Abstract
Attribute-based medicine is essential for patient-centered medicine. To date, the groups of patients with chronic kidney disease (CKD) requiring urate-lowering therapy are clinically unknown. Herein, we evaluated the efficacy of febuxostat using a cross-classification, attribute-based research approach. We performed post hoc analysis of multicenter, randomized, double-blind, placebo-controlled trial data for 395 patients with stage 3 CKD and asymptomatic hyperuricemia. Participants were divided into febuxostat or placebo groups and subcohorts stratified and cross-classified by proteinuria and serum creatinine concentrations. In patients stratified based on proteinuria, the mean eGFR slopes were significantly higher in the febuxostat group than in the placebo group (P = 0.007) in the subcohort without proteinuria. The interaction between febuxostat treatment and presence of proteinuria in terms of eGFR slope was significant (P for interaction = 0.019). When cross-classified by the presence of proteinuria and serum creatinine level, the mean eGFR slopes significantly differed between the febuxostat and placebo groups (P = 0.040) in cross-classified subcohorts without proteinuria and with serum creatinine level ≥ median, but not in the cross-classified subcohorts with proteinuria and serum creatinine level < median. Febuxostat mitigated the decline in kidney function among stage 3 CKD patients with asymptomatic hyperuricemia without proteinuria.
Subject terms: Diseases, Nephrology
Introduction
The definition of chronic kidney disease (CKD) has been widely disseminated and generally accepted1. However, some controversies remain in CKD research2,3, including the effects of nephrosclerosis4 and urate-lowering therapy for CKD5. Although urate-lowering therapy is not generally approved for patients with asymptomatic hyperuricemia6, pharmacological treatment of hyperuricemia in patients with CKD is recommended in Japan7.
As risk factors and pathological conditions for CKD progression differ with the etiology and patient attributes, studies are needed to consider these factors. Nephrosclerosis is characterized by the absence of or minimal proteinuria associated with slowly progressing kidney disease4, and the number of patients with end-stage kidney disease due to nephrosclerosis is increasing with the aging population6. Interestingly, hyperuricemia in patients with CKD has gained attention owing to the global spread of arteriosclerosis7,8 and is associated with kidney arteriolopathy9,10. Animal studies have reported a direct negative effect of uric acid on endothelial11 and smooth muscle cells12,13 in kidney arterial/arteriolar vessels. The observation period of several clinical trials was short because of the associated expenses, making it difficult to detect the outcomes of CKD involving slowly progressive nephrosclerotic kidney diseases.
The Febuxostat Versus Placebo Randomized Controlled Trial Regarding Reduced Renal Function in Patients With Hyperuricemia Complicated by Chronic Kidney Disease Stage 3 (FEATHER) study (follow-up, 108 weeks) failed to confirm that febuxostat alleviated the decline in the estimated glomerular filtration rate (eGFR) in stage 3 CKD patients with hyperuricemia without gout in the entire cohort. However, the FEATHER study reported substantial suppression of eGFR decline in patients without proteinuria (P = 0.005) or a serum creatinine level lower than the median (P = 0.009) after febuxostat treatment14.
Patient-centered medicine15 and personalized evidence-based medicine (EBM)16 have recently garnered attention. Although randomized controlled trials (RCTs) are the cornerstone of EBM with the highest-quality evidence when setting clinical guidelines and policies for patient care17, ingenuity in adapting RCT results to individual patients is required18. Although subgroup analyses of the heterogeneity of treatment effects will provide valuable information for patient care15 and can lead to personalized EBM16,19, the characteristics of patients may influence the efficacy of therapy; thus, conventional subgroup analyses are typically not informative16,20. This is because RCT results are generally examined through conventional subgroup analyses, contrasting effects in groups of patients, and defining one variable or attribute at a time18,21. Such single-attribute comparisons intrinsically result in subcohorts that are more similar to the total cohort because of the averaging of the multiple characteristics of patients in cohorts comprising heterogeneous patients16,20. Thus, conventional subgroup (one-attribute-at-a-time) analyses under-represent heterogeneity among patients who differ in several variables16.
Two-attributes-at-a-time subgroup analysis (cross-classification approach) is frequently used in research for marketing and has expanded to various fields22,23. In cross-classified models, the main diagonal elements are responsible for direct effects and off-diagonal elements are responsible for indirect effects, which are important for discriminating subcohorts24. As shown in Fig. 1, a cross-classified structure can help identify interactions between attributes and determine the attribute (A or B) that is important by evaluating the off-diagonal values24. The primary outcome in an RCT can be influenced by baseline prognostic factors such as disease severity, referred to as chance bias25. Chance bias can be eliminated by stratified randomization or minimization25 and by adjusting the imbalance in baseline variables16,20. Notably, proteinuria and decreased kidney function are precise indices of the severity of CKD1 and major risk factors for kidney disease progression1,23,26, which should be adequately adjusted in RCTs in patients with CKD. However, the prognostic factors that affected the efficacy of febuxostat in the FEATHER study are unclear. Furthermore, the need for a plausible hypothesis for treatment heterogeneity between patients with and without proteinuria in urate-lowering therapy for CKD has been increasing5,27. Therefore, it is necessary to identify a precise subcohort suitable for febuxostat therapy. We hypothesized that the efficacy of febuxostat is affected by proteinuria, and febuxostat is more useful for populations without proteinuria, comprising patients with atherosclerosis. To overcome the limitations of the conventional one-attribute-at-a-time subgroup analysis and to adjust two major and interactive risk factors in CKD simultaneously, we performed novel cross-classification subgroup analyses of patients in the FEATHER study. We aimed to investigate whether proteinuria or kidney function affects the efficacy of febuxostat.
Figure 1.
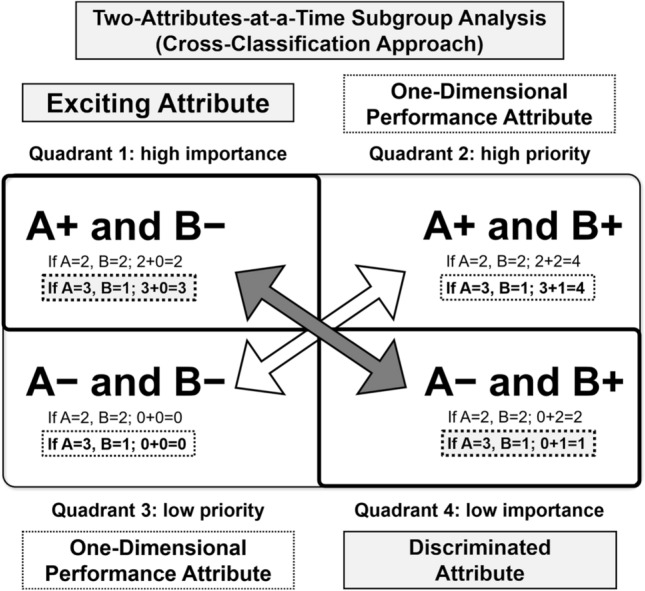
Cross-classification approach for attributes (importance grid analysis). Cross-classification of the attributes showing which attribute, A or B, is important. First, Quadrants 2 and 3, which are diagonal elements, generally become one-dimensional performance attributes with a high value in Quadrant 2 and low value in Quadrant 3. Quadrants 1 and 4, which are off-diagonal elements, can be used to discriminate the importance of A and B. When the attribute value of A is higher than that of B, Quadrant 1 becomes an exciting attribute. For example, if the attribute value of A is 3 and that of B is 1, the attribute value of A positive and B negative is 3, which is higher than Quadrant 4 (A negative and B positive, which has an attribute value of 1). Therefore, by considering differences between Quadrants 1 and 4, the difference in the importance of A and B can be found. A+, A positive; A−, A negative; B+, B positive; B−, B negative.
Results
Study patients
The baseline characteristics of the patients are presented in Table 1 and Supplementary Tables S1 and S2. Table 1 presents the overall baseline characteristics of the study participants based on febuxostat use and proteinuria presence. In total, 395 stage 3 CKD patients with hyperuricemia without gout (305 males and 90 females; mean age, 64.7 years; mean eGFR, 45.2 mL/min/1.73 m2) were treated with febuxostat (203 patients) or placebo (192 patients). The study groups were well-matched regarding eGFR, sex, age, proteinuria, and diabetes mellitus, with no significant differences in the baseline characteristics from those of the entire cohort. Patients without proteinuria were significantly older (66.4 ± 10.8 vs. 62.6 ± 12.8 years, P = 0.002) and had significantly higher eGFR (46.2 ± 9.6 vs. 43.9 ± 9.6 mL/min/1.73 m2, P = 0.02) than those with proteinuria. Supplementary Table S1 (online) presents the baseline characteristics of study participants for febuxostat treatment stratified by the presence of proteinuria, and Supplementary Table S2 (online) presents the baseline characteristics for febuxostat treatment cross-classified by the presence of proteinuria and the serum creatinine level. The patients’ baseline clinical characteristics and laboratory results were similar among the assigned treatment groups, with no significant differences between both subgroups stratified by the presence of proteinuria and those cross-classified by the presence of proteinuria/serum creatinine level.
Table 1.
Baseline characteristics of the study participants according to febuxostat treatment and presence or absence of proteinuria.
| Total (n = 395) | Placebo (n = 203) | Febuxostat (n = 192) | P-value | Proteinuria-negative (n = 218) | Proteinuria-positive (n = 177) | P-value | |
|---|---|---|---|---|---|---|---|
| Clinical characteristics | |||||||
| Male sex | 305 (77.2%) | 154 (75.9%) | 151 (78.6%) | 0.510 | 178 (81.7%) | 127 (71.8%) | 0.020 |
| Age (years) | 64.7 ± 11.9 | 64.7 ± 12.2 | 64.7 ± 11.5 | 0.978 | 66.4 ± 10.8 | 62.6 ± 12.8 | 0.002 |
| Body mass index (kg/m2) | 24.8 ± 4.1 | 24.7 ± 3.6 | 24.9 ± 4.5 | 0.641 | 24.8 ± 4.0 | 24.8 ± 4.2 | 0.960 |
| Systolic blood pressure (mmHg) | 130.6 ± 15.0 | 129.6 ± 15.0 | 131.7 ± 15.0 | 0.170 | 130.1 ± 15.1 | 131.1 ± 14.9 | 0.512 |
| Diastolic blood pressure (mmHg) | 77.9 ± 10.8 | 77.3 ± 11.1 | 78.4 ± 10.6 | 0.309 | 77.2 ± 10.5 | 78.6 ± 11.2 | 0.187 |
| Current or former smoker | 237 (60.0%) | 117 (57.6%) | 120 (62.5%) | 0.612 | 127 (58.3%) | 110 (62.1%) | 0.226 |
| Laboratory results | |||||||
| Estimated GFR (mL/min/1.73 m2) | 45.2 ± 9.6 | 45.0 ± 9.8 | 45.3 ± 9.5 | 0.775 | 46.2 ± 9.6 | 43.9 ± 9.6 | 0.020 |
| Serum creatinine (mg/dL) | 1.26 ± 0.25 | 1.26 ± 0.25 | 1.26 ± 0.24 | 0.880 | 1.24 ± 0.23 | 1.28 ± 0.26 | 0.146 |
| Serum uric acid (mg/dL) | 7.96 ± 0.63 | 7.98 ± 0.64 | 7.94 ± 0.62 | 0.539 | 7.93 ± 0.61 | 8.00 ± 0.65 | 0.293 |
| Hemoglobin A1c (%) | 6.0 ± 0.6 | 6.0 ± 0.6 | 6.0 ± 0.6 | 0.214 | 6.0 ± 0.5 | 6.0 ± 0.6 | 0.477 |
| UACR (mg/g) | 117.0 [17.2–518.0] | 120.0 [17.2–517.0] | 110.5 [18.1–521.5] | 0.958 | 24.1 [7.1–85.7] | 534.0 [224.0–981.0] | < 0.001 |
| Proteinuria | 177 (44.8%) | 93 (45.8%) | 84 (43.8%) | 0.680 | 0 (0.0) | 177 (100%) | NA |
| Coexisting conditions | |||||||
| Diabetes mellitus | 114 (28.9%) | 59 (29.1%) | 55 (28.6%) | 0.927 | 63 (28.9%) | 51 (28.8%) | 0.985 |
| Ischemic heart disease | 24 (6.1%) | 11 (5.4%) | 13 (6.8%) | 0.574 | 14 (6.4%) | 10 (5.6%) | 0.749 |
| Cerebrovascular disease | 43 (10.9%) | 17 (8.4%) | 26 (13.5%) | 0.099 | 25 (11.5%) | 18 (10.2%) | 0.680 |
| Medications | |||||||
| ACE inhibitor and/or ARB | 310 (78.5%) | 152 (74.9%) | 158 (82.3%) | 0.073 | 150 (68.8%) | 160 (90.4%) | < 0.001 |
| Statins | 150 (38.0%) | 68 (33.5%) | 82 (42.7%) | 0.059 | 70 (32.1%) | 80 (45.2%) | 0.008 |
| Antidiabetic drugs | 81 (20.5%) | 45 (22.2%) | 36 (18.8%) | 0.400 | 44 (20.2%) | 37 (20.9%) | 0.860 |
| Diuretics | 71 (18.0%) | 32 (15.8%) | 39 (20.3%) | 0.239 | 40 (18.3%) | 31 (17.5%) | 0.830 |
Values for categorical variables are given as count (percentage); values for continuous variables as mean ± standard deviation; non-normally distributed data as median [quartile 1–quartile 3].
ACE angiotensin-converting enzyme, ARB angiotensin receptor blocker, CKD chronic kidney disease, GFR glomerular filtration rate, UACR urinary albumin-creatinine ratio.
Primary endpoints
The eGFR slope analysis results are presented in Table 2. During observation for 108 weeks, statistical analysis of the population mean eGFR slope values of individual patients revealed no significant differences between the febuxostat and placebo groups (between-group difference, 0.31 mL/min/1.73 m2/year; P = 0.76). However, when stratified by proteinuria, the mean eGFR slope values significantly differed between the febuxostat and placebo groups (between-group difference, 0.72 mL/min/1.73 m2/year; P = 0.007) in the subcohort without proteinuria. The interaction between febuxostat treatment and the presence of proteinuria in terms of eGFR slope was significant (P for interaction = 0.02). When stratified by serum creatinine level, a similar tendency was observed: the mean eGFR slopes differed between the febuxostat and placebo groups (between-group difference, 0.53 mL/min/1.73 m2/year; P = 0.06) in the subcohort with serum creatinine level < median.
Table 2.
Analysis of the eGFR slopes.
| Cohort | Group | N | Mean | Between-group difference, mL/min/1.73 m2/year (95% CI) | P | P for interaction |
|---|---|---|---|---|---|---|
| Entire cohort | Placebo | 203 | − 0.46 | 0.31 (− 0.11 to 0.73) | 0.762 | NA |
| Febuxostat | 192 | − 0.15 | ||||
| Entire cohort | Proteinuria-negative | 218 | 0.20 | − 1.15 (− 1.56 to − 0.74) | < 0.001 | NA |
| Proteinuria-positive | 177 | − 0.94 | ||||
| Entire cohort | s-Cre < median | 192 | 0.19 | − 0.98 (− 1.39 to − 0.57) | < 0.001 | NA |
| s-Cre ≥ median | 203 | − 0.79 | ||||
| Proteinuria | 0.019 | |||||
| Negative | Placebo | 110 | − 0.15 | 0.72 (− 0.20 to 1.24) | 0.007 | |
| Febuxostat | 108 | 0.60 | ||||
| Positive | Placebo | 93 | − 0.82 | − 0.25 (− 0.89 to 0.39) | 0.439 | |
| Febuxostat | 84 | − 1.08 | ||||
| s-Cre | 0.283 | |||||
| < Median | Placebo | 98 | − 0.07 | 0.53 (− 0.02 to 1.09) | 0.061 | |
| Febuxostat | 94 | 0.47 | ||||
| ≥ Median | Placebo | 105 | − 0.83 | 0.09 (− 0.52 to 0.69) | 0.780 | |
| Febuxostat | 98 | − 0.74 | ||||
| Cross-classified cohort | 0.064 | |||||
| Proteinuria-negative and s-Cre < median | Placebo | 52 | 0.45 | 0.64 (− 0.09 to 1.38) | 0.086 | |
| Febuxostat | 55 | 1.09 | ||||
| Proteinuria-negative and s-Cre ≥ median | Placebo | 58 | − 0.69 | 0.72 (0.03 to 1.41) | 0.040 | |
| Febuxostat | 53 | 0.03 | ||||
| Proteinuria-positive and s-Cre < median | Placebo | 46 | − 0.65 | − 0.24 (− 0.54 to 1.01) | 0.543 | |
| Febuxostat | 39 | − 0.41 | ||||
| Proteinuria-positive and s-Cre ≥ median | Placebo | 47 | − 0.99 | − 0.65 (− 0.33 to 1.64) | 0.191 | |
| Febuxostat | 45 | − 1.65 |
CI confidence interval, eGFR estimated glomerular filtration rate, s-Cre serum creatinine.
When cross-classified by proteinuria and serum creatinine level, the mean eGFR slopes significantly differed between the febuxostat and placebo groups (between-group difference, 0.72 mL/min/1.73 m2/year; P = 0.04) in the cross-classified subcohorts without proteinuria and with the serum creatinine level ≥ median, but not in the cross-classified subcohorts with proteinuria and serum creatinine level < median (between-group difference, − 0.24 mL/min/1.73 m2/year; P = 0.5), indicating that the presence of proteinuria exhibited an association stronger than a high serum creatinine level with febuxostat efficacy (Fig. 2). Interactions between febuxostat treatment and a proteinuria/serum creatinine level ≥ median in terms of the eGFR slope were significant (P for interaction = 0.06).
Figure 2.
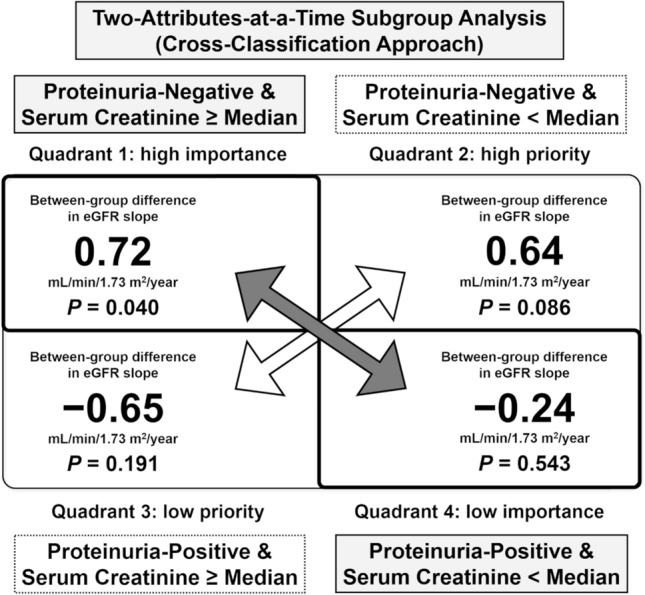
Cross-classification approach (importance grid analysis) for attributes regarding between-group differences in the estimated glomerular filtration rate (eGFR) slope. Quadrant 1: without proteinuria and serum creatinine level ≥ median; Quadrant 2: without proteinuria and serum creatinine level < median; Quadrant 3: with proteinuria and serum creatinine level ≥ median; Quadrant 4: with proteinuria and serum creatinine level < median. Quadrants 1 and 4 can be used to discriminate the importance of the absence of proteinuria and a serum creatinine level < median for the efficacy of febuxostat in patients with chronic kidney disease. As the between-group difference in the eGFR slope in quadrant 1 was significant for the efficacy of febuxostat, despite the fact that the serum creatinine level was ≥ median, the absence of proteinuria is a more important attribute compared to the serum creatinine level being < median. Accordingly, the absence of proteinuria is an attribute affecting the efficacy of febuxostat in patients with chronic kidney disease (all values were derived from Table 1).
Secondary endpoints
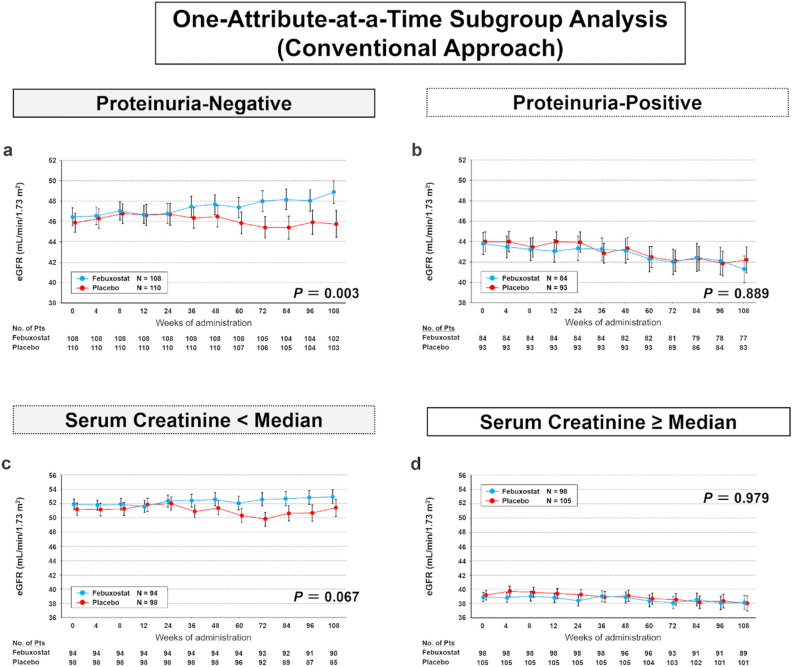
Supplementary Figs. S2 and S3 show time-course changes in the eGFRs from weeks 0 to 108 of febuxostat treatment. Between-group differences in the mean eGFR increased at week 24 or later in the entire cohort (Supplementary Fig. S2) and significantly increased in the subcohort without proteinuria (Fig. 3a, P = 0.003); however, this increase was not observed in the subgroup with proteinuria (Fig. 3b). When stratified by the serum creatinine level, between-group differences in the mean eGFR increased in the subcohort with a serum creatinine level < median (Fig. 3c), which was not observed in the subgroup with a serum creatinine level ≥ median (Fig. 3d).
Figure 3.
Time-course changes in the estimated glomerular filtration rates (eGFRs) from week 0 through week 108 of treatment. (a) Subcohort of patients without proteinuria. (b) Subcohort of patients with proteinuria. (c) Subcohort of patients with serum creatinine level < median. (d) Subcohort of patients with serum creatinine level ≥ median. The mean eGFR in the two groups is shown at different timepoints during the trial. Error bars indicate the standard error. The mean eGFR is shown for participants with available levels at each timepoint. P-values were obtained by a test of the trend profile using a mixed model.
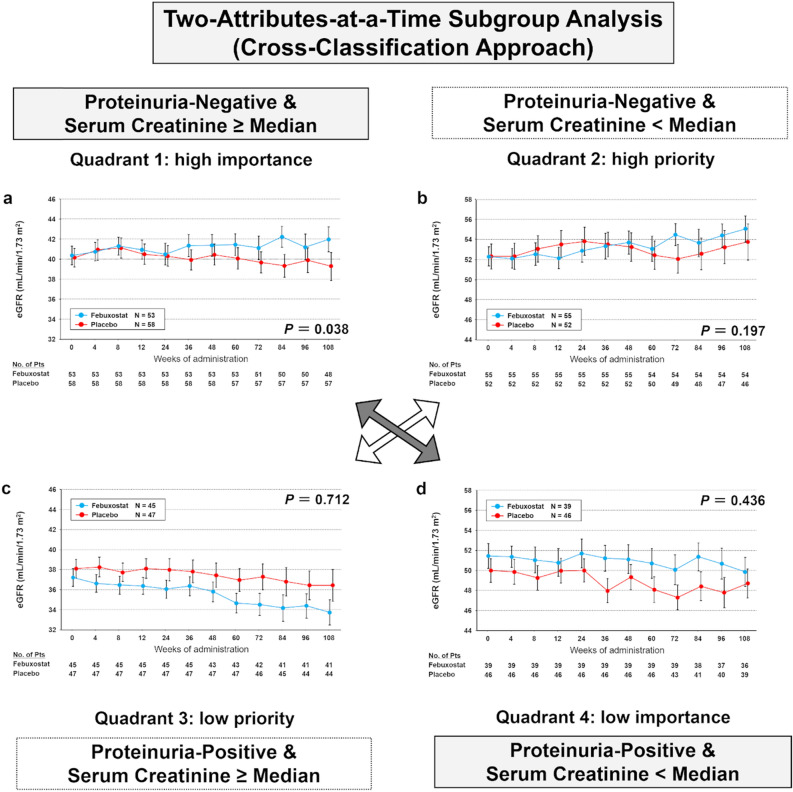
When cross-classified by the presence of proteinuria and serum creatinine level (Fig. 4), between-group differences in the mean eGFR significantly increased in the cross-classified subcohorts without proteinuria and serum creatinine level ≥ median (Fig. 4a, P = 0.04). In contrast, an increase was not observed in subcohorts with proteinuria and serum creatinine level < median (Fig. 4d), which was similar to the primary endpoint. These results indicate that the presence of proteinuria is more crucial than the serum creatinine level for febuxostat efficacy.
Figure 4.
Time-course changes in estimated glomerular filtration rates (eGFRs) from week 0 through week 108 of treatment in subcohorts according to cross-classification. (a) Quadrant 1: without proteinuria and a serum creatinine level ≥ median. (b) Quadrant 2: without proteinuria and a serum creatinine level < median. (c) Quadrant 3: with proteinuria and a serum creatinine level ≥ median. (d) Quadrant 4: with proteinuria and a serum creatinine level < median. The mean eGFR in the two groups is shown at different timepoints during the trial. Error bars indicate the standard error. The mean eGFR is shown for participants with available levels at each timepoint. P-values were obtained by a test of the trend profile using a mixed model. Similar to Fig. 2, quadrants 1 and 4 can be used to discriminate the importance of the absence of proteinuria and a serum creatinine level < median for the efficacy of febuxostat in patients with chronic kidney disease. As the trend profile in the time-course changes in the eGFRs in quadrant 1 was significant for the efficacy of febuxostat, despite the fact that the serum creatinine level was ≥ median, the absence of proteinuria is a more important attribute compared to the serum creatinine level being < median. Accordingly, the absence of proteinuria is an attribute affecting the efficacy of febuxostat in patients with chronic kidney disease.
Discussion
The pathophysiology and progression of CKD vary substantially across certain subcohorts based on the patients’ proteinuria status, diabetes status, CKD stage, and ethnicities1,23,28. Thus, the prognosis for slowly progressive kidney disease caused by hyperuricemia with febuxostat treatment should be assessed in cohorts presenting similar conditions, etiology, and pathophysiology. In the present post hoc analysis of the FEATHER study results, we clarified the differences between patients with and without proteinuria in terms of the efficacy of febuxostat in patients with stage 3 CKD using a cross-classification approach. To the best of our knowledge, this is the first study to elucidate that the presence of proteinuria rather than the serum creatinine level affects the efficacy of febuxostat. Febuxostat was useful for patients with stage 3 CKD without proteinuria, and its efficacy was confirmed even in the subcohort with a serum creatinine level ≥ median.
Recently, an RCT in patients with stage 3–4 CKD (Controlled Trial of Slowing of Kidney Disease Progression from the Inhibition of Xanthine Oxidase trial: CKD-FIX; follow-up, 104 weeks) failed to confirm the benefits of allopurinol on kidney outcomes29. However, an RCT does not provide a basis for extrapolation beyond the involved etiology, period, or drugs. The major differences between the present post hoc FEATHER study and the CKD-FIX study were the CKD stage (stage 3 vs. stages 3–4), eGFR (45.2 vs. 31.7 mL/min/1.73 m2), eGFR slope in the placebo group (− 0.31 vs. − 3.33 mL/min/1.73 m2/year), urinary albumin-to-creatinine ratio (117.0 vs. 716.9 mg/g), proportion of patients with diabetes (29% vs. 45%), ethnicity of the participants (100% Asian vs. 5% Asian), and the agent used (febuxostat vs. allopurinol). The major reason for the difference in efficacy of urate-lowering agents for patients with CKD is unknown, but may involve the presence or absence of proteinuria.
Proteinuria is an important risk factor for kidney diseases, and several patients with glomerulonephritis and diabetic nephropathy present with proteinuria26. However, in clinical settings, patients without proteinuria show progressive kidney diseases30 and develop end-stage renal disease. Most of these patients are clinically considered to have nephrosclerosis, which shows a slow progression of kidney dysfunction and is referred to as benign nephrosclerosis31. However, it has been recently shown that patients with nephrosclerosis have a poor long-term prognosis32. Nephrosclerosis is pathologically characterized as arterionephrosclerosis and subsequent tubulointerstitial inflammation/fibrosis. Arterionephrosclerosis is considered associated with systemic atherosclerosis. It arises because of various risk factors, including obesity, oxidative stress, and chronic inflammation, and has two pathological populations4,31. One is interstitial inflammatory fibrosis caused by ischemia/hypoxia/oxidative stress33 and the other is glomerular hypertrophy caused by losing autoregulation of renal blood flow resulting from an impairment of the afferent arteriole myogenic response31,34.
Notably, hyperuricemia has been associated with arteriosclerosis and arterionephrosclerosis/arteriolopathy in human9,10 and animal13,35,36 studies. Experimental studies of serum and intracellular hyperuricemic models have found that hyperuricemia causes renal arterionephrosclerosis/arteriolopathy owing to the activation of the renin–angiotensin–aldosterone system35,37 and decreased nitric oxide bioavailability35,37. This results in ischemic injury with tubulointerstitial inflammation/fibrosis12,35,37 caused by impaired renal ischemia/hypoxia/intrarenal oxidative stress, leading to glomerular hypertrophy12,38. Thus, hyperuricemia is considered to directly and indirectly cause nephrosclerosis.
Febuxostat, a non-purine selective inhibitor of xanthine oxidoreductase (XOR), inhibits both the reduced and oxidized forms of XOR and has been reported to attenuate experimental atherosclerosis in mice39. Xanthine oxide (XO) and XOR, both sources of reactive oxygen species, are associated with arteriolopathy and tubulointerstitial inflammation/fibrosis40. In animal models, febuxostat has been found to attenuate kidney injuries caused by impaired renal ischemia/hypoxia/intrarenal oxidative stress12,41, such as renal arterionephrosclerosis/arteriolopathy12,41,42, tubulointerstitial inflammation/fibrosis12,41,42, and glomerular hypertrophy12. Thus, hyperuricemia and increased XO and XOR activities in animal models result in arteriolopathy and tubulointerstitial inflammation/fibrosis, which is sometimes associated with arteriosclerosis-related diseases without excessive proteinuria; these data are consistent with the findings of human observational studies10,43. Therefore, febuxostat is expected to be effective for such conditions in humans44, and to the best of our knowledge, the present study is the first to show the effects of febuxostat in patients without proteinuria after six months. The reason for the lack of a febuxostat effect in less than six months is unclear, but may be related to variable glomerular size31,45,46. There are two types of nephrosclerosis, glomerular collapse and glomerular hypertrophy4,31, and the response to febuxostat may differ at individual nephron levels. Some glomeruli may increase the single-nephron GFR by improving ischemia and some glomeruli may decrease it by improving glomerular hyperfiltration/hypertrophy. Because of this difference, it may take six months for the average renoprotective effect of febuxostat to be detected. Furthermore, improvements in chronic tubular interstitial injury due to renal ischemia/hypoxia/intrarenal oxidative stress may require a long recovery time to restore the tissue damage47.
In RCTs for CKD, substantial and identifiable heterogeneity of risk in the trial population is pathophysiologically anticipated owing to the nature of the kidney disease, which can cause a chance bias16,25; therefore, we consider that the analysis should be adjusted for baseline attributes25. Patient-centered medicine and research have recently attracted attention15. In patient-centered research, it is important to evaluate a homogeneous patient group to overcome individual heterogeneity in human studies. Attribute-based studies of homogeneous patient groups are essential for patient-centered medicine, and the two-attributes-at-a-time subgroup analysis (cross-classification approach) can harmonize with RCTs in patients with CKD. Thus, the cross-classification approach will expand and contribute to subgroup analyses in future RCTs.
This study had limitations. As described in a previous study14, patients with stage 4 or 5 CKD were excluded. The number of patients was relatively low, and there were more male participants than female participants (males accounted for nearly four-fifths of the entire cohort). Since we adjusted the baseline characteristics using proteinuria and the serum creatinine level (which were confirmed using their interactions with kidney outcome in the original FEATHER study [RCT for CKD]), the attributes for which interaction had not been confirmed, including diuretics, were not completely adjusted. The proportion of patients using diuretics in this study was 18%, which is considered to have little effect on the results. However, the effect of diuretic-related hyperuricemia on kidney outcome remains to be studied. Patients without proteinuria were likely to have clinical nephrosclerosis; however, those who participated in this study were not diagnosed by kidney biopsy. Finally, the association between hyperuricemia and kidney outcomes may not be generalizable to other populations, as all participants were Japanese.
In conclusion, the cross-classification approach revealed that the presence or absence of proteinuria rather than the level of serum creatinine affects febuxostat efficacy. Compared with the placebo, febuxostat mitigated the decline in kidney function among patients with stage 3 CKD asymptomatic hyperuricemia without proteinuria.
Methods
Study design
We conducted a post hoc analysis of the results of the FEATHER study, which was a multicenter, randomized, double-blind, parallel-group, placebo-controlled, prospective cohort study in Japan. Patients were enrolled between November 2012 and January 2014, and their last visits were between March 2013 and February 2016. The FEATHER study was registered at the University Hospital Medical Information Network Clinical Trials Registry under UMIN000008343. The design and methods of the FEATHER study have been published previously14,48. The study protocol was approved by the ethics committee of each participating medical institution (Tokyo Women’s Medical University, No. 130902), and the study was conducted in accordance with the tenets of the Declaration of Helsinki. The pre-specified subgroups were based on the level of kidney function (median serum creatinine level determined in the primary FEATHER study) and presence or absence of proteinuria.
Participants
The screening criteria in the FEATHER study have been described previously48. In brief, the major inclusion criteria were age > 20 years, hyperuricemia (uric acid concentration > 7.0–10.0 mg/dL), stage 3 CKD, no history of gout, and patients agreed to provide written informed consent. The major exclusion criteria were uncontrolled diabetes mellitus or blood pressure and ≥ 50% variation in serum creatinine concentration within 12 weeks before eligibility confirmation. After screening in the primary FEATHER study, 443 patients from 55 nephrology centers were randomly assigned to the febuxostat or placebo group, and the data of 441 eligible patients were analyzed14. In this study, to adjust for an imbalance in the follow-up, 46 individuals who were observed for < 1 year and < 4 timepoints were excluded from the study cohort by the enrollment center (the Japan Clinical Research Support Unit, a not-for-profit organization in Tokyo, Japan). The data of the remaining 395 patients were distributed to an independent statistical expert for analyses (Supplementary Fig. S1).
Study endpoint
The primary endpoint was the eGFR slope (mL/min/1.73 m2/year) or annual change in the eGFR calculated using a linear mixed-effects model with repeated GFR estimates over time for each patient. Mean values and their standard errors for eGFR were also examined from baseline to week 108 to test the trend using a mixed model.
Statistical analysis
Data are presented as the mean ± standard deviation, median with interquartile range, or proportion. Group differences were evaluated using unpaired Student’s t-test, Wilcoxon rank-sum test, chi-square test, or Fisher’s exact test. Time-course changes in the eGFR from baseline through week 108 in the study population and subcohorts were analyzed by testing the trend profile using a mixed model. Analysis of the inconsistency in febuxostat treatment effects in pre-specified subgroups was explored regarding the primary endpoints by adding interaction terms to the generalized linear model. Statistical significance was set at P < 0.05. An independent statistical data center (STATZ Institute, Tokyo, Japan) performed statistical analyses using SAS ver. 9.4 software (SAS Institute, Cary, NC, USA).
Supplementary Information
Acknowledgements
The authors thank the members of the independent data monitoring committee, Tadao Akizawa, Tamio Teramoto, Hiroshi Kasanuki, and Kenichi Yoshimura, for data monitoring and Yoji Mitadera for administrative assistance. We appreciate the advice of Dr. Takahiro Mochizuki (deceased June 25, 2017) on the cross-classification approach and his contribution to medical care and medical research in Japan. Finally, we appreciate the support provided by Katsunori Shimada, Ph.D. (STATZ Institute, Tokyo, Japan), who provided expert assistance with the statistical analysis. FEATHER Study Investigators; Febuxostat Versus Placebo Randomized Controlled Trial Regarding Reduced Renal Function in Patients With Hyperuricemia Complicated by Chronic Kidney Disease Stage 3 (FEATHER) Study Investigators.
Author contributions
Conceptualization: H.K.; writing—original draft: H.K.; validation: Y.T., N.I., and R.Y.; supervision: T.M., M.O., K.T., K.N., K.K., and T.H. All authors have approved the final manuscript.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Competing interests
H.K. and T.M. belong to an endowed department sponsored by Otsuka Pharmaceutical Co., Chugai Pharmaceutical Co., Kyowa Hakko Kirin Co., and JMS Co. T.M. received honoraria for lectures from Otsuka Pharmaceutical Co. K.K. received personal fees from Public Health Research Foundation during the conduct of the study, as well as personal fees from Sanwa Kagaku Co, Ltd, Teijin Pharma Co., Ltd., Sekisui Medical Co., Ltd., Arkray Co., Ltd., Tanabe Mitsubishi Co, Ltd, Chugai Seiyaku Co, Ltd, Kowa Souyaku Co., Ltd., Nippon Tobacco Sangyo Co., Ltd., Fuji Yakuhin Co., Ltd., Dai-Nippon Sumitomo Seiyaku Co., Ltd., and Mylan Co., Ltd. outside the work described here. T.H. received personal fees from Public Health Research Foundation while conducting the study. All other authors have no conflicts to declare.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Hiroshi Kataoka, Email: kataoka@twmu.ac.jp.
the FEATHER Investigators:
Kenjiro Kimura, Tatsuo Hosoya, Sadayoshi Ito, Masaaki Inaba, Yasuhiko Tomino, Shunya Uchida, Hirofumi Makino, Seiichi Matsuo, Hisashi Yamanaka, Tetsuya Yamamoto, Iwao Ohno, Yugo Shibagaki, Satoshi Iimuro, Naohiko Imai, Masanari Kuwabara, Hiroshi Hayakawa, Tadao Akizawa, Tamio Teramoto, Hiroshi Kasanuki, Kenichi Yoshimura, Kenjiro Kimura, Tatsuo Hosoya, Yugo Shibagaki, Iwao Ohno, Hiroshi Sato, Shunya Uchida, Satoshi Horikoshi, Syoichi Maruyama, Masahiko Inaba, Yuji Moriwaki, Haruhito Uchida, Nagayuki Kaneshiro, Naohiko Imai, Hidekazu Moriya, Yasuhiro Komatsu, Shinya Kaname, Kazunari Hanaoka, Makoto Ogura, Masato Ikeda, Kenji Kasai, Akira Sugiura, Kazushi Takahashi, Kenichiro Kojima, Kosaku Nitta, Hirofumi Tamai, Hiroshi Nagaya, Senji Okuno, Ryusuke Kakiya, Hiroya Takeoka, Kyouji Hirata, Kenichiro Asano, Yasuo Fukaya, Yasushi Iwaida, Yasuo Tsuneda, Shigeaki Nishimura, Takeyuki Hiramatsu, Yoshitaka Isaka, Takafumi Ito, Yukio Yuzawa, Kunihiro Yamagata, Tadashi Sofue, Yoshimi Jinguji, Keita Hirano, Kazuhiro Matsuyama, Teruhiko Mizumoto, Yuko Shibuya, Masahiro Sugawara, Moritoshi Kadomura, Yasuaki Teshima, Hiroshi Ohtani, Hiroki Kamata, Susumu Okawara, Masaki Fukushima, Katsumi Takemura, Eriko Kinugasa, Masami Kogure, and Yoichi Ehara
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-07737-9.
References
- 1.Levey AS, et al. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 2.Babitt JL, et al. Controversies in optimal anemia management: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Conference. Kidney Int. 2021;99:1280–1295. doi: 10.1016/j.kint.2021.03.020. [DOI] [PubMed] [Google Scholar]
- 3.Levey AS, et al. The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 4.Meyrier A. Nephrosclerosis: A term in quest of a disease. Nephron. 2015;129:276–282. doi: 10.1159/000381195. [DOI] [PubMed] [Google Scholar]
- 5.Feig DI. Urate-lowering therapy and chronic kidney disease progression. N. Engl. J. Med. 2020;382:2567–2568. doi: 10.1056/NEJMe2015886. [DOI] [PubMed] [Google Scholar]
- 6.Wakasugi M, Kazama JJ, Narita I. Anticipated increase in the number of patients who require dialysis treatment among the aging population of Japan. Ther. Apher. Dial. 2015;19:201–206. doi: 10.1111/1744-9987.12266. [DOI] [PubMed] [Google Scholar]
- 7.Hsu CY, Iribarren C, McCulloch CE, Darbinian J, Go AS. Risk factors for end-stage renal disease: 25-year follow-up. Arch. Intern. Med. 2009;169:342–350. doi: 10.1001/archinternmed.2008.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kataoka, H. et al. Sex differences in time-series changes in pseudo-R(2) values regarding hyperuricemia in relation to the kidney prognosis. J. Pers. Med. 10 (2020). [DOI] [PMC free article] [PubMed]
- 9.Kohagura K, et al. An association between uric acid levels and renal arteriolopathy in chronic kidney disease: A biopsy-based study. Hypertension Res. 2013;36:43–49. doi: 10.1038/hr.2012.135. [DOI] [PubMed] [Google Scholar]
- 10.Momoki K, Kataoka H, Moriyama T, Mochizuki T, Nitta K. Hyperuricemia as a predictive marker for progression of nephrosclerosis: Clinical assessment of prognostic factors in biopsy-proven arterial/arteriolar nephrosclerosis. J. Atheroscler. Thromb. 2017;24:630–642. doi: 10.5551/jat.37523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanchez-Lozada LG, et al. Uric acid-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP concentrations. Nephron Exp. Nephrol. 2012;121:e71–78. doi: 10.1159/000345509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Omizo H, et al. Cardio-renal protective effect of the xanthine oxidase inhibitor febuxostat in the 5/6 nephrectomy model with hyperuricemia. Sci. Rep. 2020;10:9326. doi: 10.1038/s41598-020-65706-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanchez-Lozada LG, et al. Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int. 2005;67:237–247. doi: 10.1111/j.1523-1755.2005.00074.x. [DOI] [PubMed] [Google Scholar]
- 14.Kimura K, et al. Febuxostat therapy for patients with stage 3 CKD and asymptomatic hyperuricemia: A randomized trial. Am. J. Kidney Dis. 2018;72:798–810. doi: 10.1053/j.ajkd.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 15.Sacristan JA. Patient-centered medicine and patient-oriented research: Improving health outcomes for individual patients. BMC Med. Inform. Decis. Mak. 2013;13:6. doi: 10.1186/1472-6947-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kent DM, Steyerberg E, van Klaveren D. Personalized evidence based medicine: Predictive approaches to heterogeneous treatment effects. BMJ Clin. Res. 2018;363:k4245. doi: 10.1136/bmj.k4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N. Engl. J. Med. 2000;342:1887–1892. doi: 10.1056/NEJM200006223422507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kent DM, et al. The predictive approaches to treatment effect heterogeneity (PATH) statement. Ann. Intern. Med. 2020;172:35–45. doi: 10.7326/M18-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothwell PM, Mehta Z, Howard SC, Gutnikov SA, Warlow CP. Treating individuals 3: from subgroups to individuals: general principles and the example of carotid endarterectomy. Lancet. 2005;365:256–265. doi: 10.1016/S0140-6736(05)17746-0. [DOI] [PubMed] [Google Scholar]
- 20.Hayward RA, Kent DM, Vijan S, Hofer TP. Multivariable risk prediction can greatly enhance the statistical power of clinical trial subgroup analysis. BMC Med. Res. Methodol. 2006;6:18. doi: 10.1186/1471-2288-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kent DM, Hayward RA. Limitations of applying summary results of clinical trials to individual patients: The need for risk stratification. JAMA. 2007;298:1209–1212. doi: 10.1001/jama.298.10.1209. [DOI] [PubMed] [Google Scholar]
- 22.Jin, Y. & Park, Y. An integrated approach to determining rural tourist satisfaction factors using the IPA and conjoint analysis. Int. J. Environ. Res. Public Health. 16 (2019). [DOI] [PMC free article] [PubMed]
- 23.Kataoka H, et al. A body mass index-based cross-classification approach for the assessment of prognostic factors in chronic kidney disease progression. Kidney Blood Press. Res. 2019;44:362–383. doi: 10.1159/000501021. [DOI] [PubMed] [Google Scholar]
- 24.Glasser J, Feng Z, Moylan A, Del Valle S, Castillo-Chavez C. Mixing in age-structured population models of infectious diseases. Math Biosci. 2012;235:1–7. doi: 10.1016/j.mbs.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Roberts C, Torgerson DJ. Understanding controlled trials: Baseline imbalance in randomised controlled trials. BMJ (Clin. Res. Ed.) 1999;319:185. doi: 10.1136/bmj.319.7203.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma S, Smyth B. From proteinuria to fibrosis: An update on pathophysiology and treatment options. Kidney Blood Press. Res. 2021;46:411–420. doi: 10.1159/000516911. [DOI] [PubMed] [Google Scholar]
- 27.Badve SV, Pascoe EM. Allopurinol and chronic kidney disease. Reply. N. Engl. J. Med. 2020;383:1691–1692. doi: 10.1056/NEJMc2026125. [DOI] [PubMed] [Google Scholar]
- 28.Russo, E. et al. Association of uric acid with kidney function and albuminuria: The Uric Acid Right for heArt Health (URRAH) Project. J. Nephrol. (2021). [DOI] [PMC free article] [PubMed]
- 29.Badve SV, et al. Effects of allopurinol on the progression of chronic kidney disease. N. Engl. J. Med. 2020;382:2504–2513. doi: 10.1056/NEJMoa1915833. [DOI] [PubMed] [Google Scholar]
- 30.Manabe S, et al. Maximum carotid intima-media thickness in association with renal outcomes. J. Atheroscler. Thromb. 2021;28:491–505. doi: 10.5551/jat.57752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bohle A, Ratschek M. The compensated and the decompensated form of benign nephrosclerosis. Pathol. Res. Pract. 1982;174:357–367. doi: 10.1016/S0344-0338(82)80017-4. [DOI] [PubMed] [Google Scholar]
- 32.Ovrehus MA, et al. Clinical phenotypes and long-term prognosis in white patients with biopsy-verified hypertensive nephrosclerosis. Kidney Int. Rep. 2020;5:339–347. doi: 10.1016/j.ekir.2019.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neusser MA, et al. Human nephrosclerosis triggers a hypoxia-related glomerulopathy. Am. J. Pathol. 2010;176:594–607. doi: 10.2353/ajpath.2010.090268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyrier A. Nephrosclerosis: Update on a centenarian. Nephrol. Dial. Transplant. 2015;30:1833–1841. doi: 10.1093/ndt/gfu366. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez-Lozada LG, et al. Role of oxidative stress in the renal abnormalities induced by experimental hyperuricemia. Am. J. Physiol. Renal Physiol. 2008;295:F1134–1141. doi: 10.1152/ajprenal.00104.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang DH, et al. A role for uric acid in the progression of renal disease. J. Am. Soc. Nephrol. 2002;13:2888–2897. doi: 10.1097/01.asn.0000034910.58454.fd. [DOI] [PubMed] [Google Scholar]
- 37.Mazzali M, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38:1101–1106. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- 38.Nakagawa T, et al. Hyperuricemia causes glomerular hypertrophy in the rat. Am. J. Nephrol. 2003;23:2–7. doi: 10.1159/000066303. [DOI] [PubMed] [Google Scholar]
- 39.Nomura J, et al. Xanthine oxidase inhibition by febuxostat attenuates experimental atherosclerosis in mice. Sci. Rep. 2014;4:4554. doi: 10.1038/srep04554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gwinner W, Scheuer H, Haller H, Brandes RP, Groene HJ. Pivotal role of xanthine oxidase in the initiation of tubulointerstitial renal injury in rats with hyperlipidemia. Kidney Int. 2006;69:481–487. doi: 10.1038/sj.ki.5000121. [DOI] [PubMed] [Google Scholar]
- 41.Kim HS, et al. The protective effect of febuxostat on chronic tacrolimus-induced nephrotoxicity in rats. Nephron. 2017;135:61–71. doi: 10.1159/000449289. [DOI] [PubMed] [Google Scholar]
- 42.Sanchez-Lozada LG, et al. Effect of febuxostat on the progression of renal disease in 5/6 nephrectomy rats with and without hyperuricemia. Nephron Physiol. 2008;108:p69–78. doi: 10.1159/000127837. [DOI] [PubMed] [Google Scholar]
- 43.Tsai CW, Lin SY, Kuo CC, Huang CC. Serum uric acid and progression of kidney disease: A longitudinal analysis and mini-review. PLoS ONE. 2017;12:e0170393. doi: 10.1371/journal.pone.0170393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chou HW, et al. Comparative effectiveness of allopurinol, febuxostat and benzbromarone on renal function in chronic kidney disease patients with hyperuricemia: A 13-year inception cohort study. Nephrol. Dial. Transplant. 2018;33:1620–1627. doi: 10.1093/ndt/gfx313. [DOI] [PubMed] [Google Scholar]
- 45.Kataoka H, Ohara M, Honda K, Mochizuki T, Nitta K. Maximal glomerular diameter as a 10-year prognostic indicator for IgA nephropathy. Nephrol. Dial. Transplant. 2011;26:3937–3943. doi: 10.1093/ndt/gfr139. [DOI] [PubMed] [Google Scholar]
- 46.Kataoka H, Mochizuki T, Nitta K. Large renal corpuscle: Clinical significance of evaluation of the largest renal corpuscle in kidney biopsy specimens. Contrib. Nephrol. 2018;195:20–30. doi: 10.1159/000486931. [DOI] [PubMed] [Google Scholar]
- 47.Zeisberg M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 2010;21:1819–1834. doi: 10.1681/ASN.2010080793. [DOI] [PubMed] [Google Scholar]
- 48.Hosoya T, et al. The effect of febuxostat to prevent a further reduction in renal function of patients with hyperuricemia who have never had gout and are complicated by chronic kidney disease stage 3: Study protocol for a multicenter randomized controlled study. Trials. 2014;15:26. doi: 10.1186/1745-6215-15-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.