Abstract
The use of lure-and-kill, large-volume ovitraps to control Aedes aegypti and Aedes albopictus populations has shown promise across multiple designs that target gravid females (adulticidal) or larvae post-oviposition (larvicidal). Here we report on a pilot trial to deploy 10 L yeast-baited ovitraps at select sites in Curepe, Trinidad, West Indies during July to December, 2019. Oviposition rates among ovitraps placed in three Treatment sites were compared to a limited number of traps placed in three Control areas (no Aedes management performed), and three Vector areas (subjected to standard Ministry of Health, Insect Vector Control efforts). Our goal was to gain baseline information on efforts to saturate the Treatment sites with ovitraps within 20–25 m of each other and compare oviposition rates at these sites with background oviposition rates in Control and Vector Areas. Although yeast-baited ovitraps were highly attractive to gravid Aedes females, a primary limitation encountered within the Treatment sites was the inability to gain access to residential compounds for trap placement, primarily due to residents being absent during the day. This severely limited our intent to saturate these areas with ovitraps, indicating that future studies must include plans to account for these inaccessible zones during trap placement.
Subject terms: Urban ecology, Biotechnology, Diseases
Introduction
Aedes aegypti (L.) and to a lesser extent Aedes albopictus (Skuse) are the primary global mosquito vectors of multiple arboviruses, such as dengue, chikungunya, Zika, and yellow fever that significantly impact human health1–4. Efforts to prevent transmission or provide treatment for these arbovirus diseases by development of effective vaccines or antiviral drugs remain largely ineffective5–8. Disease prevention or reduction, globally, of these arboviruses has historically depended on efforts directed at controlling the mosquito vectors, most often by breeding site reduction and insecticide applications9. Notably resistance to all commonly used chemical insecticides is widespread among Aedes populations, thus limiting their effectiveness10. Therefore, a range of alternative methods that target larval or adult stages are being developed and tested11,12.
Gravid Aedes females actively seek to oviposit in man-made containers in and around human dwellings13,14, with a majority of mosquito breeding often seen in neighborhoods with low and medium socioeconomic levels15. The unreliability of piped water services in these areas, encourages active water storage in drums and tanks, as well as discarded container availability around houses, which combine to facilitate mosquito breeding16,17. Gravid Aedes females are known to practice ‘skip oviposition’, wherein they prefer to distribute eggs across multiple containers if given a choice, thereby maximizing the numbers of larval positive containers that mosquito control programs need to target18,19. Still, uncovered water storage drums and tanks are often the most prolific sources of Aedes mosquitoes in neighborhoods14,19–22.
Ovitraps consisting of small containers (usually ~ 500 ml) with water and an oviposition substrate have been employed as Aedes surveillance tools for decades23–25. More recently, concerted efforts have been directed at developing lethal ovitraps that: (1) are larger volume, (2) often spiked with attractants to lure gravid females, and (3) may include physical mechanisms like sticky boards to capture females that enter the traps26. The two most advanced lethal ovitraps are the Gravid Aedes Trap (GAT) and the Aedes Gravid Ovitrap (AGO). The GAT has a 10 L volume and contains 3 L of water, with lethality typically provided by an insecticide surface spray27. The AGO has a 19 L volume and contains 10 L of water, with lethality provided by a sticky surface adhesive28. Both traps utilize a hay infusion for enhanced attraction of gravid females. These larger volume traps have been shown to out-compete smaller volume lethal ovitrap designs26.
Large volume lethal ovitraps, while effective in reducing mosquito populations, face challenges relative to insecticide resistance as well as adverse effects on non-target organisms. There is a critical need to develop biofriendly pesticides that can easily be integrated into lethal ovitrap control programs. A promising technology, the RNA interference (RNAi) pathway is active in most eukaryotic cells and has evolved to silence gene expression with high specificity by the production of small interfering RNAs (siRNAs) of short length (21–25 bp)29. This pathway can be manipulated for the biofriendly control of mosquitoes that transmit pathogens to a vertebrate host by sequence-specific design of siRNAs that target critical genes in the mosquito30. The potential for successful development of RNAi-based larvicides for mosquitoes and the biomanipulation of the yeast Saccharomyces cerevisiae to produce them as short hairpin RNAs (shRNAs) has been shown to be highly effective and specific in controlled laboratory studies30,31, as well as simulated field and semi-field trials32,33.
Our previous laboratory assays with small volume (500 ml) containers have shown that gravid A. aegypti females were significantly more likely to oviposit in those baited with yeast compared to those with water only30. Subsequent laboratory and semi-field studies in Indiana, USA, as well as small-scale field trials in Trinidad, West Indies, all conducted with large volume ovitraps (7.5 L or 10 L) confirmed a preference to oviposit in those baited with yeast by A. aegypti as well as A. albopictus females34. In this study, we conducted a preliminary, small-scale field trial in neighborhoods around Curepe, Trinidad. Our goal was to obtain information on household access for ovitrap placement, general attractiveness of inactive yeast-baited ovitraps to gravid Aedes females when placed in a natural urban environment, and numbers of eggs laid in individual traps throughout the typical breeding season. These results will be used to facilitate planning for large-scale field trials with active long-lasting yeast formulations currently being developed and tested in laboratory studies.
Results
Treatment block demographics
The three selected Treatment blocks varied in total area from 9,588 to 22,558 m2 (Table 1) and were representative of most blocks in the Curepe study area (Fig. 1). The majority of buildings were private residences (73.7–85.7%), with some apartment complexes and a small number of commercial buildings. Nearly all private residences and apartment complexes were occupied, housing from 70 to 131 total people per block. A survey to identify all potential water-holding containers, irrespective of whether they held water at the time or not, within accessible household compounds that could represent sources for Aedes breeding prior to ovitrap distribution (May 27–29, 2019) identified a broad range of container types, including tanks that could be or were being used for water storage to a wide variety of miscellaneous small containers like flower pots and trays, plastic bottles, and metal cans. Aedes breeding activity observed in these containers was minimal, with only three positive containers observed among containers that were identified across all three Treatment blocks.
Table 1.
Demographics for Treatment blocks in Curepe, Trinidad.
| Block No. | 4 | 5 | 7 |
|---|---|---|---|
| Area (m2) | 22,558 | 9588 | 17,360 |
| Total No. buildings | 36 | 19 | 28 |
| Private residence | 29 | 14 | 24 |
| Apartment building | 7 | 3 | 5 |
| Commercial building | 0 | 2 | 4 |
| Total No. occupied | 36 | 17 | 28 |
| Total No. occupants | 131 | 70 | 100 |
| Potential water containersa (%) | |||
| Tanks | 0/32 (18.9) | 0/16 (10.4) | 0/33 (6.6) |
| Drums | 0/12 (7.1) | 0/7 (4.5) | 0/10 (2.0) |
| Tubs and Basins | 2/65 (38.5) | 0/55 (35.7) | 1/107 (21.4) |
| Tires | 0/2 (1.2) | 0/5 (3.2) | 0/17 (3.4) |
| Misc. Small | 0/40 (23.7) | 0/71 (46.1) | 0/305 (61.0) |
| Indoors | 0/18 (10.7) | 0 | 0/28 (5.6) |
| Total | 2/169 | 0/154 | 1/500 |
aNo. Positive containers/Total containers inspected, May 27–29, 2019. All containers present were counted irrespective of whether they contained water or not. Data were limited to households that were accessible for container surveys.
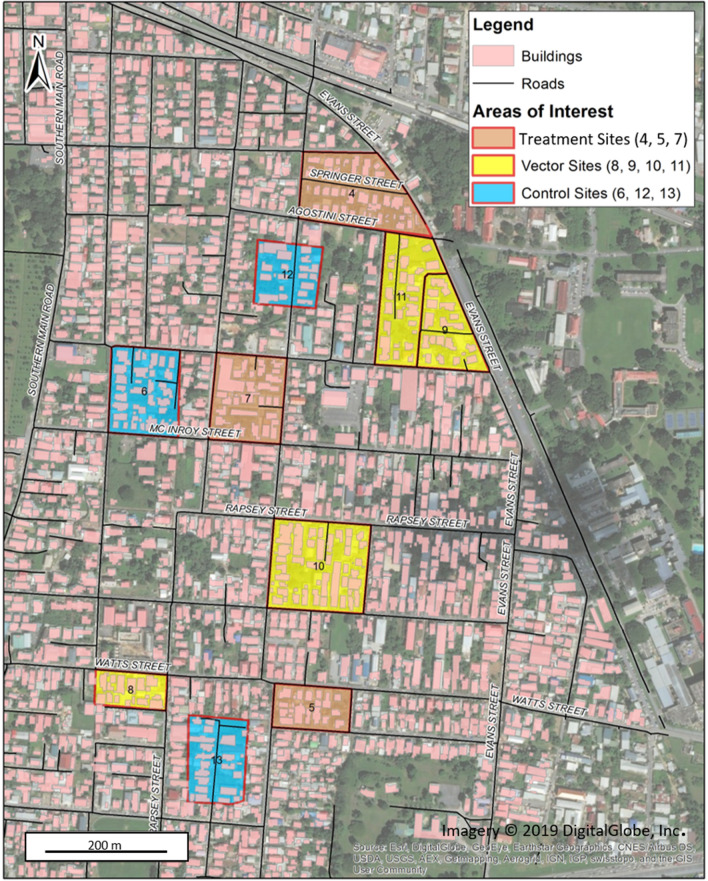
Figure 1.
Map of study area in Curepe, Trinidad, West Indies from July to December 2019. Block areas identifying sites for Control, Vector, and Treatment yeast-baited 10 L ovitrap placement are highlighted. Figure was prepared with Environmental Systems Research Institute (ESRI). "Topographic basemap.” "World Topographic Map." (Sept. 28, 2021). https://www.arcgis.com/home/item.html?id=10df2279f9684e4a9f6a7f08febac2a9. (May 21, 2019).
Vector block container surveys
Vector blocks were each surveyed for potential Aedes breeding containers five times during the study (except Block 8 that was not included in the initial June 27–28, 2019 survey) by IVCD staff (Table 2). Total container counts across survey dates for individual Vector blocks varied considerably due to limitations in accessing individual household areas as inhabitants were away when surveys were conducted. The most common container types based on the IVCD categories, were tubs and basins, and small miscellaneous. As observed in the Treatment blocks, Aedes breeding activity was minimal and mainly observed in the most common container types.
Table 2.
Potential water containers and type for Vector blocks in Curepe, Trinidad, 2019.
| Block number | Total units | Inspection date | No. units inspected | Tanks | Drums | Tubs and basins | Tires | Small Misc | Indoors | Totala |
|---|---|---|---|---|---|---|---|---|---|---|
| 8 | 39 | June 27–28 | None | |||||||
| July 23 | 12 | 8 | 1/4 | 64 | 1 | 86 | 2 | 1/164 | ||
| September 3–4 | 18 | 7 | 7 | 165 | 0 | 147 | 17 | 0/343 | ||
| October 15 | 20 | 21 | 10 | 2/58 | 1/19 | 230 | 39 | 3/377 | ||
| November 20 | 21 | 5 | 2 | 2/79 | 2/21 | 122 | 15 | 4/244 | ||
| 9/11 | 79 | June 27–28 | 38 | 43 | 4 | 99 | 5 | 139 | 9 | 0/299 |
| July 23 | 36 | 40 | 3 | 155 | 0 | 145 | 10 | 0/354 | ||
| September 3–4 | 40 | 42 | 9 | 1/173 | 0 | 170 | 19 | 1/411 | ||
| October 15 | 30 | 37 | 2 | 148 | 4 | 140 | 25 | 0/356 | ||
| November 20 | 42 | 49 | 2 | 180 | 12 | 241 | 43 | 0/518 | ||
| 10 | 82 | June 27–28 | 26 | 35 | 9 | 184 | 6 | 273 | 4 | 0/424 |
| July 23 | 52 | 31 | 24 | 1/288 | 9 | 1/514 | 32 | 2/898 | ||
| September 3 | 34 | 35 | 10 | 2/119 | 5 | 1/295 | 32 | 3/496 | ||
| October 15 | 49 | 50 | 24 | 1/131 | 10 | 1/486 | 15 | 2/714 | ||
| November 20 | 54 | 28 | 2 | 2/83 | 2 | 170 | 15 | 2/284 |
aNo. Positive containers/Total containers inspected.
Ovitrap distribution and seasonal egg counts
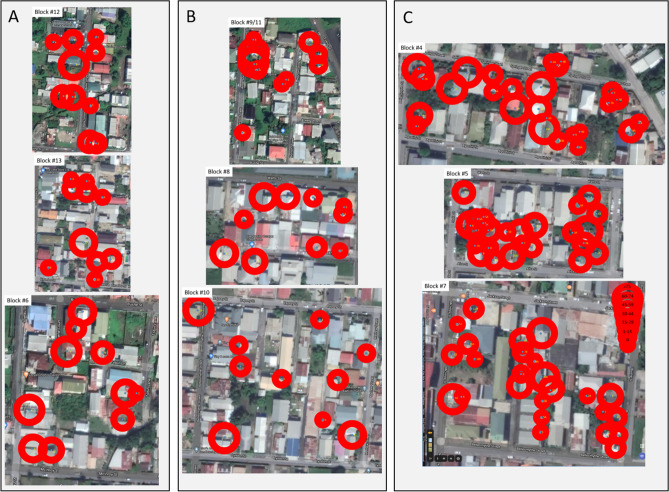
We collected data on Aedes oviposition in yeast-baited 10 L ovitraps for 23 weeks beginning June 26 and ending December 4. In addition to the 10 traps placed at each of the Control blocks (n = 3) and Vector blocks (n = 3), we were able to eventually place a total of 76 traps across the 3 Treatment blocks. One trap was removed midway during the study due to continued disturbance (block #4), leaving a total of 75 traps (n = 25 across each Treatment block). In addition, data were not collected for some weeks for some individual traps during the study due to disturbance by household pets, general household activities, flooding events, and owner absence. The complete data set is provided in Supplementary Table S1. Individual ovitrap locations and median egg counts/trap across the entire study period are shown for each block area in Fig. 2. Ovitrap placement across Treatment blocks was clearly nonrandom, primarily reflecting inaccessibility to some properties due to resident absence (presumably for work; only seven properties were inaccessible across all three Treatment blocks because the residents declined our request to place and monitor ovitraps on them), but also open mainly grassy areas and commercial buildings where ovitrap placement was unwarranted.
Figure 2.
Individual yeast-baited 10 L ovitrap locations and median egg counts/trap from July to December 2019. (A) Control blocks; (B) Vector blocks; (C) Treatment blocks.
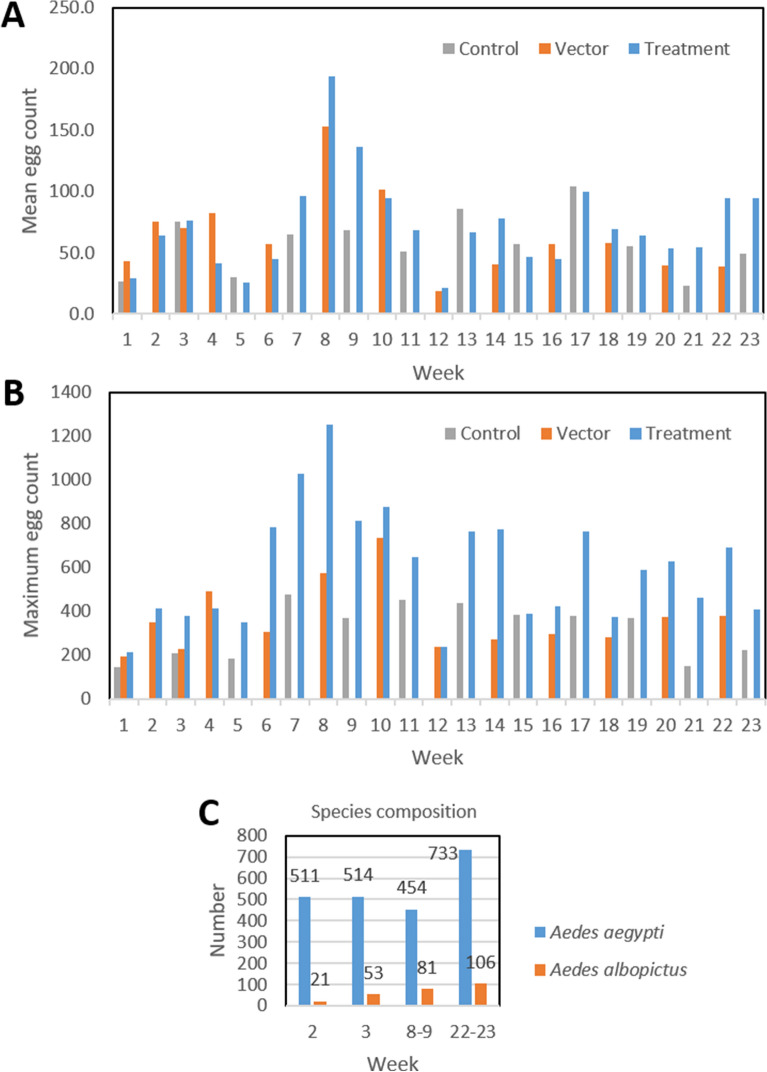
During the course of the study, a total of 157,183 eggs were removed from the 135 ovitraps. Of these, 112,170 (71.4%) were collected from the 75 Treatment ovitraps and 45,013 (28.6%) from the 60 Control and Vector ovitraps. Mean weekly egg counts per ovitrap for the study period are shown in Fig. 3A, and ranged from ~ 20 to 200 eggs per trap, with considerable variability that generally reflects the typical rainy season in Trinidad that spans May to November23,24. Maximum egg counts per week per single ovitrap also showed considerable seasonal variability (Fig. 3B). The maximum egg count was n = 1251 during week 8 (August 20–21) in ovitrap #10 located in Treatment block #7. Of note, the individual ovitrap with the maximum weekly egg count was located within one of the Treatment blocks for 22 of the 23-week study period. This observation suggests that gravid Aedes females were attracted to these areas with more densely placed ovitraps. An evaluation of species composition based on periodic rearing of pooled eggs collected during weeks 2 (July 9–10), 3 (July 16–17), 8–9 (August 20–21, 27–28), and 22–23 (November 26–27, December 3–4) revealed a mixture of A. aegypti and A. albopictus, with A. aegypti being the predominant species (83.3–96.1%) throughout the study period (Fig. 3C). The inferred number of egg laying events showed much lower temporal variance and magnitude (Supplementary Fig. S1) compared to the raw data.
Figure 3.
Summary of weekly egg collections of yeast-baited 10 L ovitraps positioned across Control, Vector, and Treatment blocks. (A) Mean egg counts; (B) Maximum egg counts; (C) A. aegypti vs. A. albopictus composition from eggs reared from select weekly samples.
Impact of increased density on index ovitraps in the Treatment block
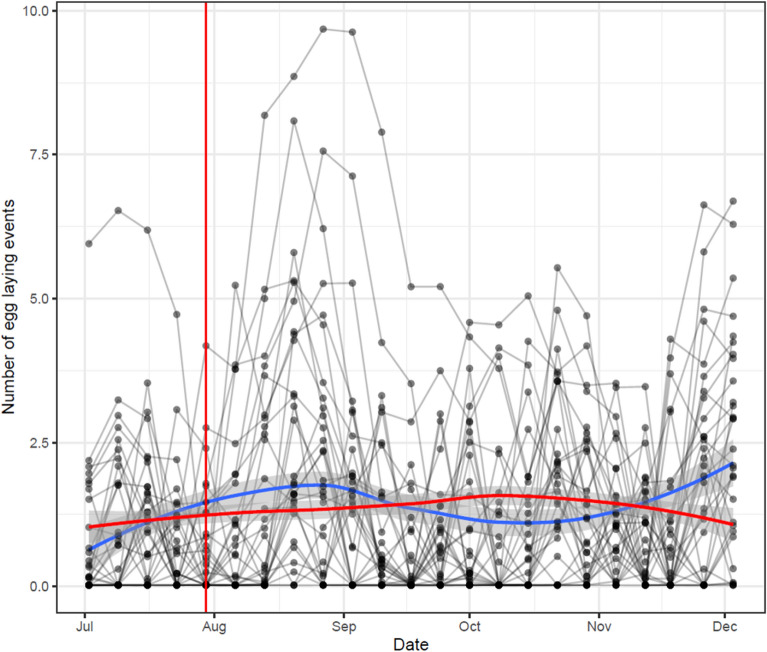
The changes in index ovitraps in the Treatment block that were intended to be measured every week are shown in Fig. 4. During the third and fourth weeks, additional traps were added and measured (therefore increasing the number of eggs that were removed from the area) in the Treatment blocks. The increase in the density of traps in the Treatment block did not lead to a decrease in the number of egg laying events in the index traps. The small increase in the number of egg laying events after the third week may have been driven by the relatively small number of ovitraps that saw an uncharacteristically large increase in the number of egg laying events. The limited effect of increasing the density of ovitraps in the Treatment blocks is supported by the very similar temporal trend in the combined data for the Control and Vector blocks (Fig. 4).
Figure 4.
The inferred number of egg laying events in the index ovitraps in the Treatment blocks. The inferred number of egg-laying events over time for each index ovitrap in the Treatment blocks are plotted as lines. The index ovitraps were measured for 3 weeks before additional ovitraps were placed in the Treatment blocks (therefore increasing the number of removed eggs in those blocks). The red vertical line indicated the time at which new ovitraps were placed. The red and blue horizontal lines represent the loess curves for the index ovitraps in the Treatment block and the ovitraps in the combined control group (Control and Vector blocks), respectively.
Impact of physical relationship between 10 L yeast-baited ovitraps and observed oviposition activity
To look more broadly for evidence of a local effect of ovitraps on the egg laying dynamics of the local mosquito population, we considered the local density of other ovitraps as a predictor of the number of egg laying events in an index ovitrap. The relative physical relationships of all 135 ovitraps is shown in Fig. 5, with the local density shown in color. As the ovitraps were not uniformly placed, they showed patterns of clustering which led to variance in the local density measures. No obvious differences were observed between egg laying events from the Control and Vector blocks (Supplementary Fig. S2), so data from both blocks were combined in this analysis.
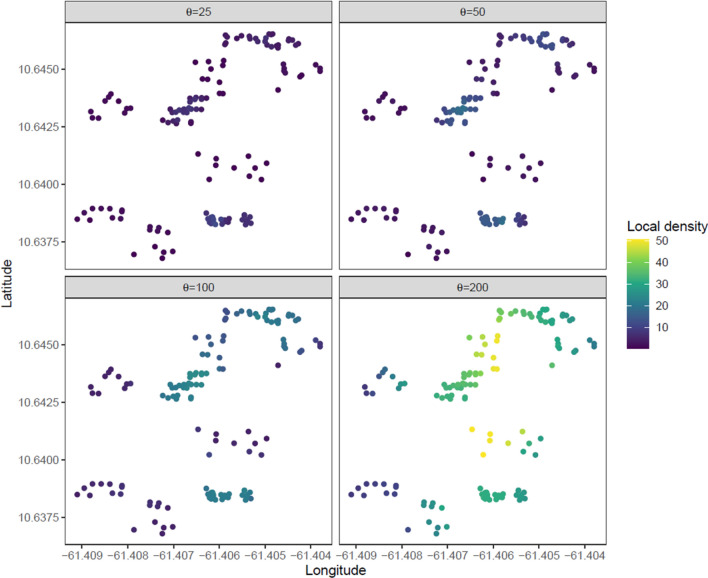
Figure 5.
Relative positions of the ovitraps in space with illustration of local density measure. The relative physical locations of ovitraps are shown as dots with colors indicating the values of the local density measure under the four parameter levels used in the analysis. Heterogeneity in the spatial distribution of traps allows us to examine the effect of varying the local density of traps on the number of observed mosquito eggs.
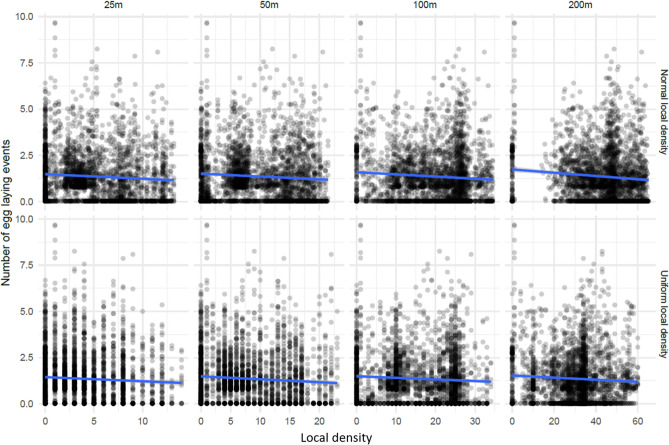
Figure 6 shows fits of a simple linear model for eight different definitions of the local density of ovitraps (details in “Methods”). In all eight cases (four parameter values for each model), the p-value for the test (i.e. no effect of local density) was found to be less than 0.01 and in all cases the sign of was negative suggesting a decrease in the number of egg laying events as the local density of traps increases however the local density is measured. However, the magnitude of the effect was very small ranging from −0.024 to −0.008. For example, in the case of the uniform local density model with parameter 25 m (=−0.022, p = 0.002), the predicted effect of adding 10 ovitraps in a 25 m radius of the index trap would, on average, reduce the number of egg laying events per week by about 0.2 or about 10 eggs per week in the index trap. Given the slight degree of heteroscedasticity in the data we also computed the p-values using robust random errors35, finding that the reported p-values were robust to the empirical degree of heteroscedasticity.
Figure 6.
Number of inferred egg laying events and the local density of ovitraps in the previous week. This plot shows the number of inferred egg-laying events by the local density of ovitraps in the previous week for all ovitraps. The rows correspond to the Normal and Uniform local density models and the columns give the distance parameter for those models. The x-axis can be loosely interpreted as the number of other ovitraps that are within about the specified number of meters of the given ovitrap. The blue line shows the predicted mean of the linear model of the data.
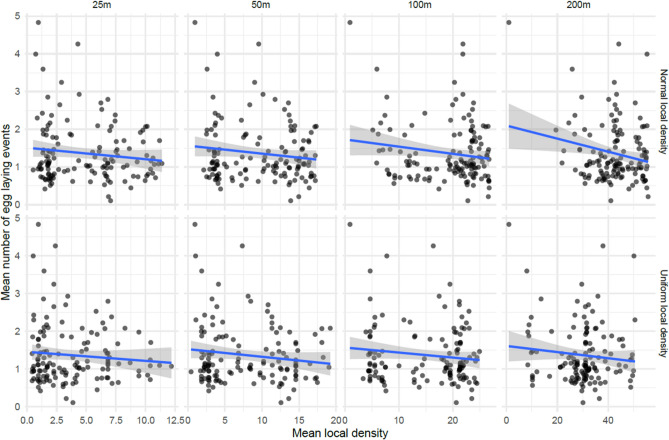
We also considered the data and local density measures in aggregate over the course of the whole study. This allowed us to relax the one-week lag assumption in the previous model, which also has the advantage of averaging over secular trends in the mosquito population levels at the cost of a much smaller dataset. Results of those model fits are shown in Fig. 6. As expected with the much smaller datasets obtained by averaging over the course of the study, the measured p-values were much larger with only the Normal local density model with parameter 200 m having a p-value less than 0.05. In general, the effect measures were similar in the weekly model and the aggregated model (range −0.008 to −0.03). However, the general trend towards small but measurable reductions in the number of egg laying events with increasing local density of other ovitraps is more apparent in the aggregated data (Fig. 7).
Figure 7.
Mean Number of inferred egg laying events and the mean local density of ovitraps in the previous week over the course of the study. This plot shows the mean number of inferred egg laying events by the mean local density of ovitraps in the previous week for all ovitraps. The rows correspond to the Normal and Uniform local density models and the columns give the distance parameter for those models. The x-axis can be loosely interpreted as the number of other ovitraps that are within about the specified number of meters of the given ovitrap. The blue line shows the predicted mean of the linear model of the data.
Discussion
In this investigation, we sought to obtain baseline information on the logistics associated with efforts directed toward large-scale deployment of yeast interfering RNA lure-and-kill ovitraps in a residential neighborhood in Curepe, Trinidad, West Indies. In a previous study conducted on the University of the West Indies campus, adjacent to Curepe, we determined that large 10 L ovitrap containers baited with yeast interfering RNA tablets were significantly more attractive than identical but unbaited containers to gravid A. aegypti and A. albopictus females34. As a proxy for simulating 100% lethality, our ovitraps were lined with seed germination papers that were removed and replaced weekly throughout the rainy season. Eggs were then counted to assess attractiveness of individual traps to gravid Aedes females and thereby also provide information on the anticipated number of larvae expected per ovitrap, particularly maximum larval numbers that our large volume lethal ovitraps will need to accommodate.
In general, the rainy season oviposition patterns in 10 L ovitraps distributed in the Curepe neighborhood were similar to those observed in a previous study on the adjacent University of the West Indies campus, with the greatest average egg numbers per trap occurring during August34. This pattern is also consistent with that observed for St. Joseph, Trinidad in 1988 using 400 ml ovitraps23,24. However, the maximum number of eggs collected in a single 10 L ovitrap during the current study was nearly double (1251 vs. 645) what we observed in the previous study, indicating that maximum oviposition rates in these traps are extremely variable and that effective yeast interfering RNA larvicide formulations need to consider this. As observed in Hapairai et al.34, both A. aegypti and A. albopictus were represented in ovitraps throughout the rainy season, although A. aegypti was predominant throughout the current study. This likely reflects the more urban environment in Curepe where the abundance of manmade containers is more conducive to A. aegypti, whereas the University of the West Indies campus likely offered fewer manmade containers and more natural breeding sites that are more conducive to A. albopictus36,37.
As the maximum number of eggs laid in individual traps was frequently over 400, it is also clear that multiple Aedes females were ovipositing in these traps. Previous molecular genetic analyses have confirmed that an average of 4.7 to 6.2 families were generally represented in individual containers, apparently independent of container size18,38, with up to nine families represented in a single container18. In addition, it is likely that individual females deposited eggs across multiple containers, a well-known behavior termed ‘skip-oviposition’18,19,38–40. Further, molecular analyses have also shown that individual females distributed eggs in containers separated up to at least 100 m18.
The observed small effect of higher-levels of ovitraps being near an index case is consistent with the reasonable assumption that removing eggs from circulation has an effect of reducing the local population of egg-laying adults. However, at the ovitrap densities obtained in this study the effects were statistically negligible. Of note too, median egg counts for individual ovitraps across the rainy season indicated that certain traps consistently attracted gravid Aedes females (Fig. 3). This phenomenon has previously been reported from other studies41,42, with the suggestion that microhabitat conditions around individual ovitraps likely plays an important role in egg laying behavior. However, Barrera et al.42 further showed that ovitrap attractiveness was mainly correlated with presence and attractiveness of nearby traps, rather than local microhabitat factors, including variables such as water volume, water temperatures, and trap exposure to rain and sun conditions. Thus, it has been concluded that microhabitat conditions play a less important role than deploying sufficient ovitraps to provide adequate coverage of neighborhoods28,42. Again, this may relate to the propensity of gravid Aedes females to exhibit skip oviposition among containers within a given area18,19,38–40.
Our results here and previously34 provide consistent evidence that our yeast-baited 10 L ovitraps are highly attractive to gravid Aedes females in an urban field setting. They also suggest that larger volume ovitraps may offer a better alternative for assessment and subsequent management of mosquito populations, including larviciding. That is, general surveillance programs have historically been based on monitoring oviposition activity using small (~ 500 ml) containers that, while readily attractive to gravid females, consistently collect fewer eggs than we have observed with larger volume containers. For example, previous studies in Trinidad with small volume ovitraps most frequently collected less than 60 eggs per week with a maximum of ~ 75 eggs23,24. This general pattern has remained consistent across multiple studies in various geographic regions that deployed similar-sized ovitraps43–48.
Consistent with other studies46,49–51, our results clearly highlight the need to include efforts directed at community participation and integrated control activities across neighborhoods. A primary difficulty we encountered in this study was in gaining access to residential properties in our efforts to saturate neighborhoods with ovitraps, even with direct assistance by staff of the IVCD. In most cases, the limitation was a consequence of the residents all likely being away for employment during the work day, and these residential compounds were typically protected by security fencing. The effects of this are evident in Fig. 2, where major areas of each Treatment block were inaccessible for ovitrap placement. This highlights the need to consider property inaccessibility in planning for future studies and how best to develop strategies that at a minimum would include efforts directed at more dense trap placements around the perimeters of these properties. We did simultaneously conduct several community engagement activities across Trinidad, including the Curepe area, prior to this study52,53, wherein we found that most residents were knowledgeable about mosquito biology and disease transmission, and also supportive of our yeast interfering RNA larvicides and their use in lethal ovitraps. Thus, it seems likely that including expanded community engagement activities prior to, and during future ovitrap trials, could promote increased neighbor-to-neighbor interactions that would facilitate access to and placement of ovitraps more effectively.
Methods
Study site
Field studies were conducted across sites in Curepe, Trinidad, West Indies (10o37′59.99″ N–61o23′59.99″ W) from June 26 until December 4, 2019. The town is located in the Tunapuna-Piarco Regional Corporation of Trinidad, covers ~ 4.34 km2, and is largely suburban with single and multi-family housing units mixed with small businesses, and a population of ~ 8,700 people. The study area encompassed an ~ 1.32 km2 portion of Curepe that is bordered by four major roads: the Priority Bus Route to the north, University Drive to the east, Churchill Roosevelt Highway to the south, and the Southern Main Road to the west (Fig. 1). The dry season typically occurs during December to May, and the wet season during May to November54.
Study design
To gain preliminary information on the logistics associated with yeast-baited lethal ovitrap placement, effects of simulated 100% larval mortalities in our yeast-baited ovitraps, as well as rainy season oviposition activity in these traps by Aedes females, we selected three discrete block areas defined as ‘Treatment’ within the Curepe area (Fig. 1). With collaboration of staff from the Insect Vector Control Division (IVCD) at the Ministry of Health (MoH), the Treatment blocks were surveyed for: total number of buildings and building use, total number of people living in occupied buildings, and total number of potential Aedes breeding containers and container types irrespective of whether they contained water at the time or not. Based on these data, Treatment blocks were chosen based on human population densities (e.g., primarily residential vs. commercial buildings) and physical isolation from each other. Thereafter, we sought to place inactive yeast-baited ovitraps in areas outside occupied buildings. For the first three weeks, 10 traps were distributed across each Treatment block to obtain baseline data on oviposition activity. During weeks four and five, our goal was to eventually place individual ovitraps within 20–25 m of each other throughout these outdoor areas, such that gravid Aedes females would have ready access to one for oviposition within easy flight range. This distribution plan was based on mark-release-recapture studies conducted under similar environmental conditions in Florida and Puerto Rico, wherein results showed that gravid Aedes females dispersal distances are inversely correlated with ready access to oviposition sites, and that they typically travel less than 50 m from release sites if oviposition sites are available55,56. Ovitrap positions were selected to provide protection from wind, rainfall, and direct sunlight.
To gain information on background oviposition activity throughout the Curepe study area, we selected three additional blocks each as: (1) areas defined as ‘Control’ where no active Aedes control measures were implemented, and (2) areas defined at ‘Vector’ where personnel from IVCD performed periodic surveillance that involved identification of and either emptying or chemical treatment of potential breeding containers. These blocks were selected such that a Control and Vector was located within the adjacent areas of each Treatment block. In these areas, we distributed 10 yeast-baited ovitraps to obtain background information on oviposition rates throughout the rainy season, but not remove enough eggs to significantly impact the local mosquito populations.
Heat-inactivated yeast tablets were prepared using a transiently transformed Saccharomyces cerevisiae control construct30 as previously described57. A single ~ 70 mg tablet was placed in each ovitrap. This construct expresses a short hairpin RNA (shRNA) that has no identified target in mosquitoes or any other organism. This construct was used because our yeast-baited large ovitraps are highly attractive to gravid females,34 and the experiment design was not intended to directly test for larvicidal activity. Individual ovitraps in Treatment areas were serviced weekly, while those in the Control and Vector areas were serviced every two weeks and staggered such that servicing of the two types was performed on alternate weeks. Dark blue 10 L plastic buckets were used as ovitraps and were set up with ~ 3.5 L of tap water, the perimeter lined with seed germination paper (Anchor Paper Company, Saint Paul, MN, USA) as an oviposition substrate (egg paper), and covered with a 10 mm mesh wire screen. Detailed ovitrap descriptions were provided elsewhere34. During weekly servicing of individual ovitraps, egg papers were removed, ovitraps emptied and thoroughly rinsed with fresh tap water, refilled and provided with a clean egg paper and a new yeast tablet. Following collection, egg papers were transported to an insectary at the University of the West Indies, where they were allowed to dry at room temperature, and then total Aedes egg counts were performed for each. As both A. aegypti and A. albopictus are present in Trinidad34,58, we periodically hatched, reared, and identified adults to species from pooled samples of individual egg papers following our standard conditions59.
Data analysis
Number of egg laying events
The total number of eggs laid in an ovitrap each week was expected to be highly variable based on our previous study34. High variability in signal can lead to spurious results, although this is mitigated by the long duration of the study. Because we want to measure the effect of local ovitrap density on the unobserved number of egg laying events rather than the raw counts of eggs in each trap, which are highly stochastic due to a variable number of eggs being laid in each laying event40, we inferred the number of egg laying events based on the observed number of laid eggs in each trap per week. This has the joint advantages of both naturally reducing the potential influence of outliers in the data—therefore reducing the likelihood of false positive results—and imputing missing values in the data. The model assumes the number of observed eggs in a given trap-week, , is distributed as a Negative Binomial random variable conditional on the inferred number of egg laying events, : . Further, the model assumes a simple autoregressive process of the form . The R library rstan 2.21.160 was used to estimate the number of unobserved egg laying events and the variance of the Normal autogressive process, . This is the same methodology as used in Haipairai et al.34.
Local density
We defined the local density of an ovitrap as a function of the number of other ovitraps within a given radius that were measured in the previous week. That is local density of ovitrap at time was calculated as
where A is the set of all ovitraps, qa is an indicator function that takes value 1 or 0 if ovitrap a was measured at time t − 1 or not respectively, and is a function with parameter of the Euclidian distance between a pair of ovitraps. We considered two definitions of local density by choosing different . For the measure “local density, uniform”, is an indicator function that takes value 1 if the distance between the two ovitraps is less than and for “local density, normal” is the normal density of with standard deviation . Note that to make the uniform and normal measures more comparable we regularized the normal density such that the maximum value was always equal to 1, therefore both of these measures have the loose interpretation of being “how many ovitraps are nearby”. We considered the following values of : 25, 50, 100, and 200 m. Because there is temporal variance in the frequency in which traps were measured, the local density of an ovitrap can vary from week to week.
Statistical model
We considered two statistical models for the effect of local density on the number of egg laying events in a given week. The first model assumes that the effect of the local density of oviraps on the number of egg laying events has a simple linear form, , where the parameter is the change in number of egg laying events per week associated with an increase in the local density of ovitraps and is the standard deviation of the error. The second model aggregates over the time series by modeling the average number of egg laying events with the average local density of ovitraps, where and are the average number of weekly egg laying events over the study period and the average local density of ovitraps in the previous week over the course of the study period respectively.
Ethics approval and informed consent to participate
Approval of field studies in Trinidad, West Indies was given by the Southwest Regional Health Authority, a division of the Trinidad and Tobago Ministry of Health. Household consent was provided verbally during IVCD routine surveillance visits to participating households, during which time IVCD staff gained permission from residents for both staff and the research team members to access the residences. Research team members then explained the nature of the study to the homeowners, who were able to approve or reject ovitrap placement. Ovitraps were clearly marked with a label indicating that the devices were being used in conjunction with a research study, and which included the name and local contact information for Dr. A. Mohammed. This work was funded by U.S. Department of Defense Awards W81XWH-17-1-0294 to MDS and W81XWH-17-1-0295 to DWS.
Supplementary Information
Acknowledgements
We thank the residents of Curepe who welcomed us onto their property for the six-month duration of this study. We thank Nicole Achee, John Grieco, and Limb Hapairai for helpful input about study design. We are grateful for the support and field assistance of the Ministry of Health, Insect Vector Control Division, including Suruj Redoy, Cliff Ifill, Godfrey Cummings, Mohan Kolahal, Robert White and Deo Ragbir. From the UWI, Department of Life Sciences we thank Brent Daniel, Christopher de Caires-Glenn, Vishal Rangersammy, Doneisha Daniel, and Anand Hanuman for assistance in the field; Anton Manoo, Rajindra Mahabir and Jason Andrews for technical assistance; Renee Ali, Sarah Evelyn, Marcelle Boissoi and Adesh Ramsubhag, for lab assistance and technical advice; and Roshan Seeramsingh and Hamish Asmath for additional field advice. We thank Shem Manickchand from the Department of Geomatics and Land Management, UWI for graphical map assistance.
Author contributions
L.D.J., N.W., A.T.M.S., and R.S.F. conducted the field studies. E.R.S. and D.W.S. analyzed the data. N.N., A.M., M.D.S., and D.W.S. designed and coordinated the project. D.W.S. drafted and revised the manuscript. All authors contributed to revisions of the manuscript and approved the final version.
Funding
Funding was provided by U.S. Department of Defense (Grant Nos. W81XWH-17-1-0294, W81XWH-17-1-0295).
Data availability
All data generated or analyzed during this study are included in this article.
Competing interests
MDS and DWS filed a patent application with the U. S. Patent Office. All other authors have no conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-07910-0.
References
- 1.Bhatt S, et al. The global distribution and burden of dengue. Nature. 2013;496:504–509. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gratz NG. Critical review of the vector status of Aedes albopictus. Med. Vet. Entomol. 2004;18:215–227. doi: 10.1111/j.0269-283X.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- 3.Weaver SC, Charlier C, Nasilakis N, Lecuit M. Zika, chikungunya, and other emerging vector-borne viral diseases. Annu. Rev. Med. 2018;69:1–14. doi: 10.1146/annurev-med-050715-105122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilder-Smith A, et al. Epidemic arboviral diseases: priorities for research and public health. Lancet Infect. Dis. 2017;17:e101–e106. doi: 10.1016/S1473-3099(16)30518-7. [DOI] [PubMed] [Google Scholar]
- 5.Felicetti T, Manfroni G, Cecchetti V, Cannalire R. Broad-spectrum flavivirus inhibitors: a medicinal chemistry point of view. Chem. Med. Chem. 2020;15:2391–2419. doi: 10.1002/cmdc.202000464. [DOI] [PubMed] [Google Scholar]
- 6.da Silveira LTC, Bernardo T, Santos M. Systemic review of dengue vaccine efficacy. BMC Inf. Dis. 2019;19:750. doi: 10.1186/s12879-019-4369-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katzelnich LC, et al. Zika virus infection enhances future risk of severe dengue disease. Science. 2020;369:1123–1128. doi: 10.1126/science.abb6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rezza G, Weaver SC. Chikungunya as a paradigm for emerging viral diseases: evaluating disease impact and hurdles to vaccine development. PLoS Negl. Trop. Dis. 2019;13:e0006919. doi: 10.1371/journal.pntd.0006919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reiter P, Gubler DJ. Surveillance and control of urban dengue vectors. In: Gubler DJ, Kuno G, editors. Dengue and dengue hemorrhagic fever. CAB International; 1997. pp. 425–462. [Google Scholar]
- 10.Moyes CL, et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl. Trop. Dis. 2017;11:e0005625. doi: 10.1371/journal.pntd.0005625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowman LR, Donegan S, McCall PJ. Is dengue vector control deficient in effectiveness or evidence?: systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2016;10:e0004551. doi: 10.1371/journal.pntd.0004551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erlanger TE, Keiser J, Utzinger J. Effect of dengue vector control interventions on entomological parameters in developing countries: a systematic review and meta-analysis. Med. Vet. Entomol. 2008;22:203–221. doi: 10.1111/j.1365-2915.2008.00740.x. [DOI] [PubMed] [Google Scholar]
- 13.Banerjee S, Aditya G, Saha GK. Household disposables as breeding habitats of dengue vectors: linking wastes and public health. Waste Manag. 2013;33:233–239. doi: 10.1016/j.wasman.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Chadee DD, Doon R, Severson DW. Surveillance of dengue fever cases using a novel Aedes aegypti population sampling method in Trinidad, West Indies: the cardinal points approach. Acta Trop. 2007;104:1–7. doi: 10.1016/j.actatropica.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Barrera R, Acevedo V, Amador M. Role of abandoned and vacant houses on Aedes aegypti productivity. J. Med. Entomol. 2020;104:145–150. doi: 10.4269/ajtmh.20-0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chadee DD, Rahaman A. Use of water drums by humans and Aedes aegypti in Trinidad. J. Vector Ecol. 2000;25:28–35. [PubMed] [Google Scholar]
- 17.Padmanabha H, Soto E, Mosquera M, Lord CC, Lounibos LP. Ecological links between water storage behaviors and Aedes aegypti production: implications for dengue vector control in variable climates. EcoHealth. 2010;7:78–90. doi: 10.1007/s10393-010-0301-6. [DOI] [PubMed] [Google Scholar]
- 18.Colton YM, Chadee DD, Severson DW. Natural skip oviposition of the mosquito Aedes aegypti indicated by codominant genetic markers. Med. Vet. Entomol. 2003;17:195–204. doi: 10.1046/j.1365-2915.2003.00424.x. [DOI] [PubMed] [Google Scholar]
- 19.Davis TJ, Kaufman PE, Hogsette JA, Kline DI. The effects of larval habitat quality on Aedes albopictus skip oviposition. J. Am. Mosq. Control Assoc. 2015;31:321–328. doi: 10.2987/moco-31-04-321-328.1. [DOI] [PubMed] [Google Scholar]
- 20.David MR, Lourenco-de-Oliveira R, de Freitas RM. Container productivity, daily survival rates and dispersal of Aedes aegypti mosquitoes in a high income dengue epidemic neighbourhood of Rio de Janeiro: presumed influence of differential urban structure on mosquito biology. Mem. Inst. Oswaldo Cruz. 2009;104:927–932. doi: 10.1590/s0074-02762009000600019. [DOI] [PubMed] [Google Scholar]
- 21.Focks DA, Chadee DD. Pupal survey: an epidemiologically significant surveillance method for Aedes aegypti: an example using data from Trinidad. Am. J. Trop. Med. Hyg. 1997;56:159–167. doi: 10.4269/ajtmh.1997.56.159. [DOI] [PubMed] [Google Scholar]
- 22.Morrison AC, et al. Temporal and geographic patterns of Aedes aegypti (Diptera: Culicidae) production in Iquitos, Peru. J. Med. Entomol. 2004;41:1123–1142. doi: 10.1603/0022-2585-41.6.1123. [DOI] [PubMed] [Google Scholar]
- 23.Chadee DD. Oviposition strategies adopted by gravid Aedes aegypti (L.) (Diptera: Culicidae) as detected by ovitraps in Trinidad, West Indies (2002–2006) Acta Trop. 2009;111:279–283. doi: 10.1016/j.actatropica.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Chadee DD. Seasonal incidence and horizontal distribution patterns of oviposition by Aedes aegypti in an urban environment in Trinidad, West Indies. J. Am. Mosq. Control Asso. 1992;8:281–284. [PubMed] [Google Scholar]
- 25.Fay RW, Eliason DA. A preferred oviposition site as a surveillance method for Aedes aegypti. Mosq. News. 1966;26:531–535. [Google Scholar]
- 26.Johnson BJ, Ritchie SA, Fonseca DM. The state of the art of lethal oviposition trap-based mass interventions for arboviral control. Insects. 2017;8:5. doi: 10.3390/insects8010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eiras AE, Buhagiar TS, Ritchie SA. Development of the gravid Aedes trap for the capture of adult female container-exploiting mosquitoes (Diptera: Culicidae) J. Med. Entomol. 2014;51:200–209. doi: 10.1603/me13104. [DOI] [PubMed] [Google Scholar]
- 28.Mackay AJ, Amador M, Barrera R. An improvied autocidal gravid ovitrap for the control and surveillance of Aedes aegypti. Parasit. Vectors. 2013;6:225. doi: 10.1186/1756-3305-6-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olson KE, Blair CD. Arbovirus-mosquito interactions: RNAi pathway. Curr. Opin. Virol. 2015;15:119–126. doi: 10.1016/j.coviro.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hapairai LK, et al. Lure-and-kill yeast interfering RNA larvicides targeting neural genes in the human disease vector mosquito Aedes aegypti. Sci. Rep. 2017;7:13223. doi: 10.1038/s41598-017-13566-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mysore K, et al. Yeast interfering RNA larvicides targeting neural genes induce high rates of Anopheles larval mortality. Malaria J. 2017;16:461. doi: 10.1186/s12936-017-2112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mysore K, et al. Characterization of a broad-based mosquito yeast interfering RNA larvicide with a conserved target site in mosquito semaphorin-1a genes. Parasit. Vectors. 2019;12:256. doi: 10.1186/s13071-019-3504-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mysore K, et al. Characterization of a yeast interfering RNA larvicide with a target site conserved in the synaptotagmin gene of multiple disease vector mosquitoes. PLoS Negl. Trop. Dis. 2019;13:e0007422. doi: 10.1371/journal.pntd.0007422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hapairai LK, et al. Evaluation of large volume yeast interfering RNA lure-and-kill ovitraps for attraction and control of Aedes mosquitoes. Med. Vet. Entomol. 2021;35:361–370. doi: 10.1111/mve.12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeileis A, Hothorn T. Diagnostic checking in regression relationships. R News. 2002;2:7–10. [Google Scholar]
- 36.Braks MAH, Honorio NA, Lourenco-de-Oliveira R, Juliano SA, Lounibos LP. Convergent habitat segregation of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in southeastern Brazil and Florida. J. Med. Entomol. 2003;40:785–794. doi: 10.1603/0022-2585-40.6.785. [DOI] [PubMed] [Google Scholar]
- 37.Kumari R, Kumar K, Chauhan LS. First dengue virus detection in Aedes albopictus from Delhi, India: Its breeding ecology and role in dengue transmission. Trop. Med. Int. Health. 2012;16:949–954. doi: 10.1111/j.1365-3156.2011.02789.x. [DOI] [PubMed] [Google Scholar]
- 38.Apostol BL, Black WC, IV, Reiter P, Miller BR. Use of randomly amplified polymorphic DNA amplified by polymerase chain reaction markers to estimate the number of Aedes aegypti families at oviposition sites in San Juan, Puerto Rico. Am. J. Trop. Med. Hyg. 1994;51:89–97. doi: 10.4269/ajtmh.1994.51.89. [DOI] [PubMed] [Google Scholar]
- 39.Corbet PS, Chadee DD. An improved method for detecting substrate preferences shown by mosquitoes that exhibit ‘skip oviposition’. Physiol. Entomol. 1993;18:114–118. [Google Scholar]
- 40.Reinbold-Wasson DD, Reiskind MH. Comparative skip-oviposition behavior among container breeding Aedes spp. mosquitoes (Diptera: Culicidae) J. Med. Entomol. 2021 doi: 10.1093/jme/tjab084. [DOI] [PubMed] [Google Scholar]
- 41.Barrera R. Spatial stability of adult Aedes aegypti populations. Am. J. Trop. Med. Hyg. 2011;85:1087–1092. doi: 10.4269/ajtmh.2011.11-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barrera R, Amador M, Ruiz-Valcarcel J, Acevedo V. Factors modulating captures of gravid Aedes aegypti females. J. Am. Mosq. Control Assoc. 2020;36:66–73. doi: 10.2987/20-6931.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moura MBCM, et al. Spatio-temporal dynamics of Aedes aegypti and Aedes albopictus oviposition in an urban area of northeastern Brazil. Trop. Med. Int. Health. 2020;25:1510–1521. doi: 10.1111/tmi.13491. [DOI] [PubMed] [Google Scholar]
- 44.Crawford JE, et al. Efficient production of male Wolbachia-infected Aedes aegypti mosquitoes enables large-scale suppression of wild populations. Nat. Biotechnol. 2020;38:482–492. doi: 10.1038/s41587-020-0471-x. [DOI] [PubMed] [Google Scholar]
- 45.Lau KW, et al. Vertical distribution of Aedes mosquitoes in multiple story buildings in Selangor and Kuala Lumpur, Malaysia. Trop. Biomed. 2013;30:36–45. [PubMed] [Google Scholar]
- 46.Perich MJ, et al. Field evaluation of a lethal ovitrap against dengue vectors in Brazil. Med. Vet. Entomol. 2003;17:205–210. doi: 10.1046/j.1365-2915.2003.00427.x. [DOI] [PubMed] [Google Scholar]
- 47.Serpa LLN, et al. Study of the the distribution and abundance of the eggs of Aedes aegypti and Aedes albopictus according to the habitat and meteorlogical variables, municipality of Sao Sebastiao, Sao Paulo state, Brazil. Parasit. Vectors. 2014;6:321. doi: 10.1186/1756-3305-6-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sithiprasasna R, et al. Field evaluation of a lethal ovitrap for the control of Aedes aegypti (Diptera: Culicidae) in Thailand. J. Med. Entomol. 2003;40:455–462. doi: 10.1603/0022-2585-40.4.455. [DOI] [PubMed] [Google Scholar]
- 49.Barrera R, Amador M, Munoz J, Acevedo V. Integrated vector control of Aedes aegypti mosquitoes around target houses. Parasit. Vectors. 2018;11:88. doi: 10.1186/s13071-017-2596-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naranjo DP, et al. Vector control programs in Saint Johns County, Florida and Guayas, Ecuador: Successes and barriers to integrated vector management. BMC Public Health. 2014;14:674. doi: 10.1186/1471-2458-14-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Regis LN, et al. Sustained reduction of the dengue vector population resulting from an integrated control strategy applied in two Brazilian cities. PLoS ONE. 2013;8:e67682. doi: 10.1371/journal.pone.0067682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stewart ATM, et al. Community acceptance of yeast interfering RNA larvicide technology for control of Aedes mosquitoes in Trinidad. PLoS ONE. 2020;15:e0237675. doi: 10.1371/journal.pone.0237675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winter N, et al. Assessment of Trinidad community stakeholder perspectives on the use of yeast interfering RNA-baited ovitraps for biorational control of Aedes mosquitoes. PLoS ONE. 2021;16:e0252997. doi: 10.1371/journal.pone.0252997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chadee DD, Corbet PS. Seasonal incidence and diel patterns of oviposition in the field of the mosquito, Aedes aegypti (L.) (Diptera: Culicidae) in Trinidad, West Indies: a preliminary study. Ann. Trop. Med. Parasitol. 1987;81:151–161. doi: 10.1080/00034983.1987.11812107. [DOI] [PubMed] [Google Scholar]
- 55.Edman JD, et al. Aedes aegypti (Diptera: Culicdae) movement influenced by availability of oviposition sites. J. Med. Entomol. 1998;35:578–583. doi: 10.1093/jmedent/35.4.578. [DOI] [PubMed] [Google Scholar]
- 56.Reiter P, Amador MA, Anderson RA, Clark GG. Short report: dispersal of Aedes aegypti in an urban area after blood feeding as demonstrated by rubidium-marked eggs. Am. J. Trop. Med. Hyg. 1995;52:177–179. doi: 10.4269/ajtmh.1995.52.177. [DOI] [PubMed] [Google Scholar]
- 57.Mysore K, et al. Preparation and use of a yeast shRNA delivery system for gene silencing in mosquito larvae. Methods Mol. Biol. 2019;1858:213–231. doi: 10.1007/978-1-4939-8775-7_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chadee DD, Fat FH, Persad RC. First record of Aedes albopictus from Trinidad, West Indies. J. Am. Mosq. Control Assoc. 2003;19:438–439. [PubMed] [Google Scholar]
- 59.Clemons A, Mori A, Haugan M, Severson DW, Duman-Scheel M. Culturing and egg collection of Aedes aegypti. Cold Spring Harb. Protoc. 2010 doi: 10.1101/pdb.prot5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stan Developmental Team. Stan Modeling Language Users Guide and Reference Manual, v.2.22.1https://mc-stan.org (2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article.