Abstract
Background:
Previous studies have suggested that the coronavirus disease 2019 (COVID-19) pandemic was associated with a decreased rate of acute exacerbation of chronic obstructive pulmonary disease (AECOPD). Data on how the COVID-19 pandemic has influenced mortality, seasonality of, and susceptibility to AECOPD in the chronic obstructive pulmonary disease (COPD) population is scarce.
Methods:
We conducted a national population-based retrospective study using data from the Health Insurance Institute of Slovenia from 2015 to February 2021, with 2015–2019 as the reference. We extracted patient and healthcare data for AECOPD, dividing AECOPD into severe, resulting in hospitalisation, and moderate, requiring outpatient care. The national COPD population was generated based on dispensed prescriptions of inhalation therapies, and moderate AECOPD events were analysed based on dispensed AECOPD medications. We extracted data on all-cause and non-COVID mortality.
Results:
The numbers of severe and moderate AECOPD were reduced by 48% and 34%, respectively, in 2020. In the pandemic year, the seasonality of AECOPD was reversed, with a 1.5-fold higher number of severe AECOPD in summer compared to winter. The proportion of frequent exacerbators (⩾2 AECOPD hospitalisations per year) was reduced by 9% in 2020, with a 30% reduction in repeated severe AECOPD in frequent exacerbators and a 34% reduction in persistent frequent exacerbators (⩾2 AECOPD hospitalisations per year for 2 consecutive years) from 2019. The risk of two or more moderate AECOPD decreased by 43% in 2020. In the multivariate model, pandemic year follow-up was the only independent factor associated with a decreased risk for severe AECOPD (hazard ratio [HR]: 0.71; 95% confidence interval [CI]: 0.61–0.84; p < 0.0001). In 2020, non-COVID mortality decreased (−15%) and no excessive mortality was observed in the COPD population.
Conclusion:
In the pandemic year, we found decreased susceptibility to AECOPD across severity spectrum of COPD, reversed seasonal distribution of severe AECOPD and decreased non-COVID mortality in the COPD population.
Keywords: acute exacerbation, COPD, COVID-19 pandemic, seasonality
Introduction
Acute exacerbation of chronic obstructive pulmonary disease (AECOPD) is a common feature of COPD and leads to increased morbidity and mortality. 1 Infections are the most common trigger, 2 with half of all AECOPD events linked to respiratory virus infection (RVI). 3 AECOPD show seasonality; exacerbations in winter are more frequent than in summer, and a similar trend has been observed for mortality.4,5 The seasonality of AECOPD is explained, at least partly, by the seasonal variation in RVIs. 6
In 2020, the world was confronted with a new coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), characterised by high transmissibility. 7 Countries have attempted to contain the virus with varying degrees of stringent public health measures. 8 These public health measures reduced the circulation of all respiratory viruses in the community, 9 demonstrated most notably by the severely reduced influenza burden during the pandemic year. 10
A meta-analysis published recently found that all included studies reported a reduction in hospitalizations due to AECOPD in the first COVID-19 wave compared to the pre-pandemic period, and the pooled data showed overall reductions of 50% in hospital admission for COPD. 11 A subset of included studies also found a reduction in RVI, suggesting a potential mechanism underlying decreased AECOPD, although overall analysis failed to confirm a link between public healthcare restrictions and reductions in AECOPD. Concerning moderate AECOPD, the meta-analysis revealed no conclusive results regarding the impact of the pandemic. However, a population-based study from Scotland and Wales found a reduction of primary care consultation for AECOPD 12
In addition to reduced RVI rates, other factors may have contributed to the observed decrease in AECOPD. 11 Among these, improved medication adherence,13,14 limited access to healthcare, 15 modified behaviours, and reluctance to seek medical care have previously been reported.13,16
All studies published to date examined the first months of the pandemic, and the long-term effects of the pandemic on AECOPD rates and on the seasonality of AECOPD are unknown. A pandemic effect on seasonality of AECOPD in relation to restrictions could indicate that reduction of RVI is the main cause of observed reductions in AECOPD. 17 In addition, data on mortality in COPD patients can further elucidate the overall impact of the pandemic.
Therefore, our national population-based study aimed to analyse the pandemic’s impact on the seasonal distribution of severe AECOPD and overall susceptibility to AECOPD. Furthermore, we assessed the effect of the pandemic on moderate AECOPD and all-cause and non-COVID mortality rates in the COPD population.
Methods
Study design and data extraction
We conducted a nationwide retrospective observational study using routinely collected data on hospitalisations, medication use, and general insuree information from the Health Insurance Institute of Slovenia (HIIS) from 2015 onwards (a detailed description in the Supplement). The Slovenian health insurance system is compulsory for all citizens (population 2.1 million) and is managed by the HIIS.
All comparisons were made between annual periods from March to February, as the COVID-19 epidemic was officially declared on March 12, 2020; at this time, Slovenia started implementing public health measures, as evidenced by The Oxford COVID-19 Government Response Tracker (OxCGRT) stringency index. 18
The results are reported according to the Reporting of Studies Conducted using Observational Routinely collected Health Data (RECORD) guidelines. 19 The study was approved by the National Medical Ethics Committee (approval no. 0120-52/2021/4).
Analysis of severe AECOPD
We defined severe AECOPD as those requiring hospitalisation. We included all admissions of patients with a primary discharge diagnosis of COPD (J44.0-9) or acute respiratory failure (J96.0) combined with a secondary diagnosis of COPD with acute exacerbation. We excluded patients without long-acting inhalation therapy during the study period.
We extracted data on length of hospital stay (LOS), level of ventilatory support (no support, noninvasive, invasive – the highest level of support was recorded), sex, age, discharge diagnoses, and date of death (if applicable). The first hospitalisation was defined as hospitalisation for AECOPD after no AECOPD-related hospitalisation occurred in the previous 2 years. Patients were categorised according to exacerbation status as follows: (1) frequent exacerbators (⩾2 AECOPD-related hospitalisations per year) and (2) persistent frequent exacerbators (⩾2 AECOPD-related hospitalisations per year for 2 consecutive years).
To compare changes in AECOPD hospitalisation numbers in 2020 to hospitalisation numbers for other diseases in order to control for the disease non-specific effects of the pandemic (especially reluctance to seek medical care and decreased access to the healthcare system), we analysed hospitalisations for heart failure, asthma, and influenza as the primary diagnoses and all hospitalisations for COPD as the secondary diagnosis (International Classification of Diseases, 10th revision, (ICD-10) codes in the Supplement).
To examine the effect of the pandemic year on patients after hospitalisation for AECOPD, we analysed rehospitalisation for AECOPD and mortality during follow-up for admitted patients from November 1 to February 28 of the study period.
Analysis of moderate AECOPD
Definition of COPD population
A national population of COPD patients was generated based on dispensed COPD medications from the medication database of the HIIS. We considered patients to have COPD if they were older than 45 years and redeemed two prescriptions for a long-acting muscarinic antagonist (LAMA), long-acting β-agonist (LABA), or both within the past year. Furthermore, a patient was considered to have COPD if they received triple inhalation therapy (a LAMA, LABA, and inhaled corticosteroid (ICS)). We excluded patients who received montelukast or ICS monotherapy (full criteria in the Supplement). In addition, we included patients in the COPD population if they were hospitalised in the analysis year when COPD was coded in the discharge diagnosis. We recorded sex, age, and time of death for all patients in the COPD population.
Definition and analysis of moderate AECOPD
We considered patients to have moderate AECOPD if they received antibiotics, oral corticosteroids, or both in an outpatient setting. We extracted data on medication prescriptions from HIIS. Only antibiotics typically used for AECOPD were included (details in the Supplement). To control for reduced access to healthcare, we also extracted data on dispensed antibiotics for urinary tract infection (UTI) in the same population of patients.
The COPD populations were defined separately each year; moderate AECOPD events were analysed for the following year.
To test the validity of our results, we analysed a subset of patients for whom COPD was coded as the primary or secondary diagnosis during hospitalisation. Furthermore, we performed sensitivity analyses with varying criteria to define COPD to ensure that the results were robust (Supplement). Last, to check for increased AECOPD rates with greater COPD severity (based on inhalation therapy), we performed subgroup analyses including surviving COPD patients with specific therapies: dual bronchodilatation and triple inhalation therapy (for 2018–2020).
Analysis of mortality in the COPD population
For survival analysis, we compared all-cause and non-COVID monthly/yearly mortality rates in the COPD population. We extracted the data of patients with COPD who died from COVID-19 in the hospital or within 30 days of discharge. Excessive mortality in the COPD population was calculated and compared with that in the general population. Data on mortality in the general population were acquired from the Statistical Office of the Republic of Slovenia SiStat Database. 20
Study endpoints
The primary endpoints of the study were changes in severe AECOPD (hospitalisations and rehospitalisations), seasonality pattern, and changes in the proportion of frequent exacerbators in 2020 compared to those in the pre-pandemic years. We further analysed moderate AECOPD event rates and all-cause mortality in the COPD population including and excluding those who died from COVID-19.
Secondary endpoints were changes in in-hospital mortality, and the rate of ventilatory support in 2020 compared to that in the pre-pandemic period. We also compared changes in hospitalisations for AECOPD and hospitalisations for heart failure, asthma, influenza, and for health failure and pneumonia in which COPD was the secondary diagnosis.
Statistical analysis
Data are expressed as absolute numbers and proportions (percentages), mean values with standard deviations (SDs) or median values with interquartile ranges (IQRs), as appropriate. The annual incidence of exacerbations was calculated as a per-person per-year (PY) rate for moderate AECOPD and as an age-standardised number per 100,000 population for AECOPD hospitalisations. Age and sex were standardised using a direct standardisation method with 5-year age groups and the Slovenian population as the reference. Changes in all relevant indicators in the pandemic year were observed relative to 2015–2019 averages. If, however, exponential trend analysis revealed a strong (R2 > 0.5) upwards or downwards trend in the 2015–2019 period and at the same time the observed values for 2019 were aligned with the trend values, changes in the pandemic year were observed relative to 2019. To assess the seasonality of AECOPD, 31-day moving averages were calculated based on daily hospitalisation data. Furthermore, to analyse the effect of follow-up in the pandemic year on the cumulative incidence of COPD rehospitalisations, we used a competing risk approach; we considered death before hospitalisation a competing risk. We constructed multivariable Fine-Grey regression models to adjust for the following important covariates: age, sex, LOS, ventilatory support at index hospitalization, and year of follow-up. We calculated the relative risk of moderate AECOPD in the pandemic year relative to exacerbations in the pre-pandemic period.
Mortality in the overall COPD population was calculated as the number of persons who died per 1000 person-years (PY) or person-months (PM), and mortality was compared between annual periods. Excessive mortality was calculated as the percentage change in the observed monthly/yearly death rate compared to that estimated based on the preceding year’s average. For mortality rate and excessive mortality analysis, we separated all-cause and non-COVID all-cause mortality.
All analyses were performed with STATA V15.1 (Stata Corp, College Station, Texas, USA, 77845) or R version 4.0.2 and its affiliated packages. P values of <.05 were considered statistically significant.
Results
Severe AECOPD
During the study period (2015–2020), a total of 12,210 hospitalisations for AECOPD occurred (0.60% of hospitalisations in Slovenia) among 5859 patients. We excluded 343 patients due to the lack of inhalation therapy. In the same period, 15,638 patients were hospitalised with either a primary or secondary diagnosis of COPD.
The baseline characteristics of the patients and AECOPD hospitalisations are summarised in Table 1. In 2020, a decline in the number of AECOPD hospitalisations (−48%) and patients (−44%) occurred (Table 1) compared to the average of preceding years. Patients in 2020, on average, were slightly older, and the mean LOS decreased by over 1 day (−11%).
Table 1.
Baseline characteristics of patients hospitalised for AECOPD during the study period.
| 2015 | 2016 | 2017 | 2018 | 2019 | Average, 2015–2019 | 2020 | |
|---|---|---|---|---|---|---|---|
| No. of patients | 1529 | 1586 | 1473 | 1597 | 1495 | 1538 | 867 |
| Age, years | 71.3 ± 9.7 | 71.1 ± 9.4 | 70.9 ± 9.3 | 70.8 ± 9.7 | 71.0 ± 9.1 | 71.0 ± 9.4 | 71.8 ± 9.1 |
| Sex, female | 513 (33.6) | 544 (34.3) | 518 (35.2) | 611 (38.3) | 575 (38.5) | 552 (36.0) | 324 (37.4) |
| No. of surviving patients in current year | 1253 | 1281 | 1228 | 1308 | 1240 | 1264 | 674 |
| No. of hospitalizations | 2153 | 2334 | 2122 | 2314 | 2129 | 2210 | 1158 |
| Age-standardised hospitalisation rate | 110.4 | 116.4 | 103.9 | 110.3 | 99.3 | 52.9 | |
| Length of stay, mean, days | 9.6 ± 9.0 | 10.2 ± 10.7 | 9.6 ± 10.2 | 9.8 ± 10.6 | 9.8 ± 10.4 | 9.8 ± 10.2 | 8.7 ± 9.5 |
| Length of stay, median, days | 7.1 (7.7) | 7.1 (7.6) | 6.9 (7.2) | 6.9 (6.9) | 7.0 (6.9) | 7.0 (7.4) | 6.7 (6.2) |
| Proportion of hospitalizations with no ventilatory support | 92.1 | 88.2 | 89.1 | 87.2 | 85.3 | 88.3 | 84.5 |
| Proportion of noninvasive ventilation | 5.1 | 7.4 | 7.4 | 8.4 | 11.8 | 8.0 | 12.3 |
| Proportion of invasive ventilation | 2.8 | 4.5 | 3.5 | 4.4 | 3.0 | 3.7 | 3.2 |
| Specific subgroups of patients | |||||||
| Frequent exacerbators a | 557 (35.1) | 567 (38.5) | 560 (35.1) | 548 (36.7) | 558 b (36.3) | 286 (33.0) | |
| Frequent exacerbators a surviving in current year | 424 (33.1) | 453 (36.9) | 427 (32.6) | 433 (34.9) | 434 b (34.4) | 211 (31.3) | |
| Frequent exacerbators a in previous year, hospitalised at least once in current year | 212 (38.1) | 226 (39.9) | 207 (37.0) | 215 c (38.3) | 147 (26.8) | ||
| Frequent exacerbators a in previous year, hospitalised at least once in current year, and surviving in current year | 153 (36.1) | 167 (36.9) | 151 (35.4) | 157 c (36.1) | 116 (26.8) | ||
| Persistent frequent exacerbators d | 127 (22.8) | 118 (20.8) | 112 (20) | 119 c (21.2) | 76 (13.9) | ||
| Persistent frequent exacerbators d surviving in current year | 93 (21.9) | 81 (17.9) | 80 (18.7) | 85 c (19.5) | 55 (12.7) | ||
Data are presented as the no. (%), mean ± SD, or median (interquartile range), unless otherwise indicated;
Patients with ⩾ 2 AECOPD events.
2016–2019.
2017–2019.
Patients with ⩾2 AECOPD events in two consecutive years.
Changes in the numbers of comparator diagnoses hospitalisations in 2020 are shown in Figure 1. The reduction in heart failure–related hospitalisations was only half of that of COPD- and asthma-related hospitalisations. In the subgroup of hospitalisations for which COPD was the secondary diagnosis, hospitalisations for pneumonia (−57%) decreased more than those for heart failure (−28%). We found the greatest reduction in influenza hospitalisation numbers. All 157 hospitalisations in 2020 were during the 2019/2020 influenza season. Of the 17,489 patients in the 2020 COPD cohort, 401 (2.3%) were hospitalised for COVID-19 in the 12 pandemic months, accounting for 2.6% of all COVID-19 hospitalisations.
Figure 1.
Relative changes in the pandemic year relative to the 2015–2019 average. (a) hospitalizations across diseases and (b) selected indicators for COPD hospitalizations.
H, hospitalizations; LOS, length of stay.
a Negative exponential trend, average annual growth rate: −10.3%, R2 = 0.978.
b Positive exponential trend, average annual growth rate: 14.1%, R2 = 0.8321.
c Negative exponential trend; average annual growth rate: −5.3%; R2 = 0.5648.
In the pre-pandemic years, the proportion of AECOPD hospitalisations in winter was higher than that in summer (29% vs 21%). In 2020, the proportions were reversed, with 20% vs 30%, respectively. Figure 2 depicts the daily variations in admissions for AECOPD in 2020 compared to the average rates in 2015–2019; alongside, the OxCGRT stringency index is shown. The pattern of seasonality was reversed in 2020. Pre-pandemic AECOPD-related hospitalisation numbers peaked in midwinter and were lowest in late summer, while in 2020, AECOPD hospitalisation numbers were sustainably elevated from late May to early September. Daily rates of AECOPD admissions were; however, lower throughout 2020 compared to 2015–2019.
Figure 2.
Comparison of the seasonality of AECOPD hospitalizations in the pandemic year vs previous years.
The number and proportion of hospitalisations of frequent exacerbators were stable over the pre-pandemic period (Table 1); however, in 2020, significant declines in both the number and proportion were observed. We found a 9% relative reduction in the proportion of frequent exacerbators in 2020, with a 30% reduction in the proportion of repeated severe AECOPD in 2020 (in the group of frequent exacerbators in 2019). The most striking decrease in the proportion, at 34%, occurred in the group of persistent frequent exacerbators.
There was a positive trend for the use of noninvasive ventilation (NIV) across years, with only a slight additional increase in 2020. The proportion of invasive ventilation (IV) remained stable throughout the years. In 2020, the proportion of hospitalisations requiring ventilatory support remained relatively stable throughout the year (summer 16.9% vs winter 15.6%). In-hospital mortality increased in 2020 (+53%), with the largest increase in those with NIV, at 133%, while mortality in those receiving IV increased by 38%. The absolute number of patients who died during AECOPD-related hospitalisation was lower in 2020 than the average in 2015 to 2019 (89 vs 116, respectively).
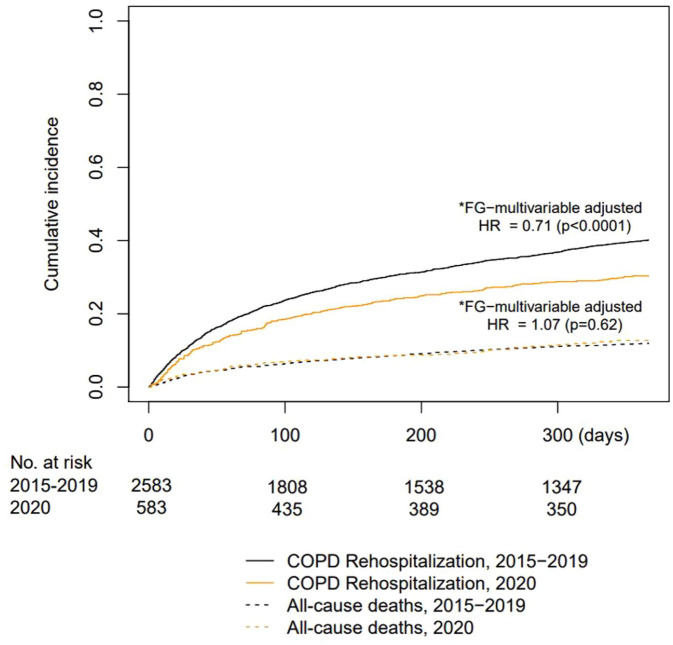
Figure 3 shows the Kaplan–Meier (KM) curves for COPD rehospitalisation (primary outcome) and all-cause mortality. When comparing the pre-pandemic years (2015–2019) to 2020, the rehospitalisation risk after the index hospitalisation was significantly lower in 2020. In the adjusted multivariable Fine-Grey model, the only significant predictor of rehospitalisation was follow-up in the pandemic year (hazard ratio [HR]: 0.71; 95% confidence interval [CI], 0.61–0.84; p < 0.0001) (Table in Supplement). However, all-cause mortality in those who were not rehospitalised for AECOPD remained constant across years. The significant predictors of all-cause mortality according to the adjusted multivariable Fine-Grey model were sex (male), age, and LOS.
Figure 3.
Cumulative incidence of COPD rehospitalizations and all-cause mortality (competing risk) according to the follow-up period.
Moderate AECOPD
We estimated the COPD patient population in Slovenia from 2016 to 2020 as described; the COPD population increased from 14,128 in 2016 to 17,489 in 2020 (Table 2). The average age of patients remained stable across years; the majority of patients were men, although the proportion of women increased steadily.
Table 2.
Moderate AECOPD during the study period.
| Population analysis for moderate exacerbations | 2016 | 2017 | 2018 | 2019 | 2020 | 2020/2019 (%) or RR a |
|---|---|---|---|---|---|---|
| COPD population b | 14,128 | 15,462 | 16,376 | 17,050 | 17,489 | |
| Female sex (%) | 35.5 | 36.6 | 37.5 | 38.3 | 39.2 | |
| Age, years (mean ± SD) | 69.4 ± 10.0 | 69.4 ± 9.9 | 69.5 ± 9.8 | 69.5 ± 9.7 | 69.6 ± 9.6 | |
| All events c (No./COPD population) | 0.85 | 0.78 | 0.80 | 0.77 | 0.51 | –33.8 |
| Antibiotics | 0.62 | 0.56 | 0.58 | 0.55 | 0.34 | –38.9 |
| Systemic corticosteroids | 0.13 | 0.13 | 0.13 | 0.12 | 0.12 | –6.1 |
| Antibiotics and corticosteroids | 0.10 | 0.09 | 0.09 | 0.09 | 0.05 | –41.1 |
| No. events/exacerbator d | 1.81 | 1.80 | 1.77 | 1.76 | 1.65 | –6.1 |
| Risk of experiencing at least 1 event | 0.469 | 0.431 | 0.450 | 0.438 | 0.309 | 0.692 a |
| Risk of experiencing at least 2 events | 0.215 | 0.195 | 0.202 | 0.193 | 0.115 | 0.575 a |
| Specific subgroups of patients | ||||||
| COPD coded in hospitalisation | ||||||
| Population | 5218 | 6271 | 7080 | 7594 | 7528 | |
| Events/population | 1.18 | 1.06 | 1.05 | 1.02 | 0.71 | –29.8 |
| Risk of experiencing at least 1 event | 0.599 | 0.534 | 0.539 | 0.529 | 0.398 | 0.728 a |
| Risk of experiencing at least 2 events | 0.312 | 0.280 | 0.280 | 0.263 | 0.174 | 0.620 a |
| Patients receiving triple therapy 2018–2020 | ||||||
| Population | 4510 | 4510 | 4510 | |||
| Events/population e | 1.05 | 0.98 | 0.68 | –32.9 f | ||
| Risk of experiencing at least 1 event | 0.550 | 0.516 | 0.380 | 0.713 a | ||
| Risk of experiencing at least 2 events | 0.276 | 0.254 | 0.173 | 0.651 a | ||
| Patients receiving dual bronchodilatation 2018–2020 | ||||||
| Population | 2665 | 2665 | 2665 | |||
| Events/population e | 0.51 | 0.50 | 0.33 | –34.4 f | ||
| Risk of experiencing at least 1 event | 0.345 | 0.341 | 0.232 | 0.674 a | ||
| Risk of experiencing at least 2 events | 0.119 | 0.103 | 0.650 | 0.585 a | ||
| Population analysis for comparator event (UTI) | ||||||
| UTI events/COPD population | 0.076 | 0.077 | 0.079 | 0.085 | 0.078 | –8.0 |
COPD, chronic obstructive pulmonary disease; SD, standard deviation; UTI, urinary tract infection.
Relative risk (risk in 2020 relative to the 2016-2019 or 2018-2019 weighted average risk).
Estimated separately for each year and based on COPD medication claims and hospitalisation events (e-Appendix 1).
Based on medication claims (antibiotics, oral corticosteroids, or both – e-Appendix 3).
Rates for patients with exacerbations.
Rates from 2018 on analysis of stable surviving subpopulation of dual/triple maintenance therapy (e-Appendix 2).
Compared to the 2018–2019 average.
Predictably, the baseline risk of moderate AECOPD increased with the severity of COPD; the risk was lower in the dual bronchodilatation subgroup and higher in the post-hospitalisation/triple-therapy subgroup (Table 2). The rate of moderate AECOPD events in the whole COPD population declined slightly during 2016–2019 and significantly in 2020, which was 66.2% of that in 2019. This decrease was mainly due to fewer antibiotic prescriptions, while the number of oral corticosteroid prescriptions remained unchanged. For comparison, the rate of UTI events was relatively stable during 2016–2020.
The risk of having at least one moderate AECOPD event decreased by 30.8% in 2020 relative to the 2016–2019 weighted average. In specific COPD patient subgroups, we observed similar decreases in risks, by 27.2% in previously hospitalised COPD patients, 28.7% in patients on triple therapy and 32.6% in patients on dual bronchodilatation. The risk of two or more moderate AECOPD decreased in 2020 overall by 42.5% (Table 2).
Mortality in COPD population
The average yearly mortality rates in the COPD population in the pre-pandemic years were 78.9/1000 (±5.0) PY; in 2020, it was 75.8/1000 PY and non-COVID mortality 66.8/1000 PY. The monthly mortality rate during the study period is represented in Figure 4. All-cause mortality in the pre-pandemic period showed a seasonal peak, with the highest mortality in winter (January and February). In 2020, it peaked in parallel with the second COVID-19 wave (October to December). During the first COVID-19 wave, all-cause mortality in the COPD population was decreased compared to previous years. Yearly excessive mortality in the 2020 annual period was +21.7% in the general population, −4.0% in the overall COPD population and −15.3% for non-COVID mortality in the COPD population.
Figure 4.
Mortality rate in the COPD population in pandemic vs pre-pandemic years: (a) monthly mortality rate over 12 months and (b) excessive mortality in the COPD population compared to excessive mortality in the general population.
Discussion
In our nationwide population study, we found that the seasonality of severe AECOPD was reversed and that the susceptibility of COPD patients to exacerbate and reexacerbate substantially decreased in the pandemic year. Furthermore, non-COVID mortality decreased in the COPD population.
Severe AECOPD
Analysing the full pandemic year allowed us to examine the seasonality of severe AECOPD. In the pre-pandemic years, we observed characteristic Northern Hemisphere seasonality. However, the pattern was reversed in 2020, with increased numbers of AECOPD in the summer. Nevertheless, reductions in exacerbations occurred throughout all seasons of the pandemic year, culminating in a 48% decrease in AECOPD hospitalisation numbers, thus confirming and expanding the results of previous, shorter studies. 11 AECOPD hospitalisation variation followed the reverse pattern of public health restrictions, suggesting a causal relationship between viral transmission prevention (restrictions) and AECOPD reduction.
Several studies and public health agency reports found that the emergence of SARS-CoV-2 and the resulting shutdowns were associated with significant reductions in the circulation of seasonal respiratory viruses.9,21–26 In fact, also in Slovenia, no patient tested positive for influenza in the 2020/21 season and respiratory syncytial virus infections were starkly reduced.22,23 These reductions in seasonal RVI caused by enforcement of public health measures likely substantially contributed to reductions in severe AECOPD. However, factors other than RVI have been linked to the seasonality of AECOPD. 27 Although we were not able to adjust for environmental factors, our finding of reversed seasonality in 2020 suggest that the major driving force of seasonal variation in AECOPD is the variation in RVI and that factors linked to winter (i.e. cold temperatures and increased pollution) are probably of lesser importance. 28
Further supporting the significant role of RVI in the pathogenesis of AECOPD (and in the pathogenesis of exacerbations of other respiratory diseases) is the fact that we found a disproportionately lower decrease in hospitalisations for heart failure (−20%) compared to diagnoses with a known infectious trigger that is, COPD (−48%) and asthma (−47%). Similar results were also observed in other studies.29–31 A lesser reduction in heart failure hospitalisations further indicates that fear and reduced access to healthcare played a minor role in the observed reduction in AECOPD. A misclassification of AECOPD as COVID-19 hospitalizations could also have contributed to the reduction in AECOPD hospitalisations. However, most patients with COPD hospitalised for COVID-19 have been found to have pneumonia and not AECOPD. 32
When analysing patients with the highest risk for COPD hospitalisation, especially frequent exacerbators, we found a decreased risk of AECOPD hospitalisation in 2020 by approximately 30%. In recently hospitalised patients, follow-up in 2020 was the only independent predictor of this reduction. In addition, we found a substantially decreased proportion of patients with two or more moderate AECOPDs. Our results suggest that the pandemic year influenced the susceptibility of patients to exacerbate and reexacerbate. The most plausible explanation for our findings is that lockdown measures with social distancing and shielding significantly impeded viral transmission and the likelihood of COPD patients acquiring an RVI, which reduced the risk of AECOPD. Our results further suggest that intrinsic susceptibility to exacerbation, which is likely of lesser importance in the pathogenesis of AECOPD, 33 requires, at least in some patients, an additional extrinsic factor to manifest as AECOPD.
In-hospital mortality due to AECOPD was increased (+53%) in 2020 compared to the previous years. Other studies have shown mixed results regarding the effect of the pandemic on in-hospital deaths.12,34 Increased in-hospital mortality is likely due to a combination of more severe patients being admitted (potentially due to fear of using health care services) and the health system being overwhelmed with COVID-19 patients.
Interestingly, in patients with COPD hospitalisations for pneumonia decreased disproportionately more than those for heart failure. This finding potentially reflects the importance of RVI as a trigger for secondary bacterial infection in COPD thus corroborating the results of other studies.35–37
Moderate AECOPD
We found an overall reduction of 33.8% in the exacerbation medication prescription, indicating a significantly lower incidence of moderate AECOPD. As the difference in the UTI antibiotic prescription rate in 2020 compared to previous years, was small, we believe that the reduced access to healthcare during the pandemic year probably played only a minor role in the reduction of AECOPD medication prescriptions. Furthermore, unmet moderate AECOPD treatment needs would likely lead to increased hospitalisation rates. Our results are consistent with the results of other populational studies that also observed reductions in moderate AECOPD exacerbations following the introduction of restrictions. 12
Mortality in COPD population
We found a substantially decreased non-COVID all-cause mortality in COPD patients in 2020. Despite the fact that Slovenia was heavily impacted by the second COVID-19 wave, with one of the highest COVID-19 mortality rates worldwide at the time, 38 no excessive mortality occurred in the COPD population during the pandemic annual period, whereas substantial excessive mortality was detected (+21%) in the general population. Several studies have found that COPD patients have an increased risk for adverse COVID-19 outcomes, including death. 39 Better compliance with lockdown measures in the COPD population could account for this difference.
Several studies have reported increased mortality due to heart disease in the first pandemic wave, indicating increased out-of-hospital mortality due to poor access to health services.40,41 When comparing heart disease and COPD outcomes during the pandemic, a difference emerges. The reductions in AECOPD at all levels in our study likely translated into a decrease in non-COVID mortality, suggesting an actual protective effect of pandemic measures on COPD and causality. The reverse is true for heart disease, where proportionally smaller reductions in health care utilisation, compared to that for COPD, were associated with increased overall mortality.
Strengths and limitations
Our population-based study has several strengths. This is one of only a few studies to examine the seasonality of severe AECOPD and long-term outcomes with complete national data, contributing to the Generalisability of the results. Patient-level information allowed us an in-depth analysis of patients with the highest risk. In addition, large data sets enabled us to simultaneously study the effect on AECOPD across the severity spectrum as well as overall and non-COVID mortality in the same population, providing unique insights into the pandemic’s effect on the COPD population.
Our study is subject to the limitations of large administrative data research. In the hospitalisation database, some degree of inaccuracy in reporting and COPD overdiagnosis could have occurred. However, we previously found, that overdiagnosis of COPD was low (less than 8%). 42 In addition, we improved the accuracy by excluding patients without inhalation therapy. Moreover, we believe inaccurate reporting was constant across years and thus did not affect our main results. The analysis of moderate AECOPD was limited by medication refills being used as a proxy for both defining the COPD population and moderate AECOPD events. However, both methods have been used previously.13,43 Moreover, the sensitivity analyses of different criteria for defining COPD showed no effect on our results.
Our detected COPD population increased over the years, similar to other studies, 44 potentially influencing the validity of comparisons across years. However, the overall rate of moderate AECOPD events and the average patient age did not differ substantially across the yearly COPD populations, suggesting similar severity among patients, validating the comparison.
Last, due to our population-based design, we could not address the underlying mechanism of the AECOPD reduction directly. Although alternative explanations to observed reductions of AECOPD that were outlined elsewhere (improved adherence to medication, decreased pollution, and fear of using healthcare services) 11 are possible and likely contributed to observed reductions, we believe our data, taken together strongly indicate that the reduction in AECOPD was mainly due to reduction in RVI, achieved with pandemic measures. Further research is necessary to confirm this notion.
Conclusion
Using complete national data, we report a sustained significant reduction in the number of AECOPD events and reversed seasonality of severe AECOPD during the first pandemic year. The pandemic year was associated with decreased tendencies in exacerbations and reexacerbations and with a decreased number of frequent exacerbators. Non-COVID all-cause mortality in the COPD population decreased compared to the preceding years. Our findings have important implications for our understanding of susceptibility to exacerbation as well as future strategies for AECOPD prevention. The pandemic, in our view, demonstrated the potential of RVI prevention and control. New approaches involving targeted behaviour modifications and the prospect of new vaccination technologies could promote the benefits of pandemic measures without the harm of lockdown.
Supplemental Material
Supplemental material, sj-docx-1-tar-10.1177_17534666221081047 for Mortality, seasonal variation, and susceptibility to acute exacerbation of COPD in the pandemic year: a nationwide population study by Irena Sarc, Alesa Lotric Dolinar, Tina Morgan, Joze Sambt, Kristina Ziherl, Dalibor Gavric, Julij Selb, Ales Rozman and Petra Dosenovic Bonca in Therapeutic Advances in Respiratory Disease
Acknowledgments
The authors thank the Health Insurance Institute of Slovenia for giving them access to their administrative health care database.
Footnotes
Author contributions: Irena Sarc: Conceptualisation; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Visualisation; Writing – original draft; Writing – review & editing.
Alesa Lotric Dolinar: Data curation; Formal analysis; Investigation; Methodology; Software; Supervision; Validation; Visualisation; Writing – review & editing.
Tina Morgan: Conceptualisation; Data curation; Formal analysis; Investigation; Methodology; Validation; Visualisation; Writing – review & editing.
Joze Sambt: Data curation; Formal analysis; Methodology; Software; Validation; Writing – review & editing.
Kristina Ziherl: Data curation; Formal analysis; Methodology; Project administration; Validation; Visualisation; Writing – review & editing.
Dalibor Gavric: Data curation; Methodology; Validation; Writing – review & editing.
Julij Selb: Formal analysis; Methodology; Software; Writing – review & editing.
Ales Rozman: Conceptualisation; Methodology; Project administration; Writing – review & editing.
Petra Dosenovic Bonca: Conceptualisation; Data curation; Formal analysis; Methodology; Resources; Software; Supervision; Validation; Visualisation; Writing – review & editing.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Irena Sarc  https://orcid.org/0000-0001-6053-7406
https://orcid.org/0000-0001-6053-7406
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Irena Sarc, Noninvasive Ventilation Department, University Clinic of Respiratory and Allergic Diseases Golnik, Golnik 36, 4204 Golnik, Slovenia Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia.
Alesa Lotric Dolinar, Academic Unit for Mathematics, Statistics and Operations Research, School of Economics and Business, University of Ljubljana, Ljubljana, Slovenia.
Tina Morgan, University Clinic of Respiratory and Allergic Diseases Golnik, Golnik, Slovenia.
Joze Sambt, Academic Unit for Mathematics, Statistics and Operations Research, School of Economics and Business, University of Ljubljana, Ljubljana, Slovenia.
Kristina Ziherl, Noninvasive Ventilation Department, University Clinic of Respiratory and Allergic Diseases Golnik, Golnik, Slovenia; Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia.
Dalibor Gavric, The Health Insurance Institute of Slovenia, Ljubljana, Slovenia.
Julij Selb, Faculty of Medicine, University of Ljubljana, Ljubljana, SloveniaUniversity Clinic of Respiratory and Allergic Diseases Golnik, Golnik, Slovenia.
Ales Rozman, Faculty of Medicine, University of Ljubljana, Ljubljana, SloveniaUniversity Clinic of Respiratory and Allergic Diseases Golnik, Golnik, Slovenia.
Petra Dosenovic Bonca, Academic Unit for Economics, School of Economics and Business, University of Ljubljana, Ljubljana, Slovenia.
References
- 1. Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet 2007; 370: 786–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ko FW, Chan KP, Hui DS, et al. Acute exacerbation of COPD. Respirology 2016; 21: 1152–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Linden D, Guo-Parke H, Coyle PV, et al. Respiratory viral infection: a potential ‘missing link’ in the pathogenesis of COPD. Eur Respir Rev 2019; 28: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rabe KF, Fabbri LM, Vogelmeier C, et al. Seasonal distribution of COPD exacerbations in the prevention of exacerbations with tiotropium in COPD trial. Chest 2013; 143: 711–719. [DOI] [PubMed] [Google Scholar]
- 5. Wise RA, Calverley PMA, Carter K, et al. Seasonal variations in exacerbations and deaths in patients with COPD during the TIOSPIR® trial. Int J COPD 2018; 13: 605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. George ŜN, Garcha DS, Mackay AJ, et al. Human rhinovirus infection during naturally occurring COPD exacerbations. Eur Respir J 2014; 44: 87–96. [DOI] [PubMed] [Google Scholar]
- 7. Petersen E, Koopmans M, Go U, et al. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect Dis 2020; 20: e238–e244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. COVID-19: stringency index, https://ourworldindata.org/grapher/covid-stringency-index?tab=table (accessed 12 July 2021).
- 9. Poole S, Brendish NJ, Clark TW. SARS-CoV-2 has displaced other seasonal respiratory viruses: results from a prospective cohort study. J Infect 2020; 81: 966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Noh JY, Seong H, Yoon JG, et al. Social distancing against COVID-19: implication for the control of influenza. J Korean Med Sci 2020; 35: 10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alqahtani JS, Oyelade T, Aldhahir AM, et al. Reduction in hospitalised COPD exacerbations during COVID-19: a systematic review and meta-analysis. PLoS ONE 2021; 16: e0255659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alsallakh MA, Sivakumaran S, Kennedy S, et al. Impact of COVID-19 lockdown on the incidence and mortality of acute exacerbations of chronic obstructive pulmonary disease: national interrupted time series analyses for Scotland and Wales. BMC Med 2021; 19: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McAuley H, Hadley K, Elneima O, et al. COPD in the time of COVID-19: an analysis of acute exacerbations and reported behavioural changes in patients with COPD. ERJ Open Res 2021; 7: 00718-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaye L, Theye B, Smeenk I, et al. Changes in medication adherence among patients with asthma and COPD during the COVID-19 pandemic. J Allergy Clin Immunol Pract 2020; 8: 2384–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Di Bidino R, Cicchetti A. Impact of SARS-CoV-2 on provided healthcare. Evidence from the emergency phase in Italy. Front Public Health 2020; 8: 583583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Czeisler MÉ, Marynak K, Clarke KEN, et al. Delay or avoidance of medical care because of COVID-19–related concerns – United States, June 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Donaldson GC, Wedzicha JA. The causes and consequences of seasonal variation in COPD exacerbations. Int J Chron Obstruct Pulmon Dis 2014; 9: 1101–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. COVID-19: stringency index, https://ourworldindata.org/grapher/covid-stringency-index?tab=chart&time=2020-03-01..2021-02-28&country=~SVN (accessed 12 July 2021).
- 19. Benchimol EI, Smeeth L, Guttmann A, et al. The reporting of studies conducted using observational routinely-collected health data (RECORD) statement. PLoS Med 2015; 12: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Presežna umrljivost po: Mesec Meritve, https://pxweb.stat.si/SiStatData/pxweb/sl/Data/-/05L1020S.px/ (accessed 12 July 2021).
- 21. RSV National Trends – NREVSS| CDC, https://www.cdc.gov/surveillance/nrevss/rsv/natl-trend.html (accessed 12 July 2021).
- 22. Surveillance atlas of infectious diseases, https://atlas.ecdc.europa.eu/public/index.aspx (accessed 12 July 2021).
- 23. Tedensko spremljanje gripe in drugih akutnih okužb dihal v sezoni 2020/2021, https://www.nijz.si/sl/tedensko-spremljanje-gripe-in-drugih-akutnih-okuzb-dihal-v-sezoni-20202021 (accessed 12 July 2021).
- 24. Human Parainfluenza National Trends – NREVSS| CDC, https://www.cdc.gov/surveillance/nrevss/human-paraflu/natl-trend.html (accessed 12 July 2021).
- 25. Weekly U.S. Influenza Surveillance Report. CDC, https://www.cdc.gov/flu/weekly/index.htm (accessed 12 July 2021).
- 26. Coronavirus shutdowns have suppressed other viruses, but there will be a rebound. The Washington Post, https://www.washingtonpost.com/health/2021/01/12/covid-shutdowns-viruses/ (accessed 12 July 2021).
- 27. Hicks A, Healy E, Sandeman N, et al. A time for everything and everything in its time – exploring the mechanisms underlying seasonality of COPD exacerbations. Int J Chron Obstruct Pulmon Dis 2018; 13: 2739–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Almagro P, Hernandez C, Martinez-Cambor P, et al. Seasonality, ambient temperatures and hospitalizations for acute exacerbation of COPD: a population-based study in a metropolitan area. Int J Chron Obstruct Pulmon Dis 2015; 10: 899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Berghaus TM, Karschnia P, Haberl S, et al. Disproportionate decline in admissions for exacerbated COPD during the COVID-19 pandemic. Respir Med 2022; 191: 106120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chan KPF, Ma TF, Kwok WC, et al. Significant reduction in hospital admissions for acute exacerbation of chronic obstructive pulmonary disease in Hong Kong during coronavirus disease 2019 pandemic. Respir Med 2020; 171: 106085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Birkmeyer JD, Barnato A, Birkmeyer N, et al. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff (Millwood) 2020; 39: 2010–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Graziani D, Soriano JB, Del Rio-Bermudez C, et al. Characteristics and prognosis of COVID-19 in patients with COPD. J Clin Med 2020; 9: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hurst JR, Donaldson GC, Wilkinson TMA, et al. Epidemiological relationships between the common cold and exacerbation frequency in COPD. Eur Respir J 2005; 26: 846–852. [DOI] [PubMed] [Google Scholar]
- 34. Bodilsen J, Nielsen PB, Søgaard M, et al. Hospital admission and mortality rates for non-covid diseases in Denmark during covid-19 pandemic: nationwide population based cohort study. BMJ 2021; 373: n1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. D’Anna SE, Balbi B, Cappello F, et al. Bacterial–viral load and the immune response in stable and exacerbated COPD: significance and therapeutic prospects. Int J Chron Obstruct Pulmon Dis 2016; 11: 445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mallia P, Footitt J, Sotero R, et al. Rhinovirus infection induces degradation of antimicrobial peptides and secondary bacterial infection in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 186: 1117–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang H, Anthony D, Selemidis S, et al. Resolving viral-induced secondary bacterial infection in COPD: a concise review. Front Immunol 2018; 9: 2345–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Coronavirus (COVID-19) deaths – statistics and research – our world in data, https://ourworldindata.org/covid-deaths (accessed 12 July 2021).
- 39. Alqahtani JS, Oyelade T, Aldhahir AM, et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS ONE 2020; 15: e0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nef HM, Elsässer A, Möllmann H, et al. Impact of the COVID-19 pandemic on cardiovascular mortality and catherization activity during the lockdown in central Germany: an observational study. Clin Res Cardiol 2021; 110: 292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Garcia S, Albaghdadi MS, Meraj PM, et al. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the united states during COVID-19 pandemic. J Am Coll Cardiol 2020; 75: 2871–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sarc I, Jeric T, Ziherl K, et al. Adherence to treatment guidelines and long-term survival in hospitalized patients with chronic obstructive pulmonary disease. J Eval Clin Pract 2011; 17: 737–743. [DOI] [PubMed] [Google Scholar]
- 43. Hansen ESH, Aasbjerg K, Moeller AL, et al. Hormone replacement therapy and development of new asthma. Chest 2021; 160: 45–52. [DOI] [PubMed] [Google Scholar]
- 44. Lange P, Tøttenborg SS, Sorknæs AD, et al. Danish register of chronic obstructive pulmonary disease. Clin Epidemiol 2016; 8: 673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tar-10.1177_17534666221081047 for Mortality, seasonal variation, and susceptibility to acute exacerbation of COPD in the pandemic year: a nationwide population study by Irena Sarc, Alesa Lotric Dolinar, Tina Morgan, Joze Sambt, Kristina Ziherl, Dalibor Gavric, Julij Selb, Ales Rozman and Petra Dosenovic Bonca in Therapeutic Advances in Respiratory Disease