Abstract
Aims:
To estimate effects of brief substance use interventions delivered in general medical settings.
Methods:
A systematic review and meta-analysis of randomized trials conducted since 1990 of brief substance use interventions in patients of any age or severity level recruited in general medical settings. Primary outcomes were any measure of substance use or substance-related consequences (indexed with Hedges’ g and risk ratios). Mixed-effects meta-regressions were used to estimate overall effects and predictors of effect variability. Analyses were conducted separately by brief intervention (BI) target substance: alcohol only or drugs.
Findings:
116 trials (64,439 participants) were identified; 111 (62,263 participants) provided effect size data and were included in the meta-analysis. Drug-targeted BIs yielded significant small improvements in multiple drug/mixed substance use (Hedges’ g () = 0.08, 95% confidence interval (CI) [0.002, 0.15), but after adjusting for multiple comparisons, did not produce significant effects on cannabis use ( = 0.06, 95% CI [0.001, 0.12), alcohol use ( = 0.08, 95% CI [−0.0003, 0.17]), or consequences ( = 0.05, 95% CI [0.01, 0.10]). Drug-targeted BIs yielded larger improvements in multiple drug/mixed substance use when delivered by a general practitioner ( = 0.19, 95% CI [0.187, 0.193]). Alcohol-targeted BIs yielded small beneficial effects on alcohol use ( = 0.12, 95% CI [0.08, 0.16), but no evidence of an effect on consequences ( = 0.05, 95% CI [−0.04, 0.13). However, alcohol-targeted BIs only had beneficial effects on alcohol use when delivered in general medical settings ( = 0.17, 95% CI [0.10, 0.24]); the findings were inconclusive for those delivered in emergency department/trauma centers ( = 0.05, 95% CI [0.00, 0.10]).
Conclusions:
When delivered in general medical settings, alcohol-targeted brief interventions may produce small beneficial reductions in drinking (equivalent to a reduction in 1 drinking day per month). There is limited evidence regarding the effects of drug-targeted brief interventions on drug use.
Keywords: Brief intervention, emergency department, primary care, meta-analysis, systematic review
Given the detrimental sequelae associated with heavy episodic drinking and other drug use,(1–5) a growing body of research has evaluated preventive interventions targeting alcohol and other drug use (referred herein collectively as “substance use”; whereas “drug use” is used to refer specifically to any licit/illicit substance use other than alcohol). One approach is the brief intervention (BI), defined here as a talk or counseling intervention delivered in a circumscribed time frame that aims to promote substance-related behavior change.(6) BIs are often incorporated into a Screening, Brief Intervention, and Referral to Treatment model: patients are initially screened to identify unhealthy use, with BIs then tailored to patients’ substance use levels and supplemented with treatment and/or referrals to other substance-related services. By definition, BIs are short in contact time, typically lasting less than one hour;(7,8) otherwise, BIs can vary in structure, targets, clinician/interventionist, and intervention philosophy.
BIs may be well-suited for delivery in some general medical (GM) settings, where a range of non-specialized healthcare services are provided to patients of various ages with a wide array of health conditions. In these settings, clinicians have numerous opportunities to screen and discuss alcohol and other drug use with non-treatment seeking patients, but limited time to deliver services beyond treatment of the presenting condition. Given the brevity of screening and BIs in GM settings, if effective, these interventions offer a potentially cost-efficient method for addressing unhealthy substance use, particularly among non-treatment seeking patients.(9–13). Recent estimates suggest alcohol screening and BIs delivered in primary care may result in a cost-effectiveness ratio of €5,400 per Quality Adjusted Life Year gained(14), with BIs offering similar benefits, but lower cost, than more intensive brief treatments.(15) Because BIs delivered in GM settings have the potential advantages of low cost and minimal clinician effort,(16,17) an important question is whether and under what circumstances they meaningfully reduce alcohol and other drug use.
Despite extensive research on drug and alcohol BIs delivered in GM settings, findings of this research have not been summarized in a rigorous evidence synthesis that includes both alcohol- and other drug-targeted BIs and includes GM settings other than primary care settings. Further, to date, there has not been a comprehensive synthesis that focuses on variability in effects of BIs. Prior reviews indicate that alcohol BIs delivered in healthcare settings can be effective in reducing self-reported alcohol use, but effect sizes vary widely and many studies report null findings.(8,18–25) Moreover, there are fewer existing trials evaluating the efficacy of BIs targeting drugs other than alcohol.(26,27) In the absence of quantitative syntheses, prior narrative reviews have highlighted the inconsistency of BI effects on drug use,(18,26,28–31) leading for calls to reconsider how unhealthy substance use is addressed in primary care settings.(32)
Effects of BIs may therefore vary depending on whether the intervention targets alcohol or drugs, but findings may also vary depending on characteristics of the BIs, patients, clinicians, settings, and study methodology. For instance, BIs may be more effective when they feature clinician advice,(33,34) or incorporate decisional balance and goal-setting exercises when targeting alcohol use among adolescents(20) but not necessarily among adult populations.(35,36) BIs may also be more effective among younger adults, males, and Latino patients, although results have varied significantly across trials.(8,20,37,38) Prior reviews also suggest BIs may be more effective when delivered in primary care versus hospital/emergency department settings.(20,39) Finally, variation in effects—and null findings in particular—may be attributable to whether trials rigorously assessed BI efficacy or instead tested real-world clinical effectiveness,(40–43) or to other aspects of trial quality(44,45).
A comprehensive and up-to-date synthesis examining whether and under what conditions BIs delivered in GM settings reduce alcohol and other drug use is needed to summarize the accumulating literature on the efficacy of BIs for reducing drug use and associated consequences.
Study Objectives
This systematic review and meta-analysis sought to (1) estimate the overall efficacy of alcohol and other drug BIs in GM settings; (2) determine whether outcome domain, study methodology (comparator type, reporting quality, and risk of bias), intervention features (target [alcohol or other drug], setting, and duration), and participant characteristics (age, gender, and race/ethnicity composition) were associated with BI efficacy; and (3) assess study quality and publication bias in this literature.
Methods
Protocol and Registration
The protocol for this review was pre-registered in the PROSPERO registry #CRD42018086832(46) and the analysis plan was pre-registered on the Open Science Framework.(47)
Eligibility Criteria
Eligible studies were those using a randomized controlled trial (RCT) design to evaluate the effects of an alcohol or other drug BI delivered in a GM setting relative to a less active comparison condition (e.g., no treatment, sham, treatment as usual). Trials had to be reported in 1990 or later and report at least one post-BI outcome of substance use or substance-related consequences. BIs must have been delivered in four or fewer sessions to participants recruited in a GM setting (defined as emergency departments, community based, university based, outpatient, inpatient, private provider, student health centers, or other GM settings such as primary care). BIs delivered in specialized clinics, substance use treatment facilities, or pharmacies were outside the review scope. Given our focus of examining overall efficacy and variability in effects, eligible BIs were not required to implement a screening assessment prior to the BI (although 97% of included studies used patient samples screened as having problematic substance use; see Table 1). Eligible samples included patients of all ages and baseline severity levels.
Table 1.
Characteristics of studies, participant samples, and interventions included in qualitative and quantitative syntheses (k = 116).
| Study and design characteristics | % (n) a |
|---|---|
| Country/region b | |
| Asia | 4 (5) |
| Australia/New Zealand | 8 (9) |
| South Africa | 4 (5) |
| South America | 3 (3) |
| U.S./Canada | 59 (68) |
| U.S. Midwest | 22 (15) |
| U.S. Northeast | 43 (29) |
| U.S. South | 10 (7) |
| U.S. West | 21 (14) |
| Multiple U.S. regions | 4 (3) |
| Western Europe | 21 (24) |
| Multiple | 2 (2) |
| Sample type b | |
| Screened/elevated risk | 97 (112) |
| Universal/unscreened | 3 (4) |
| Design b | |
| RCT | 90 (104) |
| Cluster RCT | 10 (12) |
| Comparison group type c | |
| Treatment as usual/usual care | 45 (55) |
| General health booklet | 34 (41) |
| Sham intervention | 7 (8) |
| No pretest assessment usual care | 3 (4) |
| Other | 11 (14) |
| Follow-up timing (wks.), M (SD) d | 34.8 (43.0) |
| Percent attrition, M (SD) b | |
| Overall | 25.0 (17.7) |
| Differential | 5.8 (7.0) |
| Implementation monitoring b | |
| Yes | 61 (71) |
| No | 4 (5) |
| Not reported | 35 (40) |
| Implementation problems b | |
| Yes | 14 (16) |
| Possible | 18 (21) |
| Not reported | 68 (79) |
| Intention-to-treat analysis b | |
| Yes | 51 (59) |
| Possible | 23 (26) |
| No | 26 (30) |
| CONSORT diagram b | |
| Yes | 83 (94) |
| No | 17 (20) |
| Intervention features | % (n) a |
| Setting b | |
| Emergency department | 41 (48) |
| Community | 34 (39) |
| University | 22 (25) |
| Outpatient | 11 (13) |
| Inpatient | 5 (6) |
| Private provider | 9 (11) |
| Student health center | 6 (7) |
| Other | 18 (21) |
| Modality c | |
| In-person | 75 (88) |
| Computer/tablet/smartphone | 20 (24) |
| Telephone | 5 (6) |
| Booster c | |
| Booster delivered | 33 (40) |
| No. boosters, median (range) e | 1 (1–4) |
| Duration (minutes), M (SD) c | 26.2 (25.7) |
| Components c, f | |
| Advice | 62 (75) |
| Information booklet | 59 (71) |
| Decisional balance exercise | 32 (39) |
| Goal-setting exercise | 54 (66) |
| Homework activity | 4 (5) |
| Personalized normative feedback | 75 (89) |
| Training | 16 (19) |
| Referrals | 26 (30) |
| Video | 4 (5) |
| Website | 5 (6) |
| Other | 37 (46) |
| Clinician/interventionist characteristics | % (n) |
| Typical clinician/interventionist c | |
| General practitioner (non-primary clinician) | 10 (12) |
| Primary care provider | 19 (23) |
| Behavioral specialist | 28 (34) |
| Other specialist clinician | 2 (2) |
| Peer | 7 (9) |
| Graduate student/trainee | 2 (2) |
| Other interventionist | 33 (41) |
| Study and design characteristics | % (n) |
| Overall risk of bias b | |
| Unclear | 75 (87) |
| High | 25 (29) |
| Participant characteristics | M (SD) h |
| Average age b | 34.1 (12.4) |
| Sample age group, % (n) b | |
| Adolescent/young adult | 24 (28) |
| Mixed or adult only | 76 (88) |
| Percent female composition b | 38.7 (22.7) |
| Race/ethnicity composition b | |
| Percent Asian | 13.9 (30.9) |
| Percent Black | 28.9 (25.0) |
| Percent Latinx | 24.2 (27.2) |
| Percent white | 59.9 (27.6) |
| Clinician/interventionist characteristics | % (n) |
| Profession c | |
| Medical doctor | 21 (26) |
| Physician’s assistant | 1 (1) |
| Nurse | 11 (14) |
| Other medical specialist | 2 (2) |
| Psychologist | 8 (10) |
| Social worker | 6 (8) |
| Other behavioral health specialist | 13 (16) |
| Other lay provider | 16 (20) |
| Could not tell | 23 (29) |
Notes. k = number of studies.
Percentages and counts shown unless otherwise indicated.
Estimates calculated at study level.
Estimates calculated at intervention or comparison group level, as appropriate.
Estimates calculated at effect size level.
Number (No.) of boosters calculated only among studies delivering boosters; one study (Córdoba et al., 1998) provided a variable number of boosters and is not included in estimates.
Interventions could use multiple components; percentages reflect proportion of all intervention groups using each component.
Means and standard deviations shown unless otherwise indicated. See Supplemental Material S1 for a version of this table with descriptive details disaggregated by BI target (i.e., by trials of drug-targeted or alcohol-targeted BIs).
Search Strategy
We used a comprehensive search to identify trials. The following databases (hosts) were searched from 1990 through March 31, 2020: PubMed; Nursing/Academic Edition (EBSCO); ERIC, Applied Social Sciences Index and Abstracts, Dissertations & Theses Global, Social Services Abstract (ProQuest); PsycINFO (PsycNET); Cochrane Central Register of Controlled Trials; WHO International Clinical Trials Registry; and NIH RePORTER. See Supplemental Material for full PubMed search strategy. We checked the bibliographies of all identified studies, including prior reviews and meta-analyses, and conducted hand-searches of the 1990–2020 tables of contents in Addiction, Addictive Behaviors, Campbell Systematic Reviews, and Journal of Studies on Alcohol and Drugs.
Study Selection and Data Extraction
Study selection and data extraction were conducted by research assistants trained and supervised by the first author. At the first stage of data collection, two reviewers independently screened titles/abstracts to eliminate clearly irrelevant studies. Any study deemed potentially relevant by at least one reviewer proceeded to the second stage, during which two reviewers independently screened the full text to make a final eligibility determination. At the third stage, two reviewers independently extracted data for all eligible trials. Any disagreements at the second and third stages of screening and coding were resolved by the first author. All data extraction followed a standardized coding protocol using the procedures defined below (see Supplemental Material for the coding manual).
Effect sizes
For continuous outcomes, effect sizes were represented with the small-sample corrected standardized mean difference (Hedges’ g), with positive values indicating beneficial BI effects.(48) For binary outcomes, effects were represented with risk ratios (relative risks), coded such that values greater than 1.0 indicated beneficial BI effects. All outlying effect sizes were double-checked for accuracy and Winsorized to less extreme values (see analysis plan in Supplemental Materials). For the 12 trials that used cluster RCT designs, standard errors of effect sizes were adjusted to account for the design effect.(49)
Study reporting quality, risk of bias, and design assessment
Our coding of study reporting quality, risk of bias, and study design is detailed in the study protocol.(47) Risk of bias assessments were collected using the Cochrane Collaboration’s risk of bias tool for RCTs,(50) with high, low, or unclear risk of bias in six domains. Ratings across domains were summarized in an overall risk of bias categorization, defined as: low (all domains rated low), high (any domain rated high), and unclear (any domain rated unclear and no domains rated high).
Study-level moderators
We collected data on effect size moderators related to interventionist/setting, BI features, and aggregate study demographics. For interventionist/setting, we measured GM setting type (emergency department/trauma center vs. other GM setting) and interventionist delivering most of the BI (general practitioner, primary care clinician, behavioral specialist, other clinician/interventionist). For BI features, we measured delivery modality (in-person, telephone, or electronic), intervention duration (in minutes), and presence/absence of booster sessions. For aggregate study demographics, we measured age group (adolescent/young adult [up to age 25] only vs. mixed ages/adult only), percent female, and percent white.
Statistical Methods
All meta-analyses were conducted using meta-regression models with robust variance estimates to accommodate dependent effect sizes.(51) Random-effects meta-regression models were used to estimate overall mean effect sizes, and mixed-effects meta-regression models (i.e., random study-level effects and fixed moderator effects) were used for all moderator analyses. Results are only presented for statistical models with adequate degrees of freedom after accounting for small sample adjustments to the robust variance estimates.(52) All analyses were conducted separately by the BI target substance: alcohol only or drugs; the drug-targeted BIs included those targeting a single drug (e.g., cannabis), multiple drugs depending on patient screening results (e.g., cannabis, cocaine), or a combination of both alcohol and other drugs (e.g., alcohol and cannabis). Analyses were also conducted separately by outcome domain (alcohol, cannabis, mixed alcohol/other drug use, substance-related consequences). All main effects meta-analyses were conducted separately for continuous and binary outcome measures, with results adjusted for pretest values. The Benjamini-Hochberg procedure was used to control Type I error rates for all analyses within an outcome domain.(53)
To maximize analytic sample sizes, all moderator meta-analyses pooled continuous and binary outcomes simultaneously by converting risk ratios into standardized mean differences using the Cox transformation.(54) Moderation models included a control variable indicating whether the effect size was derived from continuous/binary data. Differences between moderator subgroups were assessed for statistical significance using the coefficients from the mixed-effects meta-regression models, with results presented as predicted subgroup means from those models. Heterogeneity was assessed using τ2, I2, and 95% prediction intervals.(55) The prediction interval (PI) provides a useful representation of the variability in effects across settings/contexts, and provides an expected range of true effects in future similar trials.(55,56) Publication bias was assessed using contour-enhanced funnel plots and Egger regression tests.(57,58) Sensitivity analyses were conducted to assess the impact of Winsorizing effect sizes, imputation of missing cluster size and intraclass correlation values (for cluster-RCTs), and the approximated within-study effect size correlation. Mean effect estimates were not found to be sensitive to any of these modeling decisions. Trial authors were contacted to obtain any missing statistical information needed for effect size estimation. Because missing data on effect size moderators was limited and a missing at random assumption could not be reasonably justified, imputation was not used to recover missing values.
Results
Study Characteristics
A total of 116 trials (37 drug-targeted; 79 alcohol-targeted) with 64,439 participants met eligibility criteria and were included in the overall synthesis (Figure 1; see Supplemental Material for references to included trials); 111 of those 116 trials (62,263 participants; 22,966 drug-targeted; 39,297 alcohol-targeted) provided effect size data and were included in the meta-analysis. The majority (81%) of drug-targeted interventions were general substance use interventions targeting the patients’ primary drug of choice, with only 19% targeting a specific drug (e.g., cannabis, prescription opioids, benzodiazepines). Most trials were conducted in the United States or Canada (59%). Nearly all (97%) trials used a screened or elevated risk sample, most (90%) used an individually randomized design, and about half (45%) used a treatment as usual comparison. The average age of participants was 34.1 years (SD = 12.4), and samples were composed of majority male (mean proportion female = 38.7%, SD = 22.7) and white (mean proportion = 59.9%, SD = 27.6) participants. One-quarter (25%) of trials were rated as high overall risk of bias, with 75% rated as unclear (Table 1).
Figure 1.
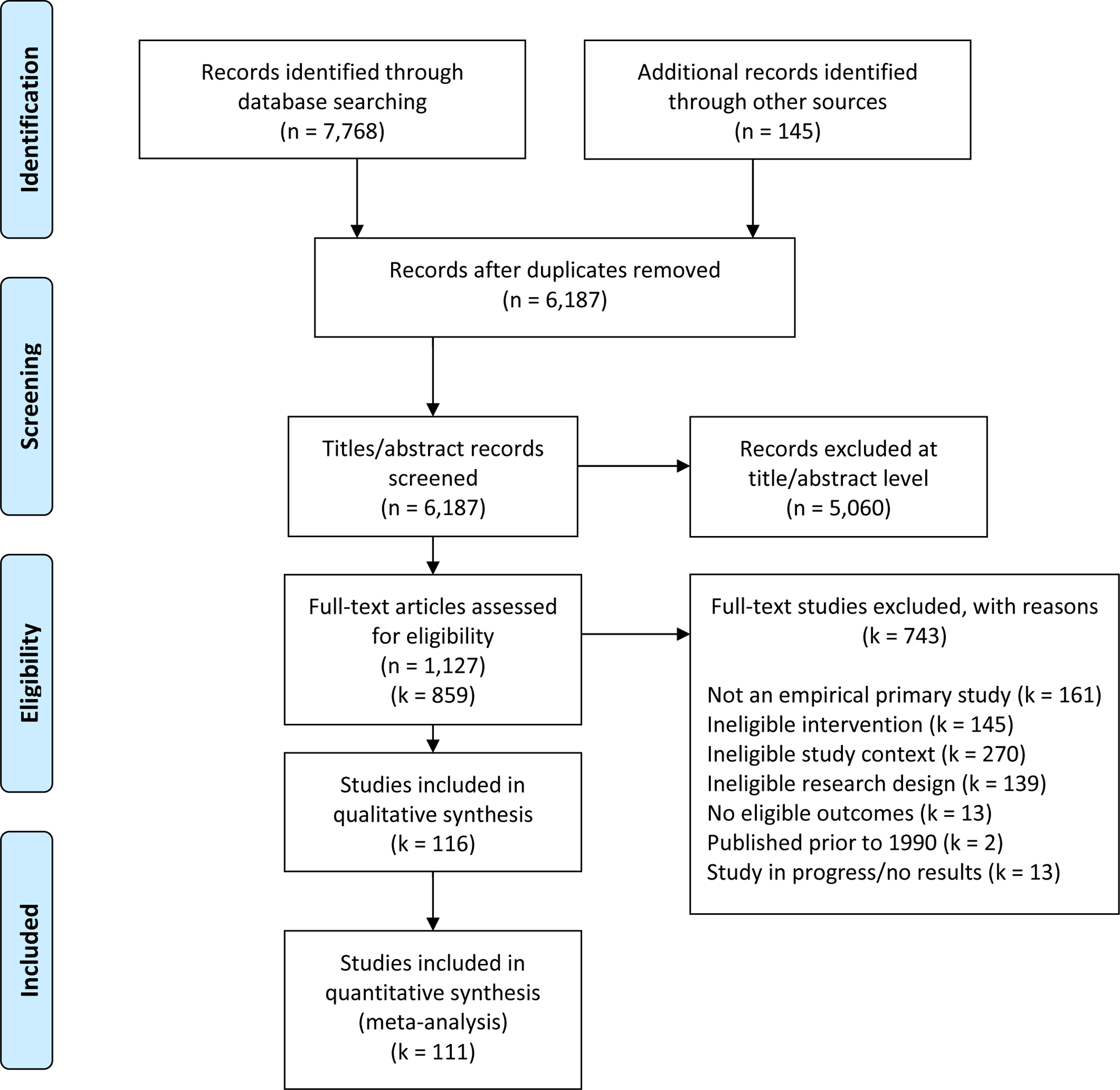
PRISMA Flow Diagram Displaying Numbers of Reports and Studies Included in Review
Forty-one percent of BIs were delivered in emergency departments, 34% in community healthcare settings, and 22% in university-based healthcare settings. Most BIs (75%) were delivered in-person, and one-third (33%) were delivered with at least one booster session. The average duration of BIs was 26 minutes (SD = 25.7); the most common intervention components reported were personalized normative feedback (75%), prescriptive advice (62%), information booklets (59%), and goal-setting exercises (54%). The BI interventionist was most commonly a behavioral specialist (28%), primary care clinician (19%), or other general practitioner other than the patient’s primary care clinician (10%).
Average Effects
Drug-targeted interventions
Overall, there was no evidence that BIs targeting drugs had a significantly beneficial or harmful effect on participants’ cannabis use, alcohol use, or substance-related consequences after adjusting for multiple comparisons (Table 2). These meta-analyses likely had limited statistical power, however, given the small number of trials evaluating drug-targeted BIs (k = 16). The mean effect for continuously measured multiple drug/mixed substance use outcomes was statistically significant ( = 0.08, 95% CI [0.002, 0.15]; k = 16; n = 93), suggesting that on average, drug-targeted BIs yielded significant but small reductions in participants’ mixed substance use. Between-study heterogeneity was minimal (τ2 = 0.01), although 27% of observed variability was attributable to true heterogeneity, so considerable variation in effects could be expected in similar future trials (95% PI [−0.10, 0.25]).
Table 2.
Pretest-adjusted mean effect sizes, 95% confidence intervals, and heterogeneity statistics by brief intervention target, outcome domain, and effect size type.
| Drug-targeted interventions | Alcohol-targeted interventions | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome domain Effect size type | [95% CI] | [95% PI] | τ 2 | I2 (%) | [95% CI] | τ2 [95% PI] | τ 2 | I2 (%) |
| Alcohol use | ||||||||
| SMD | 0.08 [−0.0003, 0.17]12, 71 | [−0.09, 0.26] | 0.00 | 27.17 | 0.12 [0.08, 0.16] 60, 396 | [−0.12, 0.37] | 0.01 | 51.21 |
| RR | 1.01 [0.92, 1.11]8, 39 | [0.93, 1.10] | 0.00 | 0.00 | 1.11 [1.04, 1.19] 27, 105 | [1.05, 1.19] | 0.00 | 0.00 |
| Cannabis use | ||||||||
| SMD | 0.06 [0.001, 0.12]13, 53 | [0.004, 0.11] | 0.00 | 0.00 | — | — | — | |
| RR | — | — | — | — | — | — | ||
| Multiple drug/mixed substance use | ||||||||
| SMD | 0.08 [0.002, 0.15] 16, 93 | [−0.10, 0.25] | 0.01 | 27.28 | — | — | — | |
| RR | — | — | — | — | — | — | ||
| Consequences | ||||||||
| SMD | 0.05 [0.01, 0.10]12, 80 | [−0.21, 0.31] | 0.01 | 48.33 | 0.05 [−0.04, 0.13]15, 64 | [−0.10, 0.19] | 0.00 | 18.41 |
| RR | — | — | — | 1.06 [0.94, 1.18]8, 38 | [0.95, 1.17] | 0.00 | 0.00 | |
Notes. Results in boldface are significantly different from null value (i.e., indicating no difference between intervention and comparison conditions) after Benjamini-Hochberg adjustment for multiple comparisons. = average effect size. CI = 95% confidence interval with robust standard errors. PI = 95% prediction interval. SMD = bias-adjusted standardized mean difference (Hedges’ g). RR = risk ratio.
indicates results not available due to inadequate effect sizes and/or degrees of freedom. Subscripts indicate k (number of studies), n (number of effect sizes).
Alcohol-targeted interventions
For alcohol-targeted BIs, syntheses were only estimable for two outcome domains: alcohol use and substance-related consequences (Table 2). Overall, alcohol-targeted BIs were associated with significant but small reductions in alcohol use; this was consistent in the models pooling effects from continuous ( = 0.12, 95% CI [0.08, 0.16]; k = 60; n = 396) and dichotomous data (RR = 1.11, 95% CI [1.04, 1.19]; k = 27, n = 105). Between-study heterogeneity was minimal (τ2 = 0.01; τ2 = 0.00), but 51% of the observed variability was attributable to true heterogeneity, suggesting considerable variation in effects could be expected in similar future trials (95% PIg [−0.12, 0.37]). Finally, there was no evidence that alcohol-targeted BIs were associated with substance-related consequences, although there was a small amount of heterogeneity in these effects and results should be interpreted cautiously given the small number of contributing trials.
Moderation Effects
Drug-targeted interventions
There was limited availability of effect sizes from drug-targeted BI trials across moderator levels, resulting in a small number of interpretable findings. Moreover, there was generally insufficient power to reliably assess whether drug-targeted BIs were significantly more (or less) effective in reducing alcohol use, cannabis use, or consequences outcomes across the measured moderators. Nonetheless, results from the planned moderator analyses indicated that after adjusting for multiple comparisons, drug-targeted BIs yielded larger improvements in multiple drug/mixed substance use outcomes when delivered by a general practitioner ( = 0.19, 95% CI [0.187, 0.193]) compared to other interventionists ( = 0.21, 95% CI [−0.26, 0.68] for primary care providers; = 0.04, 95% CI [−0.34, 0.42] for behavioral specialists; = 0.05, 95% CI [−0.88, 0.97] for peer providers; = 0.06, 95% CI [−0.04, 0.17] for others).
Drug-targeted BI effects on multiple drug/mixed substance use outcomes were significant and positive when delivered without a booster ( = 0.12, 95% CI [0.05, 0.19]) vs. with a booster ( = 0.07, 95% CI [−0.08, 0.22]); when no implementation problems were reported ( = 0.11, 95% CI [0.05, 0.17]) vs. with possible problems ( = 0.05, 95% CI [−1.77, 1.87]); and when compared to a treatment as usual condition ( = 0.15, 95% CI [0.06, 0.23]) vs. other control ( = 0.08, 95% CI [−0.02, 0.18]); however, none of these group contrasts was statistically significant after adjusting for multiple comparisons. Similarly, effects were slightly larger in studies that did not use ITT analyses ( = 0.12, 95% CI [0.08, 0.16] vs. = 0.11, 95% CI [−0.001, 0.22]) but again, this group contrast was not statistically significant after adjusting for multiple comparisons.
Finally, drug-targeted BIs were associated with significantly worse (i.e., higher) levels of substance use consequences when delivered by a primary care provider ( = −0.05, 95% CI [−0.06, −0.049]) compared to other interventionists ( = −0.15, 95% CI [−0.26, 0.68] for general practitioners; = 0.06, 95% CI [−0.03, 0.15] for behavioral specialists; = 0.11, 95% CI [−0.27, 0.49] for peer providers; = 0.08, 95% CI [0.01, 0.15] for others).
Alcohol-targeted interventions
Study reporting and design characteristics
Overall, the effects of alcohol-targeted BIs did not substantially differ across trials based on their comparison group type, attrition level, use of implementation monitoring, reporting of implementation problems, or use of an ITT analysis (Table 3). However, results indicated a substantially smaller reduction in alcohol use in trials that reported a CONSORT diagram ( = 0.08, 95% CI [0.04, 0.12]) versus those that did not ( = 0.24, 95% CI [0.07, 0.41]), and in studies with a high overall risk of bias ( = 0.02, 95% CI [−0.07, 0.11]) compared to those with unclear overall risk ( = 0.14, 95% CI [0.08, 0.19]).
Table 3.
Moderator effects for alcohol-targeted interventions, by outcome domain.
| Outcome domain | ||
|---|---|---|
| Alcohol use | Consequences | |
| Categorical moderators | ||
| Comparison group type | ||
| Other types | 0.12 [0.06, 0.19] 62, 501 | 0.03 [−0.13, 0.20]20, 102 |
| Treatment as usual | 0.10 [0.03, 0.17] 62, 501 | 0.06 [0.00, 0.13]20, 102 |
| Implementation monitoring | ||
| Yes | 0.10 [0.05, 0.15] 62. 501 | 0.06 [−0.01, 0.13]20, 102 |
| Not reported | 0.14 [0.05, 0.23] 62, 501 | — |
| Implementation problems | ||
| Yes | 0.15 [−0.04, 0.35]62, 501 | — |
| Possible | 0.05 [−0.02, 0.13]62, 501 | — |
| Not reported | 0.11 [0.06, 0.16] 62, 501 | — |
| Intention-to-treat analysis | ||
| Yes | 0.10 [0.05, 0.15] 61, 497 | 0.09 [0.02, 0.16]20, 102 |
| Possible | 0.11 [−0.02, 0.24]61, 497 | — |
| No | 0.14 [0.03, 0.26] 61, 497 | — |
| CONSORT diagram reported | ||
| Yes | 0.08 [0.04, 0.12] 62, 501 | 0.03 [−0.04, 0.11]20, 102 |
| No | 0.24 [0.07, 0.41] 62, 501 | — |
| Overall risk of bias | ||
| High | 0.02 [−0.07, 0.11]62, 501 | — |
| Unclear | 0.14 [0.08, 0.19] 62, 501 | 0.07 [0.01, 0.14]20, 102 |
| Setting | ||
| Emergency department | 0.05 [0.00, 0.10]62, 501 | 0.00 [−0.08, 0.08]20, 102 |
| General medical setting | 0.17 [0.10, 0.24] 62, 501 | 0.11 [0.02, 0.20]20, 102 |
| Modality | ||
| In-person | 0.14 [0.08, 0.20] 61, 497 | — |
| Computer/tablet/smartphone | 0.10 [0.01, 0.18]61, 497 | — |
| Telephone | 0.01 [−0.07, 0.10]61, 497 | — |
| Booster delivered | ||
| Yes | 0.16 [0.07, 0.25] 62, 501 | — |
| No | 0.10 [0.04, 0.15] 62, 501 | 0.03 [−0.04, 0.11]20, 102 |
| Age group | ||
| Adolescent/young adult | 0.11 [0.04, 0.18] 62, 501 | — |
| Mixed or adult only | 0.12 [0.06, 0.17] 62, 501 | 0.02 [−0.06, 0.10]20, 102 |
| Continuous moderators | ||
| Overall attrition | −0.28 [−0.78, 0.22]58, 493 | 0.15 [−0.33, 0.64]20, 102 |
| Differential attrition | 0.47 [−0.82, 1.77]52, 371 | 0.19 [−4.60, 4.99]16, 69 |
| Efficacy-to-effectiveness scale score | −0.01 [−0.03, 0.01]62, 501 | −0.02 [−0.05, 0.02]20, 102 |
| Duration | 0.00 [−0.003. 0.004]54, 443 | 0.00 [−0.01, 0.003]16, 90 |
| Proportion female | −0.21 [−0.52, 0.09]58, 487 | 0.24 [−0.05, 0.52]19, 100 |
| Proportion white | 0.02 [−0.23, 0.28]33, 280 | — |
Notes. Predicted mean effect sizes and 95% CIs presented for categorical moderators. Unstandardized coefficients and 95% CIs presented for continuous moderators. Results in boldface are significantly different from zero after Benjamini-Hochberg adjustment for multiple comparisons. k (number of studies), n (number of effect sizes). — not estimable due to limited degrees of freedom.
Intervention features
Alcohol-targeted BIs yielded no evidence of a significant effect on alcohol use outcomes when delivered in emergency departments/trauma centers ( = 0.05, 95% CI [−0.00, 0.10]) compared to other GM settings ( = 0.17, 95% CI [0.10, 0.24]). This pattern also emerged for substance use consequences. In-person delivery of alcohol-targeted BIs appeared to be associated with a larger reduction in alcohol use ( = 0.14, 95% CI [0.08, 0.20]) compared to delivery using a computer, tablet, or smartphone ( = 0.10, 95% CI [0.01, 0.18]), although this difference was not statistically significant (p = .38). Use of an intervention booster was associated with a larger reduction in alcohol use ( = 0.16, 95% CI [0.07, 0.25]) compared to BIs without a booster ( = 0.10, 95% CI [0.04, 0.15]). There was no evidence that duration of the BI significantly moderated efficacy on alcohol use or consequences.
Participant characteristics
A similar reduction in alcohol use was observed in studies that tested alcohol-targeted BIs in adolescent/young adult samples (all participants ≤ 30; = 0.11, 95% CI [0.04, 0.18]) and in adult or mixed-age sample ( = 0.12, 95% CI [0.06, 0.17]). Alcohol-targeted BI effects on alcohol use did not significantly vary by aggregate proportion of female or white participants.
Publication Bias
Contour-enhanced funnel plots and regression tests for funnel plot asymmetry suggested potential publication bias (also known as small study bias) for alcohol and cannabis use outcomes, but not for alcohol and other drug use and consequences outcomes (see (47)). Meaningful interpretation of funnel plots was not possible, however, given limited variability in sample sizes across trials, large sample sizes in most trials, and observed heterogeneity in effects.(59)
Discussion
This review synthesized findings from 116 trials and 64,439 total participants to estimate variability in the efficacy of alcohol/other drug focused BIs delivered in GM settings. We found few trials evaluating the efficacy of drug-targeted BIs; synthesizing evidence from 16 studies, we found no evidence that BIs targeting drug use significantly improved or worsened patients’ use or substance-related consequences. These null effects were generally consistent across the settings, participants, and study design characteristics assessed, but results should be interpreted cautiously given the smaller body of literature on drug-targeted BIs (versus alcohol-targeted BIs). Our results suggested potential small beneficial effects when drug-targeted BIs were delivered by a general practitioner. For alcohol-targeted BIs, after synthesizing evidence from 60 studies, results were consistent with prior literature indicating small beneficial reductions in self-reported alcohol use, roughly equivalent to a reduction from 11.6 to 10.7 drinking days per month. Effects were larger for alcohol BIs delivered in-person or in a general medical setting (vs. emergency department/trauma center settings) but were also larger in trials with higher risk of bias. These small effects of alcohol BIs may not be clinically meaningful at the individual level given that similar future trials are expected to yield effects no larger than 0.25 standard deviation reductions for alcohol use; however, these small effects may still be clinically meaningful at the population level. Future research should consider the population effects of BIs, distinct from individual effects, where small mean changes over large numbers of people could potentially reduce population level harms. Further, effects of this magnitude could be clinically meaningful for certain population subgroups, such as adolescents and young adults, if a one-day reduction in drinking could interrupt a trajectory from experimentation to an alcohol use disorder.
Consistent with prior work, our results provide limited evidence that screening and BIs for drug use are efficacious for reducing drug use(27–29,60) despite consistent evidence of small beneficial effects for alcohol BIs.(8,18,20) Although identification and management of drug use may be reasonable to consider in clinical practice (for diagnosis/management of other health conditions, creating opportunities for future conversations, and inviting care-seeking), given the lack of evidence regarding the efficacy of drug BIs, new strategies and approaches should also be explored for addressing patients’ drug use. And although our finding that drug BIs may have small beneficial effects when delivered without booster sessions could be spurious, it does accord with prior literature suggesting that adding boosters may not improve the efficacy of BIs.(61–64)
Our results also suggested alcohol BIs may have the smallest effects when delivered in emergency departments or trauma centers, versus other GM settings. This is likely due to differences in expectations/relationships between clinicians and patients in other GM settings that include primary care settings (e.g., patients may have longer duration and more trusting relationships with primary care providers than with emergency care providers), as well as differences across settings in patient baseline substance use severity, physical and mental health, and socioeconomic status.(65) Finally, our results indicated that any beneficial effects of alcohol BIs may be upwardly biased in trials with poorer reporting quality, so practitioners and policymakers should exercise caution when considering the magnitude of alcohol BI effects without first evaluating trial reporting quality.
The findings from this meta-analysis should be interpreted in light of several limitations. First, given the small number of trials examining drug-targeted BIs (k = 16), we did not have adequate power to assess heterogeneity in effects for several of the moderators of interest. Moreover, as telehealth models have become increasingly prevalent due to the COVID-19 pandemic, future research may need to examine variability in BI effects delivered via telehealth. Second, because patient characteristic moderators were collected at the study level (e.g., proportion female), we were unable to assess whether BIs were more or less effective for certain types of patients (e.g., by demographics or level of substance use severity) given the risk of ecological fallacy when using aggregate level data to make inferences about individuals. Future syntheses using individual participant data meta-analysis approaches will be better suited for examining variability in BI effects across individual patient characteristics such as age, race, ethnicity, comorbidities, and baseline substance use.(66) Third, given inconsistent reporting in individual trials, it was not possible to estimate subgroup effects for specific types of GM settings (e.g., primary care vs. general hospital) or patient age groups (e.g., adolescents only). Finally, this synthesis was limited to evidence on the efficacy of BIs delivered in GM settings and did not examine efficacy in other settings (e.g., alcohol or drug treatment centers, reproductive clinics).
Conclusion
Unhealthy alcohol and other drug use continues to be a widespread public health concern, necessitating the identification of effective preventive approaches for decreasing risk (for which there is modest evidence in the existing literature) and for interrupting trajectories toward clinical substance use disorders (for which there is no evidence in the literature). Given their brevity, low cost, and minimal clinician effort, BIs have emerged as a promising approach for addressing substance use. When delivered in GM settings, alcohol-targeted BIs are likely to produce small reductions in drinking, but the literature does not include enough trials of drug-targeted BIs to ascertain whether they consistently reduce drug use or associated consequences. Clinical research should continue testing alcohol and drug BIs, as well as developing other novel approaches for addressing alcohol and drug use in healthcare settings, particularly those using telehealth models that maximize patient reach when in-person intervention is not possible.(67) Research on telehealth approaches could also determine whether providing ongoing support, referrals, and other resources available by telehealth after a BI is delivered leads to increased intervention efficacy.
Supplementary Material
Acknowledgements
This work was supported by the National Institute on Drug Abuse (R01DA043589). The content is solely the responsibility of the authors and does not necessarily represent the official position or policy of the National Institute on Drug Abuse, National Institutes of Health, or Department of Veterans Affairs. We would like to acknowledge the research assistants and research associates at the University of Oregon who assisted with data collection. Any opinions, findings, and conclusions expressed in this manuscript are those of the authors and do not necessarily reflect the views of these colleagues.
Declarations of Competing Interest:
ETS, NP, and MSC have no conflicts of interest to declare. RS reports personal fees from University of Oregon to support this work. RS also reports non-financial support from Alkermes, personal fees from American Society of Addiction Medicine, American Medical Association, National Council on Behavioral Healthcare, Kaiser Permanente, UpToDate/Wolters Kluwer, Yale University, National Committee on Quality Assurance, Oregon Health Sciences University, RAND Corporation, Leed Management Consulting/Harvard Medical School, Partners, Beth Israel/Deaconess Hospital, American Academy of Addiction Psychiatry, Group Health Cooperative, Checkup & Choices, International Network on Brief Interventions for Alcohol and Other Drugs (INEBRIA) supported via funds from Systembolaget, Smart Recovery, Karolinska Institutet, Institute for Research and Training in the Addictions, Medical Malpractice Expert Witness, Charles University, Brandeis University, Massachusetts Medical Society outside the submitted work; research consulting to ABT Corporation (not remunerated). RS is an author of primary studies included in this review but was not involved in the data extraction or data analysis of those studies for this review.
Footnotes
Protocol Registration Details: The protocol for this review was pre-registered in PROSPERO #CRD42018086832 and the analysis plan was pre-registered in OSF#osf.io/m48g6.
Ethical Approval
The University of Oregon Institutional Review Board (IRB) reviewed the protocol for this review and determined that the study activities did not meet the definition of research with human subjects according to Title 45 CFR 46.102 (d-f) and thus did not require IRB approval.
CRediT Authorship Roles
Tanner-Smith: Conceptualization, Methodology, Investigation, Writing – Original Draft, Writing – Review & Editing, Supervision, Project administration, Funding acquisition. Parr: Methodology, Formal analysis, Investigation, Writing – Original Draft, Writing – Review and Editing, Visualization. Schweer-Collins: Methodology, Investigation, Writing – Original Draft, Writing – Review and Editing. Saitz. Conceptualization, Writing – Review & Editing, Supervision, Funding acquisition.
References
- 1.Boden JM, Fergusson DM. Alcohol and depression. Addiction. 2011;106(5):906–14. [DOI] [PubMed] [Google Scholar]
- 2.Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. Am J Prev Med. 2011;41(5):516–24. [DOI] [PubMed] [Google Scholar]
- 3.Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet. 2012;379(9810):55–70. [DOI] [PubMed] [Google Scholar]
- 4.United Nations Office on Drugs and Crime. World drug report 2012. Vienna: United Nations Office on Drugs and Crime; 2012. [Google Scholar]
- 5.Cherpitel CJ, Martin G, Macdonald S, Brubacher JR, Stenstrom R. Alcohol and drug use as predictors of intentional injuries in two emergency departments in British Columbia: Alcohol and drug use as predictors. Am J Addict. 2013;22(2):87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanner-Smith EE, Grant SP. Brief interventions as evidence-based prevention strategies. In: Sloboda Z, Petras H, Robertson E, Hingson R, editors. Prevention of Substance Use [Internet]. Cham: Springer International Publishing; 2019. p. 181–92. Available from: http://link.springer.com/10.1007/978-3-030-00627-3_11 [Google Scholar]
- 7.Tanner-Smith EE, Risser MD. A meta-analysis of brief alcohol interventions for adolescents and young adults: variability in effects across alcohol measures. Am J Drug Alcohol Abuse. 2016;42(2):140–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaner EF, Beyer FR, Muirhead C, Campbell F, Pienaar ED, Bertholet N, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev. 2018; 2(2): CD004148. Available from: http://doi.wiley.com/10.1002/14651858.CD004148.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleming MF, Mundt MP, French MT, Manwell LB, Stauffacher EA, Barry KL. Benefit-cost analysis of brief physician advice with problem drinkers in primary care settings. Med Care. 2000;38(1):7–18. [DOI] [PubMed] [Google Scholar]
- 10.Neighbors CJ, Barnett NP, Rohsenow DJ, Colby SM, Monti PM. Cost-effectiveness of a motivational intervention for alcohol-involved youth in a hospital emergency department. J Stud Alcohol Drugs. 2010;71(3):384–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wutzke SE, Shiell A, Gomel MK, Conigrave KM. Cost effectiveness of brief interventions for reducing alcohol consumption. Soc Sci Med. 2001;52(6):863–70. [DOI] [PubMed] [Google Scholar]
- 12.Kunz F, French M, Bazargan-Hejazi S. Cost-effectiveness analysis of a brief intervention delivered to problem drinkers presenting at an inner-city hospital emergency department. J Stud Alcohol. 2004;65(3):363–70. [DOI] [PubMed] [Google Scholar]
- 13.Angus C, Scafato E, Ghirini S, Torbica A, Ferre F, Struzzo P, et al. Cost-effectiveness of a programme of screening and brief interventions for alcohol in primary care in Italy. BMC Fam Pract. 2014;15:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tariq L, van den Berg M, Hoogenveen RT, van Baal PH. Cost-effectiveness of an opportunistic screening programme and brief intervention for excessive alcohol use in primary care. PLoS One. 2009;4(5):e5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbosa C, Cowell A, Dowd W, Landwehr J, Aldridge A, Bray J. The cost-effectiveness of brief intervention versus brief treatment of Screening, Brief Intervention and Referral to Treatment (SBIRT) in the United States: Cost-effectiveness analysis of SBIRT. Addiction. 2017;112:73–81. [DOI] [PubMed] [Google Scholar]
- 16.Mundt MP. Analyzing the costs and benefits of brief intervention [Internet]. NIAAA Publications. 2006. Available from: https://pubs.niaaa.nih.gov/publications/arh291/34-36.htm [PMC free article] [PubMed] [Google Scholar]
- 17.Estee S, Wickizer T, He L, Shah MF, Mancuso D. Evaluation of the Washington state screening, brief intervention, and referral to treatment project: Cost outcomes for Medicaid patients screened in hospital emergency departments. Med Care. 2010;48(1):18–24. [DOI] [PubMed] [Google Scholar]
- 18.O’Donnell A, Anderson P, Newbury-Birch D, Schulte B, Schmidt C, Reimer J, et al. The impact of brief alcohol interventions in primary healthcare: A systematic review of reviews. Alcohol Alcohol. 2014;49(1):66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patton R, Deluca P, Kaner E, Newbury-Birch D, Phillips T, Drummond C. Alcohol screening and brief intervention for adolescents: The how, what and where of reducing alcohol consumption and related harm among young people. Alcohol Alcohol. 2014;49(2):207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanner-Smith EE, Lipsey MW. Brief alcohol interventions for adolescents and young adults: A systematic review and meta-analysis. J Subst Abuse Treat. 2015;51:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnett E, Sussman S, Smith C, Rohrbach LA, Spruijt-Metz D. Motivational interviewing for adolescent substance use: A review of the literature. Addict Behav. 2012;37(12):1325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beich A, Thorsen T, Rollnick S. Screening in brief intervention trials targeting excessive drinkers in general practice: Systematic review and meta-analysis. BMJ. 2003;327(7414):536–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertholet N, Daeppen JB, Wietlisbach V, Fleming M, Burnand B. Reduction of alcohol consumption by brief alcohol intervention in primary care: Systematic review and meta-analysis. Arch Intern Med. 2005;165(9):986–95. [DOI] [PubMed] [Google Scholar]
- 24.Donoghue K, Patton R, Phillips T, Deluca P, Drummond C. The effectiveness of electronic screening and brief intervention for reducing levels of alcohol consumption: A systematic review and meta-analysis. J Med Internet Res. 2014;16(6):e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elzerbi C, Donoghue K, Boniface S, Drummond C. Variance in the efficacy of brief interventions to reduce hazardous and harmful alcohol consumption between injury and noninjury patients in emergency departments: A systematic review and meta-analysis of randomized controlled trials. Ann Emerg Med. 2017;70(5):714–723.e14. [DOI] [PubMed] [Google Scholar]
- 26.Saitz R. Screening and brief intervention for unhealthy drug use: Little or no efficacy. Front Psychiatry. 2014;5:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saitz R, Alford DP, Bernstein J, Cheng DM, Samet J, Palfai T. Screening and brief intervention for unhealthy drug use in primary care settings: Randomized clinical trials are needed. J Addict Med. 2010;4(3):123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young MM, Stevens A, Galipeau J, Pirie T, Garritty C, Singh K, et al. Effectiveness of brief interventions as part of the Screening, Brief Intervention and Referral to Treatment (SBIRT) model for reducing the nonmedical use of psychoactive substances: A systematic review. Syst Rev. 2014;3(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imtiaz S, Roerecke M, Kurdyak P, Samokhvalov AV, Hasan OSM, Rehm J. Brief interventions for cannabis use in healthcare settings: Systematic review and meta-analyses of randomized trials. J Addict Med. 2020;14(1):78–88. [DOI] [PubMed] [Google Scholar]
- 30.Halladay J, Scherer J, MacKillop J, Woock R, Petker T, Linton V, et al. Brief interventions for cannabis use in emerging adults: A systematic review, meta-analysis, and evidence map. Drug Alcohol Depend. 2019;204:107565. [DOI] [PubMed] [Google Scholar]
- 31.Lynch T, Ryan C, Hughes CM, Presseau J, Allen ZM, Bradley CP, et al. Brief interventions targeting long‐term benzodiazepine and Z‐drug use in primary care: A systematic review and meta‐analysis. Addiction. 2020;115(9):1618–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hingson R, Compton WM. Screening and brief intervention and referral to treatment for drug use in primary care. JAMA. 2014;312(5):488–9. [DOI] [PubMed] [Google Scholar]
- 33.Fleming MF, Barry KL, Manwell LB, Johnson K, London R. Brief physician advice for problem alcohol drinkers. A randomized controlled trial in community-based primary care practices. JAMA. 1997;277(13):1039–45. [PubMed] [Google Scholar]
- 34.Wallace P, Cutler S, Haines A. Randomised controlled trial of general practitioner intervention in patients with excessive alcohol consumption. BMJ. 1988;297(6649):663–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collins SE, Carey KB. Lack of effect for decisional balance as a brief motivational intervention for at-risk college drinkers. Addict Behav. 2005;30(7):1425–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaume J, McCambridge J, Bertholet N, Daeppen JB. Mechanisms of action of brief alcohol interventions remain largely unknown - a narrative review. Front Psychiatry. 2014;5:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Field CA, Caetano R, Harris TR, Frankowski R, Roudsari B. Ethnic differences in drinking outcomes following a brief alcohol intervention in the trauma care setting: Ethnicity and brief intervention. Addiction. 2010;105(1):62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gelberg L, Andersen RM, Afifi AA, Leake BD, Arangua L, Vahidi M, et al. Project QUIT (Quit Using Drugs Intervention Trial): A randomized controlled trial of a primary care-based multi-component brief intervention to reduce risky drug use. Addiction. 2015;110(11):1777–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merz V, Baptista J, Haller DM. Brief interventions to prevent recurrence and alcohol-related problems in young adults admitted to the emergency ward following an alcohol-related event: A systematic review. J Epidemiol Community Health. 2015;69(9):912–7. [DOI] [PubMed] [Google Scholar]
- 40.Kaner EF, Beyer F, Dickinson HO, Pienaar E, Campbell F, Schlesinger C, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev. 2007;(2):CD004148. Available from: http://doi.wiley.com/10.1002/14651858.CD004148.pub3 [DOI] [PubMed] [Google Scholar]
- 41.Saitz R, Palfai TPA, Cheng DM, Alford DP, Bernstein JA, Lloyd-Travaglini CA, et al. Screening and brief intervention for drug use in primary care: The ASPIRE randomized clinical trial. JAMA. 2014;312(5):502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heather N Interpreting null findings from trials of alcohol brief interventions. Front Psychiatry. 2014;5:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heather N The efficacy-effectiveness distinction in trials of alcohol brief intervention. Addict Sci Clin Pract. 2014;9(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kypri K Methodological issues in alcohol screening and brief intervention research. Subst Abus. 2007;28(3):31–42. [DOI] [PubMed] [Google Scholar]
- 45.Newton AS, Dong K, Mabood N, Ata N, Ali S, Gokiert R, et al. Brief emergency department interventions for youth who use alcohol and other drugs: A systematic review. Pediatr Emerg Care. 2013;29(5):673–84. [DOI] [PubMed] [Google Scholar]
- 46.Tanner-Smith E, et al. Brief substance use counseling interventions to reduce consumption and consequences among patients in general healthcare settings: Understanding variability in effects [Internet]. 2018. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42018086832
- 47.Tanner-Smith E, Saitz R, Darlington T, Parr N, Schweer-Collins M, Frankel L. Brief substance use interventions in general healthcare settings meta-analysis [Internet]. OSF; 2020. Available from: osf.io/m48g6 [Google Scholar]
- 48.Hedges LV. Distribution theory for Glass’s estimator of effect size and related estimators. J Educ Behav Stat. 1981;6(2):107–28. [Google Scholar]
- 49.Higgins JPT, Eldridge S, Li T. Chapter 23: Including variants on randomized trials. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from: www.training.cochrane.org/handbook [Google Scholar]
- 50.Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from: www.training.cochrane.org/handbook [Google Scholar]
- 51.Hedges LV, Tipton E, Johnson MC. Robust variance estimation in meta-regression with dependent effect size estimates. Res Synth Method. 2010;1(1):39–65. [DOI] [PubMed] [Google Scholar]
- 52.Tipton E Small sample adjustments for robust variance estimation with meta-regression. Psychol Methods. 2015;20(3):375–93. [DOI] [PubMed] [Google Scholar]
- 53.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57(1):289–300. [Google Scholar]
- 54.Sánchez-Meca J, Marín-Martínez F, Chacón-Moscoso S. Effect-size indices for dichotomized outcomes in meta-analysis. Psychol Methods. 2003;8(4):448–67. [DOI] [PubMed] [Google Scholar]
- 55.Parr NJ, Schweer-Collins ML, Darlington TM, Tanner-Smith EE. Meta-analytic approaches for examining complexity and heterogeneity in studies of adolescent development. J Adolesc. 2019;77:168–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.IntHout J, Ioannidis JPA, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6(7):e010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61(10):991–6. [DOI] [PubMed] [Google Scholar]
- 59.Lau J, Ioannidis JPA, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. 2006;333(7568):597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saitz R Screening for unhealthy drug use: Neither an unreasonable idea nor an evidence-based practice. JAMA. 2020;323(22):2263–5. [DOI] [PubMed] [Google Scholar]
- 61.Hatch-Maillette MA, Donovan DM, Laschober TC. Dosage of booster phone calls following an SBIRT intervention in the emergency department for reducing substance use. J Subst Abuse Treat. 2020;116:108043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bogenschutz MP, Donovan DM, Mandler RN, Perl HI, Forcehimes AA, Crandall C, et al. Brief intervention for patients with problematic drug use presenting in emergency departments: A randomized clinical trial. JAMA Internal Medicine. 2014. Nov 1;174(11):1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blow FC, Walton MA, Bohnert ASB, Ignacio RV, Chermack S, Cunningham RM, et al. A randomized controlled trial of brief interventions to reduce drug use among adults in a low-income urban emergency department: the HealthiER You study. Addiction. 2017;112(8):1395–405. [DOI] [PubMed] [Google Scholar]
- 64.Bernstein E, Edwards E, Dorfman D, Heeren T, Bliss C, Bernstein J. Screening and brief intervention to reduce marijuana use among youth and young adults in a pediatric emergency department. Acad Emerg Med. 2009;16(11):1174–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O’Grady MA, Kapoor S, Kwon N, Morley J, Auerbach M, Neighbors CJ, et al. Substance use screening and brief intervention: Evaluation of patient and implementation differences between primary care and emergency department settings. J Eval Clin Pract. 2019;25(3):441–7. [DOI] [PubMed] [Google Scholar]
- 66.Sutton AJ, Higgins JPT. Recent developments in meta-analysis. Statist Med. 2008;27(5):625–50. [DOI] [PubMed] [Google Scholar]
- 67.Oesterle TS, Kolla B, Risma CJ, Breitinger SA, Rakocevic DB, Loukianova LL, et al. Substance use disorders and telehealth in the COVID-19 pandemic era: A new outlook. Mayo Clin Proc. 2020;S0025619620311952. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


