Abstract
Background and Aims.
Smoking is associated with higher morbidity and mortality, including increased risk for brain aging/atrophy and dementia. Few studies examine early associations with brain aging. We hypothesized that adult men with a history of heavier smoking in early midlife would have older than predicted brain age 16 to 28 years later.
Design.
Prospective cohort observational study, utilizing smoking pack years data from average age 40 (early midlife) predicting predicted brain age difference scores (PBAD) at average ages 56, 62 (later midlife) and 68 (early old age). Early midlife alcohol use was also evaluated.
Setting.
Population-based United States sample.
Participants/Cases.
Participants were male twins of predominantly European ancestry who served in the United States military sometime between 1965 and 1975. Structural magnetic resonance imaging (MRI) began at average age 56. Subsequent study waves included most baseline participants; attrition replacement subjects were added at later waves.
Measures.
Self-reported smoking information was used to calculate pack years smoked at ages 40, 56, 62, and 68. MRIs were processed with the BARACUS program to create PBAD scores (chronological age – predicted brain age) acquired at average ages 56 (N=493; 2002–08), 62 (N=408; 2009–14), and 68 (N=499; 2016–19).
Findings.
In SEM models, age 40 pack years predicted more advanced age 56 PBAD (β = −0.144, p = 0.012, 95%CI −0.257, −0.032). Age 40 pack years did not additionally predict PBAD at later ages. Age 40 alcohol consumption, but not a smoking-by-alcohol interaction, predicted more advanced PBAD at age 56 (β = −0.166, p=0.001, 95%CI −0.261,−0.070) with additional influences at age 62 (β = −0.115, p = 0.005, 95%CI −0.195,−0.036). Age 40 alcohol did not predict age 68 PBAD. Within-twin pair analyses suggested some genetic mechanism partially underlying effects of alcohol, but not smoking, on PBAD.
Conclusions.
Heavier smoking and alcohol consumption by age 40 predicted advanced brain aging by age 56 in men. Although we cannot confirm causality, smoking and alcohol consumption in young adulthood and early middle age may accelerate brain aging.
INTRODUCTION
According to a World Health Organization 2020 report, over 1.3 billion people currently smoke cigarettes worldwide, with an estimated 8 million people dying from smoking-related illness each year.1 Smoking is a global health risk, associated with higher morbidity and mortality and increased risk for cognitive impairment and Alzheimer’s disease (AD).2,3 Neuroimaging studies of cigarette smoking and brain regions of interest (ROIs) consistently show that higher levels of cigarette smoking are related to thinner cortex and smaller subcortical volumes.4–9 A UK Biobank study comprising approximately 20,000 participants reported smaller total gray matter volume in long-term smokers.10 Smokers seeking treatment for tobacco use have shown smaller cerebellar volumes relative to nonsmokers.11 Associations between smoking, the entorhinal cortex, fusiform gyrus, and inferior temporal lobe provide additional evidence of links between smoking behaviors and pathological brain aging.6 A study of middle-aged adults found that individuals who had ever smoked had brain signatures more similar to adults with AD than non-smokers.12 Currently there are gaps in the literature as to whether smoking earlier in adulthood is related to later brain aging or how early in the life course those associations manifest, and whether the degree of brain aging continues to advance with continued aging.
Machine learning algorithms applied to magnetic resonance imaging (MRI) data have been developed to create neuroimaging-derived biomarkers of estimated ‘brain age’ that can be used to predict a person’s age based on the morphometry of their brain relative to chronological age or the brain of one’s peers.13–16 In contrast to approaches focused on specific ROIs, the brain age approach contextualizes a person’s brain age by their chronological age group, thereby summarizing a large amount of complex information into a single metric—the difference between one’s chronological age and estimated brain age. This allows for inferences about advanced brain aging and global brain integrity for one’s age. This approach comprises a powerful tool for identifying associations between premature/advanced brain aging and potential contributing factors or clinically relevant consequences. In predominantly cross-sectional studies, older brain age relative to chronological age is significantly associated with neurodegenerative diseases such as AD13,17–19, poorer cognitive performance15,20–22, health, lifestyle factors, and stress.13,23–26 Smoking has been associated with an older predicted brain age.21,24,25,27 Brains of individuals who smoked or consumed alcohol frequently appear to exhibit older predicted brain age compared to those of their peers.24 Some researchers have also proposed synergistic effects between smoking and alcohol.8,11 Importantly, the majority of studies have been cross-sectional or only included a single time point for brain imaging indices and had limited covariates.
In the present study we expand on the existing literature on relationships between smoking and brain aging by a) isolating cigarette smoking history (e.g. smoking pack years) at age 40 and b) incorporating longitudinal MRI data. Smoking data were available at average ages 40, 56, 62, and 68. We created estimates of brain age at ages 56, 62, and 68 based on the MRI data. We predicted that a history of heavier smoking by early midlife would be associated with more advanced brain aging later in life. Second, because some previous studies report co-occurrence of smoking and alcohol consumption, we conducted sensitivity analyses by additionally examining early midlife alcohol consumption and the interaction between smoking and alcohol consumption.
METHODS
Participants.
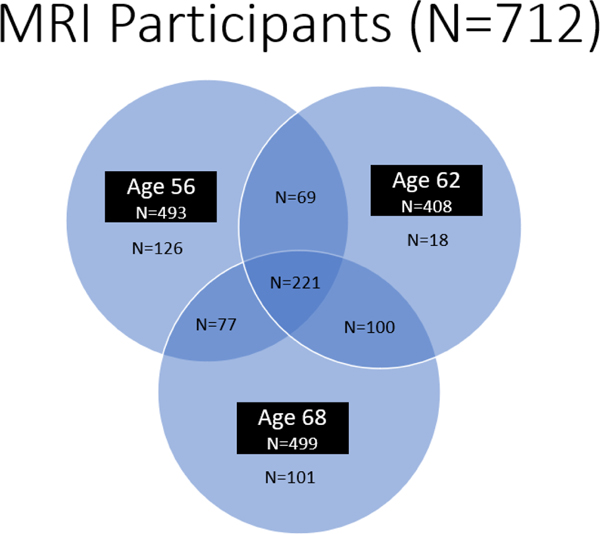
We recruited the original Vietnam Era Twin Study of Aging (VETSA) 1 (age 56; 2002–2008) participants28 from the Vietnam Era Twin Registry (VETR) members who participated in the Harvard Twin Study of Substance Abuse (HTSSA).29 All VETR members were eligible to participate in the HTSSA and were not selected for any disorder or substance use. The VETR is a large nationally distributed registry of male-male twin pairs who served in the United States military at some point between 1965 and 1975;30,31 most (80%) did not experience combat. VETSA 1 MRI (2004–2008) substudy eligibility requirements included enrolling in the VETSA parent study starting in 2004, being between the ages of 51 and 59 at enrollment, both members of a twin pair agreeing to participate, and passing the MRI safety screen (N=493). There were no significant differences between participants who had an MRI and those who did not. VETSA 2 (age 62; 2009–2014) and VETSA 3 (age 68; 2016–2019) data collections included the majority of the original participants as well as attrition replacement participants recruited from the same VETR cohort (Figure 1 shows the enrollment in the MRI study over time). Time between VETSA 1 and 2 was approximately 5.6 years, and 5.7 years between VETSA 2 and 3. Details of the sample ascertainment and data collections are described in elsewhere.28,32,33
Figure 1.
Enrollment in the VETSA MRI study at different waves and overlap over time.
The majority of participants (90.8%) were non-Hispanic White, with average lifetime education of 13.88 years. On average, participants were age 56 at VETSA 1 (M=56.2, SD=2.6, range 51–60), age 62 at VETSA 2 (M=61.8, SD=2.6, range 56–66), and age 68 at VETSA 3 (M=67.5, SD=2.6, range 66–73) (see Table 1 for descriptive statistics). Participants had comparable health and lifestyle characteristics to American men in their age range.34 We excluded participants if they had a history of seizures, multiple sclerosis, HIV/AIDS, or schizophrenia.
Table 1.
Descriptive Information on the VETSA Participants with Magnetic Resonance Imaging (MRI).
| Age 40 | Age 56 | Age 62 | Age 68 | X 2 | F-value | P-Value | |
|---|---|---|---|---|---|---|---|
| Participants with MRI (N) | 493 | 408 | 499 | ||||
| Age (yrs) | 40.03 (2.69) | 56.20 (2.62) | 61.82 (2.62) | 67.53 (2.61) | |||
| Age Change (yrs) | |||||||
| Age 40 to first MRI | 16.20 (0.87) | ||||||
| Education (years completed) | 13.88 (2.09) | 13.82 (2.12) | 13.74 (2.03) | 13.98 (2.07) | 1.108 | 0.344 | |
| Ethnicity | 2.030 | 0.512 | |||||
| White non-Hispanic | 90.76% | 89.86% | 88.24% | 88.58% | |||
| Other | 9.24% | 10.14% | 11.76% | 11.42% | |||
| Pack Years (if smoker) | 20.29 (14.94) | 27.20 (21.25) | 28.78 (22.84) | 28.57 (23.51) | 13.030 | <.001 | |
| Smoking Status | 56.828 | <.001 | |||||
| Current | 29.48% | 23.12% | 18.63% | 13.45% | |||
| Former | 31.79% | 40.77% | 43.38% | 48.39% | |||
| Never | 38.73% | 36.11% | 37.99% | 38.15% | |||
| Alcohol (Drinks/14 days) | 12.14 (21.73) | 10.91 (20.09) | 12.39 (22.09) | 11.37 (20.95) | 0.498 | 0.684 | |
| Alcohol Status | 2.282 | 0.892 | |||||
| None | 35.30% | 35.50% | 34.31% | 37.95% | |||
| Light | 50.86% | 52.33% | 51.72% | 50.20% | |||
| Heavy | 13.84% | 12.17% | 13.97% | 11.85% | |||
| Body Mass Index (BMI) | 25.61 (3.19) | 28.72 (4.15) | 28.89 (4.25) | 29.24 (4.25) | 108.900 | <.001 | |
| Heath Issues (% with condition) | |||||||
| Stroke | 0.31% | 1.42% | 1.72% | 4.61% | 2038.900 | <.001 | |
| Hypertension | 15.99% | 55.17% | 61.03% | 70.14% | 400.360 | <.001 | |
| Diabetes | 1.39% | 9.53% | 14.46% | 24.05% | 147.770 | <.001 |
Abbreviations. MRI=Magnetic Resonance Imaging
Procedures.
VETSA in-person assessments involved questionnaires, medical history interviews, neuropsychological testing, and structural MRI of the brain. Assessments occurred at two sites (University of California, San Diego and Boston University [with MRIs at Massachusetts General Hospital], but MRIs were conducted only in San Diego in VETSA 3. In addition, we accessed previously collected data from the VETR archive for these participants. First, from mean age 20 (SD=1.31, range 17–26), we used a cognitive assessment participants completed at their induction into the military as a measure of early adult general cognitive ability (GCA). Second, we utilized data from a mailed health survey conducted when participants were, on average, 40 years old (SD=2.7, range 33–44, 1990)30,31 which queried about cigarette smoking, alcohol consumption, health problems, and demographics. Approximately 16 years elapsed between the first assessment of smoking at age 40 and the first MRI at age 56. Measures assessed at each wave of data collection are depicted in Table 2.
Table 2.
Assessment Timeline of Key Variables
| Average Age at Assessment | ||||
|---|---|---|---|---|
|
| ||||
| 20 | 40 | 56 | 62 | 68 |
| Regular Variables | ||||
|
| ||||
| General Cognitive Ability | Smoking Consumption | PBAD | PBAD | PBAD |
| Alcohol Consumption | Age | |||
| BMI | Ethnicity | |||
| Cardiovascular health problems | Education | |||
| Hypertension | ||||
| Depression | ||||
| Respiratory health problems | ||||
|
| ||||
| Change Scores | ||||
|
| ||||
| Change in Alcohol Consumption (56–40) | ||||
| Change in Smoking Consumption (56–40) | ||||
| Age Change (62–56) | ||||
| Age Change (68–56) | ||||
Abbreviations: PBAD=predicted brain age difference score.
The studies were approved by local institutional review boards at the participating institutions, and participants provided written informed consent. From this point forward we refer to data collections by the mean participant age at time of assessment: 20, 40, 56, 62, and 68.
Measures.
Smoking pack years.
At ages 40, 56, and 68, participants responded to the same questions evaluating start and end dates of smoking and number of cigarettes smoked, if they had smoked more than 100 cigarettes in their lifetime. By age 40, 39% of participants had never smoked and 29% currently smoked (Table 1). At age 62, participants were asked whether their smoking habits had changed since the last data collection. If they smoked and indicated no change then the values for number of cigarettes smoked and initiation data at age 56 were used; if they indicated a change they reported their current smoking information.
Pack years is a standard measure of exposure risk which combines duration plus intensity of cigarette smoking. The pack years a person had smoked at each time point were calculated by multiplying the number of packs (# cigarettes/20) smoked per day by the number of years the person smoked). Pack years were highly correlated over time, from r=0.96 (age 40 with age 68) to r=0.99 (age 62 with age 68). Change in smoking was calculated by subtracting pack years at age 40 from pack years at age 56 where values of the change score represent gain in pack years. Due to non-normal distributions both pack years at age 40 and the pack years change score were subsequently square-root transformed.
Magnetic resonance imaging acquisition and predicted brain age.
Structural MRIs of the brain were acquired at age 56 (N=493) using 1.5 tesla (1.5T) MRI scanners, and at ages 62 (N=408) and 68 (N=499) using 3 tesla (3T) MRI scanners; 221 participants were scanned at all three times (Figure 1). Full MRI methods are provided in Supplement 1.35,36 We used Brain-Age Regression Analysis and Computation Utility software (BARACUS) v0.9.415,26 linear support vector regression models derived from each individual’s cortical thickness, cortical surface area, and subcortical volume data to create the composite predicted brain age score. Predicted brain age is subtracted from chronological age creating the predicted brain age difference score (PBAD).26 A negative PBAD indicates brain age that is estimated to be older than one’s chronological age. We then used residualized PBAD scores that were adjusted for scanner.
Covariates.
Covariates included age, race/ethnicity, education, GCA assessed at age 20, and alcohol consumption, cardiovascular health, respiratory health, hypertension, body mass index (BMI), and depression at age 40. Participants completed the age 20 GCA measure—the Armed Forces Qualification Test (AFQT). The AFQT is a 100-item multiple-choice test37,38 that is highly correlated with other tests of GCA such as Wechsler Adult Intelligence Scale (r=0.84); average intelligence of the VETSA sample is estimated at 105.39
Health variables at age 40 were self-reported. Alcohol consumption was based on consumption of wine, beer, and/or hard liquor during the past 14 days (total of number of days drank*number of drinks per day). We computed a change in alcohol consumption score by calculating the difference in drinks per 14 days at age 56 minus age 40. Due to a non-normal distribution, alcohol consumption at age 40 was square-root transformed, while the alcohol consumption change score was left in its original units. BMI was calculated as (weight (lbs.)/height (in)2)*703, hypertension was self-reported (yes/no). The cardiovascular disease (CVD) index composite included heart attack, angina, heart failure, rheumatic heart disease, mitral valve prolapse, stroke, heart surgery, peripheral vascular disease, atrial fibrillation, and diabetes. Respiratory health was a composite of history of asthma, chronic bronchitis, chronic obstructive pulmonary disease, and emphysema. At age 40, about 3.9% of participants had any CVD, 5.4% reported respiratory issues, and 7.8% said a doctor had ever told them they had depression.
We were primarily interested in modeling how smoking history at age 40 smoking predicts later PBAD, but this could be affected by change in several measures from age 40. We therefore included several change variables as covariates in various statistical models. Change scores simply subtract a participant’s smoking pack years, alcohol consumption, or age between respective assessments. Change in smoking pack years age range from 0 to a positive value; alcohol change could be positive or negative. Age change variables were the elapsed number of years between assessments. Note that age 56, 62, and 68 are averages, so the actual number of elapsed years varies across individuals. We included both education and age 20 GCA in order to adjust for social class/opportunity (e.g., education) and early adult cognitive ability, both of which may play a role in smoking initiation and continuity.
Data Analyses.
Descriptive statistics tested for participant differences at assessment waves by using ANOVA or chi-square tests (Table 1). Structural equation modeling (SEM) used Full Information Maximum Likelihood (FIML) with robust standard errors (MLR) because twin data were nested in families in these non-twin analyses.
We used MPlus 8.4 (Muthen & Muthen, 2019) to construct three SEM models: (1) Model 1 is the base—most simple—model where smoking pack years and change in smoking pack years predicted PBAD at ages 56, 62, and 68, and included demographic covariates and age 20 GCA; (2) Model 2 added age 40 alcohol consumption and change in alcohol consumption predicting PBAD at ages 56, 62, and 68, plus age 40 health covariates to Model 1; (3) Model 3 added the smoking-by-alcohol consumption interaction to Model 2.
Models controlled for a variety of covariates directly on age 56 PBAD and indirectly on age 62 and age 68 PBAD, which include education, race/ethnicity, age, age changes (between VETSA 1 and 2, and between VETSA 2 and 3), age 40 health covariates, and age 20 GCA. Key predictors that directly predicted age 56, 62, and 68 PBAD included smoking pack years at age 40, alcohol consumption at age 40, age tested at the age 56 assessment, and the change in smoking pack years between age 40 and age 56 assessments, and change in alcohol consumption between age 40 and age 56 assessments. Additionally, elapsed age in years between age 56 and age 62 assessments (AgeChange56to62) and between the age 56 and age 68 assessments (AgeChange56to68 ) predicted age 56 and age 68 PBAD, respectively. All three models adjusted for family since these are non-twin analyses.
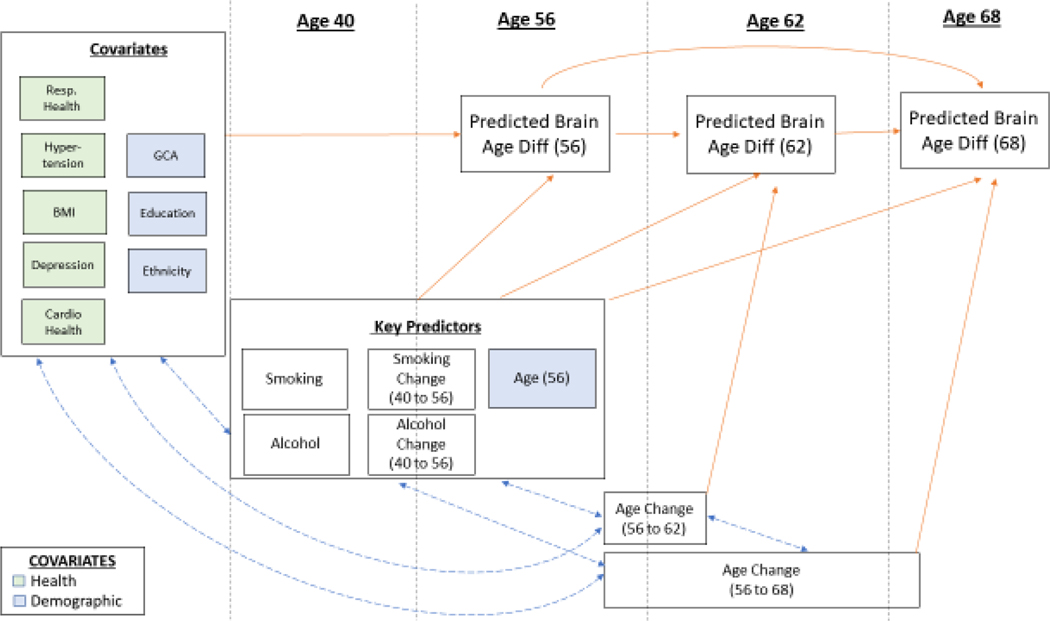
From a data visualization perspective, in Figure 2 we show simplified models, using blue paths to display correlations and orange paths to display predictor variables. In this figure, we also broke up variables into how they were modeled. These included covariates (correlated with other variables and only predict age 56 PBAD), key predictors (correlated with other variables and predict age 56, 62, and 68 PBAD), and age change variables (correlated with other variables, but only predict age 62 or 68 PBAD). Age change scores account for amount of elapsed time between assessments.
Figure 2.
Measures in Model 2: Smoking and Alcohol Predicting PBAD, with Covariates. Abbreviations: GCA=General Cognitive Ability; Resp=Respiratory; BMI=body mass index; Diff=difference. GCA, education, ethnicity, respiratory health, hypertension, BMI, cardiovascular health, and depression were correlated with each other. Key predictors (smoking, smoking change [age 40 to 56], alcohol consumption, and alcohol consumption change [age 40 to 56]) were correlated with each other. Age change at 56 to 62 and 56 to 68 were correlated with each other. Then, covariates, key predictors, and age change intercorrelations were modeled. Age 56 PBAD was regressed onto covariates. All three PBAD scores were regressed onto key predictors. Age 62 PBAD was regressed onto age change (56 to 62), and age 68 PBAD was regressed onto age change (56 to 68). Age 68 PBAD was regressed onto both age 56 PBAD and age 62 PBAD, and age 62 PBAD was regressed onto age 56 PBAD.
We applied a FIML approach where we added all covariates, key predictors, and age change variables as stand-alone variables in the models, estimating their individual variances. FIML allows for all available data to be used and produces unbiased parameters assuming data are missing at random with variables relevant to missingness included.40 Models adjusted for chronological age to control for effects of regression dilution bias in the PBAD measures.41 FIML raw continuous data methods used here are very robust to data missing at random (MAR). In contrast, if data are missing not at random, then missing values of a given variable may be related to the values of the variable itself. For example, subjects who report higher substance use or show signs of advanced brain ageing may experience poorer health preventing study participation, which results in non-random attrition. Capitalizing on this being a twin sample, for each of the six variables of interest (smoking pack years, pack years change, alcohol consumption and PBAD at Waves 1–3), we tested for distributional differences between complete and incomplete twin pair data using tests of mean and variance homogeneity.42 To the extent that higher substance use or advanced brain aging is (a) familial and (b) predicts subject attrition, then data from twin singletons (whose co-twin did not participate) would reveal different response distributions when compared to data from complete twin pairs. After false discovery rate (FDR) correction for multiple testing 43, we found no significant differences (see Supplement 4, Table S4–1). Thus, there does not appear to be non-random attrition related to our variables of interest.
We report standardized beta estimates. Significance levels are two-tailed. Age change covariates were considered as control variables of no interest. The analysis was not pre-registered.
In secondary analyses that further capitalized on this being a twin sample, we compared within-twin pair differences in monozygotic (MZ) and dizygotic (DZ) pairs—a type of cotwin-control analysis—to assess whether effects of pack years or alcohol on PBAD were due to environmental influences or due to genetic confounding.44,45 Detailed methods of this approach are provided in Supplement 3. Briefly, because MZ twins are genetically identical, only environmental factors can make them different.45 Thus, smaller within-pair differences in MZs compared with DZs would suggest that some genetic influences are at work. A lack of within-pair difference between MZ and DZ pairs would suggest environmental influences. A table of results for these analyses is provided in Supplement 3.
Goodness of Fit analyses
Multiple measures were used to assess fit46,47: Tucker-Lewis index (TLI)48; comparative fit index (CFI);49 root-mean square of approximation (RMSEA); and chi-square statistic50, where significance suggests lack of fit. Because these models are nested, chi-square tests were adjusted with a scaling factor.51 Good RMSEA values are typically <0.06; good-fitting CFI and TLI values are typically >0.95.52 Finally, the Akaike Information Criterion (AIC) is used to compare two models, where lower values indicate better fit.53 See Supplement 2, Table S2–3 for goodness of fit results.
RESULTS
Preliminary analyses.
Correlations among key measures are provided in Supplement 2 Table S2–1. Smoking pack years by age 40 were negatively correlated with PBAD at ages 56, 62, and 68 (r = −0.12, p = 0.005; r = −0.09, p = 0.06; r = −0.15, p = 0.001, respectively). Alcohol consumption at age 40 was also negatively correlated with PBAD at ages 56, 62, and 68 (r = −0.18, p <0.001; r = −0.22, p <0.001, r = −0.22, p <0.001, respectively). Thus, participants who smoked or drank more heavily had older than expected brains for their age, indicating more advanced brain aging. PBAD scores correlated highly across the 12 years of assessment (rs = 0.75 – 0.79, ps < 0.001). At age 40, heavier smokers consumed higher amounts of alcohol (r = 0.25, p <.001).
Structural Equation Models (SEM).
We focus here on the results for the main effects of age 40 smoking pack years and alcohol consumption, and their interaction. Full results for all the variables in the models, with confidence intervals, are shown in Supplement 2 Table S2–2.
Model 1 (Base Model).
Age 40 pack years were associated with age 56 PBAD (β = −0.165, p=0.004, 95%CI −0.276, −0.053), indicating that heavier smoking was associated with older brain age than expected (see Table 3). Age 40 pack years were not associated with PBAD at age 62 (β = 0.010, p = 0.839, 95%CI −0.090,0.111) or age 68 PBAD (β = −0.041, p=0.297, 95%CI −0.117,0.036). Age 56 PBAD was significantly associated with age 62 and age 68 PBAD (β = 0.736, p =0.000, 95%CI 0.673,0.798; β = 0.363, p=0.000, 95%CI 0.244,0.482 respectively) and age 62 PBAD was significantly associated with age 68 PBAD (β = 0.503, p=0.000, 95%CI 0.385,0.621. Neither age 20 GCA, education, nor change in smoking from age 40 to age 56 was associated with age 56 PBAD. Model 1 demonstrated good fit compared to a saturated model (see Supplement 2, Table S2–3).
Table 3.
Structural Equation Model Results for Key Measures from Best Fitting Model (Model 2).
| PBAD Age 56 | PBAD Age 62 | PBAD Age 68 | |
|---|---|---|---|
| Standardized estimate (se) p-value | Standardized estimate (se) p-value | Standardized estimate (se) p-value | |
| Smoking pack years age 40 | −0.144 (0.06) p=0.012 | 0.030 (0.05) p=0.563 | −0.043 (0.04) p=0.286 |
| Change in smoking | 0.130 (0.07) p=0.059 | −0.020 (0.06) p=0.723 | −0.014 (0.05) p=0.775 |
| Alcohol consumption age 40 | −0.166 (0.06) p=0.001 | −0.115 (0.04) p=0.005 | −0.028 (0.04) p=0.471 |
| Change in alcohol | −0.042 (0.05) p=0.359 | −0.009 (0.04) p=0.806 | 0.018 (0.03) p=0.534 |
| PBAD Age 56 | 0.721 (0.03) p<0.0001 | 0.361 (0.06) p<0.0001 | |
| PBAD Age 62 | 0.499 (0.06) p<.0001 |
Abbreviations: PBAD=Predicted Brain Age Difference. Model 2 variables include: smoking pack years at age 40, average drinks of alcohol in past 14 days at age 40, demographics (age, ethnicity, education), age 20 general cognitive ability, age 40 BMI, hypertension, cardiovascular disease index, respiratory index, depression (yes/no), changes in age (56 to 62; 56 to 68), changes in smoking (from 40 to 56), changes in alcohol consumption (40 to 56). (Full models including confidence intervals are provided in Supplement 2 Table S2–2).
Model 2.
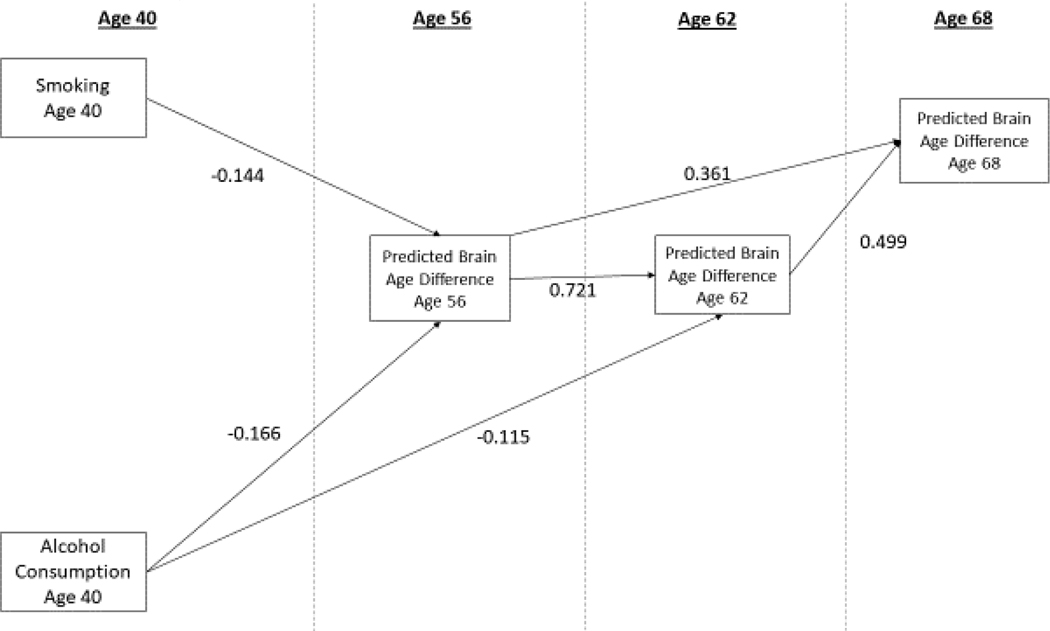
Model 2 added age 40 alcohol consumption measures and health covariates to Model 1. Age 40 pack years were associated with age 56 PBAD (β = −0.144, p = 0.012, 95%CI −0.257, −0.032; see Table 3 & Figure 3), but not with age 62 or age 68 PBAD. Age 40 alcohol consumption was associated with both age 56 PBAD and age 62 PBAD, but not with age 68 PBAD (β =−0.028, p=0.471, 95% CI −0.105,0.048). Thus, heavier smoking and alcohol consumption at age 40 were independently associated with having an older than predicted brain at age 56, and—for alcohol—also at age 62. Neither change in smoking nor change in alcohol consumption contributed significantly to Model 2. The model demonstrated good fit compared to a saturated model (see Supplement 2, Table S2–3). Of the age 40 covariate health measures, only hypertension was associated with age 56 PBAD (β = −0.125, SE = 0.036, p<0.0001) indicating that early hypertension was independently associated with having an older than expected brain age at age 56.
Figure 3.
shows significant main effects of age 40 smoking pack years and alcohol consumption on PBAD for the best-fitting model (Model 2). Model 2 adjusts for all covariates as indicated in Figure 2 except the smoking by alcohol interaction. Shown are standardized parameter estimates for significant paths (see Supplement 2 Table S2–2 for full results, including the confidence intervals). We constrained paths with the interaction term in Model 3 to zero, and the Model 2 AIC was then compared to Model 3. Model 3’s AIC was higher than the constrained model (37,797.452 vs 37,793.782), indicating that the more parsimonious Model 2, without the interaction, is a better fitting model.
Model 3.
Model 3 added the age 40 smoking-by-alcohol consumption interaction to Model 2. Neither age 40 smoking (β = −0.102, p = 0.122, 95%CI −0.231,0.027), alcohol (β = −0.095, p=0.23, 95%CI −0.249,0.06), nor their interaction (β = −0.11, p = 0.222, 95%CI −0.286, 0.066) were significantly associated with age 56 PBAD. Neither were they associated with age 62 or age 68 PBAD (see Supplement 2, Table S2–2). Although Model 3 demonstrated good fit (chi-square (𝜒2(20) = 21.48, p = 0.3694, RMSEA = 0.010, CFI = 0.998, TLI = 0.995), comparisons of Model 3 with Model 2 by constraining paths with the interaction term in Model 3 to zero showed that Model 3’s AIC was higher than the constrained model (37,797.452 vs 37,793.782). Thus, the more parsimonious Model 2—without the interaction—is a better fitting model.
Cotwin control analyses.
We compared within-twin pair differences in MZ and DZ pairs—a type of cotwin-control analysis—to assess whether effects of pack years or alcohol on PBAD were due to environmental influences or due to genetic confounding. A lack of MZ-DZ pair differences indicated that smoking effects on PBAD were due to environmental exposure. Because MZ twins are genetically identical, only environmental factors can make them different (see Supplement 3 for detailed methods and Table S3–1 for results). Although underpowered for a definitive conclusion, the lower MZ than DZ between-pair difference did suggest some genetic confounding of alcohol consumption effects on PBAD.
DISCUSSION
Our major finding was that smoking history at age 40 was associated with more advanced predicted brain age 16 years later at age 56 and that alcohol consumption independently contributed to advanced predicted brain age at both ages 56 and 62. Individuals with lower cognitive ability in young adulthood and fewer years of education were more likely to have higher pack years and higher alcohol consumption, but age 20 GCA and education were not directly associated with predicted brain age after accounting for their associations with alcohol consumption and smoking history at age 40. Hypertension was the only early midlife health factor that was associated with PBAD. Consistent with prior cross-sectional results, high blood pressure was associated with worse predicted brain age; however, our results were across a 16-year period.54 Studies of much older adults have reported associations between smoking and alcohol consumption, and associations of advanced predicted brain age with mild cognitive impairment (MCI) and dementia6,17,18,27,55. Our finding that age 40 smoking and alcohol consumption independently predicted PBAD as early as age 56 suggests that their associations with brain aging begin earlier than previously identified.
There are a number of distinguishing features of the present study. First, we had information on history of smoking and alcohol consumption at age 40—16 years before the brain age measures—and we had access to a broad array of risk/protective factors. It is a particular strength of the study that we are able to adjust for age 20 GCA since higher GCA is typically associated with having a larger and healthier brain as well as with less smoking and alcohol consumption.12,22 Previous findings about education are likely confounded with GCA and measures of premorbid GCA are seldom available in studies of older adults. With the data back to age 20 and use of SEM, we were able to adjust for interrelationships among these measures over a long period of time. The PBAD measure is also a strength of the study. In contrast to ROI-based measures, the brain-age approach contextualizes a person’s overall brain morphometry by their age group. This creates a single metric that summarizes a large amount of complex information across the brain, thus allowing for inferences about advanced brain aging and global brain morphometry. Having multiple measures of PBAD from age 56 to age 68 as well as smoking history assessed at multiple time points allowed us to examine the timing of the effects of smoking on brain aging. We were surprised, however, that, with the exception of age 40 hypertension, age 40 health problems—including the cardiovascular disease index—were not associated with this metric of brain aging. Lane et al. (2020) for instance, reported that higher Framingham cardiovascular risk scores at age 36, 53, and 69 were associated with smaller whole brain volume at age 69.56 It may be that the low prevalence of diseases at age 40 in our sample or the reliance on self-report reduced our ability to find these associations. However, while the original Framingham index may pick up on aggregated risk factors prior to disease onset, it may also obscure the relative contribution of separate risk factors at different times since smoking is one component of the Framingham index.
Smoking and alcohol consumption are presumed to affect brain health through multiple pathways involving cardiovascular risk and neurotoxic effects.11,57–60 In addition, smoking and alcohol consumption in early midlife could increase risk for dementia through their effects on brain aging.27 Smoking and alcohol consumption as environmental exposure effects on brain aging are intuitive. Interestingly, however, our within-twin pair analyses also suggested some genetic effects underlying the association of alcohol consumption and PBAD. Whether these genetic effects are related to genes that influence susceptibility to or amount of alcohol consumption, or genes that influence how the brain responds to alcohol remains to be determined. In any case, the results suggests that a partial mechanism underlying this effect is genetic differences. Further follow-up will also be needed to determine the extent to which earlier smoking, alcohol consumption, and PBAD modulate risk for AD or MCI.
Limitations.
The study has limited generalizability to women and ethnic minorities. Ample data on deleterious effects of smoking and alcohol are suggestive of causality, but without age 40 MRI data, definitive causal inferences cannot be made from our observational study. Although smoking pack years is an imperfect measure, it provides perspective on lifetime risk and exposure.61 Thus, smoking pack years at age 40 helps to anchor risk in early midlife. Also, due to a change in smoking questions only at age 62, the age 62 report of smoking required that the participant be able to compare their current smoking with smoking at age 56. This may introduce some bias due to recall at age 62 for those who were still smoking. The original BARACUS formulas were developed with data from 3T scanners but our age 56 data were from 1.5T scanners.15 However, the high intercorrelations among the PBAD measures across the three waves support the validity of the age 56 measure. Finally, genetic influences underlie both smoking behavior and brain aging. Our results suggest the interesting conclusion that alcohol’s effect on brain aging is partially genetic, but due to insufficient power, that conclusion must be considered preliminary.
Conclusions.
Meta-analyses suggest that a brain health risk reduction agenda could be effective in reducing risk for dementia, and smoking and alcohol consumption are among the top modifiable risk factors for dementia.3,62,63 The 2020 Lancet Dementia Prevention, Intervention, and Care Commission reported that ~40% of dementia incidence can be attributed to modifiable risk factors2, although this remains to be fully supported by clinical trials.64 The suggestion of genetic confounding for the effects of alcohol suggest that risk assessment and optimal intervention strategies regarding alcohol might differ for different genetic subgroups. The Lancet Commission life course model recommended targeting alcohol consumption during middle age and smoking in old age. Our findings regarding age 40 smoking and alcohol consumption extend life course research in this area and suggest that harm associated with these modifiable lifestyle behaviors was evident as early as midlife in men.
Supplementary Material
ACKNOWLEDGEMENTS.
The content is the responsibility of the authors and does not necessarily represent official views of the NIA, NIH, or VA. The U.S. Department of Veterans Affairs, Department of Defense; National Personnel Records Center, National Archives and Records Administration; National Opinion Research Center; National Research Council, National Academy of Sciences; and the Institute for Survey Research, Temple University provided invaluable assistance in the creation of the VET Registry. The Cooperative Studies Program of the U.S. Department of Veterans Affairs provided financial support for development and maintenance of the Vietnam Era Twin Registry. We would also like to acknowledge the continued cooperation and participation of the members of the VET Registry and their families.
FUNDING
This work was supported by the National Institute on Aging at the National Institutes of Health grant numbers R01s AG050595, AG022381, AG037985, AG062483, and P01 AG055367.
Footnotes
Declarations of competing interest: No competing interests to declare.
REFERENCES.
- 1.World Health Organization. Tobacco. https://www.who.int/news-room/fact-sheets/detail/tobacco. Published 2020. Updated May 2020. Accessed 12/22/2020, 2020.
- 2.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orgeta V, Mukadam N, Sommerlad A, Livingston G. The Lancet Commission on Dementia Prevention, Intervention, and Care: a call for action. Ir J Psychol Med. 2019;36(2):85–88. [DOI] [PubMed] [Google Scholar]
- 4.Sutherland MT, Riedel MC, Flannery JS, et al. Chronic cigarette smoking is linked with structural alterations in brain regions showing acute nicotinic drug-induced functional modulations. Behav Brain Funct. 2016;12(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durazzo TC, Meyerhoff DJ, Nixon SJ. Interactive effects of chronic cigarette smoking and age on hippocampal volumes. Drug Alcohol Depend. 2013;133(2):704–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durazzo TC, Meyerhoff DJ, Yoder KK. Cigarette smoking is associated with cortical thinning in anterior frontal regions, insula and regions showing atrophy in early Alzheimer’s Disease. Drug Alcohol Depend. 2018;192:277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durazzo TC, Meyerhoff DJ, Yoder KK, Murray DE. Cigarette smoking is associated with amplified age-related volume loss in subcortical brain regions. Drug Alcohol Depend. 2017;177:228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elbejjani M, Auer R, Jacobs DR Jr., et al. Cigarette smoking and gray matter brain volumes in middle age adults: the CARDIA Brain MRI sub-study. Transl Psychiatry. 2019;9(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prom-Wormley E, Maes HH, Schmitt JE, et al. Genetic and environmental contributions to the relationships between brain structure and average lifetime cigarette use. Behav Genet. 2015;45(2):157–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gray JC, Thompson M, Bachman C, Owens MM, Murphy M, Palmer R. Associations of cigarette smoking with gray and white matter in the UK Biobank. Neuropsychopharmacology. 2020;45(7):1215–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardenas VA, Hough CM, Durazzo TC, Meyerhoff DJ. Cerebellar Morphometry and Cognition in the Context of Chronic Alcohol Consumption and Cigarette Smoking. Alcohol Clin Exp Res. 2020;44(1):102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neth BJ, Graff-Radford J, Mielke MM, et al. Relationship Between Risk Factors and Brain Reserve in Late Middle Age: Implications for Cognitive Aging. Front Aging Neurosci. 2019;11:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franke K, Gaser C. Ten Years of BrainAGE as a Neuroimaging Biomarker of Brain Aging: What Insights Have We Gained? Front Neurol. 2019;10:789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franke K, Ziegler G, Kloppel S, Gaser C, Alzheimer’s Disease Neuroimaging I. Estimating the age of healthy subjects from T1-weighted MRI scans using kernel methods: exploring the influence of various parameters. Neuroimage. 2010;50(3):883–892. [DOI] [PubMed] [Google Scholar]
- 15.Liem F, Varoquaux G, Kynast J, et al. Predicting brain-age from multimodal imaging data captures cognitive impairment. Neuroimage. 2017;148:179–188. [DOI] [PubMed] [Google Scholar]
- 16.Cole JH, Marioni RE, Harris SE, Deary IJ. Brain age and other bodily ‘ages’: implications for neuropsychiatry. Mol Psychiatry. 2019;24(2):266–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaser C, Franke K, Kloppel S, Koutsouleris N, Sauer H, Alzheimer’s Disease Neuroimaging I. BrainAGE in Mild Cognitive Impaired Patients: Predicting the Conversion to Alzheimer’s Disease. PLoS One. 2013;8(6):e67346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowe LC, Gaser C, Franke K, Alzheimer’s Disease Neuroimaging I. The Effect of the APOE Genotype on Individual BrainAGE in Normal Aging, Mild Cognitive Impairment, and Alzheimer’s Disease. PLoS One. 2016;11(7):e0157514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Knol MJ, Tiulpin A, et al. Gray Matter Age Prediction as a Biomarker for Risk of Dementia. Proc Natl Acad Sci U S A. 2019;116(42):21213–21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beheshti I, Maikusa N, Daneshmand M, et al. Classification of Alzheimer’s Disease and Prediction of Mild Cognitive Impairment Conversion Using Histogram-Based Analysis of Patient-Specific Anatomical Brain Connectivity Networks. J Alzheimers Dis. 2017;60(1):295–304. [DOI] [PubMed] [Google Scholar]
- 21.Cole JH. Multimodality neuroimaging brain-age in UK biobank: relationship to biomedical, lifestyle, and cognitive factors. Neurobiol Aging. 2020;92:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elliott ML, Belsky DW, Knodt AR, et al. Brain-age in midlife is associated with accelerated biological aging and cognitive decline in a longitudinal birth cohort. Mol Psychiatry. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franke K, Gaser C, Manor B, Novak V. Advanced BrainAGE in older adults with type 2 diabetes mellitus. Front Aging Neurosci. 2013;5:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ning K, Zhao L, Matloff W, Sun F, Toga AW. Association of relative brain age with tobacco smoking, alcohol consumption, and genetic variants. Sci Rep. 2020;10(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith SM, Vidaurre D, Alfaro-Almagro F, Nichols TE, Miller KL. Estimation of brain age delta from brain imaging. Neuroimage. 2019;200:528–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hatton SN, Franz CE, Elman JA, et al. Negative fateful life events in midlife and advanced predicted brain aging. Neurobiol Aging. 2018;67:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Habes M, Janowitz D, Erus G, et al. Advanced brain aging: relationship with epidemiologic and genetic risk factors, and overlap with Alzheimer disease atrophy patterns. Transl Psychiatry. 2016;6:e775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kremen WS, Thompson-Brenner H, Leung YM, et al. Genes, environment, and time: the Vietnam Era Twin Study of Aging (VETSA). Twin Res Hum Genet. 2006;9(6):1009–1022. [DOI] [PubMed] [Google Scholar]
- 29.Tsuang MT, Bar JL, Harley RM, Lyons MJ. The Harvard Twin Study of Substance Abuse: What we have learned. Harv Rev Psychiatry. 2001;9:267–279. [PubMed] [Google Scholar]
- 30.Goldberg J, Curran B, Vitek ME, Henderson WG, Boyko EJ. The Vietnam Era Twin Registry. Twin Research. 2002;5(5):476–481. [DOI] [PubMed] [Google Scholar]
- 31.Henderson WG, Eisen SE, Goldberg J, True WR, Barnes JE, Vitek M. The Vietnam Era Twin Registry: A resource for medical research. Public Health Rep. 1990;105:368–373. [PMC free article] [PubMed] [Google Scholar]
- 32.Kremen WS, Franz CE, Lyons MJ. VETSA: the Vietnam Era Twin Study of Aging. Twin Res Hum Genet. 2013;16(1):399–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kremen WS, Franz CE, Lyons MJ. Current Status of the Vietnam Era Twin Study of Aging (VETSA). Twin Res Hum Genet. 2019;22(6):783–787. [DOI] [PubMed] [Google Scholar]
- 34.Schoenborn CA, Heyman KM. Health characteristics of adults aged 55 years and over: United States, 2004–2007. In: U.S. Department of Health and Human Services CfDCaP, ed. Vol 16. Hyattsville, MD: 2009. [PubMed] [Google Scholar]
- 35.Eyler LT, Chen CH, Panizzon MS, et al. A comparison of heritability maps of cortical surface area and thickness and the influence of adjustment for whole brain measures: a magnetic resonance imaging twin study. Twin Res Hum Genet. 2012;15(3):304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orme DR, Brehm W, Ree MJ. Armed Forces Qualification Test as a measure of premorbid intelligence. Military Psychology. 2001;13(4):187–197. [Google Scholar]
- 38.Uhlaner JE. Development of the Armed Forces Qualification Test and predecessor army screening tests, 1946–1950. PRB Rep. 1952. [Google Scholar]
- 39.Lyons MJ, York TP, Franz CE, et al. Genes determine stability and the environment determines change in cognitive ability during 35 years of adulthood. Psychol Sci. 2009;20(9):1146–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Enders CK, Bandalos DL. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural equation modeling. 2001;8:430–457. [PubMed] [Google Scholar]
- 41.de Lange AM, Cole JH. Correction procedures in brain-age prediction. Neuroimage: Clinical. 2020;26:102229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gillespie NA, Hatton SN, Hagler DJ, et al. The genetic etiology of longitudinal measures of predicted brain ageing in a population-based sample of mid to late-age males. bioRxiv. 2021. [Google Scholar]
- 43.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995;B 57:289–300. [Google Scholar]
- 44.Carlin JB, Gurrin LC, Sterne JA, Morley R, Dwyer T. Regression models for twin studies: a critical review. Int J Epidemiol. 2005;34(5):1089–1099. [DOI] [PubMed] [Google Scholar]
- 45.McGue M, Osler M, Christensen K. Causal Inference and Observational Research: The Utility of Twins. Perspect Psychol Sci. 2010;5(5):546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hutchinson SR, Olmos A. Behavior of Descriptive Fit Indexes in Confirmatory Factor Analysis Using Ordered Categorical Data. Struct Equ Modeling. 1998;5(4):344–364. [Google Scholar]
- 47.Schermelleh-Engel K, Moosbrugger H. Evaluating the fit of structural equation models: Tests of signficance and descriptive goodness-of-fit measures. Methods of Psychological Research. 2003;8:23–74. [Google Scholar]
- 48.Bentler PM, Bonett DG. Significance Tests and Goodness of Fit in the Analysis of Covariance-Structures. Psychol Bull. 1980;88(3):588–606. [Google Scholar]
- 49.Bentler PM. Comparative Fit Indexes in Structural Models. Psychol Bull. 1990;107(2):238–246. [DOI] [PubMed] [Google Scholar]
- 50.Bollen KA. A New Incremental Fit Index for General Structural Equation Models. Sociol Method Res. 1989;17(3):303–316. [Google Scholar]
- 51.Satorra A, Bentler PM. Ensuring positiveness of the scaled difference chi-square test statistic. Psychometrika. 2010;75:243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu LT, Bentler PM. Cutoff Criteria for Fit Indexes in Covariance Structure Analysis: Conventional Criteria Versus New Alternatives. Struct Equ Modeling. 1999;6(1):1–55. [Google Scholar]
- 53.Akaike H. Factor-Analysis and Aic. Psychometrika. 1987;52(3):317–332. [Google Scholar]
- 54.de Lange AG, Anaturk M, Suri S, et al. Multimodal brain-age prediction and cardiovascular risk: The Whitehall II MRI sub-study. Neuroimage. 2020;222:117292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chetelat G. Multimodal Neuroimaging in Alzheimer’s Disease: Early Diagnosis, Physiopathological Mechanisms, and Impact of Lifestyle. J Alzheimers Dis. 2018;64(s1):S199–S211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lane CA, Barnes J, Nicholas JM, et al. Associations Between Vascular Risk Across Adulthood and Brain Pathology in Late Life: Evidence From a British Birth Cohort. JAMA Neurol. 2020;77(2):175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mazzone P, Tierney W, Hossain M, Puvenna V, Janigro D, Cucullo L. Pathophysiological impact of cigarette smoke exposure on the cerebrovascular system with a focus on the blood-brain barrier: expanding the awareness of smoking toxicity in an underappreciated area. Int J Environ Res Public Health. 2010;7(12):4111–4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matloff WJ, Zhao L, Ning K, Conti DV, Toga AW. Interaction effect of alcohol consumption and Alzheimer disease polygenic risk score on the brain cortical thickness of cognitively normal subjects. Alcohol. 2020;85:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mende MA. Alcohol in the Aging Brain - The Interplay Between Alcohol Consumption, Cognitive Decline and the Cardiovascular System. Front Neurosci. 2019;13:713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Starke RM, Thompson JW, Ali MS, et al. Cigarette Smoke Initiates Oxidative Stress-Induced Cellular Phenotypic Modulation Leading to Cerebral Aneurysm Pathogenesis. Arterioscler Thromb Vasc Biol. 2018;38(3):610–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lubin JH, Caporaso NE. Misunderstandings in the misconception on the use of pack-years in analysis of smoking. Br J Cancer. 2013;108(5):1218–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hussenoeder FS, Riedel-Heller SG. Primary prevention of dementia: from modifiable risk factors to a public brain health agenda? Soc Psychiatry Psychiatr Epidemiol. 2018;53(12):1289–1301. [DOI] [PubMed] [Google Scholar]
- 63.World Health Organization. Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines. Geneva: 2019. [PubMed] [Google Scholar]
- 64.Montero-Odasso M, Ismail Z, Livingston G. One third of dementia cases can be prevented within the next 25 years by tackling risk factors. The case “for” and “against”. Alzheimers Res Ther. 2020;12(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.