Abstract
Background
Long-term, continuous treatment with medication like buprenorphine is the gold standard for opioid use disorder (OUD). As high deductible health plans (HDHPs) become more prevalent in the commercial insurance market, they may pose financial barriers to people with OUD.
Objective
To estimate the impact of HDHPs on continuity of buprenorphine treatment, concurrent visits for counseling/psychotherapy and OUD-related evaluation and management, and out-of-pocket spending.
Design
Difference-in-differences analysis comparing trends in outcomes among enrollees whose employers offer an HDHP (treatment group) to enrollees whose employers never offer an HDHP (comparison group).
Participants
Enrollees with OUD from a national sample of commercial health insurance plans during 2007–2017 who initiate buprenorphine treatment.
Main Measures
Number of days of continuous buprenorphine treatment; probabilities of continuous buprenorphine treatment ≥30, ≥90, ≥180, and ≥365 days; probability of concurrent (i.e., within the same month) behavioral therapy (i.e., counseling or psychotherapy); probability of concurrent OUD-related evaluation and management visits; proportions of buprenorphine treatment episodes with counseling/psychotherapy and evaluation and management visits; and out-of-pocket (OOP) spending on buprenorphine, behavioral therapy, and evaluation and management visits.
Key Results
HDHPs were associated with an average increase of $98 (95% CI: $48, $150) on OOP spending on buprenorphine per treatment episode but no change in the number of days of continuous buprenorphine treatment or concurrent use of related services.
Conclusions
HDHPs do not reduce continuity of buprenorphine treatment among commercially insured enrollees with OUD but may increase financial burden for this population.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11606-021-07094-9.
KEY WORDS: buprenorphine, opioid use disorder, insurance, financing, high deductible health plans
INTRODUCTION
The underuse of FDA-approved medications for opioid use disorder (OUD) in the USA is one of the key obstacles to ameliorating the overdose crisis.1 These medications relieve withdrawal symptoms and cravings that drive continued opioid use.2,3 Randomized controlled trials4,5 and observational research1,6–9 demonstrate that buprenorphine and methadone, the two medications with the strongest evidence base, substantially reduce overdose mortality. Gold standard OUD treatment involves use of one of these medications within a chronic disease framework1 in which long-term medication use is associated with reduced problematic use of opioids and improved health outcomes.9–15 As with other chronic health conditions, changes in financial or insurance status may disrupt treatment; given the risks of opioid overdose, discontinuation of OUD medication can have lethal consequences.3,9,14
Financial barriers can inhibit continuous OUD medication treatment and may be exacerbated by insurance products with higher levels of cost sharing. Over the last two decades, high deductible health plans (HDHPs) have become an increasingly prominent type of insurance product in the commercial market. In 2020, 31% of covered workers were enrolled in HDHPs, a six-fold increase from 2006.16 In exchange for lower monthly premiums, enrollees with HDHPs pay the costs of all plan-covered services out-of-pocket (OOP) until reaching their deductible, at which point the insurer portion of covered services kicks in.
Limited research to date has examined how particular insurance products with different cost-sharing arrangements affect continuity of OUD medication treatment despite the increasing prevalence of HDHPs.17 The literature on HDHPs’ impact on medication use for chronic medical conditions is equivocal.18–20 A recent study focused on enrollees with bipolar disorder found no impact of HDHPs on use of medications or visits with psychiatrists, but did find reductions in non-psychiatrist mental health provider visits.21 Research on the impact of HDHPs on medication treatment for substance use disorder is limited.22 A recent study by our team found that HDHPs are associated with a lower probability of any use of medication for substance use disorder but did not assess the impact of these plans on continuity of medication treatment.23
The confluence of the opioid overdose crisis, the underuse of OUD medications, and increasing HDHP enrollment underscore the critical need for research at this intersection. Given that commercial insurers cover methadone less consistently than buprenorphine24–26 and more commercially insured enrollees with OUD receive treatment with buprenorphine than methadone,27 our analysis concentrates on continuity of buprenorphine treatment specifically. In this study, we seek to understand the impact of HDHPs on the length of time individuals with OUD initiating treatment with buprenorphine continue to receive buprenorphine and out-of-pocket spending (OOP) on buprenorphine. Building on prior research indicating that HDHPs may differentially impact prescriber and non-prescriber visits for behavioral health conditions,21 we also assess the impact of these plans on enrollee use of behavioral therapy (i.e., counseling or psychotherapy visits for substance use treatment) and OUD-related evaluation and management visits, including medication management, as well as OOP spending on these services.
METHODS
Data and Study Groups
We used de-identified data from the OptumLabs® Data Warehouse. These data include enrollment and benefit design information, blinded employer identifiers, and medical, behavioral health (inclusive of mental health and substance use services), and pharmacy claims for the years 2007–2017. The blinded employer identifier was essential to our analytic approach because it allowed us to determine whether enrollees’ employers offered an HDHP, enabling us to code HDHP exposure at the employer level rather than individual level by grouping enrollees offered the same health insurance plan options.
Our inclusion criteria required enrollees to be at an employer that we could categorize as a treatment or comparison employer. Individuals who decide to enroll in HDHPs are different from those who do not across a number of dimensions not observable in administrative claims.28,29 Therefore, rather than directly measuring individual enrollment in an HDHP and producing potentially biased effect estimates due to unmeasured individual confounders, our intent-to-treat-type analysis defines the treatment at the employer level by identifying enrollees at employers that did not offer an HDHP option and then began offering this option during our study period. Our comparison group included enrollees at employers that never offered HDHPs.
We identified employers offering HDHPs through a multi-stage process. First, we limited the analytic sample to individuals aged 12–64 years enrolled for at least 11 months during the calendar year with medical, behavioral health, and pharmacy benefits and no missing benefit design data. Second, we eliminated employers with large fluctuations in number of enrollees (>50% change) from year-to-year to ensure that enrollees were not shifting to plans offered by insurers unobserved in the OptumLabs data. If an HDHP offer led to disproportionate plan switching among enrollees with serious health conditions, like OUD, the analytic sample composition might change after HDHP offer, potentially biasing model estimates. We evaluate this by comparing standardized mean differences between covariates pre-post HDHP offer in the treatment group. In sensitivity analyses, we re-estimated models using analytic samples requiring employer size changes no greater than 25% and no greater than 75% from year-to-year. Third, we identified employers as treatment employers if the percentage of enrollees in an HDHP increased from less than 5% to greater than 5% in consecutive years. We varied the threshold to 3% and 20% in sensitivity analyses. Fourth, we identified comparison employers as those with 0% HDHP enrollment for all years in which the employer is observed in the data. We excluded enrollees at employers always offering an HDHP.
Our unit of analysis was the buprenorphine treatment episode. To identify individuals with OUD, we required at least one claim with a diagnosis of OUD (Appendix)7 during the 60 days prior to initiation of buprenorphine treatment or during the buprenorphine treatment episode. Following other studies examining buprenorphine treatment length, we distinguished initiation of a new buprenorphine treatment episode by requiring no buprenorphine medical administration or pharmacy claims in the 60 days prior to the index claim.10,12,30 We used National Drug Codes (NDC) in pharmacy claims to identify buprenorphine, excluding short-term injectable formulations, and procedure codes in medical claims to identify buprenorphine administration (Appendix).
To ensure that we did not misattribute discontinuation of buprenorphine to loss of insurance coverage, we required continuous enrollment for at least 60 days following the discontinuation of buprenorphine except for those with buprenorphine treatment extending to 365 days or beyond because we did not measure continuous treatment beyond 365 days. We defined discontinuation of buprenorphine treatment as occurring when the individual had no buprenorphine claim within 30 days after the prior buprenorphine prescription days’ supply should have ended based on days’ supply or after the prior medication administration claim indicated treatment would have expired.17,31,32
We applied a wash-out period by excluding buprenorphine treatment episodes that began within the 365 days prior to the date that the enrollee’s employer began offering an HDHP and episodes that overlapped the HDHP offer date. To avoid introducing bias in the length of buprenorphine treatment episodes between the HDHP treatment and comparison groups, we applied equivalent inclusion criteria to both groups. Given that comparison employers did not have an HDHP offer date, we used the pool of HDHP treatment employers’ offer dates to assign artificial offer dates at random to comparison employers. We then excluded buprenorphine treatment episodes at comparison employers that began within 365 days preceding or that overlapped the artificially assigned offer date so that we could apply the same exclusion criteria used for enrollees at HDHP treatment employers. Our analytic sample included 7853 treatment episodes.
Measures
Our primary outcome of interest was the number of continuous days of buprenorphine in the treatment episode. In a sensitivity analysis, we excluded 12.6% of episodes that were 14 days or less to ensure our sample focused on individuals initiating buprenorphine for maintenance treatment rather than withdrawal management. We also examined four separate binary outcomes measuring whether the buprenorphine treatment episode extended beyond 30 days, 90 days, 180 days, or ended at or beyond 365 days. Although a National Quality Forum measure designates 6 months of continuous buprenorphine treatment as a key quality indicator, research suggests that buprenorphine treatment exceeding 6 months produces superior health outcomes.10,12
We also included four non-medication measures of utilization. To determine whether a person received concurrent behavioral therapy during their buprenorphine treatment episode, we identified claims with a diagnosis of substance use disorder (SUD) and a procedure code indicating psychotherapy or counseling.27 We constructed a binary measure of whether an enrollee had any behavioral therapy use during their buprenorphine treatment episode and also calculated the proportion of months in the episode during which they had at least one claim for behavioral therapy. To measure OUD-related evaluation and management visits, we identified claims with a primary or secondary diagnosis of OUD and an evaluation and management (E&M) or medication management procedure code. We then constructed a binary measure of whether any evaluation and management visit occurred during the buprenorphine treatment episode and a measure of the proportion of months in the episode during which the enrollee also had an evaluation and management visit.
We calculated total out-of-pocket spending within each treatment episode separately for each utilization category: buprenorphine medication; behavioral therapy visits; and OUD-related evaluation and management visits.
Covariates included in the difference-in-differences models described below included enrollee age, an indicator for female, an indicator for white race, an indicator of Bachelor’s degree educational attainment or greater (based on Census block characteristics), a 5-category measure of household income (<$40,000, $40,000–$75,000, $75,000–$125,000, $125,000–$200,000, and >$200,000) imputed from consumer data, a 4-category measure of Census region (Northeast, South, Midwest, West), and number of chronic medical conditions (0, 1–2, or 3 or more), calculated using the Chronic Conditions Warehouse.33 Twenty-two percent of buprenorphine treatment episodes were among individuals contributing multiple episodes to our analytic sample; we included a variable to indicate if the episode was one of several for the same person in our analytic dataset. We controlled for employer size and whether the employer was self-insured, meaning that it, rather than the insurer, bore the financial risk of coverage.
Statistical Analysis
We calculated standardized mean differences for all covariates to assess differences between the HDHP treatment and comparison groups (Appendix). Given that most standardized mean differences fell below 0.1 and all fell below 0.2, a standard threshold for assessing good covariate balance across groups,34 we opted not to apply inverse probability of treatment weights. We also examined standardized mean differences in the HDHP offer group between the pre- and post-periods (Appendix) to ensure that the composition of the sample did not change in response to employer offer of HDHP. We used a difference-in-differences approach to compare trends in outcomes pre-post HDHP offer in the HDHP treatment group to trends in the comparison group. We included year fixed effects. Our estimator of interest was the interaction of a variable indicating that the enrollee was at an HDHP offer employer versus a comparison employer with the post- versus pre-HDHP offer indicator variable. The models also controlled for individual-, employer-, and area-level characteristics described above.
Given the right-censoring of our primary outcome, number of continuous days receiving buprenorphine treatment, we estimated tobit models to account for the truncation of this variable at 365 days. For the models with binary outcomes (e.g., ≥30 days of treatment), we estimated generalized linear models specifying a binomial family with log link. To model the proportion outcomes, we estimated fractional logit models. For OOP spending outcomes, we used two-part models in which the first part predicted any OOP spending and the second part predicted spending conditional on any spending.35 These models estimated the impact of HDHP offer on unconditional OOP spending, the product of the two parts. In all models, we adjusted standard errors for clustering within employer. All analyses were conducted in Stata version 16.36 The Johns Hopkins Bloomberg School of Public Health Institutional Review Board approved this study.
RESULTS
Individuals with buprenorphine treatment episodes at employers offering an HDHP were similar to individuals with buprenorphine treatment episodes at comparison employers during the pre- and post-HDHP offer periods (Table 1). The mean age across groups was 34 years and slightly greater than a third of enrollees were female. The majority of treatment episodes were among white enrollees (73%) and residents of the southern Census region (47–54% across groups). Most treatment episodes were among enrollees with zero chronic medical conditions (55–57% across groups).
Table 1.
Characteristics of Individuals with Opioid Use Disorder Treated with Buprenorphine, 2007–2017
| Offered HDHP | Not offered HDHP ‡‡ | |||
|---|---|---|---|---|
| Pre-period | Post-period | Pre-period | Post-period | |
| Age, mean (SD) | 34.8 (11.7) | 34.5 (12.3) | 34.3 (11.8) | 34.3 (12.0) |
| Female (%) | 38.9 | 38.3 | 39.0 | 38.3 |
| Race and ethnicity (%) | ||||
| Asian | 1.0 | 0.8 | 1.6 | 1.4 |
| Black | 7.3 | 7.3 | 6.6 | 5.5 |
| Hispanic | 5.7 | 4.3 | 6.2 | 5.6 |
| White | 73.1 | 72.4 | 73.0 | 72.8 |
| Unknown | 12.9 | 15.1 | 12.6 | 14.6 |
| Census region (%) | ||||
| Northeast | 19.5 | 12.0 | 14.5 | 16.0 |
| South | 48.1 | 54.1 | 47.0 | 48.5 |
| Midwest | 16.1 | 17.3 | 20.0 | 22.2 |
| West | 16.2 | 16.5 | 18.4 | 13.4 |
| Household income (%) | ||||
| < $40,000 | 11.2 | 12.3 | 12.1 | 13.0 |
| ≥$40,000 to < $74,000 | 23.2 | 25.1 | 27.3 | 26.2 |
| ≥ $75,000 to < $124,000 | 29.9 | 29.6 | 28.5 | 29.2 |
| ≥ $125,000 to < $199,000 | 16.6 | 16.2 | 14.4 | 15.0 |
| ≥ $199,000 | 11.9 | 10.1 | 7.8 | 10.0 |
| Unknown | 7.2 | 6.8 | 9.9 | 6.7 |
| Census block education level (%) | ||||
| Bachelor’s or more | 14.8 | 14.9 | 13.3 | 16.0 |
| Less than Bachelor’s or unknown | 85.2 | 85.1 | 86.7 | 84.0 |
| Number of chronic conditions† (%) | ||||
| 0 | 55.2 | 57.4 | 58.5 | 58.5 |
| 1 or 2 | 34.0 | 30.1 | 30.3 | 28.8 |
| 3 or more | 10.8 | 12.5 | 11.2 | 12.7 |
| Employer-level factors | ||||
| Firm size, mean (SD) | 18284 (29021) | 32988 (38791) | 3821 (6139) | 4419 (7206) |
| Self-insured (vs. fully insured) (%) | 49.8 | 59.1 | 32.1 | 39.8 |
| Days of buprenorphine treatment, mean (SD) | 155.0 (143.9) | 133.9 (135.2) | 153.6 (141.4) | 134.7 (136.2) |
| >1 buprenorphine treatment episode (%) | 22.6 | 23.4 | 18.4 | 23.3 |
| Concurrent behavioral therapy use†† (%) | 16.4 | 15.2 | 15.2 | 14.4 |
| Concurrent OUD-related E/M visit ‡ (%) | 54.3 | 50.6 | 53.9 | 49.9 |
| Out-of-pocket (OOP) buprenorphine spending | 293.7 (339.4) | 305.9 (435.9) | 281.5 (306.5) | 218.9 (265.5) |
| Out-of-pocket spending on prescriber visits, mean (SD) | 97.5 (216.5) | 99.2 (224.4) | 88.8 (203.9) | 80.1 (199.9) |
| Out-of-pocket spending on psychotherapy visits, mean (SD) | 44.4 (193.9) | 38.7 (195.4) | 29.6 (135.1) | 44.8 (296.0) |
| N | 1307 | 2864 | 564 | 3118 |
Data from OptumLabs Data Warehouse.
†Number of chronic conditions calculated using the Chronic Condition Warehouse (CCW) software.
††Visits with a primary or secondary diagnosis of substance use disorder and with a procedure code for psychotherapy or counseling.
‡Visits with a primary diagnosis of opioid use disorder and with an evaluation and management or medication management procedure code.
‡‡To assign pre-post dates to the group never offered an HDHP, we assigned pre-post dates at random using the set of pre-post dates among employers offering an HDHP and overlapping in time with comparison employers.
Figure 1 displays the distribution of number of days of buprenorphine treatment across all episodes included in the analytic sample. The mean number of days of buprenorphine treatment was 139 days and the median number of days was 72 with an interquartile range of 30 to 270. Twenty-three percent of treatment episodes were less than 30 days and 69% were less than 180 days (i.e., 6 months). Twenty-one percent of treatment episodes continued for at least 365 days.
Figure 1.
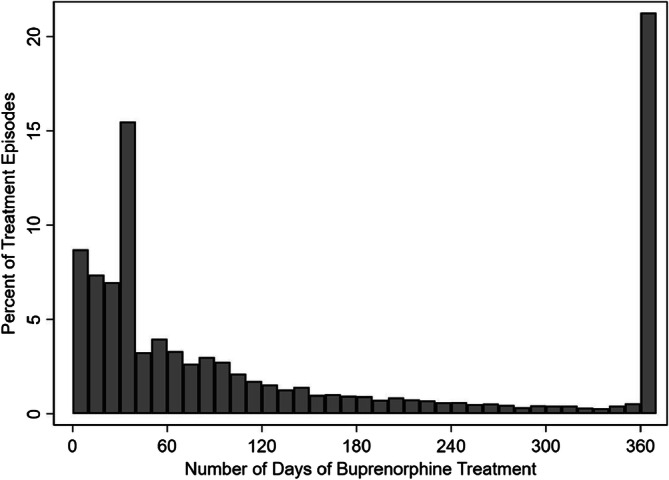
Distribution of number of days of continuous treatment across buprenorphine treatment episodes among enrollees with opioid use disorder. Figure notes: The histogram columns display the distribution of number of days across buprenorphine treatment episodes using 10-day bins. N=7853. Data from OptumLabs Data Warehouse.
We found no impact of HDHP offer on the mean number of days of buprenorphine treatment and no change in the probability of having a buprenorphine treatment episode extending beyond 30 days, 90, days, 180 days, or 365 days (Table 2). We also estimated no change in the probability of having behavioral therapy or evaluation and management visits during the buprenorphine treatment episode attributable to HDHP offer (Table 3).
Table 2.
Impact of High Deductible Health Plan (HDHP) Offer on Length of Buprenorphine Treatment Among Enrollees with Opioid Use Disorder, 2007–2017
| Offered HDHP | Never offered HDHP | Estimated effect attributable to HDHP offer | |||
|---|---|---|---|---|---|
| Pre-period | Post-period | Pre-period | Post-period | ||
| Predicted number of days | Change in number of days (95% CI) | ||||
| Number of days receiving buprenorphine | 176.0 | 149.9 | 173.8 | 150.8 | 2.4 (−19.1, 23.9) |
| Predicted probability | Change in probability (95% CI) | ||||
| Buprenorphine treatment episode ≥30 days | 0.80 | 0.76 | 0.79 | 0.76 | −0.00 (−0.05, 0.05) |
| Buprenorphine treatment episode ≥90 days | 0.49 | 0.44 | 0.51 | 0.44 | 0.02 (−0.04, 0.08) |
| Buprenorphine treatment episode ≥180 days | 0.36 | 0.30 | 0.35 | 0.30 | 0.00 (−0.05, 0.06) |
| Buprenorphine treatment episode ≥365 days | 0.25 | 0.20 | 0.24 | 0.21 | 0.00 (−0.04, 0.05) |
Estimated effect of HDHP offer is the difference in the difference in the number of days of continuous buprenorphine treatment (or in the probability of continuing buprenorphine treatment beyond a certain number of days) before and after HDHP offer in the HDHP offer versus comparison group. N=7853. Data from OptumLabs Data Warehouse.
Table 3.
Impact of High Deductible Health Plan (HDHP) Offer on Related Visits Among Enrollees with Opioid Use Disorder Receiving Buprenorphine Treatment, 2007–2017
| Offered HDHP | Never offered HDHP | Estimated effect attributable to HDHP offer | |||
|---|---|---|---|---|---|
| Pre-period | Post-period | Pre-period | Post-period | ||
| Predicted probability | Change in probability (95% CI) | ||||
| Any receipt of behavioral therapy during buprenorphine treatment | 0.16 | 0.15 | 0.15 | 0.14 | −0.00 (−0.05, 0.04) |
| Any OUD-related E/M visit during buprenorphine treatment | 0.54 | 0.51 | 0.54 | 0.50 | 0.01 (−0.05, 0.08) |
| Predicted proportion | Change in proportion (95% CI) | ||||
| Proportion of months with concurrent behavioral therapy use | 0.09 | 0.08 | 0.07 | 0.08 | −0.02 (−0.05, 0.01) |
| Proportion of months with OUD-related E/M visit | 0.35 | 0.33 | 0.34 | 0.33 | 0.00 (−0.05, 0.05) |
Estimated effect of HDHP offer is the difference in the difference in the probabilities of receipt of behavioral therapy or an OUD-related evaluation and management (E/M) visit (or in the proportion of months with a concurrent behavioral therapy or E/M visit) before and after HDHP offer in the HDHP offer versus comparison groups. Behavioral therapy encompasses any substance use disorder–related psychotherapy or counseling. OUD-related E/M visits encompass visits with a primary diagnosis of OUD and a procedure code for an evaluation and management service or medication management service. N=7853. Data from OptumLabs Data Warehouse.
HDHP offer was associated with an average increase of $98.73 (95% CI: $47.51, $141.95) in OOP spending on buprenorphine medication per treatment episode. We estimated no changes in OOP spending on behavioral therapy or evaluation and management visits associated with HDHP offer (Table 4).
Table 4.
Impact of High Deductible Health Plan (HDHP) Offer on Average Out-of-Pocket Spending per Buprenorphine Treatment Episode Among Enrollees with Opioid Use Disorder, 2007–2017
| Offered HDHP | Never offered HDHP | Estimated effect attributable to HDHP offer | |||
|---|---|---|---|---|---|
| Pre-period | Post-period | Pre-period | Post-period | ||
| Mean out-of-pocket (OOP) spending ($) per treatment episode |
Change in OOP spending ($) (95% CI) |
||||
| OOP spending on buprenorphine | 290.91 | 307.39 | 284.61 | 218.52 |
98.73* (47.51, 149.95) |
| OOP spending on concurrent behavioral therapy | 45.21 | 39.39 | 27.92 | 44.63 |
−19.26 (−41.65, 3.13) |
| OOP spending on OUD-related E/M visits | 96.76 | 99.27 | 90.59 | 80.08 |
13.13 (−13.86, 40.11) |
Estimated effect of HDHP offer is the difference in the difference in average OOP spending per buprenorphine treatment episode on each category of service before and after HDHP offer in the HDHP offer versus comparison groups. Asterisk (*) indicates p-value <0.05. N=7853. Data from OptumLabs Data Warehouse.
Results were robust to varying the thresholds used to identify employers offering HDHP plans and excluding short episodes (≤14 days) of buprenorphine treatment (Appendix).
DISCUSSION
In this difference-in-differences analysis estimating the impact of HDHP offer among commercially insured enrollees, we found that HDHP offer was associated with significantly increased average OOP spending on buprenorphine medication. Given that 43.9% of treatment episodes in our HDHP offer group were among those who enrolled in an HDHP, we estimate that actual HDHP enrollment translated to an average increase of $225 in OOP spending on buprenorphine per treatment episode. Nevertheless, we found no evidence that HDHPs reduced the length of time enrollees with OUD receive continuous buprenorphine treatment nor altered their use of related services, such as behavioral therapy.
This study adds to the limited literature on HDHPs and care for people with substance use disorders. Related work by our team found that HDHPs were associated with lower probability of any use of substance use disorder medication.23 This raises concerns that HDHPs could inhibit initiation of life-saving medications, a serious risk as powerful synthetic opioids like fentanyl escalate the lethality of drug use. Given that most people with OUD do not receive treatment with medication,1 our analysis is limited to the subset who do, a group that may not be generalizable to the broader population of candidates for buprenorphine treatment. Among this group, the present study suggests that even though HDHPs appear to increase the financial burden of continuing buprenorphine treatment, these plans do not negatively affect retention in medication treatment. These findings are surprising yet are also robust to numerous sensitivity analyses. It is possible that people receiving buprenorphine prioritize this medication over other health care services in the face of high deductibles due to the high risks of discontinuing treatment (e.g., recurrence of opioid use, overdose) and the anticipated pain of withdrawal.
No other research has evaluated the impact of HDHPs on continuity of treatment among people with OUD. Recent work focused on commercially insured enrollees with bipolar disorder found no impact of HDHPs on psychiatric medication use but a small reduction in behavioral therapy use.21 An accompanying qualitative study found that enrollees with bipolar disorder facing high cost-sharing intentionally and consciously prioritized their medications, viewing them as essential, and reduced use of non-pharmacologic mental health treatment like psychotherapy, even when they felt those cuts negatively affected their psychiatric health.37 It is possible that people with OUD make similar trade-offs when responding to greater cost-sharing. This study raises questions about what types of services people with OUD may be foregoing in the face of high deductibles to ensure their ability to continue life-saving buprenorphine medication.
The average length of buprenorphine treatment episodes in our analytic sample was somewhat shorter than other recent studies examining buprenorphine treatment in commercially insured populations.31,38–42 These studies estimated between 36 and 50% of buprenorphine treatment episodes lasted at least 6 months31,38–41 relative to 31% in our study. Additionally, in our sample, 21% of treatment episodes lasted a year or longer, while two other commercial claims-based studies estimated about 45% of buprenorphine treatment episodes extended beyond a year.41,42 The relatively shorter duration of treatment episodes in our sample could be due to the population represented in the data or methodological differences in defining discontinuity in treatment.
Findings should be considered in the context of the following set of limitations. First, this study includes a national sample of commercially insured enrollees at large employers. Generalizability may not extend to enrollees at small employers, in individual plans, or in public insurance plans. Second, the analytic approach estimates the effect of being at a firm that offers an HDHP, not the effect of participating in an HDHP, which is inherently conservative because only 43.9% of those at employers offering an HDHP enrolled in an HDHP. Third, given small numbers, we excluded enrollees with OUD receiving methadone or extended-release naltrexone treatment. It is possible that HDHPs differentially affect enrollees receiving other types of pharmacotherapy for OUD. Fourth, we did not measure health outcomes. Future research should examine the extent to which HDHPs impact critical outcomes for people receiving medication treatment for OUD, including overdose.
Although our findings suggest HDHPs do not reduce length of continuous buprenorphine treatment for commercially insured enrollees with OUD, HDHPs do appear to impose a greater financial burden on this group. Future research should examine how HDHPs affect other types of service utilization and health outcomes among commercially insured enrollees with OUD receiving medication treatment.
Supplementary Information
(DOCX 40 kb)
Funding
We gratefully acknowledge funding from the National Institute on Drug Abuse (R01DA044201).
Declarations
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.National Academies of Sciences Engineering and Medicine . Medications for Opioid Use Disorder Save Lives. Washington: The National Academies Press; 2019. [PubMed] [Google Scholar]
- 2.Connery HS. Medication-Assisted Treatment of Opioid Use Disorder. Harv Rev Psychiatry. 2015;23(2):63–75. doi: 10.1097/HRP.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 3.Connery HS, Weiss RD. Discontinuing buprenorphine treatment of opioid use disorder: What do we (not) know? Am J Psychiatry. 2020;177(2):104–106. doi: 10.1176/APPI.AJP.2019.19121245. [DOI] [PubMed] [Google Scholar]
- 4.Mattick R, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;6(2):CD002207. doi: 10.1002/14651858.CD002207.pub4. [DOI] [PubMed] [Google Scholar]
- 5.Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;3:CD002209. doi: 10.1002/14651858.CD002209.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz RP, Gryczynsi J, O’Grady KE, et al. Opioid agonist treatments and heroin overdose deaths in Baltimore, Maryland, 1995-2009. Am J Public Heal. 2013;103(5):917–922. doi: 10.2105/AJPH.2012.301049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wakeman SE, Larochelle MR, Ameli O, et al. Comparative Effectiveness of Different Treatment Pathways for Opioid Use Disorder. JAMA Netw open. 2020;3(2):e1920622. doi: 10.1001/jamanetworkopen.2019.20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: A cohort study. Ann Intern Med. 2018;169(3):137–145. doi: 10.7326/M17-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krawczyk N, Mojtabai R, Stuart EA, et al. Opioid agonist treatment and fatal overdose risk in a state-wide US population receiving opioid use disorder services. Addiction. 2020;115(9). [DOI] [PMC free article] [PubMed]
- 10.Williams AR, Samples H, Crystal S, Olfson M. Acute Care, Prescription Opioid Use, and Overdose Following Discontinuation of Long-Term Buprenorphine Treatment for Opioid Use Disorder. Am J Psychiatry. 2020;177(2):117–124. doi: 10.1176/appi.ajp.2019.19060612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo-Ciganic WH, Gellad WF, Gordon AJ, et al. Association between trajectories of buprenorphine treatment and emergency department and in-patient utilization. Addiction. 2016;111(5):892–902. doi: 10.1111/add.13270. [DOI] [PubMed] [Google Scholar]
- 12.Samples H, Williams AR, Crystal S, Olfson M. Impact Of Long-Term Buprenorphine Treatment On Adverse Health Care Outcomes In Medicaid. Health Aff (Millwood). 2020;39(5):747–755. doi: 10.1377/hlthaff.2019.01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ronquest N, Willson T, Montejano L, Nadipelli V, Wollschlaeger B. Relationship between buprenorphine adherence and relapse, health care utilization and costs in privately and publicly insured patients with opioid use disorder. Subst Abuse Rehabil. 2018;9:59–78. doi: 10.2147/sar.s150253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. doi: 10.1136/bmj.j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiellin DA, Schottenfeld R, Cutter C, Moore B, Barry D, O’Connor P. Primary care-based buprenorphine taper vs maintenance therapy for prescription opioid dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(12):1947–1954. doi: 10.1001/jamainternmed.2014.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaiser Family Foundation. 2020 Employer Health Benefits Survey. https://www.kff.org/health-costs/report/2020-employer-health-benefits-survey/. Published 2020. Accessed October 9, 2020.
- 17.Samples H, Williams AR, Olfson M, Crystal S. Risk factors for discontinuation of buprenorphine treatment for opioid use disorders in a multi-state sample of Medicaid enrollees. J Subst Abuse Treat. 2018;95(September):9–17. doi: 10.1016/j.jsat.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewey J, Gagne JJ, Franklin J, Lauffenburger JC, Brill G, Choudhry NK. Impact of High Deductible Health Plans on Cardiovascular Medication Adherence and Health Disparities. Circ Cardiovasc Qual Outcomes. 2018;11(11):e004632. doi: 10.1161/CIRCOUTCOMES.118.004632. [DOI] [PubMed] [Google Scholar]
- 19.Reiss SK, Ross-Degnan D, Zhang F, Soumerai SB, Zaslavsky AM, Wharam JF. Effect of switching to a high-deductible health plan on use of chronic medications. Health Serv Res. 2011;46(5):1382–1401. doi: 10.1111/j.1475-6773.2011.01252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fendrick AM, Buxbaum JD, Tang Y, et al. Association Between Switching to a High-Deductible Health Plan and Discontinuation of Type 2 Diabetes Treatment. JAMA Netw open. 2019;2(11):e1914372. doi: 10.1001/jamanetworkopen.2019.14372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frank Wharam J, Busch AB, Madden J, et al. Effect of high-deductible insurance on health care use in bipolar disorder. Am J Manag Care. 2020;26(6):248–255. doi: 10.37765/ajmc.2020.43487. [DOI] [PubMed] [Google Scholar]
- 22.Eisenberg MD, Du S, Sen AP, Kennedy-Hendricks A, Barry CL. Health Care Spending by EnrolleesWith Substance Use and Mental Health Disorders in High-Deductible Health Plans vs Traditional Plans. JAMA Psychiatry. 2020;77(8):872–875. doi: 10.4088/JCP.12m08156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisenberg MD, Kennedy-Hendricks A, Schilling CJ, Busch AB, Huskamp HA, Stuart EA, Meiselbach M, Barry CL. The impact of high deductible health plans on service use and spending for substance use disorders. Under review
- 24.Reif S, Creedon TB, Horgan CM, Stewart MT, Garnick DW. Commercial Health Plan Coverage of Selected Treatments for Opioid Use Disorders from 2003 to 2014. J Psychoactive Drugs. 2017;49(2):102–110. doi: 10.1080/02791072.2017.1300360.Commercial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reif S, Horgan CM, Hodgkin D, Matteucci AM, Creedon TB, Stewart MT. Access to Addiction Pharmacotherapy in Private Health Plans. J Subst Abuse Treat. 2016;66:23–29. doi: 10.1016/j.jsat.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polsky D, Arsenault S, Azocar F. Private coverage of methadone in outpatient treatment programs. Psychiatr Serv. 2020;71(3):303–306. doi: 10.1176/appi.ps.201900373. [DOI] [PubMed] [Google Scholar]
- 27.Busch AB, Greenfield SF, Reif S, Normand SLT, Huskamp HA. Outpatient care for opioid use disorder among the commercially insured: Use of medication and psychosocial treatment. J Subst Abuse Treat. 2020;115(May):108040. doi: 10.1016/j.jsat.2020.108040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdus S. The role of plan choice in health care utilization of high-deductible plan enrollees. Heal Serv Res. 2020;55(1):119–127. doi: 10.1111/1475-6773.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kullgren J, Volpp K, Polsky D. Are the healthy behaviors of US high-deductible health plan enrollees driven by people who chose these plans? Smoking as a case study. PLoS One. 2013;8(2):e56154. doi: 10.1371/journal.pone.0056154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manhapra A, Petrakis I, Rosenheck R. Three-year retention in buprenorphine treatment for opioid use disorder nationally in the Veterans Health Administration. Am J Addict. 2017;26(6):572–580. doi: 10.1111/ajad.12553. [DOI] [PubMed] [Google Scholar]
- 31.Meinhofer A, Williams AR, Johnson P, Schackman BR, Bao Y. Prescribing decisions at buprenorphine treatment initiation: Do they matter for treatment discontinuation and adverse opioid-related events? J Subst Abuse Treat. 2019;105(March):37–43. doi: 10.1016/j.jsat.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khemiri A, Kharitonova E, Zah V, Ruby J, Toumi M. Analysis of buprenorphine/naloxone dosing impact on treatment duration, resource use and costs in the treatment of opioid-dependent adults: A retrospective study of US public and private health care claims. Postgrad Med. 2014;126(5):113–120. doi: 10.3810/pgm.2014.09.2805. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Medicare & Medicaid Services. CMS Chronic Conditions Data Warehouse. www2.ccwdata.org. Published 2020. Accessed February 1, 2020.
- 34.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–3679. doi: 10.1002/sim.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belotti F, Deb P, Manning WG, Norton EC. twopm: Two-part models. Stata J. 2015;15(1):3–20. doi: 10.1177/1536867X1501500102. [DOI] [Google Scholar]
- 36.StataCorp. Stata Statistical Software: Release 16. 2019.
- 37.Carroll KM, Weiss RD. The Role of Behavioral Interventions in Buprenorphine Treatment of Opioid Use Disorders. Am J Psychiatry. 2017;174(8):738–747. doi: 10.1176/appi.ajp.2016.16070792.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saloner B, Daubresse M, Alexander GC. Patterns of Buprenorphine-Naloxone Treatment for Opioid Use Disorder in a Multistate Population. Med Care. 2017;55(7):669–676. doi: 10.1097/MLR.0000000000000727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson KE, Saloner B, Eckstein J, et al. Quality of Buprenorphine Care for Insured Adults With Opioid Use Disorder. Med Care. 2021;Publish Ah(5):393-401. 10.1097/mlr.0000000000001530 [DOI] [PMC free article] [PubMed]
- 40.Mark TL, Hinde JM, Zarkin GA, Parish W, Kluckman M. Adherence to buprenorphine treatment guidelines among individuals with an opioid use disorder who have private insurance. J Subst Abuse Treat. 2020;116(May):108062. doi: 10.1016/j.jsat.2020.108062. [DOI] [PubMed] [Google Scholar]
- 41.Agbese E, Leslie DL, Manhapra A, Rosenheck R. Early discontinuation of buprenorphine therapy for opioid use disorder among privately insured adults. Psychiatr Serv. 2020;71(8):779–788. doi: 10.1176/appi.ps.201900309. [DOI] [PubMed] [Google Scholar]
- 42.Manhapra A, Agbese E, Leslie DL, Rosenheck RA. Three-year retention in buprenorphine treatment for opioid use disorder among privately insured adults. Psychiatr Serv. 2018;69(7):768–776. doi: 10.1176/appi.ps.201700363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 40 kb)


