Abstract
Porous silica particles were prepared by sol–gel method with some modification to get wide-pore particles. These particles were derivatized with N-phenylmaleimide-methylvinylisocyanate (PMI) and styrene by reversible addition fragmentation chain transfer (RAFT) polymerization to prepare N-phenylmaleimide embedded polystyrene (PMP) stationary phases. Narrow bore stainless steel column (100 × 1.8 mm i.d) was packed by slurry packing method. The chromatographic performance of PMP column was evaluated for the separation of synthetic peptides mixture composed of five peptides (Gly-Tyr, Gly-Leu-Tyr, Gly-Gly-Tyr-Arg, Tyr-Ile-Gly-Ser-Arg, Leucine enkephalin) and tryptic digest of human serum albumin (HAS) respectively. Number of theoretical plates as high as 280,000 plates/m were obtained for peptides mixture at optimum elution condition. Separation performance of the developed column was compared with commercial Ascentis Express RP-Amide column and it was observed that separation performance of PMP column was better than commercial column in terms of separation efficiency and resolution.
Subject terms: Analytical chemistry, Biochemistry, Materials chemistry, Polymer chemistry, Chemical synthesis, Biochemistry, Chemical biology, Chemistry
Introduction
In recent years the biopharmaceutical industry has become an expanding global market with a substantially increased market share. The analysis of peptides and proteins is highly desired with the explosive growth of biopharmaceutical industry1–3. Several impurities are produced during peptide synthesis in addition to the target peptides, therefore chromatographic purification is required to get peptides of the required purity. Analysis and characterization of proteins in bodily fluids, tissues and cells is a highly challenging task owing to the presence of large number of potentially detectable species in a single sample. Even though mass spectrometry is an effective tool for peptides and proteins sequencing but if such samples are injected at once to mass spectrometer, the separation will be not satisfactory. This issue can be mitigated by the implementation of liquid chromatography (LC) separation prior to MS analysis, which will reduce the number of analytes entering to the mass spectrometer at a given time4–6. Furthermore, during the liquid phase separation the analytes can be focused within narrow zones, which concentrate these analytes and increase MS detection sensitivity. Liquid chromatography (LC) has significantly advanced over the past decade and became a prevalent technique in proteomic analysis7–10.
Reverse phase liquid chromatography (RP-LC) is widely used for the purification and separation of peptides mixture using octadecyl-modified silica (ODS) as a stationary phase11–13. However, RP stationary phases do not provide satisfactory separation of peptides and proteins due to their complex structure and amphoteric nature14,15. Therefore, specially designed stationary phases are required for the analysis of peptides and proteins that have both polar and nonpolar moieties to interact with these analytes and retain them16. Mixed-mode chromatography which offers multimodal interaction could be an alternative to RP-LC for the separation of peptides, proteins and other complex mixtures. Several mixed-mode stationary phases have been prepared and the columns packed with these stationary phases have been used for peptides and proteins separation17–21. Mixed-mode stationary phases (WAX/RPLC, HILIC/RPLC, polar embedded/RPLC) are suitable for peptides and proteins separation owing to the presence of both polar and non-polar groups22–28. Similarly, polar-embedded stationary phases with covalently bonded polar groups have shown good separation capability and a unique selectivity for polar and nonpolar analytes, because the separation depends on multimodal interactions between the analyte and the stationary phase29–32. Recently, Zhang et al.30 have prepared docosyl-terminated polyamine stationary phase and successfully separated hydrocarbons, antidepressants, flavonoids, nucleosides, estrogens and several other analytes. Polar embedded stationary have both polar and non-polar groups therefore it could be used for the separation of peptides and proteins which have both hydrophobic and hydrophilic moieties. Polar embedded columns such as amide embedded C18 columns are commercially available under the trade name Ascentis Express RP-Amide column, but these columns have only been used for the analysis of amines33.
In current study, a polar-embedded stationary phase (N-phenylmaleimide embedded polystyrene) was prepared and evaluated for the separation of peptides and tryptic digest of HSA. The following strategies were adopted for the preparation of stationary phase. Porous silica particles were prepared according to the procedure given in our previous publications with some modification in the preparation protocol31,34–39.The ratio of urea, polyethylene glycol (PEG), TMOS, water acetic acid was adjusted to prepare silica particles having large pore size. Secondly, a new ligand phenylmaleimide-methylvinyl isocyanate was synthesized and silica particles were derivatized with it to prepare polar-embedded stationary phase. The resultant stationary phase was packed into stainless steel column (100 × 1.8 mm i.d) using an optimized packing protocol. Column packing was assisted with mechanical vibration to ensure the formation of a homogenous bed inside the column. The packed column was evaluated for separation of peptides mixture composed of five peptides; (Gly-Tyr, Gly-Leu-Tyr, Gly-Gly-Tyr-Arg, Tyr-Ile-Gly-Ser-Arg, Leucine Enkephalin) and tryptic digest of Human serum albumin (HAS). It was observed that peptides mixture and tryptic digest of HSA were separated with good resolution and efficiency. The separation performance of PMP column was compared with Ascentis Express RP-Amide column. It was observed that peptides and proteins were separated with good resolution and efficiency on PMP column and the separation efficiency of PMP column was higher than Ascentis Express RP-Amide column.
Material and methods
Chemicals and reagents
PEG (polyethylene glycol), urea, acetic acid, trimethoxy orthosilicate (TMOS), Chlorotrimethylsilane(TMCS), trypsin, human serum albumin (HSA), ammonium chloride, urea, hexamethyldisilazane (HMDS), methacryloyl chloride (MC), styrene, 4-hydroxy-TEMPO, benzoyl peroxide (BPO), HPLC grade acetonitrile (ACN), methanol, 2-propanol, and acetone were purchased from Sigma–Aldrich (St. Louis, MO, USA).
Synthesis of porous silica particles
A mixture of urea (8 g), polyethylene glycol (8 g) and 8 mL 0.01 N acetic acid was stirred for 10 min and 24 mL TMOS was added into it under ice-cold condition. The reaction mixture was heated at 40 °C for 6 h followed by heating at 120 °C for 8 h in a stainless steel autoclave. Water was decanted and the residual material was dried at 70 °C for 12 h. The dried soft bulk was smoothly grounded and calcined at 550 °C for 12 h in a furnace. Three batches were prepared and characterized to check the reproducibility in terms of particle size, pore size and surface area of the particles.
Synthesis of polar-embedded RP stationary phase
A stationary phase containing both polar groups and polystyrene chain was prepared by the surface modification of silica particles with a pre-synthesized ligand phenylmaleimide-methylvinylisocyanate (PCMP) followed by radial polymerization with styrene. The preparation procedure is described below.
Preparation of phenylmaleimide-methylvinylisocyanate (PMCP)
Phenylmaleimide-methylvinylisocyanate copolymer (PMCP) was prepared by dissolving N-phenylmaleimide (200 mg) and methylvinylisocyanate (100 mg) in anhydrous toluene, then 0.1 mL of 2,2'-azoisobutyronitrile (AIBN) were added into the reaction flask through dropping funnel. The mixture was heated at 60 °C for 3 h, filtered and dried at 40 °C in oven for 3 h.
Functionalization of silica particles with PMCP
Dried silica particles (2 g) were dispersed in dry toluene (100 mL), stirred and sonicated for 10 min in 500 mL round bottom flask. PMCP (10 mg) was dissolved in toluene and added drop-wise into the reaction flask through a dropping funnel. The mixture was refluxed at 100 °C for 8 h, filtered and washed with acetone and dried at 60 °C for 3 h. Then, PMCP bonded silica particles (100 g) was dissolved in toluene (200 ml), then 4-hydroxy-TEMPO (2 mL) was added into it in the presence of 100 µL dibutyl-tin-dilaurate as a catalyst. The mixture was stirred at 50 °C for 8 h, filtered and dried at 50 °C for 3 h.
Radical Polymerization of Styrene
Styrene (1 mL), benzoyl peroxide BPO (0.5 mL) and TEMPO-PMCP attached silica particles (1.5 g) were dispersed in toluene and purged with nitrogen. The polymerization of styrene was carried out at 100 °C for 12 h. The resultant product was washed with methanol and dried at 60 °C overnight. The overall reaction scheme is given in the Fig. 1.
Figure 1.
Reaction scheme for the preparation of mixed-mode stationary phase.
Characterization of the stationary phase
The samples were degassed at 393 K for 1 h to obtain a residual pressure of less than 10−3 Torr. The amount of N2 adsorbed at a relative pressure of P/P0 = 0.99 was used to determine the total pore volume. The morphology of bare and ligand bonded silica particles was examined with scanning electron microscope (Hitachi High Technologies, Tokyo, Japan). The dried samples (bare silica and ligand bonded silica particles) were put on aluminum stubs using adhesive carbon tape. A Q150T sputter coater was used to coat gold over the sample and 5 nm Au layer was deposited over the sample. This enhances the efficiency of the process using low voltages and giving a fine-grain, cool sputtering. A Thermo Electron (Waltham, MA, USA) Flash EA1112 elemental analyzer was used to carry out elemental analysis. A Malvern (Worcestershire, UK) Mastersizer 2000 particle size analyzer was used to get particle size distribution. Bare silica particles and ligand bonded silica particles (5 mg each) were dispersed in 5 mL isopropyl alcohol, sonicate for 10 min, vortexes for 5 min, and subjected to the optical bench of Mastersizer. Thermo gravimetric analysis were carried out over a temperature range of 30 to 800 °C at 5 °C per minute.
Column packing
Glass-lined stainless-steel narrow-bore column of dimensions (100 × 1.8 mm i.d.) was packed using slurry packing method, the same procedure was applied as used in Ref.31. A stainless steel column (glass lined, 100 × 1.8 mm i.d.) with an outlet union containing 1 µm screen frit was connected to the slurry packer (Alltech Deerfield, IL, USA). The slurry of stationary phase was prepared by suspending the 150 mg stationary phase in 1.2 mL methanol, and fed into the reservoir column. Methanol was used as slurry solvent as well as pushing solvent. The columns were packed by applying a pressure sequence of 100 MP for 10 min, 80 MP for 15 min, and 60 MP for 30 min. Mechanical vibration was applied with two GC column vibrators (Alltech, Deerfield, IL, USA) during packing to ensure the homogenous packing of column. Slurry packer was closed and the pressure was released slowly to prevent any damage inside the column. Column was disconnected from the slurry packing set-up and another union was connected to the inlet and connected to LC system to check its performance.
HPLC analysis
A special µLC set-up was constructed using LC pump (10AD Shimadzu, Japan), an injector with 50nL injection loop (Valco (USA) C14 W.05), membrane degasser (Shimadzu DGU-14A), a UV–VIS capillary window detector (UV-2075), and glass lined micro-column. Very narrow and short connecting tubing were used to minimize the effect of extra column band broadening. After packing, the 1/16" outlet of the reducing union was installed with a capillary (50 μm i.d. 365 ith a capillary (50 μm) of the reducing union was installed. Data collection and chromatogram processing were accomplished with Multichro 2000 software. The UV absorbance of the test analytes was monitored at 254 nm. The chromatographic data was analyzed by OriginPro8 (Northampton, MA).
Preparation of tryptic digest of Human Serum Albumin (HSA)
Albumin from human serum, lyophilized powder, ≥ 96% (agarose gel electrophoresis) 3 mg was mixed with trypsin (1.5 mg), 4.0 M urea (1 mL), and 0.2 M ammonium bicarbonate (1 mL). The solution was stirred for 10 min and kept in water bath at 37 °C for 6 h followed by quenching with 1 mL 0.1% TFA. The solution was filtered and stored below 4 °C.
Columns evaluation
PMP column was evaluated for the separation of peptides mixture and tryptic digest of HSA respectively. PMP column was checked for the separation of peptides mixture and tryptic digest of HSA and the results were compared with Ascentis Express RP-Amide column. Numbers of theoretical plates were calculated by the following equation:
| 1 |
where w1/2 is the bandwidth at half height and tr is the retention time.
Results and discussion
Morphology of the stationary phase
The SEM images of bare silica particles and ligand bonded silica particles are shown in Fig. 2. SEM images of bare silica particles (A,B) show that these particles are spherical in shape as compared to our previous study where the particles were elongated or having an irregular symmetry. The surface of ligand bonded silica particles (C, D) are smoother than bare silica particles which may be owing to the coating of polystyrene chain over the surface of silica particles.
Figure 2.
Scanning electron microscopic images of bare silica particles (A, B) and ligand bonded silica particles (C, D).
Particle size distribution
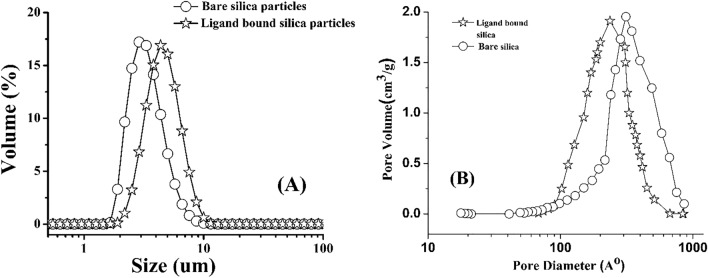
The particle size distribution of bare silica particles and ligand bonded silica particles is shown in Fig. 3(A). The volume-based particle size distribution curves show that the size of silica particles increases after chemical modification (Fig. 3A). The particles size distribution data of silica particles of the current study and previous study are compared in Table 1(A). The volume-based particle size d(0.5) of PMP is 3.36 μm while d(0.5) value of our previous study was 3.05 μm (polystyrene bound silica particles)34. The particle size distribution of this batch is narrow as compared to our previous study34 due to change in the ratio of PEG, urea, TMOS and acetic acid in reaction mixture. The particles size of PMP phase is little bigger than polystyrene bound silica particles phase of our previous study. It means that the surface functionalization of silica particles with styrene only have deposited a polystyrene layer (0.97 µm) over the silica surface while in PMP phase the layer thickness is 1.38 µm.
Figure 3.
Particle size distribution (A) and pore size distribution (B) of bare silica particles and ligand bound silica particles.
Table 1.
Particle size distribution (A) and pore size distribution (B) of bare ligands bound silica particles.
| (A) Particle size distribution | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Bare silica particles | Ligand bound silica particles (Current study) | ligand bound silica particles (Previous study)34 | |||||||
| Particle size (Volume based) | d (0.1) | d (0.5) | d(0.9) | d (0.1) | d (0.5) | d(0.9) | d (0.1) | d(0.5) | d(0.9) |
| 0.96 | 1.46 | 3.01 | 1.65 | 3.36 | 6.27 | 1.56 | 3.05 | 7.62 | |
| (B) Pore size distribution | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pore size | Bare Silica particles | Ligand bound silica particles | |||||||
| Previous studies | Current study | Previous studies | Current study | ||||||
| 40 | 41 | 34 | 40 | 41 | 34 | ||||
| Pore size (Å) | 363 | 296 | 295 | 310 | 303 | 216 | 232 | 241 | |
| Pore volume (cm3/g) | 1.09 | 0.75 | 0.73 | 0.67 | 0.95 | 0.63 | 0.61 | 0.58 | |
| Surface area (m2/g) | 110 | 126 | 124 | 116 | 83 | 115 | 111 | 105 | |
Pore size distribution of bare and ligand bound silica particles
The pore size, pore volume and surface area of silica particles of the current this study are given in Table 1(B). The PSD plots of bare silica particles and ligand bonded silica particles are shown in Fig. 3(B). The results are comparable to our previous study34. The pore size of bare and ligand bound silica particles is 310 Å and 241 Å respectively, which indicated that after chemical modification the pore size decreased by 69 Å as shown in Table 1(B) and the curve’s shift can be seen in Fig. 3(B). Similarly, the pore volume of silica particles decreased from 0.67 to 0.58 cm3/g after chemical modification. The specific surface area of silica particles of current study is 116 m2/g which is comparable to that of our previous study (124 m2/g). The surface area (m2/g) of silica particles also decreased from 116 m2/g to 105 m2/g after chemical modification as shown in Table 1(B).
Characterization of PMP stationary phase
The elemental analysis results of the stationary phase is shown in Table 2. The carbon load of current stationary phase is 6.35%, which is less than those of our previous studies, (polystyrene bonded silica particles, which were 7.93%35 and 10.21 %)42. The carbon load of the current stationary phase is low because some polar ligands such as phenylmaleimide-methylvinylisocyanate (PCMP) and 4-hydroxy-TEMPO were used in addition to styrene in the preparation of current SP. The nitrogen weight % of current stationary phase is 2.21% while that of previous studies were 0.1735 and 0.85%42 respectively. It means that the wt % of nitrogen is high in the current stationary phase owing to phenylmaleimide. Similarly the carbon loads of product (4) and (5) are 2.7% and 2.9% respectively, while the carbon load of final product (6) is 6.35% as shown in Table 2. Thermo gravimetric analysis (TGA) was carried out for PMP stationary phase to check the weight loss, and the TGA curve is shown in Fig. 4. The TGA curve shows the weight loss of 8.6% which is in good agreement with the carbon load (6.35%) since the ligand contains not only C but also N, O, and H.
Table 2.
Elemental analysis of polystyrene bound silica particles.
Figure 4.

TGA of PMP stationary phase, product 5 (a), product 6 (b).
Phenylmaleimide-methylvinylisocyanate ligand was chosen for surface modification of silica particles because it have a polar phenylmaleimide group and vinyl isocyanate group. Vinyl isocyanate group can be further reacted with styrene via living radical polymerization. The second reason was to insert a group which have moderate interaction with analyte and do not have strong electrostatic interactions between analytes and stationary phase since the phenylmaleimide moiety have no virtual charge at normal pH. The polarity of the stationary phase can be controlled by optimum amount of styrene and reaction time of radical polymerization. The last step of reaction (radical polymerization) is very critical which can change the polarity of stationary phase. Elemental analysis were carried out to check the carbon load of these stationary phases. It was observed that increasing the amount of styrene and reaction time increased the carbon load of stationary phase and vice versa. SP prepared with different concentration of styrene have different carbon loads. Similarly these stationary phases were packed in stainless steel column and their chromatographic performance (selectivity, resolution, N values etc.) were checked. Based on these experiments an optimized formulation was selected for preparation of PMP stationary phase to secure both controlled polarity and good analyte retention.
Chromatographic performance of PMP columns
Separation of synthetic peptides
The PMP column was also evaluated for the analysis of five peptides mixture (Gly-Tyr, Gly-Leu-Tyr, Gly-Gly-Tyr-Arg, Tyr-Ile-Gly-Ser-Arg, Leucine Enkephalin) using a mobile phase; 60/40 (v/v) ACN/water (0.1% TFA) at the flow rate of 80 μL/min. Numbers of theoretical plates (N) 20,000 ± 100 per column (100 × 1.8 mm i.d.) were obtained (200,000 plates/m) at optimum elution conditions. The N values of three PMP columns are given in Table 3 and the chromatograms are shown in Fig. 5A. Fast analysis was carried out at high flow rate (700 μL/min) on PMP column and five peptides were eluted within one minute with quite good N value 13,500 ± 330 per column (100 × 1.8 mm i.d.) corresponding to 135,000 plates/m (Fig. 5B). Three columns of the same dimensions (100 × 1.8 mm i.d. were packed from three different batches of PMP stationary phases to check the reproducibility. The same test mixture was separated on each column using optimum elution conditions and the number of theoretical plates N and retention times of analytes were recorded for each column. The reproducibility data of PMP columns are shown in Table 4. There is a good correlation in reproducibility of PMP columns with very low %RSD values as shown in Table 3.
Table 3.
Comparison of number of theoretical plates (N) for PMP column and Ascentis Express RP-Amide column at optimum flow rate, column dimensions (100 × 1.8 mm i.d) and elution condition: ACN: H2O 60:40 (0.1% TFA), flow rate (80 µL/min).
| Peptide | PMP Column current study | Ascentis Express RP-Amide column |
|---|---|---|
| Gly-Tyr | 30,600 ± 100 | 19,500 ± 400 |
| Gly-Leu-Tyr | 30,100 ± 200 | 19,300 ± 500 |
| Gly-Gly-Tyr-Arg | 29,100 ± 250 | 18,300 ± 300 |
| Tyr-Ile-Gly-Ser-Arg | 29,000 ± 300 | 15,200 ± 200 |
| Leucine Enkephalin | 28,100 ± 200 | 15,000 ± 100 |
Figure 5.

Separation of peptides mixture on PMP Column (B) and Ascentis Express RP-Amide column (A); mobile phase 60/40 ACN/ H2O (TFA 0.1%), PMP column dimension (100 × 1.8 mm i.d); The elution order of analytes: 1(Gly-Tyr), 2 (Gly-Leu-Tyr), 3(Gly-Gly-Tyr-Arg), 4 (Tyr-Ile-Gly-Ser-Arg) and 5 (Leucine Enkephalin).
Table 4.
Column to column & day to day reproducibility, N values of three PMP columns at optimum flow rate, column dimensions (150 × 1.8 mm i.d.) and elution condition: ACN: H2O 60:40 (0.1% TFA), flow rate (80 µL/min).
| Analyte | Reproducibility (Batch to batch) | Reproducibility (per days) | ||
|---|---|---|---|---|
| N values | %RSD | N values | %RSD | |
| Gly-Tyr | 30,900 ± 400 | 0.08 | 30,700 ± 100 | 0.10 |
| Gly-Leu-Tyr | 30,800 ± 300 | 0.14 | 30,300 ± 200 | 0.13 |
| Gly-Gly-Tyr-Arg | 30,100 ± 700 | 0.16 | 30,000 ± 400 | 0.16 |
| Tyr-Ile-Gly-Ser-Arg | 29,500 ± 400 | 0.26 | 29,300 ± 100 | 0.20 |
| Leucine Enkephalin | 29,200 ± 300 | 0.33 | 29,000 ± 200 | 0.24 |
Separation of tryptic digest of Human Serum Albumin (HSA)
PMP column (100 × 1.8 mm i.d) was evaluated for the separation of tryptic digest of human serum albumin in high performance liquid chromatography. The chromatogram in Fig. 6 shows that the sample is separated well with quite good resolution. The HSA digest was analyzed using flow rate of 100 µL/min, mobile phase 70/30 acetonitrile/water with 0.1% TFA. The HSA digest has been separated into 17 peaks as shown in the chromatogram (Fig. 6) which corresponds to 17 peptides. The separation efficiency of individual peaks from HSA digest are calculated and the values are given in Table 5.
Figure 6.
Separation of tryptic digest of HSA on PMP column (100 × 1.8 mm i.d); flow rate (100 µL/min), mobile phase 60/40 acetonitrile/ water with 0.1% TFA.
Table 5.
Retention time (Rt) and number of theoretical plates (N) values of peptides in tryptic digest of human serum albumin (HAS). Column: SMP column, column dimensions (50 × 1.8 mm i.d) and elution condition: ACN: H2O 60:40 (0.1% TFA), flow rate (80 µL/min).
| Peak number | Retention time | N value |
|---|---|---|
| 1 | 3.2 | 22,700 |
| 2 | 5.3 | 25,300 |
| 3 | 7 | 15,300 |
| 4 | 7.5 | 20,100 |
| 5 | 12.5 | 18,300 |
| 6 | 18 | 24,700 |
Permeability of PMP column
Column permeability was calculated using the following equation.
| 2 |
where L is the column length, η is the viscosity of the mobile phase, ΔP is the column back pressure and u is mobile phase linear velocity. The permeability of PMP column was 2.5 × 10−14 m2 at 25 μL/min using 60/40 v/v ACN/water. The permeability of the PMP column (100 × 1.8 mm i.d) is similar to that of our previous study Ref.34. The permeability of the columns packed with superficially porous particles were noted to be 1.7 × 10−15 for 1.3 μm particles, 3.1 × 10−15 for 1.7 μm particles, 5.2 × 10−15 for 2.6 μm particles and 2.5 × 10−14 m2 for 5 μm particles43. So, the permeability PMP phase is similar to the permeability of 5 μm core–shell particles.
Porosity of PMP column
Porosity was calculated by the following equation using methanol and chloroform as filling solvents
| 3 |
where Wx is the weight of the column filled with chloroform and Wy is the weight of column filled with methanol, ρ is the density of the solvent. Density of methanol (ρ = 0.7866) and that of chloroform (ρ = 1.484). The total porosity of SILICA PARTICLES -C18 column (100 × 1.8 mm i.d)34 and C18-Urea column31 of our previous study were found to be 0.63 and 0.55 respectively. It means that the presence of urea ligand has decreased the permeability of stationary phase. On the other hand the total porosity of PMP column (100 × 1.8 mm i.d) was 0.60. The permeability PMP column is less than C18 bonded silica particles packed column because in C18 types stationary phases the C18 ligands attached to silica particles in the form of linear chains while in polystyrene types stationary phases relatively thick layer of polymer form around silica particles. In a typical experiment the column porosity was calculated as,
| 4 |
Van Deemter plots
Figure 7A, B shows the van Deemter plot of PMP column (100 × 1.8 mm i.d) and Ascentis Express RP-Amide column (100 × 1.8 mm i.d) respectively using the same elution conditions i.e. 60/40 ACN/H2O with 0.1% TFA. Separation of the selected peptides mixture (Gly-Tyr, Gly-Leu-Tyr, Gly-Gly-Tyr-Arg, Tyr-Ile-Gly-Ser-Arg, Leucine Enkephalin) was carried out at various flow rates ranging from 20 µL/min to 800 µL/min on both columns. The minimum HETP values at optimum flow rate (80 µL/min) for PMP column and Ascentis Express RP-Amide column were found to be 2.6 µm and 3.9 µm respectively. The HETP values shows that the separation efficiency of PMP column (100 × 1.8 mm i.d) is much better than commercially available Ascentis Express RP-Amide column (100 × 1.8 mm i.d). The van Deemter plot in Fig. 7(A) shows that the decrease in N value with increase in flow rate is not significantly high as compared to our previous study34. Better separation efficiency of the PMP column (100 × 1.8 mm i.d) in comparison to Ascentis Express RP-Amide column is based on the improvement in particles shape, size and sophisticated column packing procedure used in the current work34.
Figure 7.

(A) The van Deemter plots (HETP vs mobile phase linear velocity) obtained with PMP column (100 × 1.8 mm i.d) in 60/40 ACN/H2O with 0.1% TFA. (B) The van Deemter plots (HETP vs mobile phase linear velocity) obtained with Ascentis Express RP-Amide column (100 × 1.8 mm i.d) in 60/40 ACN/H2O with 0.1% TFA.
Conclusions
A polar embedded polystyrene stationary phases was prepared and evaluated for the separation of synthetic peptides mixture and tryptic digest of human serum albumin (HAS) in high performance liquid chromatography. The chromatographic performance of PMP column for peptides mixture is excellent in terms of separation efficiency and resolution. The enhanced separation performance of PMP column is owing to several reasons such as particle size and pore size of silica particles, controlled synthesis of the stationary phase and sophisticated column packing. In addition to high separation efficiency, column back pressure was low at high flow rates, which is an additional advantage of this stationary phase. The PMP column exhibited good repeatability and could be applied for the analysis of peptides mixture and tryptic digest of various proteins. We intend to apply this column for the separation of natural products, bioactive compounds from medicinal plants and fungi extracts in liquid chromatography. In future the PMP column will also be evaluated for the separation of proteins and monoclonal antibodies.
Acknowledgements
The authors would like to thank Taif University Researchers Supporting Project number (TURSP-2020/200), Taif University, Taif, Saudi Arabia for supporting this project.
Abbreviations
- PMP
N-phenylmaleimide embedded polystyrene
- RP
Reversed phase
- HILIC
Hydrophilic interaction liquid chromatography
- ODS
Octadecyl-modified silica
- RAFT
Reversible addition fragmentation chain transfer polymerization
- SI-ATRP
Surface initiated-atom transfer radical polymerization
- DBTD
Dibutyltin dichloride
- HAS
Human serum albuim
- PMCP
Phenylmaleimide-methylvinylisocyanate copolymer
Author contributions
A.A. and S.A. wrote the main manuscript text, M.I. & A.I & B.A prepared figures, S.N and F.M revised the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Field JK, Euerby MR, Lau J, Thøgersen H, Petersson P. Investigation into reversed phase chromatography peptide separation systems part I: Development of a protocol for column characterisation. J. Chromatogr. A. 2019;1603:113–129. doi: 10.1016/j.chroma.2019.05.038. [DOI] [PubMed] [Google Scholar]
- 2.Gomes B, Augusto MT, Felício MR, Hollmann A, Franco OL, Gonçalves S, Santos NC. Designing improved active peptides for therapeutic approaches against infectious diseases. Biotechnol. Adv. 2018;36(2):415–429. doi: 10.1016/j.biotechadv.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Vlieghe P, Lisowski V, Martinez J, Khrestchatisky M. Synthetic therapeutic peptides: Science and market. Drug Discov. Today. 2010;15(1–2):40–56. doi: 10.1016/j.drudis.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Xie F, Smith RD, Shen Y. Advanced proteomic liquid chromatography. J. Chromatogr. A. 2012;1261:78–90. doi: 10.1016/j.chroma.2012.06.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu W, Song Q, Cao Y, Zhao Y, Huo H, Wang Y, Song Y, Li J, Tu P. Advanced liquid chromatography-mass spectrometry enables merging widely targeted metabolomics and proteomics. Anal. Chim. Acta. 2019;1069:89–97. doi: 10.1016/j.aca.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Chesnut SM, Salisbury JJ. The role of UHPLC in pharmaceutical development. J. Sep. Sci. 2007;30(8):1183–1190. doi: 10.1002/jssc.200600505. [DOI] [PubMed] [Google Scholar]
- 7.Wu N, Clausen AM. Fundamental and practical aspects of ultrahigh pressure liquid chromatography for fast separations. J. Sep. Sci. 2007;30(8):1167–1182. doi: 10.1002/jssc.200700026. [DOI] [PubMed] [Google Scholar]
- 8.Wren SA, Tchelitcheff P. Use of ultra-performance liquid chromatography in pharmaceutical development. J. Chromatogr. A. 2006;1119(1–2):140–146. doi: 10.1016/j.chroma.2006.02.052. [DOI] [PubMed] [Google Scholar]
- 9.Gu H, Liu Y, Wang L, Zhang B, Yin D, Zhang Q. Monolithic macroporous hydrogels prepared from oil-in-water high internal phase emulsions for high-efficiency purification of Enterovirus 71. Chem. Eng. J. 2020;401:126051. doi: 10.1016/j.cej.2020.126051. [DOI] [Google Scholar]
- 10.Shi Y, Xiang R, Horváth C, Wilkins JA. The role of liquid chromatography in proteomics. J. Chromatogr. A. 2004;1053(1–2):27–36. doi: 10.1016/S0021-9673(04)01204-X. [DOI] [PubMed] [Google Scholar]
- 11.Fekete S, Veuthey J-L, Guillarme D. New trends in reversed-phase liquid chromatographic separations of therapeutic peptides and proteins: Theory and applications. J. Pharm. Biomed. Anal. 2012;69:9–27. doi: 10.1016/j.jpba.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 12.Gilar M, Olivova P, Daly AE, Gebler JC. Two-dimensional separation of peptides using RP-RP-HPLC system with different pH in first and second separation dimensions. J. Sep. Sci. 2005;28(14):1694–1703. doi: 10.1002/jssc.200500116. [DOI] [PubMed] [Google Scholar]
- 13.Felletti S, De Luca C, Lievore G, Pasti L, Chenet T, Mazzoccanti G, Gasparrini F, Cavazzini A, Catani M. Investigation of mass transfer properties and kinetic performance of high-efficiency columns packed with C18 sub-2 μm fully and superficially porous particles. J. Sep. Sci. 2020;43(9–10):1737–1745. doi: 10.1002/jssc.202000041. [DOI] [PubMed] [Google Scholar]
- 14.Piovesana S, Capriotti AL, Cavaliere C, La Barbera G, Montone CM, Zenezini Chiozzi R, Laganà A. Recent trends and analytical challenges in plant bioactive peptide separation, identification and validation. Anal. Bioanal. Chem. 2018;410(15):3425–3444. doi: 10.1007/s00216-018-0852-x. [DOI] [PubMed] [Google Scholar]
- 15.Müller JB, Geyer PE, Colaço AR, Treit PV, Strauss MT, Oroshi M, Doll S, Virreira Winter S, Bader JM, Köhler N, Theis F, Santos A, Mann M. The proteome landscape of the kingdoms of life. Nature. 2020;582(7813):592–596. doi: 10.1038/s41586-020-2402-x. [DOI] [PubMed] [Google Scholar]
- 16.De Luca C, Lievore G, Bozza D, Buratti A, Cavazzini A, Ricci A, Macis M, Cabri W, Felletti S, Catani M. Downstream processing of therapeutic peptides by means of preparative liquid chromatography. Molecules (Basel, Switzerland) 2021;26(15):4688. doi: 10.3390/molecules26154688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Y, Geng X. Mixed-mode chromatography and its applications to biopolymers. J. Chromatogr. A. 2011;1218(49):8813–8825. doi: 10.1016/j.chroma.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Zhao G, Dong X-Y, Sun Y. Ligands for mixed-mode protein chromatography: Principles, characteristics and design. J. Biotechnol. 2009;144(1):3–11. doi: 10.1016/j.jbiotec.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Sýkora D, Řezanka P, Záruba K, Král V. Recent advances in mixed-mode chromatographic stationary phases. J. Sep. Sci. 2019;42(1):89–129. doi: 10.1002/jssc.201801048. [DOI] [PubMed] [Google Scholar]
- 20.Zhao K, Yang L, Wang X, Bai Q, Yang F, Wang F. Preparation of a novel dual-function strong cation exchange/hydrophobic interaction chromatography stationary phase for protein separation. Talanta. 2012;98:86–94. doi: 10.1016/j.talanta.2012.06.050. [DOI] [PubMed] [Google Scholar]
- 21.Zhao K, Yang F, Xia H, Wang F, Song Q, Bai Q. Preparation of a weak anion exchange/hydrophobic interaction dual-function mixed-mode chromatography stationary phase for protein separation using click chemistry. J. Sep. Sci. 2015;38(5):703–710. doi: 10.1002/jssc.201401020. [DOI] [PubMed] [Google Scholar]
- 22.Hoegger D, Freitag R. Investigation of mixed-mode monolithic stationary phases for the analysis of charged amino acids and peptides by capillary electrochromatography. J. Chromatogr. A. 2003;1004(1–2):195–208. doi: 10.1016/S0021-9673(03)00563-6. [DOI] [PubMed] [Google Scholar]
- 23.Liu C, Bults P, Bischoff R, Crommen J, Wang Q, Jiang Z. Separation of deamidated peptides with mixed-mode chromatography using phospholipid-functionalized monolithic stationary phases. J. Chromatogr. A. 2019;1603:417–421. doi: 10.1016/j.chroma.2019.05.053. [DOI] [PubMed] [Google Scholar]
- 24.Kozlik P, Vaclova J, Kalikova K. Mixed-mode hydrophilic interaction/ion-exchange liquid chromatography–Separation potential in peptide analysis. Microchem. J. 2021;165:106158. doi: 10.1016/j.microc.2021.106158. [DOI] [Google Scholar]
- 25.Wang L, Wei W, Xia Z, Jie X, Xia ZZ. Recent advances in materials for stationary phases of mixed-mode high-performance liquid chromatography. TrAC Trends Anal. Chem. 2016;80:495–506. doi: 10.1016/j.trac.2016.04.001. [DOI] [Google Scholar]
- 26.Ali F, Cheong WJ, Rafique A, AlOthman ZA, Sadia M, Muhammad M. Particle packed mixed-mode chromatographic stationary phase for the separation of peptide in liquid chromatography. J. Sep. Sci. 2021;44(7):1430–1439. doi: 10.1002/jssc.202100001. [DOI] [PubMed] [Google Scholar]
- 27.Ray S, Takafuji M, Ihara H. Chromatographic evaluation of a newly designed peptide-silica stationary phase in reverse phase liquid chromatography and hydrophilic interaction liquid chromatography: Mixed mode behavior. J. Chromatogr. A. 2012;1266:43–52. doi: 10.1016/j.chroma.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Progent F, Taverna M, Banco A, Tchapla A, Smadja C. Chromatographic behaviour of peptides on a mixed-mode stationary phase with an embedded charged group by capillary electrochromatography and high-performance liquid chromatography. J. Chromatogr. A. 2006;1136(2):221–225. doi: 10.1016/j.chroma.2006.09.095. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Zhong H, Zhou S, Zhang D, Lu R, Han H, Zhang M, Qiu H. Design and evaluation of polar-embedded stationary phases containing triacontyl group for liquid chromatography. J. Chromatogr. A. 2020;1621:461035. doi: 10.1016/j.chroma.2020.461035. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Zhong H, Zhou S, Han H, Zhang M, Qiu H. A docosyl-terminated polyamine amphiphile-bonded stationary phase for multimodal separations in liquid chromatography. J. Chromatogr. A. 2021;1642:462045. doi: 10.1016/j.chroma.2021.462045. [DOI] [PubMed] [Google Scholar]
- 31.Sun G, Ali A, Kim YS, Kim JS, An HJ, Cheong WJ. An optimized mixed-mode stationary phase based on silica monolith particles for the separation of peptides and proteins in high-performance liquid chromatography. J. Sep. Sci. 2019;42(24):3621–3630. doi: 10.1002/jssc.201900914. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Lu R, Chen M, Zhou S, Zhang D, Han H, Zhang M, Qiu H. A highly efficient acyl-transfer approach to urea-functionalized silanes and their immobilization onto silica gel as stationary phases for liquid chromatography. J. Chromatogr. A. 2020;1626:4613. doi: 10.1016/j.chroma.2020.461366. [DOI] [PubMed] [Google Scholar]
- 33.S.V. Hugh Cramer, Michael Kiselewsky (2011).
- 34.Ali A, Ali F, Cheong WJ. Sedimentation assisted preparation of ground particles of silica monolith and their C18 modification resulting in a chromatographic phase of improved separation efficiency. J. Chromatogr. A. 2017;1525:79–86. doi: 10.1016/j.chroma.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 35.Ali A, Sun G, Kim JS, Cheong WJ. Polystyrene bound silica monolith particles of reduced size as stationary phase of excellent separation efficiency in high performance liquid chromatograhy. J. Chromatogr. A. 2019;1594:72–81. doi: 10.1016/j.chroma.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Han K-M, Cheong W-J. C18 modified monolith silica particles of 3–5 μm. Bull. Korean Chem. Soc. 2008;29(11):2281–2283. doi: 10.5012/bkcs.2008.29.11.2281. [DOI] [Google Scholar]
- 37.Ko JH, Baik YS, Park ST, Cheong WJ. Ground, sieved, and C18 modified monolithic silica particles for packing material of microcolumn high-performance liquid chromatography. J. Chromatogr. A. 2007;1144(2):269–274. doi: 10.1016/j.chroma.2007.01.086. [DOI] [PubMed] [Google Scholar]
- 38.Sun G, Kim JS, Kim YS, An HJ, Cheong WJ. Styrene-N-phenylacrylamide co-polymer modified silica monolith particles with an optimized mixing ratio of monomers as a new stationary phase for the separation of peptides in high performance liquid chromatography. J. Sep. Sci. 2019;42(16):2612–2620. doi: 10.1002/jssc.201900340. [DOI] [PubMed] [Google Scholar]
- 39.Ali A, Cheong WJ. Production of raw and ligand-modified silica monolith particles in an enhanced scale and their application in high performance liquid chromatography. Bull. Korean Chem. Soc. 2017;38(8):919–927. doi: 10.1002/bkcs.11203. [DOI] [Google Scholar]
- 40.Ali F, Cheong WJ. C18-bound porous silica monolith particles as a low-cost high-performance liquid chromatography stationary phase with an excellent chromatographic performance. J. Sep. Sci. 2014;37(23):3426–3434. doi: 10.1002/jssc.201400811. [DOI] [PubMed] [Google Scholar]
- 41.Ali A, Ali F, Cheong WJ. Cheap C18-modified silica monolith particles as HPLC stationary phase of good separation efficiency. Bull. Korean Chem. Soc. 2015;36(6):1733–1736. doi: 10.1002/bkcs.10320. [DOI] [Google Scholar]
- 42.Ali F, Kim YS, Lee JW, Cheong WJ. Catalyst assisted synthesis of initiator attached silica monolith particles via isocyanate-hydroxyl reaction for production of polystyrene bound chromatographic stationary phase of excellent separation efficiency. J. Chromatogr. A. 2014;1324:115–120. doi: 10.1016/j.chroma.2013.11.027. [DOI] [PubMed] [Google Scholar]
- 43.Broeckhoven K, Cabooter D, Desmet G. Kinetic performance comparison of fully and superficially porous particles with sizes ranging between 2.7 μm and 5 μm: Intrinsic evaluation and application to a pharmaceutical test compound. J. Pharm. Anal. 2013;3(5):313–323. doi: 10.1016/j.jpha.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]