Abstract
Primary central nervous system lymphoma (PCNSL) remains a disease with poor outcome and high recurrence rate. We retrospectively analyzed the clinical data of 243 immunocompetent patients with PCNSL in Beijing Tiantan Hospital. The median age of PCNSL patients was 57 years (range 10-95 years). For induction therapy, 94.7% of patients received high-dose methotrexate (HD-MTX) containing regimens, and 59.3% received rituximab, which increased over time. The overall response rate was 72.8%, with 58.8% achieving complete response. With a median follow-up of 27.0 months (95% confidence interval 23.6-30.4), the median progression-free survival (PFS) time was 14.0 months (95% CI 9.45-18.55), and the 2-year PFS rate was 33.2%. The median overall survival (OS) was not reached (NR), with an estimated overall survival rate at 4 years of 61.6%. Among 95 patients who completed sequential consolidation chemotherapy with either pemetrexed or etoposide plus cytarabine, the median PFS was 28 months (95% CI 17.11-38.89), and the estimated overall survival at 4 years was 78.7%. In conclusion, HD-MTX based induction chemotherapy with non-myeloablative sequential consolidation chemotherapy is an alternative feasible treatment option.
Keywords: PCNSL = primary central nervous system lymphoma, HD-MTX, consolidation, chemoimmunotherapy, R-MAD regimen
Introduction
Primary central nervous system lymphoma (PCNSL) is an uncommon extranodal non-Hodgkin lymphoma, and more than 95% of PCNSLs are large B-cell subtypes. PCNSL in immunocompetent patients is distinct from systemic lymphoma in diagnosis, prognosis and therapeutic strategies (1). A population-based study reported a 5-year overall survival (OS) of 38-56% (2, 3).
High-dose methotrexate (HD-MTX) based systemic chemotherapy is widely accepted in current practice, meanwhile, promising results have been shown in several clinical trials investigating combination regimens of rituximab with cytotoxic chemotherapy (4–8). Although the importance of HD-MTX in newly developed PCNSL has become a general consensus, opinions on the optimal first-line chemotherapy regimens have not come to an agreement. While PCNSL responds to chemotherapy, about 50% of cases experience relapse in the first two years (9), emphasizing the necessity for efficient consolidation therapies. The use of whole-brain radiotherapy (WBRT) is limited by its neurotoxicity, especially in elderly patients. High-dose chemotherapy followed by autologous stem cell transplant (HCD-ASCT), however, is not available everywhere and is not suitable for elderly patients either. As a result, non-myeloablative sequential chemotherapy was considered another consolidation treatment strategy (5).
The object of this research was to summarize the characteristics among 243 PCNSL patients and analyze the efficacy of HD-MTX based induction therapy, meanwhile, explore the feasibility of nonmyeloablative sequential consolidation chemotherapy.
Methods
Patients
The clinical data of 243 immunocompetent patients with PCNSL from 10 May 2010 to 9 January 2020 were analyzed retrospectively. The inclusion criteria were as follows (1): PCNSL diagnosed by stereotactic biopsy, surgery or cerebrospinal fluid (CSF)/vitreous biopsy; (2) negative full-body CT scan or FDG-PET scan; and (3) immunocompetence and negative HIV status. Patients with primary intraocular lymphoma were excluded. The database was approved by the Beijing Tiantan Hospital Ethics Committee, and all patients gave written informed consent.
Evaluation of Response
Response was assessed according to International PCNSL Collaborative Group criteria. Enhanced MRI was routinely evaluated before each induction or consolidation treatment or any sign of potential disease progression. The overall objective response rate was defined as the sum of the complete response (CR), unconfirmed complete response (CRu) and partial response (PR) rates. Progression-free survival (PFS) was calculated from the start of treatment to the time of disease progression or death due to PCNSL. Overall survival (OS) was calculated from the date of diagnosis to the time of death from any cause.
Statistical Analysis
Clinical characteristics of patients with various induction regimens were compared using a chi square test. Probability estimates for PFS and OS were calculated by using Kaplan-Meier analysis. Cox’s regression model was applied for a multivariate survival analysis. SPSS statistics version 24.0 was utilized for all data analyses. P < 0.05 was considered significant.
Data Sharing Statement
The datasets supporting the conclusions of this study are available from the corresponding author on reasonable request.
Results
Main Characteristics at Diagnosis
In total, 243 newly diagnosed PCNSL patients were included in the analysis. Their main characteristics at diagnosis are reported in Table 1 . The median age was 57 years (range 10-95), and the median ECOG-PS (Eastern Cooperative Oncology Group performance status) score was 2 (range 0-4). Motor/sensory deficit and intracranial hypertension were predominant in 49.8% and 46.9% of the cases, respectively. Ocular involvement was diagnosed in 17 out of 119 patients who underwent ophthalmologic tests. Regarding diagnostic approaches, 94.7% received cerebral biopsy or tumor resection, and the remaining patients were diagnosed through vitrectomy, CSF cytology.
Table 1.
Main characteristics at diagnosis.
| Median (range) or n/N (%) | |
|---|---|
| No | 243 |
| Age, y | 57 (10-95) |
| 10-50 | 77 (31.7) |
| 51-60 | 70 (28.8) |
| 61-70 | 64 (26.3) |
| 71-80 | 25 (10.3) |
| >80 | 7 (2.9) |
| Sex, M/F | 138/105 (1.31) |
| ECOG-PS score | 2 (0-4) |
| Elevated serum LDH | 81/243 (33.3) |
| Symptoms at diagnosis | |
| Cognitive impairment | 69 (28.4) |
| Walking disorders | 37 (15.2) |
| Motor/sensory deficit | 121 (49.8) |
| Intracranial hypertension | 114 (46.9) |
| Epilepsy | 8 (3.3) |
| Blurred vision | 35 (14.4) |
| Ophthalmologic workup done | 119 (49) |
| Ocular involvement | 17 |
| CSF workup done | 76 (31.3) |
| CSF protein>0.5 g/L | 45/76 (59.2) |
| CSF cell count>5 cell | 32/76 (42.1) |
| Diagnostic method | |
| Cerebral biopsy | 178 (73.3) |
| Tumor resection | 52 (21.4) |
| Vitrectomy | 4 (1.6) |
| CSF | 9 (3.7) |
| Histopathologic diagnosis | 243 |
| Diffuse large B-cell lymphoma | 196 (80.7) |
| Other high-grade B-cell lymphoma | 3 (1.2) |
| Unclassifiable B-cell lymphoma | 41 (16.9) |
| T-cell lymphoma | 1 (0.4) |
| NK-T lymphoma | 1 (0.4) |
| Intravascular large B-cell lymphoma | 1 (0.4) |
| MRI | |
| Multiple lesions | 134 (55.1) |
| Infratentorial involvement | 67 (27.6) |
CSF, cerebrospinal fluid.
Induction Treatment
The main characteristics of the induction treatment are reported in Table 2 . A total of 94.7%(n=230) of the patients received HD-MTX-containing chemotherapy, 221 received at least 3g/m2 per injection. A total of 59.3% (144) used rituximab, with an increase in use over time (from 35.4% in 2010-2016 to 64.6% in 2017-2020).
Table 2.
Treatment characteristics.
| Values | |
|---|---|
| Delay first symptoms to treatment, d | 33 (0-1824) |
| Other chemotherapy | 13/243 (5.3%) |
| HD-MTX-based chemotherapy | 230/243 (94.7%) |
| Characteristics of HD-MTX treatment | |
| Dose per injection, g/m2 | 3.5 (1.5-3.5) |
| Total number of injections | 6 (1-10) |
| 3-3.5 g/m2 | 221 |
| Induction chemotherapy protocols including HD-MTX | 230 |
| M | 13/230 (5.7%) |
| MAD | 62/230 (27.0%) |
| R-MAD | 108/230 (47.0%) |
| MADD | 11/230 (4.8%) |
| R-MADD | 36/230 (15.7%) |
| Use of rituximab | 144/243 (59.3%) |
| Consolidation regimen | 95 |
| Pemetrexed and cytarabine | 35/95 (36.8%) |
| Etoposide and cytarabine | 60/95 (63.2%) |
HD-MTX, high-dose methotrexate, M, Methotrexate; R, Rituximab; MAD, Methotrexate +Ara-C +Dexamethasone; MADD, Methotrexate+ Ara-C+ Dexamethasone+ liposomal doxorubicin.
The HD-MTX containing regimen in our research mainly included the HD-MTX single regimen, HD-MTX, cytarabine (Ara-C), and dexamethasone (MAD) with or without rituximab (RTX), HD-MTX, Ara-C, liposomal doxorubicin and dexamethasone (MADD) with or without rituximab (drug dose see in Supplementary Data 1 ). Intravitreal methotrexate injection (0.04ml/400ug) were given after 2-4 cycles of intravenous HD-MTX containing chemotherapy when performance status was improved. based on suggestion of experienced ophthalmologist during the intermission of systemic chemotherapy.
Outcome of First-Line Treatment
The overall response rate (ORR) of all patients was 72.8%, with 58.8% achieving complete response after induction chemotherapy (IC). The proportion of disease progression was 20.2%. The combination of HD-MTX, cytarabine and dexamethasone-containing regimens with or without RTX was used in 70.0% of cases, and 19.3% added liposomal doxorubicin on the basis of the (R)MAD regimen ( Table 3 ). While there was no significant difference in the use of RTX among (R)MAD and (R)MADD groups (p=0.498), the additional liposomal doxorubicin led to a higher ORR of 89.3% ( Figure 1 ). Patients who didn’t achieve CR after induction therapy proceeded to salvage treatment ( Figure 2 ).
Table 3.
Outcome of first-line treatment.
| Response | All patients (n=243) | Other treatment* (n=26) | (R)MAD (n=170) | (R)MADD (n=47) |
|---|---|---|---|---|
| Complete response | 143 (58.8%) | 10 (38.5%) | 96 (56.5%) | 37 (78.7%) |
| Partial response | 34 (14.0%) | 6 (23.1%) | 23 (13.5%) | 5 (10.6%) |
| Stable disease | 17 (7.0%) | 5 (19.2%) | 11 (6.5%) | 1 (2.1%) |
| Progression disease | 49 (20.2%) | 5 (19.2%) | 40 (23.5%) | 4 (8.5%) |
| Overall response rate | 177 (72.8%) | 16 (61.5%) | 119 (70.0%) | 42 (89.3%) |
*Other treatment included HD-MTX single regimen, Temozolomide, Pemetrexed, lenalidomide regimen; (R)MAD, (Rituximab)+ Methotrexate +Ara-C +Dexamethasone; (R)MADD, (Rituximab)+ Methotrexate+ Ara-C+ Dexamethasone+ liposomal doxorubicin.
Figure 1.

Response to first-line treatment.
Figure 2.
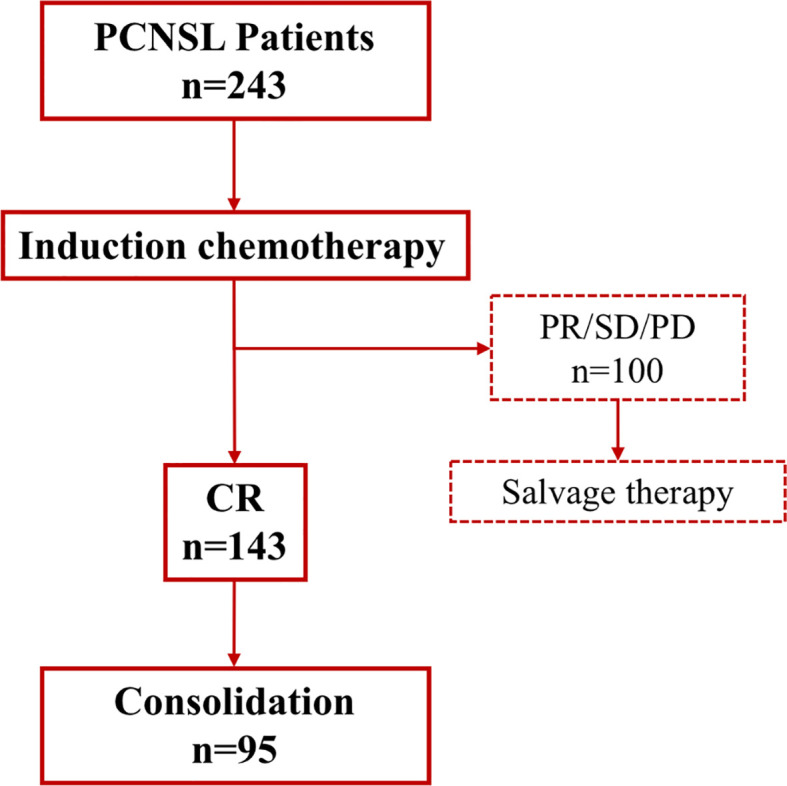
Response of induction and salvage treatment. CR, complete response; PR, partial response; PD, progressive disease; SD, stable disease.
Consolidation
Among 143 patients who achieved CR following induction chemotherapy, 95 patients proceeded to sequential consolidation chemotherapy. There was no statistical difference in age, gender and ECOG at diagnosis between patients received consolidation and those who didn’t (see in Supplementary Data 2 ). The lag time between induction therapy and consolidation was 2 months. Thirty-five patients received pemetrexed and the Ara-C regimen (PA), while sixty patients received etoposide and the Ara-C (EA) regimen. The PA regimen consisted of pemetrexed 900 mg/m2 administered on day 1, and then Ara-C was administered intravenously at 1-2 g/m2 on day 2 (oral folic acid was administered at 400μg daily 1 week before pemetrexed administration and continued for 3 weeks after the last dose). The EA regimen consisted of 100 mg/m2 etoposide on days 1-3 and 1-2 g/m2 Ara-C on days 2-3. The dose of Ara-C in both regimens depended on the patient age and ECOG-PS. Both sequential chemotherapy consolidation regimens were administered every 2 months for the first year and then every 6 months for the second year.
As expected, there’s no grade 4 toxicity in consolidation episode, 9.5% of patients experienced grade 2-3 neutropenia, 4.2% of patients experienced grade 2-3 thrombocytopenia, and 5.3% and 6.3% had grade 2 nephrotoxicity and hepatotoxicity, respectively. No grade 2-4 acute neurotoxicity was observed, detailed neurocognitive testing were not performed. There were no treatment-related deaths observed in our study (detailed toxicity see in Supplementary Data 3 ).
Survival
The median follow-up period in 243 patients was 27.0 months (95% confidence interval 23.6-30.4), and the median PFS was 14.0 months (95% CI 9.45-18.55), with a 2-year PFS rate of 33.2%. The median OS was not reached (NR), with an estimated overall survival at 4 years of 61.6%. We compared the outcomes among (R)MAD, (R)MADD and other treatments. The median OS among all cases and all three groups was not reached, and the IC regimen was not significantly associated with OS (p=0.588). The median PFS was 18.00 (95% CI 10.73-25.27), 12.00 (95% CI 6.67-17.33), and 8.00 (95% CI 0.39-15.60) for (R)MADD, (R)MAD and other treatments, respectively, without statistical significance (p=0.177).
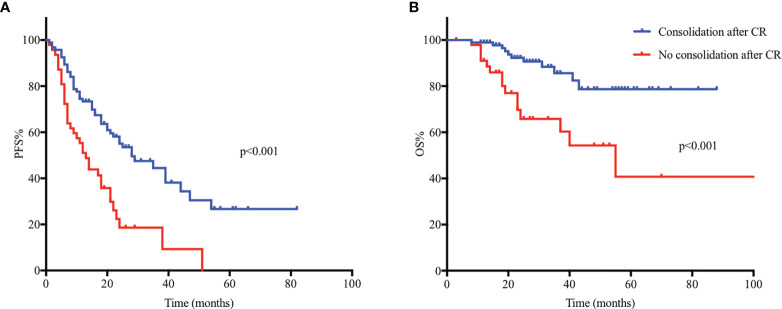
For patients who completed sequential consolidation chemotherapy (n=95) and those who did not (n=48), the median PFS was 28 months (95% CI 17.11-38.89) and 12 months (95% CI 7.74-16.26) (p<0.001), respectively. The 2-year PFS for patients who received consolidation and those who did not was 54.5% and 18.2%, respectively. The median OS for patients who completed sequential consolidation chemotherapy (n=95) was not reached, for those who did not receive consolidation (n=48), the median OS was 55 months (95% CI 29.8-80.2). The estimated OS at 4 years was 78.7% and 54.3% (p<0.001), respectively ( Figure 3 ).
Figure 3.
Kaplan-Meier analysis of progression-free survival (PFS) and overall survival (OS) in 143 PCNSL patients and comparison of PFS and OS between groups proceeded to consolidation (n=95) and who did not (n=48). (A) The median PFS was 28 months (95% CI 17.11-38.89) and 12 months (95% CI 7.74-16.26) (p < 0.001) for patients who completed sequential consolidation chemotherapy (n=95) and those who did not (n=48). (B) The median OS for patients who completed sequential consolidation chemotherapy (n=95) was not reached, for those who did not completed consolidation (n=48), the median OS was 55 months (95% CI 29.8-80.2).
Prognostic Factors
We included factors previously reported in other studies that have prognostic effects in PCNSL. Age, sex, ECOG-PS score, involvement of deep regions and serum LDH at diagnosis were factors in the IELSG score, but we did not include CSF protein because only 75/243 underwent CSF testing. The other main prognostic factors are indicated in Table 4 . A better early response was found to be associated with a longer PFS time; however, we did not find a significant correlation between PFS and other clinical variables (see in Supplementary Data 4 ). Age ≤ 60, ECOG-PS ≤ 1, early response to IC, and use of RTX were associated with prolonged OS in univariate analysis, and all the above-mentioned factors, except for the ECOG-PS score, were associated with prolonged OS in multivariate analysis ( Figure 4 ).
Table 4.
Univariate and multivariate analyses of OS.
| N | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Median OS | P value | HR (95%CI) | P value | ||
| Age ≤ 60 y | 147 | NR | 0.026 | 1.745 (1.024-2.973) | 0.041 |
| Age >60 y | 96 | 45 | |||
| Male | 138 | NR | 0.918 | ||
| Female | 105 | 59 | |||
| ECOG-PS ≤ 1 | 79 | NR | 0.049 | 1.855 (0.940-3.664) | 0.075 |
| ECOG-PS>1 | 164 | 59 | |||
| Normal blood LDH | 163 | NR | 0.608 | ||
| Elevated blood LDH | 80 | 59 | |||
| Unifocal lesion | 99 | NR | 0.359 | ||
| Diffuse lesion | 144 | NR | |||
| Absence of deep structure involvement | 76 | 56 | 0.293 | ||
| Deep structure involvement | 167 | NR | |||
| Tumor resection | 52 | NR | 0.508 | ||
| No tumor resection | 191 | NR | |||
| RTX in first-line treatment | 144 | NR | 0.012 | 2.183 (1.305-3.645) | 0.003 |
| No RTX in first-line treatment | 99 | 59 | |||
| Doxorubicin in first-line treatment | 47 | NR | 0.653 | ||
| No doxorubicin in first-line treatment | 196 | NR | |||
| Early response | 0.016 | 2.081 (1.168-3.706) | 0.013 | ||
| CR/PR | 186 | NR | |||
| SD/PD | 57 | 56 | |||
PFS, progressive free survival; RTX, rituximab; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NR, not reached.
Figure 4.
Clinical prognostic variables and their relationship to progression-free survival (PFS) and overall survival (OS). (A-D) Age ≤ 60, ECOG-PS ≤ 1, early response to induction chemotherapy (IC), and use of RTX were associated with prolonged OS in univariate analysis.
Discussion
In this single-center retrospective study, we reported the clinical characteristics, outcomes and prognostic factors of 243 PCNSL patients. Our team previously reported on outcomes of the (R)MAD regimen, showing a CR rate of 66.7% and a 2-year PFS rate of 34.0%. In this study, despite the inclusion of patients with advanced age and poor performance status, a CR rate of 58.8% and a 2-year PFS rate of 33.2% were achieved, in line with our previous report (10). This hospital-based study could add to current clinical trials and better reflect real-world PCNSL outcomes and survival.
There is little uniformity in the management of PCNSL outside of the consensus regarding the use of HD-MTX is the absolute backbone of PCNSL induction therapy. Drugs combining HD-MTX are often dictated by geographical region and drug availability. The currently used polychemotherapy regimens have an ORR of 35-74% and confer a median OS of 25-50 months (5, 9, 11–13). A previous study by AJ Ferreri proved that the addition of HD-cytarabine to HD-MTX was associated with improved activity against PCNSL. This randomized phase 2 study included 79 newly diagnosed PCNSL patients, and the ORR of the MA group was 69%, with a median PFS of 18 months (9). Our team introduced a modified dosage on the basis of the MA regimen in a retrospective study with 60 PCNSL cases and achieved a CR rate of 53.3% and a 2-year PFS rate of 34.0% (10). Other prospective trials including MA with or without thiotepa or temozolomide led to an ORR range of 53.0-92.0% due to varying consolidation strategies (4, 5, 12). With the limited activity of the MA regimen and unavailability of thiotepa, we introduced liposomal doxorubicin into the induction chemotherapy regimen.
Although doxorubicin is pivotal for the treatment of systemic aggressive lymphoma, was absent from the treatment of PCNSL due to its poor penetration of the brain-blood barrier (14, 15). Therefore, the formulation of liposomal doxorubicin was introduced to overcome this barrier (14, 16–19). Lionakis et al (20) reported the efficacy of DA-TEDDi-R in PCNSL. They measured the plasma and CSF pharmacokinetics of liposomal doxorubicin after the first cycle of induction chemotherapy and found that the CSF concentration of liposomal doxorubicin was low but with measurable concentrations during the entire treatment cycle, which suggested an accumulation effect. According to another study based on human and murine intracerebral breast cancer, liposomal doxorubicin achieved 7–17-fold higher concentrations in tumors than in the normal brain (14, 17). In this analysis, the addition of liposomal doxorubicin led to a higher ORR and tended to achieve better efficacy and survival in PCNSL. A larger sample size will be needed to prove its potential efficacy in PCNSL.
The optimal consolidation for PCNSL has yet to be elucidated. There are multiple lines of evidence supporting whole-brain radiotherapy (WBRT) and myeloablative high-dose chemotherapy followed by autologous stem cell transplant (HDC-ASCT) (21–24). Delayed neurotoxicity associated with WBRT can be a risk especially for elderly patients, reduced-dose WBRT and ASCT can initially improve cognition but developed delayed neurotoxicity after 3 years (25). As a result, for a subset of patients, predominantly elderly patients who were not candidates for HCD-ASCT and were also more likely to be susceptible to neurocognitive toxicity by WBRT, nonmyeloablative sequential consolidation chemotherapy was a feasible option. Rubenstein et al (5) conducted a study of high-dose chemotherapy consolidation in PCNSL with etoposide plus cytarabine (EA) (CALGB 50202) offering another consolidation treatment option in PCNSL without episodes of grade 3 or 4 neurotoxicity in consolidation. In agreement with CALGB 50202 and a few other retrospective studies (26, 27), our results showed that nonmyeloablative consolidation chemotherapy was feasible in PCNSL patients who achieved CR after first-line IC. The 2-year time to progression was longer in the report of Rubenstein than ours, which may be due to the exclusion of patients with PS larger than 2 in clinical trials. In our research, similar to CALGB 50202 study, no sever acute neurotoxicity was observed, detailed neurocognitive test should be explored in further trails. There is an ongoing study comparing the efficacy of myeloablative versus non-myeloablative consolidation chemotherapy in PCNSL (NCT01511562 and NCT02531841), which may provide us with more evidence in the future.
Regarding prognostic factors, two prognostic scoring systems are widely used to predict the clinical outcome of PCNSL: International Extranodal Lymphoma Study Group (IELSG) scoring (28) and Memorial Sloan Kettering Cancer Center (MSKCC) scoring (29). Age and ECOG-PS score were well-established prognostic factors in both the scoring systems above, consistent with our results. However, the use of RTX has been highly debated; in our study, administration of RTX was a favorable prognostic factor. The HOVON 105/ALLG NHL 24 trial did not observe a beneficial effect of RTX, while in IELSG32, another randomized trial, the HR for progression-free survival was 0.52 (95% CI 0.32–0.86) and for overall survival, it was 0.63 (0.42–1.02), both strongly supporting rituximab. Despite the controversy of the benefit of RTX, in most first-line studies, rituximab has remained an indispensable component of treatment for patients with primary CNS lymphoma (9, 30, 31). Serum LDH, deep structure involvement, and tumor resection were not related to outcome. With the evolution of PCNSL treatment, drugs such as ibrutinib have been included in the treatment of relapsed/refractory and even newly diagnosed PCNSL; thus, in the new drug era, new prognostic scoring systems should be explored.
This study has few limitations. First, this is a retrospective study, and 23 patients were lost to follow-up, but this number did not exceed 10% of our sample size. Induction treatment option may cause bias for patients with stronger treatments tended to have better financial situation and better tolerance to treatment. In the analysis of prognostic factors, immunohistological information on BCL-2, MYC, BCL-6 and MMSE was not included (32, 33). Data on detailed and delayed neurotoxicity and quality of life need further evaluation. Validation for the efficacy of EA sequential consolidation chemotherapy in prospective studies is needed in the future.
In conclusion, high-dose methotrexate (HD-MTX)-based induction chemotherapy with non-myeloablative sequential consolidation chemotherapy was a feasible strategy for PCNSL patients. Age and early treatment response were independent predictors of survival. Further prospective clinical trials should be conducted.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical approval was provided by Beijing Tiantan Hospital Ethics Committee, Capital Medical University (Ethical approval reference number: KY 2020-066-02). Informed consent was written obtained when patients were admitted to Department of Neurosurgery or Department of Hematology before initiation of chemotherapy. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
YL designed the study. NJ, YMW, and CG provided the patient samples. SS revised neuroimaging. XS and YCW analyzed the data and wrote the manuscript. RX, QC, HZ, and JQ performed the experiments. XB, YC, QL, JL, and WL collected and analyzed the data. All the authors have read the manuscript and approved its submission.
Funding
This study was supported by the Capital’s Funds for Health improvement and Research (NO. 2020-2-2049).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank the patients and their families.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.792274/full#supplementary-material
References
- 1. Grommes C, Rubenstein JL, DeAngelis LM, Ferreri AJM, Batchelor TT. Comprehensive Approach to Diagnosis and Treatment of Newly Diagnosed Primary CNS Lymphoma. Neuro-Oncology (2019) 21(3):296–305. doi: 10.1093/neuonc/noy192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fallah J, Qunaj L, Olszewski AJ. Therapy and Outcomes of Primary Central Nervous System Lymphoma in the United States: Analysis of the National Cancer Database. Blood Adv (2016) 1(2):112–21. doi: 10.1182/bloodadvances.2016000927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Houillier C, Soussain C, Ghesquières H, Soubeyran P, Chinot O, Taillandier L, et al. Management and Outcome of Primary CNS Lymphoma in the Modern Era: An LOC Network Study. Neurology (2020) 94(10):e1027–e39. doi: 10.1212/wnl.0000000000008900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferreri AJ, Reni M, Foppoli M, Martelli M, Pangalis GA, Frezzato M, et al. High-Dose Cytarabine Plus High-Dose Methotrexate Versus High-Dose Methotrexate Alone in Patients With Primary CNS Lymphoma: A Randomised Phase 2 Trial. Lancet (Lond Engl) (2009) 374(9700):1512–20. doi: 10.1016/s0140-6736(09)61416-1 [DOI] [PubMed] [Google Scholar]
- 5. Rubenstein JL, Hsi ED, Johnson JL, Jung SH, Nakashima MO, Grant B, et al. Intensive Chemotherapy and Immunotherapy in Patients With Newly Diagnosed Primary CNS Lymphoma: CALGB 50202 (Alliance 50202). J Clin Oncol (2013) 31(25):3061–8. doi: 10.1200/jco.2012.46.9957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Colombat P, Lemevel A, Bertrand P, Delwail V, Rachieru P, Brion A, et al. High-Dose Chemotherapy With Autologous Stem Cell Transplantation as First-Line Therapy for Primary CNS Lymphoma in Patients Younger Than 60 Years: A Multicenter Phase II Study of the GOELAMS Group. Bone Marrow Transplant (2006) 38(6):417–20. doi: 10.1038/sj.bmt.1705452 [DOI] [PubMed] [Google Scholar]
- 7. Glass J, Won M, Schultz CJ, Brat D, Bartlett NL, Suh JH, et al. Phase I and II Study of Induction Chemotherapy With Methotrexate, Rituximab, and Temozolomide, Followed By Whole-Brain Radiotherapy and Postirradiation Temozolomide for Primary CNS Lymphoma: NRG Oncology RTOG 0227. J Clin Oncol (2016) 34(14):1620–5. doi: 10.1200/jco.2015.64.8634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schaff LR, Ambady P, Doolittle ND, Grommes C. Primary Central Nervous System Lymphoma: A Narrative Review of Ongoing Clinical Trials and Goals for Future Studies. Ann Lymphoma (2021) 5:8. doi: 10.21037/aol-20-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferreri AJ, Cwynarski K, Pulczynski E, Ponzoni M, Deckert M, Politi LS, et al. Chemoimmunotherapy With Methotrexate, Cytarabine, Thiotepa, and Rituximab (MATRix Regimen) in Patients With Primary CNS Lymphoma: Results of the First Randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) Phase 2 Trial. Lancet Haematol (2016) 3(5):e217–e27. doi: 10.1016/s2352-3026(16)00036-3 [DOI] [PubMed] [Google Scholar]
- 10. Sun X, Liu J, Wang Y, Bai X, Chen Y, Qian J, et al. Methotrexate-Cytarabine-Dexamethasone Combination Chemotherapy With or Without Rituximab in Patients With Primary Central Nervous System Lymphoma. Oncotarget (2017) 8(30):49156–64. doi: 10.18632/oncotarget.17101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thiel E, Korfel A, Martus P, Kanz L, Griesinger F, Rauch M, et al. High-Dose Methotrexate With or Without Whole Brain Radiotherapy for Primary CNS Lymphoma (G-PCNSL-SG-1): A Phase 3, Randomised, Non-Inferiority Trial. Lancet Oncol (2010) 11(11):1036–47. doi: 10.1016/s1470-2045(10)70229-1 [DOI] [PubMed] [Google Scholar]
- 12. Omuro A, Chinot O, Taillandier L, Ghesquieres H, Soussain C, Delwail V, et al. Methotrexate and Temozolomide Versus Methotrexate, Procarbazine, Vincristine, and Cytarabine for Primary CNS Lymphoma in an Elderly Population: An Intergroup ANOCEF-GOELAMS Randomised Phase 2 Trial. Lancet Haematol (2015) 2(6):e251–e9. doi: 10.1016/s2352-3026(15)00074-5 [DOI] [PubMed] [Google Scholar]
- 13. Fritsch K, Kasenda B, Schorb E, Hau P, Bloehdorn J, Möhle R, et al. High-Dose Methotrexate-Based Immuno-Chemotherapy for Elderly Primary CNS Lymphoma Patients (PRIMAIN Study). Leukemia (2017) 31(4):846–52. doi: 10.1038/leu.2016.334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anders CK, Adamo B, Karginova O, Deal AM, Rawal S, Darr D, et al. Pharmacokinetics and Efficacy of PEGylated Liposomal Doxorubicin in an Intracranial Model of Breast Cancer. PLoS One (2013) 8(5):e61359. doi: 10.1371/journal.pone.0061359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wilson WH. Treatment Strategies for Aggressive Lymphomas: What Works? Hematol Am Soc Hematol Educ Program (2013) 2013:584–90. doi: 10.1182/asheducation-2013.1.584 [DOI] [PubMed] [Google Scholar]
- 16. Caraglia M, Addeo R, Costanzo R, Montella L, Faiola V, Marra M, et al. Phase II Study of Temozolomide Plus Pegylated Liposomal Doxorubicin in the Treatment of Brain Metastases From Solid Tumours. Cancer Chemother Pharmacol (2006) 57(1):34–9. doi: 10.1007/s00280-005-0001-z [DOI] [PubMed] [Google Scholar]
- 17. Koukourakis MI, Koukouraki S, Fezoulidis I, Kelekis N, Kyrias G, Archimandritis S, et al. High Intratumoural Accumulation of Stealth Liposomal Doxorubicin (Caelyx) in Glioblastomas and in Metastatic Brain Tumours. Br J Cancer (2000) 83(10):1281–6. doi: 10.1054/bjoc.2000.1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin NU, Bellon JR, Winer EP. CNS Metastases in Breast Cancer. J Clin Oncol (2004) 22(17):3608–17. doi: 10.1200/jco.2004.01.175 [DOI] [PubMed] [Google Scholar]
- 19. Vail DM, Amantea MA, Colbern GT, Martin FJ, Hilger RA, Working PK. Pegylated Liposomal Doxorubicin: Proof of Principle Using Preclinical Animal Models and Pharmacokinetic Studies. Semin Oncol (2004) 31(6 Suppl 13):16–35. doi: 10.1053/j.seminoncol.2004.08.002 [DOI] [PubMed] [Google Scholar]
- 20. Lionakis MS, Dunleavy K, Roschewski M, Widemann BC, Butman JA, Schmitz R, et al. Inhibition of B Cell Receptor Signaling by Ibrutinib in Primary CNS Lymphoma. Cancer Cell (2017) 31(6):833–43.e5. doi: 10.1016/j.ccell.2017.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Houillier C, Taillandier L, Dureau S, Lamy T, Laadhari M, Chinot O, et al. Radiotherapy or Autologous Stem-Cell Transplantation for Primary CNS Lymphoma in Patients 60 Years of Age and Younger: Results of the Intergroup ANOCEF-GOELAMS Randomized Phase II PRECIS Study. J Clin Oncol (2019) 37(10):823–33. doi: 10.1200/jco.18.00306 [DOI] [PubMed] [Google Scholar]
- 22. Omuro A, Correa DD, DeAngelis LM, Moskowitz CH, Matasar MJ, Kaley TJ, et al. R-MPV Followed by High-Dose Chemotherapy With TBC and Autologous Stem-Cell Transplant for Newly Diagnosed Primary CNS Lymphoma. Blood (2015) 125(9):1403–10. doi: 10.1182/blood-2014-10-604561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Illerhaus G, Kasenda B, Ihorst G, Egerer G, Lamprecht M, Keller U, et al. High-Dose Chemotherapy With Autologous Haemopoietic Stem Cell Transplantation for Newly Diagnosed Primary CNS Lymphoma: A Prospective, Single-Arm, Phase 2 Trial. Lancet Haematol (2016) 3(8):e388–e97. doi: 10.1016/s2352-3026(16)30050-3 [DOI] [PubMed] [Google Scholar]
- 24. Ferreri AJM, Cwynarski K, Pulczynski E, Fox CP, Schorb E, La Rosée P, et al. Whole-Brain Radiotherapy or Autologous Stem-Cell Transplantation as Consolidation Strategies After High-Dose Methotrexate-Based Chemoimmunotherapy in Patients With Primary CNS Lymphoma: Results of the Second Randomisation of the International Extranodal Lymphoma Study Group-32 Phase 2 Trial. Lancet Haematol (2017) 4(11):e510–e23. doi: 10.1016/s2352-3026(17)30174-6 [DOI] [PubMed] [Google Scholar]
- 25. Correa DD, Braun E, Kryza-Lacombe M, Ho KW, Reiner AS, Panageas KS, et al. Longitudinal Cognitive Assessment in Patients With Primary CNS Lymphoma Treated With Induction Chemotherapy Followed by Reduced-Dose Whole-Brain Radiotherapy or Autologous Stem Cell Transplantation. J Neuro Oncol (2019) 144(3):553–62. doi: 10.1007/s11060-019-03257-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nakasu Y, Mitsuya K, Hayashi N, Okamura I, Mori K, Enami T, et al. Response-Adapted Treatment With Upfront High-Dose Chemotherapy Followed by Autologous Stem-Cell Transplantation Rescue or Consolidation Phase High-Dose Methotrexate for Primary Central Nervous System Lymphoma: A Long-Term Mono-Center Study. SpringerPlus (2016) 5:307. doi: 10.1186/s40064-016-1954-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schorb E, Finke J, Ferreri AJ, Ihorst G, Mikesch K, Kasenda B, et al. High-Dose Chemotherapy and Autologous Stem Cell Transplant Compared With Conventional Chemotherapy for Consolidation in Newly Diagnosed Primary CNS Lymphoma–A Randomized Phase III Trial (MATRix). BMC Cancer (2016) 16:282. doi: 10.1186/s12885-016-2311-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ferreri AJ, Blay JY, Reni M, Pasini F, Spina M, Ambrosetti A, et al. Prognostic Scoring System for Primary CNS Lymphomas: The International Extranodal Lymphoma Study Group Experience. J Clin Oncol (2003) 21(2):266–72. doi: 10.1200/jco.2003.09.139 [DOI] [PubMed] [Google Scholar]
- 29. Abrey LE, Ben-Porat L, Panageas KS, Yahalom J, Berkey B, Curran W, et al. Primary Central Nervous System Lymphoma: The Memorial Sloan-Kettering Cancer Center Prognostic Model. J Clin Oncol (2006) 24(36):5711–5. doi: 10.1200/jco.2006.08.2941 [DOI] [PubMed] [Google Scholar]
- 30. Bromberg JEC, Issa S, Bakunina K, Minnema MC, Seute T, Durian M, et al. Rituximab in Patients With Primary CNS Lymphoma (HOVON 105/ALLG NHL 24): A Randomised, Open-Label, Phase 3 Intergroup Study. Lancet Oncol (2019) 20(2):216–28. doi: 10.1016/s1470-2045(18)30747-2 [DOI] [PubMed] [Google Scholar]
- 31. Kasenda B, Illerhaus G. CNS Border Posts Against Rituximab? Lancet Oncol (2019) 20(2):169–70. doi: 10.1016/s1470-2045(18)30829-5 [DOI] [PubMed] [Google Scholar]
- 32. Kim S, Nam SJ, Kwon D, Kim H, Lee E, Kim TM, et al. MYC and BCL2 Overexpression Is Associated With a Higher Class of Memorial Sloan-Kettering Cancer Center Prognostic Model and Poor Clinical Outcome in Primary Diffuse Large B-Cell Lymphoma of the Central Nervous System. BMC Cancer (2016) 16:363. doi: 10.1186/s12885-016-2397-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van der Meulen M, Dirven L, Bakunina K, van den Bent MJ, Issa S, Doorduijn JK, et al. MMSE Is an Independent Prognostic Factor for Survival in Primary Central Nervous System Lymphoma. J Neuro Oncol (2021) 152(2):357–62. doi: 10.1007/s11060-021-03708-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.