Abstract
Transdermal drug delivery aims to create a safe and effective method of administering drugs through the skin that attracts a lot of attention and investment due to the constant progress in the field. Transferosomes are flexible or malleable vesicles (having almost the same structure as liposomes but with better skin penetration properties) discovered initially in the early 90s. The name transferosomes, which means “carrying bodies,” is coined from the Latin phrase “Transferee,” which means “to carry through,” and the Greek term “soma,” meaning “body.” In comparison to typical herbal extracts, phytosomes (Transferosomes) are created by attaching specific herbal extracts to phosphatidylcholine, resulting in a formulation with increased solubility and, hence, better absorption, resulting in improved pharmacokinetic and pharmacodynamic features of the entrapped drugs. We are using the word phytosomes and transferosomes interchangeably as we have consolidated vesicular delivery of herbal drugs through skin. In this mini-review, we have demonstrated the enormous potential of developing nanotechnology to deliver bioactive phytochemicals, with a special emphasis on phytosomes (Transferosomes) as a unique lipid-based nanocarrier for transdermal drug delivery.
Keywords: transferosome, phytosome, complementary medicine (CAM), phytochemicals, transdermal drug delivery
Introduction
Transdermal drug delivery system (TDDS) is an appealing option to oral drug administration and is poised to give an alternative to hypodermic injection as well. People have been applying herbal extracts and chemicals to their skin for thousands of years for therapeutic purposes, and in the contemporary period, a wide range of topical formulations have been produced to treat local indications (Alkilani et al., 2015). In comparison to the oral route, transdermal administration provides several benefits. It is utilized particularly when the liver has a substantial first-pass impact that might cause medications to be metabolized early. Transdermal administration also offers advantages over hypodermic injections, which are uncomfortable, create hazardous medical waste, and risk of disease transmission through needle re-use, particularly in impoverished nations (Jeong et al., 2021).
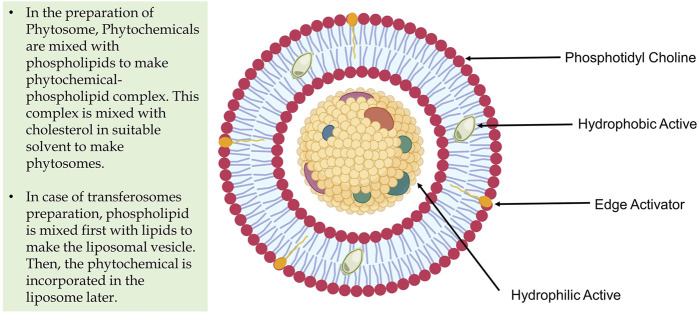
The introduction of transferosome (phytosome) nanotechnology can transform the existing state of external therapeutic phytochemicals delivery. The fundamental obstacle in translating phytochemicals’ therapeutic potential to a clinical context is their extremely low absorption rate and limited penetration through biological barriers (Alharbi et al., 2021). Phytosomes, as lipid-based vesicular nanocarriers, perform an essential role in improving the pharmacokinetic and pharmacodynamic characteristics of herbal-derived polyphenolic chemicals, making this nanotechnology a prospective tool for the creation of novel topical formulations. Because of their distinct physicochemical properties, phytochemicals may be able to penetrate biological barriers more easily with the use of this nanosized vesicular drug delivery method, and ultimately enhancing their bioavailability (More et al., 2021). Medicinal plants and their phytochemicals are currently widely used as a treatment for a variety of ailments. Nonetheless, their low bioavailability and selectivity may restrict their therapeutic use. As a result, bioavailability is regarded as a significant problem in improving bio-efficacy in transporting dietary phytochemicals (Barani et al., 2021). However, with enhanced membrane flexibility, ultra-deformability, and soft nature, the basic structure of a transferosome is ideal approach for transdermal phytochemical delivery for local and systemic drug action (Pawar et al., 2016). However, phospholipid interacts with the phytochemicals via the configuration of an H-bond in between the polar head of the phospholipid and the polar functions of the phytochemical constituents. Flexibility is achieved by incorporating an edge activator (surfactants) into the cholesterol-phospholipid bilayers (Figure 1). Transferosomes are especially effective for transporting medicines with limited solubility through the epidermis. The ability of phytoconstituents derived from herbal sources to penetrate the epidermis or any other semi-permeable barrier is relatively restricted (Chavda et al., 2021; Opatha et al., 2020).
FIGURE 1.
Structural feature of Transferosome and Phytosome.
Phytochemical Delivery Through Transferosome (Phytosome)
Transferosomes are applied topically in a non-occluded manner and have been proven to permeate into the stratum corneum (lipid lamellar areas), leading to the skin’s hydration (Benson, 2006). An osmotic gradient develops due to the evaporation of skin water by the body heat, which acts as a driving force to transfer the drug from the site of application to the targeted area, either locally or for systemic action (Sudhakar et al., 2021; Naik et al., 2006). Furthermore, transferosomes enhance the functions of the skin by improving enzyme balance, hydration, and collagen structure (Opatha et al., 2020). Because of their hydrophilicity, polyphenols have lower absorption and lipid solubility, restricting their in vivo action. Several flavonoid molecules attach firmly to the phospholipid components of phytosomes. Transferosomes are infused with phytoconstituents with limited permeability and incorporated into dosage forms for topical applications such as the transdermal patches to facilitate better bioavailability with site-specific action, which is referred to as ‘Phytosomes’. In short, phytosomes are transferosomes with an infused phytoconstituent or phytochemical (Romero and Morilla, 2013). The difference between transferosome and phytosome can also be understood by their method of preparation though the composition is identical (Figure 1). Phytosomes continue to outperform herbal extracts in improving its pharmacokinetics and pharmacodynamics (Lu et al., 2019). The transferosomes–herbal complex shows greater affinity to the skin phospholipid component, increasing the topical formulation’s lipid solubility (Alharbi et al., 2021).
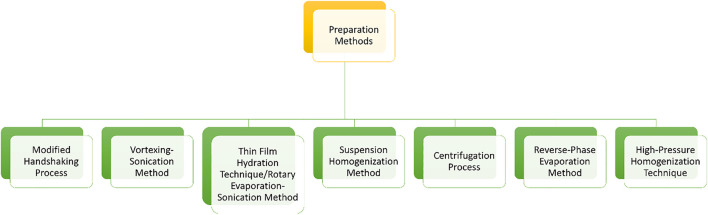
Transferosomes majorly involve the ingredients like amphipathic ingredients (combination of hydrophilic and lipophilic molecules like soy phosphatidylcholine), surface activators (e.g. surfactants), alcohol, and water (Jiang et al., 2018). The preparation method was determined based on the composition of phytosomes, such as drug-carrying capacity and suitable transporter with optimal stability and deformability (Kumar, 2018). Numerous techniques for creating phytosomes have been presented, and classified such as the film hydration method, (also known as a rotational evaporation sedimentation method) is a frequently utilized preparation process for transferosomes and phytosomes, and it enables more topical penetration through the skin as compared to other modified approaches (Figure 2) (Singh et al., 2017). Solvent evaporation is popular and widely utilized approach for generating phospholipid vesicles. In addition to the traditional procedure, methods such as freeze-thaw, centrifugation, reverse-phase evaporation, high-pressure homogenization, and suspension homogenization are used to engineer the phytochemical loaded transferosomes (Opatha et al., 2020). Transfersome characterization variables (Table 1) such as vesicle shape and size, size distribution, polydispersity index, zeta potential, number of vesicles per cubic mm, entrapment efficiency, degree of deformability, and skin permeability measurements are useful for optimising the transfersomal formulation (Opatha et al., 2020).
FIGURE 2.
Method of preparation for Transferosomes (phytosomes).
TABLE 1.
Charecterization methods for transferosome.
| Evaluation parameter | Determination method | References |
|---|---|---|
| Number of vesicles/cubic mm | (The total number of phytosomes/transfersomes counted × dilution factor)/The total number of squares counted | Chauhan and Tyagi (2018) |
| Vesicle size distribution | Photon correlation spectroscopy or Dynamic light scattering method | Chauhan and Tyagi, (2018) |
| Zeta potential | Electrophoretic mobility technique | Chauhan and Tyagi (2018) |
| Degree of deformability | D = J (rv/rp) Where rv and rp indicate the vesicle size and the barrier pore size respectively, while J remains for the amount of suspension squeezed out in 5 min | Chauhan and Tyagi (2018) |
| Entrapment efficiency (EE) | % EE = The amount of drug entrapped ×100/The total amount of drug added | Ascenso et al. (2015) |
| In vitro skin permeation studies | Human skin is ideal for permeation studies but due to less availability, various animal models and animal skin were examined for permeation study, including porcine, rat, mouse Guinea pig, primate, and snake skins. Systemic membranes such as Start M® and Franz cell model are also widely used for determination | Singh et al. (2017) |
| In vitro drug release | Extrusion method or Franz diffusion cells are used | GM et al. (2008) |
| Stability studies | The vesicle size and structure changes concerning time is taken into consideration, for that transmission electron microscope (TEM) or dynamic light scattering methods (DLS) are employed | Ascenso et al. (2015) |
Transfersomes have the potential to open up a whole new world of possibilities for efficient drug administration. The following are the benefits of using transfersomes as vesicle-based TDDS,
• High vesicle deformability allows medications to be transported through the skin without significant loss of intact vesicles and can be utilized for both topical and systemic therapies (Moawad et al., 2017).
• Transfersomes are a natural alternative for producing continuous drug release as well as predictable and prolonged activity duration of the drug (Opatha et al., 2020).
• Transfersome carriers are made up of hydrophilic and hydrophobic moieties, resulting in a one-of-a-kind drug carrier system capable of delivering therapeutic drugs with a wide range of solubility (Bnyan et al., 2018).
• Avoiding first-pass metabolism, which is a key disadvantage of oral medication delivery, results in improved drug bioavailability (Moawad et al., 2017).
• Because they are composed of natural phospholipids and edge activators, they appear to be biocompatible and biodegradable (Li et al., 2015).
• They can improve bioactive agents' site selectivity and increase transdermal flow.
• Reduce the drug’s unwanted side effects while also protecting it from metabolic destruction (Bnyan et al., 2018).
• They have the benefit of being created from pharmaceutically approved substances and using established processes, but they must be developed and optimized on an individual basis (Opatha et al., 2020).
• It is straightforward to scale up because of a rapid and simple manufacturing technique (Li et al., 2015).
Transfersomes offer several advantages, but they also have some restrictions,
• The high cost of raw materials and costly equipments for manufacturing impacts the costing of the final drug product. As a result, because it is very inexpensive, phosphatidylcholine is the most often utilized lipid component (Iskandarsyah et al., 2018).
• Another barrier for using transfersomes as a drug delivery mechanism is the difficulty in obtaining pure natural phospholipids. As a result, synthetic phospholipids might be employed as a substitute (Grit and Crommelin, 1993; Iskandarsyah et al., 2018).
• Because of their propensity for oxidative destruction, transfersomes are thought to be chemically unstable. Transfersome oxidation can be considerably reduced by storing a product at a low temperature and keeping it away from light. Transfersome storage performance can be improved with post-preparation processing such as freeze-drying and spray-drying (Grit and Crommelin, 1993).
Currently, many clinical trials are in progress for the transdermal delivery of various drugs using transferosomes as a carrier however, all are on synthetic analogues (Opatha et al., 2020). Various phytochemicals are known to be delivered with the help of transferosomes as a carrier. Avadhani et al., in 2017, formulated transferosomes of epigallocatechin-3-gallate and hyaluronic using a modified thin-film hydration method, followed by a high-pressure homogenization technique. This work resulted in the enhancement of their efficacy as antioxidants (Avadhani et al., 2017). In 2019, Wu et al. studied the antioxidant drug resveratrol (RSV) and its resveratrol loaded transferosomes, including its antioxidant assays, in vitro transdermal delivery analysis, and cell viability assay (Wu et al., 2019). Formulation containing RSV transferosomes showed enhancement in stability, bioavailability, and safety of resveratrol (Szulc-Musioł and Sarecka-Hujar, 2021). Research conducted by Jiang et al. states that the topical delivery of anticancer agents like paclitaxel in melanoma chemotherapy can be done by paclitaxel transferosomes embedded within oligopeptide hydrogels (Jiang et al., 2018). Vincristine transferosomes have shown an increase in their permeation capability through the skin and improvement in their lymph targeting ability (Lu et al., 2007; Pahwa et al., 2021). Transferosomes are found to improve the stability and the efficacy of anti-inflammatory drugs. Transferosomes embedded with curcumin were developed and have shown an increase in their permeation capability and bioavailability (Anita et al., 2021). A study on the antiarthritic activity of capsaicin-loaded transferosomes in arthritic rats showed better inhibitory activity and the desired therapeutic concentration of the drug at the target site (Jain et al., 2021). Similarly, Ines Castangia and collegues have prepared the transferosome encapsulating Jabuticaba (Myrciaria jaboticaba) extract having flavonoids having better wound-healing in human keratinocytes (Castangia et al., 2021). Caffeine transferosomes also showed greater permeation across the stratum corneum of the skin and an increase in permeation of hydrophilic caffeine through hair follicles (Abd et al., 2016). Various other phytochemicals like embelin and colchicine can also be delivered transdermally by forming its transferosomes. Numerous experts have discovered novel techniques and generated phytosome compositions (Table 2).
TABLE 2.
Patents on phytosomes for the phytochemical delivery.
| Name of patent | Development | Patent No |
|---|---|---|
| Phospholipid complexes derived from olive fruits or leaves with enhanced bioavailability | The bioavailability of olive fruit/leaf extracts is improved when it is employed in phospholipid complexes | EP1844785 |
| Curcumin phospholipid complex with improved bioavailability | Curcumin phospholipid complexes have a larger systemic level of primary agent than simple curcumin | WO 2009/101551 |
| Wound healing and skin treatment, with thymosin β-4 | Preparation is comprising thymosin β4 for wound repair | US/2007/0015698 |
| Oral formulations for cellulite therapy | Centella asiatica triterpenes, Vitis vinifera extracts, and Ginkgo biloba flavonoids in free or complexed form with phospholipids are used in topical pharmaceutical preparation | US7691422 |
| For the management of asthmatic and allergic disorders, formulations including Ginkgo biloba metabolites | Constituents of Ginkgo biloba fractions for the therapy of asthma and allergic diseases | EP1813280 |
Conclusion and Future Prospects
As new phytochemicals are discovered, studies on their medicinal advantages in a biological setting will be continually updated. Future research could look into combining phytosomes with several other phytochemicals or combining drugs and phytochemicals in the same nano-vesicle to produce stimulatory activity. Phytosomes are identical to liposomes and have equivalent skin permeability and stability profiles. In phytosomes, however, the phospholipid interfaces with the phytochemicals through the generation of an H-bond between the polar head of the phospholipid and the polar capabilities of the bioactive molecules. In compared to liposomes, this significantly improves the stability and skin penetration of phytochemicals. Clinical trials are still inadequate to judge the bioactivities of specific compositions, but the conclusive data for these compositions is positive, and experts are encouraged to continue their studies in this sector. Clinical trials on standardized products that demonstrate improved efficacy relative to non-formulated components or extracts will be critical in driving interest in these advancements. All the research works carried out in the recent past for the phyochemical loaded lipidic vesicles i.e., transferosome depicts the potential of this formulation approach to tackle the challenges associated with phyochemicals for their successful transdermal delivery for local and systemic action. Further, clinical research data of such drug delivery platform in the near future will unveils the future potential use of such drug delivery platform.
Acknowledgments
VC wants to dedicate this work to LM College of pharmacy as a part of the 75th year celebration of the college. We are also thankful to Darsh Vaghasiya, Khandu Muhammedsaad Bashirahmed and Kushal Trivedi for their help in the literature search. The figure of the manuscript is created with BioRender.Com. We are very thankful to Dr. Lalit Vora (Queens University Belfast, UK) for his support in the figure preparation.
Author Contributions
VC and Z-SC conceived the idea of the work. VC and R-PC wrote the first draft with the help of AP. VC and R-PC refined the article with the help of Z-SC.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abd E., Roberts M. S., Grice J. E. (2016). A Comparison of the Penetration and Permeation of Caffeine into and through Human Epidermis after Application in Various Vesicle Formulations. Skin Pharmacol. Physiol. 29, 24–30. 10.1159/000441040 [DOI] [PubMed] [Google Scholar]
- Alharbi W. S., Almughem F. A., Almehmady A. M., Jarallah S. J., Alsharif W. K., Alzahrani N. M., et al. (2021). Phytosomes as an Emerging Nanotechnology Platform for the Topical Delivery of Bioactive Phytochemicals. Pharmaceutics 13 (9), 1475. 10.3390/pharmaceutics13091475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkilani A. Z., McCrudden M. T., Donnelly R. F. (2015). Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the Stratum Corneum. Pharmaceutics 7, 438–470. 10.3390/pharmaceutics7040438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anita C., Munira M., Mural Q., Shaily L. (2021). Topical Nanocarriers for Management of Rheumatoid Arthritis: A Review. Biomed. Pharmacother. 141, 111880. 10.1016/j.biopha.2021.111880 [DOI] [PubMed] [Google Scholar]
- Ascenso A., Raposo S., Batista C., Cardoso P., Mendes T., Praça F. G., et al. (2015). Development, Characterization, and Skin Delivery Studies of Related Ultradeformable Vesicles: Transfersomes, Ethosomes, and Transethosomes. Int. J. Nanomedicine 10, 5837–5851. 10.2147/IJN.S86186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avadhani K. S., Manikkath J., Tiwari M., Chandrasekhar M., Godavarthi A., Vidya S. M., et al. (2017). Skin Delivery of Epigallocatechin-3-Gallate (EGCG) and Hyaluronic Acid Loaded Nano-Transfersomes for Antioxidant and Anti-aging Effects in UV Radiation Induced Skin Damage. Drug Deliv. 24, 61–74. 10.1080/10717544.2016.1228718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barani M., Sangiovanni E., Angarano M., Rajizadeh M. A., Mehrabani M., Piazza S., et al. (2021). Phytosomes as Innovative Delivery Systems for Phytochemicals: A Comprehensive Review of Literature. Int. J. Nanomedicine 16, 6983–7022. 10.2147/IJN.S318416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson H. A. (2006). Transfersomes for Transdermal Drug Delivery. Expert Opin. Drug Deliv. 3, 727–737. 10.1517/17425247.3.6.727 [DOI] [PubMed] [Google Scholar]
- Bnyan R., Khan I., Ehtezazi T., Saleem I., Gordon S., O'Neill F., et al. (2018). Surfactant Effects on Lipid-Based Vesicles Properties. J. Pharm. Sci. 107, 1237–1246. 10.1016/j.xphs.2018.01.005 [DOI] [PubMed] [Google Scholar]
- Castangia I., Manca M. L., Allaw M., Hellström J., Granato D., Manconi M. (2021). Jabuticaba (Myrciaria Jaboticaba) Peel as a Sustainable Source of Anthocyanins and Ellagitannins Delivered by Phospholipid Vesicles for Alleviating Oxidative Stress in Human Keratinocytes. Molecules 26, 6697. 10.3390/molecules26216697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan P., Tyagi B. K. (2018). Herbal Novel Drug Delivery Systems and Transfersomes. J. Drug Deliv. Ther. 8, 162–168. 10.22270/JDDT.V8I3.1772 [DOI] [Google Scholar]
- Chavda V. P., Ertas Y. N., Walhekar V., Modh D., Doshi A., Shah N., et al. (2021). Advanced Computational Methodologies Used in the Discovery of New Natural Anticancer Compounds. Front. Pharmacol. 12, 702611. 10.3389/fphar.2021.702611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Maghraby G. M., Barry B. W., Williams A. C. (2008). Liposomes and Skin: from Drug Delivery to Model Membranes. Eur. J. Pharm. Sci. 34, 203–222. 10.1016/J.EJPS.2008.05.002 [DOI] [PubMed] [Google Scholar]
- Grit M., Crommelin D. J. (1993). Chemical Stability of Liposomes: Implications for Their Physical Stability. Chem. Phys. Lipids 64, 3–18. 10.1016/0009-3084(93)90053-6 [DOI] [PubMed] [Google Scholar]
- Iskandarsyah I., Rahmi A. D., Pangesti D. M. (2018). Comparison of the Characteristics of Transfersomes and Protransfersomes Containing Azelaic Acid. Jyp 10, s11–S15. 10.5530/jyp.2018.2s.3 [DOI] [Google Scholar]
- Jain S., Vaidya A., Gupta P. K., Rosenholm J. M., Bansal K. K. (2021). Antiarthritic Activities of Herbal Isolates: A Comprehensive Review. Coatings 11, 1329. 10.3390/coatings11111329 [DOI] [Google Scholar]
- Jeong W. Y., Kwon M., Choi H. E., Kim K. S. (2021). Recent Advances in Transdermal Drug Delivery Systems: a Review. Biomater. Res. 25, 24–15. 10.1186/s40824-021-00226-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T., Wang T., Li T., Ma Y., Shen S., He B., et al. (2018). Enhanced Transdermal Drug Delivery by Transfersome-Embedded Oligopeptide Hydrogel for Topical Chemotherapy of Melanoma. ACS Nano 12, 9693–9701. 10.1021/acsnano.8b03800 [DOI] [PubMed] [Google Scholar]
- Kumar A. (2018). Transferosome: a Recent Approach for Transdermal Drug Delivery. J. Drug Deliv. Ther. 8, 100–104. 10.22270/jddt.v8i5-s.1981 [DOI] [Google Scholar]
- Li J., Wang X., Zhang T., Wang C., Huang Z., Luo X., et al. (2015). A Review on Phospholipids and Their Main Applications in Drug Delivery Systems. Asian J. Pharm. Sci. 10, 81–98. 10.1016/j.ajps.2014.09.004 [DOI] [Google Scholar]
- Lu Y., Hou S., Zhang L., Li Y., He J.-Y., Guo D.-D. (2007). Transdermal and Lymph Targeting Transfersomes of Vincristine. Yao Xue Xue Bao 42, 1097–1101. [PubMed] [Google Scholar]
- Lu M., Qiu Q., Luo X., Liu X., Sun J., Wang C., et al. (2019). Phyto-phospholipid Complexes (Phytosomes): A Novel Strategy to Improve the Bioavailability of Active Constituents. Asian J. Pharm. Sci. 14, 265–274. 10.1016/j.ajps.2018.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moawad F. A., Ali A. A., Salem H. F. (2017). Nanotransfersomes-loaded Thermosensitive In Situ Gel as a Rectal Delivery System of Tizanidine HCl: Preparation, In Vitro and In Vivo Performance. Drug Deliv. 24, 252–260. 10.1080/10717544.2016.1245369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- More M. P., Pardeshi S. R., Pardeshi C. V., Sonawane G. A., Shinde M. N., Deshmukh P. K., et al. (2021). Recent Advances in Phytochemical-Based Nano-Formulation for Drug-Resistant Cancer. Med. Drug Discov. 10, 100082. 10.1016/j.medidd.2021.100082 [DOI] [Google Scholar]
- Naik S. R., Pilgaonkar V. W., Panda V. (2006). Evaluation of Antioxidant Activity of Ginkgo biloba Phytosomes in rat Brain. Phytother. Res. 20, 1013–1016. 10.1002/ptr.1976 [DOI] [PubMed] [Google Scholar]
- Opatha S. A. T., Titapiwatanakun V., Chutoprapat R. (2020). Transfersomes: A Promising Nanoencapsulation Technique for Transdermal Drug Delivery. Pharmaceutics 12, 855. 10.3390/pharmaceutics12090855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahwa R., Pal S., Saroha K., Waliyan P., Kumar M. (2021). Transferosomes: Unique Vesicular Carriers for Effective Transdermal Delivery. J. Appl. Pharm. Sci. 11 (05), 001–008. [Google Scholar]
- Pawar A., Jadhav K. R., Chaudhari L. H. (2016). Transfersome : A Novel Technique Which. Asian J. Pharm. 10, 425–436. 10.4184/asj.2016.10.1.176 [DOI] [Google Scholar]
- Romero E. L., Morilla M. J. (2013). Highly Deformable and Highly Fluid Vesicles as Potential Drug Delivery Systems: Theoretical and Practical Considerations. Int. J. Nanomedicine 8, 3171–3186. 10.2147/IJN.S33048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Verma D., Mirza M. A., Das A. K., dudeja M., Anwer M. K., et al. (2017). Development and Optimization of Ketoconazole Loaded Nano-Transfersomal Gel for Vaginal Delivery Using Box-Behnken Design: In Vitro , Ex Vivo Characterization and Antimicrobial Evaluation. J. Drug Deliv. Sci. Techn. 39, 95–103. 10.1016/J.JDDST.2017.03.007 [DOI] [Google Scholar]
- Sudhakar K., Fuloria S., Subramaniyan V., Sathasivam K. V., Azad A. K., Swain S. S., et al. (2021). Ultraflexible Liposome Nanocargo as a Dermal and Transdermal Drug Delivery System. Nanomater. 11 (10), 2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szulc-Musioł B., Sarecka-Hujar B. (2021). The Use of Micro- and Nanocarriers for Resveratrol Delivery into and across the Skin in Different Skin Diseases-A Literature Review. Pharmaceutics 13, 451. 10.3390/pharmaceutics13040451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P. S., Li Y. S., Kuo Y. C., Tsai S. J., Lin C. C. (2019). Preparation and Evaluation of Novel Transfersomes Combined with the Natural Antioxidant Resveratrol. Molecules 24, 1–12. 10.3390/molecules24030600 [DOI] [PMC free article] [PubMed] [Google Scholar]