Abstract
Objective:
Schistosoma mansoni (S. mansoni) is endemic in Africa, the Middle East, South America, and the Caribbean. This study investigated the modulation of immune response against S. mansoni through estimation of interleukin-4 (IL-4) (Th2 cytokine) and interferon-gamma (INF-γ) (Th1 cytokine) under the effect of anti-schistosomal drugs.
Methods:
Laboratory bred female albino mice (n = 120) were divided into the following groups: untreated mice, S. mansoni infected mice, S. mansoni infected mice treated with artemisinin (ART), arachidonic acid (ARA), nifedipine or praziquantel (PZQ). Levels of IL-4 and INF-γ cytokines in the serum samples of treated and untreated mice were determined by enzyme-linked immunosorbent assay and the results were further validated by measuring the mRNA levels IL-4 and INF-γ using quantitative real-time polymerase chain reaction.
Results:
Anti-schistosomiasis drugs ART and ARA increased the levels of Th2 cytokine IL-4 (P < 0.05), whereas PZQ drug decreased the response of IL-4 (P < 0.05). However, nifedipine was found to be ineffective in modulating the response of IL-4 (P > 0.05). As far as Th-1 cytokine IFN γ was concerned, only PZQ increased its levels (P < 0.05), whereas other tested anti-schistosomiasis drugs; ART, ARA, and nifedipine were found to be infective (P > 0.05).
Conclusions:
These findings indicated that anti-schistosomiasis drugs ART, ARA, and PZQ play a role in the modulation of expression of Th2 cytokines. Whereas, only PZQ may play a role in the modulation of Th1 cytokines. These findings provide a scope for the formulation of novel anti-schistosomal drugs as well as in the therapeutic management of patients infected with S. mansoni.
Keywords: Arachidonic acid, artemisinin, interferon-gamma, interleukin-4, nifedipine, praziquantel, Schistosoma mansoni
Introduction
Schistosomiasis is considered by the World Health Organization (WHO) as the second disease worldwide that has a socioeconomic importance and the third parasitic disease in terms of public health impact. Human schistosomiasis is considered a neglected tropical disease whose burden is mainly concentrated in sub-Saharan Africa. The main schistosome species that infect humans are Schistosoma mansoni, Schistosoma haematobium, and Schistosoma japonicum.[1] S. mansoni is endemic in 54 countries including Africa, the Middle East, South America, and the Caribbean. The intermediate hosts are aquatic snails of the genus Biomphalaria (Mollusca: Gastropoda: Pulmonata: Planorbidae). Biomphalaria glabrata is recognized as the best adapted intermediate host for transmitting S. mansoni due to its wide geographical distribution, high rates of infection, and transmission efficiency.[2]
In 2010, the WHO report revealed that the total number of people needing preventive chemotherapy globally was over 237 million, of these more than 108 million were school-age children, and of which only 13% received treatment.[3] Previous reports confirmed the impairment of dendritic cell activity,[4] regulatory T-cells (Tregs)[5] and regulatory B cells[6] post schistosomiasis infection. Discovering or developing a novel treatment for potent immune responsiveness is an expensive and long process, hence, synthesizing and developing derivatives from current drugs that have already given therapeutic efficiency, is an interesting possibility.[7] Thus, new modified therapies against schistosomiasis may lead to an increase of cytokine’s expression and production of an antigen-specific proliferation.[8-10] Thus, this study was designed to determine the expression of interleukin-4 (IL-4) and interferon-gamma, (INF-g) under treatments by four common derivatives drugs as modulation of immunoassays (artemisinin [ART], praziquantel [PZQ], arachidonic acid [ARA] and nifedipine). ART is a sesquiterpene lactone with an endoperoxide group, essential to its activity.[11] Initially is used widely as an excellent antimalarial drug, recently has an excellent property against most species of human Schistosoma including juvenile stages with low toxicity.[12,13] Details extracted from in vivo studies revealed that schistosomula are especially over sensitive to ART, whereas mild worm burden reductions are still evident for adult worms.[14] PZQ is an isoquinoline derivative and considered an effective anti-helminthic drug for all species of Schistosoma.[15,16] It acts to diminish the size of granuloma[17] may be due to reduction of cellular infiltrates. Although therapeutic with this drug is effective, recurrent schistosome reinfection takes place after treatment because of the relative resistance to schistosomicidal therapies.[18] Indeed, this drug was developed by Bayer in the 1970s and is effective against parasitic flatworms, particularly schistosomes. Remarkably, schistosomiasis therapy has depended on this drug and its derivatives for over 40 years.[19,20] Since, PZQ and its derivative exhibited less toxicity[21] without genotoxic risks[22] during detection of mutagenicity[23] among animal testes. The little notice that reported accumulation of prospect mutagenic metabolites may be due to abnormalities among overwhelming evidence indicating that PZQ derivative is a safe drug.[24] On the other hand, Keiser et al., 2012; Xiao et al., 2012 have supported other drugs based on ARA and its derivatives as alternative or complementary drugs against schistosomes.[25,26] In addition, nifedipine showed significant antischistosomal activity against schistosomula when used either alone or in combination with PZQ. In contrast, PZQ showed less significant efficacy when used alone.[27]
Heavy reliance on a single drug for schistosomiasis control may promote the selection and spread of drug-resistant parasites. Indeed, S. mansoni isolates with reduced susceptibilities to PZQ have already been identified. Obviously, there is a need to develop new antischistosomal drugs with a broad spectrum of activity against all stages of the parasite.[28] Schistosomal granulomogenesis is mediated by MHC II binding CD4+ T helper cells and MHC I binding CD8+ T cells play a little role and results in a shift of the immunologic balance from a Th1 to a Th2 cell type response. A type 2 cytokine response is characterized by increased production of IL 4 and IL 5 and concurrent reduction in type 1 cytokines (IFN γ, IL 2) responses. Th2 immunity functions like a two-edged sword, on one hand protecting the host against excessive granulomatous inflammation, and on the other, causing host immune-dependent liver damage. A balance between Th1 and Th2 responses may therefore be necessary to prevent severe pathology.[29] In this study, we investigated the modulation of immune response against S. mansoni infection through estimation of IL-4 (Th2 cytokine) and INF-γ (Th1 cytokine) under the effect of anti-schistosomal drugs in experimentally infected mice with S. mansoni.
Materials and Methods
Experimental animals
This study was carried out on 120 laboratory bred female albino mice, 6–8 weeks old and weighing 24 ± 2 g. These mice were divided into two main groups; Group A: mice treated 21 days after infection with S. mansoni and Group B: mice treated after 42 days. Group A was subdivided into subgroups: normal control, untreated infected control, A1 (treated with ART, Sigma–Aldrich, ChemieGmbh, Germany), A2 (treated with ARA, Efamol Ltd., Leatherhead, Surrey, UK), A3 (treated with nifedipine, EPICO Pharmaceutical Industrial Co., Cairo, Egypt) and A4 (treated with PZQ, Alexandria Co. for Pharmaceuticals and chemical industries, Alexandria, Egypt). Similarly, group B was subdivided into four subgroups in addition to normal control and untreated infected control (B1, B2, B3, and B4), in the same way. Each group consists of 10 mice. The treated and non-treated mice were sacrificed 2 days after treatment and blood samples were collected. Samples were transferred to a 1.5 ml tube and centrifuged for serum separation. Serum samples were stored at −20°C until used.
Administration of drugs
ART was administered intraperitoneally with a single dose of 100 mg/kg of body weight diluted in 100 μl of dimethyl sulfoxide as described previously.[30] PZQ tablets were grounded and used as freshly prepared suspension in 2% Cremophore-EL by vortexing and administered by oral gavage as a single dose of 300 mg/kg corresponding to a dose volume of 0.5 ml.[31] Nifedipine was suspended in a 1% aqueous solution of Tween 80 and administered intraperitoneally as a dose of 20 mg/kg of body weight at a volume of 10 ml/kg.[32] ARA was administered as a single oral dose of 300 mg/kg corresponding to a dose volume of 0.5 ml.[33]
Enzyme linked immunosorbent assay
DuoSet kits for detection specific for IL-4 or INF- γ cytokines were purchased from R and D Systems Europe Ltd, Abingdon, UK, and specific sandwich enzyme-linked immunosorbent assays were performed as described by the supplier (R and D Systems). The plates were coated with 100 µl of capture antibodies at room temperature overnight. They were washed three times with a wash buffer (0.05% Tween 20 in phosphate buffered saline [PBS], pH 7.2). They were blocked with 200 µl of block buffer (1% bovine serum albumin, 5% sucrose in PBS with 0.05% NaN3) sealed at room temperature for 2 h and washed as before. 100 µl of each sample and standard dilution were loaded per well. Samples were run in duplicate. They were sealed and incubated for 2 h at room temperature. The plates were washed and 100 µl of detection antibodies were loaded and incubated for 2 h at room temperature. Excess antibodies were washed away with a wash buffer and a 100 µl of streptavidin-horseradish peroxidase was loaded per well. Plates were then incubated for 20 min at room temperature, away from direct light. The plates were washed as before and a 100 µl of substrate solution was added to each well and were further incubated another 20 min at room temperature, away from direct light. The reactions were then stopped with 50 µl of 2N H2SO4. Optical densities were determined immediately using a microplate reader (DYNEX MRX Revelation) set to 450 nm with wavelength correction set to 540 nm.
Gene expression assays
Extraction of RNA
Peripheral blood samples were collected in EDTA tubes from all subgroups and controls participants. RNA was isolated from these samples using a commercial RNA extraction sample in QIAzollysis solution (QIAGEN, Valencia, CA, USA) for 40 s at 33,000 rpm. The QuickGene automated system (QuickGene, USA) was implemented in keeping with the manufacturer’s guidelines to extract the total RNA. RNase-free water was subsequently used for elution of RNA of high quality. Moreover, a Thermo Scientific NanoDrop spectrophotometer (Waltham, MA, USA) was employed to monitor the quality of RNA at 260 and 280 nm. Processing was conducted solely on the RNA samples with an absorbance ratio 260/280 nm of ~2.0>. The samples of total RNA were stored at –70°C.
Reverse transcription (cDNA synthesis)
The synthesis of single-stranded cDNA was performed with 1.0 μg total RNA and the High-Capacity cDNA Reverse Transcription Kit (NEB, USA). To synthesize first-strand cDNA, a first strand cDNA synthesis kit was employed with a 24T primer (0.4 nmol/reaction) in a 25 μl reaction mix, in line with the manufacturer’s guidelines. Furthermore, before running in real-time polymerase chain reaction (RT-PCR), tenfold dilution of the cDNA products was undertaken.
Quantitative RT-PCR (RT-qPCR)
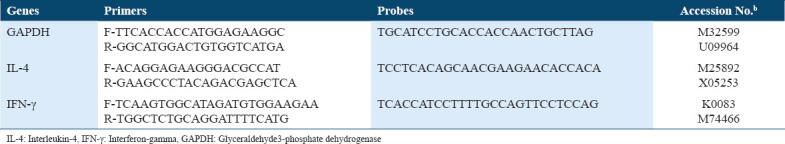
The qPCR core kit for SYBR Green (QIAGEN, USA) and StepOne RT-PCR System (Applied Biosystems, Foster City, CA) were employed to run the reaction of amplification with a 2.5 × RT-PCR master mix. The 25 μl mix used for every reaction consisted of 400 nmol of every primer and 1 μl 1:10 diluted cDNA. Table 1 provides the sequences of primers. Standard profile times were applied, namely, 15 min at 95°C in the first step, 15 s at 94°C in the second step, half minute at 60°C, and ½ min at 72°C for 40 cycles with melting curve analysis. After normalization to the equivalent glyceraldehyde3-phosphate dehydrogenase (GAPDH) (ThermovFisher, USA), the target mRNA was contrasted against untreated sample representing the control in the same plate. The 2-ΔΔCT technique was adopted to determine the values, with the discrepancy in threshold cycles for target and the housekeeping gene (GAPDH) being denoted by ΔCT and the discrepancy in ΔCT and the threshold cycles for the control being denoted by ΔΔCT.
Table 1.
Primer and probe sequences used for the murine IL-4 and IFN-γ expression in immune responses. The expression of GAPDH was used endogenous control
Statistical analysis
The data collected were tabulated and analyzed by statistical package for the social science software statistical package version 20 on International Business Machines compatible computer.[34] Two types of statistics were done: Descriptive statistics; Percentage, mean and standard deviation. Analytic statistics; One-way analysis of variance (ANOVA) (F ratio), Kruskal-Wallis test and post-hoc test. The Tukey’s honestly significant difference (HSD) procedure facilitates pairwise comparisons within ANOVA data. Probability value: P < 0.05* means significant.
Results
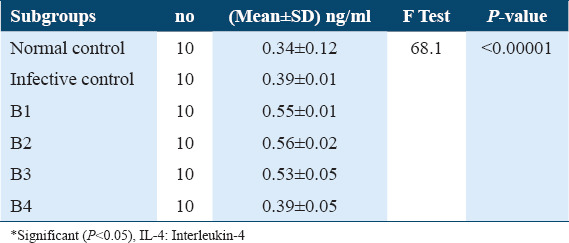
Estimation of IL-4 levels in Sera collected from treated mice after 21 days post-infection showed a highly significant increase in IL-4 levels under exposure to ART (A1) and ARA (A2) compared to untreated infective control, 0.59 ± 0.04, 0.56 ± 0.06, versus 0.38 ± 0.03, respectively, (P < 0.0001). Whereas, treatment with nifedipine (A3) did not show any significant increase in IL-4 level, 0.39 ± 0.01 versus 0.38 ± 0.03 compared to untreated infective control. Thus, treatment with PZQ (A4) showed a significantly lower IL-4 levels compared with infected untreated control group 0.36 ± 0.01 versus 0.38 ± 0.03, (P < 0.05) Table 2. On the other hand, during treatment of the infected mice after 42 days post-infection, the concentration of IL-4 in treated groups: ART (B1), ARA (B2), and nifedipine (B3) showed highly significant increase in comparison with untreated infected control (0.55 ± 0.01, 0.56 ± 0.02 and 0.39 ± 0.01), (P < 0.05), respectively. While treatment with PZQ (B4) did not show any significant change in IL 4 levels compared with untreated infected control 0.39 ± 0.01 versus 0.39 ± 0.01. These data are summarized in Table 3.
Table 2.
IL-4 levels in sera of different A subgroups among Schistosoma mansoni infected mice (21 days post-infection)
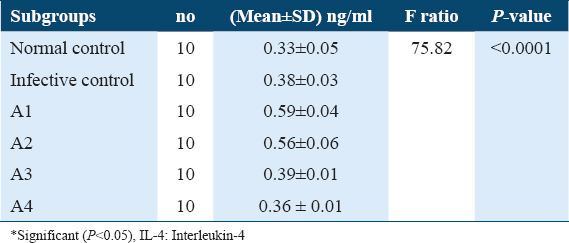
Table 3.
IL-4 levels in sera of different B subgroups among Schistosoma mansoni infected mice (42 days post-infection)
Estimation of IFN- γ levels in sera collected from treated mice after 21 days post infection didn’t show any significant increase under effect of ART (A1), ARA (A2) and nifedipine (A3) in comparison with untreated infected control, 0.34 ± 0.01, 0.35 ± 0.02, and 0.32 ± 0.01 versus 0.33±0.02, respectively, Table 4. Whereas, treatment with PZQ (A4) showed a significantly higher increase in IFN- γ levels compared with untreated infected control 0.53 ± 0.01 versus 0.33 ± 0.02 (P < 0.05; [Tables 4 and 5]). Subsequently, IFN- γ levels from sera of treated mice 42 days post-infection showed a significant increase under effect of ART (B1) ARA (B2) and PZQ (B4) in comparison with untreated infected control, (0.47 ± 0.01, 0.46 ± 0.01 and 0.54 ± 0.02 vs. 0.35 ± 0.02) (P < 0.05), respectively. Whereas, it did not show any significant change with nifedipine (B3) in comparison with untreated infected control, 0.34 ± 0.02 vs. 0.35 ± 0.02 [Tables 6 and 7].
Table 4.
INF-? levels in sera of different A subgroups among γ levels in sera of different A subgroups among Schistosoma mansoni infected mice (21 days post-infection)
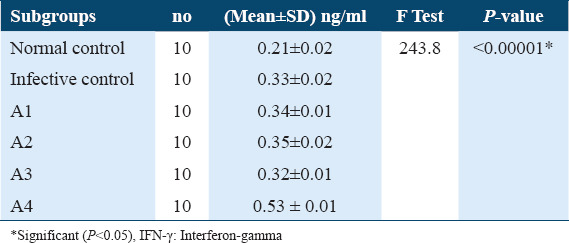
Table 5.
The Tukey’s HSD (honestly significant difference) of INF-γ levels in sera of A subgroups among Schistosoma mansoni infected mice (21 days post-infection)
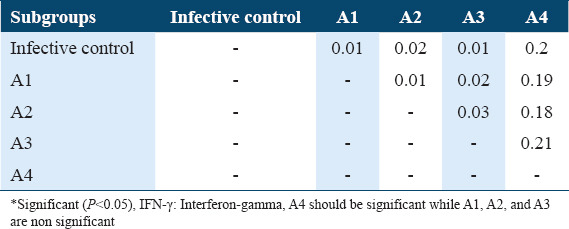
Table 6.
INF-γ levels in sera of different B subgroups among Schistosoma mansoni infected mice (42 days post-infection)
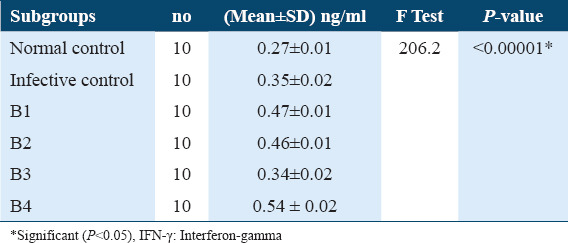
Table 7.
The Tukey’s HSD (honestly significant difference) of INF-γ levels in sera of B subgroups among Schistosoma mansoni infected mice (42 days post-infection)
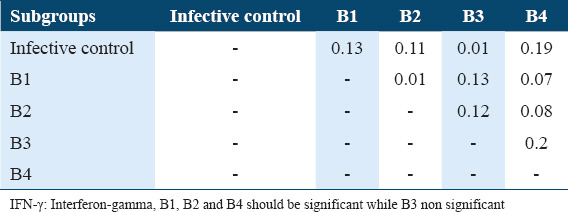
The Tukey’s HSD of IL-4 levels among the various subgroups of treatments and infection status were determined post-hoc test. It showed highly significant difference between infective control with A1, A2, and A3 as well as with B1, B2, and B3 subgroups in comparison with untreated infective control (P < 0.05) and highly significance difference between A1 subgroup with A4 subgroup, between A3 with A4 as well as between B1 with B4, between B2 and B4 and between B3 and B4 (P < 0.05). As well as a significant difference between A3 subgroups and A4 subgroups (P < 0.05). These data are summarized in Tables 8 and 9. Whereas The Tukey’s HSD of INF-g levels showed a significant difference between untreated infective group with A2 subgroup as well as with B1, B2, and B4 (P < 0.05), also there are significant difference between A1 with A3 and A4 as well as between B1 with B3 and B4 in addition between B2 with B3 and B4 and between B3 and B4 (P < 0.05) [Tables 5 and 7].
Table 8.
The Tukey’s HSD (honestly significant difference) of IL-4 levels in sera of A subgroups among Schistosoma mansoni infected mice (21 days post-infection)
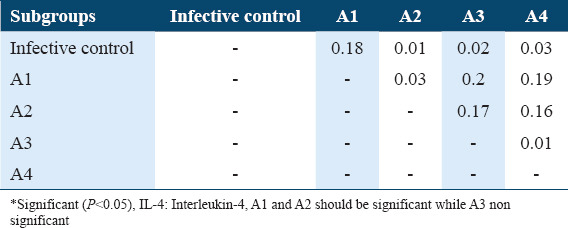
Table 9.
The Tukey’s HSD (honestly significant difference) of IL-4 level in sera of B subgroups among Schistosoma mansoni infected mice (42 days post-infection)
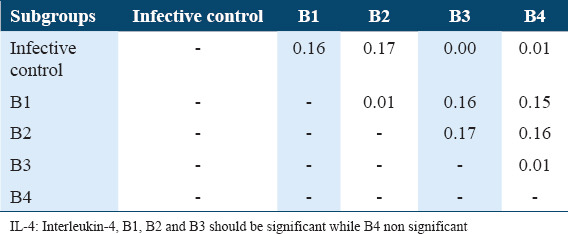
IL4 and IFN-γ Gene Expression: PCR conditions were optimized in order to validate the effectiveness and specificity of amplification of every gene expression of RT-qPCR array. The 2-DDCt technique was applied to contrast Ct values among treated, untreated control and infective control to enable assessment of gene expression (in relation to GAPDH) profile modulations in the normal control, infective control, A1, A2, A3, and A4. The relative quantity of RT-qPCR was analyzed after treatments from 21 days post-infection and revealed that the expression of the IL-4 gene increased to 1.6-fold in the infective control group before treatments, but it increased to 1.9-fold after treated with ART subgroup (A1), as well as with ARA subgroup (A2), whereas it decreased to 0.7-fold with nifedipine subgroup (A3) while slightly increased to 1.1- fold (non-significant) after treatment with PZQ subgroup (A4), in comparison with normal untreated control (1-fold) [Figure 1a]. The quantitative RT-PCR was analyzed after treatments from 42 days post-infection, revealed that the expression of the IL-4 gene increased to 2.94-fold in the infective control group, and to 3.6-fold after treated with ART subgroup (B1), also increased to 2-fold in ARA subgroup (B2), whereas it slightly increased to 1.1-fold (non-significant) after treatment with nifedipine subgroup (B3) and it decreased to 0.9- fold with PZQ subgroup (B4), in comparison with normal untreated control (1-fold) [Figure 1b].
Figure 1.
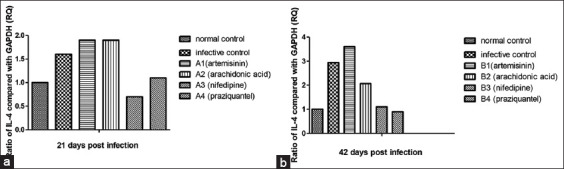
Gene expression of interleukin-4 of treatment groups compared to nontreated control group (relative to equivalent glyceraldehyde3-phosphate dehydrogenase), using the 2-DDCT method. (a) 21 days post-infection, (b) 42 days post-infection
The expression of IFN-γ gene after treatments 21 days post-infection increased to 2.05-fold in the infective control group without any treatments, but it was decreased to 0.86-fold after treated with ART subgroup (A1), to 0.91-fold with ARA subgroup (A2), and to 0.94-fold with nifedipine subgroup (A3). On the other hand, it increased to 2.58- fold after treatment with PZQ subgroup (A4), in comparison with normal untreated control (1-fold) [Figure 2a].
Figure 2.
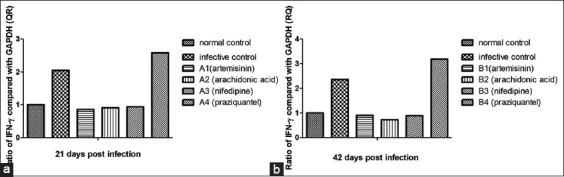
Gene expression of interferon-gamma of treatment groups compared to nontreated control group (relative to equivalent glyceraldehyde3-phosphate dehydrogenase), using the 2-DDCT method. (a) 21 days post-infection, (b) 42 days post-infection
The quantitative RT-PCR analyzed after treatments 42 days post-infection revealed that the expression of the IFN-γ gene increased to 2.36-fold in the infective control group, whereas decreased to 0.9-fold after treated with ART subgroup (B1), to 0.72-fold with ARA subgroup (B2), and to 0.89-fold after treatment with nifedipine subgroup (B3). The expression of the IFN-γ gene was increased to 3.18- fold with PZQ subgroup (B4), in comparison with normal untreated control (1-fold) [Figure 2b].
Discussion
S. mansoni is a fluke-shaped parasite that belongs to the class of Trematoda. It is the most prevalent species among the human-infecting schistosomes that cause gastrointestinal infection in humans as humans serve.[35] The main pathology related to schistosomiasis is due to cellular immune responses that are generated against this parasite by B and T cells. Specifically, the immune response is mounted against the ova laid by the female Schistosoma in the endothelial lining of the capillary walls of small inferior mesenteric blood vessels that surround the colon and caecum. The type of immune reaction implemented against the ova results in granulomatous inflammation in the tissue around the ova.[36] Hoffmann et al., (2002) confirmed the modulations in T helper cytokine profiles during prompting of granulomatous reaction with cytokines playing a main role in the regulation of fibrosis deposition and degradation.[37] In this study, the levels of IL-4 and IFN-γ cytokines were determined among the studied subgroups under treatment by ART, ARA, Nifedipine, and PZQ as anti-schistosomiasis drugs as well as among the control. This vital issue highlights the importance of an alternative drug that can be used in the treatment of schistosomiasis. Initially, IL-4 and IFN-γ levels were found to be significantly increased in untreated infected mice compared to normal control. This may be due to the deficiency of hepatic clearance function that results in increased serum cytokine levels. Thus, we found out that, pertaining to the levels of IL-4, in early treated mice (21 days post-infection), there was a significant boost in the level of IL-4 in mice that was treated by ART and ARA as compared with infected untreated mice (P < 0.05). Whereas, treatment with nifedipine did not show any significant increase in IL-4 level compared to untreated infective control. Thus, treatment with PZQ showed significantly lower IL-4 levels compared with infected untreated control groups (P < 0.05). The relative quantity of RT-qPCR revealed that the expression of the IL-4 gene increased to 1.6-fold in the infective control group and 1.9-fold under treatment with ART, and ARA while decreased to 0.7-fold with nifedipine and slightly increased to 1.1- fold (non-significant) under treatment with PZQ (in relation to GAPDH). Similar results were obtained for IL-4 levels in mice treated 42 days post-infection. Regarding the relative quantity of RT-qPCR, it revealed that the expression of IL-4 gene increased to 2.94-fold in the infective control whereas increased to 3.6-fold under treatment with ART, and ARA while slightly increased to 1.1- fold (non-significant) under treatment with nifedipine and slightly decreased to 0.9- fold (non-significant) under treatment with PZQ (in relation to GAPDH). These results were in accordance with El Sayed et al., (2016) who measured the levels of IL-4 in mice treated with silymarin and/or PZQ. Treated groups showed significant decrease in IL-4 levels compared to infected control.[38] In contrast, a study conducted by Zhongguo et al., (2014) concluded that IL-4 levels in sera were considerably elevated under the effect of PZQ treatment, as compared to control group (P < 0.05).[39] Whereas, Bardley et al., (2008) stimulated splenocytes of mice infected with S. japonicum, and reported that artemether showed a non-significant increase in IL-4 levels.[40]
Concerning IFN-γ levels among early treated mice (21 days post-infection), our results did not demonstrate any significantly increasing under the effect of ART, ARA, and nifedipine in comparison with untreated infected control. Whereas treatment with PZQ showed a significantly higher increase in IFN- γ levels compared with untreated infected control (P < 0.05). According to our results of IFN-γ expression, it was increased to 2.05-fold in the infective control group. IFN-γ expression was decreased to 0.86-fold with ART, 0.91-fold with ARA and to 0.94-fold with nifedipine while it was increased to 2.58- fold after treatment with PZQ (in relation to GAPDH), in comparison with normal untreated control (1-fold). In those mice that were treated after 42 days post-infection, IFN-γ levels showed a significant increase under the effect of ART, ARA and PZQ in comparison with untreated infected control (P < 0.05). Whereas, it did not show any significant change with nifedipine. Thus, according to our results of IFN-γ expression, were found it increased to 2.36-fold in the infective control group, but it decreased to 0.9-fold with ART, as well as to 0.89-fold with ARA and to 0.72-fold with nifedipine while it increased to 3.18- fold with PZQ (in relation to GAPDH. These results are in agreement with previous studies which reported that treatment with silymarin and/or PZQ in murine models caused a significant increase in IFN-γ levels[38] and a very highly significant increase in serum level of IFN-γ under the effect of PZQ.[39-41] Furthermore, Labuda et al., (2020) reported that in S. haematobium infected school children, levels of IFN-γ were significantly increased after PZQ treatment compared with the infected group.[42] Studies using radiation-attenuated cercariae revealed that the protection observed in mice exposed to attenuated cercariae was primarily associated with IFN-γ production and antibody production after multiple rounds of vaccination.[43] Schistosomiasis in the experimental animals such murine model, found that IFN-γ which belong to Th1 cytokines and activation macrophages have been associated with immunity. On the other hand, Th2-correlated cytokines such as IL-4, inhibit classical macrophage activation and have been implicated in granuloma formation and fibrogenesis around tissue-deposited eggs.[44] Therefore, IL-4 exhibited a highly significant increase under the effect of some anti-schistosomiasis drugs, indicating that immune responses to schistosomes may modulate and polarize toward Th2 cytokines. In the current study, there was a significant difference in the IFN-γ under the effect of some anti-schistosomiasis drugs, correlated to the inception of the immunity response. Whereas Silva et al., (2004) suggested that there is noteworthy general downregulation of Th1 responses in infected mice.[45] Caldas et al. (2008) reported that in the acute stage there is a mixed expression of Th1 and Th2 cytokines with the prevalence of Th1 in the early infection.[46] The higher increase of IFN-γ, in acute as compared to chronic schistosomiasis, partially explains the deficiency of modulation of the immune response in acute patients. Hence, the high production of IFN-γ under exposure to different drugs may be an excellent module for immune response. Furthermore, our results of the Tukey’s HSD of cytokines levels indicated the difference effect in between the different drugs against schistosomiasis, thus this study suggested that patients infected with schistosomiasis might be treated with a combination of drugs.
Conclusions
Anti-schistosomiasis drugs ART, ARA, and PZQ play a role in the modulation of expression of Th2 cytokines. Whereas, only PZQ may play a role in the modulation of Th1. Schistosomes adapt the immune response in the chronic phase of infection particularly at the stage of egg deposition in human tissues. However, there is escalating evidence that modulation of the immune response takes place at a much earlier stage even at the time infective cercariae penetrate the host skin, hence studying the impact of immune response modulation of anti-schistosomal drugs against S. mansoni is a crucial area, as it will provide a scope for the formulation of novel anti-schistosomal drugs as well as in therapeutic management of patients infected with S. mansoni.
Authors’ Declaration Statements
Ethics approval
Ethical approval was obtained from the Clinical and Molecular Parasitology Department, National Liver Institute, Menoufia University, Egypt.
Consent for publication
None.
Availability of data and material
The data used in this study are available and will be provided by the corresponding author on a reasonable request.
Competing interests
The authors declare no conflict of interest.
Funding statement
None.
Authors’ Contributions
Study conception and design and data collection: Osama F. Sharaf, Ahmed A. Ahmed, Waleed Al Abdulmonem; Analysis and interpretation of results: Asmaa F. Ibrahim, Ali Shariq, Abdullah S. Alkhamiss, Ruqaih S. Alghsham, Sami A. Althwab, Fahad A. Alhumaydhi, Rana Alghamdi, Ahmad Alshomar, Tasleem Alabdullatif, Abdulrahman Alkhulayfi; manuscript preparation and revision: Abdulrhman A. Alghunaim. All authors reviewed the results and approved the final version of the manuscript.
ORCID link of the submitting author: https://orcid.org/0000-0003-2984-9262
References
- 1.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development:Systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–25. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 2.Carvalho OS, Amaral RS, Dutra LV, Scholte RG, Guerra MA. Distribuiçioespacial de Biomphalaria glabrata, B. straminea e B. tenagophila moluscos hospedeiros intermeditrios do Schistosom amansoni no Brasil. In: Carvalho OS, Coelho PM, Lenzi HL, editors. Schistosoma mansoni e Esquistossomose:Umavisiomultidisciplinar. Vol. 1. Rio de Janeiro: Fiocruz; 2008. pp. 395–418. [Google Scholar]
- 3.Barsoum RS, Esmat G, El-Baz T. Human schistosomiasis:Clinical perspective:Review. J Adv Res. 2013;4:423–4. doi: 10.1016/j.jare.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Everts B, Adegnika AA, Kruize YC, Smits HH, Kremsner PG, Yazdanbakhsh M. Functional impairment of human myeloid dendritic cells during Schistosoma haematobium infection. PLoS Negl Trop Dis. 2010;4:e667. doi: 10.1371/journal.pntd.0000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watanabe K, Mwinzi PN, Black CL, Muok EM, Karanja DM, Secor WE, et al. T regulatory cell levels decrease in people infected with Schistosoma mansonion effective treatment. Am J Trop Med Hyg. 2007;77:676–82. [PMC free article] [PubMed] [Google Scholar]
- 6.van der Vlugt LE, Labuda LA, Ozir-Fazalalikhan A, Lievers E, Gloudemans AK, Liu KY, et al. Schistosomes induce regulatory features in human and mouse CD1d(hi) B cells:Inhibition of allergic inflammation by IL-10 and regulatory T cells. PLoS One. 2012;7:e30883. doi: 10.1371/journal.pone.0030883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corrêa SA, de Oliveira RN, Mendes TM, Dos Santos KR, Boaventura S, Jr, Garcia VL, et al. In vitro and in vivo evaluation of six artemisinin derivatives against Schistosoma mansoni. Parasitol Res. 2019;118:505–16. doi: 10.1007/s00436-018-6188-9. [DOI] [PubMed] [Google Scholar]
- 8.Grogan JL, Kremsner PG, Deelder AM, Yazdanbakhsh M. Elevated proliferation and interleukin-4 release from CD4+cells after chemotherapy in human Schistosoma haematobium infection. Eur J Immunol. 1996;26:1365–70. doi: 10.1002/eji.1830260628. [DOI] [PubMed] [Google Scholar]
- 9.Joseph S, Jones FM, Walter K, Fulford AJ, Kimani G, Mwatha JK, et al. Increases in human T helper 2 cytokine responses to Schistosoma mansoni worm and worm-tegument antigens are induced by treatment with praziquantel. J Infect Dis. 2004;190:835–42. doi: 10.1086/422604. [DOI] [PubMed] [Google Scholar]
- 10.Bourke CD, Nausch N, Rujeni N, Appleby LJ, Mitchell KM, Midzi N, et al. Integrated analysis of innate, Th1, Th2, Th17, and regulatory cytokines identifies changes in immune polarisation following treatment of human schistosomiasis. J Infect Dis. 2013;208:159–69. doi: 10.1093/infdis/jis524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ansari MT, Saify ZS, Sultana N, Ahmad I, Saeed-Ul-Hassan S, Tariq I, et al. Malaria and artemisinin derivatives:An updated review. Mini Rev Med Chem. 2013;13:1879–902. doi: 10.2174/13895575113136660097. [DOI] [PubMed] [Google Scholar]
- 12.Utzinger J, Xiao SH, Tanner M, Keiser J. Artemisinins for schistosomiasis and beyond. Curr Opin Investig Drugs. 2007;8:105–16. [PubMed] [Google Scholar]
- 13.Frezza TF, Oliveira CN, Banin TM, Rehder VL, Boaventura JS, Allegretti SM. Tegumentary changes in two different strains of Schistosoma mansoni treated with artemisinin and artesunic acid. J Trop Pathol. 2013;42:309–21. [Google Scholar]
- 14.Li HJ, Wang W, Qu GL, Li YZ, Tao YH, Xing YT, et al. Effect of the in vivo activity of dihydroartemisinin against Schistosoma mansoni infection in mice. Parasitol Res. 2012;110:1727–32. doi: 10.1007/s00436-011-2692-x. [DOI] [PubMed] [Google Scholar]
- 15.Utzinger J, Keiser J. Schistosomiasis and soil-transmitted helminthiasis:Common drugs for treatment and control. Expert Opin Pharmacother. 2004;5:263–85. doi: 10.1517/14656566.5.2.263. [DOI] [PubMed] [Google Scholar]
- 16.Trainor-Moss S, Mutapi F. Schistosomiasis therapeutics:What's in the pipeline? Expert Rev Clin Pharmacol. 2016;9:157–60. doi: 10.1586/17512433.2015.1102051. [DOI] [PubMed] [Google Scholar]
- 17.El-Ahwany EG, Nosseir MM, Aly IR. Immunomodulation of pulmonary and hepatic granulomatous response in mice immunized with purified lung-stage schistosomulae antigen. J Egypt Soc Parasitol. 2006;36:335–50. [PubMed] [Google Scholar]
- 18.Silva IM, Thiengo R, Conceição MJ, Rey L, Lenzi HL, Filho EP, et al. Therapeutic failure of praziquantel in the treatment of Schistosoma haematobium infection in Brazilians returning from Africa. Mem Inst Oswaldo Cruz. 2005;100:445–9. doi: 10.1590/s0074-02762005000400018. [DOI] [PubMed] [Google Scholar]
- 19.Caffrey CR. Schistosomiasis and its treatment. Future Med Chem. 2015;7:675–6. doi: 10.4155/fmc.15.27. [DOI] [PubMed] [Google Scholar]
- 20.Cioli D, Pica-Mattoccia L. Praziquantel. Parasitol Res. 2003;90(Suppl 1):S3–9. doi: 10.1007/s00436-002-0751-z. [DOI] [PubMed] [Google Scholar]
- 21.Frohberg H. Results of toxicological studies on praziquantel. Arzneimittelforschung. 1984;34:1137–44. [PubMed] [Google Scholar]
- 22.Montero R, Ostrosky P. Genotoxic activity of praziquantel. Mutat Res. 1997;387:123–39. doi: 10.1016/s1383-5742(97)00027-6. [DOI] [PubMed] [Google Scholar]
- 23.Kramers PG, Gentile JM, Gryseels BJ, Jordan P, Katz N, Mott KE, et al. International commission for protection against environmental mutagens and carcinogens. ICPEMC publication No. 18. Review of the genotoxicity and carcinogenicity of antischistosomal drugs;is there a case for a study of mutation epidemiology?report of a task group on mutagenic antischistosomals. Mutat Res. 1991;257:49–89. doi: 10.1016/0165-1110(91)90019-r. [DOI] [PubMed] [Google Scholar]
- 24.Doenhoff MJ, Coles GC, Pica-Mattoccia L, Wheatcroft-Franclow K. Chemotherapy and drug resistance in schistosomiasis, fascioliasis and tapeworm infections. In: Mayers D, editor. Antimicrobial Drug Resistance:Mechanism of Drug Resistance. Vol. 1. New York: Springer; 2009. pp. 629–46. [Google Scholar]
- 25.Keiser J, Tritten L, Adelfio R, Vargas M. Effect of combinations of marketed human anthelmintic drugs against Trichuris muris in vitro and in vivo. Parasit Vectors. 2012;5:292. doi: 10.1186/1756-3305-5-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao SH, Xue J, Mei JY, Jiao PY. Effectiveness of synthetic trioxolane OZ78 against Schistosoma japonicum in mice and rabbits. Parasitol Res. 2012;110:2307–14. doi: 10.1007/s00436-011-2765-x. [DOI] [PubMed] [Google Scholar]
- 27.Silva-Moraes V, Couto FF, Vasconcelos MM, Araújo N, Coelho PM, Katz N, et al. Antischistosomal activity of a calcium channel antagonist on schistosomula and adult Schistosoma mansoni worms. Mem Inst Oswaldo Cruz. 2013;108:600–4. doi: 10.1590/0074-0276108052013011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abbas A, Murphy KM, Sher a. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 29.Peine M, Rausch S, Helmstetter C, Fröhlich A, Hegazy AN, Kühl AA, et al. Stable T-bet (+) GATA-3 (+) Th1/Th2 hybrid cells arise in vivo, can develop directly from naive precursors, and limit immunopathologic inflammation. PLoS Biol. 2013;11:e1001633. doi: 10.1371/journal.pbio.1001633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Witkowski B, Lelièvre J, Nicolau-Travers ML, Iriart X, Soh PN, Bousejra-Elgarah F, et al. Evidence for the contribution of the hemozoin synthesis pathway of the murine Plasmodium yoelii to the resistance to artemisinin-related drugs. PLoS One. 2012;7:e32620. doi: 10.1371/journal.pone.0032620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gönnert R, Andrews P. Praziquantel, a new board-spectrum antischistosomal agent. Z Parasitenkd. 1977;52:129–50. doi: 10.1007/BF00389899. [DOI] [PubMed] [Google Scholar]
- 32.Luszczki JJ, Trojnar MK, Trojnar MP, Kimber-Trojnar Z, Szostakiewicz B, Zadrozniak A, et al. Effects of three calcium channel antagonists (amlodipine, diltiazem and verapamil) on the protective action of lamotrigine in the mouse maximal electroshock-induced seizure model. Pharmacol Rep. 2007;59:672–82. [PubMed] [Google Scholar]
- 33.El Ridi R, Tallima H, Salah M, Aboueldahab M, Fahmy OM, Al-Halbosiy MF, et al. Efficacy and mechanism of action of arachidonic acid in the treatment of hamsters infected with Schistosoma mansoni or Schistosoma haematobium. Int J Antimicrob Agents. 2012;39:232–9. doi: 10.1016/j.ijantimicag.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 34.Bryman A, Cramer D. Quantitative Data Analysis with IBM SPSS 17, 18 and 19:A Guide for Social Scientists. New York: Routledge; 2011. [Google Scholar]
- 35.Oliveira G, Rodrigues NB, Romanha AJ, Bahia D. Genome and genomics of schistosomes. Can J Zool. 2004;82:375–90. [Google Scholar]
- 36.Cheever AW, Hoffmann KF, Wynn TA. Immunopathology of schistosomiasis mansoni in mice and men. Immunol Today. 2000;21:465–6. doi: 10.1016/s0167-5699(00)01626-1. [DOI] [PubMed] [Google Scholar]
- 37.Hoffmann KF, Wynn TA, Dunne DW. Cytokine-mediated host responses during schistosome infections;walking the fine line between immunological control and immunopathology. Adv Parasitol. 2002;52:265–307. doi: 10.1016/s0065-308x(02)52014-5. [DOI] [PubMed] [Google Scholar]
- 38.El-Sayed NM, Fathy GM, Abdel-Rahman SA, El-Shafei MA. Cytokine patterns in experimental schistosomiasis mansoni infected mice treated with silymarin. J Parasit Dis. 2016;40:922–9. doi: 10.1007/s12639-014-0606-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang YY, Xu YX, Yuan ZY, Shen YJ, Wu Y, Liu HP, et al. Effect of toll-like receptor (TLR) 7 deficiencies on the in vivo immune response against Schistosoma japonicum. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2014;32:172–5. [PubMed] [Google Scholar]
- 40.Bartley PB, Glanfield A, Li Y, Stanisic DI, Duke M, Jones MK, et al. Artemether treatment of prepatent Schistosoma japonicum induces resistance to reinfection in association with reduced pathology. Am J Trop Med Hyg. 2008;78:929–35. [PMC free article] [PubMed] [Google Scholar]
- 41.Madbouly NA, Shalash IR, El Deeb SO, El Amir AM. Effect of artemether on cytokine profile and egg induced pathology in murine schistosomiasis mansoni. J Adv Res. 2015;6:851–7. doi: 10.1016/j.jare.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Labuda LA, Adegnika AA, Rosa BA, Martin J, Ateba-Ngoa U, Amoah AS, et al. A praziquantel treatment study of immune and transcriptome profiles in Schistosoma haematobium-infected gabonese schoolchildren. J Infect Dis. 2020;222:2103–13. doi: 10.1093/infdis/jiz641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 44.Burke ML, Jones MK, Gobert GN, Li YS, Ellis MK, McManus DP. Immunopathogenesis of human schistosomiasis. Parasite Immunol. 2009;31:163–76. doi: 10.1111/j.1365-3024.2009.01098.x. [DOI] [PubMed] [Google Scholar]
- 45.Silva LM, Oliveira SA, Ribeiro-dos-Santos R, Andrade ZA, Soares MB. Comparison of immune responses of Schistosoma mansoni-infected mice with distinct chronic forms of the disease. Acta Trop. 2004;91:189–96. doi: 10.1016/j.actatropica.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 46.Caldas IR, Campi-Azevedo AC, Oliveira LF, Silveira AM, Oliveira RC, Gazzinelli G. Human schistosomiasis mansoni:Immune responses during acute and chronic phases of the infection. Acta Trop. 2008;108:109–17. doi: 10.1016/j.actatropica.2008.05.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this study are available and will be provided by the corresponding author on a reasonable request.


