Abstract
Background
Macular edema is the most common cause of impaired vision due to uveitis. Although various medications are available, not all uveitis patients with macular edema are satisfied with the treatment results. Therefore, solving this gap becomes the utmost concern worldwide. This study attempted to use bibliometric analysis to compare the valuable information in the top 100 highly cited studies in the field of drug therapy for uveitic macular edema (UME) and then determine the research hot spots and trends in this field.
Methods
In this study, the Science Citation Index Expanded (SCIE) of Web of Science (WOS) was used to collect the top 100 most cited studies on UME and analyze the literature from different countries/regions, institutions, and journals. The visualization knowledge maps is generated by VOSviewer and Citespace software.
Results
The top 100 highly cited studies are from 34 countries/regions. The United States has the largest number of publications, followed by the England, Spain and Germany. The top three institutions publishing highly cited literature are all from the England: University of London, University College London, and Moorfields Eye Hospital NHS Foundation Trust. Ophthalmology is the most widely published journal with 14 papers. The total number of citations is 1,371, meaning that Ophthalmology is the most authoritative journal in the field of UME drug therapy. The top two articles with the most cited times are from the United States, accounting for 36.5% of the total cited times of the top 10 articles. Keywords were divided into three clusters: the corticosteroid administration pathway, biological agents, and clinical trials. Uveitis, cystoid macular edema, efficacy, dexamethasone, and triamcinolone acetonide appeared more frequently in keywords. Researches on local and long-acting drug has gradually becoming the hot spots and trends.
Conclusion
This study concludes that bibliometric analysis can intuitively and quickly obtain the frontiers and hot spots of research in the field of UME drug therapy. Corticosteroid administration, biological agents, and clinical trials are considered the potential focus of future research.
Keywords: macular edema, uveitis, citations, bibliometric analysis, drug treatment
Introduction
Macular edema is one of the most common complications of uveitis and one of the main causes of blindness in non-infectious uveitis patients (1, 2). According to literature reports, the incidence of macular edema in uveitis patients is about 20–30% or even higher, and more than 1/4 of these patients were with best-corrected visual acuity (BCVA) ≤20/50 during the 2-year follow-up (1, 3).
Nowadays, the pathologic mechanisms and diagnostic classification of UME are relatively clear. In UME, inflammation leads to the destruction of the blood–retinal barrier and increased chorioretinal vascular permeability, resulting in fluid accumulation in the macular area, which is more common in the outer plexus layer (4). Clinically, optical coherence tomography (OCT) can be used to confirm the diagnosis, which can be classified into three types (5, 6): cystoid macular edema (CME), diffuse macular edema (DME), and serous retinal detachment (RD). Among them, DME was the most common, reaching more than half (6). In addition, Iannetti et al. compared the visual impairment between CME and DME patients and found that CME has a greater impact on visual acuity (7).
In contrast, the treatment of UME is a challenging problem due to the diversity of drug therapy. At present, glucocorticoids are still the first-line therapy in clinical practice, and immunosuppressants and biological agents are also being used more and more (8). The Multicenter Uveitis Steroid Treatment Trial (MUST) research group found that intravitreal corticosteroid injections were more effective than periocular injections in the treatment of UME (9). Injectable fluocinolone acetonide implant was used to treat non-infective UME in a clinical study with an average follow-up of 19 months. The implant improved vision and was safe and long-lasting (10, 11). Other studies have shown that for uveitis patients with refractory CME, systemic treatment with interferon alpha-2b can significantly improve CME and vision (12). In recent years, much attention has been paid to the efficacy of biological agents, and there are more and more kinds of biological agents. For example, TNF-α inhibitors are used for refractory cystic macular edema associated with non-infectious uveitis (13). Although there are a variety of drugs used to treat UME in clinical practice, they still cannot meet the needs of all patients. Gaggiano et al. found that patients with juvenile idiopathic arthritis (JIA) related uveitis received a single conventional disease-modifying anti-rheumatic drugs (cDMARDs) does not achieve a good clinical response, especially concurrent UME, which may require a combination of biologic agents, such as interleukin-6 inhibitors, to achieve better therapeutic effect (14). Moreover, there is no sign of active ocular inflammation, macular edema may still persist and even develop into refractory UME, resulting in severe visual impairment. Therefore, UME needs to be further studied in terms of drug therapy.
In recent years, bibliometric analysis has received much attention from researchers. It is a method to study literature publications by using mathematics and statistics and has been widely used in various scientific fields (15). For example, some scholars have used econometric analysis of the literature to determine hot spots and trends in the study of brain inflammasome/coke death (16). Bibliometric analysis can systematically output valuable and reliable information in all relevant literature in a certain field in the form of scientific knowledge maps and tables. Bibliometric analysis is easy to operate, reliable, and reproducible and can prevent the influence of potentially subjective factors. It can help researchers quickly understand the hot spots and trends in related fields and provide new ideas for future research direction planning (4, 16). It is vital for researchers to grasp the research hot spots and emerging development trends in relevant fields in a timely and accurate manner (17). To sum up, bibliometric analysis can precisely meet the needs of researchers.
However, with the rapid development of uveal inflammatory macular edema, research on drug treatment of UME is increasing year by year, but the research hot spot and development trend in this field are still not clear. In this study, bibliometric analysis was applied for the first time in the field of uveal inflammation and macular edema, especially to grasp the hot spots and trends of drug therapy. We used VOSviewer to visualize the top 100 most cited studies in the field of drug therapy for UME in the Web of Science (WOS) database to explore the research direction and potential hot spots of UME in drug therapy.
Materials and Methods
Data Sources and Search Strategies
We used the Science Citation Index Expanded (SCIE) of WOS to collect articles. Literature search was performed on October 17, 2021 at Zhejiang University. The search query was framed as follows: (TS = (uveitis) AND TS = (macular edema) AND (TS = (drug therapy) OR TS = (biologicals)OR TS = (Biological Medicine) OR TS = (Biological Drug) OR TS = (Immunosuppressive Agents) OR TS = (Adrenal Cortex Hormones) OR TS = (Glucocorticoids) OR TS = (dexamethasone))). The date range was set from the beginning of the database to October 17, 2021. The search results were arranged by the citation counts in descending order. Then, the 100 most cited documents were selected and sorted in Excel 2019.
Data Collection
The characteristics of these documents were extracted, including title, keywords, document type, citation number, publication date, country, institutions, journals, and the 2020 impact factor of journals. VOSviewer 1.6.17 (Leiden University, Leiden, the Netherlands) (18) was used to make figures for keyword co-occurrence networks. The circles on the graph represent the keywords, and the diameter of the circles represents the frequency. The larger the diameter of the circle, the higher the frequency of the keyword and the stronger the correlation with the whole. The top 20 keywords with the strongest citation bursts is to import the data of the top 100 highly cited literatures in this field into Citespace 5.8.R3 (19) for analysis. The red bars mean some keywords cited continually; the green bars were keywords cited infrequently.
Results
Years and Types of Publication
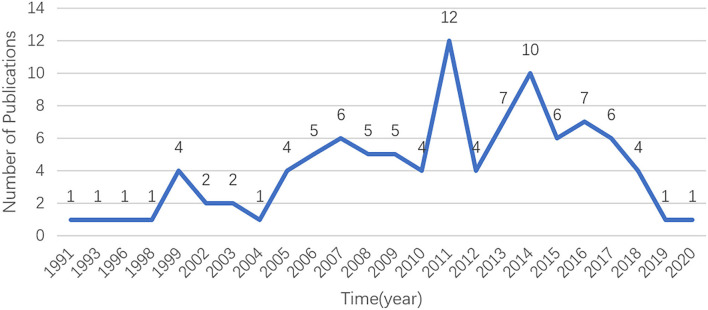
A total of 370 documents were acquired from the database by using the search terms given earlier. As shown in Figure 1, the 100 most cited documents were published from 1991 to 2020. The publication time of most of the highly cited documents was from 2005 to 2018. Among them, 12 and 10 papers were published in 2011 and 2014, respectively. The highly cited literature in these two years primarily focused on the treatment of dexamethasone intravitreal implant (DEX implant). This coincided with the time when the DEX implant (Ozurdex; Allergan, Inc., Irvine, CA, USA) was approved by the United States Food and Drug Administration (20). Interestingly, since 2016, the number of highly cited references has decreased significantly, which may be due to the lack of classical literature published in recent years. On the contrary, some studies published earlier did not have more citations due to a longer time.
Figure 1.
The number of publications in the top 100 most-cited articles per year in the field of UME drug therapy.
In terms of document types, Table 1 shows that among the 100 most cited literature studie. Among them, articles account for 60%, reviews account for 31%, proceedings paper accounted for 7%, while book chapters, and editorial materials account for 1% respectively.
Table 1.
Type of articles of the 100 most-cited articles in the field of UME drug therapy.
| Type of articles | Number |
|---|---|
| Article | 60 |
| Review | 31 |
| Article; book chapter | 1 |
| Editorial material | 1 |
| Article; proceedings paper | 7 |
Countries/Regions and Institutions Analysis
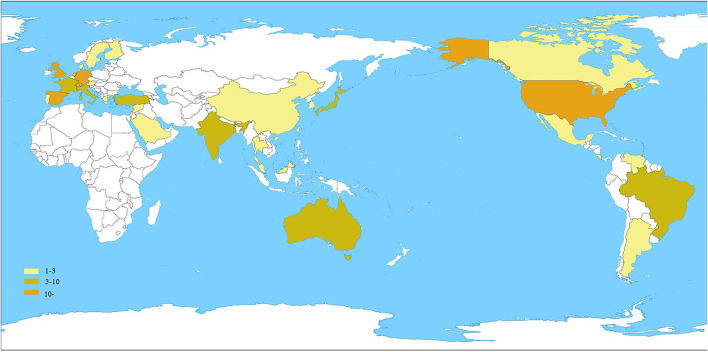
Figure 2 summarizes the spatial distribution of 100 highly cited global publications in the field of UME drug therapy. The documents came from 34 countries/regions. Table 2 shows the United States had the largest number of publications, with 46. The England, Spain, and Germany were the next countries/regions to publish more than 10 articles. It can be seen that most of the high-level literature comes from developed countries/regions.
Figure 2.
Global spatial distribution of the top 100 highly cited articles published.
Table 2.
The top 100 most-cited articles in the field of UME drug therapy by countries/regions and number of publications.
| Country/regions | Number |
|---|---|
| USA | 46 |
| England | 17 |
| Spain | 16 |
| Germany | 11 |
| France | 8 |
| Netherlands | 8 |
| Italy | 7 |
| Switzerland | 6 |
| India | 4 |
| Japan | 4 |
| Australia | 3 |
| Brazil | 3 |
| Turkey | 3 |
| Argentina | 2 |
| Greece | 2 |
| Malaysia | 2 |
| Mexico | 2 |
| Peoples R. China | 2 |
| Saudi Arabia | 2 |
| Thailand | 2 |
| Venezuela | 2 |
| Austria | 1 |
| Canada | 1 |
| Costa Rica | 1 |
| Denmark | 1 |
| Finland | 1 |
| Israel | 1 |
| Lebanon | 1 |
| Portugal | 1 |
| Scotland | 1 |
| Singapore | 1 |
| South Korea | 1 |
| Sweden | 1 |
| Syria | 1 |
Table 3 shows the top 10 institutions publishing the most cited literature on UME drug therapy and their publications. The top three, notably, are all from the England: University of London (n = 12), University College London (n = 11), and Moorfields Eye Hospital NHS Foundation Trust (n = 11). It is followed by two institutions from Spain: Hospital Clinic Barcelona (n = 10) and University of Barcelona (n = 10). Also, although the United States has the largest number of highly cited studies, the ranking of institutions in the United States is not the highest.
Table 3.
Top 10 institutions publishing highly cited literature in the field of UME drug therapy and their publications.
| Rank | Institution | No. of publications | Countries/Regions |
|---|---|---|---|
| 1 | University of London | 12 | England |
| 2 | Moorfields Eye Hospital NHS Foundation TrusT | 11 | England |
| 3 | University College London | 11 | England |
| 4 | Hospital Clinic de Barcelona | 10 | Spain |
| 5 | University of Barcelona | 10 | Spain |
| 6 | Abbvie | 9 | the United States |
| 7 | Allergan | 9 | the United States |
| 8 | University of California System | 9 | the United States |
| 9 | Assistance Publique Hopitaux Paris APHP | 5 | France |
| 10 | IDIBAPS | 5 | Spain |
Journal Analysis
Table 4 lists all journals of these 100 highly cited papers by the number of publications, and 80% of the top 10 journals are from the United States, with the other 20% from the Netherlands and the England. However, the most recent impact factors (IFs) for the top 10 journals ranged from 2.671 to 12.079, with 70% of them in Quartile 1. Of interest, the leading journal was Ophthalmology (IF = 12.079) from the United States, with 14% publications and 1,371 citations, indicating that Ophthalmology is the authoritative journal in the field of UME pharmacotherapy. The second- and third-ranked journals are Retinal-The Journal of Retinal (IF = 4.256) and American Journal of Ophthalmology (IF = 5.285). Their publications are 7 and 6, respectively, and their total citations are 305 and 475, respectively. These two journals are also from the United States. These show that the United States has a high degree of influence in this area.
Table 4.
Journals of the 100 most-cited articles in the field of UME drug therapy.
| Journal | No. of articles | Citation count | IF | Quartile in category | Countries/regions |
|---|---|---|---|---|---|
| Ophthalmology | 14 | 1,371 | 12.079 | Q1 | USA |
| Retina-The Journal of Retinal and Vitreous Diseases | 7 | 305 | 4.256 | Q1 | USA |
| American Journal of Ophthalmology | 6 | 475 | 5.285 | Q1 | USA |
| Ocular Immunology and Inflammation | 6 | 256 | 3.07 | Q2 | Netherlands |
| Journal of Ocular Pharmacology and Therapeutics | 5 | 227 | 2.671 | Q2 | USA |
| Graefes Archive for Clinical and Experimental Ophthalmology | 4 | 288 | 3.117 | Q2 | USA |
| Investigative Ophthalmology and Visual Science | 4 | 170 | 4.799 | Q1 | USA |
| Archives of Ophthalmology | 3 | 744 | 4.399 | Q1 | USA |
| British Journal of Ophthalmology | 3 | 225 | 4.638 | Q1 | England |
| Current Opinion in Ophthalmology | 3 | 152 | 3.761 | Q1 | USA |
| Eye | 3 | 98 | 3.775 | Q1 | England |
| Ophthalmologica | 3 | 159 | 3.25 | Q2 | Switzerland |
| Documenta Ophthalmologica | 2 | 158 | 2.379 | Q3 | Netherlands |
| European Journal of Ophthalmology | 2 | 56 | 2.597 | Q3 | England |
| Expert Opinion on Pharmacotherapy | 2 | 82 | 3.889 | Q2 | England |
| Ophthalmic Research | 2 | 147 | 2.892 | Q2 | Switzerland |
| Orphanet Journal of Rare Diseases | 2 | 71 | 4.123 | Q2 | England |
| Survey of Ophthalmology | 2 | 145 | 6.048 | Q1 | USA |
| Advanced Drug Delivery Reviews | 1 | 95 | 15.47 | Q1 | Netherlands |
| Advances in Therapy | 1 | 95 | 3.845 | Q3 | England |
| Annals of the New York Academy of Sciences | 1 | 34 | 5.691 | Q1 | USA |
| Arthritis Rheumatology | 1 | 78 | 10.995 | Q1 | USA |
| Biomedicine Pharmacotherapy | 1 | 59 | 6.529 | Q1 | France |
| BMC Ophthalmology | 1 | 32 | 2.209 | Q3 | England |
| Canadian Journal of Ophthalmology Journal Canadian D Ophtalmologie | 1 | 31 | 1.882 | Q4 | Canada |
| Clinical Rheumatology | 1 | 26 | 2.98 | Q3 | England |
| Cochrane Database of Systematic Reviews | 1 | 39 | 9.266 | Q1 | England |
| Current Drug Targets | 1 | 40 | 3.465 | Q2 | U Arab Emirates |
| Drugs | 1 | 34 | 9.546 | Q1 | New Zealand |
| European Journal of Pharmacology | 1 | 27 | 4.432 | Q2 | Netherlands |
| Experimental Eye Research | 1 | 46 | 3.467 | Q2 | USA |
| International Journal of Pharmaceutics | 1 | 41 | 5.875 | Q1 | Netherlands |
| International Ophthalmology Clinics | 1 | 30 | 0.469 (2001) | Q4 | USA |
| Journal of Cataract and Refractive Surgery | 1 | 40 | 3.351 | Q2 | USA |
| Journal of Drug Targeting | 1 | 32 | 5.121 | Q1 | England |
| Journal of Ophthalmology | 1 | 35 | 1.909 | Q3 | USA |
| Journal of Rheumatology | 1 | 51 | 4.666 | Q2 | Canada |
| Mediators of Inflammation | 1 | 28 | 4.711 | Q2 | England |
| Neurology | 1 | 115 | 9.91 | Q1 | USA |
| Pharmaceutical Science to Improve the Human Condition Prix Galien 2014 | 1 | 34 | 5.691 | Q1 | USA |
| Progress in Retinal and Eye Research | 1 | 167 | 21.198 | Q1 | England |
| Proteomics | 1 | 38 | 3.984 | Q2 | Germany (FED REP GER) |
| Rheumatology | 1 | 39 | 7.58 | Q1 | England |
| Scientific World Journal | 1 | 48 | 1.219 (2013) | Q2 | USA |
| Seminars in Arthritis and Rheumatism | 1 | 53 | 5.532 | Q1 | USA |
| Translational Vision Science Technology | 1 | 31 | 3.283 | Q2 | USA |
Cited Literature Analysis
Table 5 lists the top 100 most cited papers in this field, sorted by citation times. Not surprisingly, the top 10 articles of high quality are all in Quartile 1. The total number of citations for the top 10 articles is 1,953. The number of citations for the top two papers, both from the United States, accounted for 36.5% of the top 10 citations. They were, respectively, published by Lowder in 2011 and Kuppermann in 2007, both of which were related to intravitreal injection of dexamethasone in the treatment of UME (19, 20). Also, 5 of the 10 papers are from the United States, 3 from the England, 1 from the Netherlands, and 1 from Spain. We note that Nussenblatt published the earliest of 100 papers in 1991, with 133 citations. He first studied the therapeutic effect of immunosuppressive cyclosporine and steroid hormone in uveitis patients with macular edema (20).
Table 5.
Top 100 most-cited articles in UME drug therapy studies.
| First author | Title | Year | Journal | Quartile in category | IF | CIT | Type | Countries/regions |
|---|---|---|---|---|---|---|---|---|
| Lowder, Careen | Dexamethasone Intravitreal Implant for non-infectious Intermediate or Posterior Uveitis | 2011 | Archives of Ophthalmology | Q1 | 4.399 (2014) | 407 | Article | USA |
| Kuppermann, Baruch D. | Randomized controlled study of an intravitreous dexamethasone drug delivery system in patients with persistent macular edema | 2007 | Archives of Ophthalmology | Q1 | 4.399 (2015) | 306 | Article | USA |
| Chu, Colin J. | Risk factors and incidence of macular edema after cataract surgery. A database study of 81,984 eyes | 2016 | Ophthalmology | Q1 | 12.079 | 177 | Article | England |
| Kok, H. | Outcome of intravitreal triamcinolone in uveitis | 2005 | Ophthalmology | Q1 | 12.079 | 169 | Article | England |
| de Smet, Marc D. | Understanding uveitis: the impact of research on visual outcomes | 2011 | Progress in Retinal and Eye Research | Q1 | 21.198 | 167 | Article | England |
| de Boer, J. | Visual loss in uveitis of childhood | 2003 | British Journal of Ophthalmology | Q1 | 4.638 | 165 | Article | Netherlands |
| Warwar, R. E. | Cystoid macular edema and anterior uveitis associated with latanoprost use - experience and incidence in a retrospective review of 94 patients | 1998 | Ophthalmology | Q1 | 12.079 | 158 | Article | USA |
| Diaz-Llopis, Manuel | Treatment of refractory uveitis with Adalimumab: a prospective multicenter study of 131 patients | 2012 | Ophthalmology | Q1 | 12.079 | 141 | Article | Spain |
| Nussenblatt, R. B. | Randomized, double-masked study of cyclosporine compared to prednisolone in the treatment of endogenous uveitis | 1991 | American Journal of Ophthalmology | Q1 | 5.258 | 133 | Article | USA |
| Williams, George A. | Dexamethasone posterior-segment drug delivery system in the treatment of macular edema resulting from uveitis or irvine-gass syndrome | 2009 | American Journal of Ophthalmology | Q1 | 5.258 | 130 | Article | USA |
| Yang, Peizeng | Clinical features of Chinese patients with Behcet's disease | 2008 | Ophthalmology | Q1 | 12.079 | 125 | Article | Peoples R. China |
| Jain, Nieraj | Fingolimod-associated macular edema incidence, detection, and management | 2012 | Neurology | Q1 | 9.91 | 115 | Review | USA |
| Erckens, R. J. | Adalimumab successful in sarcoidosis patients with refractory chronic non-infectious uveitis | 2012 | Graefes Archive for Clinical and Experimental Ophthalmology | Q2 | 3.117 | 109 | Article | Netherlands |
| Jonas, Jost B. | Intravitreal triamcinolone acetonide: a change in a paradigm | 2006 | Ophthalmic Research | Q2 | 2.892 | 107 | Review | Germany |
| Kiddee, Weerawat | Intraocular pressure monitoring post intravitreal steroids: a systematic review | 2013 | Survey of Ophthalmology | Q1 | 6.048 | 104 | Review | Canada |
| Moroi, S. E. | Cystoid macular edema associated with latanoprost therapy in a case series of patients with glaucoma and ocular hypertension | 1999 | Ophthalmology | Q1 | 12.079 | 102 | Article; Proceedings Paper | USA |
| Yasukawa, T. | Intraocular sustained drug delivery using implantable polymeric devices | 2005 | Advanced Drug Delivery Reviews | Q1 | 15.47 | 96 | Review | Japan |
| London, Nikolas J. S. | The dexamethasone drug delivery system: indications and evidence | 2011 | Advances in Therapy | Q3 | 3.847 | 95 | Review | USA |
| Zarbin, Marco A. | Ophthalmic evaluations in clinical studies of fingolimod (FTY720) in multiple sclerosis | 2013 | Ophthalmology | Q1 | 12.079 | 89 | Article | USA |
| Diaz-Llopis, Manuel | Adalimumab therapy for refractory uveitis: a pilot study | 2008 | Journal of Ocular Pharmacology and Therapeutics | Q2 | 2.671 | 87 | Article | Spain |
| Adan, Alfredo | Tocilizumab treatment for refractory uveitis-related cystoid macular edema | 2013 | Graefes Archive for Clinical and Experimental Ophthalmology | Q2 | 3.117 | 80 | Article | Spain |
| Wolfensberger, T. J. | The role of carbonic anhydrase inhibitors in the management of macular edema | 1999 | Documenta Ophthalmologica | Q2 | 2.379 | 80 | Article; Proceedings Paper | Switzerland |
| Calvo-Rio, Vanesa | Anti-Interleukin-6 receptor tocilizumab for severe juvenile idiopathic arthritis-associated uveitis refractory to anti-tumor necrosis factor therapy a multicenter study of twenty-five patients | 2017 | Arthritis and Rheumatology | Q1 | 10.995 | 78 | Article | Spain |
| Tomkins-Netzer, Oren | Treatment with repeat dexamethasone implants results in long-term disease control in eyes with non-infectious uveitis | 2014 | Ophthalmology | Q1 | 12.079 | 78 | Article | England |
| Guex-Crosier, Y. | The pathogenesis and clinical presentation of macular edema in inflammatory diseases | 1999 | Documenta Ophthalmologica | Q2 | 2.379 | 78 | Article; Proceedings Paper | Switzerland |
| Zarranz-Ventura, Javier | Multicenter study of intravitreal dexamethasone implant in non-infectious uveitis: indications, outcomes, and reinjection frequency | 2014 | American Journal of Ophthalmology | Q1 | 5.258 | 76 | Article | England |
| Taylor, Simon R. J. | Intraocular methotrexate in the treatment of uveitis and uveitic cystoid macular edema | 2009 | Ophthalmology | Q1 | 12.079 | 76 | Article; Proceedings Paper | England |
| Malcles, Ariane | Safety of intravitreal dexamethasone implant (ozurdex) the safodex study. incidence and risk factors of ocular hypertension | 2017 | Retina-The Journal of Retinal and Vitreous Diseases | Q1 | 4.256 | 71 | Article | France |
| Muselier, Aurore | Efficacy of tocilizumab in two patients with anti-TNF-alpha refractory uveitis | 2011 | Ocular Immunology and Inflammation | Q2 | 3.07 | 71 | Article | France |
| Hsu, Jason | Drug delivery methods for posterior segment disease | 2007 | Current Opinion in Ophthalmology | Q1 | 3.761 | 70 | Article | USA |
| Plskova, Jarka | Interferon-alpha as an effective treatment for non-infectious posterior uveitis and Panuveitis | 2007 | American Journal of Ophthalmology | Q1 | 5.258 | 69 | Article | Scotland |
| Adan, Alfredo | Dexamethasone intravitreal implant for treatment of uveitic persistent cystoid macular edema in vitrectomized patients | 2013 | Retina-The Journal of Retinal and Vitreous Diseases | Q1 | 4.256 | 63 | Article | Spain |
| Taylor, Simon R. J. | New developments in corticosteroid therapy for uveitis | 2010 | Ophthalmologica | Q2 | 3.25 | 63 | Article | England |
| Rathinam, Sivakumar R. | A randomized clinical trial comparing methotrexate and mycophenolate mofetil for non-infectious uveitis | 2014 | Ophthalmology | Q1 | 12.079 | 61 | Article | India |
| Kenawy, Nihal | Abatacept: a potential therapy in refractory cases of juvenile idiopathic arthritis-associated uveitis | 2011 | Graefes Archive for Clinical and Experimental Ophthalmology | Q2 | 3.117 | 61 | Article | England |
| Nayak, Kritika | A review on recent drug delivery systems for posterior segment of eye | 2018 | Biomedicine and Pharmacotherapy | Q1 | 6.53 | 59 | Review | India |
| Mesquida, Marina | Long-term effects of tocilizumab therapy for refractory uveitis-related macular edema | 2014 | Ophthalmology | Q1 | 12.079 | 57 | Article | Spain |
| Edelman, Jeffrey L. | Differentiating intraocular glucocorticoids | 2010 | Ophthalmologica | Q2 | 3.25 | 57 | Article | USA |
| Rothova, Aniki | Inflammatory cystoid macular edema | 2007 | Current Opinion in Ophthalmology | Q1 | 3.761 | 56 | Article | Netherlands |
| Rothova, A. | Medical treatment of cystoid macular edema | 2002 | Ocular Immunology and Inflammation | Q2 | 3.07 | 54 | Review | Netherlands |
| Pato, Esperanza | Systematic review on the effectiveness of immunosuppressants and biological therapies in the treatment of autoimmune posterior uveitis | 2011 | Seminars in Arthritis and Rheumatism | Q1 | 5.532 | 53 | Review | Spain |
| Tappeiner, Christoph | Evidence for tocilizumab as a treatment option in refractory uveitis associated with juvenile idiopathic arthritis | 2016 | Journal of Rheumatology | Q2 | 4.666 | 51 | Article | Germany |
| Zhao, Min | Differential regulations of AQP4 and Kir4.1 by triamcinolone acetonide and dexamethasone in the healthy and inflamed retina | 2011 | Investigative Ophthalmology and Visual Science | Q1 | 4.799 | 50 | Article | France |
| Kato, A | Feasibility of drug delivery to the posterior pole of the rabbit eye with an episderal implant | 2004 | Investigative Ophthalmology and Visual Science | Q1 | 4.799 | 50 | Article | Japan |
| Dutra Medeiros, Marco | Dexamethasone intravitreal implant in vitrectomized versus non-vitrectomized eyes for treatment of patients with persistent diabetic macular edema | 2014 | Journal of Ocular Pharmacology and Therapeutics | Q2 | 2.671 | 49 | Article | Spain |
| Thorne, Jennifer E. | Multifocal choroiditis with panuveitis - incidence of ocular complications and of loss of visual acuity | 2006 | Ophthalmology | Q1 | 12.079 | 49 | Article | USA |
| Kiernan, Daniel F. | The use of intraocular corticosteroids | 2009 | Expert Opinion on Pharmacotherapy | Q2 | 3.889 | 48 | Review | USA |
| Sarao, Valentina | Intravitreal steroids for the treatment of retinal diseases | 2014 | Scientific World Journal | Q2 | 1.219 (2013) | 47 | Review | Italy |
| Artornsombudh, Pichaporn | Infliximab treatment of patients with birdshot retinochoroidopathy | 2013 | Ophthalmology | Q1 | 12.079 | 47 | Article | USA |
| Barcia, Emilia | Downregulation of endotoxin-induced uveitis by intravitreal injection of polylactic-glycolic acid (PLGA) microspheres loaded with dexamethasone | 2009 | Experimental Eye Research | Q2 | 3.467 | 46 | Article | Spain |
| Okhravi, N. | Cystoid macular edema in uveitis | 2003 | Ocular Immunology and Inflammation | Q2 | 3.07 | 45 | Article | England |
| Cao, Jennifer H. | Dexamethasone intravitreal implant in the treatment of persistent uveitic macular edema in the absence of active inflammation | 2014 | Ophthalmology | Q1 | 12.079 | 42 | Article | USA |
| Whitcup, Scott M. | Pharmacology of corticosteroids for diabetic macular edema | 2018 | Investigative Ophthalmology and Visual Science | Q1 | 4.799 | 41 | Review | USA |
| Rodriguez Villanueva, Javier | Pharmaceutical technology can turn a traditional drug, dexamethasone into a first-line ocular medicine. A global perspective and future trends | 2017 | International Journal of Pharmaceutics | Q1 | 5.875 | 41 | Review | Spain |
| Fardeau, C. | Uveitic macular edema | 2016 | Eye | Q1 | 3.775 | 41 | Review | France |
| Nguyen, Quan Dong | Treating chronic non-infectious posterior segment uveitis: the impact of cumulative damage - Proceedings of an expert panel roundtable discussion | 2006 | Retina-The Journal of Retinal and Vitreous Diseases | Q1 | 4.256 | 41 | Article | USA |
| Schumer, R. A. | Putative side effects of prostaglandin analogs | 2002 | Survey of Ophthalmology | Q1 | 6.048 | 41 | Review | USA |
| Becerra, E. M. | Clinical evidence of intravitreal triamcinolone acetonide in the management of age-related macular degeneration | 2011 | Current Drug Targets | Q3 | 3.465 | 40 | Review | Argentina |
| Tappeiner, C. | Rituximab as a treatment option for refractory endogenous anterior uveitis | 2007 | Ophthalmic Research | Q2 | 2.892 | 40 | Article | Germany |
| Negi, A. K. | Single perioperative triamcinolone injection versus standard postoperative steroid drops after uneventful phacoemulsification surgery - Randomized controlled trial | 2006 | Journal of Cataract and Refractive Surgery | Q1 | 3.351 | 40 | Article; Proceedings Paper | England |
| Ozguler, Yesim | Management of major organ involvement of Behcet's syndrome: a systematic review for update of the EULAR recommendations | 2018 | Rheumatology | Q1 | 7.58 | 39 | Review | Turkey |
| Juthani, Viral V. | Non-steroidal anti-inflammatory drugs versus corticosteroids for controlling inflammation after uncomplicated cataract surgery | 2017 | Cochrane Database of Systematic Reviews | Q1 | 9.289 | 39 | Review | USA |
| Yong, Tao | Intravitreal Triamcinolone | 2011 | Ophthalmologica | Q2 | 3.25 | 39 | Review | Germany |
| Theodossiadis, Panagiotis G. | Intravitreal administration of the anti-TNF monoclonal antibody Infliximab in the rabbit | 2009 | Graefes Archive for Clinical and Experimental Ophthalmology | Q2 | 3.117 | 38 | Article | Greece |
| Deeg, Cornelia A. | Down-regulation of pigment epithelium-derived factor in uveitic lesion associates with focal vascular endothelial growth factor expression and breakdown of the blood-retinal barrier | 2007 | Proteomics | Q2 | 3.984 | 38 | Article | Germany |
| Vegas-Revenga, Nuria | Anti-IL6-receptor tocilizumab in refractory and non-infectious uveitic cystoid macular edema: multicenter study of 25 patients | 2019 | American Journal of Ophthalmology | Q1 | 5.258 | 37 | Article | Spain |
| Denniston, Alastair K. | Heterogeneity of primary outcome measures used in clinical trials of treatments for intermediate, posterior, and panuveitis | 2015 | Orphanet Journal of Rare Diseases | Q2 | 4.123 | 36 | Review | England |
| Minos, Evangelos | Birdshot chorioretinopathy: current knowledge and new concepts in pathophysiology, diagnosis, monitoring and treatment | 2016 | Orphanet Journal of Rare Diseases | Q2 | 4.123 | 35 | Review | England |
| Cabrera, Mariana | Sustained-release corticosteroid options | 2014 | Journal of Ophthalmology | Q3 | 1.909 | 35 | Review | USA |
| Saraiya, Nehali V. | Dexamethasone for ocular inflammation | 2011 | Expert Opinion on Pharmacotherapy | Q2 | 3.889 | 35 | Editorial Material | USA |
| Blumenkranz, Mark S. | Correlation of visual acuity and macular thickness measured by optical coherence tomography in patients with persistent macular edema | 2010 | Retina-The Journal of Retinal and Vitreous Diseases | Q1 | 4.256 | 35 | Article | USA |
| Mesquida, Marina | Twenty-four month follow-up of tocilizumab therapy for refractory uveitis- related macular edema | 2018 | Retina-The Journal of Retinal and Vitreous Diseases | Q1 | 4.256 | 34 | Article | Spain |
| Whitcup, Scott M. | Development of a dexamethasone intravitreal implant for the treatment of non-infectious posterior segment uveitis | 2015 | Pharmaceutical Science to Improve the Human Condition: Prix Galien 2014 | / | / | 34 | Article; Book Chapter | USA |
| Gulati, Nishi | Vascular endothelial growth factor inhibition in uveitis: a systematic review | 2011 | British Journal of Ophthalmology | Q1 | 4.638 | 34 | Review | USA |
| Becker, M. D. | Management of sight-threatening uveitis - New therapeutic options | 2005 | Drugs | Q1 | 9.546 | 34 | Review | Germany |
| Chen, Hongming | Recent developments in ocular drug delivery | 2015 | Journal of Drug Targeting | Q1 | 5.121 | 32 | Review | USA |
| Bhagat, Rahul | Comparison of the Release Profile and Pharmacokinetics of Intact and Fragmented Dexamethasone Intravitreal Implants in Rabbit Eyes | 2014 | Journal of Ocular Pharmacology and Therapeutics | Q2 | 2.671 | 32 | Article | USA |
| Tempest-Roe, Shenzhen | Local therapies for inflammatory eye disease in translation: past, present and future | 2013 | BMC Ophthalmology | Q3 | 2.209 | 32 | Review | England |
| Goldstein, Debra A. | Suprachoroidal corticosteroid administration: a novel route for local treatment of non-infectious uveitis | 2016 | Translational Vision Science and Technology | Q2 | 3.283 | 31 | Article | USA |
| Cunningham, Emmett T., Jr. | Practical approach to the use of corticosteroids in patients with uveitis | 2010 | Canadian Journal of Ophthalmology-Journal Canadien D Ophtalmologie | Q4 | 1.882 | 31 | Article; Proceedings Paper | USA |
| Mruthyunjaya, Prithvi | Efficacy of low-release-rate fluocinolone acetonide intravitreal implants to treat experimental uveitis | 2006 | Archives of Ophthalmology | Q1 | 4.399 (2016) | 31 | Article | USA |
| Schilling, H. | Long-term effect of acetazolamide treatment of patients with uveitic chronic cystoid macular edema is limited by persisting inflammation | 2005 | Retina-The Journal of Retinal and Vitreous Diseases | Q1 | 4.256 | 31 | Article | Germany |
| Holland, G. N. | Immune recovery uveitis | 1999 | Ocular Immunology and Inflammation | Q2 | 3.07 | 31 | Article; Proceedings Paper | USA |
| Khurana, Rahul N. | Efficacy and safety of dexamethasone intravitreal implant for persistent uveitic cystoid macular edema | 2015 | Retina-The Journal of Retinal and Vitreous Diseases | Q1 | 4.256 | 30 | Article | USA |
| Mudumba, Sri | Tolerability and pharmacokinetics of intravitreal sirolimus | 2012 | Journal of Ocular Pharmacology and Therapeutics | Q2 | 2.671 | 30 | Article | USA |
| Samiy, N. | The role of non-steroidal antiinflammatory drugs in ocular inflammation | 1996 | International Ophthalmology Clinics | Q4 | 0.469 (2001) | 30 | Article | USA |
| Nussenblatt, R.B. | A masked, randomized, dose-response study between cyclosporine-a and cyclosporine-g in the treatment of sight-threatening uveitis of non-infectious origin | 1993 | American Journal of Ophthalmology | Q1 | 5.258 | 30 | Article | USA |
| Bansal, Pooja | Posterior segment drug delivery devices: current and novel therapies in development | 2016 | Journal of Ocular Pharmacology and Therapeutics | Q2 | 2.671 | 29 | Review | India |
| Zierhut, Manfred | Therapy of ocular behcet disease | 2014 | Ocular Immunology and Inflammation | Q2 | 3.07 | 29 | Review | Germany |
| Ghosn, Corine R. | Treatment of experimental anterior and intermediate uveitis by a dexamethasone intravitreal implant | 2011 | Investigative Ophthalmology and Visual Science | Q1 | 4.799 | 29 | Article | USA |
| Hogewind, B. F. T. | Intravitreal triamcinolone for the treatment of refractory macular edema in idiopathic intermediate or posterior uveitis | 2008 | European Journal of Ophthalmology | Q3 | 2.597 | 29 | Article | Netherlands |
| Arcieri, E. S. | The effects of prostaglandin analogs on the blood aqueous barrier and corneal thickness of phakic patients with primary open-angle glaucoma and ocular hypertension | 2008 | Eye | Q1 | 3.775 | 29 | Article | Brazil |
| Pelegrin, L. | Long-term evaluation of dexamethasone intravitreal implant in vitrectomized and non-vitrectomized eyes with macular edema secondary to non-infectious uveitis | 2015 | Eye | Q1 | 3.775 | 28 | Article | Spain |
| Wang, Jillian | Drug delivery implants in the treatment of vitreous inflammation | 2013 | Mediators of Inflammation | Q2 | 4.711 | 28 | Review | USA |
| Dibas, Adnan | Glucocorticoid therapy and ocular hypertension | 2016 | European Journal of Pharmacology | Q3 | 2.597 | 27 | Article | USA |
| Goldberg, I. | A 5-year, randomized, open-label safety study of latanoprost and usual care in patients with open-angle glaucoma or ocular hypertension | 2008 | European Journal of Ophthalmology | Q3 | 2.597 | 27 | Article | Australia |
| Rajesh, Bindu | Safety of 6,000 intravitreal dexamethasone implants | 2020 | British Journal of Ophthalmology | Q1 | 4.638 | 26 | Article | Singapore |
| Lopalco, Giuseppe | IL-6 blockade in the management of non-infectious uveitis | 2017 | Clinical Rheumatology | Q3 | 2.98 | 26 | Review | Italy |
| Adeniran, Janelle M. Fassbender | Common and rare ocular side-effects of the dexamethasone implant | 2017 | Ocular Immunology and Inflammation | Q2 | 3.07 | 26 | Review | USA |
| Pearce, William | Advances in drug delivery to the posterior segment | 2015 | Current Opinion in Ophthalmology | Q1 | 3.761 | 26 | Review | USA |
Keyword Analysis
Table 6 lists the top 20 keywords with the highest frequency and strongest correlation strength in the 100 studies. Among them, the top 10 keywords are uveitis (n = 37), cystoid macular edema (n = 33), macular edema (n = 24), therapy (n = 16), efficacy (n = 15), dexamethasone (n = 12), posterior uveitis (n = 12), retinal vein occlusion (n = 12), diabetic macular edema (n = 11), and triamcinolone acetonide (n = 11). In general, the higher the occurrence frequency of keywords, the higher the corresponding overall correlation strength. From the perspective of these high-frequency keywords, the research hot spot in this field is mainly reflected in the effect of UME drug therapy, and the research on steroid hormones is relatively active.
Table 6.
The top 20 keywords with the highest occurrence and strongest overall association strength in articles related to UME drug therapy research.
| Keywords | Occurrences | Total link strength |
|---|---|---|
| Uveitis | 37 | 216 |
| Cystoid macular edema | 33 | 189 |
| Macular edema | 24 | 148 |
| Therapy | 16 | 75 |
| Efficacy | 15 | 83 |
| Dexamethasone | 12 | 84 |
| Retinal vein occlusion | 12 | 97 |
| Posterior uveitis | 12 | 78 |
| Diabetic macular edema | 11 | 79 |
| Triamcinolone acetonide | 11 | 64 |
| Triamcinolone | 10 | 76 |
| Endothelial growth factor | 10 | 73 |
| Intravitreal triamcinolone acetonide | 10 | 68 |
| Corticosteroids | 10 | 67 |
| Disease | 10 | 50 |
| Standardization | 9 | 52 |
| Dexamethasone intravitreal implant | 9 | 51 |
| Pars plana vitrectomy | 8 | 66 |
| Intermediate | 8 | 50 |
| Fluocinolone acetonide implant | 8 | 45 |
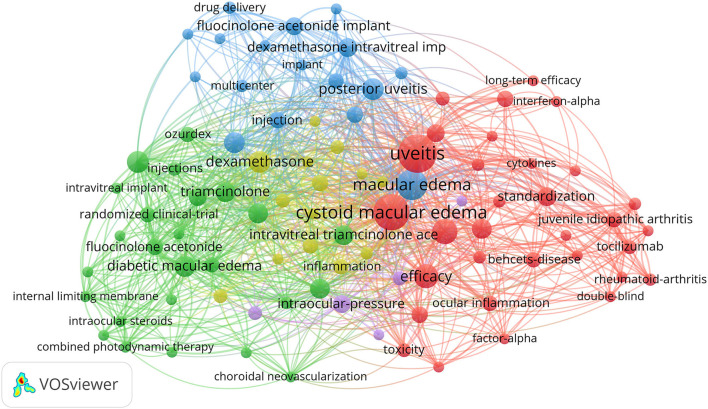
VOSviewer software was used to make a keyword co-occurrence network diagram, which can be very intuitive and quickly obtain the frontier and hot spots of a certain field of research. As shown in Figure 3, in the top 100 studies, a total of 93 keywords appeared more than three times. Obviously, these keywords are mainly divided into three clusters and are represented in different colors. The three clusters represent (1) the corticosteroid administration pathway in green, such as intravitreal implant, intravitreal triamcinolone acetonide, corticosteroids, and intraocular steroids; (2) biological agents in red, such as therapy, efficacy, infliximab, and interleukin-6; and (3) clinical trials in blue, such as trial, drug delivery, and multicenter. Also, there are two clusters of lower frequencies of purple and yellow, ocular hypertension, acetazolamide, and open-angle glaucoma. Recent hot spots and trends are reflected in these three aspects.
Figure 3.
VOSviewer visualization of keywords from the top 100 highly cited articles in the field of UME drug therapy.
Figure 4 reflects the top 20 most-cited keywords in UME drug therapy field at different stages by years. We found that the research hot spot in the field of UME drug therapy has shifted from systemic to local treatment, such as oral dexamethasone into intraocular steroid injection Meanwhile, the research hot spots of drug types is also shifting from short-acting to long-acting, for example, intraocular steroid injections from short-acting triamcinolone to long-acting fluocinolone acetonide implant.
Figure 4.
Top 20 keywords with the strongest citation bursts from the top 100 highly cited articles in the field of UME drug therapy.
Discussion
In this study, we identified the top 100 cited studies in the field of UME pharmacotherapy in the WOS Core database from the beginning of the database to 2021. Bibliometrics analysis was innovatively used to evaluate the national institutions, citations, journals, keywords, and other valuable information in these literature studies, especially the hot spots and trends in drug treatment.
A medical treatment for UME was first proposed 30 years ago (21). To this day, the volume of literature published in this field is increasing year by year. Interestingly, the top institutions for publishing these highly cited articles are mainly located in London (the England) and Barcelona (Spain), but the country with the largest number of articles is the United States. This indicates that in the field of UME drug therapy, the research institutions in the England and Spain are relatively concentrated, while in the United States, they are relatively dispersed. However, this may also be related to different national conditions and research institutions. We also found that among the 100 highly cited papers, the most published papers, the most cited times, and the top three journals were all from the United States, followed by the England, the Netherlands, and Spain. All of these indicate that in the field of UME drug therapy, the United States leads in the quality of academic papers, publications, and high-impact journals. We believe this is because of three reasons as follows: (1) newly developed and produced related drugs were first marketed in the United States, such as Ozurdex and adalimumab; (2) the United States has an advantage in economic level; and (3) the US government has made policies favorable to scientific research.
Through the analysis of high-frequency keywords, it can be found that the current attention on drug treatment of UME mainly focuses on the pathway of corticosteroid administration, biological agents, and clinical verification. Despite the rapid development of immunosuppressive and biologic therapies in recent years, corticosteroids are still the main treatment for UME (22). As the field of UME drug therapy advances, the research focus of corticosteroid therapy is reflected in the way of administration. According to our research, the way steroid hormones are used is improving in a certain direction. We conclude the following two aspects: (1) in terms of drug efficacy, the focus of intraocular steroid injection has gradually shifted from short-acting drug form to long-acting sustained-release drug form, reducing the injection frequency; and (2) in terms of safety, attention is gradually shifting from systematic to local drug use, which can significantly reduce systemic side effects of patients and significantly improve safety. In particular, for patients with systemic drug intolerance or poor efficacy, intravitreal injection of corticosteroids has become a new effective choice, such as triamcinolone acetonide (TA) or DEX implant (22–24). Some studies show that the effect of intravitreal injection of TA was short-lived, but the therapeutic effect of a single injection of DEX implant lasted for more than 6 months (22, 25). Therefore, the research hot spot in the field of UME drug therapy has changed from systemic treatment to local treatment, the type of drugs has changed from short-acting to long-acting, and the clinical validation of various drugs is also the focus. Furthermore, the shift in focus from systemic treatment to local treatment may be due to the latter's less invasive nature and fewer systemic side effects. The shift from short-acting to long-acting drugs has also brought benefits to patients, such as less frequent use and less pain. At the same time, the diversification of drug types requires perfect clinical trials to ensure drug safety. Our study reveals this latest practice trend and opens up a new perspective for readers to think about.
In recent years, the research trend in the field of UME drug therapy has gradually shifted to biologic therapy, and its role has been paid more and more attention by scholars. Aleksandra Radosavljevic et al. found that biological agents such as anti-IL-6 and interferon showed positive efficacy in the majority of patients with non-infectious severe or refractory UME (26). Deuter et al. found that tozizumab can be considered for chronic UME even if immunoregulatory therapy fails (27). Thomas found that TNF-α inhibitors such as Adalimumab and infliximab may be considered as first-line therapy for Uveitis associated with Behcet disease and juvenile idiopathic arthritis (28). Diaz-Llopis et al. found that adalimumab reduces inflammatory activity and is well-tolerated in patients with refractory uveitis who are tolerant to prednisone or an immunosuppressant, and it also reduces the dose of steroids used (29). In addition, a multicenter study reported that anti-IL-6 receptor tocilizumab (TCZ) was effective in treating refractory and non-infectious uveitis CME (30). A new clinical study suggests that interferon alpha-2a is safe and well-tolerated in the treatment of refractory uveitis and macular edema, and it is an option for patients with persistent refractory UME (31). In summary, biologic therapy has many advantages, such as the rapid onset of action, rapid control of active inflammation, longer-lasting efficacy than steroid hormones, high topical safety, and fewer side effects. However, there may be cases of relapse after withdrawal, requiring multiple injections. This is one of the hot points of this study.
Although the use of intravitreal corticosteroids and biologics is becoming more common, a large number of multicenter, randomized controlled trials are still needed to evaluate the safety, efficacy, and durability of these emerging therapies due to the large individual variation in these patients. A recent multicenter steroid therapy (MUST) trial for uveitis and a follow-up study of up to 7 years showed that 94% of patients ended up with UME gone, although they were still at risk for recurrence (32). There are a wide variety of drugs to treat UME, and their risks and side effects should be considered as well as their efficacy. For example, although tocilizumab (TCZ) is effective in treating refractory UME, side effects such as nausea, viral conjunctivitis, and bullous impetigo were still observed in a few patients (27). Therefore, bibliometric analysis shows that clinical verification of drug therapy for UME is still a hot topic in future research.
Strengths and Limitations
This article analyzes global research of UME, for the first time, by using the bibliometric analysis method. At the same time, we use the WOS database and bibliometric analysis software, both of which are objective, convincing, and more common than other databases or software. However, in our research, there are some unavoidable shortcomings. We use only one database, which may lead to the omission of some citations and documents. It is worth mentioning that due to the characteristics of the subject, the total number of articles in this field is not huge, which may be the most important reason for the limitations of this study.
Conclusion
In this study, we used bibliometric analysis to determine the current research status and hot trends in the field of UME drug therapy. At present, the research focus of UME drug therapy is often the route of corticosteroid administration, biological agents, and clinical trials. A timely understanding of the hot trends of UME drug therapy can better help scholars to clarify the future research direction and continuously discover and solve the urgent difficulties in this field.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author Contributions
LF and SC designed this study. JK performed the search and collected data. SC, JK, and LF rechecked data. SC performed analysis. SC and JK wrote the manuscript. LF reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The work was supported by grants from the National Nature Science Foundation of China (Nos. 81870648 and 82070949).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Lardenoye CW, van Kooij B, Rothova A. Impact of macular edema on visual acuity in uveitis. Ophthalmology. (2006) 113:1446–9. 10.1016/j.ophtha.2006.03.027 [DOI] [PubMed] [Google Scholar]
- 2.Cunningham ET, Zierhut M. Uveitic macular edema. Ocul Immunol Inflamm. (2018) 26:987–90. 10.1080/09273948.2018.1529466 [DOI] [PubMed] [Google Scholar]
- 3.Tomkins-Netzer O, Talat L, Bar A, Lula A, Taylor SR, Joshi L, et al. Long-term clinical outcome and causes of vision loss in patients with uveitis. Ophthalmology. (2014 D) 121:2387–92. 10.1016/j.ophtha.2014.07.007 [DOI] [PubMed] [Google Scholar]
- 4.Ma C, Su H, Li H. Global research trends on prostate diseases and erectile dysfunction: a bibliometric and visualized study. Front Oncol. (2020) 10:627891. 10.3389/fonc.2020.627891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Accorinti M, Okada AA, Smith JR, Gilardi M. Epidemiology of macular edema in uveitis. Ocul Immunol Inflamm. (2019) 27:169–80. 10.1080/09273948.2019.1576910 [DOI] [PubMed] [Google Scholar]
- 6.Markomichelakis NN, Halkiadakis I, Pantelia E, Peponis V, Patelis A, Theodossiadis P, et al. Patterns of macular edema in patients with uveitis: qualitative and quantitative assessment using optical coherence tomography. Ophthalmology. (2004) 111:946–53. 10.1016/j.ophtha.2003.08.037 [DOI] [PubMed] [Google Scholar]
- 7.Iannetti L, Accorinti M, Liverani M, Caggiano C, Abdulaziz R, Pivetti-Pezzi P. Optical coherence tomography for classification and clinical evaluation of macular edema in patients with uveitis. Ocul Immunol Inflamm. (2008) 16:155–60. 10.1080/09273940802187466 [DOI] [PubMed] [Google Scholar]
- 8.Teper SJ. Update on the management of uveitic macular edema. J Clin Med. (2021) 10:4133. 10.3390/jcm10184133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thorne JE, Sugar EA, Holbrook JT, Burke AE, Altaweel MM, Vitale AT, et al. Periocular triamcinolone vs. intravitreal triamcinolone vs intravitreal dexamethasone implant for the treatment of uveitic macular edema: the periocular vs Intravitreal corticosteroids for uveitic macular edema (point) trial. Ophthalmology. (2019) 126:283–95. 10.1016/j.ophtha.2018.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weber LF, Marx S, Auffarth GU, Scheuerle AF, Tandogan T, Mayer C, et al. Injectable 0.19-mg fluocinolone acetonide intravitreal implant for the treatment of non-infectious uveitic macular edema. J Ophthalmic Inflamm Infect. (2019) 9:3. 10.1186/s12348-019-0168-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stout NL, Alfano CM, Belter CW, Nitkin R, Cernich A, Lohmann Siegel K, et al. A bibliometric analysis of the landscape of cancer rehabilitation research (1992-2016). J Natl Cancer Inst. (2018) 110:815–24. 10.1093/jnci/djy108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butler NJ, Suhler EB, Rosenbaum JT. Interferon alpha 2b in the treatment of uveitic cystoid macular edema. Ocul Immunol Inflamm. (2012) 20:86–90. 10.3109/09273948.2011.645989 [DOI] [PubMed] [Google Scholar]
- 13.Schaap-Fogler M, Amer R, Friling R, Priel E, Kramer M. Anti-tnf-α agents for refractory cystoid macular edema associated with noninfectious uveitis. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle. Ophthalmologie. (2014) 252:633–40. 10.1007/s00417-013-2552-8 [DOI] [PubMed] [Google Scholar]
- 14.Gaggiano C, Rigante D, Tosi GM, Vitale A, Frediani B, Grosso S, et al. Treating juvenile idiopathic arthritis (jia)-related uveitis beyond tnf-α inhibition: a narrative review. Clin Rheumatol. (2020) 39:327–37. 10.1007/s10067-019-04763-3 [DOI] [PubMed] [Google Scholar]
- 15.Thompson DF, Walker CK. A descriptive and historical review of bibliometrics with applications to medical sciences. Pharmacotherapy. (2015) 35:551–9. 10.1002/phar.1586 [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Li Y, Guo L, Hong J, Zhao W, Hu X, et al. Bibliometric analysis of the inflammasome and pyroptosis in brain. Front Pharmacol. (2020) 11:626502. 10.3389/fphar.2020.626502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma D, Yang B, Guan B, Song L, Liu Q, Fan Y, et al. A bibliometric analysis of pyroptosis from 2001 to 2021. Front Immunol. (2021) 12:731933. 10.3389/fimmu.2021.731933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Eck NJ, Waltman L. Software survey: Vosviewer, a computer program for bibliometric mapping. Scientometrics. (2010) 84:523–38. 10.1007/s11192-009-0146-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci USA. (2004) 101(Suppl 1):5303–10. 10.1073/pnas.0307513100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghosn CR Li Y, Orilla WC, Lin T, Wheeler L, Burke JA, et al. Treatment of experimental anterior and intermediate uveitis by a dexamethasone intravitreal implant. Invest Ophthalmol Vis Sci. (2011) 52:2917–23. 10.1167/iovs.10-5939 [DOI] [PubMed] [Google Scholar]
- 21.Nussenblatt RB, Palestine AG, Chan CC, Stevens G, Mellow SD, Green SB. Randomized, double-masked study of cyclosporine compared to prednisolone in the treatment of endogenous uveitis. Am J Ophthalmol. (1991) 112:138–46. 10.1016/S0002-9394(14)76692-9 [DOI] [PubMed] [Google Scholar]
- 22.Lowder C, Belfort R, Lightman S, Foster CS, Robinson MR, Schiffman RM, et al. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol. (2011) 129:545–53. 10.1001/archophthalmol.2010.339 [DOI] [PubMed] [Google Scholar]
- 23.Couch SM, Bakri SJ. Intravitreal triamcinolone for intraocular inflammation and associated macular edema. Clin Ophthalmol. (2009) 3:41–7. 10.2147/OPTH.S4477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong Z, Namba K, Kitaichi N, Goda C, Kitamura M, Ohno S. Efficacy and complications of intravitreal injection of triamcinolone acetonide for refractory cystoid macular edema associated with intraocular inflammation. Jpn J Ophthalmol. (2008) 52:374–9. 10.1007/s10384-008-0574-2 [DOI] [PubMed] [Google Scholar]
- 25.Khurana RN, Porco TC. Efficacy and safety of dexamethasone intravitreal implant for persistent uveitic cystoid macular edema. Retina. (2015) 35:1640–6. 10.1097/IAE.0000000000000515 [DOI] [PubMed] [Google Scholar]
- 26.Radosavljevic A, Agarwal M, Bodaghi B, Smith JR, Zierhut M. Medical therapy of uveitic macular edema: biologic agents. Ocul Immunol Inflamm. (2020) 28:1239–50. 10.1080/09273948.2019.1709648 [DOI] [PubMed] [Google Scholar]
- 27.Deuter CME, Zierhut M, Igney-Oertel A, Xenitidis T, Feidt A, Sobolewska B, et al. Tocilizumab in uveitic macular edema refractory to previous immunomodulatory treatment. Ocul Immunol Inflamm. (2017) 25:215–20. 10.3109/09273948.2015.1099680 [DOI] [PubMed] [Google Scholar]
- 28.Thomas AS. Biologics for the treatment of noninfectious uveitis: Current concepts and emerging therapeutics. Curr Opin Ophthalmol. (2019) 30:138–50. 10.1097/ICU.0000000000000562 [DOI] [PubMed] [Google Scholar]
- 29.Diaz-Llopis M, Salom D, Garcia-de-Vicuna C, Cordero-Coma M, Ortega G, Ortego N, et al. Treatment of refractory uveitis with adalimumab: A prospective multicenter study of 131 patients. Ophthalmology. (2012) 119:1575–81. 10.1016/j.ophtha.2012.02.018 [DOI] [PubMed] [Google Scholar]
- 30.Vegas-Revenga N, Calvo-Río V, Mesquida M, Adán A, Hernández MV, Beltrán E, et al. Anti-il6-receptor tocilizumab in refractory and noninfectious uveitic cystoid macular edema: multicenter study of 25 patients. Am J Ophthalmol. (2019) 200:85–94. 10.1016/j.ajo.2018.12.019 [DOI] [PubMed] [Google Scholar]
- 31.De Simone L, Sangiovanni A, Aldigeri R, Mastrofilippo V, Bolletta E, Invernizzi A, et al. Interferon alpha-2a treatment for post-uveitic refractory macular edema. Ocul Immunol Inflamm. (2020) 28:322–8. 10.1080/09273948.2019.1589526 [DOI] [PubMed] [Google Scholar]
- 32.Tomkins-Netzer O, Lightman SL, Burke AE, Sugar EA, Lim LL, Jaffe GJ, et al. Seven-year outcomes of uveitic macular edema: the multicenter uveitis steroid treatment trial and follow-up study results. Ophthalmology. (2021) 128:719–28. 10.1016/j.ophtha.2020.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.