Abstract
Pallister-Hall syndrome, typically caused by germline or de novo variants within the GLI3 gene, has key features of hypothalamic hamartoma and polydactyly. Recently, a few similar cases have been described with bi-allelic SMO variants. We describe two siblings born to non-consanguineous unaffected parents presenting with hypothalamic hamartoma, post-axial polydactyly, microcephaly amongst other developmental anomalies. Previous clinical diagnostic exome analysis had excluded a pathogenic variant in GLI3. We performed exome sequencing re-analysis and identified bi-allelic SMO variants including a missense and synonymous variant in both affected siblings. We functionally characterised this synonymous variant showing it induces exon 8 skipping within the SMO transcript. Our results confirm bi-allelic SMO variants as an uncommon cause of Pallister-Hall syndrome and describe a novel exon-skipping mechanism, expanding the molecular architecture of this new clinico-molecular disorder.
Subject terms: Disease genetics, Disease genetics, Genetics of the nervous system
Introduction
Hypothalamic hamartoma is a benign congenital lesion of the hypothalamus that is associated with a refractory gelastic epilepsy of early life. The prevalence of hypothalamic hamartoma is estimated to be between 1 in 50,000 to 100,000 people [1]. Hypothalamic hamartoma can be non-syndromic or occur as a feature of several disorders including Pallister-Hall (PHS) and Oral-Facial-Digital VI (OFD VI) syndromes [2]. PHS, clinically characterized by hypothalamic hamartoma and polydactyly [2], is predominately familial and usually arises as a consequence of germline or de novo variants within the GLI3 gene [3, 4]. OFD VI is a recessively inherited condition, where hypothalamic hamartomas are seen in about 25% of cases [5], that is most commonly associated with compound heterozygous variants in the cilia gene CPLANE1 [6]. Very recently, three patients with hypothalamic hamartoma, polydactyly and other developmental anomalies have been reported with bi-allelic germline SMO variants [7, 8].
Here we describe phenotypic and genetic analyses of two siblings with hypothalamic hamartoma, post-axial polydactyly, microcephaly, and shortening of the long bones.
Methods
Please refer to the Supplementary Materials for sample collection, bioinformatics, and molecular analyses methodologies.
Results
Case histories
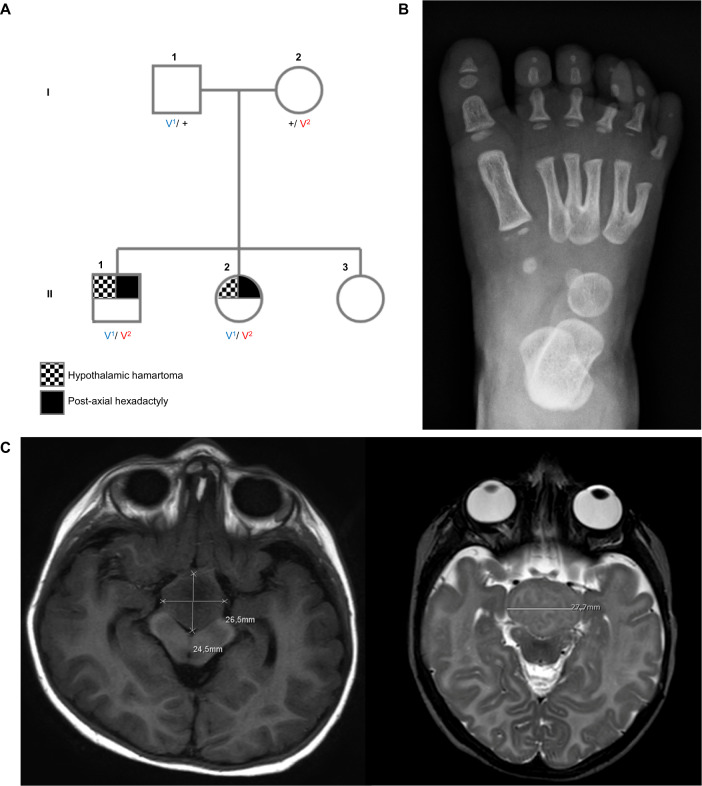
Two siblings from a non-consanguineous Italian family (Fig. 1A) were assessed because of multiple developmental anomalies. The male proband (II-1) was the product of a normal pregnancy and birth with normal Apgar scores. He was small at birth (height, weight, head circumference all < 3rd centile) and at last assessment, aged 7 years and 9 months height (112 cm; −2.94 SD), weight (21.7 kg; −1.37 SD) and head circumference (46.5 cm; −4.8 SD) were all low. He was dysmorphic with plagiobrachycephalus, deep-set eyes, retrognathia, hexadactyly of both feet (Fig. 1B) and a small skin appendage on the right hand, hypospadias with circular prepuce and penile shaft curvature. He had severe motor, speech and cognitive delay. He began to walk at age 5 years and at 7 years spoke only a few single words. Ultrasonography and MRI showed a large suprasellar hypothalamic lesion (Type IIb of Valdueza [9]; See Fig. 1C). At the age of 11 months, he developed central precocious puberty and the GnRH analogue leuprolide was given.
Fig. 1. Pedigree and clinical imaging.
A Pedigree of family. V1: SMO c.1199 G > A; p.Arg400His, V2: SMO c.1416 C > T; p.Tyr472Tyr B Right foot X-ray of the proband (II-1) at age 12 months showing post-axial polydactyly. C Axial MRI brain image of the male proband (II-1) at age 2 years showing a 2.5 cm diameter hypothalamic lesion extending into the interpeduncular fossa (left). Axial MRI brain image of his younger female sibling (II-2) at age 2 months showing a similar large hypothalamic lesion (right).
At age 24 months, gelastic seizures (up to 50/day) and less frequent generalized convulsive seizures developed that were refractory to anti-seizure medication. At 38 months partial resection and disconnection of the lesion was performed. Post-operatively he improved but seizures continued at 0–6/day, and he was on three anti-seizure medications.
His younger sister (II-2), aged 6 years and 5 months, was also small at birth (height, weight, head circumference all < 3rd centile) with current height (102 cm; −3.6 SD), weight (13.3 kg −4.3 SD), and head circumference (44.5 cm −5.24 SD) all being low. MRI also revealed a suprasellar lesion (type IIb of Valdueza [9], Fig. 1C). She had hexadactyly of the left foot, bilateral skin appendages of the little fingers. She had short stature but no evidence of precocious puberty by age 6 years. She had mild motor and speech delay and she was speaking 2-word phrases at age 6 years. From the age of 22 months, gelastic seizures (0–3/day) developed but convulsive seizures were not reported.
Cardiac, renal, and thoracic anomalies were not detected in either child. Facial dysmorphism was present only in the brother.
Genomic analysis
We re-analysed quad clinical exome data from the two affected siblings and both their unaffected parents. We searched for rare germline candidate variants that were shared between the two affected offspring (II-1 & II-2). Following the exclusion of variants within the known disease genes, GLI3 or CPLANE1, we considered candidate variants only within the 50-known sonic-hedgehog (SHH) pathway genes, as classified by the Kyoto Encyclopedia of Genes and Genomes (https://www.genome.jp/kegg/) [10], given the key SHH transcription factor GLI3 is the cause of classic PHS.
Our initial germline analysis of non-synonymous or splice site variants that alter amino acid sequence revealed an ultra-rare (gnomAD frequency: 7.95 × 10−6) [11] missense variant in exon 6 of SMO (NM_005631.5: c.1199G > A; p.Arg400His). This variant was inherited from the unaffected father (I-1), was predicted to be deleterious to the SMO protein by multiple in silico tools, and is present only at low frequency in gnomAD consistent with recessive inheritance [11] (Fig. 1, Table S1).
Since SMO pathogenic variants were reported to be recessively inherited in two studies [7, 8], and there was no other obvious rare non-synonymous variant we searched for a second allele in trans by interrogating intronic and synonymous variants that were filtered out in our initial exome analysis. This revealed an ultra-rare (gnomAD frequency: 1.59 × 10−5) [11] synonymous variant (NM_005631.5: c.1416C>T; p.Tyr472Tyr) in exon 8 of SMO in both affected offspring inherited in trans from their unaffected mother (I-2, Fig. 1, Table S1). Human Splicing Finder (https://www.genomnis.com/access-hsf) predicted the synonymous variant will result in alternate splicing of the SMO transcript through disruption of exonic splice enhancer (ESE) and silencer (ESS) motifs within exon 8 [12].
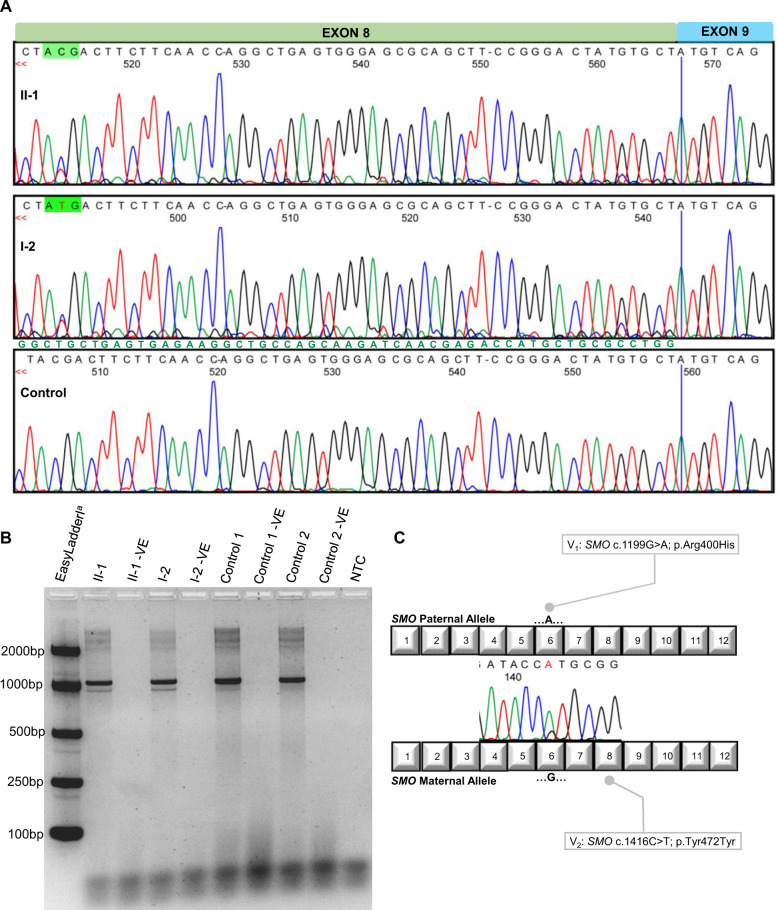
To interrogate the effect of this synonymous variant we analysed fibroblast-derived RNA samples obtained from the mother and affected son (I-2 & II-1). Sequencing of cDNA confirmed exon 8 skipping evidenced by underlying exon 9 sequence beginning at the junction of exon 7-8, and exon 7 sequence beginning at the junction of exon 8-9, shown in the forward and reverse directions (Fig. 2A). Gel electrophoresis of the same RT-PCR products demonstrates alternate splicing in the variant carriers I-2 and II-1 with the presence of two alleles (Fig. 2B). The exclusion of exon 8 leads to a frameshift and premature stop codon (p.Gly453AspfsTer6) which is predicted to lead to nonsense-mediated decay since exon 8 is not one of the last two exons of the SMO gene.
Fig. 2. Molecular analyses.
A Sequencing of patient derived cDNA demonstrates the underlying exon 7 sequence beginning at the junction of exon 8 and 9 of the SMO transcript within individuals I-2 & II-1. The underlying exon 7 sequence is annotated in green text. The sequencing displayed is in the reverse direction. B Gel electrophoresis of RT-PCR products demonstrating the two alleles for I-2 and II-1 representing the canonically spliced SMO transcripts (1069 bp) and the alternatively spliced SMO transcript not containing exon 8 (960 bp). aMeridian Bioscience EasyladderI (Cincinnati, OH, USA). -VE: No SuperScript® III/RNaseOUTTM Enzyme Mix (Thermo Fisher Scientific, Waltham, MA, USA) added; NTC: No Template Control. C RT-PCR demonstrates the paternal allele containing the missense variant is the most abundant normally spliced mRNA transcript, confirming a partial mis-splicing of the maternal allele housing the synonymous variant.
We investigated the mis-splicing further by interrogating allele specific contributions to the SMO transcript in the affected son (II-1). We demonstrated there is a significant reduction in normally spliced mRNA from the maternal allele that carries the synonymous variant, compared to the paternal allele housing the missense variant (Fig. 2C). This confirmed partial rather than complete mis-splicing of exon 8 from the maternal allele.
Discussion
The two siblings we report here show phenotypic concordance with the previously described bi-allelic SMO cases sharing the clinical features of hypothalamic hamartoma, polydactyly, and microcephaly as well as shortening of the long bones (Table 1) [7, 8]. The male proband was more severely affected with facial dysmorphism and more severe developmental disabilities. Bi-allelic SMO variants were found in both siblings, inherited in trans from their unaffected parents (Fig. 1). Both variants are ultra-rare and neither have been reported in gnomAD as homozygotes. The synonymous variant is predicted to disrupt cis-regulatory ESE and ESS motifs that are critical for the recruitment of spliceosome proteins to process pre-mRNA [13]. We confirmed this synonymous variant leads to partial mis-splicing resulting in exon 8 skipping in patient-derived RNA samples (Fig. 2). Several previous reports have identified equivalent pathogenic synonymous variants in different genes confirmed at the RNA level to lead to exon skipping [14–17].
Table 1.
| Le et al. [8] | This report | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| F1-II:1 | F2-II:1a | F2-II:2a | F3-II:1 | F4-II:4 | F5-II:2 | F5-II:3 | II-1 | II-2 | |
| Sex | M | M | M | M | M | M | F | M | F |
| Psychomotor development | normal | normal | normal | mild delay | normal | NR | normal | severe delay | mild delay |
| Gelastic seizures | + | + | + | − | − | − | − | + | + |
| Hypothalamic hamartoma | + | + | + | − | − | NR | NR | + | + |
| Microcephaly | − | + | − | − | − | − | − | + | + |
| Dysmorphic facial features | + | + | + | + | − | − | − | + | − |
| Chest and rib abnormalities | − | − | − | + | moderate | − | − | − | − |
| Postaxial polydactyly | + | + | + | + | + | + | + | + | + |
| Syndactyly | − | 5/6 | 2/3 | 5/6 | − | 2/3 | 2/3 | 3/4 | − |
| Cardiac defect | − | − | − | − | + | + | + | − | − |
| Shortening of long bones | − | − | − | + | − | − | − | + | + |
We report two cases (II-1 & II-2) that share the clinical features of hypothalamic hamartoma, gelastic seizures, developmental delay, microcephaly (<3rd centile), post-axial polydactyly and shortening of the long bones. The male proband (II-1) had precocious puberty and hypospadias.
NR not reported.
aPreviously reported [7].
SMO is a member of the SHH signalling pathway and encodes a seven-transmembrane protein [18]. The SMO protein, known as ‘Smoothened’, is a frizzled class receptor of the SHH ligand that localises to the primary cilium along with other SHH constituents such as the GLI1/2/3 transcription factors to facilitate SHH signalling during development [18].
Germline SMO variants have recently been reported in seven patients from five families presenting with a variety of developmental anomalies including syndromic hypothalamic hamartoma in three cases (Table 1) [7, 8]. Functional analysis of primary cilia within fibroblasts of these reported cases has demonstrated non-inducible GLI1 or PTCH1 expression, mis-localised SMO protein, and ligand-independent localisation of GLI2 protein, which together disrupt normal SHH signalling [8].
It is important to acknowledge that hypothalamic hamartoma is not reported in all patients with bi-allelic SMO variants, as is the case for individuals with Greig’s syndrome due to GLI3 mutations [3, 8]. Intriguingly, the patients with hypothalamic hamartoma and bi-allelic SMO variants have been shown to have more severe mis-localization of SMO protein within primary cilia than those without hypothalamic hamartoma. However, this observation was only made based on a relatively small number of cases [8].
In summary, we report two familial hypothalamic hamartoma cases with developmental abnormalities and bi-allelic germline SMO variants. We characterise a rare genetic mechanism of a synonymous variant triggering exon skipping. This highlights the importance of genetic analyses extending beyond non-synonymous coding variants when routine clinical diagnostic genetic testing is unrevealing. Our findings further support that hypothalamic hamartoma is caused by dysregulation of SHH signalling during development. This recognition will provide the impetus to consider additional SHH pathway genes as candidates for other unsolved hypothalamic hamartoma syndromes, and encourage clinical trials of SMO inhibitors already under development for patients with these broad developmental disorders.
Supplementary information
Acknowledgements
We would like to thank the family for their participation in this study.
Author contributions
TEG and MFB performed bioinformatic analysis. TEG and MSH performed molecular genetics experiments. SS and JRL performed the fibroblast cell culturing. MS and SFB conducted clinical phenotyping. TEG, SFB, and MSH drafted the paper. All authors discussed the results and commented on the final manuscript.
Funding
We are grateful to the Hope for Hypothalamic Hamartoma Foundation for their generous support of our study. This work was made possible through Victorian State Government Operational Infrastructure Support and Australian Government NHMRC IRIISS.
Data availability
The identified variants are publicly available in the Lieden Open Variation Database (LOVD). SMO NM_005631.5: c.1199G > A; p.Arg400His: Variant #0000791113, URL: https://databases.lovd.nl/shared/variants/0000791113#00019464 and SMO NM_005631.5: c.1416C > T; p.Tyr472Tyr: Variant #0000791114, URL: https://databases.lovd.nl/shared/variants/0000791114#00019464.
Competing interests
The authors declare no competing interests.
Ethical approval
The study was approved by the Human Research Ethics Committee of Austin Health (Heidelberg, Victoria, Australia). Written informed consent for the study was obtained from both parents.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Michael S. Hildebrand, Samuel F. Berkovic.
Contributor Information
Michael S. Hildebrand, Email: michael.hildebrand@unimelb.edu.au
Samuel F. Berkovic, Email: s.berkovic@unimelb.edu.au
Supplementary information
The online version contains supplementary material available at 10.1038/s41431-021-01023-4.
References
- 1.Khawaja AM, Pati S, Ng Y-T. Topical review: management of epilepsy due to hypothalamic hamartomas. Pediatr Neurol. 2017;75:29–42. doi: 10.1016/j.pediatrneurol.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Biesecker LG. Pallister-Hall syndrome In: GeneReviews. University of Washington, Seattle; 1993-2021. 2000 [updated May 18th 2017]. https://www.ncbi.nlm.nih.gov/books/NBK1465/?report=classic. Accessed 27 July 2021. [PubMed]
- 3.Johnston JJ, Olivos-Glander I, Killoran C, Elson E, Turner JT, Peters KF, et al. Molecular and clinical analyses of Greig Cephalopolysyndactyly and Pallister-Hall syndromes: robust phenotype prediction from the type and position of GLI3 mutations. Am J Hum Genet. 2005;76:609–22. doi: 10.1086/429346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang S, Graham JM, Jr., Olney AH, Biesecker L. GLI3 frameshift mutations cause autosomal dominant Pallister-Hall syndrome. Nat Genet. 1997;15:266–8. doi: 10.1038/ng0397-266. [DOI] [PubMed] [Google Scholar]
- 5.Bonnard C, Shboul M, Tonekaboni SH, Ng AYJ, Tohari S, Ghosh K, et al. Novel mutations in the ciliopathy-associated gene CPLANE1 (C5orf42) cause OFD syndrome type VI rather than Joubert syndrome. Eur J Hum Genet. 2018;61:585–95. doi: 10.1016/j.ejmg.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Lopez E, Thauvin-Robinet C, Reversade B, Khartoufi NE, Devisme L, Holder M, et al. C5orf42 is the major gene responsible for OFD syndrome type VI. Hum Genet. 2014;133:367–77. doi: 10.1007/s00439-013-1385-1. [DOI] [PubMed] [Google Scholar]
- 7.Rubino S, Qian J, Pinheiro-Neto CD, Kenning TJ, Adamo MA. A familial syndrome of hypothalamic hamartomas, polydactyly, and SMO mutations: a clinical report of 2 cases. J Neurosurg Pediatr. 2019: 98-103. [DOI] [PubMed]
- 8.Le T-L, Sribudiani Y, Dong X, Huber C, Kois C, Baujat G, et al. Bi-allelic variations of SMO in humans cause a broad spectrum of developmental anomalies due to abnormal hedgehog signaling. Am J Hum Genet. 2020;106:779–92. doi: 10.1016/j.ajhg.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valdueza JM, Cristante L, Dammann O, Bentele K, Vortmeyer A, Saeger W, et al. Hypothalamic hamartomas: with special reference to gelastic epilepsy and surgery. Neurosurgery. 1994;34:949–58. doi: 10.1227/00006123-199406000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Kanehisa M, Goto S. KEGG: Kyoto Encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alfoldi J, Wang Q, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–43. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desmet F-O, Hamroun D, Lalande M, Collod-Béroud G, Claustres M, Béroud C. Human Splicing Finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009;37:e67. doi: 10.1093/nar/gkp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, Burge CB. Splicing regulation: from a parts list of regulatory elements to an integrated splicing code. RNA. 2008;14:802–13. doi: 10.1261/rna.876308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christian LL, Eric H, Elliot JA, Brunhilde W, Single A. Nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc Natl Acad Sci USA. 1999;96:6307–11. doi: 10.1073/pnas.96.11.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li M, Lu X, Dong J, Yao Z, Wu Y, Rao H, et al. A synonymous mutation in exon 39 of FBN1 causes exon skipping leading to Marfan syndrome. Genomics. 2020;112:3856–61. doi: 10.1016/j.ygeno.2020.06.024. [DOI] [PubMed] [Google Scholar]
- 16.Li N, Xu Y, Yu T, Yao R, Chen J, Luo C, et al. Further delineation of bone marrow failure syndrome caused by novel compound heterozygous variants of MYSM1. Gene. 2020;757:144938. doi: 10.1016/j.gene.2020.144938. [DOI] [PubMed] [Google Scholar]
- 17.Oliveira J, Soares-Silva I, Fokkema I, Gonçalves A, Cabral A, Diogo L, et al. Novel synonymous substitution in POMGNT1 promotes exon skipping in a patient with congenital muscular dystrophy. J Hum Genet. 2008;53:565–72. doi: 10.1007/s10038-008-0263-5. [DOI] [PubMed] [Google Scholar]
- 18.Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DYR, Reiter JF. Vertebrate smoothened functions at the primary cilium. Nature. 2005;437:1018–21. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The identified variants are publicly available in the Lieden Open Variation Database (LOVD). SMO NM_005631.5: c.1199G > A; p.Arg400His: Variant #0000791113, URL: https://databases.lovd.nl/shared/variants/0000791113#00019464 and SMO NM_005631.5: c.1416C > T; p.Tyr472Tyr: Variant #0000791114, URL: https://databases.lovd.nl/shared/variants/0000791114#00019464.