and CN (bilateral cerebellum) and subcortical network (striatum and thalamus) (see Table 1 and Figure 2 for details).
Table 1.
Results of meta-analysis of altered resting-state functional connectivity in α-Synucleinopathy compared with healthy control.
| Seeds network | Connected region (peak location) | BA | Voxels | SDM-Z | MNI coordinate | Located network |
|---|---|---|---|---|---|---|
| FPN | Hyperconnectivity | |||||
| R-MPFC | 10 | 303 | 4.386 | 4,50,0 | DMN | |
| R-SMA | 6 | 191 | 4.019 | 2,16,54 | SMN | |
| Hypoconnectivity | ||||||
| L-cerebellum IV / V | 19 | 3053 | -6.642 | -16,-56,-16 | CN | |
| R-IPG | 40 | 289 | -4.746 | 50,-48,44 | FPN | |
| R-cerebellum IV / V | 37 | 211 | -4.354 | 20,-50,-28 | CN | |
| R-anterior thalamic projections | NA | 213 | -4.165 | 12,12,6 | subcortical network | |
| L-striatum | NA | 132 | -3.997 | -14,20,-2 | subcortical network | |
| DMN | Hypoconnectivity | |||||
| R-STG | NA | 1131 | -5.307 | 48,8,-12 | DMN | |
| L-rolandic operculum | 48 | 966 | -4.705 | -48,6,2 | VAN | |
| L-MPFC | 32 | 94 | -3.975 | 2,34,28 | DMN | |
| R-insula | 48 | 81 | -3.831 | 36,18,2 | VAN | |
| CN | Hypoconnectivity | |||||
| R-striatum | 48 | 568 | -6.241 | 32,-6,8 | subcortical network | |
| R-insula/operculum | 48 | 459 | -6.22 | 36,-2,12 | VAN | |
| R-STG/MTG/ITG | 20/21/22 | 500 | -6.978 | 48,-10,-12 | DMN | |
| R-STG/angular/IPG | 42/39/40 | 1248 | -6.9 | 52,-42,18 | DMN/FPN | |
| R-Precuneus/PCC | 23 | 676 | -6.044 | 4,-46,38 | DMN | |
| ACC/OFC | 10/11 | 676 | -5.675 | -4,42,-4 | DMN | |
| R-DLPFC | 9 | 118 | -4.676 | 22,-10,-26 | FPN | |
|
Subcortical Network |
Hyperconnectivity | |||||
| L-postCG | 4 | 85 | 4.999 | -26,-30,64 | SMN | |
| Hypoconnectivity | ||||||
| R-Precuneus | 23 | 364 | -4.688 | 2,-34,32 | DMN | |
| L-insula | 47 | 166 | -3.873 | -26,20,-6 | VAN | |
| L-STG | 42 | 122 | -3.967 | -58,-46,20 | DMN | |
| SMN | Hypoconnectivity | |||||
| R-lenticular nucleus, putamen | 48 | 240 | -3.961 | 30,-4,0 | subcortical network | |
| L-lenticular nucleus, putamen | 106 | -3.738 | -28,0,0 | subcortical network | ||
| VAN | Hypoconnectivity | |||||
| R-PHG | 30 | 229 | -4.261 | 18,-20,-20 | LN | |
| L-insula | 48 | 157 | -3.79 | -36,-16,10 | VAN | |
| L-IFG, triangular part | 45 | 84 | -3.319 | -48,16,0 | VAN | |
| R-ACC | 24 | 54 | -3.145 | 6,12,38 | VAN | |
| LN | Hyperconnectivity | |||||
| Middle cerebellar peduncles | 212 | 4.314 | 12,-28,-30 | CN | ||
| Hypoconnectivity | ||||||
| L-sgACC | 25 | 54 | -3.292 | -2,24,-4 | VAN | |
| R-rolandic operculum | 48 | 50 | -3.214 | 46,-12,10 | VAN |
Abbreviations: BA, Brodmann area; SDM-Z, seed-based d mapping-z value; MNI, Montreal Neurological Institute; FPN, frontoparietal network; R-, the right; L-, the left; DMN, default mode network; CN, cerebellar network; SMN, somatomotor network; VAN, ventral attention network; LN, limbic network, MPFC, medial prefrontal cortex, SMA, supplementary motor area, IPG, inferior parietal gyrus; STG, superior temporal gyrus; MTG, middle temporal gyrus; ITG, inferior temporal gyrus; DLPFC, dorsolateral prefrontal cortex; sgACC, subgenual anterior cingulate cortex; OFC, orbital prefrontal cortex; postCG, posterior central gyrus; PCC, posterior cingulate cortex; PHG, parahippocampal gyrus.
Figure 2.
Meta-analysis of abnormal resting-state functional connectivity in α-Synucleinopathy. The brain regions of altered resting-state functional connectivity for each network in α-synucleinopathy (α-Syn) patients are compared with healthy controls (HC). Red refers to hyperconnectivity (α-Syn >HC), and blue refers to hypoconnectivity (α-Syn < HC). Abbreviations: FPN, frontoparietal network, DMN, default mode network, CN, cerebellar network, SMN, somatomotor network, VAN, ventral attention network, LN, limbic network.
Reduced connectivity of cognition related networks
As reported above, α-Syn patients showed hypoconnectivity between the subcortical network /CN seeds and executive control systems (FPN and VAN); these seeds also presented with reduced connectivity to the posterior DMN (the superior temporal gyrus, the precuneus and the posterior cingulate gyrus). In addition, reduced connectivity within DMN, i.e., between DMN seeds and posterior DMN (the superior temporal gyrus) is also observed in α-Syn patients comparing to HC (see Table 1 and Figure 2 for details).
Reduced connectivity of attention related networks
Comparing to HC, hypoconnectivity was also present in networks involved in externally (VAN) or internally (DMN) oriented attention in α-Syn patients. Firstly, decreased connectivity was observed between DMN seeds and anterior DMN (medial prefrontal cortex) as well as VAN regions (insula and operculum). Secondly, decreased connectivity was also observed within VAN, i.e., between VAN seeds and insula, the triangular part of left inferior frontal, and the anterior cingulate cortex (ACC) (see Table 1 and Figure 2 for details).
Altered connectivity of emotion related networks
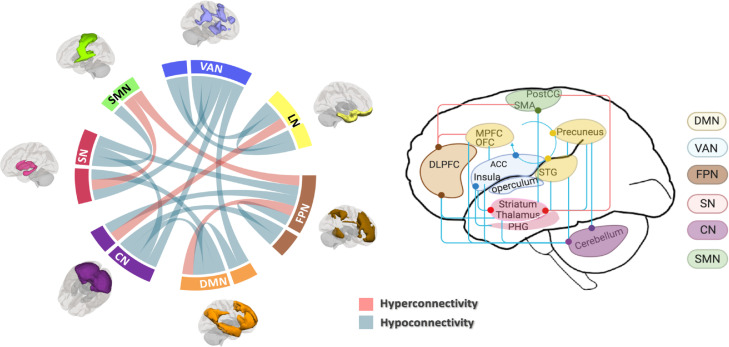
The present meta-analysis revealed increased connectivity between FPN seeds and anterior DMN region (medial prefrontal cortex) involved in “top-down” emotional regulation, and decreased connectivity between VAN seeds and LN (parahippocampal gyrus) as well as between LN seeds and VAN (subgenual ACC and operculum) involved in “bottom-up” emotional processing (see Table 1 and Figure 2 for details). A schematic overview of these disrupted functional architecture is shown in Figure 3.
Figure 3.
Schematic overview of within- and between-network connectivity changes in α-Synucleinopathy. For both graphs, pink line indicates hyperconnectivity and blue line indicates hypoconnectivity; different networks were marked with different colors. Abbreviations: FPN, frontoparietal network; DMN, default mode network; CN, cerebellar network; SN, subcortical network; SMN, somatomotor network; VAN, ventral attention network; LN, limbic network; MPFC, medial prefrontal cortex; SMA, supplementary motor area; IPG, inferior parietal gyrus; STG, superior temporal gyrus; DLPFC, dorsolateral prefrontal cortex; ACC, anterior cingulate cortex; OFC, orbital prefrontal cortex; postCG, posterior central gyrus; PHG, parahippocampal gyrus.
Subgroup analyses and meta-regression analyses
For medication-naive/OFF experiments, only FPN, DMN, CN and subcortical network seeds have sufficient datasets for quantitative meta-analysis. As for medication-ON experiments, only FPN, DMN, and subcortical network seeds have sufficient datasets for quantitative meta-analysis. The results of medication-naïve/OFF experiments remained largely the same as main results, except hypoconnectivity between subcortical network and the right cerebellum in medication-ON experiments that was not present in the main results (Figs. S3,4 in supplementary materials).
Overall, it indicated there was no major medication influence on the main results.
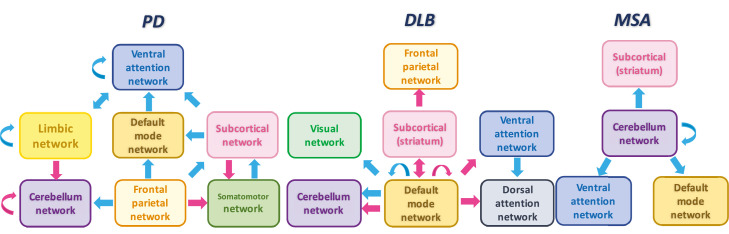
Among all the listed articles of α-Syn, PD accounted for the largest proportion and explains most of the abnormalities. Quantitative analysis for each network was available in PD, while in MSA, CN seeds were the most frequently used and could be included into quantitative analyses. However, the literature of DLB was limited and could not be included into quantitative analyses. Thus, we summarized the findings of network abnormalities in DLB (Figure 4). Generally speaking, the effect regions of DMN and FPN in PD were similar to that of converging results, but with smaller effect size and cluster size. For CN abnormalities, PD exhibited hyperconnectivity within CN and hypoconnectivity between CN and FPN, while MSA exhibited hypoconnectivity between CN and VAN. There was common hypoconnectivity in PD and MSA between CN and precuneus (located in DMN). Hyperconnectivity between subcortical network and postCG had a larger effect size and cluster size in PD than converging results (Figs. 4 and S6 in the supplementary materials).
Figure 4.
Illustration of the network dysfunction models of Parkinson's disease (PD), dementia with Lewy body (DLB) and multiple system atrophy (MSA). Hyper/hypoconnectivity is presented with pink/blue arrows. PD and MSA models are outlined from quantitative analysis, while DLB model is summarized from included studies.
Meta-regression analyses showed that the UPDRS-III scores of patients were negatively correlated with connectivity between FPN seeds and CN (the left cerebellum). In other words, as motor function became worse, their rsFC would decrease. There was no other effect of clinical variables on rsFC changes.
Sensitivity analysis, heterogeneity analysis and publication bias
The jackknife sensitivity analyses revealed that all clusters of main results were highly replicable. Details of the results of the sensitivity analysis were listed in Table S7 (in the supplementary materials). Moreover, τ2, H2, I2 statistics indicate there were small heterogeneities observed in the main results (see Table S7 in the supplementary materials). In addition, none of the clusters showed significant publication bias based on Egger's test (p > 0.05). Funnel plots were presented in Figs. S8–38 in the supplementary materials.
Quality assessment
The quality of each study was considered as good, with mean overall score of 18.84. See Figure S2 (in the supplementary materials) for further specification of the criteria of each study.
Discussion
Network models have been increasingly recognized as useful tools to study core intrinsic activity features and clinical biomarkers of neurodegenerative disorders.102 This study provided a meta-analytic overview of abnormal rsFC in patients with α-Syn, which implicated widespread abnormal crosstalk across brain networks involved in motor, cognition, attention, and emotion. These findings supported the neurodegenerative models103 in which network dysfunction is tightly linked to motor and non-motor signs observed in α-Syn patients.
Imbalanced connectivity in networks involved in motor and “top-down” motor control
The present meta-analysis revealed alteration of connectivity in α-Syn patients: (1) both increased and decreased connectivity between subcortical network and SMN; (2) decreased connectivity between CN and subcortical network; (3) decreased connectivity between CN/subcortical network and FPN/VAN. These findings correspond to the current understanding of parkinsonian symptoms as related to an impairment of connectivity in motor, top-down control networks, and their interactions.104,105
The subcortical network and the SMN formed the so called corticobasal ganglia–thalamocortical loop, which is a fundamental circuit for motor ability and is critically related to the parkinsonism.106 We found an increased connectivity of the corticobasal ganglia–thalamocortical network between subcortical network and SMN (the postCG), which has also been well-documented in PD in previous meta-analysis of rsFC studies.9 However, we also found decreased connectivity in this network, i.e., between SMN seeds and bilateral putamen that has not been reported before, which suggested an imbalanced network functioning influenced by the pathophysiology of parkinsonism.107 The cerebellum has also been known to play an important role in the pathogenesis of α-Syn.108,109 The decreased cerebellar-subcortical network connectivity is likely to be a reflection of abnormal signals from the basal ganglia to influence cerebellar function, which has also influenced connectivity between the motor subsystem and large-scale cortical networks.110 The FPN and VAN are important in cognitive control, specifically impact motor execution.111 Previous rs-fMRI study suggested that reorganization of FPN, VAN, and CN can contribute to problems with self-initiation and task-set maintenance in PD.112 Hence, the current pattern of imbalanced or poorly coordinated functioning of networks involved in motor and “top-down” control in α-Syn patients may reflect the deficits in self-initiated, maintaining, and goal-directed movements as well as disorganized executive control.113 Our meta-regression results of the correlation of motor dysfunction severity to the hypoconnectivity between FPN and cerebellum also supports this hypothesis. Moreover, these abnormalities were robust in the subgroup of those medication-off patients, while they were not present in those medication-ON patients, which indicates that dopaminergic treatment may reverse some of these abnormalities. However, α-Syn could be treated with different medications depending on their symptoms. For example, motor symptoms were primarily treated with dopaminergic treatments, while non-motor symptoms such as dementia, depression and psychosis were treated with cholinesterase inhibitors, antidepressants and antipsychotics, respectively.114,115 So different active neuropharmacological interventions may have divergent effects on FC changes in α-Syn patients which should be considered more in future studies.
Reduced connectivity between networks involved in cognitive impairments
Our results also demonstrated hypoconnectivity between regions in subcortical network, CN, and posterior DMN. This reinforced the current knowledge that subcortical rs-FC changes were not only attributed to a loss of dopaminergic neurons that was related to parkinsonism but was also associated with cognitive deficits.116 For example, recent studies showed that specific striatal (caudate) rsFC changes are associated with cognitive decline in PD.53,116 Cerebellum, as well, has a significant role beyond sensorimotor control in cognition, based on the proposed concept of the universal cerebellar transform of cerebello-basal ganglia-thalamo-cortical circuits.117 The posterior DMN is believed to play an important role in various cognitive functions,118 whose deficits were related to more rapid cognitive decline according to longitudinal studies in PD.119 Our findings appear to highlight the role of decreased synchronicity and connectivity between subcortical network, CN, and posterior DMN in impaired cognition in α-Syn patients. An interesting question is whether the FC deficiency is a cause or consequence of cognitive deficits in α-Syn? Future longitudinal studies and image-genetics analysis may map the chronological order of network dysfunction and behavioral deficits to answer this question more comprehensively. In addition, these findings indicate that another exciting direction for future research is to examine the relationship between network changes and various clinical phenotypes of α-Syn.
Reduced connectivity between networks involved in attentional control
The present meta-analysis also provided evidence of hypoconnectivity between brain networks involved in attentional control. A recent hypothesis proposed that dysfunction across attentional networks, especially in VAN and DMN, may explain visual misperception and hallucinations (VH),120 which occur frequently in PD and DLB. Dysfunction within the VAN could result in difficulties in orienting attention to relevant stimuli,121 leading to a relative inability to “shut down” resting activity within the DMN, which is typically associated with the retrieval and manipulation of semantic and episodic memories.122 Indeed, both neuropathological and neuroimaging studies of PD and DLB patients have reported anomalies that were associated with a number of VAN and DMN regions in the presence of VH.123,124 More interestingly, our finding shows reduced connectivity between VAN and parahippocampal gyrus, the region with direct relationship to visual hallucination.125 Thus, the demonstration of hypoconnectivity across these networks is in line with theoretical models, suggesting either a failure to recall appropriate information or select inappropriate stored information, as well as inability to communicate in the presence of an ambiguous percept, which might be the neural underpinning of VH.120
Imbalanced connectivity between networks involved in emotion processing
The present meta-analysis revealed α-Syn patients showed increased connectivity between FPN and anterior DMN, and decreased connectivity between VAN and limbic regions. These networks are critically involved in emotional regulation and adaptive behaviors.126 Abnormal connections of these regions are demonstrated in meta-analyses of psychiatric disorders, such as depression7,127 and bipolar disorder.128 Decreased LN to VAN (subgenual ACC) connectivity might reflect an inability or interference of bottom-up emotion processing, which may constitute an increase in top-down emotional regulation reflected by hyperconnectivity between the FPN and anterior DMN.129 This pattern of activity might also explain affective symptomatology observed in α-Syn patients.
Limitations and future directions
The major challenge is the relative rarity of studies with DLB and MSA, so the findings might be overshadowed by PD. Further studies of DLB and MSA are needed in order to better understand the clinical and neuropathological associations by transcending all α-Syn clinical phenotypes. Furthermore, in neurodegenerative diseases with similar signs, such as PD dementia, DLB, and Alzheimer's disease, a network-based taxonomy can possibly cut across traditional diagnostic boundaries and, instead, include distinct subtypes of neural network dysfunction that might better reflect the clinical homogeneity or heterogeneity of neurodegeneration.
Secondly, important clinical information such as parkinsonism laterality, autonomic and visual assessments were not systematically provided in all the included studies which have limited our analyses. Future studies should systematically study clinical symptoms in α-Syn patients to help delineate network-guided dimensions of psychopathology across clinical diagnostic categories, which could serve as a foundation for developing network-based biomarkers in α-Syn.
Finally, the age range and illness duration of included participants was quite wide which would potentially influence the results,130 albeit we have controlled the age effect during analyses. Also, we have looked at the illness duration as a regressor in the meta-regression analysis, the result showed that there was no significant association with FC changes. A good way to understand the trajectory of rsFC changes during disease progression is to track α-Syn longitudinally.131 As rsFC changes might emerge before the clinical diagnose of α-Syn,132 i.e., the prodromal stage of α-Syn, it would be important to identify imaging markers at the prodromal stage. Currently, idiopathic REM sleep behavior disorder (iRBD) is considered as the most distinct prodromal phenotype of α-Syn which has been identified to highly predict the subsequent development of PD, DLB and MSA.133,134 As some recent studies have shown that FC changes in iRBD patients were similar to that found in α-Syn and were associated with some cognitive aspects,135,136 it is anticipated that prospective rsFC study in iRBD subjects and their high-risk relatives137 might help to monitor disease progression and phenoconversion to α-Syn. While rsFC is highly variable even within individuals, in this concern, future study using more sophisticated FC methods such as dynamic/hierarchical analyses are needed to capture the variation of FC changes in α-Syn.
Conclusion
To our knowledge, this study provides the first meta-analytic overview of large-scale network dysfunction in patients with α-Syn, including imbalanced connectivity among networks involved in motor function and executive control, decreased connectivity among networks involved in cognition and attention, and imbalanced connectivity among networks involved in emotion processing and regulation, which could potentially correlate to the motor, cognitive, and psychiatric symptoms. These findings also support the proposal to view the subcortical, cortex and cerebellum as an integrated system to understand the involvement of these areas in α-Syn.
Contributors
S.T. contributed to the conception and design of the study. S.T. and YL.W. performed study selection, data extraction and statistical analyses. S.T. and YL.W. have verified the underlying data. S.T. drafted the manuscript. Y.L., S.C. and YK.W. participated in discussion of analyses and results. J.C., W.C., J.A., and V.M. reviewed the manuscript and provided comments. Y.L. and YK.W. revised the manuscript. All authors have read and approved the final version of the submitted manuscript.
Data sharing statement
This study does not involve public datasets or codes. The data supporting the findings in this study might be requested via the corresponding author of this article upon reasonable request.
Declaration of interests
J.C. received personal fees from Eisai Co.,Ltd for joining an insomnia expert forum, which is outside the submitted work.
YK.W. received personal fees from Eisai Co., Ltd for lecture, travel support from Lundbeck HK Limited, which are outside the submitted work.
Other authors have nil conflict of interest to disclose.
Acknowledgments
This study was partially supported by the Research Grants Council of Hong Kong (Grant No. RGC14116121). We would like to thank the colleagues of Li Chiu Kong Family Sleep Assessment Unit of Shatin Hospital and the Department of Imaging & Interventional Radiology for their support and help to this work. We would also like to thank the participants and researchers in the included studies.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.103915.
Appendix. Supplementary materials
References
- 1.McCann H., Stevens C.H., Cartwright H., Halliday GM. α-Synucleinopathy phenotypes. Parkinsonism Relat Disord. 2014;20(Suppl 1):S62–S67. doi: 10.1016/S1353-8020(13)70017-8. [DOI] [PubMed] [Google Scholar]
- 2.Azizi S.A., Azizi S.A. Synucleinopathies in neurodegenerative diseases: accomplices, an inside job and selective vulnerability. Neurosci Lett. 2018;672:150–152. doi: 10.1016/j.neulet.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Biswal B., Yetkin F.Z., Haughton V.M., Hyde J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 4.Raichle M.E. The brain's default mode network. Annu Rev Neurosci. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- 5.Smith S.M., Fox P.T., Miller K.L., et al. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106(31):13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeo B.T., Krienen F.M., Sepulcre J., et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaiser R.H., Andrews-Hanna J.R., Wager T.D., Pizzagalli D.A. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72(6):603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tahmasian M., Bettray L.M., van Eimeren T., et al. A systematic review on the applications of resting-state fMRI in Parkinson's disease: does dopamine replacement therapy play a role? Cortex. 2015;73:80–105. doi: 10.1016/j.cortex.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Ji G.J., Hu P., Liu T.T., et al. Functional connectivity of the corticobasal ganglia-thalamocortical network in Parkinson disease: a systematic review and meta-analysis with cross-validation. Radiology. 2018;287(3):973–982. doi: 10.1148/radiol.2018172183. [DOI] [PubMed] [Google Scholar]
- 10.Koga S., Aoki N., Uitti R.J., et al. When DLB, PD, and PSP masquerade as MSA: an autopsy study of 134 patients. Neurology. 2015;85(5):404–412. doi: 10.1212/WNL.0000000000001807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Insel T.R., Cuthbert B.N. Medicine. Brain disorders? Precisely. Science. 2015;348(6234):499–500. doi: 10.1126/science.aab2358. [DOI] [PubMed] [Google Scholar]
- 12.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. Clin Res Ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes A.J., Daniel S.E., Kilford L., Lees A.J. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Postuma R.B., Berg D., Stern M., et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord. 2015;30(12):1591–1601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 15.McKeith I.G., Dickson D.W., Lowe J., et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 16.McKeith I.G., Boeve B.F., Dickson D.W., et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89(1):88–100. doi: 10.1212/WNL.0000000000004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilman S., Wenning G.K., Low P.A., et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71(9):670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Müller V.I., Cieslik E.C., Laird A.R., et al. Ten simple rules for neuroimaging meta-analysis. Neurosci Biobehav Rev. 2018;84:151–161. doi: 10.1016/j.neubiorev.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi E.Y., Yeo B.T., Buckner R.L. The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol. 2012;108(8):2242–2263. doi: 10.1152/jn.00270.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albajes-Eizagirre A., Solanes A., Vieta E., Radua J. Voxel-based meta-analysis via permutation of subject images (PSI): theory and implementation for SDM. Neuroimage. 2019;186:174–184. doi: 10.1016/j.neuroimage.2018.10.077. [DOI] [PubMed] [Google Scholar]
- 21.Hedges L.V. Distribution theory for glass's estimator of effect size and related estimators. J Educ Stat. 1981;6(2):107–128. [Google Scholar]
- 22.Radua J., Mataix-Cols D., Phillips M.L., et al. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur Psychiatry. 2012;27(8):605–611. doi: 10.1016/j.eurpsy.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Li K.H., Meng X.L., Raghunathan T.E., Rubin D.B. Significance levels from repeated p-values with multiply-imputed data. Stat Sin. 1991:65–92. [Google Scholar]
- 24.Goetz C.G., Stebbins G.T., Tilley B.C. Calibration of unified Parkinson's disease rating scale scores to Movement Disorder Society-unified Parkinson's disease rating scale scores. Mov Disord. 2012;27(10):1239–1242. doi: 10.1002/mds.25122. [DOI] [PubMed] [Google Scholar]
- 25.Radua J., Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry. 2009;195(5):393–402. doi: 10.1192/bjp.bp.108.055046. the journal of mental science. [DOI] [PubMed] [Google Scholar]
- 26.Wolfgang V. Bias and efficiency of meta-analytic variance estimators in the random-effects model. J Educ Behav Stat. 2005;30(3):261–293. [Google Scholar]
- 27.Albajes-Eizagirre A., Radua J.J.N. What do results from coordinate-based meta-analyses tell us? 2018;176:550–3. [DOI] [PubMed]
- 28.Egger M., Smith G.D., Phillips A.N. Meta-analysis: principles and procedures. BMJ. 1997;315(7121):1533–1537. doi: 10.1136/bmj.315.7121.1533. (Clinical research ed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwabuchi S.J., Krishnadas R., Li C., Auer D.P., Radua J., Palaniyappan L. Localized connectivity in depression: a meta-analysis of resting state functional imaging studies. Neurosci Biobehav Rev. 2015;51:77–86. doi: 10.1016/j.neubiorev.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Bezdicek O., Ballarini T., Albrecht F., et al. SERIAL-ORDER recall in working memory across the cognitive spectrum of Parkinson's disease and neuroimaging correlates. J Neuropsychol. 2020 doi: 10.1111/jnp.12208. [DOI] [PubMed] [Google Scholar]
- 31.Dong W., Qiu C., Jiang X., et al. Can the executive control network be used to diagnose Parkinson's disease and as an efficacy indicator of deep brain stimulation? Parkinson's Dis. 2020;2020 doi: 10.1155/2020/6348102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang P., Guan X., Guo T., et al. Damaged insula network contributes to depression in Parkinson's disease. Front Psychiatry. 2020;11:119. doi: 10.3389/fpsyt.2020.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee Y.H., Bak Y., Park C.H., et al. Patterns of olfactory functional networks in Parkinson's disease dementia and Alzheimer's dementia. Neurobiol Aging. 2020;89:63–70. doi: 10.1016/j.neurobiolaging.2019.12.021. [DOI] [PubMed] [Google Scholar]
- 34.Li M.G., He J.F., Liu X.Y., Wang Z.F., Lou X., Ma L. Structural and functional thalamic changes in Parkinson's disease with mild cognitive impairment. J Magn Reson Imaging JMRI. 2020;52(4):1207–1215. doi: 10.1002/jmri.27195. [DOI] [PubMed] [Google Scholar]
- 35.Liao H., Fan J., Shen Q., et al. Alterations of interhemispheric functional connectivity in Parkinson's disease with depression: a resting-state functional MRI study. Front Hum Neurosci. 2020;14:193. doi: 10.3389/fnhum.2020.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maiti B., Koller J.M., Snyder A.Z., et al. Cognitive correlates of cerebellar resting-state functional connectivity in Parkinson disease. Neurology. 2020;94(4):e384–ee96. doi: 10.1212/WNL.0000000000008754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruppert M.C., Greuel A., Tahmasian M., et al. Network degeneration in Parkinson's disease: multimodal imaging of nigro-striato-cortical dysfunction. Brain J Neurol. 2020;143(3):944–959. doi: 10.1093/brain/awaa019. [DOI] [PubMed] [Google Scholar]
- 38.Siciliano M., De Micco R., Giordano A., et al. Supplementary motor area functional connectivity in "drug-naïve" Parkinson's disease patients with fatigue. J Neural Transm. 2020;127(8):1133–1142. doi: 10.1007/s00702-020-02219-6. [DOI] [PubMed] [Google Scholar]
- 39.Walpola I.C., Muller A.J., Hall J.M., et al. Mind-wandering in Parkinson's disease hallucinations reflects primary visual and default network coupling. Cortex. 2020;125:233–245. doi: 10.1016/j.cortex.2019.12.023. [DOI] [PubMed] [Google Scholar]
- 40.Bharti K., Suppa A., Pietracupa S., et al. Abnormal cerebellar connectivity patterns in patients with Parkinson's disease and freezing of gait. Cerebellum. 2019;18(3):298–308. doi: 10.1007/s12311-018-0988-4. [DOI] [PubMed] [Google Scholar]
- 41.Chen X., Hou X., Luo X., et al. Altered intra- and inter-regional functional connectivity of the anterior cingulate gyrus in patients with tremor-dominant Parkinson's disease complicated with sleep disorder. Front Aging Neurosci. 2019;11:319. doi: 10.3389/fnagi.2019.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiang P.L., Chen H.L., Lu C.H., et al. Interaction of systemic oxidative stress and mesial temporal network degeneration in Parkinson's disease with and without cognitive impairment. J Neuroinflammation. 2018;15(1):281. doi: 10.1186/s12974-018-1317-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hou Y., Yang J., Luo C., et al. Resting-state network connectivity in cognitively unimpaired drug-naïve patients with rigidity-dominant Parkinson's disease. J Neurol Sci. 2018;395:147–152. doi: 10.1016/j.jns.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Li M.G., Chen Y.Y., Chen Z.Y., et al. Altered functional connectivity of the marginal division in Parkinson's disease with mild cognitive impairment: a pilot resting-state fMRI study. J Magn Reson Imaging JMRI. 2019;50(1):183–192. doi: 10.1002/jmri.26548. [DOI] [PubMed] [Google Scholar]
- 45.Multani N., Taghdiri F., Anor C.J., et al. Association between social cognition changes and resting state functional connectivity in frontotemporal dementia, alzheimer's disease, Parkinson's disease, and healthy controls. Front Neurosci. 2019;13:1259. doi: 10.3389/fnins.2019.01259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang N., Zhang L., Yang H., Liu H., Luo X., Fan G. Similarities and differences in cerebellar grey matter volume and disrupted functional connectivity in idiopathic Parkinson's disease and multiple system atrophy. Neuropsychologia. 2019;124:125–132. doi: 10.1016/j.neuropsychologia.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 47.Ballarini T., Růžička F., Bezdicek O., et al. Unraveling connectivity changes due to dopaminergic therapy in chronically treated Parkinson's disease patients. Sci Rep. 2018;8(1):14328. doi: 10.1038/s41598-018-31988-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee Y., Ham J.H., Cha J., et al. The cholinergic contribution to the resting-state functional network in non-demented Parkinson's disease. Sci Rep. 2018;8(1):7683. doi: 10.1038/s41598-018-26075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manes J.L., Tjaden K., Parrish T., et al. Altered resting-state functional connectivity of the putamen and internal globus pallidus is related to speech impairment in Parkinson's disease. Brain Behav. 2018;8(9):e01073. doi: 10.1002/brb3.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen Y.T., Li J.Y., Yuan Y.S., et al. Disrupted amplitude of low-frequency fluctuations and causal connectivity in Parkinson's disease with apathy. Neurosci Lett. 2018;683:75–81. doi: 10.1016/j.neulet.2018.06.043. [DOI] [PubMed] [Google Scholar]
- 51.Tuovinen N., Seppi K., de Pasquale F., et al. The reorganization of functional architecture in the early-stages of Parkinson's disease. Parkinsonism Relat Disord. 2018;50:61–68. doi: 10.1016/j.parkreldis.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 52.Wang H., Chen H., Wu J., et al. Altered resting-state voxel-level whole-brain functional connectivity in depressed Parkinson's disease. Parkinsonism Relat Disord. 2018;50:74–80. doi: 10.1016/j.parkreldis.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 53.Anderkova L., Barton M., Rektorova I. Striato-cortical connections in Parkinson's and Alzheimer's diseases: relation to cognition. Mov Disord. 2017;32(6):917–922. doi: 10.1002/mds.26956. [DOI] [PubMed] [Google Scholar]
- 54.Boord P., Madhyastha T.M., Askren M.K., Grabowski T.J. Executive attention networks show altered relationship with default mode network in PD. NeuroImage Clin. 2017;13:1–8. doi: 10.1016/j.nicl.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen B., Wang S., Sun W., et al. Functional and structural changes in gray matter of Parkinson's disease patients with mild cognitive impairment. Eur J Radiol. 2017;93:16–23. doi: 10.1016/j.ejrad.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 56.Gallea C., Ewenczyk C., Degos B., et al. Pedunculopontine network dysfunction in Parkinson's disease with postural control and sleep disorders. Mov Disord. 2017;32(5):693–704. doi: 10.1002/mds.26923. [DOI] [PubMed] [Google Scholar]
- 57.Gu Q., Cao H., Xuan M., et al. Increased thalamic centrality and putamen-thalamic connectivity in patients with Parkinsonian resting tremor. Brain Behav. 2017;7(1):e00601. doi: 10.1002/brb3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hou Y., Luo C., Yang J., et al. Default-mode network connectivity in cognitively unimpaired drug-naïve patients with rigidity-dominant Parkinson's disease. J Neurol. 2017;264(1):152–160. doi: 10.1007/s00415-016-8331-9. [DOI] [PubMed] [Google Scholar]
- 59.Hu X., Jiang Y., Jiang X., et al. Altered functional connectivity density in subtypes of Parkinson's disease. Front Hum Neurosci. 2017;11:458. doi: 10.3389/fnhum.2017.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lucas-Jiménez O., Ojeda N., Peña J., et al. Altered functional connectivity in the default mode network is associated with cognitive impairment and brain anatomical changes in Parkinson's disease. Parkinsonism Relat Disord. 2016;33:58–64. doi: 10.1016/j.parkreldis.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 61.Markovic V., Agosta F., Canu E., et al. Role of habenula and amygdala dysfunction in Parkinson disease patients with punding. Neurology. 2017;88(23):2207–2215. doi: 10.1212/WNL.0000000000004012. [DOI] [PubMed] [Google Scholar]
- 62.Shen B., Gao Y., Zhang W., et al. Resting state fMRI reveals increased subthalamic nucleus and sensorimotor cortex connectivity in patients with Parkinson's disease under medication. Front Aging Neurosci. 2017;9:74. doi: 10.3389/fnagi.2017.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thibes R.B., Novaes N.P., Lucato L.T., et al. Altered functional connectivity between precuneus and motor systems in Parkinson's disease patients. Brain Connect. 2017;7(10):643–647. doi: 10.1089/brain.2017.0534. [DOI] [PubMed] [Google Scholar]
- 64.Yao Q., Zhu D., Li F., et al. Altered functional and causal connectivity of cerebello-cortical circuits between multiple system atrophy (Parkinsonian Type) and Parkinson's disease. Front Aging Neurosci. 2017;9:266. doi: 10.3389/fnagi.2017.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Borchert R.J., Rittman T., Passamonti L., et al. Atomoxetine enhances connectivity of prefrontal networks in Parkinson's Disease. Neuropsychopharmacology. 2016;41(8):2171–2177. doi: 10.1038/npp.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hou Y., Luo C., Yang J., et al. A resting-state fMRI study on early-stage drug-naïve Parkinson's disease patients with drooling. Neurosci Lett. 2016;634:119–125. doi: 10.1016/j.neulet.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 67.Hou Y., Yang J., Luo C., et al. Patterns of striatal functional connectivity differ in early and late onset Parkinson's disease. J Neurol. 2016;263(10):1993–2003. doi: 10.1007/s00415-016-8211-3. [DOI] [PubMed] [Google Scholar]
- 68.Peraza L.R., Colloby S.J., Firbank M.J., et al. Resting state in Parkinson's disease dementia and dementia with Lewy bodies: commonalities and differences. Int J Geriatr Psychiatry. 2015;30(11):1135–1146. doi: 10.1002/gps.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Simioni A.C., Dagher A., Fellows L.K. Compensatory striatal-cerebellar connectivity in mild-moderate Parkinson's disease. NeuroImage Clin. 2016;10:54–62. doi: 10.1016/j.nicl.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang M., Jiang S., Yuan Y., et al. Alterations of functional and structural connectivity of freezing of gait in Parkinson's disease. J Neurol. 2016;263(8):1583–1592. doi: 10.1007/s00415-016-8174-4. [DOI] [PubMed] [Google Scholar]
- 71.Wang Z., Chen H., Ma H., Ma L., Wu T., Feng T. Resting-state functional connectivity of subthalamic nucleus in different Parkinson's disease phenotypes. J Neurol Sci. 2016;371:137–147. doi: 10.1016/j.jns.2016.10.035. [DOI] [PubMed] [Google Scholar]
- 72.Yang W., Liu B., Huang B., et al. Altered resting-state functional connectivity of the striatum in Parkinson's disease after levodopa administration. PLoS One. 2016;11(9) doi: 10.1371/journal.pone.0161935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang J.R., Feng T., Hou Y.N., Chan P., Wu T. Functional connectivity of vim nucleus in tremor- and akinetic-/rigid-dominant Parkinson's disease. CNS Neurosci Ther. 2016;22(5):378–386. doi: 10.1111/cns.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baggio H.C., Segura B., Sala-Llonch R., et al. Cognitive impairment and resting-state network connectivity in Parkinson's disease. Hum Brain Mapp. 2015;36(1):199–212. doi: 10.1002/hbm.22622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carriere N., Lopes R., Defebvre L., Delmaire C., Dujardin K. Impaired corticostriatal connectivity in impulse control disorders in Parkinson disease. Neurology. 2015;84(21):2116–2123. doi: 10.1212/WNL.0000000000001619. [DOI] [PubMed] [Google Scholar]
- 76.Fernández-Seara M.A., Mengual E., Vidorreta M., et al. Resting state functional connectivity of the subthalamic nucleus in Parkinson's disease assessed using arterial spin-labeled perfusion fMRI. Hum Brain Mapp. 2015;36(5):1937–1950. doi: 10.1002/hbm.22747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ham J.H., Cha J., Lee J.J., et al. Nigrostriatal dopamine-independent resting-state functional networks in Parkinson's disease. Neuroimage. 2015;119:296–304. doi: 10.1016/j.neuroimage.2015.06.077. [DOI] [PubMed] [Google Scholar]
- 78.Luo C., Guo X., Song W., et al. The trajectory of disturbed resting-state cerebral function in Parkinson's disease at different Hoehn and Yahr stages. Hum Brain Mapp. 2015;36(8):3104–3116. doi: 10.1002/hbm.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mathys C., Caspers J., Langner R., et al. Functional connectivity differences of the subthalamic nucleus related to Parkinson's disease. Hum Brain Mapp. 2016;37(3):1235–1253. doi: 10.1002/hbm.23099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Müller-Oehring E.M., Sullivan E.V., Pfefferbaum A., et al. Task-rest modulation of basal ganglia connectivity in mild to moderate Parkinson's disease. Brain Imaging Behav. 2015;9(3):619–638. doi: 10.1007/s11682-014-9317-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Festini S.B., Bernard J.A., Kwak Y., et al. Altered cerebellar connectivity in Parkinson's patients ON and OFF L-DOPA medication. Front Hum Neurosci. 2015;9:214. doi: 10.3389/fnhum.2015.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sunwoo M.K., Cha J., Ham J.H., et al. Olfactory performance and resting state functional connectivity in non-demented drug naïve patients with Parkinson's disease. Hum Brain Mapp. 2015;36(5):1716–1727. doi: 10.1002/hbm.22732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang D., Liu X., Chen J., Liu B., Wang J. Widespread increase of functional connectivity in Parkinson's disease with tremor: a resting-state FMRI study. Front Aging Neurosci. 2015;7:6. doi: 10.3389/fnagi.2015.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Agosta F., Caso F., Stankovic I., et al. Cortico-striatal-thalamic network functional connectivity in hemiparkinsonism. Neurobiol Aging. 2014;35(11):2592–2602. doi: 10.1016/j.neurobiolaging.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 85.Luo C., Song W., Chen Q., et al. Reduced functional connectivity in early-stage drug-naive Parkinson's disease: a resting-state fMRI study. Neurobiol Aging. 2014;35(2):431–441. doi: 10.1016/j.neurobiolaging.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 86.Luo C., Chen Q., Song W., et al. Resting-state fMRI study on drug-naive patients with Parkinson's disease and with depression. J Neurol Neurosurg Psychiatry. 2014;85(6):675–683. doi: 10.1136/jnnp-2013-306237. [DOI] [PubMed] [Google Scholar]
- 87.Rektorova I., Krajcovicova L., Marecek R., Mikl M. Default mode network and extrastriate visual resting state network in patients with Parkinson's disease dementia. Neurodegener Dis. 2012;10(1–4):232–237. doi: 10.1159/000334765. [DOI] [PubMed] [Google Scholar]
- 88.Wu T., Long X., Wang L., et al. Functional connectivity of cortical motor areas in the resting state in Parkinson's disease. Hum Brain Mapp. 2011;32(9):1443–1457. doi: 10.1002/hbm.21118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baudrexel S., Witte T., Seifried C., et al. Resting state fMRI reveals increased subthalamic nucleus-motor cortex connectivity in Parkinson's disease. Neuroimage. 2011;55(4):1728–1738. doi: 10.1016/j.neuroimage.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 90.Seibert T.M., Murphy E.A., Kaestner E.J., Brewer J.B. Interregional correlations in Parkinson disease and Parkinson-related dementia with resting functional MR imaging. Radiology. 2012;263(1):226–234. doi: 10.1148/radiol.12111280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kwak Y., Peltier S., Bohnen N.I., Müller M.L., Dayalu P., Seidler R.D. Altered resting state cortico-striatal connectivity in mild to moderate stage Parkinson's disease. Front Syst Neurosci. 2010;4:143. doi: 10.3389/fnsys.2010.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Helmich R.C., Derikx L.C., Bakker M., Scheeringa R., Bloem B.R., Toni I. Spatial remapping of cortico-striatal connectivity in Parkinson's disease. Cereb Cortex. 2010;20(5):1175–1186. doi: 10.1093/cercor/bhp178. (New York, NY: 1991) [DOI] [PubMed] [Google Scholar]
- 93.Chabran E., Noblet V., Loureiro de Sousa P., et al. Changes in gray matter volume and functional connectivity in dementia with Lewy bodies compared to Alzheimer's disease and normal aging: implications for fluctuations. Alzheimer's Res Ther. 2020;12(1):9. doi: 10.1186/s13195-019-0575-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kenny E.R., O'Brien J.T., Firbank M.J., Blamire A.M. Subcortical connectivity in dementia with Lewy bodies and Alzheimer's disease. Br J Psychiatry. 2013;203(3):209–214. doi: 10.1192/bjp.bp.112.108464. the journal of mental science. [DOI] [PubMed] [Google Scholar]
- 95.Kenny E.R., Blamire A.M., Firbank M.J., O'Brien J.T. Functional connectivity in cortical regions in dementia with Lewy bodies and Alzheimer's disease. Brain J Neurol. 2012;135(Pt 2):569–581. doi: 10.1093/brain/awr327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Galvin J.E., Price J.L., Yan Z., Morris J.C., Sheline Y.I. Resting bold fMRI differentiates dementia with Lewy bodies vs Alzheimer disease. Neurology. 2011;76(21):1797–1803. doi: 10.1212/WNL.0b013e31821ccc83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhao B., Liu H., Li H., Shang X. Abnormal functional connectivity of the amygdala is associated with depressive symptoms in patients with multiple system atrophy. Neuropsychiatr Dis Treat. 2018;14:3133–3142. doi: 10.2147/NDT.S178657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang H., Luo X., Yu H., et al. Altered resting-state voxel-level whole-brain functional connectivity in multiple system atrophy patients with cognitive impairment. Clin Neurophysiol. 2020;131(1):54–62. doi: 10.1016/j.clinph.2019.09.026. [DOI] [PubMed] [Google Scholar]
- 99.Yang H., Wang N., Luo X., Lv H., Liu H., Fan G. Altered functional connectivity of dentate nucleus in parkinsonian and cerebellar variants of multiple system atrophy. Brain Imaging Behav. 2019;13(6):1733–1745. doi: 10.1007/s11682-019-00097-5. [DOI] [PubMed] [Google Scholar]
- 100.Ren S., Zhang H., Zheng W., et al. Altered functional connectivity of cerebello-cortical circuit in multiple system atrophy (Cerebellar-Type) Front Neurosci. 2018;12:996. doi: 10.3389/fnins.2018.00996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kawabata K., Hara K., Watanabe H., et al. Alterations in cognition-related cerebello-cerebral networks in multiple system atrophy. Cerebellum. 2019;18(4):770–780. doi: 10.1007/s12311-019-01031-7. [DOI] [PubMed] [Google Scholar]
- 102.Hohenfeld C., Werner C.J., Reetz K. Resting-state connectivity in neurodegenerative disorders: is there potential for an imaging biomarker? NeuroImage Clin. 2018;18:849–870. doi: 10.1016/j.nicl.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Seeley W.W., Crawford R.K., Zhou J., Miller B.L., Greicius M.D. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62(1):42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sabatini U., Boulanouar K., Fabre N., et al. Cortical motor reorganization in akinetic patients with Parkinson's disease: a functional MRI study. Brain J Neurol. 2000;123(Pt 2):394–403. doi: 10.1093/brain/123.2.394. [DOI] [PubMed] [Google Scholar]
- 105.Yu H., Sternad D., Corcos D.M., Vaillancourt D.E. Role of hyperactive cerebellum and motor cortex in Parkinson's disease. Neuroimage. 2007;35(1):222–233. doi: 10.1016/j.neuroimage.2006.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Alexander G.E., DeLong M.R., Strick P.L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 107.Hacker C.D., Perlmutter J.S., Criswell S.R., Ances B.M., Snyder A.Z. Resting state functional connectivity of the striatum in Parkinson's disease. Brain J Neurol. 2012;135(Pt 12):3699–3711. doi: 10.1093/brain/aws281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wu T., Hallett M. The cerebellum in Parkinson's disease. Brain J Neurol. 2013;136(Pt 3):696–709. doi: 10.1093/brain/aws360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim H.J., Jeon B., Fung V.S.C. Role of magnetic resonance imaging in the diagnosis of multiple system atrophy. Mov Disord Clin Pract. 2017;4(1):12–20. doi: 10.1002/mdc3.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bostan A.C., Dum R.P., Strick P.L. The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci U S A. 2010;107(18):8452–8456. doi: 10.1073/pnas.1000496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Husárová I., Mikl M., Lungu O.V., Mareček R., Vaníček J., Bareš M. Similar circuits but different connectivity patterns between the cerebellum, basal ganglia, and supplementary motor area in early Parkinson's disease patients and controls during predictive motor timing. J Neuroimaging. 2013;23(4):452–462. doi: 10.1111/jon.12030. [DOI] [PubMed] [Google Scholar]
- 112.Tinaz S., Lauro P., Hallett M., Horovitz S.G. Deficits in task-set maintenance and execution networks in Parkinson's disease. Brain Struct Funct. 2016;221(3):1413–1425. doi: 10.1007/s00429-014-0981-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Herz D.M., Eickhoff S.B., Løkkegaard A., Siebner H.R. Functional neuroimaging of motor control in Parkinson's disease: a meta-analysis. Hum Brain Mapp. 2014;35(7):3227–3237. doi: 10.1002/hbm.22397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Armstrong M.J., Okun M.S. Diagnosis and treatment of Parkinson disease: a review. JAMA. 2020;323(6):548–560. doi: 10.1001/jama.2019.22360. [DOI] [PubMed] [Google Scholar]
- 115.Fanciulli A., Wenning G.K. Multiple-system atrophy. N Engl J Med. 2015;372(3):249–263. doi: 10.1056/NEJMra1311488. [DOI] [PubMed] [Google Scholar]
- 116.Manza P., Zhang S., Li C.S., Leung H.C. Resting-state functional connectivity of the striatum in early-stage Parkinson's disease: cognitive decline and motor symptomatology. Hum Brain Mapp. 2016;37(2):648–662. doi: 10.1002/hbm.23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Guell X., Gabrieli J.D.E., Schmahmann J.D. Triple representation of language, working memory, social and emotion processing in the cerebellum: convergent evidence from task and seed-based resting-state fMRI analyses in a single large cohort. Neuroimage. 2018;172:437–449. doi: 10.1016/j.neuroimage.2018.01.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Buckner R.L., DiNicola L.M. The brain's default network: updated anatomy, physiology and evolving insights. Nat Rev Neurosci. 2019;20(10):593–608. doi: 10.1038/s41583-019-0212-7. [DOI] [PubMed] [Google Scholar]
- 119.Olde Dubbelink K.T., Schoonheim M.M., Deijen J.B., Twisk J.W., Barkhof F., Berendse H.W. Functional connectivity and cognitive decline over 3 years in Parkinson disease. Neurology. 2014;83(22):2046–2053. doi: 10.1212/WNL.0000000000001020. [DOI] [PubMed] [Google Scholar]
- 120.Shine J.M., Halliday G.M., Naismith S.L., Lewis S.J. Visual misperceptions and hallucinations in Parkinson's disease: dysfunction of attentional control networks? Mov Disord. 2011;26(12):2154–2159. doi: 10.1002/mds.23896. [DOI] [PubMed] [Google Scholar]
- 121.Muller A.J., Shine J.M., Halliday G.M., Lewis S.J. Visual hallucinations in Parkinson's disease: theoretical models. Mov Disord. 2014;29(13):1591–1598. doi: 10.1002/mds.26004. [DOI] [PubMed] [Google Scholar]
- 122.Greicius M.D., Krasnow B., Reiss A.L., Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Harding A.J., Broe G.A., Halliday G.M. Visual hallucinations in Lewy body disease relate to Lewy bodies in the temporal lobe. Brain J Neurol. 2002;125(Pt 2):391–403. doi: 10.1093/brain/awf033. [DOI] [PubMed] [Google Scholar]
- 124.Franciotti R., Delli Pizzi S., Perfetti B., et al. Default mode network links to visual hallucinations: a comparison between Parkinson's disease and multiple system atrophy. Mov Disord. 2015;30(9):1237–1247. doi: 10.1002/mds.26285. [DOI] [PubMed] [Google Scholar]
- 125.Mégevand P., Groppe D.M., Goldfinger M.S., et al. Seeing scenes: topographic visual hallucinations evoked by direct electrical stimulation of the parahippocampal place area. J Neurosci. 2014;34(16):5399–5405. doi: 10.1523/JNEUROSCI.5202-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Comte M., Schön D., Coull J.T., et al. Dissociating bottom-up and top-down mechanisms in the cortico-limbic system during emotion processing. Cereb Cortex. 2016;26(1):144–155. doi: 10.1093/cercor/bhu185. (New York, NY: 1991) [DOI] [PubMed] [Google Scholar]
- 127.Tang S., Lu L., Zhang L., et al. Abnormal amygdala resting-state functional connectivity in adults and adolescents with major depressive disorder: a comparative meta-analysis. EBioMedicine. 2018;36:436–445. doi: 10.1016/j.ebiom.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang Y., Gao Y., Tang S., et al. Large-scale network dysfunction in the acute state compared to the remitted state of bipolar disorder: a meta-analysis of resting-state functional connectivity. EBioMedicine. 2020;54 doi: 10.1016/j.ebiom.2020.102742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ochsner K.N., Gross J.J. The cognitive control of emotion. Trends Cogn Sci. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 130.Farras-Permanyer L., Mancho-Fora N., Montalà-Flaquer M., et al. Age-related changes in resting-state functional connectivity in older adults. Neural Regen Res. 2019;14(9):1544–1555. doi: 10.4103/1673-5374.255976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Boon L.I., Hepp D.H., Douw L., et al. Functional connectivity between resting-state networks reflects decline in executive function in Parkinson's disease: a longitudinal fMRI study. NeuroImage Clin. 2020;28 doi: 10.1016/j.nicl.2020.102468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Postuma R.B. Resting state MRI: a new marker of prodromal neurodegeneration? Brain J Neurol. 2016;139(Pt 8):2106–2108. doi: 10.1093/brain/aww131. [DOI] [PubMed] [Google Scholar]
- 133.Dauvilliers Y., Schenck C.H., Postuma R.B., et al. REM sleep behaviour disorder. Nat Rev Dis Prim. 2018;4(1):19. doi: 10.1038/s41572-018-0016-5. [DOI] [PubMed] [Google Scholar]
- 134.Feng H., Chen L., Liu Y., et al. Rest-activity pattern alterations in idiopathic REM sleep behavior disorder. Ann Neurol. 2020;88(4):817–829. doi: 10.1002/ana.25853. [DOI] [PubMed] [Google Scholar]
- 135.Byun J.I., Kim H.W., Kang H., et al. Altered resting-state thalamo-occipital functional connectivity is associated with cognition in isolated rapid eye movement sleep behavior disorder. Sleep Med. 2020;69:198–203. doi: 10.1016/j.sleep.2020.01.010. [DOI] [PubMed] [Google Scholar]
- 136.Campabadal A., Abos A., Segura B., et al. Disruption of posterior brain functional connectivity and its relation to cognitive impairment in idiopathic REM sleep behavior disorder. NeuroImage Clin. 2020;25 doi: 10.1016/j.nicl.2019.102138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu Y., Zhang J., Lam S.P., et al. A case-control-family study of idiopathic rapid eye movement sleep behavior disorder. Ann Neurol. 2019;85(4):582–592. doi: 10.1002/ana.25435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.