Abstract
Background
Frailty is often cited as a factor influencing oral anticoagulation (OAC) prescription in patients with non-valvular atrial fibrillation (NVAF). We sought to determine the prevalence of frailty and its association with OAC prescription in older veterans with NVAF.
Methods
We used ICD-9 codes in Veterans Affairs (VA) records and Medicare claims data to identify patients with NVAF and CHA2DS2VASC ≥2 receiving care between February 2010 and September 2015. We examined rates of OAC prescription, further stratified by direct oral anticoagulant (DOAC) or vitamin K antagonist (VKA). Participants were characterized into 3 categories: non-frail, pre-frail, and frail based on a validated 30-item EHR-derived frailty index. We examined relations between frailty and OAC receipt; and frailty and type of OAC prescribed in regression models adjusted for factors related to OAC prescription.
Results
Of 308,664 veterans with NVAF and a CHA2DS2VASC score ≥2, 121,839 (39%) were prescribed OAC (73% VKA). The mean age was 77.7 (9.6) years; CHA2DS2VASC and ATRIA scores were 4.6 (1.6) and 5.0 (2.9) respectively. Approximately a third (38%) were frail, another third (32%) were pre-frail, and the remainder were not frail. Veterans prescribed OAC were younger, had higher bleeding risk, and were less likely to be frail than participants not receiving OAC (all p’s<0.001). After adjustment for factors associated with OAC use, pre-frail (OR: 0.89, 95% CI: 0.87–0.91) and frail (OR: 0.66, 95% CI: 0.64–0.68) veterans were significantly less likely to be prescribed OAC than non-frail veterans. Of those prescribed OAC, pre-frail (OR:1.27, 95% CI: 1.22–1.31) and frail (OR: 1.75, 95% CI: 1.67–1.83) veterans were significantly more likely than non-frail veterans to be prescribed a DOAC than a VKA.
Conclusions
There are high rates of frailty among older veterans with NVAF. Frailty using an EHR-derived index is associated with decreased OAC prescription.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11606-021-06834-1.
KEY WORDS: atrial fibrillation, oral anticoagulation, frailty
INTRODUCTION
Anticoagulation (AC) decision-making in older individuals with atrial fibrillation (AF) is complex since age is associated both with an elevated risk for stroke from untreated AF and bleeding complications with oral anticoagulation (OAC).1 In addition to advancing age, stroke risk relates strongly to comorbid conditions that are prevalent among older adults, including hypertension and diabetes.2 Despite the fact that older AF patients are frequently at higher risk for stroke than younger patients, up to 40% of patients above the age of 63 years remain untreated with OAC in national registries.3 Furthermore, for each decade after age 75, 15% lower rates of OAC prescription are observed, suggesting that aging-related factors strongly influence OAC decision-making.3,4 When surveyed about reasons for withholding OAC in older adults, clinicians frequently point to concern for falls and perceived frailty as key factors that influence bleeding risk.3
Frailty is a syndrome associated with aging that results from depleted physiological reserve and is associated with increased vulnerability to illness in the face of stressors.5–8 Since cardiovascular disease can cause multiorgan impairment, it can contribute directly to frailty.9 Whether frailty is associated with OAC prescribing patterns is unclear, with several studies suggesting that frail AF patients are less likely to receive OAC than non-frail patients,10–12 and other studies finding no association between frailty and OAC prescribing.13,14 Furthermore, recent observational studies suggest that older AF patients may have fewer complications if they are treated with direct oral anticoagulants (DOACs) than vitamin K antagonists (VKA),15,16 but associations between frailty and type of OAC prescribed are not well established.
The recent validation of a frailty assessment using structured data in the VA electronic health record enables us to examine relations between frailty and OAC prescribing in a large contemporary cohort of veterans.17 We sought to assess the relations between OAC prescription and frailty among older patients with AF and evaluate the use of VKA vs. DOAC medications in this population.
METHODS
Study Population
This study included VA beneficiaries who had recorded a diagnostic code for non-valvular AF between 2010 and 2015, during an inpatient or ambulatory visit, based on the International Classification of Diseases, Ninth Revision (ICD 9) code 427.31. The study years were selected to begin when DOACs were approved for use and after the adoption of the CHA2DS2VASC risk score for stroke risk classification.18,19 We excluded participants with mitral stenosis, rheumatic heart valve disease, mechanical heart valves, or other indications for anticoagulation such as deep vein thrombosis or pulmonary embolism (Supplementary eTable 1). We restricted the cohort to participants with a CHA2DS2VASC ≥2 to restrict to participants eligible to receive OAC according to the 2011 American College of Cardiology/American Heart Association Consensus Guidelines on the Management of Atrial Fibrillation.19 The study was approved by the Institutional Review Boards of the University of Massachusetts Medical School and the Bedford VA Medical Center, with a waiver of informed consent.
Frailty Status Calculation
Frailty was defined using a validated VA-frailty index (VA-FI) which is based on the cumulative deficit method.17,20 We removed AF from the original VA-FI definition to avoid complete saturation of the cohort with that variable.20 Hence, a total of 30 deficits were used for the calculation (Supplementary eTable 2). The total number of deficits for an individual was counted and divided by 30 (the total number of possible deficits) to give a VA-FI score between 0 and 1. To ensure adequate calculation of frailty, we included relevant claims codes in the same year as AF diagnosis (time of entry into the cohort) and 2 years prior.17 For example, if the AF diagnosis was made first in 2014, we used the period between 2012 and 2014 to query ICD9 codes for frailty status determination. This ensures the capture of deficits even for veterans who only sought care at irregular intervals and allows for diagnoses to drop off rather than perpetually assuming all codes relate to active conditions. For example, if cancer were diagnosed in 2010, treated in 2011, and no longer coded in 2012, it would not enter the frailty index calculation. A similar approach was taken to determine CHA2DS2VASC scores and ATRIA scores at the time of AF diagnosis. Based on consensus in the literature and prior work,21–24,25 we used pre-defined cut points to divide the cohort into three categories based on the frailty index: non-frail (frailty index between 0 and 0.1), pre-frail (frailty index from 0.11 to 0.2), and frail (frailty index > 0.2).
Data Abstraction
Demographic, clinical, treatment, and laboratory characteristics were obtained from the EHR at the time of study enrollment. These data included participants’ age, sex, race, VA entitlement status, comorbidities relevant to stroke and bleeding risk (e.g., diabetes, hypertension, heart failure, anemia, chronic kidney disease), cardiovascular treatments (i.e., use of anti-platelet agents), and total number of medications. Information about key laboratories, including serum creatinine and hemoglobin, was also abstracted from the health record. CHA2DS2VASC and ATRIA bleeding risk scores were calculated based on relevant clinical data in the electronic health record. We also calculated a count of Elixhauser Comorbidities for each patient.20 The electronic health record was queried for any of the following medication names to determine whether the participant was prescribed an OAC: warfarin, coumadin, jantoven, coumarin for VKA, as well dabigatran/Pradaxa, edoxaban/Savaysa, rivaroxaban/Xarelto, eliquis/Apixaban for DOACs. Those patients whose initial OAC prescription occurred within 30 days of an inpatient admission were characterized as inpatient-initiation and others were characterized as having had outpatient-initiation of OAC. Aspirin prescription data was excluded from the analyses since most patients purchase it over the counter and it is not reliably captured in VA data.
Statistical Analysis
We compared the characteristics of the participants with NVAF prescribed OAC vs. those were who were not using analysis of variance (ANOVA) for continuous variables and the χ2 test for categorical variables. Similarly, we compared the characteristics of participants who were prescribed a VKA vs. those prescribed a DOAC. In logistic regression models, we examined the association of frailty with OAC treatment (yes/no) and type (VKA/DOAC) using prespecified covariates that were known to be associated with the prescription of OAC but not included in the frailty index, ATRIA, or CHA2DS2VASC scores.4,10–14,16 We limited the number of covariates in the models to avoid over-fitting. The prespecified covariates included for adjustment in fully adjusted regression models for both prescription of OAC and type of OAC included gender, race, marital status, depression, smoking, heart failure, vascular disease, hypertension, diabetes, intracranial bleeding, stroke, alcohol abuse, VA entitlement, CKD stage, clopidogrel use, medication count, body mass index, hospital admission within the prior 4 weeks, prescription of prasugrel, ticagrelor, statin, beta-blocker, or angiotensin-converting enzyme inhibitor or angiotensin receptor blocker. To analyze how FI correlated with other existing scores in literature, we computed the Pearson correlation coefficient between the frailty score and three existing standard scores: Atria score, Elixhauser Index, and CHA2DS2VASC score. Since our frailty score is between 0 and 1, we normalized the other scores also by diving with the max possible value for each score. All analyses were conducted using SAS, version 9.4 (SAS Corporation, Cary, NC).
RESULTS
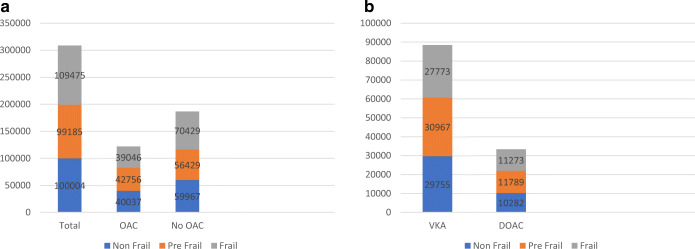
The baseline characteristics of the 308,664 veterans with NVAF included in our sample are shown in Table 1. The mean age of all participants was 77.7 years (SD 9.6) and 2.1% were women. The mean CHA2DS2VASC score was 4.6 (SD 1.6) and the mean ATRIA score was 5 (2.9) (Table 1). Most (86%) participants were white. Cardiovascular and non-cardiovascular comorbidities, including major bleeding (37%), were prevalent among both OAC-treated and untreated veterans with NVAF. Just over one-third of study participants were frail (n=109,475, based on FI>0.2) and an additional one-third were pre-frail (n= 99,185, 0.1>FI>0.2, Fig. 1a). There was a weak correlation among the prespecified pairs i.e., FI-CHA2DS2VASC: 0.45; FI-ATRIA: 0.41; and FI-Elixhauser: 0.44.
Table 1.
Characteristics of Participants with Non-valvular Atrial Fibrillation and Elevated Stroke Risk by Status of Oral Anticoagulation Prescription
| Oral anticoagulant | p value | ||
|---|---|---|---|
| Yes N= 121,839 (39%) |
No N= 186,825 (61%) |
||
| Age, M (SD) | 75.7 (9.1) | 79.0 (9.7) | <.0001 |
| Age category | |||
| <75 years | 59,368 (49) | 63,990 (34) | <.0001 |
| 75–84 years | 34,564 (28) | 53,208 (28) | |
| >=85 years | 27,907 (23) | 69,627 (37) | |
| Female | 21922 | 4324 (2) | <.0001 |
| CHA2DS2VASC score mean (SD) | 4.6 (1.5) | 4.6 (1.6) | 0.12 |
| ATRIA score mean (SD) | 4.7 (2.8) | 5.2 (2.9) | <.0001 |
| Body mass index category | |||
| <25 | 26,909 (22) | 54,771 (29) | |
| 25–30 | 38,496 (32) | 59,771 (32) | |
| 31–35 | 28,411 (23) | 35,874 (19) | |
| >35 | 25,144 (21) | 22,938 (12) | <.0001 |
| Race/ethnicity | |||
| Asian/Hispanic | 15521 | 23681 | <.0001 |
| Black | 10,839 (9) | 13,161 (7) | |
| Native American | 752 (0.6) | 1,141 (0.6) | |
| White | 103,585 (85) | 162,333 (87) | |
| Marital status | |||
| Divorced | 30,304 (25) | 34,852(19) | <.0001 |
| Married | 68,503 (56) | 111,560 (60) | |
| Single | 71 (0.06) | 144 (0.08) | |
| Widowed | 15,715 (13) | 30,470 (16) | |
| Admissions 4 weeks prior | 7315 (6) | 8835 (5) | <.0001 |
| Medical history | |||
| Dementia | 15,774 (13) | 37,492 (20) | <.0001 |
| Depression | 37,632 (31) | 54,923 (29) | <.0001 |
| Current smoker | 32,655 (27) | 40,855 (22) | <.0001 |
| Heart failure | 65,013 (53) | 90,737 (49) | <.0001 |
| Peripheral vascular disease | 28,038 (23) | 36,475 (20) | <.0001 |
| Hypertension | 117,413 (96) | 175,830 (94) | <.0001 |
| Diabetes | 68,651 (56) | 91,988 (49) | <.0001 |
| Liver disease | 31,945 (16) | 18,343 (15) | .01 |
| Major bleeding | 45,068 (37) | 69,598 (37) | 0.33 |
| GI bleeding | 23,646 (19) | 36,961 (20) | 0.036 |
| Intracranial bleeding | 3273 (3) | 6559 (4) | <.0001 |
| Stroke | 32,040 (26) | 47,224 (25) | <.0001 |
| Alcohol abuse | 16,239 (13) | 21,112 (11) | <.0001 |
| Anemia | 51,716 (42) | 91,832 (49) | <.0001 |
| Asthma/COPD | 54,837 (45) | 83,152 (44) | 0.0241 |
| Renal failure | 44,096 (36) | 70,822 (37) | <.0001 |
| Elixhauser Comorbidity Index, mean (SD) | 6.09 (3.3) | 5.30 (3.5) | <.0001 |
| VA entitlement | |||
| Service connected or aid and attendance (no copayment) | 61,229 (50) | 88,185 (47) | <0.001 |
| Non-service connected | 60,504 (50) | 98,305 (53) | <0.001 |
| Laboratory values | |||
| Creatinine (mg/dL), mean (SD) | 1.43 (3.2) | 1.49 (9.0) | 0.26 |
| Hemoglobin (g/DL), mean (SD) | 12.3 (2.6) | 12.0 (2.6) | 0.06 |
| Frailty category | |||
| Non-frail | 40,037(33) | 59,967 (32) | <.0001 |
| Pre-frail | 42,756 (35) | 56,429 (30) | |
| Frail | 39,046 (32) | 70,429 (38) | |
| Region | |||
| Mid-west | 30,133 (25) | 43,565 (23) | <.0001 |
| North east | 16,842 (14) | 34,481 (18) | |
| South | 48,322 (40) | 70,525 (38) | |
| West | 26,542 (22) | 38,254 (20) | |
Note: All values are N (%) unless otherwise noted
Figure 1.
a Breakdown of study participants (n=308,664) by frailty categories based on oral anticoagulation prescription status. b Breakdown of study participants (n=121,839) by frailty categories based on the type of oral anticoagulation prescribed. OAC, oral anticoagulation; VKA, vitamin K antagonist; DOAC, direct oral anticoagulant.
Of the patients prescribed OAC, 96,282 (79%) received their initial OAC prescription in the ambulatory setting whereas 25,557 (21%) prescriptions were initiated in the context of an in-hospital stay (Supplementary eTable 3). Compared to patients with NVAF not prescribed OAC, veterans who were prescribed an OAC were significantly younger had a higher average Elixhauser Comorbidity Index, as well as higher rates of cardiovascular risk factors and diseases, including stroke, heart failure, and diabetes (Table 1). Participants receiving OAC had, on average, a lower ATRIA bleeding risk score, as well as lower rates of GI bleeding, intracranial bleeding, anemia, and bleeding risk factors (i.e., renal failure, Table 1). Notably, stroke risk (CHA2DS2VASC) did not differ by OAC treatment status in our cohort.
Approximately 40% (n=121,839) of veterans with NVAF treated between 2010 and 2015 were prescribed an OAC, out of which 88,495 (71%) were prescribed a VKA and 33,344 (29%) received a DOAC. Figure 1b shows the breakdown of participants by frailty class stratified by type of OAC prescribed. Among those receiving a DOAC, 11,003 (33%) were on apixaban, 13,004 (39%) were on dabigatran, 9336 (28%) were on rivaroxaban, and 12 (<1%) were prescribed edoxaban. Among veterans with NVAF treated with an OAC, those who were prescribed VKA had, on average, a higher ATRIA bleeding risk score, higher rates of anemia and renal failure, and a higher Elixhauser comorbidity than did veterans with NVAF who were prescribed a DOAC (Table 2). Those prescribed a VKA also had significantly higher rates of major bleeding, intracranial bleeding, and gastrointestinal bleeding than did NVAF patients treated with a DOAC. Stroke risk did not differ by type of OAC prescribed.
Table 2.
Characteristics of Participants with Non-valvular Atrial Fibrillation and Elevated Stroke Risk by Types of Oral Anticoagulant
| Warfarin N=88,495 (71%) |
Direct oral anticoagulant N= 33,344 (29%) |
p value | |
|---|---|---|---|
| Age, M (SD) | 75.6 (9.2) | 75.9 (8.7) | <.0001 |
| Age category | |||
| <75 years | 43,155 (49) | 16,213 (49) | 0.005 |
| 75–84 years | 24,908 (28) | 9656 (29) | |
| >=85 years | 20,432 (23) | 7475 (22) | |
| Female | 15892 | 603 (2) | 0.88 |
| CHA2DS2VASC score (M, SD) | 4.69 (1.5) | 4.42 (1.6) | 0.12 |
| ATRIA score (M, SD) | 4.84 (2.9) | 4.27 (2.7) | <.0001 |
| Body mass index category | |||
| <25 | 19,873 (23) | 7036 (20) | |
| 25–30 | 26,404 (31) | 12,092 (34) | |
| 31–35 | 19,495 (23) | 8916 (25) | |
| >35 | 17,980 (21) | 7,164 (20) | <.0001 |
| Race/ethnicity | |||
| Asian/Hispanic | 11371 | 415 (1) | |
| Black | 8665 (10) | 21746 | |
| Native American | 561 (0.6) | 191 (1) | |
| White | 74,319 (84) | 29,266 (88) | <.0001 |
| Marital status | |||
| Divorced | 23,287 (26) | 7017 (21) | <.0001 |
| Married | 47,546 (54) | 20,957 (63) | |
| Single | 54 (0.06) | 17 (0.05) | |
| Widowed | 12,069 (14) | 3646 (11) | |
| Admission 4 weeks prior | 5153 (6) | 21626 | <.0001 |
| Medical history | |||
| Dementia | 11,866 (14) | 3908 (11) | <.0001 |
| Depression | 12,193(14) | 3581 (11) | <.0001 |
| Current smoker | 24,741 (28) | 7914 (24) | <.0001 |
| Heart failure | 50,198 (57) | 14,815 (44) | <.0001 |
| Peripheral vascular disease | 22,139 (25) | 5899 (18) | <.0001 |
| Hypertension | 85,236 (96) | 32,177 (97) | 0.13 |
| Diabetes | 51,354 (58) | 17,297(52) | <.0001 |
| Liver disease | 13,354(15) | 4989(15) | 0.5778 |
| Major bleeding | 33,833 (38) | 11,235 (34) | <.0001 |
| GI bleeding | 17,853 (20) | 5793 (17) | <.0001 |
| Intracranial bleeding | 26723 | 601 (2) | <.0001 |
| Stroke | 24,262 (27) | 7778 (23) | <.0001 |
| Alcohol abuse | 11,974 (14) | 4265 (13) | 0.0007 |
| Anemia | 39,237 (44) | 12,479 (37) | <.0001 |
| Asthma/COPD | 41,441 (47) | 13,396 (40) | <.0001 |
| Renal failure | 34,555 (39) | 9541 (29) | <.0001 |
| Elixhauser Comorbidity Index, mean (SD) | 6.58 (3.3) | 4.90 (3.2) | <.0001 |
| VA entitlement | |||
| Service connected or aid and attendance (no copayment) | 61,229 (50) | 88,185 (47) | <0.0001 |
| Non-service connected | 60,504 (50) | 98,305 (53) | <0.0001 |
| Creatinine (mg/dL), mean (SD) | 1.50 (2.7) | 1.24 (4.1) | 0.27 |
| Hemoglobin (g/DL), mean (SD) | 12.3 (2.6) | 12.5 (2.6) | 0.0006 |
| Frailty category | |||
| Non-frail | 29,755 (34) | 10,282 (31) | <.0001 |
| Pre-frail | 30,967(35) | 11,789 (35) | |
| Frail | 27,773 (31) | 11,273 (34) | |
| Region | |||
| Mid-west | 21,723 (25) | 8410 (25) | 0.0002 |
| North east | 12,113 (14) | 4729 (14) | |
| South | 35,146 (40) | 13,176 (40) | |
| West | 19,513 (22) | 7029 (21) | |
Note: All values are N (%) unless otherwise noted
In the fully adjusted models, pre-frail (OR: 0.89; 95% CI: 0.87, 0.91) and frail (OR: 0.66; 95% CI: 0.64, 0.68) patients with NV AF were significantly less likely to be prescribed an OAC than were non-frail NVAF patients (Table 3). Among patients with NVAF who were prescribed an OAC, pre-frail (OR: 1.27; 95% CI: 1.22, 1.31) and frail (OR: 1.75; 95% CI: 1.67, 1.83) patients were significantly more likely than non-frail patients to be prescribed a DOAC as opposed to VKA (Table 4).
Table 3.
Association Between Frailty Status and Prescription of Oral Anticoagulation, among Those with Non-valvular Atrial Fibrillation (N = 308664)
| Frailty status | N | Percent on oral anticoagulation | Odds ratio (95% confidence interval) for anticoagulation use | |
|---|---|---|---|---|
| Crude | Adjusted* | |||
| Non-frail | 100,004 | 40 | Reference | Reference |
| Pre-frail | 99,185 | 43 | 1.14 (1.12, 1.16) | 0.89 (0.87, 0.91) |
| Frail | 109,475 | 37 | 0.83 (0.82, 0.85) | 0.66 (0.64, 0.68) |
*Model adjusted for gender, race, marital status, depression, current smoker, heart failure, vascular disease, hypertension, diabetes, intracranial bleeding, GI bleeding, anemia, stroke, alcohol abuse, VA entitlement, CKD stage, medication count, BMI, admission 4 weeks prior, clopidogrel, prasugrel, ticagrelor, statin, beta blocker, and ACEI/ARB. Values in Bold indicate p<0.05
Table 4.
Association between Frailty Status and Use of DOAC vs. Warfarin, among Those Receiving OAC (N=121839)
| Frail status | Number of patients | Percent on DOAC | Odds ratio (95% confidence interval) for DOAC | |
|---|---|---|---|---|
| Crude | Adjusted* | |||
| Non-frail | 40,037 | 26 | Reference | Reference |
| Pre-frail | 42,756 | 28 | 1.12 (1.08,1.15) | 1.27 (1.22, 1.31) |
| Frail | 39,046 | 29 | 1.25 (1.21, 1.29) | 1.75 (1.67, 1.83) |
*Model adjusted for gender, race, marital status, depression, current smoker, heart failure, vascular disease, hypertension, diabetes, intracranial bleeding, GI bleeding, anemia, stroke, alcohol abuse, VA entitlement, CKD stage, medication count, BMI, admission 4 weeks prior, clopidogrel, prasugrel, ticagrelor, statin, beta blocker, and ACEI/ARB. Values in Bold indicate p<0.05
DISCUSSION
In this well-characterized cohort of VA beneficiaries with NVAF and a CHA2DS2VASC stroke risk score ≥2, we applied a validated frailty index which incorporates variables from the electronic medical records. We found that two-thirds of older veterans with NVAF were either pre-frail or frail and that these vulnerable patients were significantly less likely to be prescribed an OAC as compared to their non-frail counterparts. Among NVAF patients who were prescribed an OAC, pre-frail and frail status were associated with higher odds of being prescribed a DOAC than a VKA agent.
In this cohort, we found a high prevalence and severity of frailty akin to that reported previously in populations with cardiovascular disease (10–60%), including among prior studies of veterans.9–14 It has been reported that compared to the general American population, Veterans tend to have a higher incidence of medical comorbidities such as hypertension, diabetes, cancer, and mental and cognitive deficits such as post-traumatic stress disorder and depression.26,27,28 Half of our cohort had heart failure, majority had hypertension, half had diabetes, and about one-fifth had dementia, which is higher than reported in other cohorts, and consistent with VA data.11,13,27 We found a weak correlation between FI and other contemporary indices for stroke prediction (CHA2DS2VASC score), bleeding (ATRIA score), and comorbidity (Elixhauser comorbidity index). We believe this; although these indices share some common variables, other clinically important variables such as dementia, incontinence, auditory and vision deficits, use of durable medical equipment, etc. that are included in the FI are not part of the other indices suggesting that FI is a more comprehensive assessment tool.
Furthermore, we observed low rates (39%) of AC prescription among those with NVAF and a CHA2DS2VASC risk score ≥2. Although rates of OAC use are increasing nationally,18 our findings are consistent with other contemporary studies outside of the VA showing relatively low rates of OAC use among older NVAF (< 60%).4,11,29 It is possible, but unlikely, that we underestimated the true rate of DOAC prescription (i.e., OAC prescribed outside of the VA health system) since the copayment for medications at VA is a flat $9 per month for any medication, which would remove the barrier of cost for DOACs within VA pharmacy. With such a low copayment inside the VA system, the prospect of a dually eligible patient receiving a DOAC outside the VA seems unlikely.
To our knowledge, this is the largest study evaluating the association of frailty with OAC prescription in patients with NVAF. Our data suggest that frail veterans are less likely to be prescribed OAC than non-frail veterans. This is consistent with previous studies, including a cross-sectional study of 682 older AF patients, where frailty (defined based on a clinical frailty scale) was associated with threefold lower odds of OAC prescription.12 In a meta-analysis of 6 studies, the authors showed that frailty was associated with lower OAC prescription at hospital admission but not at discharge whereas a community-dwelling study showed increased OAC prescription with frailty.10 Similarly, in an analysis of 9749 patients from the ORBIT-AF registry, both frailty and cognitive impairment were associated with a significantly lower likelihood of AC prescription.11 However, several other studies show no association between frailty status and use of OAC in NVAF patients.13,14 Notably, the methods used to define frailty varied across the studies, including the cardiovascular health study frailty scale and the Edmonton frailty scale. To the best of our knowledge, our investigation is the only study to date to employ a digital approach to assess frailty in NVAF patients and relate it to OAC prescribing. Importantly, the frailty index is a global measure of physical disability, falls, deficits in cognition, and other aging-related domains than other individual comorbidity indices such as cognition, memory, and gait speed. One of the reasons for these discrepant findings may be the variable rates of OAC prescribing observed across the studies, as low as 17% in a nursing home cohort30 whereas, in other contemporary ambulatory cohorts, up to 85% are prescribed OAC.13
Despite the fact that DOACs were FDA approved for NVAF near the onset of our cohort, the high prevalence of renal dysfunction, and high rates of polypharmacy observed in our cohort, DOACs were prescribed at similar rates compared to other contemporary NVAF cohorts.13 This may be aided by the fact that the copayment for DOACs in VA was relatively low ($9/month). Furthermore, we found that frail veterans with NVAF were more likely to be prescribed a DOAC than warfarin. Since the FI includes geriatric conditions, including mobility issues (i.e., the use of a walker), clinicians may favor DOACs because they have fewer burdensome issues than warfarin (i.e., no need for INR draws), less dietary restrictions, and improved compliance. Since the first DOAC was approved in 2010, there has been an increased utilization of DOACs and a decrease in VKA use.18,31 In a post hoc analysis from the ARISTOTLE trial where patients were characterized based on multi-morbidity, the safety and efficacy of apixaban were preserved in the group with high multi-morbidity,32 suggesting that these patients may be eligible for DOACs.
Our study also has several limitations. We included only veterans, which limits generalizability. The participants were mostly white men, which also limits the application of these findings to women and people of other races and ethnicities. The frailty index evenly weights 30 diagnoses, which may not accurately reflect the full degree of disability of a participant. For example, stroke may be more disabling than anemia and may be considered by healthcare providers when they prescribe OAC therapy. However, the cumulative deficit theory of frailty posits that if one morbidity is significantly impacting other body systems and frailty domains, these will be counted, such that the FI weights itself.33 Certain conditions, such as dementia and depression, are known to be under-reported in the medical record and participants with cognitive impairment or depressive symptoms may have not been characterized accurately by relying on EHR diagnoses. In our models, we included items that were also in the FI, which may introduce endogeneity. However, other studies of frailty have suggested that the aggregate FI value is more important than the individual components, and when compared to a phenotype definition, results are similar.34 We were not able to characterize switching from one type of OAC to another during the study timeframe which may have influenced our findings. Characterization of OAC initiation as inpatient vs. outpatient may be inaccurate as there may be misclassification of patients who may have had their OAC initiated at non-VA hospitals. Frailty may have a linear correlation with OAC prescription and perhaps with outcomes in AF patients. Our study looked at the relation with absolute presence or absence of frailty (based on the FI) with OAC prescription rather than stratifying frailty into arbitrary sub-groups. This was done to define a clinically meaningful measure (frail vs. non-frail) which can be used in future studies to enhance risk stratification and clinical outcomes. Lastly, although our cohort is in line with other contemporary data reported in the literature, we acknowledge that we restricted participants to those who had the diagnosis of AF prior to 2015. These data may not accurately reflect OAC prescription practices since 2015.
Conclusion
Our findings suggest that a frailty index calculated from electronic health records was associated with lower odds of OAC prescription and higher odds of receiving a DOAC vs. a VKA, suggesting that frailty impacts NVAF care and that an electronic risk score may correlate with clinician estimates of frailty. Further research is needed to determine if frailty relates to outcomes among patients with NVAF and whether the addition of an objective measure of frailty to contemporary stroke and bleeding risk scores can enhance outcome risk prediction.
Supplementary Information
(DOCX 29 kb)
Funding
This manuscript was supported by grants R01HL137794 and R01HL125089 from the National Heart, Lung, and Blood Institute. DDM’s time was also supported by grants R01HL126911, R01HL13660, and R01HL141434, also from the National Heart, Lung, and Blood Institute. HY’s time was also supported by grants R01DA045816,R01HL125089, 1R01MH125027, R01HL137794, R01HL135219, and R01LM012817. ARO is supported by National Heart, Lung, and Blood Institure grant 5R01HL137794-02 VA CSR&D CDA-2 IK2CX001800-01A1 and National Institute on Aging grant R03-AG060169. AK's time was supported by National Heart, Lung, and Blood institute grant R01HL137794-01A1. Support for VA/CMS data is provided by the Department of Veterans Affairs, VA Health Services Research and Development Service, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004).
Declarations
Conflict of Interest
DDM has received research grant support from Apple Computer, Bristol-Myers Squibb, Boehringer-Ingelheim, Pfizer, Flexcon, Samsung, Philips Healthcare, Biotronik, has received consultancy fees from Bristol-Myers Squibb, Pfizer, Flexcon, Boston Biomedical Associates, Avania. AK has received research grant support from Pfizer and Bristol-Myers Squibb.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke Statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg BA, Kim S, Thomas L, et al. Lack of concordance between empirical scores and physician assessments of stroke and bleeding risk in atrial fibrillation: results from the outcomes registry for better informed treatment of atrial fibrillation (ORBIT-AF) registry. Circulation. 2014;129(20):2005–2012. doi: 10.1161/CIRCULATIONAHA.114.008643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GY. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010;123(7):638–645. doi: 10.1016/j.amjmed.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 5.Clegg A, Young J, Lliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–62. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried LP, Tangen CM, Watson J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M 146–56. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 7.Rockwood K. Conceptual model of frailty: accumulation of deficits. Can J Cardiol. 2016;32(9):1046–1050. doi: 10.1016/j.cjca.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 8.Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm: issues and controversies. J Gerontol A Biol Sci Med Sci. 2007;62:731–7. doi: 10.1093/gerona/62.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. 2014;63(8):747–62. doi: 10.1016/j.jacc.2013.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilkinson C, Todd O, Clegg A, Gale CP, Hall M. Management of atrial fibrillation for older people with frailty: a systemic review and meta-analysis. Age and ageing. 2019;48:196–203. doi: 10.1093/ageing/afy180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madhavan M, Holmes DN, Piccini JP, et al. Association of frailty and cognitive impairment with benefits of oral anticoagulation in patients with atrial fibrillation. Am Heart J. 2019;211:77–89. doi: 10.1016/j.ahj.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Lefebvre MD, St-Onge M, Glazer-Cavanagh M, et al. The effect of bleeding risk and frailty status on anticoagulation patterns in octogenarians with atrial fibrillation: the FRAIL-AF study. Canadian Journal of Cardiology. 2016;32(2):169–176. doi: 10.1016/j.cjca.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Saczynski JS, Sanghai SR, Kiefe CI, et al. Geriatric elements and Oral anticoagulant prescribing in older atrial fibrillation patients: SAGE-AF. J Am Geriatr Soc. 2009;00:1–8. doi: 10.1111/jgs.16178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen TN, Cumming RG, Hilmer SN. Atrial fibrillation in older inpatients: are thereany differences in clinical characteristics and pharmacological treatment between the frail and the non-frail? Intern Med J. 2016;46(1):86–95. doi: 10.1111/imj.12912. [DOI] [PubMed] [Google Scholar]
- 15.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 16.Makam RCP, Hoaglin DC, McManus DD, et al. Efficacy and safety of direct oral anticoagulants approved for cardiovascular indications: systematic review and meta-analysis. PLoS ONE. 2018;13:e0197583. doi: 10.1371/journal.pone.0197583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orkaby AR, Nussbaum L, Ho YK et al. The Burden of Frailty Among U.S. Veterans and Its Association With Mortality, 2002-2012. J Gerontol A Biol Sci Med Sci. 2018. doi: 10.1093/gerona/gly232 [DOI] [PMC free article] [PubMed]
- 18.Barnes GD, Lucas E, Alexander GC, Goldberger ZD. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:1300–130. doi: 10.1016/j.amjmed.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.2011 Writing Group Members. Wann LS, Curtis AB, January CT, Ellenbogen KA, et al. 2011 ACCF/AHA/HRS Focused Update on the Management of Patients With Atrial Fibrillation (Updating the 2006 Guideline): A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:104–123. doi: 10.1161/CIR.0b013e3181fa3cf4. [DOI] [PubMed] [Google Scholar]
- 20.Searle SD, Mitnitski A, Gahbauer EA, et al. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626–633. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 22.Pajewski NM, Williamson JD, Applegate WB, et al. Characterizing frailty status in the Systolic Blood Pressure prevention trial. J Gerontol A Biol Sci Med Sci. 2016;71:649–655. doi: 10.1093/gerona/glv228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc. 2013;61:1537–1551. doi: 10.1111/jgs.12420. [DOI] [PubMed] [Google Scholar]
- 24.Rockwood K, Song X, Mitnitski A. Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian national population health survey. CMAJ. 2011;183(8):E487–94. doi: 10.1503/cmaj.101271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selim AJ, Berlowitz DR, Fincke G, et al. The health status of elderly veteran enrollees in the Veterans Health Administration. J Am Geriatr Soc. 2004;52(8):1271–6. doi: 10.1111/j.1532-5415.2004.52355.x. [DOI] [PubMed] [Google Scholar]
- 26.Eibner C, Krull H, Brown KM, et al. Current and Projected Characteristics and Unique Health Care Needs of the Patient Population Served by the Department of Veterans Affairs. RAND health. 2016;5(4):13. doi: 10.1097/MLR.0b013e31827da95a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waldo AL, Becker RC, Tapson VF, et al. Hospitalized patients with atrial fibrillation and a high risk of stroke are not being provided with adequate anticoagulation. J Am Coll Cardiol. 2005;46(9):1729–36. doi: 10.1016/j.jacc.2005.06.077. [DOI] [PubMed] [Google Scholar]
- 28.O’Caoimh R, Igras E, Ramesh A, Power B, O’Connor K, Liston R. Assessing the appropriateness of oral anticoagulation for atrial fibrillation in advanced frailty: the use of stroke and bleeding risk-prediction models. J Frailty Aging. 2017;6:46–52. doi: 10.14283/jfa.2016.118. [DOI] [PubMed] [Google Scholar]
- 29.Alalwan AA, Voils SA, Hartzema AG. Trends in utilization of warfarin and direct oral anticoagulants in older adult patients with atrial fibrillation. Am J Health Syst Pharm. 2017;74(16):1237–1244. doi: 10.2146/ajhp160756. [DOI] [PubMed] [Google Scholar]
- 30.Alexander P, Brouwer MA, Mulder H, et al. Outcomes of apixaban versus warfarin in patients with atrial fibrillation and multi-morbidity Insights from the ARISTOTLE trial. Am Heart J. 2019;208:123–131. doi: 10.1016/j.ahj.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 31.Mitnitksi A, Song X, Rockwood K. Assessing biological aging: the origin of deficit accumulation. Biogerontology. 2013;14(6):709–717. doi: 10.1007/s10522-013-9446-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orkaby A, Lunetta KL, Sun FJ et al. Cross-sectional association of frailty and arterial stiffness in community-dwelling older adults: The Framingham Heart Study. The Journals of Gerontology 2018; 73(3): 376-379. 10.1093/gerona/gly134 [DOI] [PMC free article] [PubMed]
- 33.Hoover M, Rotermann M, Sanmartin C, Bernier J. Validation of an index to estimate the prevalence of frailty among community-dwelling seniors. Health Rep. 2013;24(9):10–7. [PubMed] [Google Scholar]
- 34.Orkaby AR, Hshieh TT, Gaziano JM, Djousse L, Driver JA. Comparison of two frailty indices in the physicians' health study. Arch Gerontol Geriatr. 2017;71:21–7. doi: 10.1016/j.archger.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 29 kb)