Abstract
Background
Treatments for non-small cell lung cancer (NSCLC) have improved tremendously, but therapeutic resistance is a common and major clinical challenge in treatment. Methyltransferase-like 3 (METTL3) is a ribonucleic acid (RNA) methyltransferase that has crucial functions in the development and progression of cancers, including drug resistance, by regulating N6-methyladenosine (m6A) modification. However, the role of METTL3 in the progression and drug resistance of NSCLC is poorly understood.
Methods
The expression levels of METTL3 and AKT serine/threonine kinase 1 (AKT1) in NSCLC tissues were detected using quantitative real-time PCR (RT-qPCR), Western blots, and immunohistochemical assays. The m6A levels of AKT1 messenger RNA (mRNA) in NSCLC tissues were detected using m6A methylated RNA immunoprecipitation–quantitative polymerase chain reaction.
Results
The expression levels of METTL3 and the AKT1 protein were significantly increased in NSCLC tissues, and m6A expression levels of AKT1 mRNA were dramatically upregulated in NSCLC tissues. Additionally, METTL3, AKT1 protein, and m6A levels of AKT1 mRNA were overexpressed in chemoresistant NSCLC samples, and high expression levels of METTL3 and AKT1 were correlated with poor patient survival, especially in chemoresistant NSCLC patients. Further, AKT1 protein expression and m6A levels of AKT1 mRNA were positively correlated with METTL3 expression, and AKT1 protein expression was positively correlated with m6A levels of AKT1 mRNA. Moreover, METTL3 and AKT1 protein expression levels were significantly associated with cisplatin susceptibility, tumor, node, metastasis stage, and lymph node metastasis.
Conclusions
Taken together, our results indicate that METTL3 contributes to the progression and chemoresistance of NSCLC by promoting AKT1 protein expression through regulating AKT1 mRNA m6A levels, and may provide an efficient therapeutic intervention target for overcoming chemoresistance in NSCLC.
Keywords: Non-small cell lung cancer (NSCLC), methyltransferase-like 3 (METTL3), AKT serine/threonine kinase 1 (AKT1), chemoresistance, biomarker
Introduction
Lung cancer is a malignant cancer with the highest mortality rate in the world (1). Non-small cell lung cancer (NSCLC) is one of the biggest causes of cancer mortality worldwide (2), and accounts for >80% of all lung cancer cases (3). Chemotherapy is a traditional therapy for NSCLC (4), and cisplatin (DDP) is a clinical drug used for chemotherapy and has become the standard treatment for NSCLC patients due to its good performance (5). Unfortunately, long-term cisplatin treatment leads to drug resistance, which in turn usually leads to NSCLC treatment failure (6). Inducing DDP susceptibility is a sound strategy for improving the treatment of NSCLC patients.
Epigenetics refers to the differential expression of genes based on changes in non-gene sequences, including chromatin conformation, deoxyribonucleic acid (DNA) methylation, and (ribonucleic acid) RNA methylation changes (7-11). In the last few decades, epigenetics has been found to be closely related to tumorigenesis and development (12,13). DNA methylation has been found involved in cancer progression and chemoresistance (14,15), and played as potential target for cancer treatment (16). The LINE-1 methylation level in hepatocellular carcinomas (HCC) tissues was significantly lower than in matched normal liver tissues and reduces the disease-free survival rates of HCC patients, the results suggested that LINE-1 methylation level might play as a biomarker for identifying patients who will experience an unfavorable clinical outcome (17). Zhang et al. found that the methylation-induced downregulation of HOXA11 might be a diagnostic and prognostic marker and contrite to cisplatin resistance in patients with lung adenocarcinoma (18). Recently, more and more studies have focused on RNA methylation, which was discovered to be a post-transcription epigenetic modification (19,20). N6-methyladenosine (m6A) is an important, and the most abundant, type of RNA methylation modification, and regulates RNA translation, stability, splicing, and nucleation (21,22). Recently, studies have shown that m6A methylation is bound up with the occurrence, progression, and drug resistance of human cancers (23-25). In recently, studies have revealed that the tumor microenvironment (TME) is associated with m6A. It has been reported that m6A enhances the anti-cancer response of tumor-infiltrating CD8+ T cells, improves the therapeutic effect of anti-PDL1 receptor blockers (26), and Liu et al. demonstrated that m6A modification plays an important role in tumorigenesis and TME infiltration characterization of low-grade gliomas (27). Also, Wang et al. found that an m6A score based on IGF2BP2, IGF2BP3, KIAA1429, METTL3, EIF3H and LRPPRC expression were proposed as an indicator of tumor microenvironment status and were instrumental in predicting the prognosis of pancreatic cancer patients (28). Furthermore, the tumor microenvironment characterization revealed that the identified m6A patterns were highly consistent with immune-inflamed, immune-excluded and immune-desert, and patients with lower m6Sig scores showed a better immune responses and durable clinical benefits in three independent immunotherapy cohorts (29). m6A methylation is catalyzed by component “writer” methyltransferase complexes [methyltransferase-like 3 (METTL3), methyltransferase-like 14, and WT1 associated protein (WTAP)] and is removed by “eraser” demethylases [FTO alpha-ketoglutarate dependent dioxygenase (FTO) and alkB homolog 5 (ALKBH5)] (30).
METTL3 has been identified as the main methyltransferase complex and plays a critical role in m6A methylation (31). The dysregulation of METTL3 regulates the total m6A methylation level, which directly affects the decay and translation of messenger RNA (mRNA) and microRNA biogenesis, which contribute to human diseases (32-35). METTL3 was found to be upregulated in gastric cancer, and the high expression of METTL3 is predictive of a poor prognosis (36). Peng et al. found that the upregulation of METTL3 results in abnormal m6A modification and is positively correlated with tumor metastasis in colorectal cancer (37). Additionally, METTL3 serves as an oncogenic gene in bladder cancer by interacting with DGCR8 microprocessor complex subunit (DGCR8) and positively regulating the pri-miR221/222 process (38). In NSCLC, METTL3 promotes the Warburg effect of NSCLC by promoting ABHD11-AS1 expression via installing the m6A modification and enhancing ABHD11-AS1 transcript stability (39). The m6A reader YT521-B homology domain containing 2 (YTHDC2) is frequently downregulated in lung adenocarcinoma and low expression of YTHDC2 was associated with poor clinical outcome (40). The m6A mRNA methylation initiated by METTL3 promotes YAP mRNA translation via recruiting YTHDF1/3 and eIF3b to the translation initiation complex and increases YAP mRNA stability through regulating the MALAT1-miR-1914-3p-YAP axis. The increased YAP expression and activity induce NSCLC drug resistance and metastasis (41). The results of previous studies showed different roles of m6A mRNA methylation in progression of NSCLC. However, few studies have reported on the functions of RNA methylation in lung cancer drug resistance, and very little is known about the important role of METTL3 in the chemoresistance of NSCLC.
In this study, we found that METTL3 was upregulated in NSCLC tissues, and its expression levels in chemoresistant NSCLC patients were significantly higher than those of chemosensitive NSCLC patients. Further, higher expression levels of METTL3 were also highly associated with shorter overall survival (OS). We also showed that AKT1 protein expression was negatively correlated with METTL3 expression. Our findings show the important role of the METTL3-AKT1 axis in the progression and chemoresistance of NSCLC, and may provide a therapeutic intervention target for NSCLC treatment.
We present the following article in accordance with the MDAR checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-21-6608/rc).
Methods
Patients and tissue samples
The data of 180 NSCLC patients, who were treated with DDP therapy at the Sun Yat-sen University Cancer Center from March 2015 to September 2016, were collected. During the DDP treatment, these patients were evaluated once every 2 weeks for drug effectiveness. Recurrent NSCLC was defined (solid tumors, RECIST 1.1) as a 5 mm or 20% increase in the total lesion diameter from the baseline (42,43). The NSCLC tissues and adjacent normal tissues (>3 cm from tumor tissues) were collected during the surgical resection, and a pathological puncture biopsy was performed. Ninety NSCLC tissues were identified as sensitive NSCLC tissues (primary), and another 90 were identified as resistant NSCLC tissues (recurrent). Written informed consent was obtained from all the patients before the study. This study was approved by the Ethics Committee of Sun Yat-sen University Cancer Center (No. GZR2020-297) and performed in accordance with the Declaration of Helsinki (as revised in 2013).
RT-qPCR
Total RNA from tissues were isolated using TRIzol reagent (TransGen Biotech, Beijing, China) and be quantitated using NanoDrop™ 2000/2000c Spectrophotometers (Thermo Scientific™). The reverse transcription of 2 μg of total RNA into first-strand complementary DNA (cDNA) was performed using the PrimeScript™ RT Master Mix (Takara Biotechnology, Beijing, China). RT-qPCR assay was performed using the SYBR Green qPCR Master Mix (MedChemExpress) in the Applied Biosystems 7500 Real-Time PCR system (ABI, USA) under the following reaction conditions: a denaturation step for 5 min at 94 °C, 38 cycles of 30 s at 94 °C, 30 s at 60 °C, and 30 s at 72 °C. The human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as an internal control, and the data were analyzed using the 2−∆∆Ct method. The primers for qPCR were as follows: forward primer for METTL3: 5'-AGAGTGTCGGAGGTGATT-3' and reverse primer for METTL3: 5'-TAGTACGGGTATGTTGAGC-3'; forward primer for AKT1: 5'-TCTTTGCCGGTATCGTGT-3' and reverse primer for AKT1: 5'-TGTCATCTTGGTCAGGTGGT-3'; and forward primer for GAPDH: 5'-CTCCTCCTGTTCGACAGTCAGC-3' and reverse primer for GAPDH: 5'-CCCAATACGACCAAATCCGTT-3'.
m6A methylated RIP‐qPCR
The methylated RNA immunoprecipitation–quantitative polymerase chain reaction (RIP-qPCR) analyses were performed as previously described (44). Briefly, the extracted RNA was fragmented by RNA fragmentation reagents (Thermo Scientific™) and 2 μg of RNA was mixed with protein A beads (Thermo Fisher Scientific, Waltham, MA) and an anti-m6A antibody (Abcam, ab151230) in an immunoprecipitation buffer for m6A-immunoprecipitation. Later, the beads were eluted with elution buffer containing 20 mg/mL of proteinase K (10401ES60, Yeasen, Shanghai, China) twice. After elution and recovery, RNA enrichment was analyzed by qPCR.
Western blot
Total protein from tissues were isolated using the cell-lysis buffer for the Western blot containing 1 mM of phenylmethanesulfonyl fluoride (Catalog number: 36978, Thermo Scientific™), and was quantified using a Bicinchoninic Acid Assay (BCA) Protein Assay Kit (P0012S, Beyotime Biotechnology, Shanghai, China). Western blot was performed as previously described (45). Primary antibodies, including METTL3 antibody (ab195352, dilution 1:1,000), AKT1 antibody (ab233755, dilution 1:1,000), and GAPDH antibody (ab9485, Dilution 1:1,000), were purchased from Abcam. Second antibodies, including Horseradish Peroxidase (HRP) goat anti-mouse (or anti-rabbit) IgG (BA1051 and BA1054), were purchased from Boster Biological Technology (Wuhan, China).
Immunohistochemical assay
Immunohistochemical (IHC) assays were performed to analyze the expression of METTL3 and AKT1 as previously described (45). The tissues were cut into 5-μm thick sections and deparaffinized with xylene. METTL3 (1:500 dilution; ab195352) and AKT1 (1:500 dilution; ab233755) antibodies were used in the study.
Statistical analysis
The data are showed as mean ± standard deviation, and were statistically analyzed using SPSS (standard version 20.0; SPSS, Chicago, IL) software. The χ2-test was used to analyze the correlations between METTL3 or AKT1 expression and the clinicopathologic features of NSCLC patients. The correlation between METTL3 expression levels and AKT1 expression levels or m6A levels of AKT1 mRNA in NSCLC tissues was determined by Pearson correlation coefficients. A univariate survival analysis was conducted using the Kaplan-Meier method, and the log-rank test was used to evaluate differences between the survival curves. The independent sample T test was used for statistical analyses between 2 groups. A P value >0.05 was considered s statistically significant.
Results
METTL3 expression is correlated with chemotherapy resistance and poor prognosis in NSCLC patients
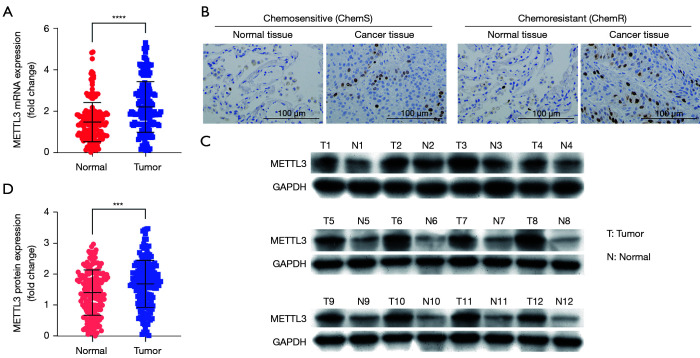
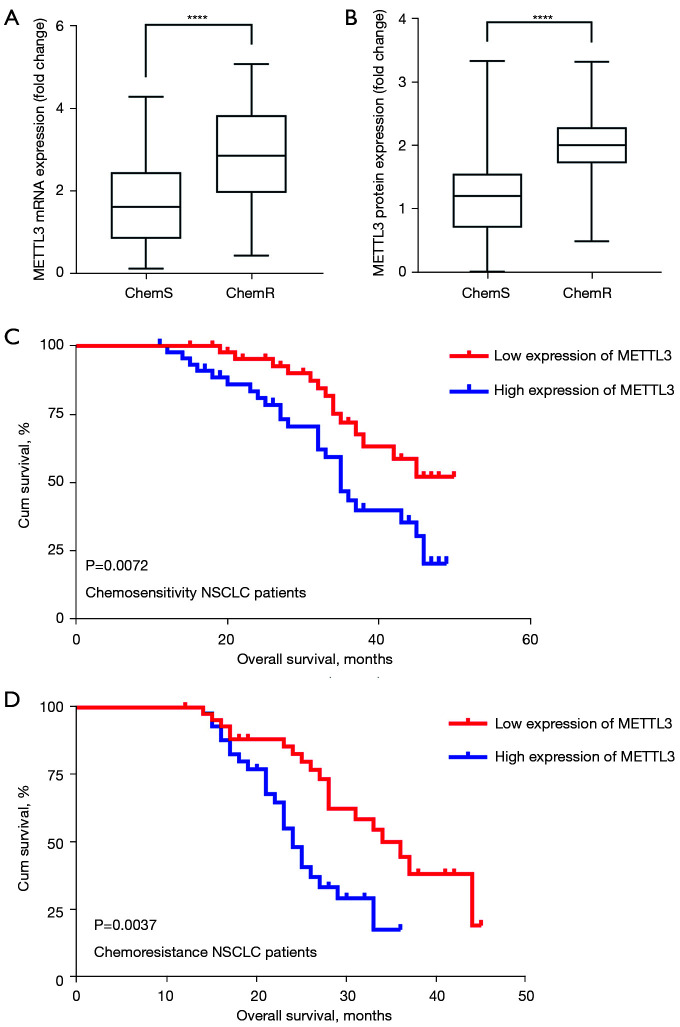
To investigate whether METTL3 expression was related to the progression of NSCLC and chemosensitivity in patients, we evaluated the expression levels of METTL3 in 180 pairs of NSCLC tissues. The qPCR results showed that the expression levels of METTL3 mRNA were dramatically increased in cancer tissues (n=180; P<0.0001; see Figure 1A). Additionally, the IHC and Western blot results revealed that METTL3 protein expression levels in cancer tissues were increased (P<0.001; see Figure 1B-1D). Further, the expression of METTL3 in chemoresistant NSCLC tissues and chemosensitive tissues were quantified, and the mRNA and protein expression levels of METTL3 were higher in the chemoresistant NSCLC tissues (n=90) than the chemosensitive NSCLC tissues (n=90; P<0.0001; see Figure 2A,2B).
Figure 1.
METTL3 is upregulated in NSCLC tissues. (A) The RNA levels of METTL3 were analyzed by qPCR assay; (B) IHC staining images of METTL3 from NSCLC tissues and adjacent normal tissues; (C,D) Western blot analysis of METTL3 protein expression. ***P<0.001, ****P<0.0001. METTL3, methyltransferase-like 3; NSCLC, non-small cell lung cancer.
Figure 2.
METTL3 is correlated with poor OS in NSCLC patients. (A,B) Quantification of METTL3 mRNA and protein expression in chemoresistant and chemosensitive NSCLC tissues; (C,D) the relationship between METTL3 expression and OS of NSCLC patients was analyzed using Kaplan-Meier survival curves. ****P<0.0001. METTL3, methyltransferase-like 3; OS, overall survival; NSCLC, non-small cell lung cancer.
Additionally, the association between METTL3 protein expression and the clinicopathological features of NSCLC patients was also explored. To assess the statistical significance, NSCLC patients were divided into 2 groups (high and low groups) according to the median (cut-off =1.729) of METTL3 protein expression. Our results showed that METTL3 protein expression was significantly associated with a number of clinicopathological features, including DDP susceptibility (P<0.0001), tumor, node, metastasis (TNM) stage (P<0.0001), and lymph node metastasis (P<0.0001; see Table 1). Additionally, the Kaplan-Meier analysis showed that the high protein expression of METTL3 was correlated with shorter OS, especially in chemoresistant NSCLC patients (see Figure 2C,2D). Thus, METLT3 is a potential biomarker that can predict chemotherapy resistance and the outcomes of NSCLC patients.
Table 1. Correlation between the protein expression of METTL3 and clinicopathological features in 180 cases of NSCLC.
| Factors | METTL3 protein expression (n=180) | P value | |
|---|---|---|---|
| Low expression (n=90) | High expression (n=90) | ||
| Age | 0.5509 | ||
| <60 years | 46 | 42 | |
| ≥60 years | 44 | 48 | |
| Gender | 0.7656 | ||
| Male | 46 | 44 | |
| Female | 44 | 46 | |
| TNM stage | <0.0001 | ||
| I–II | 64 | 25 | |
| III–IV | 26 | 65 | |
| DDP | <0.0001 | ||
| Sensitive | 78 | 12 | |
| Resistant | 12 | 78 | |
| Lymph node metastasis | <0.0001 | ||
| Yes | 18 | 70 | |
| No | 72 | 20 | |
| Smoking status | 0.8815 | ||
| Smoker | 46 | 45 | |
| Non-smoker | 44 | 45 | |
METTL3, methyltransferase-like 3; NSCLC, non-small cell lung cancer; DDP, cisplatin.
AKT1 expression is correlated with chemotherapy resistance and a poor prognosis in NSCLC patients
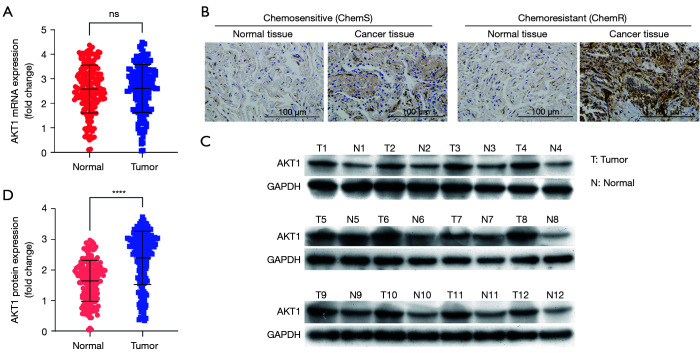
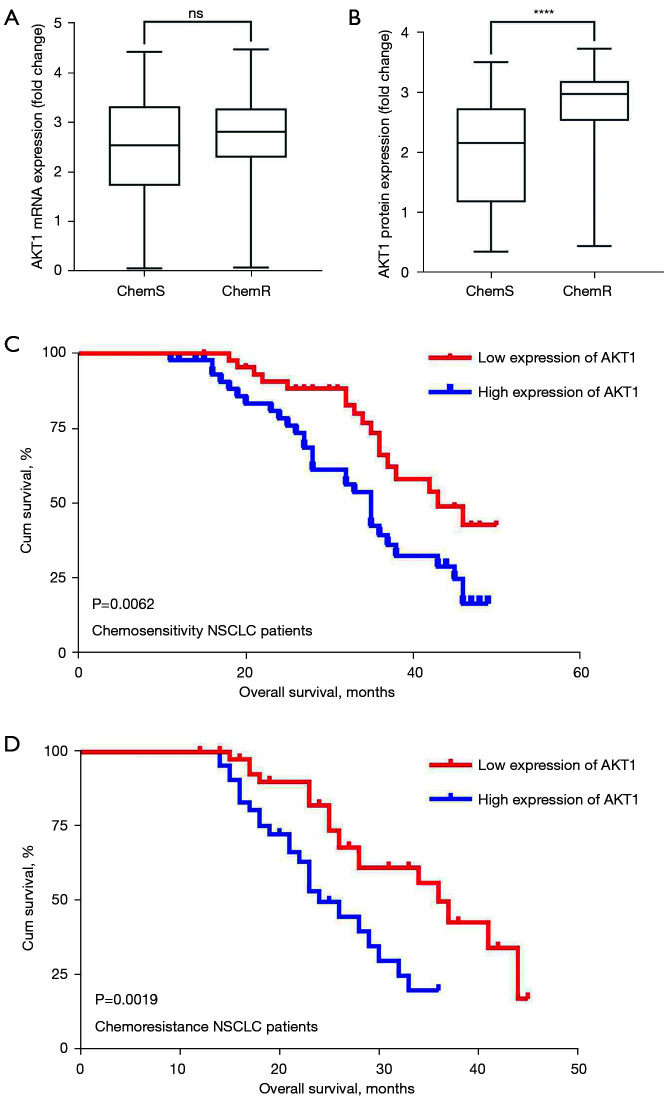
A previous study indicated that m6A mRNA methylation regulates AKT1 activity, and promotes the tumorigenicity of endometrial cancer (46). However, it is not yet known whether AKT1 is involved in the progression of NSCLC and chemoresistance. We evaluated the expression levels of AKT1 in 180 pairs of NSCLC tissues. The qPCR results showed that there was no statistically significant difference between NSCLC tissues and tumor-adjacent normal tissues in terms of AKT1 mRNA expression (see Figure 3A). Notably, the IHC and Western blot results revealed increased AKT1 protein expression in NSCLC tissues (P<0.0001; see Figure 3B-3D). Further, the expression of AKT1 in chemoresistant NSCLC tissues and chemosensitive tissues were quantified, and the protein expression levels of AKT1 were higher in the chemoresistant NSCLC tissues (n=90) than the chemosensitive NSCLC tissues (n=90), but no significant difference in mRNA levels was found (see Figure 4A,4B).
Figure 3.
AKT1 is upregulated in NSCLC tissues. (A) The RNA level of AKT1 was analyzed by qPCR assays; (B) IHC staining images of AKT1 from NSCLC tissues and adjacent normal tissues; (C,D) Western blot analysis of AKT1 protein expression. ****P<0.0001. AKT1, AKT serine/threonine kinase 1; NSCLC, non-small cell lung cancer.
Figure 4.
AKT1 protein is correlated with the poor OS of NSCLC patients. (A,B) AKT1 mRNA and protein were quantified in chemoresistant and chemosensitive NSCLC tissues; (C,D) the relationship between AKT1 expression and the OS of NSCLC patients was analyzed using Kaplan-Meier survival curves. ****P<0.0001. AKT1, AKT serine/threonine kinase 1; OS, overall survival; NSCLC, non-small cell lung cancer.
Additionally, the association between AKT1 protein expression and the clinicopathological features of NSCLC patients was also explored. To assess the statistical significance, the NSCLC patients were divided into high and low AKT1 protein expression groups according to the median (cut-off =2.642) of AKT1 protein expression. As Table 2 shows, AKT1 protein expression was significantly associated with clinicopathological features, including DDP susceptibility (P<0.0001), TNM stage (P=0.0006), and lymph node metastasis (P<0.0001). Additionally, the high protein expression of AKT1 was significantly correlated with poor survival, especially in chemoresistant NSCLC patients (see Figure 4C,4D).
Table 2. Correlation between protein expression of AKT1 and clinicopathological features in 180 cases of NSCLC.
| Factors | ATK1 protein expression (n=180) | P value | |
|---|---|---|---|
| Low expression (n=90) | High expression (n=90) | ||
| Age | 0.1797 | ||
| <60 years | 49 | 40 | |
| ≥60 years | 41 | 50 | |
| Gender | >0.9999 | ||
| Male | 46 | 46 | |
| Female | 44 | 44 | |
| TNM stage | 0.0006 | ||
| I–II | 56 | 33 | |
| III–IV | 34 | 57 | |
| DDP | <0.0001 | ||
| Sensitive | 64 | 26 | |
| Resistant | 26 | 64 | |
| Lymph node metastasis | <0.0001 | ||
| Yes | 29 | 59 | |
| No | 61 | 31 | |
| Smoking status | 0.2967 | ||
| Smoker | 49 | 42 | |
| Non-smoker | 41 | 48 | |
AKT1, AKT serine/threonine kinase 1; NSCLC, non-small cell lung cancer; DDP, cisplatin.
Correlation between METTL3 protein expression and AKT1 expression in NSCLC
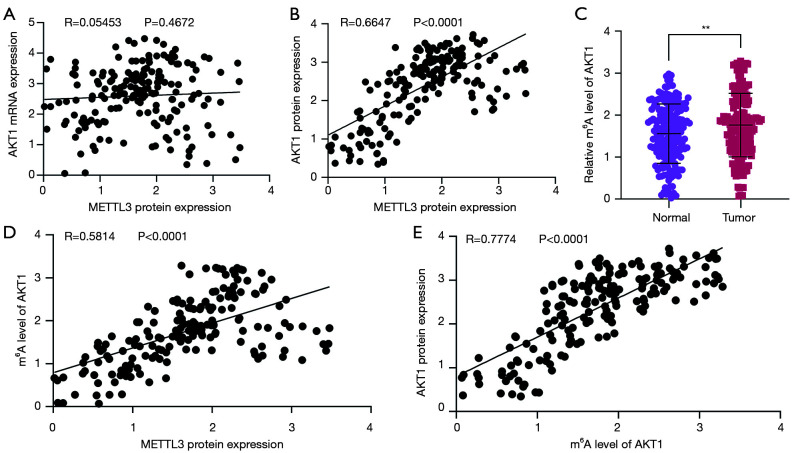
Based on our findings, we examined whether METTL3 regulated AKT1 expression through m6A. First, we analyzed the correlation between METTL3 and AKT1 expression levels using Pearson correlation coefficients, and found that the mRNA expression of AKT1 was not correlated with METTL3 protein expression (R=0.05453, P=0.4672; see Figure 5A), but the protein expression of AKT1 was positively correlated with METTL3 protein expression (R=0.6647, P<0.0001; see Figure 5B). Further, we evaluated the m6A levels of AKT1 in 180 NSCLC tissues using the MeRIP-qPCR method, and found the m6A levels of AKT1 were significantly upregulated in NSCLC tissues (P<0.01; see Figure 5C). Moreover, the m6A levels of AKT1 were positively correlated with METTL3 protein expression (R=0.5814, P<0.0001; see Figure 5D), and were significantly positively correlated with the m6A levels of AKT1 (R=0.7774, P<0.0001; see Figure 5E). All above, our results indicated that METTL3 contributes to the progression and chemoresistance of NSCLC by promoting AKT1 protein expression via the regulation of AKT1 mRNA m6A levels.
Figure 5.
Correlation between METTL3 protein expression and AKT1 expression in NSCLC. (A) The relationship between the METTL3 protein and AKT1 mRNA in NSCLC tissues was analyzed; (B) the relationship between the METTL3 protein and AKT1 protein in NSCLC tissues was analyzed; (C) the m6A levels of ATK1 mRNA were analyzed by MeRIP-qPCR assays; (D) the relationship between the METTL3 protein and m6A levels of ATK1 mRNA in NSCLC tissues was analyzed; (E) the relationship between the AKT1 protein and m6A levels of ATK1 mRNA in NSCLC tissues was analyzed. **P<0.01. METTL3, methyltransferase-like 3; AKT1, AKT serine/threonine kinase 1; NSCLC, non-small cell lung cancer.
Discussion
In this study, we examined the prognostic effect of METTL3 expression levels in 180 NSCLC patients. METTL3 plays a critical role in m6A methylation and regulates the total m6A methylation level, which contributes to human diseases (32-35). This study showed that METTL3 was overexpressed in NSCLC cancer tissues, and its expression levels in chemoresistant NSCLC patients were significantly higher than those in chemosensitive NSCLC patients, and higher METTL3 expression levels were significantly correlated with a poor prognosis. The results suggest that METTL3 is a potential biomarker for identifying NSCLC patients who will experience unfavorable clinical outcomes and develop chemoresistance. Additionally, METTL3 expression levels were correlated with AKT1 protein expression.
The relationship between METTL3 expression levels and prognosis has been examined in different types of human cancer (47-50). METTL3 was shown to be downregulated in glioma tissues, and the downregulation of METTL3 was shown to stimulate the malignant development of glioma (51). METTL3 expression was also found to be significantly increased in clear cell renal cell cancer (ccRCC), and higher METTL3 expression predicted shorter OS in ccRCC patients (49). Further, METTL3-mediated autophagy was shown to revise gefitinib resistance in NSCLC cells (52). In this study, we demonstrated that the expression of METTL3 is correlated with a poor prognosis and chemoresistance in NSCLC patients.
METTL3 plays a critical role in regulation of m6A methylation, which directly affects the decay and translation of mRNA and microRNA biogenesis. A previous study indicated that m6A mRNA methylation regulates AKT1 activity, which promotes the tumorigenicity of endometrial cancer (46). Protein kinase B (AKT) is a pleckstrin homology (PH) domain comprising a serine threonine kinase (53). AKT1 is an important isoform of AKT, which acts as a vital subtype and is closely related to many types of human tumors (54-56). Wang et al. found that the overexpression of MNAT1 component of CDK activating kinase (MAT1) mediates the upregulation of AKT1 expression, which promotes the lung metastasis of osteosarcoma (57). However, the chemokine receptor C-X-C motif chemokine receptor 2 (CXCR2) moderates AKT1 results by suppressing breast cancer metastasis and chemoresistance (58). In the progression of bone marrow mesenchymal stem cell (BMSC) adipogenesis, overexpression or knockdown of METTL3 decreased or increased AKT1 expression at the mRNA level, respectively. The results showed that METTL3 could negatively regulate AKT protein expression in MSCs by mediating the m6A modification of AKT1‐mRNA (59). In the current study, AKT1 protein (not RNA expression) was increased in NSCLC tissues and positively related with METTL3 expression, and more highly expressed in chemoresistant NSCLC tissues than chemosensitive tissues. To evaluate the potential difference in METTL3 mediates AKT1 expression, we predicted m6A sites in AKT1‐mRNA (NM_005163.2) using SRAMP (http://www.cuilab.cn/sramp/). The results revealed 10 m6A sites (see Figure S1) of which three high confidence sites in 5'UTR, four sites (one high confidence site, one moderate confidence site and two low confidence sites) in CDS region, and three sites (two moderate confidence sites and one low confidence site) in 3'UTR of AKT1‐mRNA. Further investigation is needed to determine which of the predicted sites are functional to measure the difference in METTL3 mediates AKT1 expression.
m6A is one of the most common RNA modifications. Gene expression can be regulated by the quite prevalent and dynamic regulation of m6A modifications (31,60). Recently, studies have demonstrated that m6A is a potential biomarker for predicting the progression of cancer (61,62). We found that the m6A levels of AKT1 were significantly upregulated in the NSCLC tissues. Additionally, the m6A levels of AKT1 were positively correlated with METTL3 protein expression, and the expression of AKT1 protein was positively correlated with the m6A levels of AKT1. Our results indicate that METTL3 may promote AKT1 protein expression by regulating AKT1 mRNA m6A levels.
Accumulating evidence suggests that m6A RNA methylation greatly impacts RNA metabolism and is involved in the pathogenesis of cancers (63). The rapidly evolving research on m6A modifications can help reveal the biological mechanisms underlying cancer development and may provide new targets for cancer treatment. Although fully understand the underlying mechanism of m6A modification is still far off, the future research on m6A modifications will be focused on regulatory network of m6A in a single cancer and screening factors for early diagnosis and prognosis in large number of clinical specimens, and development of potential m6A-related targets for cancer treatment.
Conclusions
In summary, our results indicate that METTL3 contributes to the progression and chemoresistance of NSCLC by activating AKT1 protein via the regulation of m6A levels of AKT1 mRNA. METTL3 is overexpressed in chemoresistant NSCLC tissues; thus, the METTL3-AKT1 axis may provide an efficient therapeutic intervention target for overcoming chemoresistance in NSCLC.
Highlights
METTL3 expression is elevated in NSCLC tissues.
METTL3 expression is higher in chemoresistant than chemosensitive NSCLC tissues.
METTL3 promotes AKT1 protein expression by regulating AKT1 mRNA m6A levels.
METTL3 is correlated with NSCLC chemoresistance and OS.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: The research was supported by grants from the National Natural Science Foundation of China (82074159 and 81874381) and the Natural Science Foundation of Guangdong Province of China (2021A1515011611 and 2021A1515010491).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from all the patients before the study. This study was approved by the Ethics Committee of Sun Yat-sen University Cancer Center (No. GZR2020-297) and performed in accordance with the Declaration of Helsinki (as revised in 2013).
Reporting Checklist: The authors have completed the MDAR checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-21-6608/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-21-6608/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-21-6608/coif). The authors have no conflicts of interest to declare.
(English Language Editor: L. Huleatt)
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 3.Wang S, Zhang X, Yang C, et al. MicroRNA-198-5p inhibits the migration and invasion of non-small lung cancer cells by targeting fucosyltransferase 8. Clin Exp Pharmacol Physiol 2019;46:955-67. 10.1111/1440-1681.13154 [DOI] [PubMed] [Google Scholar]
- 4.Duma N, Santana-Davila R, Molina JR. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin Proc 2019;94:1623-40. 10.1016/j.mayocp.2019.01.013 [DOI] [PubMed] [Google Scholar]
- 5.Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature 2018;553:446-54. 10.1038/nature25183 [DOI] [PubMed] [Google Scholar]
- 6.Sun CY, Zhang QY, Zheng GJ, et al. Phytochemicals: Current strategy to sensitize cancer cells to cisplatin. Biomed Pharmacother 2019;110:518-27. 10.1016/j.biopha.2018.12.010 [DOI] [PubMed] [Google Scholar]
- 7.Arruabarrena-Aristorena A, Maag JLV, Kittane S, et al. FOXA1 Mutations Reveal Distinct Chromatin Profiles and Influence Therapeutic Response in Breast Cancer. Cancer Cell 2020;38:534-50.e9. 10.1016/j.ccell.2020.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harvey ZH, Chen Y, Jarosz DF. Protein-Based Inheritance: Epigenetics beyond the Chromosome. Mol Cell 2018;69:195-202. 10.1016/j.molcel.2017.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Unnikrishnan A, Freeman WM, Jackson J, et al. The role of DNA methylation in epigenetics of aging. Pharmacol Ther 2019;195:172-85. 10.1016/j.pharmthera.2018.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L, Lu Q, Chang C. Epigenetics in Health and Disease. Adv Exp Med Biol 2020;1253:3-55. 10.1007/978-981-15-3449-2_1 [DOI] [PubMed] [Google Scholar]
- 11.Traube FR, Carell T. The chemistries and consequences of DNA and RNA methylation and demethylation. RNA Biol 2017;14:1099-107. 10.1080/15476286.2017.1318241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones PA, Ohtani H, Chakravarthy A, et al. Epigenetic therapy in immune-oncology. Nat Rev Cancer 2019;19:151-61. 10.1038/s41568-019-0109-9 [DOI] [PubMed] [Google Scholar]
- 13.Miranda Furtado CL, Dos Santos Luciano MC, Silva Santos RD, et al. Epidrugs: targeting epigenetic marks in cancer treatment. Epigenetics 2019;14:1164-76. 10.1080/15592294.2019.1640546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang S, Shen Y, Chen B, et al. H3K27me3 induces multidrug resistance in small cell lung cancer by affecting HOXA1 DNA methylation via regulation of the lncRNA HOTAIR. Ann Transl Med 2018;6:440. 10.21037/atm.2018.10.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Köhler F, Rodríguez-Paredes M. DNA Methylation in Epidermal Differentiation, Aging, and Cancer. J Invest Dermatol 2020;140:38-47. 10.1016/j.jid.2019.05.011 [DOI] [PubMed] [Google Scholar]
- 16.Liang G, Weisenberger DJ. DNA methylation aberrancies as a guide for surveillance and treatment of human cancers. Epigenetics 2017;12:416-32. 10.1080/15592294.2017.1311434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harada K, Baba Y, Ishimoto T, et al. LINE-1 methylation level and patient prognosis in a database of 208 hepatocellular carcinomas. Ann Surg Oncol 2015;22:1280-7. 10.1245/s10434-014-4134-3 [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Yuan Y, Li Y, et al. An inverse interaction between HOXA11 and HOXA11-AS is associated with cisplatin resistance in lung adenocarcinoma. Epigenetics 2019;14:949-60. 10.1080/15592294.2019.1625673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer KD, Saletore Y, Zumbo P, et al. Comprehensive analysis of mRNA methylation reveals enrichment in 3' UTRs and near stop codons. Cell 2012;149:1635-46. 10.1016/j.cell.2012.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He C. Grand challenge commentary: RNA epigenetics? Nat Chem Biol 2010;6:863-5. 10.1038/nchembio.482 [DOI] [PubMed] [Google Scholar]
- 21.Yue Y, Liu J, He C. RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes Dev 2015;29:1343-55. 10.1101/gad.262766.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Zhao BS, Roundtree IA, et al. N(6)-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell 2015;161:1388-99. 10.1016/j.cell.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ban Y, Tan P, Cai J, et al. LNCAROD is stabilized by m6A methylation and promotes cancer progression via forming a ternary complex with HSPA1A and YBX1 in head and neck squamous cell carcinoma. Mol Oncol 2020;14:1282-96. 10.1002/1878-0261.12676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo X, Li K, Jiang W, et al. RNA demethylase ALKBH5 prevents pancreatic cancer progression by posttranscriptional activation of PER1 in an m6A-YTHDF2-dependent manner. Mol Cancer 2020;19:91. 10.1186/s12943-020-01158-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin Z, Niu Y, Wan A, et al. RNA m(6) A methylation regulates sorafenib resistance in liver cancer through FOXO3-mediated autophagy. EMBO J 2020;39:e103181. 10.15252/embj.2019103181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Q, Li P, Wu W. A systematic analysis of immune genes and overall survival in cancer patients. BMC Cancer 2019;19:1225. 10.1186/s12885-019-6414-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu W, Li C, Wu Y, et al. Integrating m6A Regulators-Mediated Methylation Modification Models and Tumor Immune Microenvironment Characterization in Caucasian and Chinese Low-Grade Gliomas. Front Cell Dev Biol 2021;9:725764. 10.3389/fcell.2021.725764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L, Zhang S, Li H, et al. Quantification of m6A RNA methylation modulators pattern was a potential biomarker for prognosis and associated with tumor immune microenvironment of pancreatic adenocarcinoma. BMC Cancer 2021;21:876. 10.1186/s12885-021-08550-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chong W, Shang L, Liu J, et al. m(6)A regulator-based methylation modification patterns characterized by distinct tumor microenvironment immune profiles in colon cancer. Theranostics 2021;11:2201-17. 10.7150/thno.52717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer KD, Jaffrey SR. Rethinking m(6)A Readers, Writers, and Erasers. Annu Rev Cell Dev Biol 2017;33:319-42. 10.1146/annurev-cellbio-100616-060758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin S, Choe J, Du P, et al. The m(6)A Methyltransferase METTL3 Promotes Translation in Human Cancer Cells. Mol Cell 2016;62:335-45. 10.1016/j.molcel.2016.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qin Y, Li L, Luo E, et al. Role of m6A RNA methylation in cardiovascular disease (Review). Int J Mol Med 2020;46:1958-72. 10.3892/ijmm.2020.4746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Z, Shi Y, Lu M, et al. METTL3 counteracts premature aging via m6A-dependent stabilization of MIS12 mRNA. Nucleic Acids Res 2020;48:11083-96. 10.1093/nar/gkaa816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi Y, Zheng C, Jin Y, et al. Reduced Expression of METTL3 Promotes Metastasis of Triple-Negative Breast Cancer by m6A Methylation-Mediated COL3A1 Up-Regulation. Front Oncol 2020;10:1126. 10.3389/fonc.2020.01126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wen L, Sun W, Xia D, et al. The m6A methyltransferase METTL3 promotes LPS-induced microglia inflammation through TRAF6/NF-κB pathway. Neuroreport 2020. [Epub ahead of print]. doi: . 10.1097/WNR.0000000000001550 [DOI] [PubMed] [Google Scholar]
- 36.Yue B, Song C, Yang L, et al. METTL3-mediated N6-methyladenosine modification is critical for epithelial-mesenchymal transition and metastasis of gastric cancer. Mol Cancer 2019;18:142. 10.1186/s12943-019-1065-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng W, Li J, Chen R, et al. Upregulated METTL3 promotes metastasis of colorectal Cancer via miR-1246/SPRED2/MAPK signaling pathway. J Exp Clin Cancer Res 2019;38:393. 10.1186/s13046-019-1408-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han J, Wang JZ, Yang X, et al. METTL3 promote tumor proliferation of bladder cancer by accelerating pri-miR221/222 maturation in m6A-dependent manner. Mol Cancer 2019;18:110. 10.1186/s12943-019-1036-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xue L, Li J, Lin Y, et al. m(6) A transferase METTL3-induced lncRNA ABHD11-AS1 promotes the Warburg effect of non-small-cell lung cancer. J Cell Physiol 2021;236:2649-58. 10.1002/jcp.30023 [DOI] [PubMed] [Google Scholar]
- 40.Ma L, Chen T, Zhang X, et al. The m(6)A reader YTHDC2 inhibits lung adenocarcinoma tumorigenesis by suppressing SLC7A11-dependent antioxidant function. Redox Biol 2021;38:101801. 10.1016/j.redox.2020.101801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin D, Guo J, Wu Y, et al. m(6)A mRNA methylation initiated by METTL3 directly promotes YAP translation and increases YAP activity by regulating the MALAT1-miR-1914-3p-YAP axis to induce NSCLC drug resistance and metastasis. J Hematol Oncol 2019;12:135. 10.1186/s13045-019-0830-6 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Patil T, Mushtaq R, Marsh S, et al. Clinicopathologic Characteristics, Treatment Outcomes, and Acquired Resistance Patterns of Atypical EGFR Mutations and HER2 Alterations in Stage IV Non-Small-Cell Lung Cancer. Clin Lung Cancer 2020;21:e191-e204. 10.1016/j.cllc.2019.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aras M, Erdil TY, Dane F, et al. Comparison of WHO, RECIST 1.1, EORTC, and PERCIST criteria in the evaluation of treatment response in malignant solid tumors. Nucl Med Commun 2016;37:9-15. 10.1097/MNM.0000000000000401 [DOI] [PubMed] [Google Scholar]
- 44.Lin X, Chai G, Wu Y, et al. RNA m(6)A methylation regulates the epithelial mesenchymal transition of cancer cells and translation of Snail. Nat Commun 2019;10:2065. 10.1038/s41467-019-09865-9 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Xia T, Wu X, Cao M, et al. The RNA m6A methyltransferase METTL3 promotes pancreatic cancer cell proliferation and invasion. Pathol Res Pract 2019;215:152666. 10.1016/j.prp.2019.152666 [DOI] [PubMed] [Google Scholar]
- 46.Liu J, Eckert MA, Harada BT, et al. m(6)A mRNA methylation regulates AKT activity to promote the proliferation and tumorigenicity of endometrial cancer. Nat Cell Biol 2018;20:1074-83. 10.1038/s41556-018-0174-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu S, Makeudom A, Sun X, et al. Overexpression of Methyltransferase-Like 3 and 14 in Oral Squamous Cell Carcinoma. J Oral Pathol Med 2021. [Epub ahead of print]. doi: . 10.1111/jop.13256 [DOI] [PubMed] [Google Scholar]
- 48.Wang X, Yu J, Chen J, et al. Copy number variation analysis of m(6) A regulators identified METTL3 as a prognostic and immune-related biomarker in bladder cancer. Cancer Med 2021;10:7804-15. 10.1002/cam4.3981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi Y, Dou Y, Zhang J, et al. The RNA N6-Methyladenosine Methyltransferase METTL3 Promotes the Progression of Kidney Cancer via N6-Methyladenosine-Dependent Translational Enhancement of ABCD1. Front Cell Dev Biol 2021;9:737498. 10.3389/fcell.2021.737498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zou J, Zhong X, Zhou X, et al. The M6A methyltransferase METTL3 regulates proliferation in esophageal squamous cell carcinoma. Biochem Biophys Res Commun 2021;580:48-55. 10.1016/j.bbrc.2021.05.048 [DOI] [PubMed] [Google Scholar]
- 51.Han J, Du S, Wu C, et al. METTL3 participates in glioma development by regulating the methylation level of COL4A1. J buon 2021;26:1556-62. [PubMed] [Google Scholar]
- 52.Liu S, Li Q, Li G, et al. The mechanism of m(6)A methyltransferase METTL3-mediated autophagy in reversing gefitinib resistance in NSCLC cells by β-elemene. Cell Death Dis 2020;11:969. 10.1038/s41419-020-03148-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nie Y, Hu Y, Yu K, et al. Akt1 regulates pulmonary fibrosis via modulating IL-13 expression in macrophages. Innate Immun 2019;25:451-61. 10.1177/1753425919861774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang X, Wang S, Wang H, et al. Circular RNA circNRIP1 acts as a microRNA-149-5p sponge to promote gastric cancer progression via the AKT1/mTOR pathway. Mol Cancer 2019;18:20. 10.1186/s12943-018-0935-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao F, Alwhaibi A, Artham S, et al. Endothelial Akt1 loss promotes prostate cancer metastasis via β-catenin-regulated tight-junction protein turnover. Br J Cancer 2018;118:1464-75. 10.1038/s41416-018-0110-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu G, Wang H, Yuan D, et al. RUNX1-activated upregulation of lncRNA RNCR3 promotes cell proliferation, invasion, and suppresses apoptosis in colorectal cancer via miR-1301-3p/AKT1 axis in vitro and in vivo. Clin Transl Oncol 2020;22:1762-77. 10.1007/s12094-020-02335-5 [DOI] [PubMed] [Google Scholar]
- 57.Wang J, Ni J, Song D, et al. MAT1 facilitates the lung metastasis of osteosarcoma through upregulation of AKT1 expression. Life Sci 2019;234:116771. 10.1016/j.lfs.2019.116771 [DOI] [PubMed] [Google Scholar]
- 58.Xu H, Lin F, Wang Z, et al. CXCR2 promotes breast cancer metastasis and chemoresistance via suppression of AKT1 and activation of COX2. Cancer Lett 2018;412:69-80. 10.1016/j.canlet.2017.09.030 [DOI] [PubMed] [Google Scholar]
- 59.Pan Z-P, Wang B, Hou D-Y, et al. METTL3 mediates bone marrow mesenchymal stem cell adipogenesis to promote chemoresistance in acute myeloid leukaemia. FEBS open bio 2021;11:1659-72. 10.1002/2211-5463.13165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Y, Li Y, Yue M, et al. N(6)-methyladenosine RNA modification regulates embryonic neural stem cell self-renewal through histone modifications. Nat Neurosci 2018;21:195-206. 10.1038/s41593-017-0057-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen B, Li Y, Song R, et al. Functions of RNA N6-methyladenosine modification in cancer progression. Mol Biol Rep 2019;46:2567-75. 10.1007/s11033-019-04655-4 [DOI] [PubMed] [Google Scholar]
- 62.Sun T, Wu R, Ming L. The role of m6A RNA methylation in cancer. Biomed Pharmacother 2019;112:108613. 10.1016/j.biopha.2019.108613 [DOI] [PubMed] [Google Scholar]
- 63.Wang T, Kong S, Tao M, et al. The potential role of RNA N6-methyladenosine in Cancer progression. Molecular cancer 2020;19:88. 10.1186/s12943-020-01204-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as