Abstract
Background:
Acute kidney injury (AKI) survivors are at heightened risk for poor short- and long-term health outcomes. Even among those who recover after an AKI episode, the risk for chronic kidney disease is 4- to 6-fold higher than in patients without AKI, underscoring the importance of identifying methods to improve AKI survivorship.
Objective:
The purpose of this report was to describe the development and feasibility of a novel multidisciplinary approach to caring for AKI survivors at care transitions (ACT).
Design:
Observational process improvement initiative.
Setting:
Single academic medical center in the United States.
Patients:
The studied population was adults with stage 3 AKI not discharging on dialysis who were established with a primary care provider (PCP) at our institution.
Methods:
An electronic health record tool was developed prior to implementation to identify AKI survivors. The ACT program encompassed engaging patients in the hospital, delivering education by nephrology-trained nurses before discharge, completing recommended laboratory testing after discharge, and conducting structured kidney-focused follow-up with a pharmacist and a PCP within 7 to 14 days after discharge. Patients could be referred for nephrology evaluation at the discretion of the PCP.
Results:
Preliminary data demonstrated that most AKI survivors of interest could be identified, educated, and followed up with this model. This strategy appeared feasible, scalable, and maximized the unique expertise of each member of the multidisciplinary team.
Limitations:
Small sample size, future assessment of process, clinical, and patient-reported outcomes needed.
Conclusions:
The multidisciplinary ACT workflow supported by clinical decision support was feasible and addressed gaps in existing care transition models. Team-based care delivery in primary care appears to be a mechanism to extend the capacity for kidney health monitoring for AKI survivors.
Keywords: acute kidney injury, outcomes, care transitions, multidisciplinary care, team-based care, primary care, kidney disease, quality improvement
Abrégé
Contexte:
Les patients qui survivent à un épisode d’insuffisance rénale aiguë (IRA) courent un risque plus élevé de mauvais résultats cliniques à court et à long terme. Même chez les patients qui se rétablissent, le risque de progression vers l’insuffisance rénale chronique (IRC) demeure de quatre à six fois plus élevé que chez les patients n’ayant jamais eu d’épisode d’IRA. Il est donc essentiel d’identifier des méthodes permettant d’améliorer la survie à un épisode d’IRA.
Objectif:
L’objectif de cette étude était de décrire l’élaboration et la faisabilité d’une nouvelle approche multidisciplinaire pour la prise en charge des survivants d’un épisode d’IRA en transition de soins (Approche multidisciplinaire en Transition de Soins—AmTS).
Type d’étude:
Initiative d’amélioration des processus menée par observation
Cadre:
Un seul centre médical universitaire aux États-Unis
Sujets:
La population étudiée était constituée d’adultes atteints d’IRA de stade 3 sans traitements de dialyse à leur sortie et qui avaient été mis en contact avec un fournisseur de soins primaires (FSP) dans l’établissement.
Méthodologie:
Avant la mise en œuvre de l’intervention, un outil de dossier de santé électronique a été développé pour identifier les survivants à un épisode d’IRA. Le programme de l’AmTS comprenait la participation des patients pendant leur séjour à l’hôpital, une formation donnée par des infirmières formées en néphrologie avant le congé, les tests de laboratoire recommandés après la sortie de l’hôpital et un suivi structuré axé sur la santé rénale avec un pharmacien et un FSP dans les 7 à 14 jours suivant la sortie de l’hôpital. Il a été laissé à la discrétion des FSP d’aiguiller ou non leurs patients pour une évaluation en néphrologie.
Résultats:
Des données préliminaires ont démontré qu’il était possible d’identifier, d’informer et d’assurer le suivi de la plupart des sujets d’intérêt (des survivants à un épisode d’IRA) avec ce modèle. Cette stratégie a semblé réalisable, évolutive et apte à optimiser l’expertise individuelle des membres de l’équipe multidisciplinaire.
Limites:
Faible taille de l’échantillon; une évaluation future du processus, des résultats cliniques et des résultats rapportés par les patients est nécessaire.
Conclusion:
Le processus de cette AmTS soutenue par une aide à la prise de décision clinique s’est avéré réalisable et a permis de combler les lacunes des modèles de transition des soins existants. Dans le contexte des soins primaires, la prestation de soins en équipe semble être un mécanisme permettant d’étendre la capacité de surveillance de la santé rénale des survivants à un épisode d’IRA.
Introduction
Acute kidney injury (AKI) is associated with 6-month mortality rates exceeding 20% and chronic morbidity, high health care costs, and decreased quality of life for survivors.1-3 Approaches to limit morbidity in AKI survivors are being evaluated. One such effort to improve post-AKI care is through a nephrologist-directed AKI survivor clinic. This model has been associated with improved self-reported kidney health knowledge 4 and process outcomes (ie, kidney-related laboratory assessments), 5 but barriers to feasibility exist, such as patients’ reluctance to add more doctors to the health care team, concerns about distance/scheduling to attend follow-up visits, lack of nephrology specialists in underserved locations, and potential for care fragmentation. 5
There is therefore a need to develop alternative models for post-AKI care delivery, particularly those that involve other health care professionals to enhance capacity. 6 Primary care providers (PCPs) have a vested interest in facilitating care continuity and minimizing fragmentation to improve the patient experience and health outcome. The purpose of this brief report was to characterize the preliminary feasibility of a multidisciplinary approach to AKI survivorship at care transitions (ACT) piloted at Mayo Clinic within primary care.
Methods
Setting
Mayo Clinic in Rochester, Minnesota, is a tertiary care center with a robust primary care practice. Primary care clinics employ a team-based care model overseen by physician leads. Trained ambulatory care pharmacists are embedded in these clinics and consult with patients in collaboration with the PCP. There are 5 inpatient nephrology consultative services that include nurse liaisons who deliver education to hospitalized patients, focusing on patients being discharged on dialysis. No nephrologist-directed dedicated AKI survivor clinic, as has been described, 7 existed at the time the ACT workflow was created.
Participants
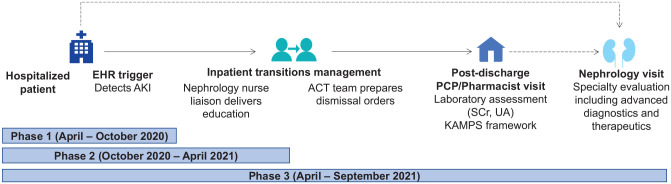
The multidisciplinary ACT pilot began in April 2020 and was rolled out in 3 phases over 18-months (Figure 1). It was developed and implemented as part of a hospital practice change (Table S1), and patients were invited to participate in a related institutional review board (IRB)-approved research study (NCT04505891). The ACT pilot targeted individuals with stage 3 AKI (Table S2) for feasibility reasons. Individuals discharging on dialysis, hospice care, to a skilled nursing facility or without a Mayo Clinic Rochester PCP were also excluded.
Figure 1.
ACT workflow.
Note. The AKI in Care Transitions (ACT) workflow was rolled out in 3 distinct phases separated by time. Phase 1 involved development of an electronic health record indicator of AKI. Phase 2 involved phase 1 + a nephrology nurse liaison education visit prior to dismissal. Phase 3 involved phases 1 and 2 + prepared dismissal orders and follow-up in the outpatient setting. Patients with abnormal serum creatinine or estimated glomerular filtration rate (eGFR) at follow-up were recommended to have a repeat assessment within 3 months. In cases where the postdischarge urinalysis with microscopy revealed an elevated protein osmolality ratio or hematuria, a repeat assessment and urine albumin-to-creatinine ratio were recommended within 3 months. Nephrology referral for follow-up in the outpatient setting was not protocolized and could occur for any patient at any time during the hospitalization or at the direction of the patient’s PCP (as demarcated by the dashed arrows). Follow-up after the immediate PCP transition of care visit coordinated through ACT was nonprotocolized. AKI = acute kidney injury; EHR = electronic health record; SCr = serum creatinine; UA = urinalysis; PCP = primary care provider; KAMPS = kidney follow-up framework (see also Table S3). 8
ACT Intervention
An electronic health record (EHR) AKI indicator was created to identify patients with stage 3 AKI using the KDIGO creatinine and urine output criteria. As hospital dismissal approached, nephrology nurse liaisons delivered focused education on post-AKI care (Table S3). Dismissal orders for serum creatinine, urinalysis with microscopy, and a posthospital follow-up visit with a PCP/pharmacist were scheduled. Preference was given to scheduling this posthospital follow-up as a combined visit, where the patient would meet with the pharmacist first, a verbal hand-off between the pharmacist and the provider would occur, and then the provider would complete the remainder of the encounter with the patient. If the patient or clinician’s schedules could not accommodate this approach, separate visits with electronic communication between the care team members using chart notes or secure messages was an option. Virtual visits were available upon request. The EHR clinical decision support tools were developed that included a failsafe alert to prompt these dismissal orders and enrollment in a “care path.” The care path provided kidney-focused prompts and resources for providers caring for these patients in the inpatient and outpatient settings (Figures S1-3). The posthospital PCP/pharmacist visit included components from the KAMPS framework (Table S4). 8 Pharmacist visits were guided by a best practice document which included a detailed review of renally eliminated and nephrotoxic medications, including nonprescription agents such as non-steroidal anti-inflammatory drugs, an evaluation of fluid status and diuretic management, and an assessment of the risks and benefits of nephroprotective therapies such as renin-angiotensin system inhibitors and sodium-glucose co-transporter 2 inhibitors. If deemed appropriate, nephrology referral was coordinated by the PCP, but was not protocolized.
Data Collection and Analysis
Data were collected on 3 distinct phases which included unique patients. The EHR AKI indicator development and testing (phase 1) included review of patients flagged by the EHR tool for validation of AKI diagnosis using manual chart review (to identify false positives). Inpatient nephrology consult service lists were also reviewed to identify any additional patients with AKI (false negatives with the EHR AKI indicator). For nephrology nurse liaison education visits (phase 2), feasibility was measured by the number of identified patients who completed education. As nephrology nurse liaisons have other responsibilities and are available Monday through Friday 8:00 to 16:00, we hypothesized a proportion of patients would not receive education. A qualitative analyst performed direct observations of interactions between nurses and patients. A focus group with the 4 nephrology nurse liaisons occurred to debrief about the intervention and workflow. Detailed notes were taken using developed guides (Table S5) and themes summarized. We also calculated the proportion of patients where follow-up appointments were scheduled (labs and provider visit) and completed (phase 3).
Results
Phase 1 (April-October 2020)
In total, 73% of alerts in the first week of testing (8/11) and 33% in the second week (4/12) were false positives. Each false positive was attributed to urine output charting without other clinical/laboratory features of AKI. The alert was adjusted to decrease the urine output threshold to ≤0.2 mL/kg/h (from <0.3 mL/kg/h), omit counted voids without quantified urinary volume, and use adjusted weight for patients with a body mass index >40 kg/m2. Follow-up evaluation during the fourth week resulted in 3 alerts, all of which were confirmed as stage 3 AKI. One of the 3 (33%) was followed by the nephrology consult service, whose rosters included 43 individuals at the time of the evaluation. No additional patients with AKI were identified upon review (false negatives).
Phase 2 (October 2020-April 2021)
Of the 42 individuals with an alert who were approached for participation in phase 2 of ACT, 18 consented to participate. A nephrology nurse liaison successfully visited all 18 of these patients targeted for education. The nephrology consultation service, and by extension the nurse liaison, was not previously following 7 (39%) of these individuals. Direct observation of 3 education visits and a focus group with the nursing team revealed key themes about workflow and interaction. Nurses observed that several patients were unaware of their AKI diagnosis. It could not be determined whether the patients were never told or were told but did not remember the discussion. The need to focus AKI education on the potential for recovery and risk of reinjury emerged. Nephrology nurse liaisons observed that components of typical education for patients with chronic kidney disease or end-stage kidney disease (ie, dietary modifications) were perceived as overwhelming for AKI survivors. Finally, we observed that successful response to teach-back questions, a surrogate for information retention, was more successful when home caregivers were present with the AKI survivor. Themes were summarized and communicated to nephrology nurse liaisons, who adjusted their strategies accordingly.
Phase 3 (April-September 2021)
At the time of the report, 17 individuals were approached for participation in phase 3, and 11 consented to be placed on the AKI survivor care path. Of these individuals, 1 died prior to discharge, 1 discharged on dialysis, and 1 did not receive education from the nurse liaisons before discharge. In the remaining 8 patients (Table S6), 100% completed an encounter with the PCP, a serum creatinine evaluation, and a urinalysis within 2 weeks of discharge (median time to follow-up 2 [interquartile range 1-6] days). Seven of the 8 patients (88%) had laboratory assessments performed on the same day as the PCP encounter. The eighth patient had laboratory assessments performed 2 days later. Six (75%) individuals completed an encounter with a pharmacist 5 (1-10) days after discharge. All encounters were in person. Two (25%) patients consulted with an outpatient nephrologist at day 1 and day 22 after discharge, respectively.
Discussion
The long-term morbidity and mortality of AKI have prompted efforts to enhance AKI survivor care. We described the development of an EHR tool to identify AKI survivors before dismissal, deliver inpatient education, and facilitate evidence-based laboratory monitoring and multidisciplinary follow-up within primary care. This pilot leveraged technology, spanned the care continuum, and prioritized scalable, sustainable, multidisciplinary care for AKI survivors.
There are several strengths of the ACT concept. First is the electronic identification of potential candidates for involvement. Historically, manual screening of inpatient service rosters has been used to identify individuals for enrollment in post-AKI clinics, with as little as 7% of targeted individuals appropriate for AKI survivor follow-up. 5 We identified many stage 3 AKI survivors who are not followed by nephrology specialists who would be missed by this method. Use of an informatics-enabled solution based on serum creatinine and urine output facilitates identification of eligible patients with AKI, particularly when not already receiving nephrology care. Second, the ACT pilot focused on the transition of care from inpatient to outpatient environments using clinical decision support. Facilitated care transition processes in other settings have reduced unplanned visits, care costs, and improved quality of life and self-rated health. 9 The care path clinical decision support is rule-based, stays with the patient after a hospital encounter until the PCP visit occurs, and minimizes interruptive alerts for busy clinicians. Third, engagement of a nurse, PCP, and pharmacist to deliver components of post-AKI care with escalation to specialty nephrology consultation as needed maximizes the skills of the multidisciplinary team, to support the limited available resources within nephrology.5,10 PCPs are well-positioned to deliver patient-centered care, address AKI alongside other health needs, and minimize care fragmentation, a barrier raised by patients regarding AKI follow-up. 5 Clinical and laboratory evaluation by a PCP may streamline a future nephrologist encounter if referred. Moreover, the ACT approach shortens the time that patients could be exposed to modifiable determinants of kidney decline (ie, nephrotoxins) without intervention. Existing literature demonstrated a median time of 48 days to nephrologist follow-up, 5 whereas in this study, PCP/pharmacist visits were completed within 1 week of discharge. Future studies should aim to evaluate the perceptions of patients and caregivers about novel AKI survivor care workflows like ACT.
Conclusion
Innovative interventions are urgently needed to mitigate the risk of adverse outcomes in AKI survivors. The ACT workflow, which combines multidisciplinary collaboration with technological advancements, addresses gaps in existing care transition models to expand access to kidney care follow-up. Further exploration of outcomes is underway to assess the impact of this model at the patient and institutional levels.
Supplemental Material
Supplemental material, sj-docx-1-cjk-10.1177_20543581221081258 for Development and Feasibility of a Multidisciplinary Approach to AKI Survivorship in Care Transitions: Research Letter by Erin F. Barreto, Heather P. May, Diana J. Schreier, Laurie A. Meade, Brenda K. Anderson, Megan E. Rensing, Kari L. Ruud, Andrea G. Kattah, Andrew D. Rule, Rozalina G. McCoy, Dawn M. Finnie, Joseph R. Herges and Kianoush B. Kashani in Canadian Journal of Kidney Health and Disease
Acknowledgments
We would like to acknowledge the valuable contributions of Dale Young, Shelley Preble, Kate Mayhew, and Sophea Seng to this project.
Footnotes
Ethics Approval and Consent to Participate: The study was approved by the Mayo Clinic Institutional Review Board (IRB #20-004204) and all participants provided written informed consent.
Consent for Publication: All authors consent for publication of the manuscript in its current form.
Availability of Data and Materials: No additional data and materials are available.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: E.F.B. consults for FAST Biomedical and Wolters Kluwer, unrelated.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by the 2020 Mayo Midwest Clinical Practice Committee Innovation Award, the National Center for Advancing Translational Sciences (grant UL1 TR002377), the National Institute of Allergy and Infectious Diseases (grant K23 AI143882, PI: E.F.B.), the Agency for Healthcare Research and Quality (HS028060-01, PI: E.F.B.), the National Institute of Diabetes and Digestive and Kidney Diseases (K23DK114497, PI: R.G.M.).
ORCID iDs: Erin F. Barreto  https://orcid.org/0000-0002-0996-1487
https://orcid.org/0000-0002-0996-1487
Andrea G. Kattah  https://orcid.org/0000-0001-7228-9876
https://orcid.org/0000-0001-7228-9876
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Susantitaphong P, Cruz DN, Cerda J, et al. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol. 2013;8(9):1482–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10:193–207. http://www.ncbi.nlm.nih.gov/pubmed/24445744. Accessed February 11, 2022. [DOI] [PubMed] [Google Scholar]
- 3. Villeneuve PM, Clark EG, Sikora L, Sood MM, Bagshaw SM. Health-related quality-of-life among survivors of acute kidney injury in the intensive care unit: a systematic review. Intensive Care Med. 2016;42(2):137–146. [DOI] [PubMed] [Google Scholar]
- 4. Ortiz-Soriano V, Alcorn JL, III, Li X, et al. A Survey Study of self-rated patients’ knowledge about AKI in a post-discharge AKI clinic. Can J Kidney Health Dis. 2019;6:2054358119830700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Silver SA, Adhikari NK, Bell CM, et al. Nephrologist Follow-Up versus Usual Care after an Acute Kidney Injury Hospitalization (FUSION): a randomized controlled trial. Clin J Am Soc Nephrol. 2021;16:1005–1014. https://cjasn.asnjournals.org/lookup/doi/10.2215/CJN.17331120. Accessed February 11, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Silver SA, Siew ED. Follow-up care in acute kidney injury: lost in transition. Adv Chronic Kidney Dis. 2017;24:246–252. doi: 10.1053/j.ackd.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 7. Silver SA, Goldstein SL, Harel Z, et al. Ambulatory care after acute kidney injury: an opportunity to improve patient outcomes. Can J Kidney Health Dis. 2015;2:36–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kashani K, Rosner MH, Haase M, et al. Quality improvement goals for acute kidney injury. Clin J Am Soc Nephrol. 2019;14:941–953. http://www.ncbi.nlm.nih.gov/pubmed/31101671. Accessed February 11, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takahashi PY, Naessens JM, Peterson SM, et al. Short-term and long-term effectiveness of a post-hospital care transitions program in an older, medically complex population. Healthcare. 2016;4:30–35. doi: 10.1016/j.hjdsi.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 10. Parker MG, Pivert KA, Ibrahim T, Molitoris BA. Recruiting the next generation of nephrologists. Adv Chronic Kidney Dis. 2013;20:326–335. http://www.ncbi.nlm.nih.gov/pubmed/23809285. Accessed February 11, 2022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cjk-10.1177_20543581221081258 for Development and Feasibility of a Multidisciplinary Approach to AKI Survivorship in Care Transitions: Research Letter by Erin F. Barreto, Heather P. May, Diana J. Schreier, Laurie A. Meade, Brenda K. Anderson, Megan E. Rensing, Kari L. Ruud, Andrea G. Kattah, Andrew D. Rule, Rozalina G. McCoy, Dawn M. Finnie, Joseph R. Herges and Kianoush B. Kashani in Canadian Journal of Kidney Health and Disease