Abstract
A nationwide susceptibility surveillance study of beta-hemolytic streptococcal isolates from pharyngeal swabs obtained in 11 Spanish hospitals between May 1996 and April 1997 against 12 antibiotics was carried out. Of the isolates 86% (786 of 914 isolates) were group A and 8.4% (77 of 914 isolates) were group C. No resistance was found to β-lactam antibiotics, but significant differences (P < 0.001) with respect to lack of susceptibility to macrolides were found between groups (27% for group A and 12% for group C) and between seasons (13.2% in summer and 31.7% in winter). Most of these isolates displayed the M phenotype (low-level resistance to erythromycin and susceptibility to clindamycin).
Penicillin remains the drug of choice in the treatment of streptococcal pharyngitis (3), although communications increasingly report treatment failure frequencies of up to 30% (2, 4). This has been attributed to copathogenicity with β-lactamase-producing microorganisms, generating doubt as to the use of penicillin as empiric treatment in recurrent infections (2). Instead, other antibiotics not subject to inactivation by β-lactamases, i.e., amoxicillin clavulanate, oral cephalosporins, or erythromycin, are favored by some, the latter being considered the drug of choice in penicillin-allergic patients (2).
In vitro penicillin resistance has not yet been described in group A beta-hemolytic streptococci (Streptococcus pyogenes) (3, 7, 8). Resistance to macrolides is more common but remains at <5% among group A streptococci in most countries of the world (8). In recent years, erythromycin resistance frequencies in Spain have ranged from 1 to 10% (3, 6, 15). As more data are gradually accumulated on antimicrobial agent use and resistance in the community (10, 17), careful surveillance is required (8).
The aim of this study was to describe the susceptibility of beta-hemolytic streptococci in a nationwide antimicrobial surveillance study carried out in Spain. Included in this prospective surveillance study were all consecutive clinical isolates of beta-hemolytic streptococci from pharyngeal swabs collected between May 1996 and April 1997 at 11 hospital centers selected on the basis of geographical location.
Once a month at each center the isolates, after being kept at −70°C, were thawed, seeded onto an enriched transport medium, incubated overnight at 35 to 37°C, and shipped to a central laboratory (Instituto Valenciano de Microbiología, Valencia, Spain), where confirmation of identification and serogroup typing (as group A, C, F, or G) with an immunoagglutination test (Streptest; Murex, Chantillon, France) were performed.
Susceptibility testing was performed by a semiautomated microdilution method (microtiter plates were manufactured by Accumed International, East Grinstead, United Kingdom) following the guidelines of the National Committee for Clinical Laboratory Standards (11), with antimicrobials commonly used in empiric therapy in Spain (penicillin, amoxicillin, amoxicillin clavulanate, cefixime, cefaclor, cefuroxime, cefotaxime, ceftriaxone, erythromycin, azithromycin, clarithromycin, and ciprofloxacin). The breakpoints employed for the calculations of percentages of antimicrobial resistance are shown in a footnote to Table 1 (11). Haemophilus influenzae ATCC 49247, Streptococcus pneumoniae ATCC 49619, Staphylococcus aureus ATCC 29213, and Escherichia coli ATCC 25922 were used as control strains. The mechanism of resistance by S. pyogenes to erythromycin was evaluated with a double diffusion disk test, as described elsewhere (18), with erythromycin (15 μg) and clindamycin (2 μg) disks placed 20 mm apart onto 5% defibrinated horse blood agar and incubated overnight at 35°C in a 5% carbon dioxide atmosphere (14).
TABLE 1.
MIC90, range of MICs, and susceptibility for 786 isolates of S. pyogenes obtained from pharyngeal exudate samples
| Anti-microbial | MIC90 (μg/ml) | Range of MICs (μg/ml) | No. (%) of strains
|
||
|---|---|---|---|---|---|
| Susceptible | Intermediatec | Resistantc | |||
| Penicillina | ≤0.015 | ≤0.015–0.06 | 786 (100.0) | 0 (0) | 0 (0) |
| Cefotaxime | ≤0.25 | ≤0.25–≤0.25 | 786 (100.0) | 0 (0) | 0 (0) |
| Cefaclor | ≤1 | ≤1–≤1 | −a | −a | −a |
| Cefixime | ≤0.25 | ≤0.25–4 | NAb | NAb | NAb |
| Cefuroxime | ≤0.25 | ≤0.25–≤0.25 | −a | −a | −a |
| Erythromycin | 4 | ≤0.1–≥16 | 573 (72.9) | 3 (0.4) | 210 (26.7) |
| Ciprofloxacin | 1 | ≤0.5–4 | NAb | NAb | NAb |
All the strains susceptible to penicillin can be susceptible to amoxicillin, amoxicillin clavulanate, cefuroxime, and cefaclor (11). Amoxicillin clavulanate was tested on a 2:1 basis.
NA, not applicable. No breakpoint criteria have been established by the National Committee for Clinical Laboratory Standards.
The breakpoints (μg/ml) for intermediate strains and resistant strains were respectively 0.5 and ≥1 for erythromycin and clarithromycin, 1 and ≥2 for azithromycin, ceftriaxone, and cefotaxime, and 0.25 and ≥4 for penicillin (11).
Statistical analysis of data was performed by the chi-square test, with the Yates correction when necessary. The data were analyzed with Epi-Info version 6.04 (5).
A total of 914 isolates of beta-hemolytic streptococci were obtained, of which 86% (786 isolates) were group A, 8.4% (n = 77) were group C, 4.5% (n = 41) were group G, and 1.1% (n = 10) were group F. The two most common groups (A and C) exhibited the same in vitro susceptibility (i.e., MIC at which 90% of the isolates were inhibited [MIC90], MIC range, and resistance prevalence) to β-lactam antibiotics and to ciprofloxacin but not to macrolides. The percentages of isolates that were nonsusceptible (intermediate strains plus resistant strains) to macrolides were 27.1% (213 of 786 isolates) for group A and 11.7% (9 of 77 isolates) for group C (P = 0.001).
Table 1 shows the in vitro susceptibility of the 786 S. pyogenes isolates. All β-lactam antibiotics exhibited similar in vitro activities, with MIC90s of <1 μg/ml. The penicillins and parenteral cephalosporins exhibited similar activities with respect to MIC90, MIC range, and resistance prevalence. With respect to oral cephalosporins, only cefixime showed MIC of >1 μg/ml (for 8 of 786 strains). Macrolides elicited resistance by around 27% of the isolates, as stated above, with MIC90 values ranging from 4 μg/ml (of erythromycin or clarithromycin) to 8 μg/ml (of azithromycin). The MIC90 of ciprofloxacin was 1 μg/ml.
This study reveals an increase in erythromycin resistance prevalence in Spain (27%) compared to the resistance rates of up to 10% found in previous studies (3, 6, 15), which is due in part to the use of different breakpoints for erythromycin in the present study and in the previous ones. Were the breakpoint (≥8 μg/ml) (12) employed in the previous studies (3, 6, 15) used, the erythromycin resistance prevalence in this study would be 6.1%.
When a cutoff value of ≥1 μg/ml was used for highly resistant strains (3, 13) resistance was found in only 3% of strains isolated in 1991 and 1992 (3) or 4.7% of those isolated in 1994 (13). The overall erythromycin resistance frequency of 27% is due in part to an increase in the isolation rate of very highly resistant strains; 6.1% of the isolates had MIC of ≥8 μg/ml, contrasted with 1% of those examined in 1991 and 1992 (3). This may be explained in part by the confirmed increase of macrolide use in Spain (1) as well as in other countries (16, 17, 19). This phenomenon has also been suggested to occur in the case of S. pneumoniae (9).
In this study fewer group A isolates were obtained in summer (91 of 786 isolates [11.6%]) than in other seasons, for which the frequency ranged from 28 to 30%. Seasonal variations have been previously described in Spain (13), and lower isolation rates in summer have been noted. The frequency of macrolide-resistance exhibited a seasonal pattern, being 13.2, 25.0, 31.7, and 31.3% in summer, autumn, winter, and spring, respectively (P < 0.001). Antibiotic consumption patterns may contribute to resistance seasonality. In Spain community antibiotic consumption accounts for 90% of total consumption, and 17% of it is consumption of macrolides (1). Another possible explanation is the existence of a seasonal clone variation of new phenotypes with respect to virulence and antibiotic susceptibility.
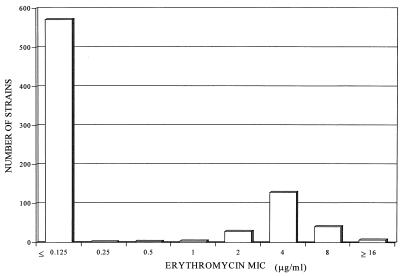
Figure 1 shows the erythromycin MIC distribution. With respect to S. pyogenes erythromycin resistance phenotypes (18), 93% (198 of 213 isolates) of strains belong to phenotype M (presumed efflux, with low-level resistance to erythromycin and susceptibility to clindamycin), as was found in previous studies in our country (6, 14), 6% (13 of 213 isolates) belong to the constitutive phenotype, and 1% (2 of 213 isolates) belong to the inducible phenotype. The increase in macrolide resistance observed is due to the M phenotype, with cross-resistance between C14 (erythromycin and clarithromycin) and C15 (azithromycin) macrolides.
FIG. 1.
Erythromycin MIC distribution.
Careful surveillance is required (8) for streptococcal isolates in countries where macrolide antibiotics are frequently prescribed (10) or high resistance rates for macrolides exist (as in Spain), as these antibiotics are the most widely used alternatives to oral β-lactams in the empiric treatment of streptococcal pharyngitis.
Acknowledgments
This study was supported by a grant from SmithKline Beecham Pharmaceuticals, Madrid, Spain.
REFERENCES
- 1.Baquero F the Task Force of the General Direction for Health Planning of the Spanish Ministry of Health. Antibiotic resistance in Spain: what can be done? Clin Infect Dis. 1996;23:819–823. doi: 10.1093/clinids/23.4.819. [DOI] [PubMed] [Google Scholar]
- 2.Bass J W. Antibiotic management of group A streptococcal pharyngotonsillitis. Pediatr Infect Dis J. 1991;10:543–549. doi: 10.1097/00006454-199110001-00010. [DOI] [PubMed] [Google Scholar]
- 3.Betriu C, Sánchez A, Gómez M, Cruceyra A, Picazo J J. Antibiotic susceptibility of group A streptococci: a 6-year follow-up study. Antimicrob Agents Chemother. 1993;37:1717–1719. doi: 10.1128/aac.37.8.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bisno A L. Streptococcus pyogenes. In: Mandell G L, Bennet J E, Dolin I R, editors. Principles and practice of infectious diseases. New York, N.Y: Churchill Livingstone, Inc.; 1995. pp. 1786–1798. [Google Scholar]
- 5.Dean A, Dean J, Coulombier D, Brendel K, Smith D, Burton A, Dicker R, Sullivan K, Fagan R, Arner T. Epi-Info version 6.04: a word processing, database and statistics program for epidemiology on microcomputers. Atlanta, Ga: Centers for Disease Control and Prevention; 1994. [Google Scholar]
- 6.García-Bermejo I, Cacho J, Orden B, Alós J I, Gómez-Garcés J L. Emergence of erythromycin-resistant, clindamycin-susceptible Streptococcus pyogenes isolates in Madrid, Spain. Antimicrob Agents Chemother. 1998;42:989–990. doi: 10.1128/aac.42.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerber M A. Antibiotic resistance in group A streptococci. Pediatr Clin N Am. 1995;42:539–551. doi: 10.1016/s0031-3955(16)38978-7. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan, E. L. 1997. Recent evaluation of antimicrobial resistance in beta-hemolytic streptococci. Clin. Infect. Dis. 24(Suppl. 1):S89–S92. [DOI] [PubMed]
- 9.Liñares J, Pallarés R, Alonso T, Pérez L, Ayats J, Gudiol F, Viladrich P F, Martin R. Trends in antimicrobial resistance of clinical isolates of Streptococcus pneumoniae in Bellvitge Hospital, Barcelona, Spain (1979–1990) Clin Infect Dis. 1992;15:99–105. doi: 10.1093/clinids/15.1.99. [DOI] [PubMed] [Google Scholar]
- 10.Maruyama S, Yoshioka H, Fujita K, Takimoto M, Satake Y. Sensitivity of group A streptococci to antibiotics: prevalence of resistance to erythromycin in Japan. Am J Dis Child. 1979;133:1143–1145. doi: 10.1001/archpedi.1979.02130110051007. [DOI] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 4th ed. Approved standard M7-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing; fourth informational supplement. Document M100-S4. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1992. [Google Scholar]
- 13.Orden B, Martínez R, López A, Franco A. Resistencia antibiotica a eritromicina, clindamicina y tetraciclina de 573 cepas de Streptococcus pyogenes (1992–1994) Enferm Infecc Microbiol Clin. 1996;14:86–89. [PubMed] [Google Scholar]
- 14.Orden B, Pérez-Trallero E, Montes M, Martínez R. Erythromycin resistance of Streptococcus pyogenes in Madrid. Pediatr Infect Dis J. 1988;17:470–473. doi: 10.1097/00006454-199806000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Pérez-Trallero E, García J M, Urbieta M. Erythromycin resistance in streptococci. Lancet. 1989;ii:444–445. doi: 10.1016/s0140-6736(89)90618-1. [DOI] [PubMed] [Google Scholar]
- 16.Phillips G, Parratt D, Orange G V, Harper I, McEwan H, Young N. Erythromycin-resistant Streptococcus pyogenes. J Antimicrob Chemother. 1990;25:723–724. doi: 10.1093/jac/25.4.723. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 17.Seppälä H, Klaukka T, Vuopio-Varkila J, Muotiala A, Helenius H, Lager K, Huovinen P. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. N Engl J Med. 1997;337:441–446. doi: 10.1056/NEJM199708143370701. [DOI] [PubMed] [Google Scholar]
- 18.Seppälä H, Nissinen A, Yu Q, Huovinen P. Three different phenotypes of erythromycin-resistant Streptococcus pyogenes in Finland. J Antimicrob Chemother. 1993;32:885–891. doi: 10.1093/jac/32.6.885. [DOI] [PubMed] [Google Scholar]
- 19.Zackrisson G, Lind L, Roos K, Larson P. Erythromycin resistant β-hemolytic streptococci group A in Göteborg, Sweden. Scand J Infect Dis. 1988;20:419–420. doi: 10.3109/00365548809032478. [DOI] [PubMed] [Google Scholar]