Abstract
The emergence of 5-Fluorouracil (5-FU) resistance is the barrier to effective clinical outcomes for colorectal cancer (CRC) patients. Autophagy was found to be involved in protecting tumor cells from 5-FU. However, the specific role of autophagy-related genes in CRC 5-FU resistance remains unclear. In this study, HSPB8 among 34 differentially expressed ARGs in CRC was identified to be the hub ARGs in 5-FU resistant which was down-regulated in CRC samples when compared with normal samples but up-regulated in CRC samples with relatively higher lymphatic invasion, later stages and poor prognosis of CRC. Mechanistic analysis demonstrated that due to the recruitment of CAFs, HSPB8 expression was enhanced in CRC cells so that HSPB8 could act together with its co-chaperone BAG3 in autophagy drived 5-FU resistance. Furthermore, the augmented expression level of HSPB8 was found to be significantly correlated to the immune cell infiltration such as Treg cells, macrophages, monocyte and dendritic cells and so on. Our results suggested CAFs driving HSPB8 induced CRC 5-FU resistance by promoting tumor autophagy would provide a new strategy in seeking potential CRC therapeutic target.
Keywords: Colorectal cancer, Autophagy, HSPB8, 5-Fluorouracil resistance
Colorectal cancer; Autophagy; HSPB8; 5-fluorouracil resistance.
1. Introduction
Data collected in 2020 by the Global Cancer Observatory (GCO, https://gco.iarc.fr), colorectal cancer (CRC) is the third most common form of cancer worldwide after lung and breast cancers [1]. CRC Patients at early stages of development and premalignant adenomatous polyps are commonly asymptomatic, but often present evident symptoms at advanced CRC. Drug adjuvant therapy is often applied to improve surgical outcomes and to prevent tumor recurrence for patients with advanced and metastatic CRC. 5-fluorouracil (5-FU), as the original member of fluoropyrimidines, is the first-line drug for both single-drug therapy and chemotherapy in CRC [2]. 5-FU-based therapies, such as FOLFOX (5-FU, leucovorin, and oxaliplatin) or FOLFIRI (5-FU, leucovorin, and irinotecan), have been used as the standard therapy for advanced CRC. However, due to the emergence of drug resistance, the effectiveness of 5-FU treatment is greatly reduced which has caused high mortality of CRC patients [3, 4]. So studying the mechanism of 5-FU resistance may be a key factor in improving colorectal cancer survival.
Autophagy is a catabolic process that is crucial for cell development and can occurs in response to nutrient deprivation or metabolic disorders [5]. In tumor cells, autophagy plays a dual role which serves as a tumor suppressor during the initial stages but later protects tumor cells from chemo- and radioresistance, hypoxia and the immune defense system [6]. Increasing studies in tumor therapy demonstrate the modulation of autophagic flux might be a potential therapeutic strategy for cancer [7]. Inhibition of autophagy could be a promising strategy enhancing the anticancer effect of 5-FU in CRC [8].
In this study, 34 differentially expressed autophagy-related genes (ARGs) were obtained from the expression data of patients in the CRC cohort in The Cancer Genome Atlas (TCGA) database, and the biological functions of these differentially expressed ARGs were analyzed. Then HSPB8 was determined to be the key ARG in enhancing CRC 5-FU resistance. Higher HSPB8 expression usually correlated to a lower time of overall survival (OS) and disease-free survival (DFS). Mechanistically, cancer-associated fibroblasts (CAFs) were found to promote tumor HSPB8 expression and increased HSPB8 expression could recruit Treg, macrophage and neutrophil cells which would further enhance tumor 5-FU resistance. All our results would provide a new strategy in seeking potential CRC diagnostic biomarkers and therapeutic targets.
2. Results
2.1. Enrichment analysis of differently expressed ARGs in CRC
Through analyzing TCGA RNA sequences data of 473 CRC samples and matched 41 solid tissue normal samples, 12781 abnormal expressed genes were obtained firstly. By comparing the TCGA database, 34 of 232 ARGs was identified with FDR< 0.05 and log2 FC > 1which showed 13 ARGs were up-regulated and 21 ARGs were down-regulated in CRC tissues (Figure 1a). Then functional enrichment analysis was performed with the 34 differentially expressed ARGs (Figure 1b). In the biological processes, the ARGs were mainly enriched in extrinsic apoptotic signaling pathway via death domain receptors, mitophagy, regulation of mitotic cell cycle, negative regulation of neuron apoptotic process and negative regulation of apoptotic process. In the cellular components, the ARGs were mainly enriched in cytosol, autophagosome membrane, autophagosome, mitochondrion, protein complex and cytoplasmic vesicle. In the molecular functions, the ARGs were mainly enriched in ubiquitin protein ligase binding, kinase activity and protein phosphatase 2A binding. In the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways, the ARGs were mainly enriched in ErbB signaling pathway, p53 signaling pathway, pathways in cancer, apoptosis, HIF-1 signaling pathway, PI3K-Akt signaling pathway, colorectal cancer and other functional pathways (Figure 1b). In addition, CancerSEA was performed to predict the ARGs associated functional states in CRC using scRNA-seq datasets. As showed in Figure 1c, the up-regulated ARGs were mainly enriched in DNA repair and cell stemness while down-regulated ARGs were mainly enriched in cell cycle (p < 0.05).
Figure 1.
Enrichment analysis of differently expressed ARGs in CRC. a: 232 ARGs were obtained from the HADb and 12781 abnormal expressed genes were obtained from TCGA. By comparing with each other, 34 of 232 ARGs was identified with FDR< 0.05 and log2 FC > 1which showed 13 ARGs were up-regulated and 21 ARGs were down-regulated in CRC tissues. b: The results of identified 34 ARGs in Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis. c: The distinct functional states of 13 up-regulated ARGs and 21 down-regulated ARGs in cancer cells at single-cell resolution were analyzed on CancerSEA.
2.2. Identification of hub ARGs in CRC 5-FU resistance
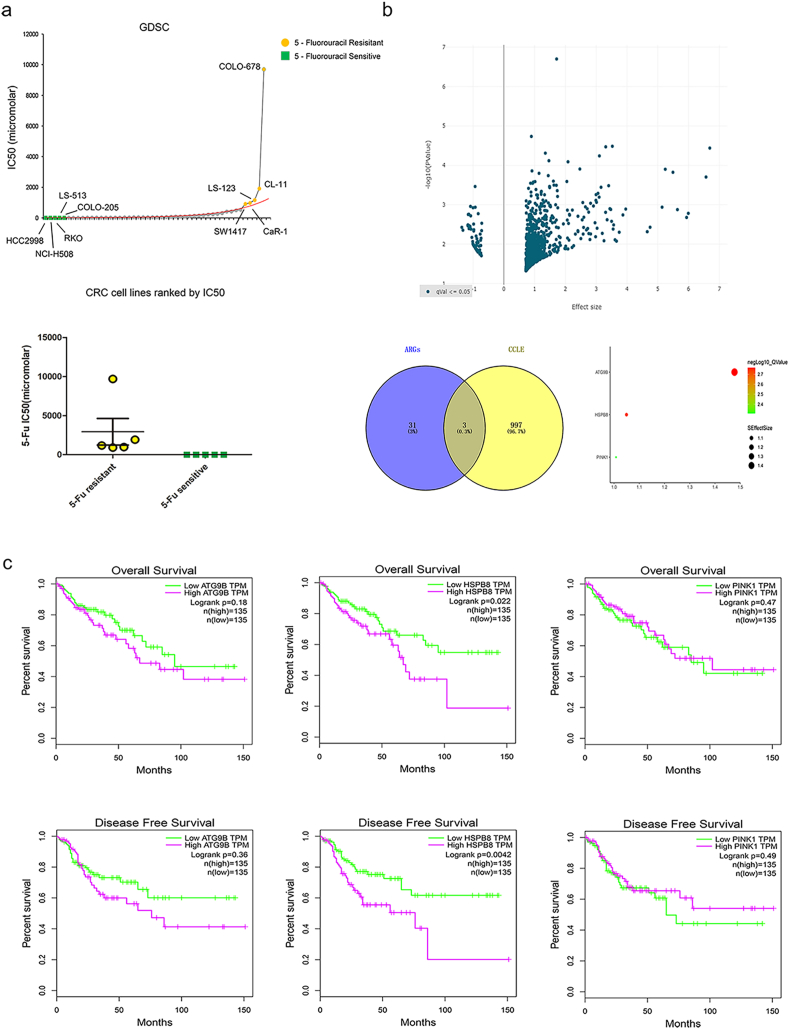
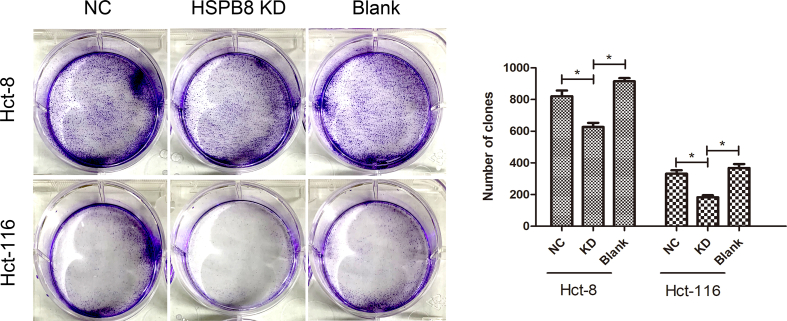
The IC50 5-FU response data of 44 CRC cell lines were analyzed in GDSC database. As showed in Figure 2a, The IC50 value was significant higher in SW1116, LS-123, HT55, HT-115, SW1417 cells but lower in SNU-407, RKO, HCT-116, LS-411N, LS-513 cells (p < 0.05). According to 5-FU IC50, cells were separated into 5-FU resistance and 5-FU sensitive groups and their genomic information were further compared in CCLE. Consequently, volcano plot of 936 up-regulated and 64 down-regulated genes were presented in Figure 2b (p < 0.05). By verification of 34 ARGs expression in CCLE, high expression of ATG9B, HSPB8 and PINK1 were determined to be the hub ARGs correlated to 5-FU resistance. Moreover, the association between hub ARGs and CRC overall survival (OS) and disease-free survival (DFS) were further explored in GEPIA. Though difference were not found in CRC patients with different ATG9B or PINK1 expression (figure2c, p > 0.05), CRC patients with high HSPB8 expression were found to have a significant shorter OS and DFS time than patients with low HSPB8 expression (figure 2c, p < 0.05). As expected, CRC cells with HSPB8 knock down were also found to show a significant lower colony forming efficiency than other cells when cultured with 5-FU (supplementary figure 1, p < 0.05).
Figure 2.
The identification of hub ARGs in CRC 5-FU resistance. a: The IC50 5-FU response data of 44 CRC cell lines in GDSC database. The IC50 value was significant higher in SW1116, LS-123, HT55, HT-115, SW1417 cells but lower in SNU-407, RKO, HCT-116, LS-411N, LS-513 cells (p < 0.05). b: The genomic information of CRC cells with different 5-FU IC50 response. By verification of 34 ARGs expression in CCLE, high expression of ATG9B, HSPB8 and PINK1 were determined to be the hub ARGs correlated to 5-FU resistance. c: The association between hub ARGs expression and CRC overall survival (OS) and disease-free survival (DFS). CRC patients with high HSPB8 expression were found to have a significant shorter OS and DFS time than patients with low HSPB8 expression (p < 0.05).
2.3. The relationship between HSPB8 expression and CRC clinical characteristics
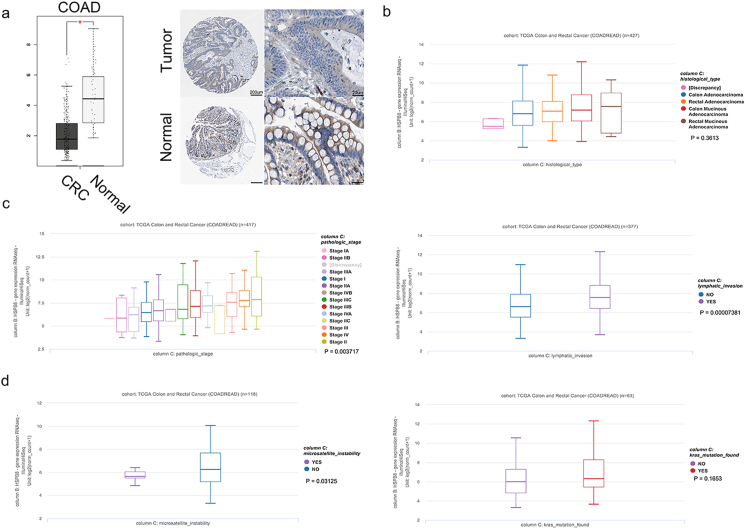
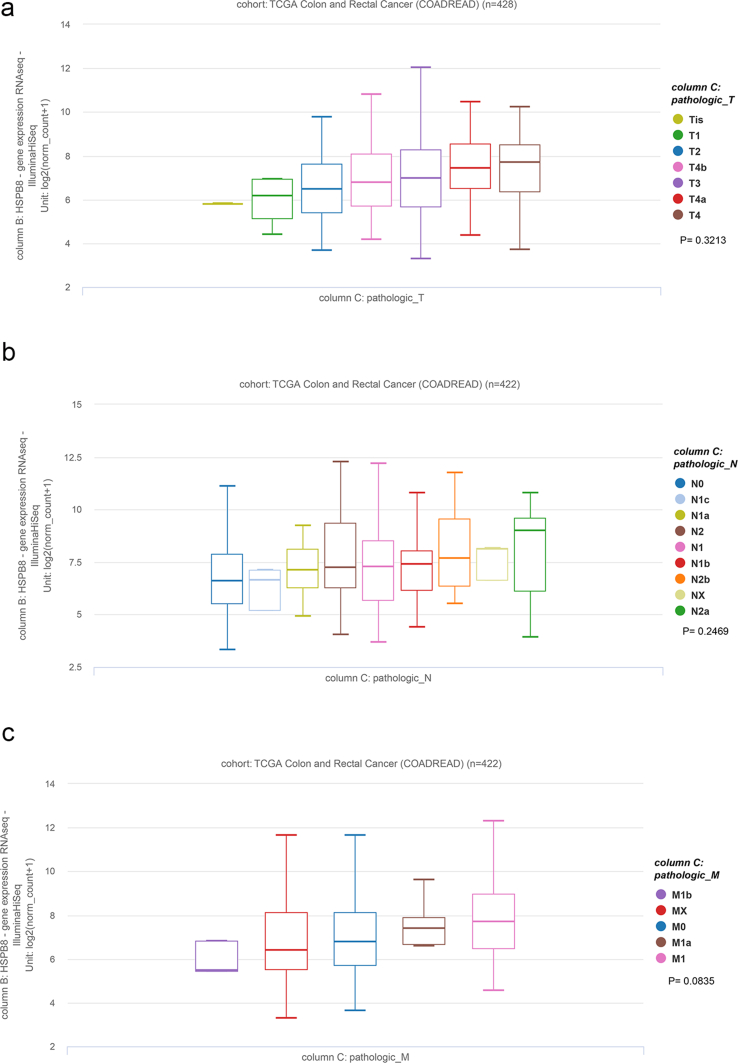
As showed in GEPIA and HPA database, both HSPB8 mRNA and protein were found to be down-regulated in CRC tissues (Figure 3a, p < 0.05). Then Xena was performed to verify the correlation between HSPB8 and CRC clinical characteristics. The results demonstrated that HSPB8 expression had no relation to CRC TNM stage or tumor histological type (Figure 3b and Supplementary figure 2, p < 0.05). Fortunately, high HSPB8 expression was found to have a higher tumor stage and lymphatic invasion (p < 0.05, Figure 3c). Additionally, the HSPB8 expression of CRC patients with different MSI and KRAS mutation were also compared. The results showed that CRC patients without MSI seemed to have a higher HSPB8 (p < 0.05), while HSPB8 expression difference was not found in patients with or without KRAS mutation (figure 3c, p > 0.05).
Figure 3.
The correlation between HSPB8 expression and CRC clinical characteristics. a: The expression of HSPB8 in CRC tissues. The HSPB8 showed a significant higher expression in tumor tissues than normal tissues (p < 0.05). b: The association between HSPB8 expression and CRC histological type. The results demonstrated that HSPB8 expression had no relation to tumor histological type (p < 0.05). c: The relationship between HSPB8 expression and CRC tumor stage and lymphatic invasion. High HSPB8 expression was found to have a higher tumor stage and lymphatic invasion (p < 0.05). d: The comparison results of HSPB8 expression in CRC patients with different MSI and KRAS mutation. The results showed that CRC patients without MSI seemed to have a higher HSPB8 (p < 0.05), while HSPB8 expression difference was not found in patients with or without KRAS mutation (p > 0.05).
2.4. Co-expression and gene interaction analyses of HSPB8 in patients with CRC
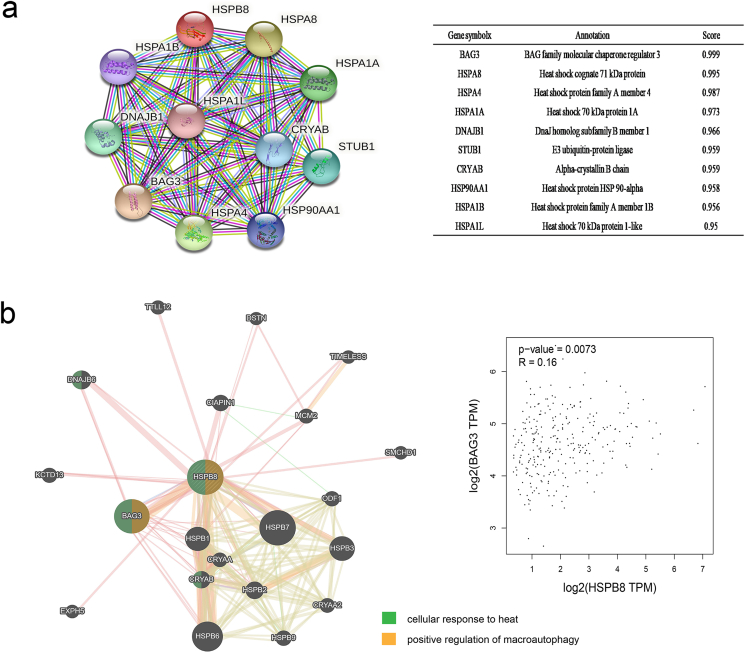
PPI network analysis of HSPB8 was conducted with STRING. As presented, 11 nodes and 54 edges were obtained in the PPI network (Figure 4a). Then GeneMANIA was used to explore the potential interaction between HSPB8 and other genes. By comparing the results of STRING, BAG3 and CRYAB were identified to be the highest frequency genes associated with HSPB8. Function analysis of these genes revealed that they were mainly focus on cellular response to heat but the interaction between HSPB8 and BAG3 was found to play an important role in positive regulation of macroautophagy. In addition, a co-expression of HSPB8 and BAG3 was also found in CRC tissues by GEPIA (figure 4b, p < 0.05).
Figure 4.
Co-Expression and gene interaction analyses of HSPB8 in CRC. a: The PPI network analysis of HSPB8 by STRING. b: The prediction results of the potential interaction between HSPB8 and other genes. The interaction between HSPB8 and BAG3 was found to play an important role in positive regulation of macroautophagy. In addition, a co-expression of HSPB8 and BAG3 was also found in CRC tissues by GEPIA (p < 0.05).
2.5. Correlation analysis between HSPB8 expression and cancer associated fibroblasts (CAFs)
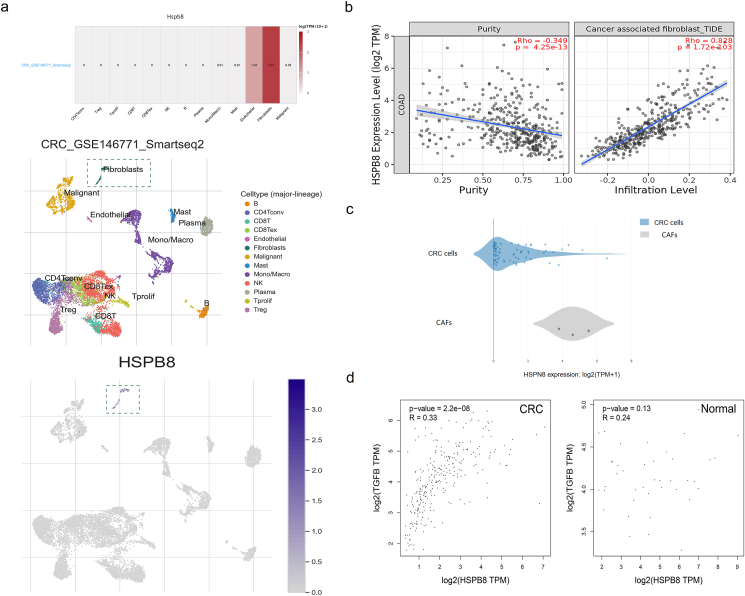
The HSPB8 expression of CRC tissues was lower than normal tissues whereas patients with down-regulated HSPB8 usually related to a better outcome. Previous studies had reported patient-derived CAFs can strikingly reprogram the metabolic machinery of cancer cells [9]. Therefore, the correlation between HSPB8 expression and CAFs was analyzed in TISCH. The results displayed that comparing with other cells, HSPB8 was found to mainly express in CAFs (Figure 5a, p < 0.05). As expected, TIMER database was conducted which also demonstrated that a significant correlation was found between HSPB8 expression and CAFs (figure 5b, p < 0.05). Then the HSPB8 expression of CRC and CAFs was analyzed which demonstrated a higher HSPB8 expression was found in CAFs (figure 5c, p < 0.05). As the recruitment factor of CAFs, transforming growth factor β (TGF- β) was included in the study to detect its association with HSPB8. Fortunately, a co-expression of HSPB8 and TGF-β was observed in CRC tissues but not in normal tissues (figure 5d, p < 0.05).
Figure 5.
Correlation analysis between HSPB8 expression and CAFs. a: The TISCH results of the association between HSPB8 expression and different cells in tumor microenvironment. b: The verification results of the correlation between HSPB8 expression and CAFs by TIMER. c: The comparison of HSPB8 expression between CRC cells and CAFs in CCLE. d: The co-expression between HSPB8 and TGF-β expression in CRC cells.
2.6. Immune cell infiltration of HSPB8 in CRC patients
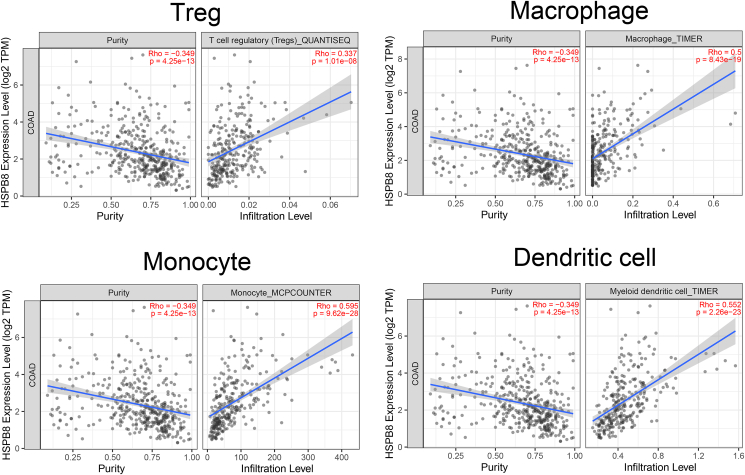
Recently, immune cells were reported to have a great affection on CRC patients’ response to treatment [10]. Thus immune marker sets, which were widely accepted as corresponding symbols of different immunocytes were applied to explore their association with HSPB8 expression in TIMER and GEPIA databases. The results showed that the immune markers of treg cells, macrophages, monocyte and dendritic cells were significantly associated with HSPB8 expression (Table 1, p < 0.05). Moreover, tumor-associated macrophages, especially for M2 macrophages were found to be highly related to HSPB8 expression (Table 1, p < 0.05). Additionally, the TIMER database was used to confirm the relationships between HSPB8 expression and tumor immune cell infiltration such as Treg cells, macrophages, monocyte, dendritic cells and so on. As expected, the increased expression level of HSPB8 was significantly correlated to the infiltration of Treg cells, macrophages, monocyte and dendritic cells (Figure 6, p < 0.05).
Table 1.
The correlation between HSPB8 expression and immune cells.
| Cell type | Gene marker | None |
Purity |
Tumor |
Normal |
||||
|---|---|---|---|---|---|---|---|---|---|
| Cor | P | Cor | P | R | P | R | P | ||
| B | CD19 | 0.263 | <0.001 | 0.149 | 0.0026 | 0.12 | 0.055 | -0.25 | 0.12 |
| KRT20 | 0.006 | 0.893 | -0.006 | 0.908 | -0.053 | 0.38 | -0.4 | 0.0099 | |
| CD38 | 0.34 | <0.001 | 0.225 | <0.001 | 0.12 | 0.053 | -0.35 | 0.024 | |
| CD4+T | CD4 | 0.568 | <0.001 | 0.489 | <0.001 | 0.25 | <0.001 | -0.21 | 0.18 |
| CCR6 | 0.093 | 0.0467 | 0.049 | 0.325 | -0.017 | 0.78 | -0.3 | 0.056 | |
| CD8+ T | CD8A | 0.301 | <0.001 | 0.195 | <0.001 | 0.29 | 0.063 | -0.16 | 0.32 |
| CD8B | 0.285 | <0.001 | 0.23 | <0.001 | 0.29 | 0.064 | -0.14 | 0.39 | |
| Treg | FOXP3 | 0.505 | <0.001 | 0.425 | <0.001 | 0.25 | <0.001 | -0.18 | 0.25 |
| IL2RA | 0.437 | <0.001 | 0.341 | <0.001 | 0.19 | 0.002 | -0.16 | 0.31 | |
| CCR8 | 0.522 | <0.001 | 0.451 | <0.001 | 0.29 | <0.001 | -0.15 | 0.36 | |
| Macrophage | CD68 | 0.467 | <0.001 | 0.4 | <0.001 | 0.2 | <0.001 | -0.23 | 0.14 |
| ITGAM | 0.611 | <0.001 | 0.563 | <0.001 | 0.25 | <0.001 | 0.38 | 0.015 | |
| M1 | NOS2 | -0.206 | <0.001 | -0.26 | <0.001 | -0.078 | 0.19 | -0.087 | 0.59 |
| IRF5 | 0.34 | <0.001 | 0.354 | <0.001 | 0.11 | 0.069 | -0.29 | 0.065 | |
| PTGS2 | 0.283 | <0.001 | 0.222 | <0.001 | 0.048 | 0.43 | 0.4 | 0.0092 | |
| M2 | ARG1 | 0.06 | 0.203 | 0.036 | 0.467 | 0.058 | 0.34 | -0.11 | 0.48 |
| MRC1 | 0.51 | <0.001 | 0.444 | <0.001 | 0.21 | <0.001 | 0.084 | 0.6 | |
| TAM | MS4A4A | 0.571 | <0.001 | 0.503 | <0.001 | 0.26 | <0.001 | 0.041 | 0.8 |
| CCL2 | 0.693 | <0.001 | 0.639 | <0.001 | 0.39 | <0.001 | 0.21 | 0.18 | |
| CD80 | 0.447 | <0.001 | 0.372 | <0.001 | 0.13 | 0.03 | -0.075 | 0.64 | |
| CD86 | 0.59 | <0.001 | 0.524 | <0.001 | 0.24 | <0.001 | -0.11 | 0.5 | |
| CCR5 | 0.507 | <0.001 | 0.418 | <0.001 | 0.19 | 0.001 | -0.19 | 0.23 | |
| Monocyte | CD14 | 0.564 | <0.001 | 0.488 | <0.001 | 0.25 | <0.001 | 0.02 | 0.9 |
| FCGR3B | 0.308 | <0.001 | 0.253 | <0.001 | 0.089 | 0.14 | 0.17 | 0.29 | |
| CSF1R | 0.603 | <0.001 | 0.537 | <0.001 | 0.31 | <0.001 | 0.036 | 0.82 | |
| Neutrophil | CD66b | -0.13 | 0.0054 | -0.111 | 0.0256 | 0.023 | 0.7 | -0.19 | 0.22 |
| FUT4 | -0.074 | 0.113 | -0.078 | 0.116 | -0.13 | 0.034 | -0.34 | 0.032 | |
| ITGAM | 0.611 | <0.001 | 0.563 | <0.001 | 0.25 | <0.001 | 0.38 | 0.015 | |
| Natural killer cell | XCL1 | 0.255 | <0.001 | 0.208 | <0.001 | 0.13 | 0.029 | -0.14 | 0.37 |
| CD7 | 0.305 | <0.001 | 0.168 | <0.001 | 0.052 | 0.39 | -0.41 | 0.0086 | |
| KIR3DL1 | 0.178 | <0.001 | 0.112 | 0.0244 | 0.013 | 0.83 | 0.053 | 0.74 | |
| Dendritic cell | CD1C | 0.495 | <0.001 | 0.426 | <0.001 | 0.24 | <0.001 | 0.01 | 0.67 |
| CD14 | 0.564 | <0.001 | 0.488 | <0.001 | 0.25 | <0.001 | 0.02 | 0.9 | |
| CD11c | 0.607 | <0.001 | 0.538 | <0.001 | 0.2 | 0.001 | -0.26 | 0.095 | |
The [bold] in the table refered to that the immune markers of treg cells, macrophages, monocyte and dendritic cells were significantly associated with HSPB8 expression.
Figure 6.
The relationships between HSPB8 expression and tumor immune cell infiltration. The increased expression level of HSPB8 was significantly correlated to the infiltration of Treg cells, macrophages, monocyte and dendritic cells (p < 0.05).
3. Discussion
Since the 1950s, 5-FU-based chemotherapy has obtained to be the mainstay of therapy for CRC patients. Although combinations of FOLFOX or FOLFIRI have conferred survival benefits greater than the use of 5-FU alone, nearly half of CRCs are resistant to 5-FU-based chemotherapies [8]. Therefore, identifications on the characterization of the biological factors involved in mediating resistance to 5-FU based therapy are urgently needed. In this study, 34 differentially expressed ARGs associated with CRC progression in 473 CRC tissues and 41 adjacent normal tissues were screen from TCGA database and found that HSPB8 was important in CRC 5-FU resistant. Due to the recruitment of CAFs, HSPB8 expression was enhanced in CRC cells so that HSPB8 acted together with its co-chaperone BAG3 promoting autophagy drived 5-FU resistance. Furthermore, the augmented expression level of HSPB8 was found to be significantly correlated to the immune cell infiltration such as Treg cells, macrophages, monocyte and dendritic cells and so on.
5-FU is a heterocyclic aromatic organic compound which is similar to the pyrimidine molecules of DNA and RNA. Therefore, 5-FU can induce cytotoxicity and tumor cell death by interfering with the metabolism of nucleoside and being incorporated into RNA and DNA [11]. Autophagy, a self-degradative mechanism, maintains cellular homeostasis and cell survival under both physiological and pathological conditions by removing damaged DNA, dysfunctional proteins and defective organelles [12]. In addition to its housekeeping roles, autophagy also involves in protecting tumor cells from anticancer agents such as 5-FU [13]. Accumulating evidence illustrated that a suppression of autophagy would greatly enhances 5-FU induced tumor cell death [14]. In this study, 34 abnormal expressed ARGs were identified and analyzed their functions including GO analysis and KEEG pathways. Preliminary analysis showed that the expression of these ARGs is mostly enriched in ErbB signaling pathway, p53 signaling pathway, HIF-1 signaling pathway, PI3K-Akt signaling pathway which functioned as negative regulator of apoptotic process. Further analysis of AGRs in single cancer cell function by CancerSEA showed a consistent result that theses ARGs played an important role in tumor cell DNA repair and maintaining cell stemness and cell cycle.
HSPB8, a member of the HSPBs, is deeply involved in the modulation of the appearance and the progression of many types of cancer in humans [15, 16, 17]. The hypothesis that many of the HSPB8 effects in tumors are due to its ability to facilitate autophagy [18]. Early studies had also evidenced the key role of up-regulated HSPB8 in the development of tamoxifen resistance in breast cancer by inducing cell autophagy [19]. In this study, the chemoresistance role of HSPB8 was also found that CRC cells with higher 5-FU IC50 usually company with an increased HSPB8 expression and patients with up-regulated HSPB8 inevitably showed a poor prognosis of CRC not only in OS but also RFS. Early studies reported in cooperation with BAG3, the chaperone activity of HSPB8 could deliver the misfolded proteins to the autophagy machinery [20, 21]. Fortunately, the strong combination between HSPB8 and BAG3 expression was also found in this study which positive regulate cell autophagy. By the way, a dual role of HSPB8 was found that CRC patients usually showed a lower HSPB8 expression than normal tissues but patients with lower HSPB8 expression showed a higher 5-FU sensitivity and better outcome than other patients. By reviewing existing studies, HSPB8 activity could be either beneficial or detrimental for cancer cell growth, migration, and death [18]. The opposite effects were also found in CHAC1 of kidney renal cell (KIRC) carcinoma which is down-regulated in KIRC samples when compared with normal samples but up-regulated in KIRC samples with relatively higher malignancy and later stages [22]. However, the mechanism of these controversial findings still remained unclear.
CAFs, as an important part of the tumor microenvironment (TME), play a key role in cancer progression by contributing to extracellular matrix deposition and remodeling, extensive crosstalk with cancer cells [23]. In this study, HSPB8 was showed to be mainly enriched in CAFs compared with other TME cells and an enhanced HSPB8 expression in CRC cells was also found to be correlated to higher CAFs infiltration. Additionally, we observed a significant co-expression of HSPB8 and TGF- β in CRC tissues whereas correlation between them was not detected in normal tissues. As present in recent studies, TGF- β released by tumor cells could recruit CAFs which played an important role in crosstalk between cancer cells and fibroblasts [24]. Hence, our results indicated the increased HSPB8 expression in CRC cells might due to the recruiting of CAFs by TGF- β.
The tumor microenvironment (TME), comprising cellular components, such as cancer-associated fibroblasts (CAFs), immune cells, endothelial cells and adipocytes, and noncellular components such as extracellular matrix (ECM), has been recognized as a critical contributor to the treatment resistance [25, 26]. Except the noticeable association between CAFs infiltration and HSPB8 expression, the correlation between the infiltration of various immune cells and HSPB8 expression was also detected. Fortunately, the treg cells, macrophages, monocyte and dendritic cells infiltration were significantly associated with HSPB8 expression. As presented, interaction between CAFs and adaptive immune cells in the TME is known to have crucial functions in the restriction of antitumor immunity [27]. Macrophages infiltrating tumors, known as tumor associated macrophages (TAMs), are classified into two different types M1 and M2. M1-type macrophages behave as an antitumor role whereas M2-type macrophages exhibit tumor-promoting activity by contributing to the activation of tumor angiogenesis, immune suppression, invasion and metastasis of cancer cells [28]. Consistently, a strong association of HSPB8 expression and M2-type macrophages was also observed in this study.
On general, HSPB8 among 34 differentially expressed ARGs in CRC was identified to be the hub ARGs in 5-FU resistant which was down-regulated in CRC samples when compared with normal samples but up-regulated in CRC samples with relatively higher lymphatic invasion, later stages and poor prognosis of CRC. Mechanistic analysis demonstrated that due to the recruitment of CAFs, HSPB8 expression was enhanced in CRC cells so that HSPB8 acted together with its co-chaperone BAG3 promoting autophagy drived 5-FU resistance. Furthermore, the augmented expression level of HSPB8 was found to be significantly correlated to the immune cell infiltration such as Treg cells, macrophages, monocyte and dendritic cells and so on. In conclusion, HSPB8 was found to be a valid indicator for poor prognosis of CRC patients with 5-FU therapy. All these results suggested CAFs driving HSPB8 induced CRC 5-FU resistance by promoting tumor autophagy would provide a new strategy in seeking potential CRC therapeutic targets.
4. Materials and methods
4.1. Identify differently expressed ARGs in CRC
232 ARGs were obtained from the HADb (Human Autophagy Database, http://www.autophagy.lu/) and transcriptome profiling data were downloaded from TCGA Data Portal (https://tcga-data.nci.nih.gov/tcga/). Then ARGs between CRC and normal samples were selected basing on a t-test of Linear Models for Microarray Analysis package in R [29]. The fold-change (FC) of the gene expression was also calculated. The threshold criteria for the ARGs selection were P < 0.05 and |log2FC| ≥1.
4.2. Functional and pathway enrichment analysis of ARGs
Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis was conducted for the selected genes by online tools of the Database for Annotation, Visualization and Inter-grated Discovery (DAVID, https://david.ncifcrf.gov/) [30]. The cut-off value for the screening of significant functions and pathways was FDR<0.05 as statistical significance. The distinct functional states of ARGs in cancer cells at single-cell resolution were analyzed on CancerSEA [31].
4.3. The identification of hub ARGs in CRC 5-FU resistance
The 5-FU response data of CRC cells and genomic information were obtained from Genomics of Drug Sensitivity in Cancer (GDSC, https://www.cancerrxgene.org/) and Cancer Cell Line Encyclopedia (CCLE, https://sites.broadinstitute.org/ccle). The hub ARGs were identified by an overlap of comparing the obtained ARGs RNA-seq data from CCLE and TCGA.
4.4. Validation of the hub ARGs in TCGA database
The expression data of hub ARGs and their correlation to CRC prognosis were validated by Gene Expression Profiling Interactive Analysis (GEPIA) [32]. The protein expression of ARGs detected by immunohistochemistry (IHC) was obtained from The Human Protein Atlas (HPA, https://www.proteinatlas.org/). Moreover, the association between hub ARGs expression and tumor histological type, stage, TNM, lymphatic invasion, microsatellite instability (MSI) and KRAS mutation were processed by Xena [33].
4.5. Protein-protein interaction (PPI) network construction and module analysis
Search Tool for the Retrieval of Interacting Genes (STRING) database was conducted to predict the potential interactions amongst the identified ARGs from the protein level. Only the interactions containing at least one DEG were filtered out to build the PPI network, with the criterion of a combined score of >0.9. Then GeneMANIA was further applied to construct the gene-gene interaction network between ARGs and target proteins [34].
4.6. ARGs exploration in tumor microenvironment (TME)
Tumor Immune Single-cell Hub (TISCH) was firstly processed to analyze the hub ARGs expression visualization across multiple datasets at the single-cell level in CRC tissues [35]. The signifcant functional states were identified with P < 0.05 and correlation >0.2. Then the association between hub ARGs expression and immune cells was verified by TIMER with p < 0.05 [36].
Declarations
Author contribution statement
Tianyi Gao: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Dan Yuan: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Bangshun He: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Yingdong Gao: Analyzed and interpreted the data.
Caidong Liu and Huilin Sun: Performed the experiments.
Junjie Nie and Shukui Wang: Contributed reagents, materials, analysis tools or data.
Zhenlin Nie: Conceived and designed the experiments; Analyzed and interpreted the data.
Funding statement
This work was supported by the National Nature Science Foundation of China (No. 81903034) and the development of Nanjing medical science and technology foundation to Tianyi Gao (no. YKK17123).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
supplementary figure 1.
The colony assay of CRC cells with HSPB8 mRNA knock down. Hct-8 and Hct-116 cells were separated into three groups and cultured with 5-FU for 10 days. As present, CRC cells transfected with HSPB8 siRNA showed a significant lower colony forming efficiency than other groups (p<0.05). NC: CRC cells transfected with negative control; HSPB8 KD: CRC cells transfected with HSPB8 siRNA; Blank: CRC cells transfected with nothing.
Supplementary figure 2.
The association between HSPB8 expression and TNM stage of CRC patients. a: The results of the association between HSPB8 expression and invasion of primary tumor; b: The results of the association between HSPB8 expression and the numbers of lymph nodes; c: the resuts of the association between HSPB8 expression and tumor metastasis. All the results demonstrated HSPB8 expression had no relation to CRC TNM stage (p>0.05).
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.García-Alfonso P., Muñoz Martín A.J., Ortega Morán L., Soto Alsar J., Torres Pérez-Solero G., Blanco Codesido M., Calvo Ferrandiz P.A., Grasso Cicala S. Oral drugs in the treatment of metastatic colorectal cancer. Ther Adv Med Oncol. 2021;13 doi: 10.1177/17588359211009001. 17588359211009001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marin J.J.G., Macias R.I.R., Monte M.J., Herraez E., Peleteiro-Vigil A., Blas B.S., Sanchon-Sanchez P., Temprano A.G., Espinosa-Escudero R.A., Lozano E., Briz O., Romero M.R. Cellular mechanisms accounting for the refractoriness of colorectal carcinoma to pharmacological treatment. Cancers. 2020;12(9):2605. doi: 10.3390/cancers12092605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo D., Zhang Y., Yang S., Tian X., Lv Y., Guo Z., Liu X., Han G., Liu S., Wang W., Cui S., Qu X., Wan S. Design, synthesis and biological evaluation of sphingosine-1-phosphate receptor 2 antagonists as potent 5-FU-resistance reversal agents for the treatment of colorectal cancer. Eur. J. Med. Chem. 2021;225:113775. doi: 10.1016/j.ejmech.2021.113775. [DOI] [PubMed] [Google Scholar]
- 5.Mukhopadhyay S., Mahapatra K.K., Praharaj P.P., Patil S., Bhutia S.K. Recent progress of autophagy signaling in tumor microenvironment and its targeting for possible cancer therapeutics. Semin. Cancer Biol. 2021;S1044–579X(21) doi: 10.1016/j.semcancer.2021.09.003. 00227-3. [DOI] [PubMed] [Google Scholar]
- 6.Mizushima N., Levine B. Autophagy in human diseases. N. Engl. J. Med. 2020;383(16):1564–1576. doi: 10.1056/NEJMra2022774. [DOI] [PubMed] [Google Scholar]
- 7.Silva V.R., Neves S.P., Santos L.S., Dias R.B., Bezerra D.P. Challenges and therapeutic opportunities of autophagy in cancer therapy. Cancers. 2020;12(11):3461. doi: 10.3390/cancers12113461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J., Na R., Xiao C., Wang X., Wang Y., Yan D., Song G., Liu X., Chen J., Lu H., Chen C., Tang H., Zhuang G., Fan G., Peng Z. The loss of SHMT2 mediates 5-fluorouracil chemoresistance in colorectal cancer by upregulating autophagy. Oncogene. 2021;40(23):3974–3988. doi: 10.1038/s41388-021-01815-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu F., Yang J., Liu J., Wang Y., Mu J., Zeng Q., Deng S., Zhou H. Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer. Signal Transduct Target Ther. 2021;6(1):218. doi: 10.1038/s41392-021-00641-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Budithi A., Su S., Kirshtein A., Shahriyari L. Data driven mathematical model of FOLFIRI treatment for colon cancer. Cancers. 2021;13(11):2632. doi: 10.3390/cancers13112632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho Y.H., Ro E.J., Yoon J.S., Mizutani T., Kang D.W., Park J.C., Il Kim T., Clevers H., Choi K.Y. 5-FU promotes stemness of colorectal cancer via p53-mediated WNT/β-catenin pathway activation. Nat. Commun. 2020;11(1):5321. doi: 10.1038/s41467-020-19173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan X., Zhang Z., Liu P., Yao H., Shen L., Tong J.S. Inhibition of EZH2 enhances the therapeutic effect of 5-FU via PUMA upregulation in colorectal cancer. Cell Death Dis. 2020;11(12):1061. doi: 10.1038/s41419-020-03266-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Levy J.M.M., Towers C.G., Thorburn A. Targeting autophagy in cancer. Nat. Rev. Cancer. 2017;17(9):528–542. doi: 10.1038/nrc.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghafouri-Fard S., Abak A., Tondro Anamag F., Shoorei H., Fattahi F., Javadinia S.A., Basiri A., Taheri M. 5-Fluorouracil: a narrative review on the role of regulatory mechanisms in driving resistance to this chemotherapeutic agent. Front. Oncol. 2021;11:658636. doi: 10.3389/fonc.2021.658636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen W., Hu M.J., Zhong X.L., Ji L.H., Wang J., Zhang C.F., Zhang R., Lin H.M. Screening of a novel autophagy-related prognostic signature and therapeutic targets in hepatocellular carcinoma. J. Gastrointest. Oncol. 2021;12(6):2985–2998. doi: 10.21037/jgo-21-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X., Zhang H., Liu J., Li P., Sun Y. Five crucial prognostic-related autophagy genes stratified female breast cancer patients aged 40-60 years. BMC Bioinf. 2021;22(1):580. doi: 10.1186/s12859-021-04503-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng D., Jin H., Zhang X., Yan W., Xia Q., Shen S., Xie S., Cui M., Ding B., Gu Y., Wang S. Identification of autophagy-related risk signatures for the prognosis, diagnosis, and targeted therapy in cervical cancer. Cancer Cell Int. 2021;21(1):362. doi: 10.1186/s12935-021-02073-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cristofani R., Piccolella M., Crippa V., Tedesco B., Montagnani Marelli M., Poletti A., Moretti R.M. The role of HSPB8, a component of the chaperone-assisted selective autophagy machinery, in cancer. Cells. 2021;10(2):335. doi: 10.3390/cells10020335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi J.J., Chen S.M., Guo C.L., Li Y.X., Ding J., Meng L.H. The mTOR inhibitor AZD8055 overcomes tamoxifen resistance in breast cancer cells by down-regulating HSPB8. Acta Pharmacol. Sin. 2018;39(8):1338–1346. doi: 10.1038/aps.2017.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klimek C., Jahnke R., Wördehoff J., Kathage B., Stadel D., Behrends C., Hergovich A., Höhfeld J. The Hippo network kinase STK38 contributes to protein homeostasis by inhibiting BAG3-mediated autophagy. Biochim. Biophys. Acta Mol. Cell Res. 2019;1866(10):1556–1566. doi: 10.1016/j.bbamcr.2019.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li F., Xiao H., Hu Z., Zhou F., Yang B. Exploring the multifaceted roles of heat shock protein B8 (HSPB8) in diseases. Eur. J. Cell Biol. 2018;97(3):216–229. doi: 10.1016/j.ejcb.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Li D., Liu S., Xu J., Chen L., Xu C., Chen F., Xu Z., Zhang Y., Xia S., Shao Y., Wang Y. Ferroptosis-related gene CHAC1 is a valid indicator for the poor prognosis of kidney renal clear cell carcinoma. J. Cell Mol. Med. 2021;25(7):3610–3621. doi: 10.1111/jcmm.16458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu F., Yang J., Liu J., Wang Y., Mu J., Zeng Q., Deng S., Zhou H. Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer. Signal Transduct Target Ther. 2021;6(1):218. doi: 10.1038/s41392-021-00641-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao H., Wei J., Sun J. Roles of TGF-β signaling pathway in tumor microenvirionment and cancer therapy. Int. Immunopharm. 2020;89(Pt B):107101. doi: 10.1016/j.intimp.2020.107101. [DOI] [PubMed] [Google Scholar]
- 25.Mehraj U., Ganai R.A., Macha M.A., Hamid A., Zargar M.A., Bhat A.A., Nasser M.W., Haris M., Batra S.K., Alshehri B., Al-Baradie R.S., Mir M.A., Wani N.A. The tumor microenvironment as driver of stemness and therapeutic resistance in breast cancer: new challenges and therapeutic opportunities. Cell. Oncol. 2021;44(6):1209–1229. doi: 10.1007/s13402-021-00634-9. [DOI] [PubMed] [Google Scholar]
- 26.Becerril-Rico J., Alvarado-Ortiz E., Toledo-Guzmán M.E., Pelayo R., Ortiz-Sánchez E. The cross talk between gastric cancer stem cells and the immune microenvironment: a tumor-promoting factor. Stem Cell Res. Ther. 2021 Sep 9;12(1):498. doi: 10.1186/s13287-021-02562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mao X., Xu J., Wang W., Liang C., Hua J., Liu J., Zhang B., Meng Q., Yu X., Shi S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol. Cancer. 2021;20(1):131. doi: 10.1186/s12943-021-01428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He Z., Zhang S. Tumor-associated macrophages and their functional transformation in the hypoxic tumor microenvironment. Front. Immunol. 2021;12:741305. doi: 10.3389/fimmu.2021.741305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng C., Ouyang Y., Lu N., Li N. The NF-κB signaling pathway, the microbiota, and gastrointestinal tumorigenesis: recent advances. Front. Immunol. 2020;11:1387. doi: 10.3389/fimmu.2020.01387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dennis G., Jr., Sherman B.T., Hosack D.A., Yang J., Gao W., Lane H.C., Lempicki R.A. DAVID: database for annotation, visualization, and integrated Discovery. Genome Biol. 2003;4(5):P3. [PubMed] [Google Scholar]
- 31.Yuan H., Yan M., Zhang G., Liu W., Deng C., Liao G., Xu L., Luo T., Yan H., Long Z., Shi A., Zhao T., Xiao Y., Li X. CancerSEA: a cancer single-cell state atlas. Nucleic Acids Res. 2019 Jan 8;47(D1):D900–D908. doi: 10.1093/nar/gky939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang Z., Li C., Kang B., Gao G., Li C., Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98–W102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldman M.J., Craft B., Hastie M., Repečka K., McDade F., Kamath A., Banerjee A., Luo Y., Rogers D., Brooks A.N., Zhu J., Haussler D. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 2020;38(6):675–678. doi: 10.1038/s41587-020-0546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warde-Farley D., Donaldson S.L., Comes O., Zuberi K., Badrawi R., Chao P., Franz M., Grouios C., Kazi F., Lopes C.T., Maitland A., Mostafavi S., Montojo J., Shao Q., Wright G., Bader G.D., Morris Q. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010;38(Web Server issue):W214–W220. doi: 10.1093/nar/gkq537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun D., Wang J., Han Y., Dong X., Ge J., Zheng R., Shi X., Wang B., Li Z., Ren P., Sun L., Yan Y., Zhang P., Zhang F., Li T., Wang C. TISCH: a comprehensive web resource enabling interactive single-cell transcriptome visualization of tumor microenvironment. Nucleic Acids Res. 2021;49(D1):D1420–D1430. doi: 10.1093/nar/gkaa1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li T., Fu J., Zeng Z., Cohen D., Li J., Chen Q., Li B., Liu X.S. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020;48(W1):W509–W514. doi: 10.1093/nar/gkaa407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.