Abstract
Luffa is a genus of tropical and subtropical vines belonging to the Cucurbitaceae family. Sponge gourd (Luffa cylindrica) and ridge gourd (Luffa acutangula) are two important species of the genus Luffa and are good sources of human nutrition and herbal medicines. As a vegetable, aromatic luffa is more preferred by consumers than nonaromatic luffa. While the aroma trait is present in the sponge gourd, the trait is not present in the ridge gourd. In this study, we identified Luffa cylindrica’s betaine aldehyde dehydrogenase (LcBADH) as a gene associated with aroma in the sponge gourd based on a de novo assembly of public transcriptome data. A single nucleotide polymorphism (SNP: A > G) was identified in exon 5 of LcBADH, causing an amino acid change from tyrosine to cysteine at position 163, which is important for the formation of the substrate binding pocket of the BADH enzyme. Based on the identified SNP, a TaqMan marker, named AroLuff, was developed and validated in 370 F2 progenies of the sponge gourd. The marker genotypes were perfectly associated with the aroma phenotypes, and the segregation ratios supported Mendelian’s simple recessive inheritance. In addition, we demonstrated the use of the AroLuff marker in the introgression of LcBADH from the aromatic sponge gourd to the ridge gourd to improve aroma through interspecific hybridization. The marker proved to be useful in improving the aroma characteristics of both Luffa species.
Subject terms: Plant breeding, Plant sciences, Plant biotechnology, Agricultural genetics
Introduction
Luffa is a genus of tropical and subtropical vines classified as Cucurbitaceae. Sponge gourd [Luffa cylindrica (L.) Roem, syn. L. aegyptica Mill] and ridge gourd [Luffa acutangula (L.) Roxb.] are two important species under the genus Luffa commonly cultivated for their fruits, which are edible when young and have a fibrous sponge-like texture when mature. Both the sponge gourd and the ridge gourd are good sources of various minerals, carbohydrates, phosphorus, and vitamin C, which makes them important vegetables and good for human nutrition1,2. These gourds are also known for their medicinal function. Some tissues, such as leaves, seeds and fruits, are used in the treatment of various diseases, especially diabetes, inflammatory diseases, diarrhea, and viral infections3,4.
The sponge gourd and the ridge gourd are important vegetables widely grown in tropical and subtropical countries. A special type of sponge gourd with a pleasant “pandan-like” aroma is also available in some countries, such as Thailand and Vietnam. Aromatic sponge gourd is more preferred by consumers than nonaromatic sponge gourd. Unlike the sponge gourd, there is no report of the existence of an aromatic type of ridge gourd. Aroma is a value-added trait in several food crops, such as rice and vegetable soybean5,6. Products with aroma have a higher demand and can achieve a higher price than products without aroma6. The 2-acetyl-1-pyrroline (2AP) is generally believed to be the main component responsible for the “popcorn-like” or “pandan-like” aroma of plants7. A variety of plants are known to synthesize 2AP, including pandan (Pandanus amaryllifolius Roxb.)8, rice (Oryza sativa L.)5,8–10, bread flowers (Vallaris glabra Ktze)11, soybean (Glycine max L.)12,13, sorghum (Sorghum bicolor L.)14, cucumber (Cucumis sativus L.)15, Bassia latifolia Roxb16, winter melon (Benincasa hispida)17 and coconut (Cocos nucifera)18,19.
The gene that plays an important role in the 2AP biosynthetic pathway of crops was first identified in rice20 and named BAD2 or BADH2 based on sequence similarity to the previously identified betaine aldehyde dehydrogenase (BADH). Later, the gene was synonymized to aminoaldehyde dehydrogenase (AMADH), a member of plant ALDH10 that catalyzes a whole range of aminoaldehydes12,21. The functional BADH/AMADH catalyzes the oxidation of gamma-aminobutyraldehyde, which is the substrate of 2AP, leading to the synthesis of gamma-aminobutyric acid (GABA). The loss of BADH2/AMADH2 function leads to the accumulation of gamma-aminobutyraldehyde, which is subsequently converted to 2AP20. The study of aroma genes in crop plants is then gradually expanding, as orthologs of this gene have been characterized as being responsible for aroma phenotypes in several crops, i.e., soybean12,22, sorghum14, and crops in the Cucurbitaceae family, such as cucumber15 and winter melon17. The study of the gene has also extended to tree plants such as coconut18,19. Several allelic variations have also been reported for this gene in several plants, such as in rice23,24, soybean12,22 and coconut18,19.
Although the gene and molecular mechanism associated with 2AP biosynthesis have been well elucidated in various crops, the detection of the gene and molecular mechanism has not been reported in Luffa, making it difficult to improve an elite variety with the aroma trait. In this study, we identified the gene associated with aroma in the sponge gourd (Luffa cylindrica) based on public transcriptome analysis. In addition, we developed a functional marker based on the SNP identified in the gene, validated it in an F2 population of sponge gourd, and used it to select the desired plants in an interspecific population (sponge gourd × ridge gourd). The marker proved useful in improving the aroma trait of Luffa.
Results
Confirmation of 2AP as a potent volatile compound contributing to the aroma of aromatic sponge gourd
The aromatic sponge gourd (Luffa cylindrica) has a “popcorn-like” or “pandan-like” aroma that is absent in its nonaromatic counterpart. As reported in other plants, this aroma is due to the potent volatile compound 2-acetyl-1-pyrroline (2AP). To confirm that the aroma in sponge gourd is also due to 2AP, we analyzed the 2AP content in the fruit from an aromatic inbred line, PB-00493, and a nonaromatic inbred line, PB-00492, using gas chromatography/mass spectrometry (GC/MS). As expected, 2AP was detected at a high concentration (7.33 ppm) in PB-00493, while it was found at a negligible level (0.39 ppm) in PB-00492 (Supplementary Fig. S1). This result indicates that the aroma of the aromatic sponge gourd is due to the presence of 2AP.
Identification of the BADH2 ortholog in sponge gourd based on a de novo transcriptome assembly
The key gene responsible for 2AP in plants is described as a nonfunctional version of betaine aldehyde dehydrogenase 2 (BADH2), also called aminoaldehyde dehydrogenase (AMADH)12,15,17–19. We hypothesized that the production of 2AP in the sponge gourd is also linked to BADH2. We attempted to identify the gene using public transcriptome data, which we assembled into a de novo transcript assembly using Trinity. As a result, 63,392 transcript contigs were assembled, of which 50,146 contained coding sequences (CDSs) and 21,977 had a BLAST hit (Supplementary Table S1). We then used the winter melon BhAMADH17 as a search query (tblastn similarity search) against these assembled contigs to identify the BADH2 ortholog in sponge gourd. As a result, a 2235-bp-long transcript contig (c16191_g1_i1) was identified as the best match, with 94.43% identity to the winter melon query gene at the amino acid level (Supplementary Fig. S2). This candidate has a 1512 bp long coding sequence (CDS) that can be translated into 503 amino acids (Supplementary Fig. S3). The gene has been named and will be referred to as LcBADH hereafter.
LcBADH gene structure and sequence variation
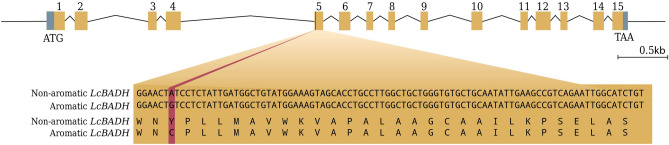
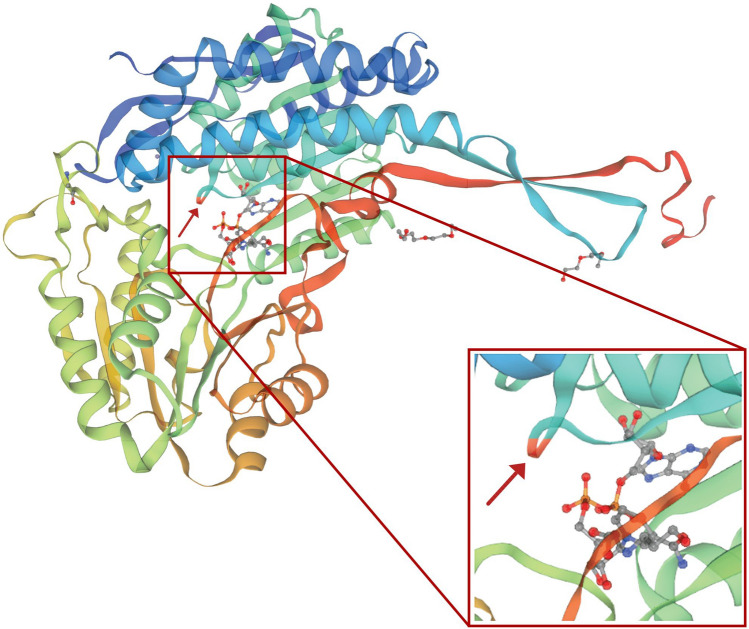
The structure of LcBADH was characterized by alignment of transcript contig c16191_g1_i1 with the cDNA sequence and genomic DNA sequence of BhAMADH from winter melon (Benincasa hispida). As a result, LcBADH was annotated with 15 exons separated by 14 introns (Supplementary Fig. S4). To determine the sequence variation in the LcBADH gene, we first sequenced the entire gene and then aligned the sequences comparing an aromatic (PB-00493) and a nonaromatic (PB-00492) sponge gourd inbred line. To sequence the entire LcBADH, we used a set of primers to amplify the genomic DNA of the sponge gourd inbred lines. The primers were designed to generate DNA fragments covering the entire LcBADH gene (Supplementary Fig. S5). The amplified fragments were sequenced using Sanger sequencing, and then the sequences were assembled into a full-length gene and aligned between aromatic and nonaromatic sponge gourd. Sequence alignment revealed a base substitution (A > G) in exon 5 causing a change in the amino acid tyrosine to cysteine (Y > C) at position 163 of the protein sequence in aromatic sponge gourd compared to nonaromatic sponge gourd (Fig. 1). Amino acid Y-163 is one of the six residues predicted to form the substrate binding pocket of the BADH/AMADH enzyme (Fig. 2). In addition, this position is near N-162, one of the two catalytic residues predicted to interact with substrate oxygen25.
Figure 1.
Structure of LcBADH and sequence variation of the gene showing the single nucleotide change on exon 5 associated with the aromatic phenotype on sponge gourd. The exons of the gene model are shown in a brown highlighted rectangle and the introns are represented by thin lines. The gray highlighted rectangles at the beginning and end of the gene model indicate the 5′ and 3′ UTR regions. The base substitution and amino acid change in exon 5 are shown in red.
Figure 2.
Three-dimensional protein structure homology models of LcBADH visualized using PyMOL (The PyMOL Molecular Graphics System, Version 2.5 Schrödinger, LLC). The expanded box shows the cysteine mutant at position 163 as indicated by a red arrow.
LcBADH gene expression in various tissues of aromatic and nonaromatic sponge gourd
To verify whether the SNP found in LcBADH affects gene expression, we performed quantitative RT-PCR (qRT-PCR) to analyze gene expression in different tissues (male and female flowers, leaves and fruits) and compare aromatic and nonaromatic sponge gourds. The results of qRT-PCR performed on three biological replicates showed that there was no significant difference in LcBADH gene expression when comparing aromatic and nonaromatic sponge gourd (Fig. 3), suggesting that the SNP does not affect gene expression. However, since the sequence variation in exon 5 in aromatic sponge gourd resulted in a change in the amino acid C163Y, which is important for enzyme activity, this mutation may cause a change in enzyme activity. Consequently, 2AP is enhanced.
Figure 3.
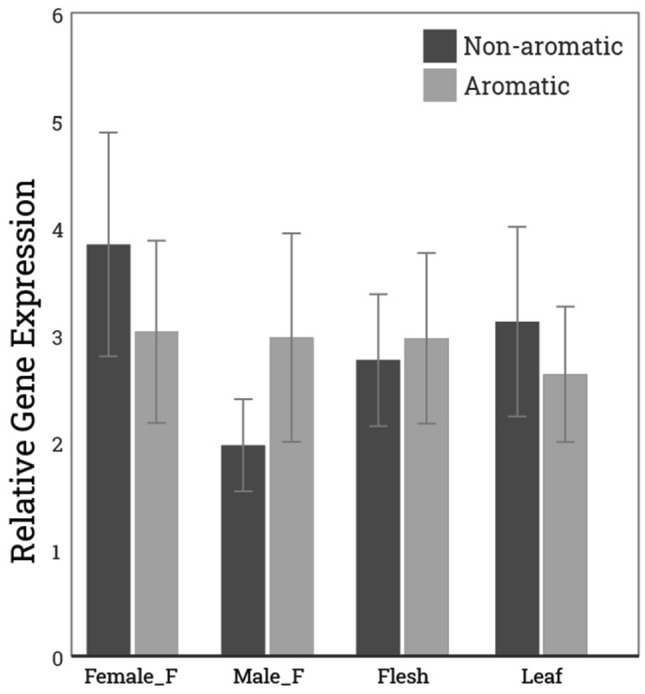
Relative normalized expression of LcBADH based on qRT-PCR in female flower, male flower, fruit, and leaf tissues compared between nonaromatic sponge gourd (PB-00492) and aromatic sponge gourd (PB-00493). Dark gray represents nonaromatic sponge gourd and pale gray represents aromatic sponge gourd. The I bars represent the standard deviation.
A functional marker of the aroma of sponge gourd
Based on the single nucleotide polymorphism (A/G) in exon 5 of LcBADH, we developed a TaqMan probe that can detect three genotypes: homozygous G/G, heterozygous A/G, and homozygous A/A (Table 1). The marker is hereafter referred to as AroLuff. To evaluate the efficacy of the AroLuff marker for the aroma trait in sponge gourd, we genotyped 370 F2 individuals derived from a cross between aromatic sponge gourd (PB-00493) and nonaromatic sponge gourd (PB-00492) and evaluated the aroma phenotype in this population using a sensory test. The genotyping results showed that 87 plants had the homozygous (G/G) genotype, 189 plants had the heterozygous (A/G) genotype, and 94 plants had the homozygous (A/A) genotype (Supplementary Fig. S6). Based on the sensory test results, all homozygous (G/G) plants were classified as aromatic, while those homozygous (A/A) and heterozygous (A/G) plants were classified as nonaromatic (Supplementary Table S2). We also performed a chi-square test for the fit of the segregating phenotypes and segregating genotypes of 370 F2 individuals. The results showed that both ratios perfectly matched the Mendelian ratio for a single recessive gene controlling the trait (Table 2). Based on these results, we confirmed with certainty that LcBADH is the key gene responsible for the aroma trait (2AP) in sponge gourd.
Table 1.
Primer and probe sequences for AroLuff TaqMan marker.
| Primer/probe name | Sequence (5′–3′) |
|---|---|
| Forward primer | CAAACGTTGCTGATGTCTGTCTTTT |
| Reverse primer | CAAGGCAGGTGCTACTTTCCA |
| Reporter 1 (VIC) | ATCAATAGAGGATAGTTCC |
| Reporter 2 (FAM) | CAATAGAGGACAGTTCC |
Table 2.
Chi-square test of aroma phenotype and genotypes.
| Number | χ2 | P value | ||
|---|---|---|---|---|
| Observed | Expected | |||
| Phenotype (expected ratio is 1:3) | ||||
| Aroma | 87 | 92.5 | ||
| Non aroma | 283 | 277.5 | ||
| Total | 370 | 0.4360 | 0.5090 | |
| Genotype (expected ratio 1:2:1) | ||||
| A/A | 94 | 92.5 | ||
| A/G | 189 | 185 | ||
| G/G | 87 | 92.5 | ||
| Total | 370 | 0.4378 | 0.8034 | |
Transfer of LcBADH from aromatic sponge gourd to ridge gourd through an interspecific hybridization and selection of the aroma using the AroLuff marker
Ridge gourd [Luffa acutangula (L.) Roxb.] is another luffa species that is also an important vegetable grown in tropical countries. To date, no aromatic ridge gourd variety has been described. Having successfully demonstrated that LcBADH is a key gene controlling the aroma trait in sponge gourd (L. cylindrica), we sought to introduce the gene from aromatic sponge gourd into ridge gourd (L. acutangula) to improve the aroma in the latter species. First, we crossed the aromatic sponge gourd (PB-00493) with ridge gourd (PB-00491) to produce F1 plants (PB-00493 × PB-00491). Then, F1 plants (L. cylindrica × L. acutangula) were used as the male parent for backcrossing with ridge gourd (PB-00491) to produce BC1F1 plants [PB-00491 × (PB-00493 × PB-00491)] (Fig. 4). We used the AroLuff marker to genotype BC1F1 plants to select plants with the heterozygous A/G genotype. Of the 66 BC1F1 plants, 16 were selected and self-pollinated to produce the BC1F2 generation. A total of 93 BC1F2 plants were generated. These plants were genotyped with the AroLuff marker, and the aroma of their fruits was evaluated with a sensory test (Supplementary Table S3). The results showed that all plants with genotype G/G were aromatic, while those with heterozygous A/G and homozygous A/A were not.
Figure 4.
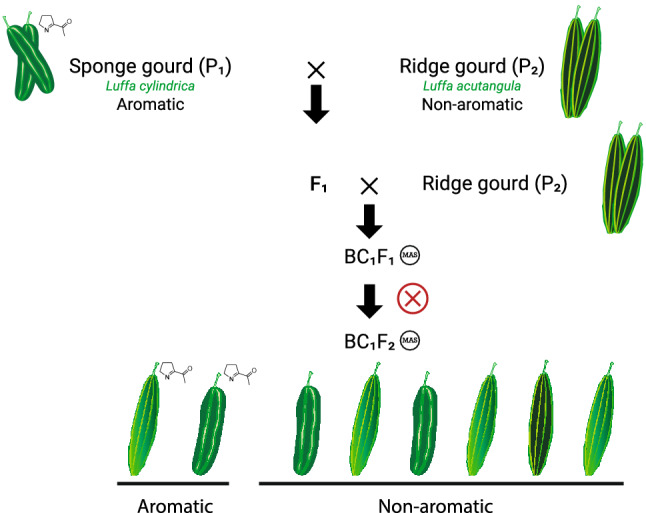
Scheme of interspecific breeding of aromatic ridge gourd.
To confirm that 2AP was produced and accumulated in the plants with genotype G/G, we analyzed the 2AP content in the fruits of some representatives of the interspecific cross in all generations, i.e., F1, BC1F1, and BC2F2, compared with the aromatic sponge gourd parent (PB-00493). As a result, a high 2AP content was observed in the aromatic plants with genotype G/G in all generations (Fig. 5).
Figure 5.
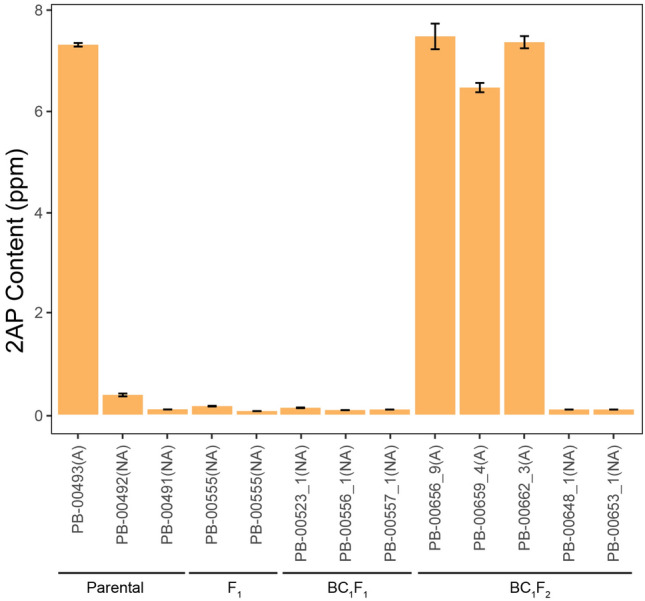
2AP analysis in parental lines and different generations of interspecific crosses. The letters A and NA after the plant names indicate aromatic and nonaromatic phenotypes, respectively.
Synteny analysis of BADH orthologous genes in some Cucurbitaceae crops
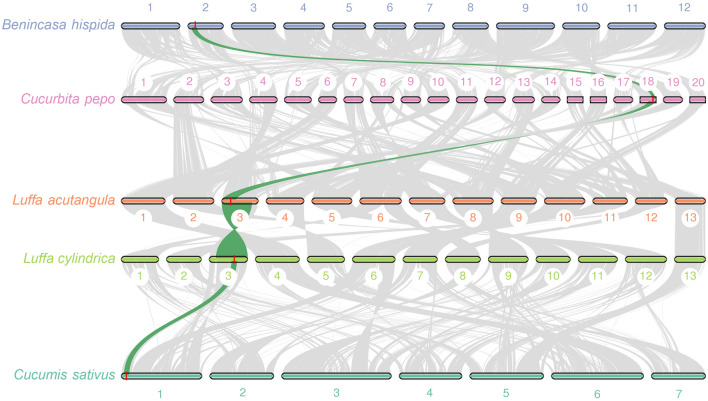
Interspecies synteny block identification was calculated using the pairwise MCScanX approach to identify BADH synteny blocks among species of Cucurbitaceae, including Benincasa hispida (2n = 24), Cucurbita pepo (2n = 40), Luffa acutangula (2n = 26), Luffa cylindrica (2n = 26) and Cucumis sativus (2n = 14). In these species, only one copy of BADH was found in their genome. The BADH gene was located on chromosome 3 in both Luffa species, whereas it was found on chromosome 2 in B. hispida, chromosome 18 in C. pepo, and chromosome 1 in C. sativus (Fig. 6). Based on the protein sequence alignment of the BADH orthologs of these species, we found high protein sequence similarity (94.83–100% similarity: Supplementary Table S6). The BADH protein sequences of both Luffa species are identical. Moreover, all major conserved residues for the catalytic sites of ALDH10 family enzymes were found to be identical in these species (Supplementary Fig. S7). The amino acid change C163Y, found exclusively in the aromatic sponge gourd, is located adjacent to the conserved N-162 residue.
Figure 6.
Syntenic relationship of BADH genes among Benincasa hispida, Cucurbita pepo, Luffa acutangula, Luffa cylindrica and Cucumis sativus visualized using MCscan (Python version (https://github.com/tanghaibao/jcvi/wiki/MCscan-(Python-version)). The green line indicates the synteny block and the red mark indicates the gene position.
Discussion
Luffa crops, sponge gourd (Luffa cylindrica) and ridge gourd (Luffa acutangula), are important vegetables in the Cucurbitaceae family. Like other crops, aromatic luffa with a pleasant aroma is preferred on the market because it has higher quality and acceptability and is preferred by consumers. Therefore, the aroma trait is attractive for breeders to include in the pipeline of the improvement of elite varieties. The volatile compound 2-acetyl-1-pyrroline (2AP) is known as the main component of the “popcorn-like” or “pandan-like” aroma in plants5,6,8,10,11,14,15,17–19. In this study, we confirmed that this compound is also a major component of aroma in aromatic sponge gourd. To date, BADH2 is the only major gene associated with 2AP biosynthesis in plants7. In this study, we identified and confirmed that LcBADH is also the key gene for the aroma trait in sponge gourd. Comparing aromatic and nonaromatic sponge gourd, the gene differed by a single nucleotide change in exon 5. Sequence variations, both SNPs and indels, identified in the coding sequence of BADH2 resulted in inactivation of BADH/AMADH enzyme activity and promoted 2AP biosynthesis5,12,25. Sequence mutations may or may not affect gene expression. For example, the 8-bp deletion in exon 7 of OsBADH2 in rice, the 2-bp deletion in exon 10 of GmAMADH2, and the 804-bp deletion in BhAMADH of winter melon resulted in a decrease in gene expression5,12,17. In contrast, the SNPs in exons 9 and 14 of CnAMADH2 in coconut had no effect on gene expression18,19. In this study, the expression of LcBADH did not differ between aromatic and nonaromatic sponge gourd according to qRT-PCR results. Thus, the mutation in exon 5 LcBADH had no effect on gene expression. However, because this mutation occurs at a position necessary for the BADH enzyme activity, we assumed that the mutation would affect protein function. Nevertheless, further study on an enzymatic assay is needed to confirm this.
Functional markers for the aroma gene have been developed for use in breeding programs in several plants 6,14,15,17,19,22. In this study, we developed the AroLuff marker based on the SNP in exon 5 of LcBADH and validated this marker in an F2 population segregating for aroma. The genotypes of the marker were perfectly associated with the aroma phenotypes. Segregation of phenotypes and genotypes in this population perfectly supported Mendelian inheritance of a single recessive gene for the aroma trait in sponge gourd. The AroLuff marker is not only useful for improving the aroma trait in sponge gourd but can also be used to improve the trait in ridge gourd. Ridge gourd and sponge gourd are two related species that have similar genome sizes (~ 760–790 Mb) and the same number of chromosomes (2n = 2x = 26). Since the aroma trait is not available in ridge gourd, we tried to transfer the trait from sponge gourd to ridge gourd by interspecific hybridization. We successfully used the AroLuff marker to select plants containing the aromatic allele of LcBADH in interspecific backcross populations of the BC1F1 and BC1F2 generations. We also confirmed that BC1F2 plants containing the homozygous recessive genotype of LcBADH are aromatic and produce 2AP at high levels. This is the first report in which the aroma trait is transferred from one species to a closely related species by interspecific hybridization combined with marker-assisted selection.
In many plant lineages, two copies of the BADH/AMADH genes, BADH1/AMADH1 and BADH2/AMADH2, are present in the genome12,18,19. In rice and soybean, the two copies of BADH/AMADH were thought to be involved in different metabolic pathways5,12. Functional BADH1/AMADH1 is involved in the glycine-betaine biosynthetic pathway, whereas functional BADH2/AMADH2 is involved in the gamma aminobutyric acid (GABA) synthetic pathway. Most studies have reported that nonfunctional BADH2/AMADH2 is the cause of 2AP biosynthesis5,12,18,19,22,25. However, there have also been a few reports on the relationship between BADH1 and 2AP26,27. Comparing the genome synteny of five species of Cucurbitaceae, we found that all these species contain only one copy of BADH/AMADH. Mutations in BADH/AMADH were reported in Benincasa hispida and Cucumis sativus and were associated with 2AP biosynthesis15,17. In the present study, we reported a mutation in LcBADH and its association with 2AP biosynthesis in Luffa cylindrica. Therefore, it is possible that a mutation in the BADH gene on chromosome 18 of Cucubita pepo, if present, could also be associated with 2AP.
Interspecific hybridization, which involves crossing two species from the same genus, could be used to improve traits in one species by using useful genes from a closely related species or from wild species, which has helped in the introgression of important traits in many vegetable crops28,29. The nearly perfect genome synteny between L. cylindrica and L. acutangula supported our success in interspecific hybridization between the two species. Our study clearly demonstrated that the aroma trait can be transferred between the two Luffa species, and the AroLuff marker will be useful in breeding programs to improve aroma in both luffa crops.
Conclusion
The gene conferring the “pandan-like” aroma trait in sponge gourd has been characterized. A SNP (A > G) found in the aromatic allele of the LcBADH gene has been shown to be associated with the aroma of sponge gourd. The functional marker “AroLuff” developed based on this SNP has proven useful in marker-assisted (MAS) breeding for the aroma trait in ridge gourd through interspecific hybridization. This is the first time that the aroma trait has been transferred between the two closely related species.
Materials and methods
Plant materials
Three hundred and seventy F2 individuals derived from the seeds of 15 F1 plants of a cross between an aromatic (PB-00493) and a nonaromatic (PB-00492) inbred line of sponge gourd (Luffa cylindrica) were used to evaluate aroma and validate the marker. An inbred line of ridge gourd (Luffa acutangula: PB-00491) was used to produce interspecific F1 progeny by crossing with aromatic sponge gourd (PB-00493) and used as a recurrent parent to produce BC1F1 progeny. Sixty-six BC1F1 plants were genotyped with the marker to select the plants with a heterozygous genotype in the gene LcBADH, and the selected plants were then self-pollinated to produce BC1F2 progeny. The sponge gourd and ridge gourd cultivars used as parents for the above populations were used together with representatives of F1 (PB-00555), F2 (PB-00522), BC1F1 (PB-00557), and BC1F2 plants to determine the content of 2-acetyl-1-pyrroline (2AP). Aromatic (PB-00493) and nonaromatic (PB-00492) sponge gourds were also used for sequencing the full-length LcBADH gene and analysis of gene expression. Field experiments were conducted at the research station of Hortigenetics Research (S.E. Asia) Limited, Suphanburi, Thailand, between July 2017 and November 2018. All plant materials used in the study were provided by Hortigenetics Research (S.E. Asia). All experiments complied with the current Biosafety guidelines of the country in which the experiments were performed.
Identification of 2AP using gas chromatography/mass spectrometry (GC/MS)
The 2AP analysis was performed on fruit from three F1 (PB-00493 × PB-00492), five aromatic, and six nonaromatic representative F2 plants, two interspecific F1 (PB-00493 × PB-00491) plants, three interspecific BC1F1, five interspecific BC1F2 plants, and the parental lines PB-00491 (ridge gourd), PB-00492 (nonaromatic sponge gourd) and PB-00493 (aromatic sponge gourd). The GC/MS system was an Agilent GC 6890 and 6850-MS 5973 (Agilent Technology, Palo Alto, CA). Extraction, detection, and quantification of 2AP from fruit flesh of sponge gourd and ridge gourd were performed according to a method described previously17.
Sensory test for aroma evaluation
The aroma characteristics of 370 F2 progenies of sponge gourd and 93 interspecific BC1F2 were evaluated using a sensory test method modified from30. One gram of fresh flesh of the 20-day-old fruit was cut into small pieces and placed in a Petri dish, which was then soaked with 2 ml of 1.7% KOH. The dish was covered with a lid and set aside at room temperature for 10 min. The aroma was then scored by three trained panels. Aroma characteristics were classified as present (aromatic) or absent (nonaromatic). Three fruits from each line were scored as replicates.
DNA and RNA extraction
Genomic DNA was isolated from young leaves using a DNeasy Plant Mini Kit (Qiagen Inc., Valencia, CA, USA). DNA quality was determined using a NanoDrop 8000 (Thermo Fisher Scientific Inc., MA, USA). DNA was stored at – 20 °C until use. Total RNA for gene expression analysis was extracted from pistils, stamens, leaves and fruits (20 days after pollination) of aromatic and nonaromatic sponge gourd using the RNeasy Plant Mini Kit (Qiagen, Inc.). The quality of total RNA was determined using a NanoDrop 8000 (Thermo Fisher Scientific Inc.). The RNA was stored at − 80 °C until use.
De novo transcriptome assembly and annotation
Transcriptome sequencing data of L. cylindrica were downloaded from the National Center for Bitechnology Information (NCBI)’s Sequence Read Archive (SRA) database (Accession number SRR1023265) and subjected to de novo transcriptome assembly. The data contained 59,874,228 pairs of 150-bp paired-end Illumina sequences. Raw data (fastq) were assessed with FASTQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/) and trimmed adapters and low-quality bases with Trimmomatic31. The quality cutoff was a PHRED33 score of > 10. Only sequencing reads ≥ 100 bp were retained. Reads that contained a portion with an average PHRED33 score < 10 that spanned at least 4 bp were removed. The clean paired-end Illumina reads were subjected to the Trinity assembly pipeline (Trinity Release v2.2.2) using the default parameters for de novo transcriptome assembly32. Assembly results were assessed using the TrinityStats.pl script that comes with Trinity, and contigs were annotated by similarity search (NCBI blastx) against a nonredundant (nr) database using standalone command line BLAST.
Sequencing of the LcBADH gene in aromatic and nonaromatic varieties of sponge gourd
To obtain the full-length sequence of LcBADH in aromatic sponge gourds, a set of primers was designed using Primer333 based on the sequence of the assembled contig (Supplementary Table S4). For each primer pair, polymerase chain reaction (PCR) was performed in a total volume of 10 μl containing 2 μl of genomic DNA (50 ng/μl), 1 μl of 10 × buffer, 1 μl of 25 mM MgCl2, 2 μl of 1 mM dNTPs, 0.5 μl of each primer (5 μM), and 0.1 μl of Taq DNA polymerase (Fermentas; Life Science, USA). PCR was initiated by denaturation at 95 °C for 3 min followed by 35 cycles at 95 °C for 15 s, 55 °C for 30 s, and 72 °C for 1 min before a final incubation at 72 °C for 10 min to complete primer extension. The amplicons were purified and sequenced using a Sanger sequencing method at First BASE Laboratories Sdn Bhd, Selangor, Malaysia. The sequences of each variety were assembled and aligned using CLC Genomics Workbench (CLC Bio; Qiagen, USA).
Three-dimensional protein structure modeling
The coding sequence (CDS) of LcBADH was translated into amino acid sequences using the Translate tool on the ExPASy website (http://web.expasy.org/translate/). The Swiss-Model Server34 (https://swissmodel.expasy.org) was used to create a 3D homology model of the LcBADH protein based on the BADH protein (PDB ID: 3IWJ) from Pisum sativum as a template. The quality of the 3D structural models was assessed using PROCHECK version 3.5.435. The 3D models were visualized in PyMOL (The PyMOL Molecular Graphics System, Version 2.5 Schrödinger, LLC).
Quantitative reverse transcription PCR (qRT-PCR) analysis
qRT-PCRs were performed using iScript One-Step RT-PCR reagent with SYBR Green (Bio-Rad, USA) to analyze the relative expression of the gene in different tissues (male and female flowers, fruit flesh and leaf) of aromatic and nonaromatic sponge gourd. Three biological replicates for aromatic and nonaromatic phenotypes were used. Total RNA at a level of one ng per sample was used as a template, and the β-actin forward primer was used as an internal reference gene to normalize the variations of the total cDNA template between samples. The gene-specific primers and actin primers are listed in Supplementary Table S5. Relative gene expression was analyzed using Bio-Rad CFX Manager analysis software (Bio-Rad, USA) to compare the change in expression compared with β-actin as a control. The results were then statistically analyzed using Student’s t-test. A P value < 0.05 was considered significant.
Development of TaqMan assays for LcBADH allelic variation detection
TaqMan allele-specific assays (primers and probes) for LcBADH were designed and synthesized by Thermo Fisher Scientific (Thermo Fisher Scientific, USA). PCR for the TaqMan® assay was performed in a total volume of 5 µl containing 1.5 μl of genomic DNA (20 ng/µl), 2.5 μl of 2 × GT express, and 0.125 μl of assay probe primer (40x), adjusted to 0.875 μl with ddH2O. The cycling conditions were 95 °C for 5 min, 40x [94 °C for 30 s and 60 °C for 1 min] and 60 °C for 2 min. Genotype calling was performed using a QuanStudio™ (Thermo Fisher Scientific, USA).
Synteny block analysis
Genome assembly data of Luffa cylindrica (GCA_017139565.1), Luffa acutangula (GCA_012295215.1), Benincasa hispida (GCF_009727055.1), Cucurbita pepo (GCF_002806865.1) and Cucumis sativus (GCF_000004075.3) were obtained from the NCBI database. Synteny blocks were generated using the MCScanX36. Syntenic relationships were visualized using Python version of MCscan37 (https://github.com/tanghaibao/jcvi/wiki/MCscan-(Python-version)). The region in the genome matching the BADH gene was searched using the MCScanX36.
Supplementary Information
Acknowledgements
This work was financially supported by Thailand Science Research and Innovation (TSRI), Grant Number RDG6050145.
Author contributions
S.A., S.W., T.T. and A.V. conceived and designed the experiments. C.S. performed the bioinformatics analysis. R.D., S.R., and K.R. performed lab experiments. S.M. performed GC–MS analysis. V.R. designed the functional marker. S.R., S.J.D.H. and C.B. managed the materials in the field. S.W. and S.A. provided critical discussion. M.K.P., S.R. and C.S. wrote the manuscript. S.W. and S.A. revised the manuscript. All authors read and approved the final manuscript.
Data availability
Transcriptome data can be found in the NCBI-SRA database, with the accession number SRR1023265. The genome assembly data of Luffa cylindrica (GCA_017139565.1), Luffa acutangula (GCA_012295215.1), Benincasa hispida (GCF_009727055.1), Cucurbita pepo (GCF_002806865.1) and Cucumis sativus (GCF_000004075.3) were obtained from the NCBI database.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Samart Wanchana, Email: samart.wan@biotec.or.th.
Siwaret Arikit, Email: siwaret.a@ku.th.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-07478-9.
References
- 1.Swetha MP, Muthukumar SP. Characterization of nutrients, amino acids, polyphenols and antioxidant activity of Ridge gourd (Luffa acutangula) peel. J. Food Sci. Technol. 2016;53:3122–3128. doi: 10.1007/s13197-016-2285-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oboh IO, Aluyor EO. Luffa cylindrica—An emerging cash crop. Afr. J. Agric. Res. 2009;4:684–688. [Google Scholar]
- 3.Abdel-Salam IM, Abou-Bakr AA, Ashour M. Cytotoxic effect of aqueous ethanolic extract of Luffa cylindrica leaves on cancer stem cells CD44+/24− in breast cancer patients with various molecular sub-types using tissue samples in vitro. J. Ethnopharmacol. 2019;238:111877. doi: 10.1016/j.jep.2019.111877. [DOI] [PubMed] [Google Scholar]
- 4.Al-Snafi AE. A review on Luffa acutangula: A potential medicinal plant. Magnesium. 2019;52:53. [Google Scholar]
- 5.Vanavichit A, Yoshihashi T. Molecular aspects of fragrance and aroma in rice. Adv. Bot. Res. 2010;56:49–73. doi: 10.1016/B978-0-12-381518-7.00002-9. [DOI] [Google Scholar]
- 6.Arikit S, et al. A PCR-based marker for a locus conferring aroma in vegetable soybean (Glycine max L.) Theor. Appl. Genet. 2011;122:311–316. doi: 10.1007/s00122-010-1446-y. [DOI] [PubMed] [Google Scholar]
- 7.Wakte K, et al. Thirty-three years of 2-acetyl-1-pyrroline, a principal basmati aroma compound in scented rice (Oryza sativa L.): A status review. J. Sci. Food Agric. 2017;97:384–395. doi: 10.1002/jsfa.7875. [DOI] [PubMed] [Google Scholar]
- 8.Buttery RG, Ling LC, Juliano BO, Turnbaugh JG. Cooked rice aroma and 2-acetyl-1-pyrroline. J. Agric. Food Chem. 1983;31:823–826. doi: 10.1021/jf00118a036. [DOI] [Google Scholar]
- 9.Widjaja R, Craske JD, Wootton M. Comparative studies on volatile components of non-fragrant and fragrant rices. J. Sci. Food Agric. 1996;70:151–161. doi: 10.1002/(SICI)1097-0010(199602)70:2<151::AID-JSFA478>3.0.CO;2-U. [DOI] [Google Scholar]
- 10.Yoshihashi T. Quantitative analysis on 2-acetyl-1-pyrroline of an aromatic rice by stable isotope dilution method and model studies on its formation during cooking. J. Food Sci. 2002;67:619–622. doi: 10.1111/j.1365-2621.2002.tb10648.x. [DOI] [Google Scholar]
- 11.Wongpornchai S, Sriseadka T, Choonvisase S. Identification and quantitation of the rice aroma compound, 2-acetyl-1-pyrroline, in bread flowers (Vallaris glabra Ktze) J. Agric. Food Chem. 2003;51:457–462. doi: 10.1021/jf025856x. [DOI] [PubMed] [Google Scholar]
- 12.Arikit S, et al. Deficiency in the amino aldehyde dehydrogenase encoded by GmAMADH2, the homologue of rice Os2AP, enhances 2-acetyl-1-pyrroline biosynthesis in soybeans (Glycine max L.) Plant Biotechnol. J. 2011;9:75–87. doi: 10.1111/j.1467-7652.2010.00533.x. [DOI] [PubMed] [Google Scholar]
- 13.Wu M-L, Chou K-L, Wu C-R, Chen J-K, Huang T-C. Characterization and the possible formation mechanism of 2-acetyl-1-pyrroline in aromatic vegetable soybean (Glycine max L.) J. Food Sci. 2009;74:S192–S197. doi: 10.1111/j.1750-3841.2009.01166.x. [DOI] [PubMed] [Google Scholar]
- 14.Yundaeng C, Somta P, Tangphatsornruang S, Wongpornchai S, Srinives P. Gene discovery and functional marker development for fragrance in sorghum (Sorghum bicolor (L.) Moench) Theor. Appl. Genet. 2013;126:2897–2906. doi: 10.1007/s00122-013-2180-z. [DOI] [PubMed] [Google Scholar]
- 15.Yundaeng C, Somta P, Tangphatsornruang S, Chankaew S, Srinives P. A single base substitution in BADH/AMADH is responsible for fragrance in cucumber (Cucumis sativus L.), and development of SNAP markers for the fragrance. Theor. Appl. Genet. 2015;128:1881–1892. doi: 10.1007/s00122-015-2554-5. [DOI] [PubMed] [Google Scholar]
- 16.Wakte KV, Kad TD, Zanan RL, Nadaf AB. Mechanism of 2-acetyl-1-pyrroline biosynthesis in Bassia latifolia Roxb. flowers. Physiol. Mol. Biol. Plants. 2011;17:231–237. doi: 10.1007/s12298-011-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruangnam S, et al. A deletion of the gene encoding amino aldehyde dehydrogenase enhances the “pandan-like” aroma of winter melon (Benincasa hispida) and is a functional marker for the development of the aroma. Theor. Appl. Genet. 2017;130:2557–2565. doi: 10.1007/s00122-017-2976-3. [DOI] [PubMed] [Google Scholar]
- 18.Saensuk C, et al. De novo transcriptome assembly and identification of the gene conferring a “pandan-like” aroma in coconut (Cocos nucifera L.) Plant Sci. 2016;252:324–334. doi: 10.1016/j.plantsci.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 19.Dumhai R, et al. Discovery of a novel CnAMADH2 allele associated with higher levels of 2-acetyl-1-pyrroline (2AP) in yellow dwarf coconut (Cocos nucifera L.) Sci. Hortic. 2019;243:490–497. doi: 10.1016/j.scienta.2018.09.005. [DOI] [Google Scholar]
- 20.Bradbury LMT, Fitzgerald TL, Henry RJ, Jin Q, Waters DLE. The gene for fragrance in rice. Plant Biotechnol. J. 2005;3:363–370. doi: 10.1111/j.1467-7652.2005.00131.x. [DOI] [PubMed] [Google Scholar]
- 21.Kopečný D, et al. Plant ALDH10 family. J. Biol. Chem. 2013;288:9491–9507. doi: 10.1074/jbc.M112.443952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juwattanasomran R, et al. A SNP in GmBADH2 gene associates with fragrance in vegetable soybean variety “Kaori” and SNAP marker development for the fragrance. Theor. Appl. Genet. 2011;122:533–541. doi: 10.1007/s00122-010-1467-6. [DOI] [PubMed] [Google Scholar]
- 23.Kovach MJ, Calingacion MN, Fitzgerald MA, McCouch SR. The origin and evolution of fragrance in rice (Oryza sativa L.) Proc. Natl. Acad. Sci. USA. 2009;106:14444–14449. doi: 10.1073/pnas.0904077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Addison, C. K. et al. Characterization of haplotype diversity in the BADH2 aroma gene and development of a KASP SNP assay for predicting aroma in U.S. rice. Rice (N Y)13, 47 (2020). [DOI] [PMC free article] [PubMed]
- 25.Chen S, et al. Badh2, encoding betaine aldehyde dehydrogenase, inhibits the biosynthesis of 2-acetyl-1-pyrroline, a major component in rice fragrance. Plant Cell. 2008;20:1850–1861. doi: 10.1105/tpc.108.058917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh A, et al. SNP haplotypes of the BADH1 gene and their association with aroma in rice (Oryza sativa L.) Mol. Breed. 2010;26:325–338. doi: 10.1007/s11032-010-9425-1. [DOI] [Google Scholar]
- 27.Monkhan T, Chen X, Somta P. BADH1 is associated with fragrance in sorghum (Sorghum bicolor (L.) Moench) cultivar ‘Ambemohor’. J. Genet. 2021;100:1–7. doi: 10.1007/s12041-020-01256-0. [DOI] [PubMed] [Google Scholar]
- 28.Manzur JP, Fita A, Prohens J, Rodríguez-Burruezo A. Successful wide hybridization and introgression breeding in a diverse set of common peppers (Capsicum annuum) using different cultivated Ají (C. baccatum) accessions as donor parents. PLoS ONE. 2015;10:e0144142. doi: 10.1371/journal.pone.0144142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J-F, Adelberg J. Interspecific hybridization in Cucumis—Progress, problems, and perspectives. HortScience. 2000;35:11–15. doi: 10.21273/HORTSCI.35.1.11. [DOI] [Google Scholar]
- 30.Sood BC, Siddiq EA. A rapid technique for scent determination in rice. Indian J. Genet. Plant Breed. 1978;38:268–275. [Google Scholar]
- 31.Bolger AM, Lohse M, Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grabherr MG, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011;29:644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Untergasser A, et al. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012;40:e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: A web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 35.Laskowski RA, MacArthur MW, Moss DS, Thornton JM. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993;26:283–291. doi: 10.1107/S0021889892009944. [DOI] [Google Scholar]
- 36.Wang Y, et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40:e49. doi: 10.1093/nar/gkr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang H, et al. Synteny and collinearity in plant genomes. Science. 2008;320:486–488. doi: 10.1126/science.1153917. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Transcriptome data can be found in the NCBI-SRA database, with the accession number SRR1023265. The genome assembly data of Luffa cylindrica (GCA_017139565.1), Luffa acutangula (GCA_012295215.1), Benincasa hispida (GCF_009727055.1), Cucurbita pepo (GCF_002806865.1) and Cucumis sativus (GCF_000004075.3) were obtained from the NCBI database.