Abstract
The potential nutritional value of duckweed Lemna minor (Lemnaceae) was evaluated for common carp Cyprinus carpio fry. Fish were fed diets containing five graded levels of duckweed: 0% (LM0, control), 5% (LM5), 10% (LM10), 15% (LM15) and 20% (LM20). The final weight and specific growth rate were significantly higher in LM15 and LM20 diets fed fish compared to others. Feed conversion ratio was minimum in fish fed diet LM20. Amylase activity was significantly higher in LM0 treatment. Total protease, trypsin and chymotrypsin activities showed linear relationships with the increased level of duckweed in the diet. Protein and essential amino acids contents were significantly higher in carp fed diets LM15 and LM20 compared to others. Lipid content was significantly higher in fish fed duckweed-based diets compared to control. A direct relationship was found between the inclusion level of duckweed in the diet and n-3 long-chain polyunsaturated fatty acid (LC-PUFA) content of carp. Contents of desaturated and elongated products of dietary linolenic acid (18:3n-3) including 20:4n-3, 20:5n-3, 22:5n-3 and 22:6n-3 increased in a graded manner with increasing dietary duckweed. The monounsaturated fatty acids and n-6 PUFA contents reduced significantly in fish fed duckweed. Expression of fads2d6, elovl2, elovl5 and fas were higher in carp fed diets LM10, LM15 and LM20 compared to control fish. The inclusion of L. minor in diet enhanced the nutritional value of carp by increasing protein, lipid, amino acids and n-3 PUFA contents.
Subject terms: Zoology, Ichthyology
Introduction
Aquaculture is growing faster than other animal food-producing sectors and has raised expectations to bridge the gap between fish demand and supply1. The expansion of the aquaculture sector primarily depends on the supply of high-quality feed for cultivated fish. At present, soybean meal is one of the most commonly used alternative ingredients to replace fishmeal in aquafeed due to its high protein content and relatively well-balanced amino acid profile that can generally satisfy the requirements of many fish species2–5. Nevertheless, soybean meal is already in high demand in the human food chain both directly and indirectly in feeds for farmed terrestrial animals. This competition means soybean meal is an expensive ingredient and this may limit its use as an ingredient to meet future demands for fish feed. Therefore, there is a constant need to find other ingredients to replace both fishmeal and its main substitute, soybean meal, in feeds for farmed fish. Ideally, such ingredients should be non-conventional to avoid or minimize competition with other animal feed sectors.
The nutritional qualities of feed ingredients including amino acid and fatty acid compositions, micronutrient and fiber contents among others are major considerations when it comes to feed formulation4–6. The nutrients must be readily bioavailable to meet the energy and other physiological requirements of the fish and so the overall composition of the ingredient should be appropriate for the digestive enzyme (protease, trypsin, chymotrypsin, lipase etc.) profile of the species, which will help to maximize feed efficiency and production. However, in addition, the composition of feed itself can influence digestive enzyme activities7. Thus, feed intake and consumption rates as well as digestive enzyme profiles can be good indicators of health status of fish8,9. For plant ingredients, the presence of anti-nutritional factors is one of the potential limitations of their use in aquafeeds10,11, although processing, such as fermentation and extrusion can reduce anti-nutritional effects12–15. In this overall context, the assessment of aquatic plants as potential ingredients for fish feed is an area of renewed interest and research16,17.
Omega-3 (n-3) long-chain polyunsaturated fatty acids (LC-PUFA), eicosapentaenoic (EPA, 20:5n-3) and docosahexaenoic (DHA, 22:6n-3) acids are essential dietary nutrients for vertebrates including humans and fish18,19. They play important roles in many physiological processes like neural development, immune and inflammatory responses18. Primary production of the n-3 LC-PUFA, EPA and DHA, occurs largely in the marine environment and so dietary sources for fish feeds are limited largely to fish oil and fishmeal20. In contrast, plant meals and vegetable oils that are the main alternatives to the marine-derived ingredients are completely devoid of EPA and DHA20. However, many freshwater fishes are capable of converting the C18 PUFA found in plants, linoleic acid (LOA; 18:2n-6) and ALA, to the physiologically essential LC-PUFA arachidonic acid (ARA; 20:4n-6), EPA and DHA21,22. Consequently, dietary LOA and ALA can satisfy the essential fatty acid (EFA) requirements of fish species such as common carp and Nile tilapia Oreochromis niloticus that have been confirmed to have the metabolic capacity to convert dietary ALA to EPA and DHA19,23,24. The conversion of LOA and ALA to LC-PUFA requires a series of fatty acyl desaturase (fads) and elongation of very long-chain fatty acids (elovl) enzymes such as elovl2 and elovl521,22,25,26. Therefore, one potential option for increasing the amount of n-3 LC-PUFA available to human populations is to exploit the endogenous ability of freshwater fish species to produce EPA and DHA from ALA20.
The nutritional value of freshwater duckweed Lemna spp. is well recognized, having relatively high protein, amino acid and fatty acid contents and low fiber, and so duckweeds can be used as dietary supplements for fish and other animals16–27. Specifically, lysine and methionine contents are higher in duckweeds compared to other plants and, therefore, more suitable as ingredients for animal feed28,29. Lemna minor is also a rich source of the omega-3 (n-3) polyunsaturated fatty acid (PUFA), α-linolenic acid (ALA, 18:3n-3)16. Several studies have investigated the use of duckweeds as an ingredient in feeds for carp species, such as L. minor30 or L. minuta31,32 in feeds for common carp Cyprinus carpio. Furthermore, L. minor has been supplemented in diets of rohu Labeo rohita15, grass carp Ctenopharyngodon idella and silver carp Hypophthalmichthys molitrix33.
Common carp is an omnivorous, bottom-feeding fish belonging to the family Cyprinidae and is one of the most cultured freshwater and economically valued species due to its fast growth and disease resistance34. This, combined with the inherent ability for the endogenous biosynthesis of n-3 LC-PUFA, makes common carp an appropriate candidate species to evaluate the utility of duckweed-based diets in freshwater aquaculture.
The overall aim of the present study is to evaluate the duckweed Lemna minor as a replacement for plant meals such as soybean meal in extruded pelleted diets for common carp Cyprinus carpio. The extrusion process helps to overcome the problems associated with the presence of anti-nutritional factors in L. minor. In the present study, the effect of Lemna minor supplemented diets on the survival, growth, digestive physiology, and biochemical composition of Cyprinus carpio are evaluated. The impact of dietary L. minor on the content of EPA and DHA and expression of fads and elovl genes in fish are also studied. This helps to understand the mechanism of endogenous biosynthesis of LC-PUFA from duckweed-derived ALA in freshwater fish like common carp. The n-3 PUFA content of control diet is very less and it helps to see the effect of L. minor supplemented diets on the composition of fish.
Results
Survival and growth of fish
After 60 days of culture, the number of fish in each aquarium was counted and no mortalities recorded in any dietary treatment. The final weight of carp fed diets LM15 (2.50 ± 0.012 g) and LM20 (2.66 ± 0.023 g) were significantly higher compared to the fish fed the other diets (Table 1). Final weight was lowest in control diet fed fish (LM0, 1.69 ± 0.005 g) with no inclusion of L. minor. The final weight and SGR of fish increased linearly (p < 0.05) with the increasing level of L. minor in the diet. There was no significant difference in feed intake of fish fed with five different diets. The feed intake of fish ranged from 2.36 to 2.46 g 100 g−1 BW day−1. The FCR was lowest and highest in carp fed diets LM20 (1.02 ± 0.01) and LM0 (1.27 ± 0.06), respectively. The FCR decreased linearly with the increasing level of duckweeds in the diet of carp.
Table 1.
Initial and final weights, survival rate, specific growth rate, feed intake, feed conversion ratio and digestive enzyme activities of Cyprinus carpio fed with five different diets.
| Parameters | LM0 | LM5 | LM10 | LM15 | LM20 | Polynomial contrasts | |
|---|---|---|---|---|---|---|---|
| Linear | Quadratic | ||||||
| Initial body weight (g) | 0.48 ± 0.009a | 0.47 ± 0.008a | 0.48 ± 0.006a | 0.48 ± 0.007a | 0.47 ± 0.005a | 0.783 | 0.643 |
| Final weight (g) | 1.69 ± 0.005d | 1.94 ± 0.030c | 2.10 ± 0.009b | 2.50 ± 0.012a | 2.66 ± 0.023a | < 0.001 | 0.425 |
| Specific growth rate (g) | 2.11 ± 0.03c | 2.35 ± 0.05b | 2.48 ± 0.02b | 2.76 ± 0.03a | 2.87 ± 0.01a | < 0.001 | 0.474 |
| Feed intake (g 100 g−1 BW day−1) | 2.36 ± 0.10a | 2.46 ± 0.12a | 2.41 ± 0.04a | 2.44 ± 0.06a | 2.38 ± 0.02a | 0.925 | 0.479 |
| Feed conversion ratio | 1.27 ± 0.06a | 1.22 ± 0.07ab | 1.14 ± 0.02b | 1.08 ± 0.02c | 1.02 ± 0.01c | 0.001 | 0.969 |
| Survival (%) | 100 | 100 | 100 | 100 | 100 | ||
| Digestive enzymes | |||||||
| Amylase (mU mg−1protein min−1) | 112.44 ± 1.67a | 75.16 ± 2.06d | 87.66 ± 0.78c | 87.22 ± 0.66c | 104.10 ± 1.14b | 0.264 | < 0.001 |
| Protease (Fluorescence change unit−1) | 104.31 ± 1.77d | 107.00 ± 0.53d | 114.70 ± 1.89c | 128.80 ± 4.40b | 151.70 ± 1.23a | < 0.001 | 0.001 |
| Trypsin (µM AMC mg-1 protein min−1) | 822.95 ± 2.10d | 838.03 ± 4.65c | 847.41 ± 2.86c | 1146.73 ± 7.60b | 1320.30 ± 5.61a | < 0.001 | < 0.001 |
| Chymotrypsin (µM AMC mg−1 protein min−1) | 1772.69 ± 14.00d | 1782.77 ± 14.00d | 2075.77 ± 26.07c | 2341.07 ± 10.21a | 2128.35 ± 15.522b | < 0.001 | < 0.001 |
| Lipase (µM 4-MU mg−1 protein min−1) | 680.98 ± 4.73b | 731.04 ± 7.55a | 691.53 ± 6.96b | 642.67 ± 2.15c | 626.54 ± 11.84c | < 0.001 | < 0.001 |
Values (means ± SE, n = 3) in each row with different superscript are significantly different (p < 0.05). The polynomial orthogonal contrast was considered significant at p < 0.05 level.
LM0 = Control, soybean; LM5 = 5% L. minor; LM10 = 10% L. minor; LM15 = 15% L. minor; LM20 = 20% L. minor.
Digestive enzyme activities of fish
Amylase activity was significantly lower in carp fed the duckweed-based diets compared to fish fed control diet LM0 (Table 1). The total protease, trypsin and chymotrypsin activities increased linearly (p < 0.05) with the increased levels of duckweeds in the diet. Specially, the graded inclusion of dietary duckweed resulted in graded increased activities of protease and trypsin with highest activities recorded in fish fed diet LM20 followed by fish fed diet LM15. Chymotrypsin activity was also higher in fish fed diets LM10–LM20 compared to fish fed the control diet LM0. Lipase activity was significantly higher in fish fed diet LM5 compared to other treatments. The lowest lipase activity was observed in fish fed diet LM20.
Biochemical composition of fish
The protein and lipid contents of common carp showed linear relationships (p < 0.05) with the increased inclusions of duckweeds in the diet (Table 2). Protein content was significantly higher in carp fed diets LM15 (145.7 ± 0.52 g kg−1 of fresh weight) and LM20 (148.6 ± 0.57 g kg−1 of fresh weight) with the two highest inclusions of duckweed compared to fish fed the other diets. Significantly higher lipid content was observed in fish fed the duckweed-based diets (65.0–67.5 g kg−1 of fresh weight) compared to carp fed the control diet without duckweed. The moisture content was significantly higher in fish fed control diet LM0 (771.8 ± 1.24 g kg−1 of fresh weight) compared to fish fed the duckweed-based diets. There was no significant difference in ash content among various diets fed carp.
Table 2.
Biochemical composition of Cyprinus carpio cultured under five different feeding regimes (g kg−1 of fresh weight).
| Parameters | LM0 | LM5 | LM10 | LM15 | LM20 | Polynomial contrasts | |
|---|---|---|---|---|---|---|---|
| Linear | Quadratic | ||||||
| Moisture | 771.8 ± 1.24a | 760.6 ± 0.58b | 759.6 ± 1.85b | 757.2 ± 3.10b | 752.1 ± 4.00b | < 0.001 | 0.285 |
| Crude protein | 140.0 ± 0.92b | 140.8 ± 1.22b | 142.4 ± 0.85b | 145.7 ± 0.52a | 148.6 ± 0.57a | < 0.001 | 0.099 |
| Crude lipid | 55.1 ± 1.00b | 65.0 ± 0.34a | 65.7 ± 0.62a | 67.1 ± 0.84a | 67.5 ± 0.44a | < 0.001 | < 0.001 |
| Crude ash | 20.0 ± 0.01a | 20.3 ± 0.02a | 20.9 ± 0.07a | 21.4 ± 0.03a | 21.7 ± 0.09a | < 0.001 | 0.749 |
Values (means ± SE, n = 3) in each row with different superscript are significantly different (p < 0.05). The polynomial orthogonal contrast was considered significant at p < 0.05 level.
LM0 = Control, soybean; LM5 = 5% L. minor; LM10 = 10% L. minor; LM15 = 15% L. minor; LM20 = 20% L. minor.
Amino acid composition of fish
Total essential amino acid in fish increased linearly with the increased level of duckweed in the diet of carp (Table 3). Arginine, isoleucine and threonine contents were highest in carp fed diet LM20 while histidine, leucine and lysine levels were highest in fish fed the control diet LM0. Phenylalanine content was highest in LM15 diet fed common carp and LM20 followed this group. While there were no significant differences in total non-essential amino acid contents among fish fed the five diets, highest alanine, glycine and serine contents were found in carp fed diet LM20, whereas aspartate, cysteine and glutamic acid levels were highest in fish fed the LM0. Non-proteinogenic amino acids level was lower in fish fed the control LM0 diet compared to fish fed the diets including duckweed. Phosphoserine, taurine, cystathionine, ϒ-amino butyric acid and 1 methyl histidine levels were significantly higher in carp fed diet LM20, while β-alanine and hydroxyproline contents were significantly higher in fish fed diets LM15 and LM20, compared to fish fed the diets with lower duckweed inclusion. In carp fed diets LM0 and LM5, β-amino isobutyric acid was not detected. Like essential amino acids, total non-proteinogenic amino acids level in fish also showed a linear relationship with the increased level of duckweed in the diet.
Table 3.
Amino acid composition of Cyprinus carpio cultured under five different feeding regimes (g kg−1 of fresh weight).
| Proteinogenic amino acids | LM0 | LM5 | LM10 | LM15 | LM20 | Polynomial contrasts | |
|---|---|---|---|---|---|---|---|
| Linear | Quadratic | ||||||
| Essential amino acids | |||||||
| Arginine (Arg) | 6.74 ± 0.13d | 7.99 ± 0.12c | 11.79 ± 0.10a | 10.81 ± 0.11b | 11.91 ± 0.03a | < 0.001 | < 0.001 |
| Histidine (His) | 3.83 ± 0.01a | 3.68 ± 0.10a | 3.29 ± 0.04b | 3.38 ± 0.03b | 3.27 ± 0.03b | < 0.001 | 0.018 |
| Isoleucine (Ile) | 5.56 ± 0.07b | 5.46 ± 0.12b | 4.82 ± 0.07c | 5.57 ± 0.01b | 5.97 ± 0.02a | 0.002 | < 0.001 |
| Leucine (Leu) | 10.10 ± 0.04a | 10.05 ± 0.07a | 8.43 ± 0.04d | 9.25 ± 0.03b | 8.89 ± 0.02c | < 0.001 | < 0.001 |
| Lysine (Lys) | 12.53 ± 0.02a | 11.76 ± 0.11b | 9.70 ± 0.01d | 10.24 ± 0.02c | 10.23 ± 0.03c | < 0.001 | < 0.001 |
| Methionine (Met) | 3.30 ± 0.05c | 3.37 ± 0.06c | 3.22 ± 0.06c | 5.32 ± 0.19a | 4.08 ± 0.02b | < 0.001 | 0.303 |
| Phenylalanine (Phe) | 5.58 ± 0.03ab | 5.73 ± 0.12a | 5.42 ± 0.01b | 5.77 ± 0.02a | 5.75 ± 0.02a | 0.082 | 0.212 |
| Threonine (Thr) | 5.24 ± 0.03d | 5.40 ± 0.08c | 5.56 ± 0.01c | 6.19 ± 0.04b | 6.42 ± 0.08a | < 0.001 | 0.005 |
| Tryptophan (Trp) | 1.61 ± 0.04d | 1.04 ± 0.05d | 3.55 ± 0.03a | 2.50 ± 0.08b | 2.30 ± 0.07c | 0.007 | 0.017 |
| Valine (Val) | 6.43 ± 0.14a | 6.45 ± 0.13a | 5.46 ± 0.70b | 5.66 ± 0.05b | 6.72 ± 0.04a | 0.517 | < 0.001 |
| Total | 60.92 ± 0.50b | 60.93 ± 0.30b | 61.24 ± 0.24b | 64.69 ± 0.53a | 65.54 ± 0.49a | < 0.001 | 0.096 |
| Non-essential amino acids | |||||||
| Alanine (Ala) | 7.81 ± 0.06b | 7.11 ± 0.12d | 7.53 ± 0.06c | 7.94 ± 0.03b | 8.25 ± 0.01a | < 0.001 | < 0.001 |
| Aspartate (Asp) | 11.56 ± 0.01a | 11.39 ± 0.07a | 10.47 ± 0.07c | 11.26 ± 0.02b | 11.06 ± 0.08b | < 0.001 | < 0.001 |
| Cysteine (Cys) | 1.34 ± 0.04a | 1.48 ± 0.03a | 1.03 ± 0.03b | 1.36 ± 0.04a | 1.14 ± 0.03b | 0.001 | 0.718 |
| Glutamic acid (Glu) | 20.16 ± 0.04a | 19.57 ± 0.01b | 19.30 ± 0.06b | 18.33 ± 0.15c | 18.67 ± 0.08c | 0.001 | 0.003 |
| Glycine (Gly) | 8.29 ± 0.13d | 9.80 ± 0.08c | 10.52 ± 0.04b | 11.16 ± 0.00a | 11.33 ± 0.11a | < 0.001 | < 0.001 |
| Proline (Pro) | 18.48 ± 0.03b | 20.45 ± 0.13a | 18.05 ± 0.09c | 17.85 ± 0.08c | 16.73 ± 0.09d | < 0.001 | < 0.001 |
| Serine (Ser) | 4.06 ± 0.05e | 4.37 ± 0.08d | 4.66 ± 0.00c | 5.44 ± 0.03b | 5.74 ± 0.01a | < 0.001 | < 0.001 |
| Tyrosine (Tyr) | 3.57 ± 0.06c | 2.61 ± 0.04e | 4.92 ± 0.01a | 3.19 ± 0.01d | 3.85 ± 0.02b | < 0.001 | 0.001 |
| Total | 75.27 ± 0.36a | 76.78 ± 0.20a | 76.48 ± 0.29a | 76.53 ± 0.32a | 76.77 ± 0.38a | 0.001 | 0.014 |
| Non-proteinogenic amino acids | |||||||
| Phosphoserine (p-Ser) | 0.05 ± 0.00c | 0.12 ± 0.01b | 0.11 ± 0.02b | 0.08 ± 0.01bc | 0.20 ± 0.03a | < 0.001 | 0.035 |
| Taurine (Tau) | 1.69 ± 0.08b | 1.78 ± 0.03b | 1.29 ± 0.02c | 1.80 ± 0.01b | 2.29 ± 0.02a | < 0.001 | < 0.001 |
| Cystathionine (Cysthi) | 0.42 ± 0.01b | 0.52 ± 0.02ab | 0.49 ± 0.04ab | 0.23 ± 0.02c | 0.65 ± 0.01a | 0.244 | 0.028 |
| β Alanine ( β-Ala) | 0.05 ± 0.01b | 0.06 ± 0.01b | 0.08 ± 0.05b | 0.18 ± 0.02a | 0.19 ± 0.03a | < 0.001 | 0.012 |
| β Amino isobutyric acid ( β -AiBA) | – | – | 0.60 ± 0.01a | 0.37 ± 0.01b | 0.21 ± 0.02c | < 0.001 | 0.001 |
| ϒ Amino butyric acid ( ϒ—ABA) | 0.39 ± 0.06a | 0.09 ± 0.01b | 0.02 ± 0.00c | – | 0.42 ± 0.01a | 0.497 | < 0.001 |
| Hydroxylysine (Hylys) | 0.47 ± 0.04a | 0.41 ± 0.01a | 0.17 ± 0.01b | 0.08 ± 0.01c | 0.03 ± 0.00d | < 0.001 | 0.084 |
| 1 Methyl histidine (1 Mehis) | 0.09 ± 0.02c | 0.09 ± 0.00c | 0.05 ± 0.01d | 0.19 ± 0.01b | 0.27 ± 0.01a | < 0.001 | < 0.001 |
| Hydroxyproline (Hypro) | 0.70 ± 0.10d | 1.23 ± 0.10c | 1.87 ± 0.07b | 2.12 ± 0.03a | 2.15 ± 0.03a | < 0.001 | < 0.001 |
| Total | 3.86 ± 0.16c | 4.30 ± 0.19b | 4.68 ± 0.22b | 4.43 ± 0.08b | 6.41 ± 0.14a | < 0.001 | < 0.001 |
| Total amino acids | 140.05 ± 0.08b | 142.01 ± 1.60b | 142.41 ± 0.21b | 145.65 ± 0.37a | 148.72 ± 0.25a | < 0.001 | 0.129 |
Values (means ± SE, n = 3) in each row with different superscript are significantly different (p < 0.05). The polynomial orthogonal contrast was considered significant at p < 0.05 level.
LM0 = Control, soybean; LM5 = 5% L. minor; LM10 = 10% L. minor; LM15 = 15% L. minor; LM20 = 20% L. minor.
Fatty acid composition of fish
Total n-3 PUFA content of fish increased with the increasing levels of duckweed in the diets (Table 4). The n-3 fatty acids found in the highest proportions in common carp were eicosatetraenoic acid (ETA 20:4n-3) and DHA, but it was particularly noteworthy that all n-3 PUFA increased in a generally graded manner with the graded increase in dietary duckweed inclusion. Thus, while the increased levels of ALA, the only n-3 PUFA present in the diets, could perhaps be expected, the more interesting result was that the levels of longer, more unsaturated n-3 PUFA including ETA, EPA, docosapentaenoic acid (DPA, 22:5n-3) and DHA also increased significantly with increasing dietary inclusion of L. minor. The levels of DPA, DHA and EPA + DHA were all two-fold higher in carp fed diet LM20 with highest duckweed inclusion compared to fish fed control diet LM0 with no duckweed (Table 4). In contrast, n-6 PUFA contents that were dominated by LOA were lower in carp fed the duckweed-based diets compared to fish fed the control diet LM0 and, consequently, the n-6 PUFA: n-3 PUFA ratio decreased from around 6 in carp fed the control diet to 3.8 in fish fed the highest inclusion of duckweed (LM20). However, monounsaturated fatty acids (MUFA), dominated by oleic acid (18:1n-9) regardless of diet, were even more reduced than n-6 PUFA by dietary inclusion of duckweed. Palmitic acid (16:0) was the dominant saturated fatty acid (SFA) in the carp and significantly higher 16:0 and SFA was found in fish fed diet LM5 compared to fish fed the other treatments. Both total SFA and total MUFA decreased linearly with the increased level of duckweed in the diet of carp.
Table 4.
Fatty acid composition of Cyprinus carpio cultured under five different feeding regimes (mg 100 g−1 of fresh weight).
| Fatty acids | LM0 | LM5 | LM10 | LM15 | LM20 | Polynomial contrasts | |
|---|---|---|---|---|---|---|---|
| Linear | Quadratic | ||||||
| Saturated fatty acids (SFA) | |||||||
| 14:0 | 27.32 ± 0.89a | 23.61 ± 0.62b | 17.26 ± 0.41c | 16.39 ± 0.16c | 11.39 ± 1.26d | < 0.001 | 0.111 |
| 15:0 | 4.45 ± 0.01c | 6.10 ± 0.48b | 5.48 ± 0.98c | 7.34 ± 0.08a | 6.39 ± 0.28b | < 0.001 | 0.031 |
| 16:0 | 383.00 ± 0.01b | 434.12 ± 1.74a | 354.66 ± 6.64c | 364.53 ± 5.15c | 320.44 ± 5.60d | < 0.001 | < 0.001 |
| 18:0 | 1.90 ± 0.02c | 4.69 ± 0.20a | 3.31 ± 0.68b | 1.38 ± 0.39c | 3.15 ± 0.45b | 0.289 | 0.008 |
| 24:0 | 10.53 ± 0.07c | 8.49 ± 0.36d | 11.03 ± 0.09b | 9.79 ± 0.18c | 12.89 ± 0.65a | < 0.001 | < 0.001 |
| Total SFA | 427.21 ± 0.89b | 477.01 ± 1.31a | 391.74 ± 7.44c | 399.44 ± 4.78c | 354.26 ± 7.86d | < 0.001 | < 0.001 |
| Monounsaturated fatty acids (MUFA) | |||||||
| 16:1n-7 | 26.44 ± 0.01a | 23.46 ± 0.93b | 8.21 ± 0.67c | 4.68 ± 0.41d | 2.29 ± 0.19e | < 0.001 | < 0.001 |
| 18:1n-9 | 1014.10 ± 0.40a | 959.98 ± 0.70b | 795.25 ± 5.50c | 745.45 ± 1.29d | 608.05 ± 6.44e | < 0.001 | < 0.001 |
| 24:1 | 2.52 ± 0.03c | 2.55 ± 0.37c | 3.23 ± 0.05b | 5.26 ± 0.37a | 2.78 ± 1.12bc | 0.010 | 0.012 |
| Total MUFA | 1043.06 ± 0.42a | 985.99 ± 0.13b | 806.69 ± 6.19c | 755.39 ± 1.32d | 613.12 ± 3.13e | < 0.001 | < 0.001 |
| Polyunsaturated fatty acids (PUFA) | |||||||
| 18:2n-6 (LOA) | 1087.98 ± 0.31a | 948.68 ± 2.69d | 982.46 ± 9.67c | 1033.81 ± 8.04b | 1099.49 ± 0.08a | < 0.001 | < 0.001 |
| 20:2n-6 | 32.73 ± 1.75a | 17.43 ± 1.14c | 24.44 ± 1.47b | 26.23 ± 3.15b | 30.66 ± 1.91ab | 0.230 | < 0.001 |
| 20:3n-6 | 16.25 ± 0.92a | 11.28 ± 1.42c | 12.42 ± 0.42bc | 13.92 ± 0.25b | 10.61 ± 0.17c | < 0.001 | 0.057 |
| 20:4n-6 | 27.41 ± 0.50a | 22.39 ± 1.12b | 22.80 ± 0.63b | 26.65 ± 1.67a | 21.51 ± 0.25b | 0.002 | 0.159 |
| 22:5n-6 | 66.97 ± 1.97a | 54.07 ± 0.34b | 49.53 ± 0.13c | 56.78 ± 0.99b | 46.22 ± 0.02d | < 0.001 | < 0.001 |
| Total n-6 PUFA | 1231.34 ± 1.52a | 1053.86 ± 8.75e | 1091.65 ± 8.85d | 1157.40 ± 7.20c | 1208.48 ± 2.42b | 0.002 | < 0.001 |
| 18:3n-3 (ALA) | 15.22 ± 0.01c | 14.92 ± 0.69c | 30.05 ± 0.33b | 38.35 ± 2.14a | 38.32 ± 0.78a | < 0.001 | 0.021 |
| 20:4n-3 | 128.04 ± 1.36d | 166.65 ± 0.40a | 150.88 ± 4.39c | 161.30 ± 0.74ab | 156.96 ± 2.57bc | < 0.001 | < 0.001 |
| 20:5n-3 (EPA) | 3.95 ± 0.02c | 5.01 ± 0.34b | 5.96 ± 0.05a | 6.00 ± 0.40a | 5.28 ± 0.12b | < 0.001 | < 0.001 |
| 22:5n-3 | 5.39 ± 0.01d | 9.09 ± 0.35c | 11.54 ± 0.14a | 11.10 ± 0.01ab | 10.42 ± 0.09b | < 0.001 | < 0.001 |
| 22:6n-3 (DHA) | 53.60 ± 5.01e | 81.01 ± 1.77d | 86.01 ± 0.52c | 91.03 ± 1.61b | 107.50 ± 1.68a | < 0.001 | 0.003 |
| Total n-3 PUFA | 206.20 ± 6.54d | 276.68 ± 2.81c | 284.44 ± 6.02c | 307.78 ± 1.67b | 318.48 ± 5.21a | < 0.001 | < 0.001 |
| EPA + DHA | 57.55 ± 5.01e | 86.02 ± 2.11d | 91.97 ± 0.58c | 97.04 ± 1.21b | 112.79 ± 1.57a | < 0.001 | 0.001 |
| n-6/n-3 | 5.97 ± 0.03a | 3.80 ± 0.01b | 3.84 ± 0.03b | 3.76 ± 0.02b | 3.79 ± 0.00b | < 0.001 | < 0.001 |
Values (means ± SE, n = 3) in each row with different superscript are significantly different (p < 0.05). The polynomial orthogonal contrast was considered significant at p < 0.05 level.
LM0 = Control, soybean; LM5 = 5% L. minor; LM10 = 10% L. minor; LM15 = 15% L. minor; LM20 = 20% L. minor.
Gene expression
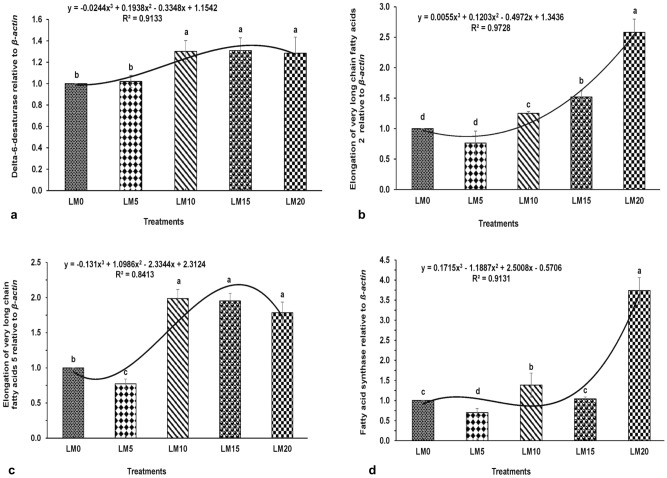
The expression levels of delta-6-desaturase (fads2d6) was significantly higher in common carp fed diets LM10, LM15 and LM20 compared to fish fed the control LM0 diet (Fig. 1a). The expression of elongation of very long-chain fatty acids protein 2 (elovl2) was significantly higher in LM20 diet fed common carp compared to others (Fig. 1b). There was up-regulation of elongation of very long chain fatty acids protein 5 (elovl5) in LM10, LM15 and LM20 diets fed fish compared to other group of fish (Fig. 1c). The fatty acid synthase (fas) expression was significantly higher in LM20 diet fed common carp compared to others (Fig. 1d). Expression levels of all the genes, except fads2d6, were lower in fish fed diet LM5 compared to carp fed the control diet. A polynomial relationship was found between the dietary inclusion levels of duckweed and expressions of various genes.
Figure 1.
Expressions of (a) delta-6-desaturase (fads2d6), (b) elongation of very long chain fatty acids protein 2 (elovl2), (c) elongation of very long chain fatty acids protein 5 (elovl5) and (d) fatty acid synthase (fas) relative to β-actin in hepatopancreas of common carp Cyprinus carpio cultured under five different feeding regimes. The polynomial (order 3) relationships were found between the diets and the expressions of fads2d6 (R2 = 0.913), elovl2 (R2 = 0.973), elovl5 (R2 = 0.841) and fas (R2 = 0.913). Bars with different superscripts are significantly (p < 0.05; n = 3) different.
Discussion
The duckweed-based diets influenced the growth of common carp in the present study. Final weight and SGR of fish increased linearly as dietary level of duckweed inclusion increased. Similar results were observed in common carp fed diets supplemented with 30–50% Lemna minuta31,32, grass carp Ctenopharyngodon idella fed water hyacinth Eichhornia crassipes leaf meal35 and rohu Labeo rohita fed 20% processed Pistia leaves36. Feeding of Nile tilapia with fermented L. minor supplemented diet (2.5%) enhanced the growth and survival rate of fish37. Fresh L. minor was supplied with a commercial diet (protein 32%) of juvenile Nile tilapia38. Higher final body weight and SGR were found in the experimental diet fed fish compared to the control diet fed one. In the present study, the FCR of carp fed the duckweed-based diets was lower compared to fish fed the control diet suggesting that replacement of soybean meal with duckweed satisfied the nutritional requirements of common carp. Similar trends in FCR were also recorded previously in common carp fed diets including L. minor and L. minuta30,32.
Digestive enzymes play a significant role in the utilization of diets39 with the activities of enzymes affecting the efficiency of nutrient absorption, and so their characterization provides key information on the digestive ability of fish to hydrolyze protein, lipid, and carbohydrate in diets40. In the present study, amylase activity reduced in carp fed the duckweed-based diets compared to the fish fed the control diet. Similarly, lower amylase activity was observed in rohu fed diets formulated with raw Pistia leaves compared to fish fed a reference diet without Pistia36. However, protein digestion in common carp was influenced by the duckweed-based diets with total protease, trypsin and chymotrypsin activities all significantly higher in fish fed the diets with the two highest levels of duckweed inclusion (LM20 and LM15) compared to fish fed the other diets. Similar results were reported in rohu fed a pelleted diet containing L. minor15. Higher activities of these enzymes suggested enhanced protein digestion in common carp fed the duckweed-based diets, which indicated that the consumed feed was used more efficiently, resulting in lower FCR and increased growth. Lower protease, trypsin, and chymotrypsin activities were recorded in carp fed the soybean-based diet in the present study. Lower enzyme activities were also observed in Atlantic salmon Salmo salar41, Nile tilapia42 and Japanese seabass Lateolabrax japonicus43 fed soybean meal-based diets although, in these studies, the reference/control feeds were based on fishmeal. The presence of anti-nutritional factors including protease inhibitors may reduce digestive enzyme activities in these fishes11,43,44.
The proximate composition of the carp showed that lipid contents were higher (and moisture lower) in fish fed the duckweed supplemented diets compared to fish fed the control diet. This was consistent with the findings of the previous studies30,32 with common carp fed diets containing duckweeds. Higher lipid content was recorded in I. aquatica leaf meal and bio-processed Pistia leaves supplemented diets fed rohu36,45. These data may reflect higher lipid (fatty acid) biosynthesis, as evidenced by the increased expression of fatty acid synthase (fas), and tissue lipid deposition. Higher expression of fas was observed in tilapia fed palm oil-based diets46.
Total amino acids contents (g kg−1) would tend to increase in the present study with increasing inclusion of duckweed reflecting the increased protein content in fish fed the highest levels of L. minor. The amino acids content varied in different ingredients and thereby, their amount in five different diets. Finally, the composition of diets influenced the amino acid contents of common carp fed with five different diets. The composition of the diets influenced digestibility of the ingested feed. The in vitro digestibility study showed that the digestibility of L. minor (6.03 ± 1.78%) was significantly higher compared to soybean meal (3.35 ± 0.01%) in common carp47. Higher protein contents were reported in the muscles of L. minor supplemented diet fed grass carp and silver carp compared to the soybean supplemented diet fed fishes33. Duckweeds exhibit a well-balanced, highly bioavailable source of amino acids for fish17,28,48, with essential amino acids such as arginine, histidine, isoleucine, leucine, phenylalanine, threonine, valine, cysteine, and methionine present in relatively high contents in L. minor15,16. Therefore, duckweeds are regarded as a rich source of essential amino acids that can generally satisfy the amino acid requirements of common carp5. In this context, it was noteworthy that carp fed diets LM15 and LM 20 had higher total levels of essential amino acids with arginine, methionine, isoleucine, threonine, tryptophan and valine generally increasing with inclusion of duckweed. Thus, feeding of common carp with duckweed-based diets improved the amino acid composition of the fish.
Fish are an almost unique source of the health beneficial n-3 LC-PUFA, EPA and DHA, for humans49,50. Previously, higher levels of EPA, DHA and n-3 PUFA were recorded in Nile tilapia fed diets including Azolla filiculoides51 and duckweeds are a good source of ALA, that is the precursor of n-3 LC-PUFA16,17,52. Therefore, in the present study, one focus was to determine if dietary duckweed can boost the levels of n-3 LC-PUFA in carp. The duckweed used in the present study was cultured with the addition of organic fertilizers that can boost lipid content to over 8% of dry weight and ALA content to over 40% of total fatty acids16. Furthermore, the inclusion of duckweed in the feeds was at the expense of soybean meal, wheat and corn flours, that are all derived from seeds where n-6 PUFA, specifically 18:2n-6, dominate the fatty acid content. Consequently, increasing inclusion of duckweed increased dietary ALA content by 14-fold while 18:2n-6 PUFA was reduced by around 20% with the n-6 PUFA: n-3 PUFA ratio in the feeds decreasing 17-fold from around 290 to 17. Thus, despite the relatively low lipid content that has precluded aquatic plants like duckweed from being regarded as dietary lipid sources, the inclusion of duckweed had a beneficial impact on the fatty acid composition of the carp feed16.
While the increased dietary ALA was also reflected in increased ALA in carp in the present study, more importantly the levels of the n-3 LC-PUFA, ETA, EPA, DPA and DHA also all increased in the carp with the graded increased inclusion of duckweed in the diet. As the feeds were free of any marine ingredients and thus devoid of n-3 LC-PUFA, this result clearly indicated that there was active bioconversion of the dietary ALA, supplied by the duckweed, to DHA in carp resulting in accumulation of n-3 LC-PUFA. This confirmed the earlier findings that freshwater fishes, both herbivorous and omnivorous have the metabolic capacity to convert dietary ALA to EPA and DHA24,53. In the present study, the compositional data was supported by the gene expression studies. Several genes including delta-6-desaturase (fads2d6), elongation of very long-chain fatty acids protein 2 (elovl2) and elongation of very long chain fatty acids protein 5 (elovl5) play significant roles in the bioconversion of fatty acids21,22,54. Previous studies on the gene expression response of fish after replacing marine ingredients with plant-based diets/vegetable oils showed that the LC-PUFA biosynthesis pathway was stimulated in liver and intestine of various fish species55–58. In the present study, the fads2d6, elovl2 and elovl5 genes were all up-regulated in the hepatopancreas of common carp fed the duckweed diets compared to fish fed the control diet. This was consistent with previous studies that showed a higher level of dietary ALA increased expression levels of fads2d6 and elovl5 in common carp59, and fads2d6 and elovl2 expression levels were up-regulated in juvenile and ongrowing rainbow trout fed plant-based diets58. Therefore, the higher levels of EPA + DHA in fish fed the duckweed-based diets confirmed the ability of common carp to synthesize LC-PUFA endogenously from dietary precursor, ALA through increased expression (and activity) of the biosynthetic desaturase and elongase enzymes.
In conclusion, the present study demonstrated that up to 20% duckweed L. minor can be included in feeds as a replacement for plant meals including soybean meal without affecting the survival or growth of common carp. The inclusion of duckweed in feeds also enhanced the nutritional value of the carp by increasing protein, lipid, and amino acids levels. Furthermore, duckweed also increased the ALA content of the feed and this enhanced the contents of n-3 LC-PUFA, including EPA and DHA of the carp.
Materials and methods
Ingredients and feed formulation
The duckweed L. minor was grown in outdoor cement tanks (150 L) fertilized with a mixture of organic manures including cattle manure, poultry wastes and mustard oil-cake as described in detail previously16. The duckweed was harvested, washed with clean water, and dried in an oven at 40 °C for 3 h. Other feed ingredients including soybean meal (Ruchi Soya Industries Limited, Mumbai, India), wheat flour (Aashirvaad Atta, ITC Limited, Bangalore, India), corn flour (Ahaar, Private Limited, New Delhi, India), sunflower oil (Fortune, Adani Wilmar Limited, Gujarat, India), amino acids (Himedia, Mumbai, India), vitamin and mineral premixes (Piramal Enterprises Limited, Mumbai) were purchased from the local market. After grinding, ingredients were sieved and stored at 4 °C prior to diet manufacture. The crude protein content of soybean meal, L. minor, wheat flour and corn flour were 50.0, 36.07, 12.0 and 12.0%, respectively. The lipid contents of soybean meal, L. minor, wheat flour and corn flour were 1.0, 8.45, 1.0 and 1.0%, respectively.
Five marine ingredient-free diets were formulated to contain 32% crude protein and 7% crude lipid using the Winfeed 2.8 software package (WinFeed (UK) Limited, Cambridge, UK) (Table 5). The control diet was based on plant meals with soybean meal as the major protein source (LM0). In four further experimental diets, duckweed was included at increasing levels of 5% (LM5), 10% (LM10), 15% (LM15) and 20% (LM20) of diet dry weight, largely at the expense of soybean meal, wheat flour and sunflower oil to maintain the diets as isonitrogenous, isolipidic and isoenergetic. Earlier study showed that in diet 20% inclusion of L. minor affected the survival rate of common carp30. Hence, in the present study the maximum inclusion of the duckweed was 20%. The amino acids viz. histidine, methionine, lysine and threonine were added in all diets5. All the dry ingredients were blended for 10 min and then mixed with the oil and warm water and feed pellets (1 mm diameter) produced using a BTPL Twin-screw-extruder (Basic Technology Private Limited, Kolkata, India). The pellets extrusion conditions were as follows: cutter rpm 134; feeder rpm 10; extrusion rpm 190; extrusion torque 9.22; heater 1 and heater 2 temperature 65 and 70 °C; final mass temperature 75 °C. The diets were dried at 40 °C before being stored at 4 °C prior to use. The proximate composition study showed that protein, lipid, carbohydrate and ash contents of L. minor were 360.70 ± 1.80, 84.50 ± 6.10, 340.70 ± 3.60 and 214.12 ± 2.00 g kg−1 (dry weight), respectively16. All essential (histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine), non-essential (alanine, arginine, aspartate, cysteine, glutamic acid, glycine, proline, serine and tyrosine) and non-proteinogenic (citrulline, hydroxiproline, taurine etc.) amino acids were found in L. minor. The fatty acid composition of L. minor was as follows: saturated fatty acids (SFA) 23–26%, monounsaturated fatty acids (MUFA) 11–12%, n-6 polyunsaturated fatty acids (PUFA), mostly linoleic acid (LOA) 17–18% and n-3 PUFA (α-linolenic acid) 41–47%. Based on this finding, the diets were prepared for the present study. The fatty acid compositions of the diets are presented in Table 6 and the amino acid compositions in Supplementary Table 1.
Table 5.
Ingredients, dietary formulations (g kg−1) and analysed proximate compositions of the control and experimental diets.
| Ingredients | LM0 | LM5 | LM10 | LM15 | LM20 | ||
|---|---|---|---|---|---|---|---|
| Soybean meal | 500.0 | 475.0 | 450.0 | 425.0 | 400.0 | ||
| Lemna minor | – | 50.0 | 100.0 | 150.0 | 200.0 | ||
| Wheat flour | 245.0 | 223.0 | 201.0 | 179.0 | 158.0 | ||
| Corn flour | 147.0 | 147.0 | 146.0 | 146.0 | 146.0 | ||
| Sunflower oil | 62.0 | 58.0 | 55.0 | 51.0 | 47.0 | ||
| Vitamin/minerals premix1 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | ||
| Mono calcium phosphate | 20.0 | 21.0 | 23.0 | 24.0 | 25.0 | ||
| Choline chloride | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||
| Histidine | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||
| Methionine | 12.0 | 12.0 | 11.0 | 11.0 | 10.0 | ||
| Lysine | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | ||
| Threonine | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Polynomial contrasts | |||||||
|---|---|---|---|---|---|---|---|
| Linear | Quadratic | ||||||
| Proximate analysis (% dry matter basis) | |||||||
| Moisture | 6.34 ± 0.08a | 6.38 ± 0.10a | 6.20 ± 0.02a | 6.27 ± 0.04a | 6.21 ± 0.5a | 0.089 | 0.902 |
| Crude protein | 31.61 ± 0.14a | 32.05 ± 0.16a | 31.42 ± 0.15a | 31.68 ± 0.21a | 31.63 ± 0.09a | 0.285 | 0.796 |
| Crude lipid | 7.42 ± 0.06a | 7.44 ± 0.12a | 7.48 ± 0.13a | 7.35 ± 0.04a | 7.32 ± 0.05a | 0.324 | 0.444 |
| Carbohydrate | 47.51 ± 0.08a | 46.59 ± 0.10a | 47.19 ± 0.09a | 47.00 ± 0.09ab | 46.02 ± 0.07b | 0.003 | 0.257 |
| Crude ash | 7.22 ± 0.22a | 7.54 ± 0.07a | 7.72 ± 0.04a | 7.70 ± 0.04a | 7.82 ± 0.08a | 0.001 | 0.077 |
| Energy value (Kcal 100 g−1) | 382.52 ± 0.36a | 381.52 ± 0.44a | 381.73 ± 0.58a | 381.79 ± 0.60a | 377.38 ± 0.31a | 0.472 | 0.075 |
LM0 = Control, soybean; LM5 = 5% L. minor; LM10 = 10% L. minor; LM15 = 15% L. minor; LM20 = 20% L. minor.
1Supradyn multivitamin tablets (Piramal Enterprises Ltd., Mumbai, India) containing minerals and trace elements (as mg/kg in diets): = Vitamin A (as acetate) 12; Cholecalciferol 0.1; Thiamine mononitrate, 40; Riboflavine 40; Pyridoxine hydrochloride, 12; Cyanocobalamin, 0.06; Nicotinamide, 400; Calcium pantothenate 65.20; Ascorbic acid 600; α-Tocopheryl acetate,100; Biotin, 1.00. Minerals: Tribasic calcium phosphate, 516; Magnesium oxide, 240; Dried ferrous sulphate, 128.16; Manganse sulphate monohydrate 8.12; Total phosphorus, 103.20. Trace elements: Copper sulphate pentahydrate 13.56; Zinc sulphate, 8.80; Sodium molybdate dihydrate, 1.00; Sodium borate 3.52.
Table 6.
Fatty acid composition of experimental and control diets (mg 100 g−1 of dry weight).
| Fatty acids | LM0 | LM5 | LM10 | LM15 | LM20 | Polynomial contrasts | |
|---|---|---|---|---|---|---|---|
| Linear | Quadratic | ||||||
| Saturated fatty acids (SFA) | |||||||
| 14:0 | 10.44 ± 0.07a | 9.55 ± 0.06a | 8.82 ± 0.04a | 10.35 ± 0.20a | 10.88 ± 2.00a | 0.361 | 0.034 |
| 15:0 | 5.20 ± 0.01a | 4.59 ± 0.17b | 2.55 ± 0.01c | 0.81 ± 0.01d | 0.04 ± 0.00e | < 0.001 | 0.981 |
| 16:0 | 581.01 ± 8.96a | 531.63 ± 8.38b | 531.73 ± 1.54b | 535.31 ± 4.55b | 538.91 ± 8.96b | 0.002 | < 0.001 |
| 18:0 | 70.09 ± 0.99a | 64.13 ± 0.93b | 56.09 ± 0.27c | 66.21 ± 1.04b | 40.68 ± 0.51d | < 0.001 | < 0.001 |
| 20:0 | 3.26 ± 0.36a | 2.98 ± 0.33a | 2.44 ± 0.08ab | 3.12 ± 0.56a | 1.85 ± 0.25b | 0.005 | 0.414 |
| 22:0 | 0.69 ± 0.01b | 0.75 ± 0.01b | 0.14 ± 0.00d | 0.31 ± 0.07c | 0.90 ± 0.10a | 0.814 | < 0.001 |
| 24:0 | 15.72 ± 2.77a | 16.77 ± 0.14a | 9.00 ± 0.46b | 8.29 ± 0.83b | 17.25 ± 2.76a | 0.250 | 0.001 |
| Total SFA | 686.41 ± 6.73a | 630.40 ± 8.97b | 610.77 ± 3.09b | 624.40 ± 3.65b | 610.51 ± 8.36b | < 0.001 | < 0.001 |
| Monounsaturated fatty acids (MUFA) | |||||||
| 18:1n-9 | 1460.56 ± 3.81a | 1336.44 ± 3.95b | 1163.59 ± 6.70c | 1163.69 ± 2.02c | 1059.34 ± 2.20d | < 0.001 | < 0.001 |
| 20:1n-9 | 7.86 ± 1.24b | 8.19 ± 0.13a | 4.09 ± 0.28c | 3.46 ± 0.33c | 3.07 ± 0.26c | < 0.001 | 0.369 |
| Total MUFA | 1468.42 ± 2.57a | 1344.63 ± 3.81b | 1167.68 ± 6.43c | 1167.15 ± 1.69c | 1062.41 ± 2.63d | < 0.001 | < 0.001 |
| Polyunsaturated fatty acids (PUFA) | |||||||
| 18:2n-6 (LOA) | 3197.60 ± 22.99a | 2925.76 ± 21.95b | 2862.71 ± 6.24b | 2769.39 ± 16.46c | 2600.36 ± 22.03d | < 0.001 | 0.008 |
| 20:2n-6 | 1.09 ± 0.02cd | 1.92 ± 0.04b | 1.38 ± 0.10c | 0.92 ± 0.12d | 3.41 ± 0.21a | < 0.001 | < 0.001 |
| 18:3n-3 (ALA) | 10.98 ± 1.45e | 51.10 ± 1.89d | 81.29 ± 1.35c | 115.44 ± 0.02b | 153.10 ± 0.26a | < 0.001 | 0.741 |
| Total PUFA | 3209.67 ± 23.42a | 2978.78 ± 22.80b | 2945.38 ± 6.50bc | 2885.75 ± 16.46c | 2756.87 ± 21.82d | < 0.001 | 0.006 |
| n-6/n-3 | 291.32 ± 11.23a | 57.29 ± 2.55b | 35.23 ± 0.51c | 23.997 ± 0.21d | 17.01 ± 0.11e | < 0.001 | < 0.001 |
LM0 = Control, soybean; LM5 = 5% L. minor; LM10 = 10% L. minor; LM15 = 15% L. minor; LM20 = 20% L. minor.
Fish culture and feeding
The Institutional Animal Ethics Committee (IAEC) of Delhi University approved the animal care and experimental procedure (DU/ZOOL/IAEC-R/2015/07). The following study was conducted in accordance with relevant guidelines and regulations. The study was carried out compliance with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guideline.
Fry of common carp were collected from the local fish farm and fed the control diet for 1 week to acclimatize to the experimental conditions. At the initiation of the experiment a total of 450 fry (average weight, 0.47–0.48 g) were distributed randomly among 15 aquaria (30 fry 60 L aquarium−1), Each aquarium was fed one of the five diets, LM0, LM5, LM10, LM15 and LM20, for 60 days, with each diet allocated randomly to three aquaria (n = 3). The fish were fed ad libitum to apparent satiation twice per day at 9.00 a.m. and 5.00 p.m. Any uneaten feed was collected after 1 h of feeding to provide an estimation of feed consumption rate. The dissolved oxygen level in the aquarium was maintained by an aerator, and an external filter (Eheim Classic 600, Germany) was employed to reduce ammonia level and maintain water quality of each aquarium. The water temperature (27.8–28.1 °C), pH (7.40–7.62), dissolved oxygen (5.5–5.82 mg L−1), ammonia (0.02–0.05 mg L−1), nitrate (0.85–1.01 mg L−1) and conductivity (380.60–427.03 μS cm−1) were regularly monitored in each aquarium using a digital water quality multi-parameter instrument (Hach HQ 40D; Loveland, Colorado, USA). The concentrations of nitrite (0.25–0.48 mg L−1) and phosphate (0.08–0.12 mg L−1) were measured regularly60. There were no significant differences in water quality parameters among the five dietary treatments throughout the study period.
Sampling
After 60 days of culture, the fish were starved for one day prior to the fish being harvested and euthanized with tricaine methane sulphonate (MS-222; Sigma-Aldrich, USA). The number of fish in each aquarium was counted and recorded to determine survival rate (SR), and the weight of individual fish was measured. The specific growth rate (SGR), feed intake (FI) and feed conversion ratio (FCR) were calculated according to the following formulae.
For carcass composition, three fish from each aquarium were collected and pooled, this pooled sample served as one biological replicate (3 replicates per dietary treatment; n = 3). The entire digestive tracts (esophageal sphincter to anus) of another two fish per aquarium were dissected and pooled to provide 3 samples per diet (2 fish per aquarium, 6 fish per diet in 3 replicates; n = 3) for the study of digestive enzymes. The fish were kept in fasting condition for 24 h before sampling and the digestive tract was empty. The hepatopancreas of three individual fish per treatment (1 fish per aquarium, 3 fish per diet; n = 3) were dissected into TRIzol reagent (Ambion, Life Technologies, USA) for the gene expression study. Fish samples (number of fish for assays) were collected following the recommendation of IAEC. All samples were stored at − 80 °C prior to analyses.
Biochemical compositions of diets and fish
The biochemical compositions of diets and whole fish were determined following the procedures of the Association of Official Analytical Chemists (AOAC) International61. Diet samples were ground and the pooled fish samples (three fish) were blended to form a homogeneous paste prior to analyses. Moisture content was determined gravimetrically after drying samples in an oven at 105 °C for 3 h. Ash content was determined after incinerating the oven dried sample in a muffle furnace at 550 °C for 8 h. Crude protein content was determined by the Kjeldahl method (Nitrogen × 6.25; Pelican Instruments, Chennai, India). Crude lipid was determined following the chloroform/methanol extraction method62. The subtraction method was used for the determination of carbohydrate contents of feeds63, and the energy value of each sample was calculated64. All samples were assayed in triplicate.
Amino acid analysis
Amino acid contents of diets and whole fish were analyzed using a dedicated Hitachi L-8900 High-Speed Amino Acid Analyzer (Hitachi Co. Ltd., Tokyo, Japan). Firstly, samples were hydrolyzed with 6 N HCl at 110 °C for 22 h under N2 atmosphere other than tryptophan that was hydrolyzed in a separate sample using 4 N methanesulfonic acid and 3-(2-aminoethyl) indole. Methionine and cysteine were oxidized with performic acid before acid digestion. After digestion, all the samples were evaporated in a Nitrogen Evaporator (PCi Analytic Private Limited, Maharashtra, India) before 0.02 N HCl was added to the dried samples to provide a protein concentration of 0.5 mg mL−1 in each sample and 1.5 mL was placed in a glass vial for the autosampler. The amino acids were separated using a cation-exchange resin column (4.6 mm ID × 60 mm L) with 3 μm particle size. The column temperature was 30–70 °C, reaction temperature was 135 °C with a ninhydrin flow rate of 0.35 mL min−1. The amino acids were monitored at 570 nm other than proline and hydroxyproline that were monitored at 440 nm. The amino acids were compared with a standard amino acid solution (Wako Pure Chemical Industries Limited, USA). All samples were assayed in triplicate and concentrations expressed as g kg−1.
Fatty acid analysis
The fatty acid compositions of diets and whole fish were measured by gas chromatography (GC). Total lipid was extracted from diet and fish paste samples after homogenization in chloroform: methanol (2:1, v/v) according62. Fatty acid methyl esters (FAME) were prepared from total lipid samples by transesterification using 1% sulphuric acid in methanol at 50 °C for 16 h65. FAME was separated on a Clarus 580 GC (PerkinElmer, USA) equipped with a ZB-wax column (60 m × 0.32 mm internal diameter × 0.25 μm; Phenomenex, UK). The data were collected from pre-installed programmed software (TotalChrom Workstation Ver6.3). The fatty acids present in diets and fish were identified by comparing the retention times of the sample peaks with a standard fatty acid mixture (Supelco FAME 37 mix, Sigma-Aldrich, USA) and published data66. All samples were assayed in triplicate and the concentration was expressed as mg 100 g−1.
Digestive enzyme assays
The samples of whole digestive tract were freeze-dried and homogenized in chilled Milli-Q® water (1:10) to maintain neutral pH of the extracts. The homogenates were centrifuged at 10,000×g at 4 °C for 30 min and the tissue supernatants collected for assay of digestive enzyme activities by fluorometric methods (Multimode reader, BioTek Synergy H1 Hybrid, USA). Amylase activity was estimated using Ultra Amylase Assay kit (Invitrogen, USA), pH of the assay mixture was 6.9. The sample was incubated at 25 °C for 25 min. The fluorescence was recorded at 485 nm for excitation and 520 nm for emission. Total protease activity was estimated with EnzChek@ Protease Assay kit (Invitrogen) using phosphate buffer (pH 7.8) at 25 °C. The fluorescence was recorded at 485 nm for excitation and 530 nm for emission. Trypsin activity was estimated following the method67 using Tris–HCl buffer (pH 8.0) and Na-benzoyl-l-arginin-methyl-coumarinylamide (Sigma-Aldrich, USA) as substrate. The sample was incubated at 30 °C for 10 min. Chymotrypsin activity was assayed using Tris–HCl buffer (pH, 7.5) and succinyl-Leu-Val-Tyr-4-methyl-coumaryl-7-aminde (Sigma-Aldrich) as substrate68. After 10 min of incubation at 37 °C, the fluorescence was recorded at 380 nm for excitation and 450 nm for emission. The substrate 4-methylumbelliferyl butyrate (4-MUB, Sigma-Aldrich) and Tris–HCl buffer (pH, 7.5) were used for the assay of neutral lipase activity69. Two sets of same samples were taken and one set was kept at 4 °C (ice bath) and the second one at 37 °C (water bath) for 10 min. The change in fluorescence was recorded at 365 nm for excitation and 450 nm for emission. The value obtained at 4 °C was subtracted from the value obtained at 37 °C. Protein content was estimated following the method using bovine serum albumin (Sigma-Aldrich) as standard70. All samples were assayed in triplicate.
Gene expression analysis
The mRNA expressions of fads2d6, elovl2, elovl5 and fas genes were studied in the hepatopancreas of common carp. Total RNA was extracted in TRIzol reagent (Ambion, Life Technologies, USA) following the manufacturer's recommendations. The quality of extracted RNA was confirmed using a spectrophotometer (NanoDrop® ND-1000, Thermo Scientific, USA) and integrity of total RNA was determined on 1% agarose gel electrophoresis. The purified RNA (1 µg) was treated with DNase I (Amplification grade 1 kit, Sigma-Aldrich) to eliminate genomic DNA contamination. The DNase-treated RNA was reverse transcribed to cDNA using a High-capacity cDNA Reverse Transcription kit (Applied Biosystems, USA). The cDNA sample for each tank was diluted and used for real-time qPCR to determine gene expression. Quantification of gene expression was performed by Quant Studio 6 Flex system (Applied Biosystems, USA) using PowerUp™ SYBR™ Green Master Mix (Applied Biosystems, USA). Primers were designed using the online primer design tool of NCBI and β‐actin was used as the reference gene (Supplementary Table 2). The reaction mixtures for qPCR (10 μL) were composed of 0.25 μL each of forward and reverse primers (2.5 μM), 1 μL of cDNA (1:3), 5 μL of 2X PowerUp™ SYBR™ Green PCR Master Mix and 3.5 μL of RNase-free water. All samples were run in duplicate with each reference gene and negative control (NTC; non template control, containing no cDNA). The cycling conditions of qPCR were as follows: initial activation step at 95 °C for 10 min followed by either 40 cycles of 95 °C for 15 s and 60 °C for 1 min (primer Tm at 60 °C) or 40 cycles of 95 °C for 15 s, 55 °C for 15 s and 72 °C for 1 min (primer Tm < 60 °C). The data of qRT‐PCR were calculated using the 2−ΔΔCT method with β‐actin as the internal control71.
Statistical analysis
Data were expressed as means ± standard error (SE) with n values as stated. The data were compared using one-way analysis of variance (ANOVA) followed where pertinent by Duncan’s multiple range test, DMR72. The polynomial orthogonal contrasts were used to determine the linear and quadratic effect of increasing levels of dietary L. minor. The significance level was considered at (p < 0.05). All statistical analyses were performed using SPSS software (version 25.0, USA).
Ethical statement
The Institutional Animal Ethics Committee (IAEC) of Delhi University approved the animal care and experimental procedure (DU/ZOOL/IAEC-R/2015/07). The following study was conducted in accordance with relevant guidelines and regulations. The study was carried out compliance with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guideline.
Supplementary Information
Acknowledgements
The authors are thankful to Department of Biotechnology (DBT), Government of India, New Delhi, India (Dy. No. 102/IFD/SAN/4678/2015-2016, dated 28.3.2016) and the Biotechnology and Biological Science Research Council (BBSRC) Newton Fund Global Research Partnership Project (BB/N005031/1) for providing financial support.
Author contributions
R.C., D.R.T., J.G.S., B.D.G.: designed the study; R.K.G., R.C., J.G.S., A.K.S., G.K.: cultured the fish and analysed samples; R.C., D.R.T., B.D.G., J.G.S., R.K.G.: wrote the manuscript; R.K.G., R.C., D.R.T., J.G.S.: prepared graphs and tables.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-07743-x.
References
- 1.FAO. The State of Food Security and Nutrition in the World 2018. Building Climate Resilience for Food Security and Nutrition. (Food and Agriculture Organization, 2018). Accessed on 29/09/2021 http://www.fao.org/3/i9553en/i9553en.pdf
- 2.Storebakken T, Shearer KD, Roem AJ. Growth, uptake and retention of nitrogen and phosphorus, and absorption of other minerals in Atlantic salmon Salmo salar fed diets with fish meal and soy-protein concentrate as the main sources of protein. Aquac. Nutr. 2000;6:103–108. doi: 10.1046/j.1365-2095.2000.00135.x. [DOI] [Google Scholar]
- 3.Trushenski JT, Kasper CS, Kohler CC. Challenges and opportunities in finfish nutrition. N. Am. J. Aquac. 2006;68:122–140. doi: 10.1577/A05-006.1. [DOI] [Google Scholar]
- 4.Gatlin DM, III, et al. Expanding the utilization of sustainable plant products in aquafeeds: A review. Aquac. Res. 2007;38:551–579. doi: 10.1111/j.1365-2109.2007.01704.x. [DOI] [Google Scholar]
- 5.National Research Council (NRC) Nutrient Requirements of Fish and Shrimp. The National Academic Press; 2011. [Google Scholar]
- 6.Glencross BD, et al. Risk assessment of the use of alternative animal and plant raw material resources in aquaculture feeds. Rev. Aquac. 2020;12:703–758. doi: 10.1111/raq.12347. [DOI] [Google Scholar]
- 7.Meetei LI, Ninawe AS, Chakrabarti R. Influence of feeding regimes on the digestive enzyme profile and ultrastructure of digestive tract of first feeding Catla catla (Hamilton) larvae. J. Aquac. Res. Dev. 2014;5:243. doi: 10.4172/2155-9546.1000243. [DOI] [Google Scholar]
- 8.Chakrabarti R, Rathore RM. Ontogenic changes in the digestive enzyme patterns and characterization of proteases in Indian major carp Cirrhinus mrigala. Aquac. Nutr. 2010;16:569–581. doi: 10.1111/j.1365-2095.2009.00694.x. [DOI] [Google Scholar]
- 9.Ahmad T, Singh SP, Khangembam BK, Sharma JG, Chakrabarti R. Food consumption and digestive enzyme activity of Clarias batrachus exposed to various temperatures. Aquac. Nutr. 2014;2:265–272. doi: 10.1111/anu.12072. [DOI] [Google Scholar]
- 10.Olsen RE, et al. Total replacement of fish meal with plant proteins in diets for Atlantic cod (Gadus morhua L.) II-Health aspects. Aquaculture. 2007;272:612–624. doi: 10.1016/j.aquaculture.2007.05.010. [DOI] [Google Scholar]
- 11.Hansen AC, Hemre GI. Effects of replacing fish meal and oil with plant resources in on-growing diets for Atlantic cod Gadus morhua L. Aquac. Nutr. 2013;19:641–650. doi: 10.1111/anu.12078. [DOI] [Google Scholar]
- 12.Bairagi A, Ghosh KS, Sen SK, Ray AK. Duckweed (Lemna polyrhiza) leaf meal as a source of feedstuff in formulated diets for rohu (Labeo rohita Ham.) fingerlings after fermentation with a fish intestinal bacterium. Bioresour. Technol. 2002;85:17–24. doi: 10.1016/S0960-8524(02)00067-6. [DOI] [PubMed] [Google Scholar]
- 13.Cruz Y, Kijora C, Wedler E, Danier J, Schulz C. Fermentation properties and nutritional quality of selected aquatic macrophytes as alternative fish feed in rural areas of the neotropics. Livest. Res. Rural Dev. 2011;23:239. [Google Scholar]
- 14.Stadtlander T, Förster S, Rosskothen D, Leiber F. Slurry-grown duckweed (Spirodela polyrhiza) as a means to recycle nitrogen into feed for rainbow trout fry. J. Clean. Prod. 2019;228:86–93. doi: 10.1016/j.jclepro.2019.04.196. [DOI] [Google Scholar]
- 15.Goswami RK, Shrivastav AK, Sharma JG, Tocher DR, Chakrabarti R. Growth and digestive enzyme activities of rohu Labeo rohita fed diets containing macrophytes and almond oil-cake. Anim. Feed Sci. Technol. 2020;263:114456. doi: 10.1016/j.anifeedsci.2020.114456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakrabarti R, et al. Mass production of Lemna minor and its amino acid and fatty acid profiles. Front. Chem. 2018;6:479. doi: 10.3389/fchem.2018.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma JG, et al. Production potential of greater duckweed Spirodela polyrhiza (L. Schleiden) and its biochemical composition evaluation. Aquaculture. 2019;513:734419. doi: 10.1016/j.aquaculture.2019.734419. [DOI] [Google Scholar]
- 18.Tocher DR. Metabolism and functions of lipids and fatty acids in teleost fish. Rev. Fish. Sci. 2003;11:107–184. doi: 10.1080/713610925. [DOI] [Google Scholar]
- 19.Tocher DR. Fatty acid requirements in ontogeny of marine and freshwater fish. Aquac. Res. 2010;41:717–732. doi: 10.1111/j.1365-2109.2008.02150.x. [DOI] [Google Scholar]
- 20.Tocher DR. Omega-3 long-chain polyunsaturated fatty acids and aquaculture in perspective. Aquaculture. 2015;449:94–107. doi: 10.1016/j.aquaculture.2015.01.010. [DOI] [Google Scholar]
- 21.Castro LFC, Tocher DR, Monroig Ó. Long-chain polyunsaturated fatty acid biosynthesis in chordates: Insights into the evolution of fads and elovl gene repertoire. Prog. Lipid Res. 2016;62:25–40. doi: 10.1016/j.plipres.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Burdge GC, editor. Polyunsaturated Fatty Acid Metabolism in Animals: Applications for Human Health and Research. Elsevier; 2018. [Google Scholar]
- 23.Tocher DR, et al. Nutritional regulation of hepatocyte fatty acid desaturation and polyunsaturated fatty acid composition in zebrafish (Danio rerio) and tilapia (Oreochromis niloticus) Fish Physiol. Biochem. 2002;24:309–320. doi: 10.1023/A:1015022406790. [DOI] [Google Scholar]
- 24.Glencross BD. Exploring the nutritional demand for essential fatty acids by aquaculture species. Rev. Aquac. 2009;1:71–124. doi: 10.1111/j.1753-5131.2009.01006.x. [DOI] [Google Scholar]
- 25.Turchini GM, Ng WK, Tocher DR, editors. Fish Oil Replacement and Alternative Lipid Sources in Aquaculture Feeds. Taylor & Francis; 2010. [Google Scholar]
- 26.Xie D, et al. Regulation of long-chain polyunsaturated fatty acid biosynthesis in teleost fish. Prog. Lipid Res. 2021;82:101095. doi: 10.1016/j.plipres.2021.101095. [DOI] [PubMed] [Google Scholar]
- 27.Aguilera-Morales ME, Canales-Martínez MM, Ávila-González E, Flores-Ortíz CM. Nutrients and bioactive compounds of the Lemna gibba and Ulva lactuca as possible ingredients to functional foods. Lat. Am. J. Aquat. Res. 2018;4:709–716. doi: 10.3856/vol46-issue4-fulltext-8. [DOI] [Google Scholar]
- 28.Rusoff LL, Blakeney EW, Culley DD. Duckweeds (Lemnaceae family): A potential source of protein and amino acids. J. Agric. Food Chem. 1980;28:848–850. doi: 10.1021/jf60230a040. [DOI] [PubMed] [Google Scholar]
- 29.Skillicorn P, Spira W, Jorney W. Duckweed aquaculture: A New Aquatic Farming System for Developing Countries. The World Bank; 1993. [Google Scholar]
- 30.Yilmaz E, Akyurt I, Gunal G. Use of duckweed, Lemna minor, as a protein feed stuff in practical diets for common carp, Cyprinus carpio, fry. Turk. J. Fish. Aquat. Sc. 2004;4:105–109. [Google Scholar]
- 31.Sirakov I, Velichkova K. The influence of aquaponically grown duckweed (Lemna minuta Kunth) used for composition of sustainable diets on hydrochemical and technological parameters in carp (Cyprinus carpio L.) Turk. J. Fish. Aquat. Sci. 2018;18:1037–1044. doi: 10.4194/1303-2712-v18_9_03. [DOI] [Google Scholar]
- 32.Velichkova K, Sirakov I, Veleva P. Use of Lemna minuta Kunth for composition of sustainable diets and influence on hydrochemical, technological and blood biochemical parameters in common carp (Cyprinus carpio L.) cultivated in aquaponics. Bulg. J. Agric. Sci. 2020;26:674–679. [Google Scholar]
- 33.Aslam S, Zuberi A, Chan MWH, Mustaquim J. Effect of Lemna minor and Glycine max on haematological parameters, glucose level, total protein content and anti-oxidant enzyme activities in Ctenopharyngodon idella and Hypophthalmichthys molitrix. Aquac. Rep. 2021;19:100616. doi: 10.1016/j.aqrep.2021.100616. [DOI] [Google Scholar]
- 34.Kriton G, et al. Impact of diets containing plant raw materials as fish meal and fish oil replacement on rainbow trout (Oncorhynchus mykiss), gilthead sea bream (Sparus aurata), and common carp (Cyprinus carpio) freshness. J. Food Qual. 2018;18:1–14. doi: 10.1155/2018/1717465. [DOI] [Google Scholar]
- 35.Mahmood S, Khan N, Iqbal KJ, Ashraf M, Khalique A. Evaluation of water hyacinth (Eichhornia crassipes) supplemented diets on the growth, digestibility and histology of grass carp (Ctenopharyngodon idella) fingerlings. J. Appl. Anim. Res. 2018;46:24–28. doi: 10.1080/09712119.2016.1256291. [DOI] [Google Scholar]
- 36.Mandal S, Ghosh K. Utilization of fermented Pistia leaves in the diet of rohu, Labeo rohita (Hamilton): Effects on growth, digestibility and whole body composition. Waste Biomass Valor. 2019;10:3331–3342. doi: 10.1007/s12649-018-0336-4. [DOI] [Google Scholar]
- 37.Herawati VE, et al. The effect of fermented duckweed (Lemna minor) in feed on growth and nutritional quality of tilapia (Oreochromis niloticus) Biodiversitas. 2020;21:3350–3358. doi: 10.13057/biodiv/d210759. [DOI] [Google Scholar]
- 38.Cipriani LA, Ha N, de Oliveira NS, Fabregat TEHP. Does ingestion of duckweed (Lemna minor) improve the growth and productive performance of juvenile Nile tilapia (Oreochromis niloticus) given formulated feeds in a recirculation system? Aquac. Int. 2021;29:2197–2205. doi: 10.1007/s10499-021-00743-0. [DOI] [Google Scholar]
- 39.Pérez-Jiménez A, Cardenete G, Morales AE, García-Alcázar A, Abellán E, Hidalgo MC. Digestive enzymatic profile of Dentex dentex and response to different dietary formulations. Comp. Biochem. Physiol. Part A. 2009;154:157–164. doi: 10.1016/j.cbpa.2009.05.126. [DOI] [PubMed] [Google Scholar]
- 40.Lemieux H, Blier P, Dutil JD. Do digestive enzymes set a physiological limit on growth rate and food conversion efficiency in the Atlantic cod (Gadus morhua)? Fish Physiol. Biochem. 1999;20:293–303. doi: 10.1023/A:1007791019523. [DOI] [Google Scholar]
- 41.Krogdahl Å, Bakke-McKellep AM, Baeverfjord G. Effects of graded levels of standard soybean meal on intestinal structure, mucosal enzyme activities, and pancreatic response in Atlantic salmon (Salmo salar L.) Aquac. Nutr. 2003;9:361–371. doi: 10.1046/j.1365-2095.2003.00264.x. [DOI] [Google Scholar]
- 42.Lin S, Luo L. Effects of different levels of soybean meal inclusion in replacement for fish meal on growth, digestive enzymes and transaminase activities in practical diets for juvenile tilapia, Oreochromis niloticus× O. aureus. Anim. Feed Sci. Technol. 2011;168:80–87. doi: 10.1016/j.anifeedsci.2011.03.012. [DOI] [Google Scholar]
- 43.Zhang C, et al. Substituting fish meal with soybean meal in diets for Japanese seabass (Lateolabrax japonicus): Effects on growth, digestive enzymes activity, gut histology and expression of gut inflammatory and transporter genes. Aquaculture. 2018;483:173–182. doi: 10.1016/j.aquaculture.2017.10.029. [DOI] [Google Scholar]
- 44.Cole DJA, Garnsworthy PC, editors. Recent Advances in Animal Nutrition. Butterworth-Heinemann; 1992. [Google Scholar]
- 45.Ali S, Kaviraj A. Aquatic weed Ipomoea aquatica as feed ingredient for rearing rohu, Labeo rohita (Hamilton) Egypt. J. Aquat. Res. 2018;44(321–325):2018. doi: 10.1016/j.ejar.2018.09.004. [DOI] [Google Scholar]
- 46.Ayisi CL, Zhao JL. Fatty acid composition, lipogenic enzyme activities and mRNA expression of genes involved in the lipid metabolism of Nile tilapia fed with palm oil. Turk. J. Fish. Aquat. Sc. 2017;17:405–415. doi: 10.4194/1303-2712-v17_2_20. [DOI] [Google Scholar]
- 47.Sharma JG, et al. In vitro digestibility study of some plant protein sources as aquafeed for carps Labeo rohita and Cyprinus carpio using pH-Stat method. Indian J. Exp. Biol. 2016;54:606–611. [PubMed] [Google Scholar]
- 48.Andriani Y, Irawan B, Iskandar I, Zidni I, Partasasmita R. Diversity of duckweed (Araceae-Lemnoideae), morphological characteristics and its potentials as food sources for herbivorous fishes in West Java, Indonesia. Biodiversitas. 2019;20:1617–1623. doi: 10.13057/biodiv/d200618. [DOI] [Google Scholar]
- 49.Alasalvar C, Taylor KDA, Zubcov E, Shahidi F, Alexis M. Differentiation of cultured and wild sea bass (Dicentrarchus labrax): Total lipid content, fatty acid and trace mineral composition. Food Chem. 2002;79:145–150. doi: 10.1016/S0308-8146(02)00122-X. [DOI] [Google Scholar]
- 50.Ruxton CHS, Reed SC, Simpson MJA, Millington KJ. The health benefits of omega-3 polyunsaturated fatty acids: A review of the evidence. J. Hum. Nutr. Diet. 2004;17:449–459. doi: 10.1111/j.1365-277X.2007.00770.x. [DOI] [PubMed] [Google Scholar]
- 51.Abou Y, Fiogbé ED, Beckers Y, Micha JC. Approximate compositional values and tissue fatty acid profiles of Nile tilapia (Oreochromis niloticus L.) fed Azolla-diets in earthen ponds. Food Nutr. Sci. 2011;2:964–973. doi: 10.4236/fns.2011.29131. [DOI] [Google Scholar]
- 52.Appenroth KJ, et al. Nutritional value of duckweeds (Lemnaceae) as human food. Food Chem. 2017;217:266–273. doi: 10.1016/j.foodchem.2016.08.116. [DOI] [PubMed] [Google Scholar]
- 53.Yu J, et al. Comparison of the growth performance and long-chain polyunsaturated fatty acids (LC-PUFA) biosynthetic ability of red tilapia (Oreochromis mossambicus ♀× O. niloticus ♂) fed fish oil or vegetable oil diet at different salinities. Aquaculture. 2021;542:736899. doi: 10.1016/j.aquaculture.2021.736899. [DOI] [Google Scholar]
- 54.Monroig Ó, et al. Evolutionary functional elaboration of the Elovl2/5 gene family in chordates. Sci. Rep. 2016;6:20510. doi: 10.1038/srep20510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leaver MJ, et al. Functional genomics reveals increases in cholesterol biosynthetic genes and highly unsaturated fatty acid biosynthesis after dietary substitution of fish oil with vegetable oils in Atlantic salmon (Salmo salar) BMC Genom. 2008;9:1–15. doi: 10.1186/1471-2164-9-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morais S, Edvardsen RB, Tocher DR, Bell JG. Transcriptomic analyses of intestinal gene expression of juvenile Atlantic cod (Gadus morhua) fed diets with Camelina oil as replacement for fish oil. Comp. Biochem. Physiol. Part B. 2012;161:283–293. doi: 10.1016/j.cbpb.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 57.Limtipsuntorn U, Haga Y, Kondo H, Hirono I, Satoh S. Microarray analysis of hepatic gene expression in juvenile Japanese flounder Paralichthys olivaceus fed diets supplemented with fish or vegetable oils. Mar. Biotechnol. 2014;16:88–102. doi: 10.1007/s10126-013-9535-y. [DOI] [PubMed] [Google Scholar]
- 58.Lazzarotto V, Médale F, Larroquet L, Corraze G. Long-term dietary replacement of fishmeal and fish oil in diets for rainbow trout (Oncorhynchus mykiss): Effects on growth, whole body fatty acids and intestinal and hepatic gene expression. PLoS ONE. 2018;13:e0190730. doi: 10.1371/journal.pone.0190730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ren HT, Yu JH, Xu P, Tang YK. Influence of dietary fatty acids on muscle fatty acid composition and expression levels of Δ6 desaturase-like and elovl5-like elongase in common carp (Cyprinus carpio var. Jian) Comp. Biochem. Physiol. B. 2012;163:184–192. doi: 10.1016/j.cbpb.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 60.APHA . Standard Methods for the Examination of Water and Waste Water. 23. American Public Health Association, American Water Works Association, Water Environment Federation; 2017. [Google Scholar]
- 61.AOAC . Official Methods of Analysis. Washington: Association of Official Analytical Chemists Inc; 2000. [Google Scholar]
- 62.Folch J, Lees M, Sloane-Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226:497–509. doi: 10.1016/S0021-9258(18)64849-5. [DOI] [PubMed] [Google Scholar]
- 63.Aksnes A, Opstvedt J. Content of digestible energy in fish feed ingredients determined by the ingredient-substitution method. Aquaculture. 1998;161:45–53. doi: 10.1016/S0044-8486(97)00255-X. [DOI] [Google Scholar]
- 64.Merrill AL, Watt BK. Energy Value of Foods: Basis and Derivation Agriculture Handbook No. 74. ARS United States Department of Agriculture; 1973. [Google Scholar]
- 65.Christie WW. Lipid Analysis. 3. Oily Press; 2003. [Google Scholar]
- 66.Tocher DR, Harvie DG. Fatty acid compositions of the major phosphoglycerides from fish neural tissues; (n-3) and (n-6) polyunsaturated fatty acids in rainbow trout (Salmo gairdneri) and cod (Gadus morhua) brains and retinas. Fish Physiol. Biochem. 1988;5:229–239. doi: 10.1007/BF01874800. [DOI] [PubMed] [Google Scholar]
- 67.Ueberschär B. Determination of the nutritional condition of individual marine fish larvae by analyzing their proteolytic enzyme activities with a highly sensitive fluorescence technique. Meeresforschung. 1988;32:144–154. [Google Scholar]
- 68.Cao MJ, et al. Purification and characterization of two anionic trypsins from the hepatopancreas of carp. Fisheries Sci. 2000;66:1172–1179. doi: 10.1046/j.1444-2906.2000.00185.x. [DOI] [Google Scholar]
- 69.Roberts IM. Hydrolysis of 4-methylumbelliferyl butyrate: A convenient and sensitive fluorescent assay for lipase activity. Lipids. 1985;20:243–247. doi: 10.1007/BF02534195. [DOI] [Google Scholar]
- 70.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. J. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 71.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 72.Montgomery DC. Design and Analysis of Experiments. Wiley; 1984. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.