Abstract
BMS-200475 was recently shown to have potent antiviral activity against hepatitis B virus (50% effective concentration = 3.7 nM; 50% cytotoxic concentration = 30 μM). In metabolic studies in both HepG2 and hepatitis B virus-transfected 2.2.15 human hepatoma cell lines, the metabolism was similar, the primary products being the di- and triphosphates. The accumulation of triphosphate was rapid and detectable down to a 5 nM concentration of added drug. When cells were labeled at 25 μM, the intracellular triphosphate concentration attained 30 pmol/106 cells (∼30 μM). The intracellular half-life of the triphosphate was about 15 h. Compared with five other nucleoside analogs of medical interest (lamivudine, penciclovir, ganciclovir, acyclovir, and lobucavir), BMS-200475 was most efficiently phosphorylated to the triphosphate in HepG2 cells.
Hepatitis B virus (HBV) is a hepadnavirus, capable of causing serious liver disorders in humans. In chronic infections, of which there are an estimated 1 million in the United States and over 300 million worldwide, there is a close association with the development of cirrhosis and hepatocellular carcinoma (1). Although approved therapies exist (alpha interferon and HBV immune globulin), they are only marginally effective and do not provide a long-term solution.
BMS-200475 (Fig. 1) was originally synthesized as an antiherpesvirus agent (SQ-34676) and displayed moderate activity against herpes simplex virus types 1 (HSV-1), HSV-2, and varicella-zoster virus (13). Activity was also seen with human cytomegalovirus, a herpesvirus lacking a thymidine kinase, but was not detected against RNA viruses such as human immunodeficiency virus or influenza (8). Recently, we discovered that BMS-200475 possesses extremely potent activity against HBV (8). In this report we extend our earlier characterization of BMS-200475 (2, 8), by describing the intracellular metabolism in both normal and HBV-infected hepatocytes. We also compare its metabolism with that of five other antiviral nucleoside analogs, three of which are also active against HBV: lamivudine, penciclovir, and lobucavir.
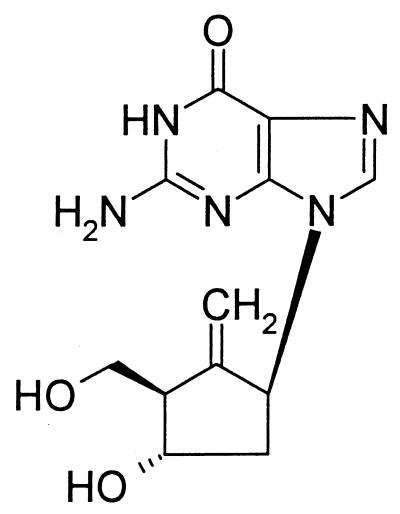
FIG. 1.
Structure of BMS-200475.
BMS-200475 (2) and [3H]BMS-200475 were prepared at Bristol-Myers Squibb. Other radiolabeled nucleosides were from Dupont/NEN, Moravek, or Amersham. The human hepatoma cell line HepG2 (American Type Culture Collection) and the HBV-transfected cell line 2.2.15 (George Acs, Mt. Sinai School of Medicine [11]) were grown on collagen-coated plates as described previously (8). Experiments to look at the intracellular metabolism of nucleoside analogs were performed with exponentially growing cells, except that when HBV activities were examined, stationary-phase 2.2.15 cells were used (12). In a typical experiment, exponential cells were plated at 2 × 105 to 5 × 105 cells/well and incubated overnight in six-well culture dishes (9.4 cm2/well). In the morning, the medium was replaced with fresh medium containing tritiated drugs (three wells per labeling condition). Following an incubation period described for each experiment, cells were extracted in situ with 60% methanol for intracellular nucleotide analysis by ion-pairing high-performance liquid chromatography (HPLC) (16). Samples were analyzed in-line with a Packard A525 radioactivity detector and concomitantly monitored by using a Waters 484 UV detector at 260 nm. The peaks in the UV profiles were integrated and used as a measure of intracellular solute mass for normalization of radioactivity in each radioactivity profile. We found that values for the integrated UV profiles increased in close parallel to the increase in cell number during the course of prolonged cell culture.
In initial experiments, HepG2 and 2.2.15 (hepatitis B-transfected) cells were plated at confluent densities (2 × 106 cells/well) in medium containing 1% fetal calf serum, to maximize the relative expression of HBV (12). These cells were incubated with 25 μM [3H]BMS-200475 for 3 days and then analyzed by HPLC for intracellular nucleotides. The profiles were qualitatively identical, with major peaks eluting at 10.5 and 22.5 min (not shown). These peaks appeared to be identical to the synthetic standards [3H]BMS-200475 and BMS-200475 triphosphate, respectively. Two minor peaks were also present, at 14 and 18.2 min. When cell extracts were treated with alkaline phosphatase prior to analysis, [3H]BMS-200475 was the only peak present. Thus, like other nucleoside analogs, BMS-200475 was metabolized by phosphorylation to its mono-, di-, and triphosphates. This metabolism appeared to be due to cellular kinases, since no difference was observed when the HBV-transfected 2.2.15 cells were used.
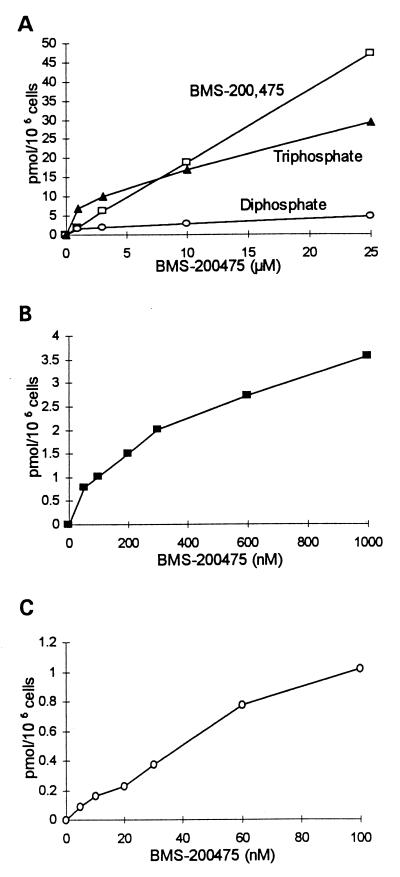
The concentration dependence of nucleoside uptake and phosphorylation was examined during a 3-day incubation in the presence of 1 to 25 μM [3H]BMS-200475 (Fig. 2A). The uptake increased linearly with the concentration of added drug, but neither the mono- nor diphosphate accumulated to an appreciable extent. The monophosphate concentration rarely exceeded 0.5 pmol/106 cells and was often undetectable. In contrast, the triphosphate accumulated to high concentrations. Phosphorylation was particularly efficient in the low-micromolar range. At 1 μM added drug, triphosphate accounted for 60 to 80% of the intracellular nucleotide label and attained levels of ∼7 μM (given an average cell volume of 10−9 ml [6]) or ∼2 μM (given a volume of 4 × 10−9 ml [15]). When the added-drug concentration was increased to 25 μM, intracellular triphosphate increased to 30 μM, nearly 10,000-fold higher than that needed for antiviral activity.
FIG. 2.
Concentration dependence of the accumulation of BMS-200475 nucleotides in HepG2 cells. (A) Cells were labeled for 3 days in the presence of 1 to 25 μM [3H]BMS-200475 (2.5 to 0.5 Ci/mmol). The graph shows the levels of intracellular nucleoside, diphosphate, and triphosphate. The monophosphate did not accumulate to appreciable levels. (B and C) Intracellular BMS-200475 triphosphate accumulation at submicromolar levels of BMS-200475 in the medium. HepG2 cells were labeled for 3 days in the presence of 50 to 1,000 nM (B) or 5 to 100 nM (C) [3H]BMS-200475 (13.9 Ci/mmol). At the lowest concentrations of [3H]BMS-200475, the triphosphate was the major detectable [3H]BMS-200475 metabolite. All data points are the average of triplicate determinations.
In further studies, the concentration of added drug was lowered to examine a range approaching the 50% effective concentration for HBV. In two successive experiments HepG2 cells were incubated for 3 days with 50 to 1,000 nM [3H]BMS-200475 and then 5 to 100 nM [3H]BMS-200475. Upon analysis by HPLC, the triphosphate was readily detected at all concentrations examined (Fig. 2B and C). It accumulated very efficiently in the low-nanomolar range, consistent with the potent antiviral activity of the drug. At 5 nM, the relative amounts of nucleoside, diphosphate, and triphosphate were 4, 16, and 80%, respectively, indicating that >95% of the intracellular nucleoside pool was phosphorylated. A similar result was obtained with the Chinese hamster ovary cell line CHO-K1, which was equally efficient in anabolizing BMS-200,475. HFF, Vero, and CV-1 cells also phosphorylated BMS-200475, but they were not tested with drug concentrations in the submicromolar range.
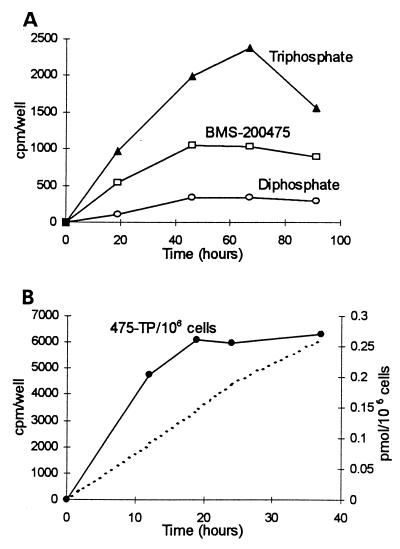
Figure 3A shows a 4-day time course for the phosphorylation of 1 μM [3H]BMS-200475 by HepG2 cells. Total uptake and accumulation of radiolabel as intracellular nucleoside, diphosphate, and triphosphate increased for 2 to 3 days before reaching a plateau. In contrast, the monophosphate was only occasionally detectable. Since the labeling medium was used continuously, the decay of triphosphate at day 4 probably reflects the exhaustion of essential nutrients. When examined on a per-cell basis, the plateau of triphosphate accumulation actually occurred earlier than shown in Fig. 3A. In the experiment shown in Fig. 3B, the phosphorylation of 50 nM [3H]BMS-200475 to the triphosphate was examined during a 36-h incubation. As shown by the dotted line, total triphosphate radioactivity per well accumulated almost linearly during the incubation. However, when corrected for the concomitant increase in cell number, the increase per cell appeared to plateau by 20 h. Thus, the phosphorylation of BMS-200475 to the triphosphate was rapid and attained a stable intracellular concentration.
FIG. 3.
Kinetics of [3H]BMS-200475 uptake and phosphorylation by HepG2 cells. (A) Cells were labeled with 1 μM [3H]BMS-200475 (2.5 Ci/mmol) for up to 4 days and then analyzed for intracellular nucleotides. The data are presented as counts per minute per well for each of the intracellular solutes. (B) Empirical and normalized data for the phosphorylation of 50 nM [3H]BMS-200475 (13.9 Ci/mmol) to the triphosphate (475-TP) during a 36-h incubation. The dotted line corresponds to the radioactivity in the triphosphate present at each time point (counts per minute per well). The solid circles correspond to these data following conversion to picomoles per 106 cells.
Given the efficient phosphorylation of BMS-200475, the intracellular half-life of the triphosphate was determined and compared in both normal and HBV-transfected cells. Cells were labeled with [3H]BMS-200475 for 2 days, washed free of radiolabel, and reincubated for up to 36 h before analysis of intracellular nucleotides. The radioactive decay curves followed monoexponential kinetics in both cell types and at labeling concentrations of 1 or 25 μM drug. The terminal half-life was ∼15 h under all conditions (n = 8).
Table 1 compares the intracellular phosphorylation of BMS-200475 with that of five other nucleoside analogs of medical importance. HepG2 cells were labeled for 3 days with either 5 or 25 μM tritiated drug and then analyzed by HPLC. Only lamivudine was phosphorylated to an extent comparable to that of BMS-200475. However, whereas lamivudine accumulated primarily as the diphosphate, BMS-200475 was more efficiently phosphorylated to the triphosphate. At a labeling concentration of 25 μM, triphosphate accumulation was about 15- to 50-fold greater for BMS-200475 than for penciclovir, ganciclovir, lobucavir, or acyclovir.
TABLE 1.
Comparison of the intracellular phosphorylation of [3H]BMS-200475 with that of several other nucleoside analogs
| Druga | Drug concn (μM) | Concn (pmol/106 cells) ofb:
|
|||
|---|---|---|---|---|---|
| Nucleoside | MP | DP | TP | ||
| Expt 1 | |||||
| BMS-200475 | 5 | 2.9 ± 0.05 | 0.16 ± 0.04 | 0.69 ± 0.08 | 7.2 ± 0.54 |
| Lamivudine | 5 | 13 ± 2.2 | ND | 21 ± 5.4 | 4.5 ± 1.4 |
| Lobucavir | 5 | 4.8 ± 0.78 | ND | 0.02 ± 0.004 | 0.03 ± 0.02 |
| Expt 2 | |||||
| BMS-200475 | 25 | 17 ± 2.3 | 1.1 ± 0.5 | 4.8 ± 1.1 | 18 ± 0.7 |
| Lamivudine | 25 | 9.7 ± 2.7 | 3.9 ± 1.2 | 29 ± 0.4 | 10 ± 0.5 |
| Penciclovir | 25 | 23 ± 2.8 | 0.52 ± 0.05 | 0.31 ± 0.002 | 1.2 ± 0.12 |
| Lobucavir | 25 | 22 ± 4.0 | 0.15 ± 0.11 | 0.23 ± 0.03 | 0.29 ± 0.08 |
| Ganciclovir | 25 | 20 ± 2.1 | 0.12 ± 0.04 | 0.22 ± 0.02 | 0.77 ± 0.04 |
| Acyclovir | 25 | 13 ± 2.1 | 0.04 ± 0.1 | 0.06 ± 0.002 | 0.34 ± 0.27 |
BMS-200475 and lamivudine were used at a specific activity of 2 Ci/mmol, while penciclovir, lobucavir, ganciclovir, and acyclovir were used at 15, 11.9, 13.1, and 15 Ci/mmol, respectively.
Data are presented as concentrations for intracellular nucleoside, monophosphate (MP), diphosphate (DP), and triphosphate (TP). For penciclovir and ganciclovir nucleotides, the values represent the sums of their respective enantiomeric species. Each data point is the average of triplicate determinations. ND, not detected.
Among nucleoside analogs with antiviral activity, BMS-200475 has a geometry of 3′- and 5′-hydroxyl groups which closely resembles that of naturally occurring d-deoxynucleosides. Thus, it was not surprising to discover that it was efficiently phosphorylated in mammalian cells, even at low-nanomolar concentrations. However, our initial interest was to learn whether an HBV-induced activity might explain the ∼1,000-fold-greater activity of BMS-200475 against this virus than herpesviruses or other viruses. HepG2 cells were compared directly with 2.2.15 cells, under growth conditions which minimized host metabolic activity and maximized the expression of HBV proteins. Even under these conditions, BMS-200475 metabolites were identical in the two cell lines, suggesting that phosphorylation was due solely to host activities. We also found that the phosphorylating activity was common to several cell lines.
In other experiments, the phosphorylation of BMS-200475 to the triphosphate was examined at concentrations from 25 μM to as low as 5 nM (Fig. 2). In all cases, the equilibrium favored the formation of triphosphate. At low-nanomolar concentrations, it was often the only detectable radiolabeled species. This observed accumulation of triphosphate at low-nanomolar concentrations was not previously observed with lobucavir, acyclovir, or ganciclovir. In our original work with lobucavir, we did not observe phosphorylation in WI-38 cells at 0.8 to 25 μM (reference 16 and unpublished data). In the current work, lobucavir triphosphate reached 0.03 pmol/106 cells when labeled at 5 μM for 3 days but only when a high specific activity (11.9 Ci/mmol) was used (Table 1). This concentration of triphosphate was the same achieved with a 100-fold-lower concentration (50 nM) of BMS-200475 during a 20-h incubation (Fig. 3B). Such a difference in phosphorylation potential between these two drugs is also consistent with the observation that lobucavir is nearly 1,000-fold less potent in 2.2.15 cells, while its triphosphate form is equivalent to BMS-200475 triphosphate against the HBV polymerase (8, 9).
In work on acyclovir, Keller et al. (10) purified the acyclovir-phosphorylating activity from rat liver as a single species, characterized as a high-Km form of cellular 5′-nucleotidase. This activity phosphorylated both acyclovir and ganciclovir in an equilibrium reaction utilizing inosine monophosphate as the phosphate donor. However, it had an extremely high Km for these nucleoside analogs (apparent Kms were ≥90 mM). Thus, it does not seem likely that this enzyme would contribute significantly to BMS-200475 phosphorylation in the low-nanomolar range (Fig. 2C). In contrast, lamivudine is known to be phosphorylated by cellular deoxycytidine kinase (4, 7), with an efficiency that parallels that of BMS-200475 (Table 1). Thus, the activity(ies) responsible for BMS-200475 phosphorylation will most likely be a nucleoside kinase(s), which uses the high energy potential of a triphosphate to drive the reaction far to the right.
In a close examination of the phosphorylation kinetics, we observed a rapid accumulation of BMS-200475 triphosphate, followed by a plateau (Fig. 3B). Since the total amount of triphosphate continued to increase for another 16 h, it appears that an equilibrium with the concentration of available nucleoside was reached. As shown in Fig. 2C, triphosphate accumulated linearly with extracellular nucleoside, suggesting the establishment of equilibrium between synthesis and degradation. To better understand this, we determined the intracellular triphosphate half-life, in both normal and HBV-infected cells and at 1 or 25 μM (∼15 h). Even when corrected for dilution due to cell growth (based on a doubling period of 1 day), this value would still be about 10 h (half-life per cell). The observation of a plateau suggests that the synthesis of triphosphate slows down considerably upon reaching this equilibrium state (Fig. 3B).
The 15-h half-life is comparable to the triphosphate half-lives of three other nucleoside analogs being developed for the treatment of HBV: lobucavir (10 h in HSV-infected WI38 cells [16]), lamivudine (12 to 15 h in peripheral blood lymphocytes [3]), and penciclovir (20 and 7 h, respectively, in HSV-2- and varicella-zoster virus-infected MRC-5 cells [5]). In light of a recent finding on the superior efficacy of famciclovir over valaciclovir in an murine model of HSV-2 infection (14), it is interesting to speculate whether the relatively long half-life of the above-described nucleoside analogs may in part augment their potent activities.
Acknowledgments
We acknowledge the help of Daniel Tenney, Carol Deminie, Min Gao, Darcy Izzarelli, and Masud Alam for providing cells lines for use in these studies, and we acknowledge Brian Terry for critical review of the manuscript.
REFERENCES
- 1.Beasley R P, Hwang L Y. Overview on the epidemiology of hepatocellular carcinoma. In: Hollinger F B, Lemon S M, Margolis M, editors. Viral hepatitis and liver disease. Baltimore, Md: The Williams & Wilkins Co.; 1991. pp. 532–535. [Google Scholar]
- 2.Bisacchi G S, Chao S T, Bachard C, Daris J P, Innaimo S, Jacobs G A, Kocy O, Lapointe P, Martel A, Merchant Z, Slusarchyk W A, Sundeen J E, Young M G, Colonno R, Zahler R. BMS-200475, a novel carbocyclic 2′-deoxyguanosine analog with potent and selective anti-hepatitis B virus activity in vitro. Bioorg Med Chem Lett. 1997;7:127–132. [Google Scholar]
- 3.Cammack N, Rouse P, Marr C L P, Reid P J, Boehme R E, Coates J A V, Penn C R, Cameron J M. Cellular metabolism of (−)enantiomeric 2′-deoxy-3′-thiacytidine. Biochem Pharmacol. 1992;43:2059–2064. doi: 10.1016/0006-2952(92)90162-c. [DOI] [PubMed] [Google Scholar]
- 4.Chang C-N, Skalski V, Zhou J H, Cheng Y-C. Biochemical pharmacology of (+)- and (−)-2′,3′-dideoxy-3′-thiacytidine as anti-hepatitis B virus agents. J Biol Chem. 1992;267:22414–22420. [PubMed] [Google Scholar]
- 5.Earnshaw D L, Bacon T H, Darlison S J, Edmonds K, Perkins R M, Vere Hodge R A. Model of antiviral action of penciclovir in MRC-5 cells infected with herpes simplex virus type 1 (HSV-1), HSV-2, and varicella-zoster virus. Antimicrob Agents Chemother. 1992;36:2747–2757. doi: 10.1128/aac.36.12.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elion G B, Furman A P, Fyfe J A, DeMiranda P, Beauchamp L, Schaeffer H J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl)guanine. Proc Natl Acad Sci USA. 1977;74:5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenberg M L, Allaudeen H S, Hershfield M S. Metabolism, toxicity and anti-HIV activity of 2′-deoxy-3′-thiacytidine (BCH-189) in T and B cell lines. Ann N Y Acad Sci. 1990;616:517–518. [Google Scholar]
- 8.Innaimo S F, Seifer M, Bisacchi G S, Standring D N, Zahler R, Colonno R J. Identification of BMS-200475 as a potent and selective inhibitor of hepatitis B virus. Antimicrob Agents Chemother. 1997;41:1444–1448. doi: 10.1128/aac.41.7.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Innaimo S F, Seifer M, Genovesi E, Clark J, Yamanaka G, Hamatake R, Terry B, Standring D, Bisacchi G, Sundeen J, Zahler R, Colonno R J. Abstracts of The 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Identification of BMS-200475 as a novel and potent inhibitor of hepatitis B virus, abstr. H24. [Google Scholar]
- 10.Keller P M, McKee S A, Fyfe J A. Cytoplasmic 5′-nucleotidase catalyzes acyclovir phosphorylation. J Biol Chem. 1985;260:8664–8667. [PubMed] [Google Scholar]
- 11.Sells M A, Chen M-L, Acs G. Production of hepatitis B virus particles in Hep G2 cells transfected with cloned hepatitis B virus DNA. Proc Natl Acad Sci USA. 1987;84:1005–1009. doi: 10.1073/pnas.84.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sells M A, Zelent A Z, Shvartsman M, Acs G. Replicative intermediates of hepatitis B virus in HepG2 cells that produce infectious virions. J Virol. 1988;62:2836–2844. doi: 10.1128/jvi.62.8.2836-2844.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slusarchyk, W. A., A. K. Field, J. A. Greytok, P. Taunk, A. V. Tuomari, M. G. Young, and R. Zahler. 1992. 4-Hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl purines and pyrimidines, a new class of anti-herpesvirus agents. Antiviral Res. 17(Suppl. 1):98.
- 14.Thackray A M, Field H J. Comparison of effects of famciclovir and valaciclovir on pathogenesis of herpes simplex virus type 2 in a murine infection model. Antimicrob Agents Chemother. 1996;40:846–851. doi: 10.1128/aac.40.4.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vere Hodge R A, Perkins R M. Mode of action of 9-(4-hydroxy-3-hydroxymethylbut-1-yl)guanine (BRL 39123) against herpes simplex virus in MRC-5 cells. Antimicrob Agents Chemother. 1989;33:223–229. doi: 10.1128/aac.33.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamanaka G, Tuomari A V, Hagen M, McGeever-Rubin B, Terry B, Haffey M, Bisacchi G S, Field A K. Selective activity and cellular pharmacology of (1R-1α,2β,3α)-9-[2,3-bis(hydroxymethyl)cyclobutyl]guanine in herpesvirus-infected cells. Mol Pharmacol. 1991;40:446–453. [PubMed] [Google Scholar]