Highlights
-
•
Trastuzumab and Pertuzumab extend the overall survival of breast cancer patients.
-
•
Unlike monoclonal antibodies B cell peptides induce immunological memory.
-
•
A multi-peptide B cell-based vaccination prevents Her-2/neu lung metastasis in mice.
-
•
The vaccination results in Her-2/neu-negative tumors, with increased PD-L1 expression.
-
•
A combination/alternating therapy for a total remission of metastases is suggested.
Keywords: Her-2/neu, Breast cancer, Lung metastases, Multi-peptide B cell vaccine, PD-L1
Abstract
In pre-clinical and clinical settings, active immunization with a Her-2/neu vaccine (HerVaxx), comprising B-cell peptide from Trastuzumab binding site, has been shown to reduce primary tumor growth via induction of polyclonal anti-tumor immune responses and immunological memory. Here, we tested the combination of HerVaxx and the recently identified B-cell epitope/mimotope of Pertuzumab, i.e. a multi-peptide B-cell vaccine, for preventing Her-2/neu lung metastases formation in a mouse model. Active immunization with the multi-peptide vaccine was associated with decreased lung weights, and histological evaluation of the lungs showed that the significant reduction of lung metastases was associated with increased CD4+ and CD8+ T cell infiltration. Notably, along with the overall reduction of lungs weights and Her-2 positive metastases, a formation of Her-2/neu-negative tumors but with increased PD-L1 expression was observed. Our results might pave the way to a multi-peptide B-cell Her-2/neu vaccine serving as a secondary intervention in adjuvant settings to prevent tumor recurrence and spread. Moreover, combination therapy targeting PD-L1 may result in total remission of metastases. Such a therapy may be used clinically to alternately target Her-2/neu and PD-L1 in metastatic breast cancer.
Introduction
Her-2/neu overexpression has been observed in approximately 15–20% of patients with breast cancer, and correlated with poor clinical outcome due to an aggressive tumor phenotype and subsequently reduced survival [1]. Due to the association of Her-2/neu with tumor progression and poor prognosis, Her-2/neu is an attractive tumor-associated target antigen for cancer therapy [2,3].
Monoclonal antibodies (mAbs) Trastuzumab and Pertuzumab which target Her-2/neu extracellular subdomains (ECDs) IV and II, respectively, have shown synergistic effects in treatment and subsequently clinical outcome of patients with Her-2/neu overexpressing breast cancer [4]. Thus, their combination has become standard of care for neoadjuvant treatment of early breast cancers as well as for first-line treatment of metastatic Her-2/neu overexpressing breast cancer [5]. Although of impressive therapeutic efficacy, frequent applications in relatively short time intervals from prolonged periods of time as well as financial considerations and toxicity pose significant disadvantages [6,7]. With respect to specificity and clinical applicability, we have earlier developed a B cell peptide-based anti-Her-2/neu vaccine, HerVaxx, containing the carrier protein CRM197 and the adjuvant Montanide, targeting Her-2/neu ECDs III and IV [8], [9], [10]. This formulated vaccine was evaluated in vivo for its anti-tumor effect and showed that active immunization with the vaccine significantly inhibited tumor growth in a mouse syngeneic model with solid tumors following engraftment of BALB/c mammary carcinoma cells expressing human Her-2/neu (D2F2/E2) [11]. In clinical Phase 1b [12] and Phase II trials [13] involving patients suffering from Her-2/neu overexpressing metastatic or advanced adenocarcinoma of the stomach, the vaccine proved to be safe, immunogenic and resulted in a prolonged progression-survival in vaccinated patients who received the vaccine plus chemotherapy, as compared to patients treated with chemotherapy only. The fact that Pertuzumab and Trastuzumab are simultaneously used for the treatment of patients with metastatic Her-2/neu - overexpressing breast cancer prompted us to test our recently identified mimotope/binding epitope of Pertuzumab [14] with or without the HerVaxx vaccine. In a recent study in a solid tumor mouse model, we showed that active immunization with the mimotope of Pertuzumab significantly reduced tumor growth to the same extent as the passive application of the corresponding mAb [14].
We describe here the simultaneous effects of the active administration of B-cell peptides directed against the Her-2/neu ECD IV and II which have been shown the potential to replace passive immunization with Trastuzumab and Pertuzumab by active vaccination, in both early as well as the advanced stage of Her-2/neu-overexpressing breast cancer. We report a significant anti-metastatic effect achieved by combined active mimotope application, as well as its potential for modulation of the disease by addition of immune checkpoint inhibitors.
Materials and methods
Animals and immunization settings
Female BALB/c mice aged 6–8 weeks at the time of delivery were purchased from Charles River (Germany), kept under conventional housing conditions, and used in the experiments detailed below. The experiments were approved by the Animal Experimentation Committee of the Medical University of Vienna and the University of Veterinary Medicine as well as by the Austrian Federal Ministry of Science and Research (BMWF-66.009/0136-WF/V/3b/2017).
In the experimental settings, mice were intraperitoneally injected with Pertuzumab (passive immunization; 100 µg of the mAb/dose), or subcutaneously injected (active immunization) with HerVaxx [[10], [11], [12],14], the recently identified and characterized mimotope of Pertuzumab, JTMP (conjugated to CRM197 and administered with Montanide ISA-51-VG (Seppic, France) [14], or the combination of both peptides (50 µg of each peptide/dose). One day after the first passive immunization or one week after the third active immunization (Fig. 1A), 105 tumor cells were injected into the mice tail-veins. Mice were sacrificed 4 weeks after the injection, and lungs were excised and evaluated for the level of metastasis by measuring the lungs’ weight and by H/E, Her-2/neu, and PD-L1 staining. To examine a possible delayed immunological effect on lung metastasis, the interval between the tumor cells injection and the lungs evaluation was extended to 7 weeks after the tumor cells injection (5 × 104 cells).
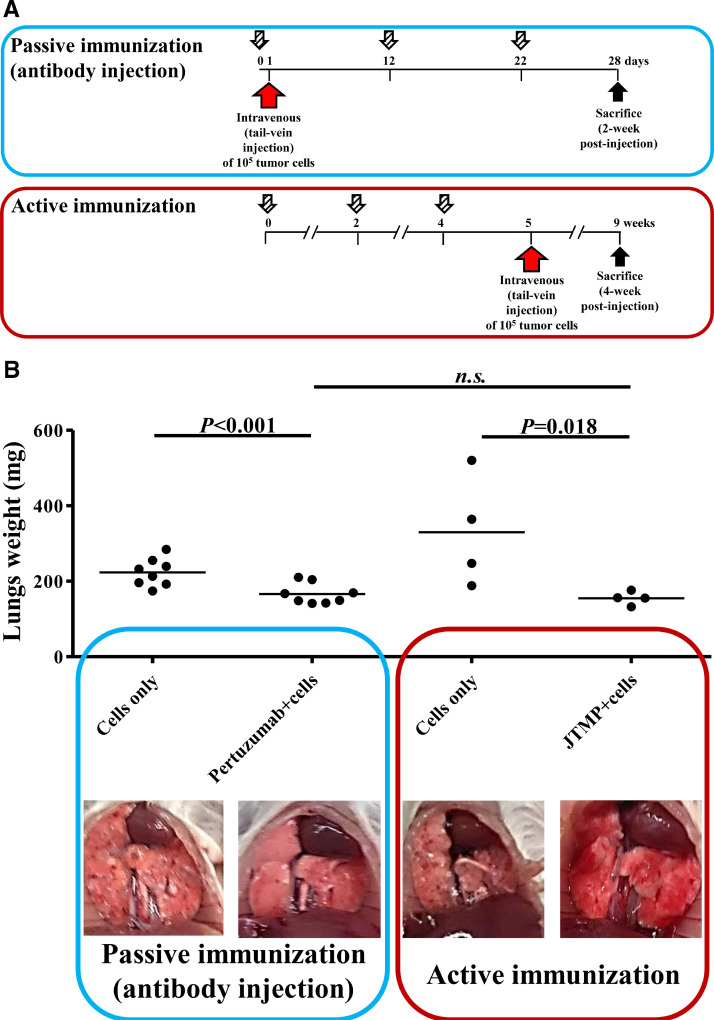
Fig. 1.
A. Immunization schedule for in vivo evaluation of metastasis prevention by Pertuzumab (passive immunization) or JTMP (active immunization). Timeline of the immunizations in the established mouse model of lung metastasis. BALB/c mice either remained non-immunized or were subcutaneously injected with JTMP as depicted (n = 4 per group), followed by tail-vein injection of the mice with 105 tumor cells and the mice sacrifice 4 weeks after. In the passive immunization schedule, mice were either remained non-immunized or intraperitoneally injected with Pertuzumab (n = 8 per group), and one day later tail-veins were injected with 105 tumor cells. The mice were immunized twice more, and sacrificed 4 weeks after the tail-vain injection.
1B The level of metastasis formation prevention in un-immunized or mice passively immunized with Pertuzumab (n = 8, per group), and in un-immunized or mice actively immunized with the mimotope of Pertuzumab, JTMP (14) (n = 4, per group). The prevention of metastases formation in lungs of the immunized mice is shown by bars expressing the weight of the lungs explanted upon sacrifice from all the mice in each group. Corresponding macroscopic images of the lungs, before excision, are shown below each bar. The results are representative of at least two repeated experiments individually involving the passive and active immunization settings. Significant differences are indicated by the respective P values. n.s.: not significant.
Immunohistochemistry and histological analysis
For histological examination, the right lung lobs and brains were excised, fixed in 4% buffered formaldehyde solution and embedded in paraffin wax. 2–3 µm thick sections, covering the entire lungs, were cut on a sliding microtome and stained with H/E. To assess the Her-2/neu and PD-L1 expression, IHC staining using primary antibodies against Her-2/neu (Cell Signaling Technology, Danvers, MA, USA; Cat# 2242, RRID: AB_331,015; dilution 1:100) and PD-L1 (Cell Signaling Technology,; Cat# 64,988, RRID: AB_2,799,672; dilution 1:200) was performed in an autostainer (Lab Vision AS 360, Thermo Scientific, Fremont, USA). IHC to detect T-cells was performed manually using primary rabbit antibodies against CD4 (Cell Signaling Technology, Cat# 25,229, RRID: AB_2,798,898; dilution 1:500) and CD8 (Cell Signaling Technology, Cat# 98,941, RRID: AB_2,756,376; dilution 1:2000). For antigen retrieval, sections were heated either in EDTA buffer pH 9 (for Her-2) or citrate buffer pH6 (for PD-L1, CD4 and CD8). Bright Vision Goat anti-Rabbit HRP-labelled antibody (ImmunoLogic, Cat# DPVR110HRP) was used as a secondary antibody and diaminobenzidine (DAB) as the chromogen. An experienced pathologist blinded to treatment regimens evaluated lungs and brains in a descriptive way with an Olympus BX-53 microscope (Olympus, Tokyo, Japan). Furthermore, the number of metastasis in whole lung sections was counted in H/E staining. The expression of Her-2 was graded in ‘strong to moderate’ or ‘weak to none’ for each metastasis present in the lung sections. . The size of the three largest Her-2-positive and -negative metastases were measured, respectively. For this evaluation an Olympus SC50 Camera was used and metastases were annotated using a 10x objective lens and Olympus CellSens Software (Olympus, Tokyo, Japan). Three images of PD-L1-, CD4- and CD8-stained lung sections were taken respectively with an Olympus DP26 camera (10x objective lens) and evaluated using the open-source software FIJI by ImageJ (RRID: SCR_003070) [15]. The tumor tissue was annotated and the PD-L1-, CD4- and CD8-stained areas were measured using consistent thresholds for each staining. Results were expressed as the percentage of stained area per area of total tumor tissue evaluated for each image. Representative images were taken with the same camera and Adobe Photoshop (RRID: SCR_014199) 2021 was used for white balance and arrangement of images.
Statistical analyses
ELISA data were log-transformed before analysis. Residuals of lung weight did not deviate from normality and were not transformed. These data were analyzed by ANOVA with linear contrasts comparing against the 'cells only' group. The percentage of areas in the metastasized lungs positive for CD4 and CD8 were logit transformed and analyzed with a mixed ANOVA with animal as random factor and experiment and group as fixed factors. The normality of residuals was tested by Kolmogorov-Smirnov tests with Lilliefors' corrected p-values. Homogeneity of variances was tested by Brown-Forsythe tests. Number of metastases per each lung was tested for outliers by Grubb's two-sided outlier test. The areas of Her2/neu positive and negative metastases were analyzed by a similar procedure. The transformation applied, however, was based on the fit of a Johnson's SB distribution.
Results
Active immunization with Pertuzumab mimotope (JTMP) prevented/reduced lung metastasis formation to the same extent as the passive administration of Pertuzumab
Studies have estimated that between 20 to 54% of malignant tumors would give rise to pulmonary metastases [16,17]. In mice, it has been shown that breast cancer metastases mainly occur in the lungs, rather than in the lymph node, liver, bone, or brain [18]. Therefore, for testing the capacity of HerVaxx, JTMP, or their combination in the prevention of Her-2/neu lung metastasis development, a Her-2/neu-expressing lung metastasis in a syngeneic breast cancer mouse model was established, as described in Supplementary Results (Supplementary Fig. S1A-D), by employing the above-mentioned Her-2-expressing D2F2/E2 cells [11].
We have shown that active immunization with the Pertuzumab mimotope (JTMP) significantly reduced solid tumor growth to a similar extent as the application (passive transfer) of the mAb [14]. Therefore, we sought to evaluate the effect of JTMP in the prevention of metastases in the established syngeneic model of lung Her-2/neu metastases, in comparison with the effect of the mAb Pertuzumab.
As shown in Fig. 1A, mice were either actively immunized with the mimotope or passively received Pertuzumab followed by tail-vein tumor cell injection, as described in Materials and Methods.
ELISAs were carried out to examine the level of antibody responses following active immunization with the mimotope. As shown in Supplementary Fig. S2, active immunization resulted in a potent induction of Her-2/neu-specific IgG antibodies compared to sham-immunized control mice.
In the mouse model, application of Pertuzumab was shown to be associated with a significantly lower weight of mice lungs (Fig. 1B). In mice actively immunized with JTMP, a reduction of lung weight and thus prevention/reduction of lung metastases was observed to the same extent as in Pertuzumab-treated mice (Fig. 1B).
Active immunization with the combination of JTMP and HerVaxx induced a strong anti-metastatic effect in a prolonged observation period of 7 weeks
We have earlier demonstrated the ability of HerVaxx to significantly reduce solid tumor growth in a syngeneic tumor mouse model [11]. Here, we evaluated the anti-metastatic effect of the combination of Pertuzumab's mimotope JTMP and HerVaxx in the prevention of lung metastases in the mouse model.
As shown in Supplementary Fig. S3A, mice were actively immunized either with the mimotope (50 µg/dose) or HerVaxx (50 µg/dose) or both (50 µg each/dose) followed by tail-vein injection of 1 × 105 tumor cells. Mice were sacrificed 4 weeks after tumor cell injection.
When compared to unvaccinated mice, active immunization with either JTMP or HerVaxx or their combination induced strong anti-Her-2/neu antibodies (Supplementary Fig. S3B). Despite the strong individual antibody responses to JTMP and HerVaxx, no significant additional increase/additive effect in Her-2/neu-specific IgG production was observed in mice receiving combined vaccination.
As shown in Fig. 2A, active immunization with JTMP, HerVaxx or their combination, was associated with a significant decrease in the lung weight, as compared to control mice who were not treated before the injection of tumor cells (Fig. 2A). No significant difference in the weight of lungs following the three examined treatments was observed. Interestingly, H/E staining of the lungs showed smaller metastases in lungs from actively immunized groups of mice (Fig. 2A; a-e).
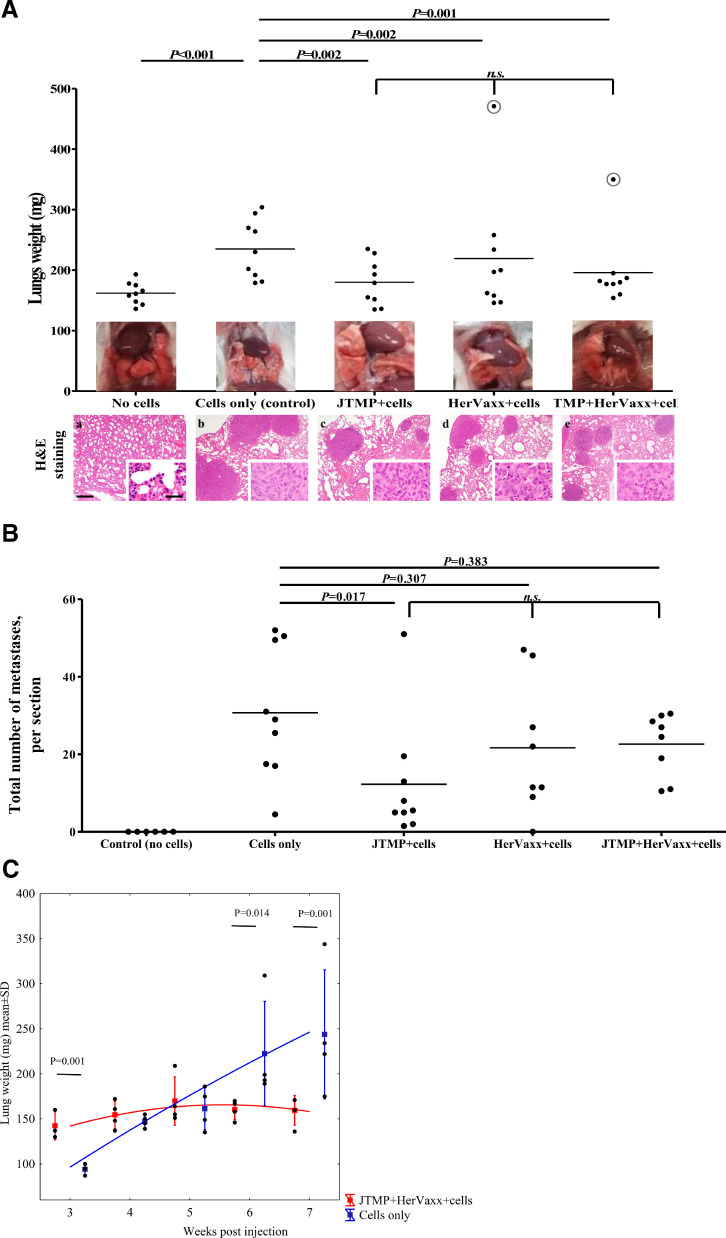
Fig. 2.
A. The level of metastasis formation prevention in the different examined groups of mice, based on lungs weight, 4 week post-injection. The level of prevention of metastasis development in mice (n = 9; sum of two independent experiments) after the examined treatments, or in the control mice, is shown based on the weight of the entire lungs explanted upon the mice sacrifice. The values circled in gray, from one mouse immunized with HerVaxx and one with the combination of JTMP and HerVaxx, were determined as outliers by Grubb's two-sided outlier test and excluded from the analysis. Corresponding macroscopic images of representative lungs, before excision, are also shown. Representative images of lungs of control and all treatment groups in H/E staining (a-e). a: Normal lung tissue without any signs of metastasis; b: In the control group multiple metastasized tumors, some of them confluent, are detectable; c, d, e: A reduced number of metastases are detectable as a result of the treatments. bar = 300 µm. Inserts show higher magnifications of tumors; bar = 30 µm.
2B The level of metastasis formation prevention in the different examined groups of mice, based on the number of metastases, 4 weeks post-injection. The number of metastases in the entire lung sections of all the mice (n = 9; sum of two independent experiments) in each group is shown.
2C. The level of the metastasis formation prevention in the different examined groups of mice (n = 40; n = 4 at each time point), based on lungs weight, at the different examined weeks following tumor cells injection. The level of prevention of lung metastasis development is shown in a long-term experiment setting as the kinetics of the lungs’ weight in mice (n = 4) either remained unimmunized (control) or were immunized with JTMP+HerVaxx. A constant increase of the lungs’ weight from the control mice (blue line) is shown, whereas a significant decrease of the lungs’ weight in the vaccinated mice is shown starting at the 6-week post-injection time point. Significant differences are indicated by the respective P values. n.s.: not significant.
In order to examine whether reduced lung weight correlated with a reduced number of metastases, histological evaluations by H/E staining were carried out. As shown in Fig. 2B, active immunization with the JTMP mimotope was shown to result in a significantly reduced number of metastasized tumors in the mice lungs, as compared to the untreated and cells-only control group. A reduced number of metastasized tumors was also shown to be associated with active immunization with HerVaxx alone or in combination with JTMP, but less pronounced, as compared to the effect induced by JTMP (Fig. 2B). These results show the capacity of the mimotopes JTMP and HerVaxx, alone or in combination, to reduce/prevent lung metastasis formation.
To examine a potential delayed immunological effect of the vaccination on metastasis formation, the observation period was prolonged in a subgroup of mice until up to 7 weeks after tumor cell injection. As shown in Fig. 2C, in immunized mice a significant and persistent reduction of lungs weight was shown to be present at the 6-week post-injection time point.
These results suggested an enhanced immunological effect and a possible synergistic anti-tumor effect following vaccination with JTMP and HerVaxx. The results further indicated the consequent prevention of metastasis formation by extending the interval between tumor cell injection and evaluation of the presence of lung metastases.
Active immunization with JTMP was associated with increased infiltration of CD4+ and CD8+ T-cells
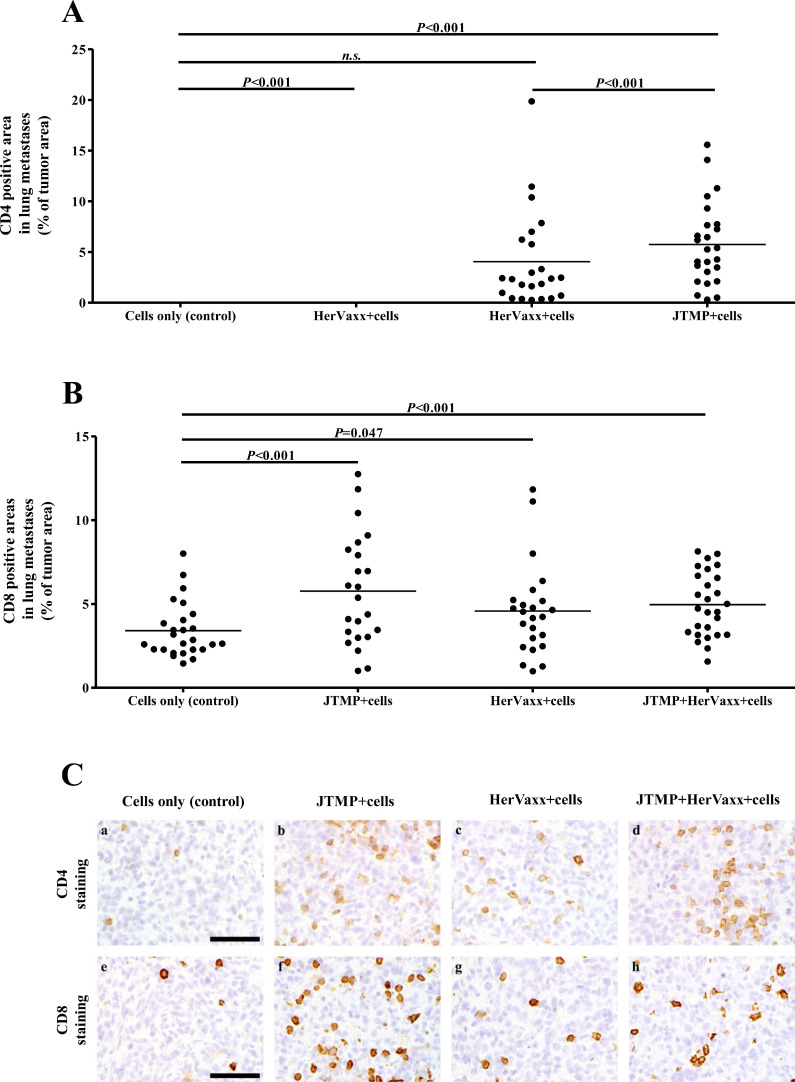
To examine whether the observed anti-metastatic effect following active immunization with JTMP was associated with tumor infiltration by lymphocytes in the tumor microenvironment, IHC staining for the evaluation of percentages of CD4+ and CD8+ T-cells in metastasized tumors was carried out. A significant increase in numbers of infiltrating CD4+ and particularly CD8+ T-cells was observed in lungs of mice actively immunized with the mimotope JTMP alone or in combination with HerVaxx, when compared to control mice (Fig. 3A-C; a, b, d, e, f, h), whereas T-cell infiltration was less prominent in lungs of mice immunized with HerAxx alone (Fig. 3A-C; a, c, e, g). These results indicated that active immunization with either JTMP or JTMP+HerVaxx, but to a lesser extent with HerVaxx alone, strongly induced infiltration of CD4+ and CD8+ T-cells thus further supporting their role in cancer cell growth.
Fig. 3.
A-C. The levels of CD4 and CD8 positive T cells infiltration in the metastases, 4 weeks post-injection. Up to 3 detected areas with CD4 (A) and CD8 (B) T-cells in the evaluated lungs, from all mice, shown as the percentage (%) of tumor areas (stained area/area of metastases). Representative images of tumor metastases in lungs of control and all treatment groups (C) in CD4 (a-d) and CD8 (e-h) staining. a, e: Control group with few CD4 and CD8 positive lymphocytes infiltrating into the tumor tissue; b, f: Significant increase in CD4 and CD8 positive T-cells in the tumor tissue after JTMP treatment; c, g: Significantly more CD8 positive infiltrating lymphocytes after HerVaxx treatment compared to control, no significant increase in CD4 positive T-cells; d, h: In the group treated with the combination of HerVaxx and JTMP a significant increase in both, CD4 and CD8 positive T-cells, within the tumor tissue was present; bar = 50 µm.
Active immunization with the combination of HerVaxx and JTMP was associated with reduced Her-2/neu expression, but increased expression of PD-L1 on metastatic tumor cells
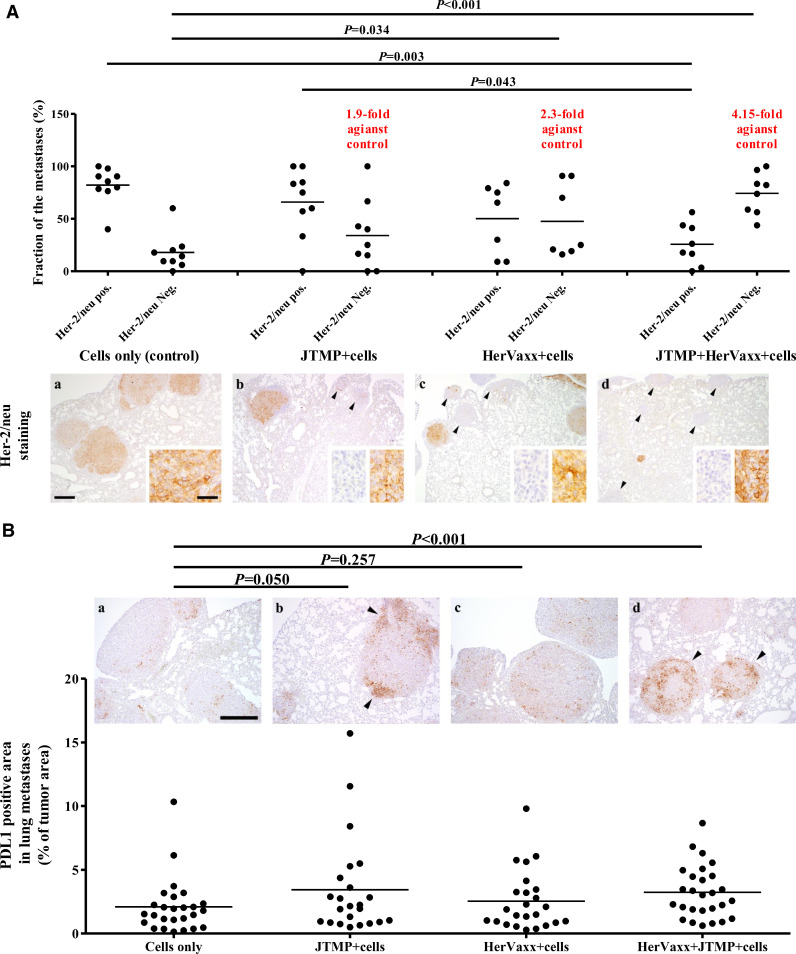
Loss of Her-2 expression in adjuvant settings after treatment with Trastuzumab has been widely reported. To examine this observation also in our model, Her-2/neu IHC staining of metastasized lungs was carried out. Following active immunization with the mimotope JTMP, a 1.9-fold increase of the fraction of metastasized tumors without Her-2/neu expression was observed, as compared to control mice (Fig. 4A). However, the fraction of Her-2/neu negative metastases was shown to be significantly higher following active immunizations with HerVaxx alone (2.3-fold) or in combination with the JTMP mimotope (4.15-fold) (Fig. 4A). This was correlated with a decrease in the number of Her-2/neu positive metastases. These observations were supported by Her-2/neu staining of lungs which showed more Her-2/neu negative metastases in the HerVaxx- or Her-Vaxx plus JTMP-treated groups (Fig. 4A; a, b, c, d). These results indicated that active immunization with the combination of JTMP and HerVaxx mimotopes led to the strongest reduction of Her-2 overexpressing metastases, but – conversely - to an increase of Her-2 negative metastases.
Fig. 4.
A. The level of Her-2/neu expression in metastases, 4 weeks post-injection, following active immunization with JTMP, a multiple B cell epitope anti-Her2/neu vaccine (HerVaxx), or with the combination of both antigens. The fraction of Her2/neu negative and positive metastases (% of all metastases in the respective lung section) (n = 9; sum of two independent experiments) after immunization with JTMP, HerVaxx, and the combination of both are shown. The average fold-increase of the number of Her-2/neu negative metastases is indicated for each examined treatment. Representative images of lungs after Her-2/neu are shown for the respective treatments. a: lung tissue with moderate Her-2/neu expression (brown signal); c, d, e: metastases with no expression of Her-2/neu are indicated with arrowheads, and their correlation with the fold increase values; bar = 300 µm. Inserts show higher magnifications of tumors with strong or no Her-2 expression, respectively; bar = 30 µm. Significant differences are indicated by the respective P values. n.s.: not significant.
4B The areas in the metastasized lungs with positive signal for PD-L1, 4 weeks post-injection. Up to 3 areas in each evaluated lung section/mouse, after PD-L1 staining, are shown as the percentage (%) of positive areas (stained area/area of metastases). Representative images of tumor metastases in lungs of control and all the treatment groups with PD-L1 staining are shown. a: Control group with few PD-L1 expressing cells; b: Strong expression in the periphery of one metastasis (arrowheads). c: Mild increase in PD-L1 expression in tumor metastases; d: Strong PD-L1 expression in two of three metastases (arrowheads); bar = 300 µm.
Referring to a recent study which has shown that the expression of Her-2/neu upon tumor cells derived from patients with non-small-cell lung cancer would be inversely correlated with PD-L1 expression [19], this aspect was also examined in the current animal model by examining mouse lungs by IHC for PD-L1 expression. As shown in Fig. 4B, a significant increase of PD-L1 expression was observed in the lung metastases of mice actively immunized with both JTMP and HerVaxx mimotopes. The significant increase of PD-L1 expression in the mimotope-treated group correlated with the significant increase of Her-2 negativity of metastatic tumor cells (Fig. 4A).
A further examination of the three largest Her-2/neu positive and negative metastases in the lungs from all the groups revealed that, compared to the control (cell-only) group, the combination treatment had resulted in a significantly reduced size of the evaluated Her-2/neu positive metastases (Supplementary Fig. S4C). The examination further showed a reduced size of Her-2/neu negative metastases compared to those expressing the receptor, in the same group (Supplementary Fig. S4C); we can speculate that the smaller size of the Her-2/neu negative metastases may be attributed to the role of the receptor in the proliferation of the tumor cells expressing the receptor [20].
When taken together, these results indicated an association between increased numbers of Her-2/neu negative metastatic tumor cells in mimotope-treated animals - particularly following active immunization with the combination of JTMP and HerVaxx - and increased PD-L1 expression.
Discussion
Visceral metastatic disease in breast cancer constitutes a prognostically detrimental situation [21]. In Her-2/neu-overexpressing breast cancer, metastatic disease has been impressively proved to be both, avoidable as well treatable by the combination of chemotherapy and Her-2/neu-directed treatment by mAbs Trastuzumab and Pertuzumab. The combined strategy is hampered, however, by the development of resistance to treatments with mAbs [22], which may potentially be due to the mAbs administered at high doses to ensure their immediate bioavailability and potency [23,24], and cardiotoxicity [25]. In the current study, we have developed this strategy one step further by asking whether a multi-peptide B cell vaccine harboring our clinically-proven Her-2/neu vaccine (HerVaxx) [10,12,13] plus a recently identified mimotope/B cell epitope of Pertuzumab, JTMP [14], for its capacity to prevent lung metastases from Her-2/neu overexpressing breast cancer cells by the using a mouse model in a pre-clinical setting.
Active immunization of mice with the mimotope of Pertuzumab significantly reduced lung metastases to a similar extent as passive immunization with the mAb, reflected by both reduced lungs weight and the number of metastases. This corresponded with a significant lung weight reduction in mice actively immunized with HerVaxx alone or in combination with JTMP. While lungs from mice immunized with the multi-peptide B cell vaccine consisting of HerVaxx and the mimotope of Pertuzumab had the lowest mass, the number of metastases was notably not significantly lower than in lungs derived from control animals. This might be explained by the short period between tumor cell injection and the assessment of lung metastases; by increasing the time interval, we have found a significantly lower number of lung metastases in mice vaccinated with JTMP and HerVaxx, as compared to controls, thus suggesting a long-term immunological effect and its potential enhancement (Fig. 2C). An additional explanation for this phenomenon might be attributed to the strong capacity of vaccine-induced anti-HerVaxx or anti-JTMP antibodies [14] to inhibit phosphorylation of intracellular Her-2/neu and downstream signaling pathways, e.g. AKT, which, in turn, may result in non-proliferative/dormant tumor cells. An in vivo dormant status was suggested to be associated with low levels of ERK and high levels of p38, an inhibitory regulator of ERK [26], resulting in tumor cells incapable of proliferation without increased apoptosis [27].
CD4+ T-cells primarily mediate anti-tumor immunity by providing help for CD8+ cytotoxic T lymphocytes and antibody responses [28]. Based on the IHC staining of the metastasized lungs, our results indicated that active immunization with HerVaxx and JTMP - alone or in combination, induced infiltration of both, CD4+ and CD8+ T-cells into metastasized lungs. Peptides used for immunizations in our study were conjugated to the carrier protein CRM197 and administered together with the adjuvant Montanide [10]. It has been shown that CD4+ T-cells were needed not only for optimal CD8+ T-cell activation but also at the effector stage within the tumor microenvironment [29]. Therefore, our results may also indicate a role of both, CD4+ and CD8+ T-cells to prevent the development of metastases following active immunization with the multi-peptide B cell vaccine. However, at the current stage, it is unknown whether infiltrating CD4+ and CD8+ T-cells are peptide- or carrier-specific.
A potential mechanism of Trastuzumab resistance is attributed to the mAb's capacity to upregulate expression of PD-L1 by the recruitment of immune effector cells and the stimulation of IFNγ secretion [30]. IHC staining of the metastasized lungs in our study showed an increased level of PD-L1 expression by metastatic cells which has developed after active immunization with the combination of HerVaxx and Pertuzumab mimotopes, but to a lesser extent achieved by each peptide alone in comparison with controls. At this point, we cannot say whether the loss of Her-2/neu is associated with increased PD-L1 expression in identical tumor cells of the assessed metastases.
It has been reported that over-expression of Her-2/neu is associated with downregulation of MHC-I molecules on tumor cells [31] and is required for tumor recognition by cytotoxic T-lymphocytes. As IFNγ, which is produced by the latter, induces PD-L1 overexpression [32], it is possible to speculate that the loss of Her-2/neu might result in increased levels of IFNγ in the tumor microenvironment which in turn induces increased expression of PD-L1. It is noteworthy in this context that the HerVaxx vaccine has been shown to induce increased levels of IFNγ [12,13].
Upregulation of PD-L1 expression upon cellular activation has been reported in lymphocytes, dendritic cells, and myeloid cells [33], [34], [35]. In our study, IHC indicated moderate to strong expression of PD-L1 in the metastases of animals following the treatment with the mimotope JTMP alone. Importantly, the expression was further increased following the treatment with Her-Vaxx and the mimotope. Furthermore, based on H/E staining, no increased number of immune cells, e.g. Macrophages, in the control and the metastasized lungs was observed (data not shown). These observations may suggest a direct link between the detected PD-L1 expression to the tumor cells. Nonetheless, the strong PD-L1 stained areas in the metastases indicate the potential role of targeting this immune checkpoint in further preventing the development of the tumor cells.
In a recent study involving patients with primary breast cancer, Trastuzumab treatment was associated with a change of Her-2/neu expression from positive to negative in 47.3% of cases with an increase to 63.2% after the addition of Pertuzumab [36]. Therefore, consecutive therapy targeting PD-L1 following the treatment with the multi-peptide B cell vaccine which targets extracellular domains identical to Pertuzumab and Trastuzumab may potentially eliminate tumor cells and be a therapeutic option for metastatic disease resulting from Her-2/neu-overexpressing breast cancers.
We have previously shown a substantial increased anti-tumor effect in a primary solid tumor model in mice actively immunized with HerVaxx in combination with passive transfer of Pertuzumab, compared to mice only treated with the vaccine [14]. In the current study, HerVaxx and the mimotope of Pertuzumab were administered together, i.e. as a mixture of the two peptides. We do not exclude the possibility that a sequential immunization of each of these two peptides may have resulted in a synergistic effect as shown by HerVaxx and Pertuzumab. Furthermore, a recent study has detected large-scale differences in the immune microenvironment of primary tumors and metastatic breast cancer cells, suggesting an immune surveillance evasion by the latter through multiple mechanisms that result in an immune-inert environment [37]. This suggests the requirement of different regimens for treating primary tumors and metastases, particularly considering metastases with the lack of Her-2/neu-expression as observed in our study.
Taken together, our study has resulted in two significant observations. Firstly, the dual treatment involving active immunization with HerVaxx and the mimotope of Pertuzumab noticeably and significantly reduced the weight of the respective lungs (Fig. 2A); this effect was further reflected based on the results from the long term-setting when the mice were sacrificed 7 weeks (Fig. 2C) after the injection of the tumor cell in comparison to the 4-week post-injection time point (short-term setting) (Fig. 2A). This observed reduction of the lungs' weight was also clearly associated with a significant reduction of Her-2/neu-expressing metastases fraction (Fig. 4A). Secondly, the combined treatment resulted in an increased fraction (number) of Her-2/neu negative metastases compared to the single treatments (Fig. 4A), suggesting the tumor cells immune-escape mechanism by not expressing Her-2/neu. However, we found that the loss of Her-2/neu expression was associated with significantly increased levels of PD-L1 in the metastases. Overall, these suggest that active immunization with the multi-peptide vaccine targeting Trastuzumab and Pertuzumab binding sites may show efficacy in the clinic in analogy to the mentioned mAbs, and PD-L1 inhibition should be included in the case of Her-2/neu-directed treatments.
Author Contributions
Conseptualization and design, data analysis, writing original draft, reviewing, editing and finalization of the manuscript: JT, UW
Development of methodology: JT, MD, SH, KA, KB, KP, ET, ASB, AS
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): JT, MD, SH, MK
Analysis and interpretation of data (e.g. statistical analysis, biostatistics, computational analysis): JT, SH, MK, UW
Reviewing and commenting: MD, KB, SH, EGS, LK, MK, CCZ
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): MD, KA, KB, PK, ET, AS
Study supervision: JT, UW
Funding
The study was supported by a research grant from Imugene Ltd to the Medical University of Vienna (until 31.10.2020), and also by the Medical University of Vienna.
Declaration of Competing Interest
JT, MD, SH, KA, KB, PK, ET, AS, EGS, LK, MK: declare no potential conflicts of interest;
CCZ: Was CSO of Imugene until June 2018; Consultancies and Speaker's Honoraria from Roche, Novartis, BMS, MSD, Imugene, Ariad, Pfizer, Merrimack, Merck KGaA, Fibrogen, AstraZeneca, Tesaro, Gilead, Servier, Shire, Eli Lilly, Athenex; Institution (CECOG): BMS, MSD, Pfizer, AstraZeneca
UW: Was CSO of Imugene until Sept. 2018 and has received from GSK, Pfizer and Themis, funding to the Institute as the principal investigator (PI) of clinical studies.
Acknowledgments
The authors would like to thank the staff at ATG:biosynthetics GmbH (Germany) for the generation of overlapping peptides that were previously employed for the identification of the mimotope used in this study. The authors also would like to thank Mrs. Michaela Schlederer for the input on IHC staining.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2022.101378.
Contributor Information
Joshua Tobias, Email: joshua.tobias@meduniwien.ac.at.
Ursula Wiedermann, Email: ursula.wiedermann@meduniwien.ac.at.
Appendix. Supplementary materials
References
- 1.Slamon D.J., Godolphin W., Jones L.A., Holt J.A., Wong S.G., Keith D.E., et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244(4905):707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 2.Tai W., Mahato R., Cheng K. The role of HER2 in cancer therapy and targeted drug delivery. J. Control Release. 2010;146(3):264–275. doi: 10.1016/j.jconrel.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Witsch E.J., Mahlknecht G., Wakim J., Sertchook R., Bublil E., Yarden Y., et al. Generation and characterization of peptide mimotopes specific for anti ErbB-2 monoclonal antibodies. Int. Immunol. 2011;23(6):391–403. doi: 10.1093/intimm/dxr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swain S.M., Miles D., Kim S.B., Im Y.H., Im S.A., Semiglazov V., et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(4):519–530. doi: 10.1016/S1470-2045(19)30863-0. [DOI] [PubMed] [Google Scholar]
- 5.Giordano S.H., Temin S., Chandarlapaty S., Crews J.R., Esteva F.J., Kirshner J.J., et al. Systemic Therapy for Patients With Advanced Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018;36(26):2736–2740. doi: 10.1200/JCO.2018.79.2697. [DOI] [PubMed] [Google Scholar]
- 6.Scolnik P.A. mAbs: a business perspective. MAbs. 2009;1(2):179–184. doi: 10.4161/mabs.1.2.7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chames P., Van Regenmortel M., Weiss E., Baty D. Therapeutic antibodies: successes, limitations and hopes for the future. Br. J. Pharmacol. 2009;157(2):220–233. doi: 10.1111/j.1476-5381.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jasinska J., Wagner S., Radauer C., Sedivy R., Brodowicz T., Wiltschke C., et al. Inhibition of tumor cell growth by antibodies induced after vaccination with peptides derived from the extracellular domain of Her-2/neu. Int. J. Cancer. 2003;107(6):976–983. doi: 10.1002/ijc.11485. [DOI] [PubMed] [Google Scholar]
- 9.Wiedermann U., Wiltschke C., Jasinska J., Kundi M., Zurbriggen R., Garner-Spitzer E., et al. A virosomal formulated Her-2/neu multi-peptide vaccine induces Her-2/neu-specific immune responses in patients with metastatic breast cancer: a phase I study. Breast Cancer Res. Tr. 2010;119(3):673–683. doi: 10.1007/s10549-009-0666-9. [DOI] [PubMed] [Google Scholar]
- 10.Tobias J., Jasinska J., Baier K., Kundi M., Ede N., Zielinski C., et al. Enhanced and long term immunogenicity of a Her-2/neu multi-epitope vaccine conjugated to the carrier CRM197 in conjunction with the adjuvant Montanide. BMC Cancer. 2017;17(1):118. doi: 10.1186/s12885-017-3098-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tobias J., Battin C., De Sousa Linhares A., Lebens M., Baier K., Ambroz K., et al. A New Strategy Toward B Cell-Based Cancer Vaccines by Active Immunization With Mimotopes of Immune Checkpoint Inhibitors. Front. Immunol. 2020;11:895. doi: 10.3389/fimmu.2020.00895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiedermann U., Garner-Spitzer E., Chao Y., Maglakelidze M., Bulat I., Dechaphunkul A., et al. Clinical and immunologic responses to a B-cell epitope vaccine in patients with HER2/neu-overexpressing advanced gastric cancer-results from phase Ib trial IMU.ACS.001. Clin. Cancer Res. 2021;27(13):3649–3660. doi: 10.1158/1078-0432.CCR-20-3742. [DOI] [PubMed] [Google Scholar]
- 13.Maglakelidze M., Ryspayeva D., Bulat I., Andric Z., Nikolic I., Chawla T., et al. A phase 1b/2 open-label study with randomization in phase 2 of Imu-131 Her2/neu peptide vaccine plus standard of care chemotherapy in patients with Her2/neu overexpressing metastatic or advanced adenocarcinoma of the stomach or gastroesophageal junction. Cancer Res. 2021;81(13_Supplement) [Google Scholar]
- 14.Tobias J., Garner-Spitzer E., Drinic M., Wiedermann U. Vaccination against Her-2/neu, with focus on peptide-based vaccines. ESMO Open. 2022;7(1) doi: 10.1016/j.esmoop.2021.100361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., et al. Fiji: an open-source platform for biological-image analysis. Nat. Method. 2012;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stella G.M., Kolling S., Benvenuti S., Bortolotto C. Lung-seeking metastases. Cancers (Basel) 2019;11(7) doi: 10.3390/cancers11071010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao X., Wen X., Wei W., Chen Y., Zhu J., Wang C. Clinical characteristics and prognoses of patients treated surgically for metastatic lung tumors. Oncotarget. 2017;8(28):46491–46497. doi: 10.18632/oncotarget.14822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim I.S., Baek S.H. Mouse models for breast cancer metastasis. Biochem. Biophys. Res. Commun. 2010;394(3):443–447. doi: 10.1016/j.bbrc.2010.03.070. [DOI] [PubMed] [Google Scholar]
- 19.Okita R., Maeda A., Shimizu K., Nojima Y., Saisho S., Nakata M. PD-L1 overexpression is partially regulated by EGFR/HER2 signaling and associated with poor prognosis in patients with non-small-cell lung cancer. Cancer Immunol. Immunother. 2017;66(7):865–876. doi: 10.1007/s00262-017-1986-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iqbal N., Iqbal N. Human epidermal growth factor receptor 2 (HER2) in cancers: overexpression and therapeutic implications. Mol. Biol. Int. 2014 doi: 10.1155/2014/852748. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medeiros B., Allan A.L. Molecular mechanisms of breast cancer metastasis to the lung: clinical and experimental perspectives. Int. J. Mol. Sci. 2019;20(9) doi: 10.3390/ijms20092272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torka P., Barth M., Ferdman R., Hernandez-Ilizaliturri F.J. Mechanisms of resistance to monoclonal antibodies (mAbs) in lymphoid malignancies. Curr. Hematol. Malig. Rep. 2019;14(5):426–438. doi: 10.1007/s11899-019-00542-8. [DOI] [PubMed] [Google Scholar]
- 23.Hendrikx J., Haanen J., Voest E.E., Schellens J.H.M., Huitema A.D.R., Beijnen J.H. Fixed dosing of monoclonal antibodies in oncology. Oncologist. 2017;22(10):1212–1221. doi: 10.1634/theoncologist.2017-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price L., Brunt A.M. Trastuzumab infusion reactions in breast cancer. Should we routinely observe after the first dose? Eur. J. Hosp. Pharm. 2018;25(6):331–333. doi: 10.1136/ejhpharm-2016-001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Z.I., Ai D.I. Cardiotoxicity associated with targeted cancer therapies. Mol. Clin. Oncol. 2016;4(5):675–681. doi: 10.3892/mco.2016.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aguirre-Ghiso J.A., Estrada Y., Liu D., Ossowski L. ERK(MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38(SAPK) Cancer Res. 2003;63(7):1684–1695. [PubMed] [Google Scholar]
- 27.Park S.Y., Nam J.S. The force awakens: metastatic dormant cancer cells. Exp. Mol. Med. 2020;52(4):569–581. doi: 10.1038/s12276-020-0423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowenfeld L., Xu S., Czerniecki B.J. CD4(+) Th1 to the rescue in HER-2+ breast cancer. Oncoimmunology. 2019;8(10) doi: 10.1080/2162402X.2015.1078062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schietinger A., Philip M., Liu R.B., Schreiber K., Schreiber H. Bystander killing of cancer requires the cooperation of CD4(+) and CD8(+) T cells during the effector phase. J. Exp. Med. 2010;207(11):2469–2477. doi: 10.1084/jem.20092450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaganty B.K.R., Qiu S., Gest A., Lu Y., Ivan C., Calin G.A., et al. Trastuzumab upregulates PD-L1 as a potential mechanism of trastuzumab resistance through engagement of immune effector cells and stimulation of IFNgamma secretion. Cancer Lett. 2018;430:47–56. doi: 10.1016/j.canlet.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maruyama T., Mimura K., Sato E., Watanabe M., Mizukami Y., Kawaguchi Y., et al. Inverse correlation of HER2 with MHC class I expression on oesophageal squamous cell carcinoma. Br. J. Cancer. 2010;103(4):552–559. doi: 10.1038/sj.bjc.6605772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia-Diaz A., Shin D.S., Moreno B.H., Saco J., Escuin-Ordinas H., Rodriguez G.A., et al. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep. 2019;29(11):3766. doi: 10.1016/j.celrep.2019.11.113. [DOI] [PubMed] [Google Scholar]
- 33.Loke P., Allison J.P. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc. Natl. Acad. Sci. U. S. A. 2003;100(9):5336–5341. doi: 10.1073/pnas.0931259100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peranzoni E., Ingangi V., Masetto E., Pinton L., Marigo I. Myeloid cells as clinical biomarkers for immune checkpoint blockade. Front. Immunol. 2020;11:1590. doi: 10.3389/fimmu.2020.01590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y., Velez-Delgado A., Mathew E., Li D., Mendez F.M., Flannagan K., et al. Myeloid cells are required for PD-1/PD-L1 checkpoint activation and the establishment of an immunosuppressive environment in pancreatic cancer. Gut. 2017;66(1):124–136. doi: 10.1136/gutjnl-2016-312078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ignatov T., Gorbunow F., Eggemann H., Ortmann O., Ignatov A. Loss of HER2 after HER2-targeted treatment. Breast Cancer Res. Treat. 2019;175(2):401–408. doi: 10.1007/s10549-019-05173-4. [DOI] [PubMed] [Google Scholar]
- 37.Szekely B., Bossuyt V., Li X., Wali V.B., Patwardhan G.A., Frederick C., et al. Immunological differences between primary and metastatic breast cancer. Ann. Oncol. 2018;29(11):2232–2239. doi: 10.1093/annonc/mdy399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.