Abstract
Objective
Fragile soft clots and stiff clots remain challenging in the treatment of acute ischemic stroke. This study aims to investigate the impact of clot stiffness on the efficacy of thrombectomy devices and a new aspiration catheter with a hydro-separator.
Methods
The Neurostar aspiration catheter has a novel hydro-separator technology that macerates clots by a stream of saline inside the catheter. The Neurostar catheter and two commercially available devices, the SOFIA aspiration catheter and Solitaire stent retriever, were tested in this study. We evaluated the efficacy of each device on clots with various stiffness in a simple in vitro model. We also assessed single-pass recanalization performance in challenging situations with large erythrocyte-rich clots and fibrin-rich clots in a realistic vascular model.
Results
We observed an inverse association between the clot stiffness and recanalization rates. The aspiration catheter, SOFIA ingested soft clots but not moderately stiff clots. When removing soft clots with the stent retriever, fragmentation was observed, although relatively stiff clots were well-integrated and removed. The Neurostar ingested soft clots similar to the aspiration catheter, and also aspirated stiff clots by continuous suction with hydro-separator. In the experiments with challenging clots, the Neurostar led to significantly higher recanalization rates than the stent retriever and aspiration catheter.
Conclusions
The stiffness of the clots affected the efficacy of endovascular thrombectomy based on the type of device. The Neurostar catheter with hydro-separator resulted in better success rates than a commercially available aspiration catheter and stent retriever in this experimental model.
Keywords: Acute ischemic stroke, endovascular thrombectomy, aspiration, clot stiffness, in vitro model
Introduction
Endovascular thrombectomy with a stent retriever or aspiration catheter has been established as the standard-of-care treatment in patients with acute ischemic stroke (AIS).1,2 Successful thrombectomies in the first attempt, known as the first-pass effect, is associated with better clinical outcomes.3,4 Despite high recanalization rates reported with the current technology, the rate of first-pass effect is approximately 30%.5,6 Therefore, there is need for future improvement of the tools for early recanalization of occluded vessels in AIS.
The properties of occlusive clots affect the technical intricacy in endovascular procedures and are thought to be a factor against achieving a high first-pass effect. A number of in vitro, in vivo and clinical studies have shown that both soft erythrocyte-rich clots and stiff fibrin-rich clots make the thrombectomy challenging.7–11 Although stiffness is believed to affect integration with stent retrievers and ingestion by aspiration catheters, 12 the research effort on the effect of clot stiffness on the procedural efficacy is quite limited, partly because the thrombi from patients are heterogenous and the analysis for stiffness needs to be performed immediately after the clot is retrieved. 13
In this study, we created artificial clot analogues with diverse stiffnesses to investigate the impact of stiffness on the efficacy of conventional endovascular thrombectomy devices and an innovative aspiration device with a hydro-separator. We assessed the performance of these devices in challenging circumstances with large soft, fragile clots and stiff fibrous clots in a tortuous vascular region.
Materials and methods
Clot analogue
Two types of clot analogues, one novel, and another conventional, were created in this study. The first clot analogue was made with agar at six different concentrations in silicone tubing with a 3 mm diameter. We created the artificial clot analogues with six stiffness variations and evaluated the efficacy of the thrombectomy devices in capturing them. The compressive modulus of the artificial clot analogues was controlled by varying concentrations of the agar.
The second conventional clot analog was made utilizing porcine blood with the dichotomy in soft erythrocyte-rich clot and firm fibrin-rich clot. The porcine blood was collected and anticoagulated with sodium citrate. The erythrocyte-rich clot was produced by adding calcium chloride to whole porcine blood. To create a fibrin-rich clot, citrated blood was centrifuged at 550 g for 15 min, and the extracted plasma was mixed with red blood cells at a volume ratio of 99:1. 14 Calcium chloride was given to the mixture to coagulate. This mixture was then injected into 6 mm or 4 mm diameter silicone tubing.
Compression test
The compression modulus is determined by how much a clot is deformed by the subjected compression force and has been previously used to represent the stiffness of human thrombi. 13 A mechanical testing machine (Instron model 5943) was used to examine compression moduli of various artificial clot analogues as previously described. 15 Samples were formed using a polydimethylsiloxane (PDMS) mold with cylindrical geometry (5 mm in diameter and 3 mm in depth). The compression test was performed at a rate of 1 mm/min with a 100 N load until the sample failed (at around 60% strain level). Testing of each clot was repeated 3 to 4 times. The compressive strain (mm) and load (N) were measured using the Bluehill Universal software and the dimensions of the samples were measured using a digital caliper. The compressive modulus was also determined based on the slope of the stress-strain curve at linear region. In addition, the compressive strength was recorded as the force with which the loaded specimen collapses.
In vitro thrombectomy
The efficacy of two commonly used devices for AIS, a stent retriever (Solitaire FR, Medtronic Neurovascular) and an aspiration catheter (SOFIA Flow Plus, MicroVention Terumo), as well as a new aspiration catheter with a hydro-separator technology (Neurostar, Irvine Neurovascular), were tested to compare single-pass recanalization performance.
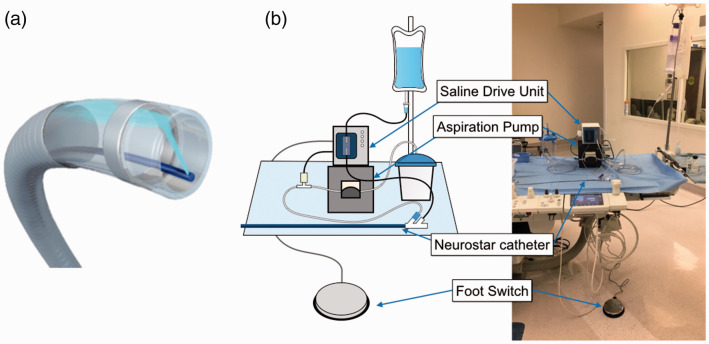
The Neurostar Thrombectomy System consists of a 6 F Neurostar catheter that is an aspiration device equipped with a hydro-separator, and a Saline Drive Unit (SDU) which creates a micro stream of saline aimed perpendicular to the long axis on the tip of the catheter (from the hydro-separator) to macerate the thrombus (Figure 1). A peristaltic pump is also incorporated in the SDU and the clots are aspirated with the maceration. The Neurostar catheter has 0.080” OD and 0.062” ID with 132 cm working length. The tip of saline delivery tubing is located < 0.5 mm from the tip of the Neurostar catheter, and the saline lumen takes approximately six percent of the cross-sectional area of the aspiration lumen. The injected saline (<40 ml/min) hits the opposing wall of the catheter and deflects distally out of the tip so that the resulting pressure that exits the tip of the catheter is <10 psi. The low pressure and low flow rate during aspiration prevents distal emboli, whereas the saline jet with high pressure inside the tip of the catheter break the clots into small pieces to be aspirated (Supplementary video).
Figure 1.
Neurostar Thrombectomy System. (a) Saline stream inside the tip of the Neurostar catheter to macerate the thrombus during aspiration (hydro-separator technology). (b) Neurostar Thrombectomy System. Aspiration is initiated by the footswitch, and negative pressure signal activates the Saline Drive Unit, creating a stream of saline on the tip of the catheter through a saline delivery tubing within the main lumen.
Two types of in vitro systems were used in this study. The first experimental system was composed of straight silicone tubing, where the agar artificial clots with various concentrations were placed. This experiment aimed to visually identify the differences in the efficacy of endovascular devices for various degrees of clot stiffness. A Solitaire FR stent retriever 4 x 20 mm was deployed across the clot and gently pulled after an embedding time of 3 minutes. A SOFIA Flow Plus aspiration catheter was placed touching the clot, and aspiration was applied by an aspiration pump (Gomco, Allied Healthcare Product) for up to 1 minute. A Neurostar catheter was also delivered proximal to the clot, and aspiration with hydro-separator was applied for five to 30 seconds. Each procedure was repeated five times and recorded with a video camera (Seiko Epson).
The second experimental system was composed of a human intracranial vascular model connected to a peristaltic pump, which was built as previously described. 16 The silicone vascular model contained internal carotid artery (ICA), middle cerebral artery (MCA), and anterior cerebral artery as well as posterior communicating artery and external carotid artery, with moderate tortuosity in the ICA and MCA. A 60/40% water/glycerin solution mixed with detergent was circulated in the system at 37°C with 100 mmHg and 240 ml/min flow in the ICA. The erythrocyte-rich clot analog in the 6 mm diameter tube was cut into 15 mm pieces. A 2 mm-deep notch was made every 3 mm to make the clot more fragile and then placed in the mid-M1 segment. The fibrin-rich clot analog in the 4 mm diameter tube was cut into 15 mm pieces, dried on surgical gauze for 3 minutes to harden and placed from the distal M1 to the proximal M2 segment. The tip of a 90 cm 6 Fr Flexor Shuttle Sheath (Cook Medical) was placed in the common carotid artery. A Solitaire FR was deployed over the clot through a 0.027-inch Phenom microcatheter and REACT68 (Medtronic Neurovascular), which was placed in the cavernous segment of the ICA. A 6 × 40 mm stent was used for the mid-M1 clot, and a 4 × 20 mm stent was used for the M1-2 clot. The stent retriever was retrieved with aspiration through REACT68. A SOFIA Flow Plus and Neurostar were navigated to the occlusion using a Phenom microcatheter. Thrombectomy with SOFIA Flow Plus was performed by aspiration for 1 minute using the Gomco pump and pulling the catheter out into the Shuttle sheath. The thrombectomy with the Neurostar system was performed over 15 to 30 seconds, with gently withdrawing the catheter into the supraclinoid ICA. In order to simulate the clinical procedural steps, all thrombectomies were performed under fluoroscopy (Artis Zeego, Siemens AG). A video clip was also taken during the thrombectomy procedures to observe the behaviors of the clot and its interaction with the device. Procedures were rated as successful if the clot was removed without visible fragmentation. Each procedure was repeated ten times.
Statistical analysis
Comparisons of successful thrombectomy between the type of thrombectomy devices and clots were made using a two-way analysis of variance (ANOVA). Comparisons of results between thrombectomy devices with each clot were then made using one-way ANOVA. Following each ANOVA, pairwise comparisons were performed by Tukey’s honestly significant difference test. The significance of statistical analyses was set at 0.05. All analyses were performed using R.
Results
Stiffness of artificial clots
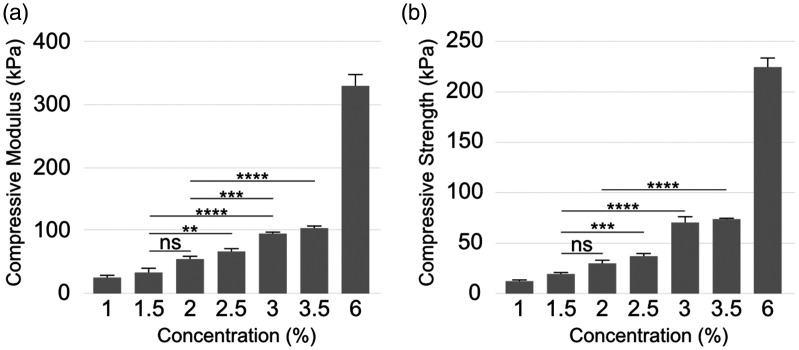
The compressive moduli of the artificial clot analogues enhanced from 23.07 ± 4.16 KPa to 327.67 ± 17.39 KPa as the agar concentration was increased from 1 to 6% (Figure 2(a)). Similarly, compressive strength, which is the force at failure, also increased with agar concentration (Figure 2(b)). The compressive moduli of the artificial clots from 1 to 6% exhibited the similar range of the stiffness of human thrombi reported before. 13 Among them, the stiffness of the clots with 1.5% and 2% concentrations showed close to the mean stiffness of human thrombi obtained by mechanical thrombectomy. 13
Figure 2.
Results of the compression test. Compression modulus (a) and compression strength (b) of artificial formed by varying agar concentrations. (Error bars indicate standard error of the means, asterisks mark significance levels of p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), and p < 0.0001 (****)).
Efficacy of thrombectomy devices by stiffness
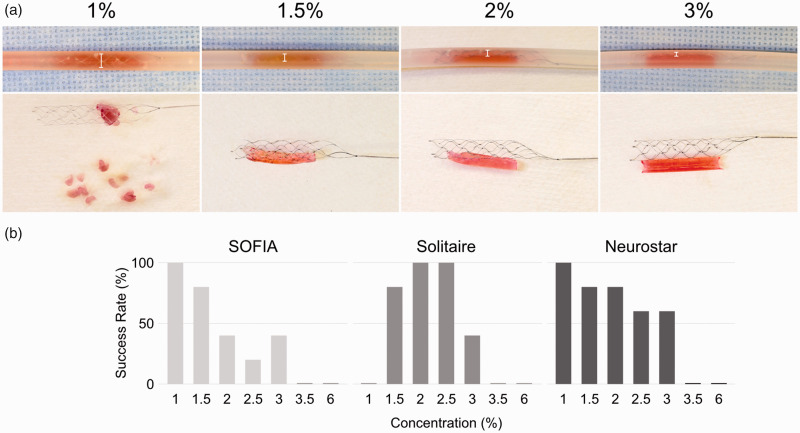
To evaluate the efficacy of each thrombectomy device by stiffness, we performed in vitro thrombectomies of artificial clot analogue at each agar concentration. The success rate of each device is shown in Figure 3(a). The SOFIA Flow Plus ingested 1% and 1.5% soft clots in less than a minute; clots above 2% were not aspirated in a minute, and the proximal part of the clot was corked into its tip. The clots were removed with a low success rate by gently pulling the entire system. The Solitaire showed high efficacy for the 1.5% to 2.5% concentration clots. The Solitaire fully expanded against 1% soft clot analogues in the tubing, but the fragmented clots remained after stent retrieval (Figure 3(b)). As the clot stiffness increased, stent expansion against the clot was gradually limited, and no integration was observed with the 3% clots. The Neurostar aspirated soft clots with 1-1.5% concentration similarly to the SOFIA Flow Plus. In addition, the 2-3% stiffer clots were also aspirated by continuous suction with the aid of the hydro-separator technology without intraluminal obstruction.
Figure 3.
Results of in vitro experiment using artificial clots with various stiffness. (a) Success rates of thrombectomy using the indicated device for clots of each agar concentration. (b) Top: Stent retriever deployed over the clot in the tubing. White bars indicate the degree of stent expansion. Bottom: Stent retriever and clot after the procedure. Fragmented clots remain in the tubing after the procedure when removing 1% clots. The integration of the stent retriever into clots reduces as the concentration increases.
Clot retrieval in human vascular model
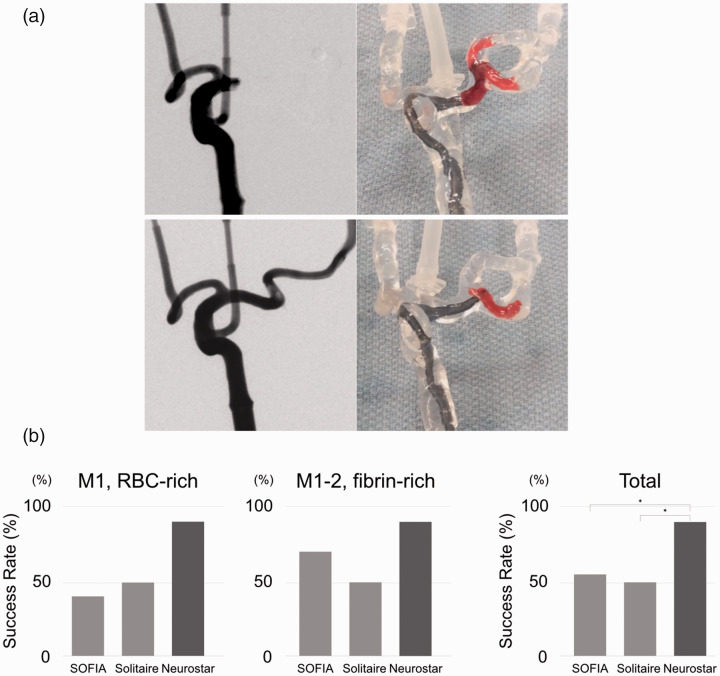
To assess the efficacy of each thrombectomy device in challenging situations, two experimental scenarios were designed: a large and fragile soft clot in the M1 segment and a stiff clot in the tortuous M1-M2 segment (Figure 4(a) and Supplementary Figure). Access to the clot was achieved by every device. The success rates of the thrombectomy devices for each clot was shown in Figure 4(b). The rate of success with the Neurostar was consistently higher than other devices. Although the results for each clot did not reach statistical significance, the total results showed significantly better thrombectomy outcomes in the Neurostar group than the SOFIA and Solitaire. The intra-procedural video showed that clot fragmentation occurred by both aspiration with SOFIA and thrombectomy with Solitaire against large M1 clots. For the M1-M2 clots, SOFIA tended to lose the clots around the tortuosity between the M1 and M2 segments in the model. Although Solitaire exhibited better outcomes than SOFIA, the stent was stretched and collapsed in the tortuous region, and the clots remained in the same place. The Neurostar catheter macerated and aspirated clots within 5 seconds in most cases, even in the tortuous regions. The Neurostar catheter was slowly withdrawn into the supraclinoid ICA, and intermittent angiograms could be performed without removing the catheter out of the guide sheath.
Figure 4.
In vitro thrombectomy in challenging scenarios using a silicone vascular model. (a) Top: large erythrocyte-rich clot in the M1 segment. Bottom: fibrin-rich clot in the tortuous M1-M2 segment. Left: digital subtraction angiogram before the procedure. Right: video taken during the procedure for the evaluation of the clot and device behavior. (b) Success rates of clot removal using each device. * p < 0.05.
Discussion
In this study, we demonstrated the stiffness of the clot analogues affected the thrombectomy performance of the three different devices in achieving first pass recanalization. The aspiration catheter was beneficial for soft clots (<50 kPa) whereas the stent retrievers effectively removed moderately stiff clots (50–100 kPa).
In addition, we showed that the new aspiration catheter with unique hydro-separator technology retrieved both the soft and moderately stiff clots. The Neurostar catheter resulted in better recanalization after first pass in challenging experimental settings reproduced with large soft, fragile clots, and stiff clots in tortuous anatomy.
Although clot stiffness is a well-known factor that affects the efficacy of the thrombectomy, there are few reports studying the stiffness of the human clots related to AIS. Chueh et al. reported that the compression moduli of the clots collected from patients with AIS and noncalcified thrombi collected during carotid endarterectomy widely varied from approximately 15 to 330 kPa. 13 To investigate the impact of stiffness, we developed simple agar clot analogues because the compositional changes of artificial blood clots affect not only stiffness but also friction, fragility and deformability. Our artificial clot analogues reproduced the observed range through adjustment of the concentration of the agar (Figure 2(a)).
Our study using the artificial clot analogues with various stiffnesses showed that increased stiffness reduces the efficacy of thrombectomy devices due to less integration and traction of the aspiration catheter and stent retriever. This finding is consistent with prior in vitro studies with soft erythrocyte-rich clots and hard fibrin-rich clots. Weafer et al. reported that fibrin-rich clots exhibited high compression moduli compared with erythrocyte-rich clots and less integration into stent struts. 17 Similarly, van der Marel demonstrated that hard clots were resistant to integration into the stent retriever as well as expansion by pushing technique. 11 In vivo and clinical studies also have shown that fibrin-rich clots are associated with multiple passes and longer procedure times.7,8,10 In this study, stiff clot analogues with compression moduli greater than 100 kPa could not be retrieved by any device. Although other factors rather than stiffness may affect the efficacy of thrombectomy devices, our results suggest that increased stiffness reduces the integration of thrombectomy devices with clots, leading to poor angiographic outcomes.
On the other hand, very soft clot analogues with compression moduli less than 30 kPa were fragmented by complete opening of the stent retriever. Concordantly, data from in vitro,11,18 animal7,10 and clinical studies 9 indicated that soft clots tend to cause fragmentation and/or distal embolization after clot removal with stent retrievers. Our results also showed that clot analogues with softness in this range could be digested and efficiently removed by the aspiration catheter. This suggests that fragment formation by stent retrievers may be prevented with the combined use of an aspiration catheter. However, current imaging techniques are unable to distinguish very soft clots (less than 30 kPa) from other erythrocyte-rich clots. Therefore, a balloon guiding catheter or a combined technique with an aspiration catheter could be a good option to prevent distal migration if soft clots are expected after imaging findings suggest erythrocyte-rich clots by hyperdense MCA signs on CT or susceptibility vessel sign on susceptibility-weighted imaging (SWI).
The Neurostar catheter could provide a relatively steady aspiration performance in the whole spectrum of the clot stiffness. The catheter is designed to remove the thrombus by aspiration while macerating clots inside the catheter by the hydro-separator technology. The experiments using artificial clot analogues showed soft to moderately stiff clots (< 100 kPa) were macerated in the catheter tip by the hydro-separator technology and removed by aspiration. In the experiments with a human vascular model, the Neurostar catheter retrieved large soft friable erythrocyte-rich clots and stiff fibrin-rich clots more successfully than in SOFIA or Solitaire. In addition to the better performance, the experiments revealed advantages of the Neurostar, including rapid clot removal and in situ treatment without removing the catheter out of the body.
This study has several limitations. We utilized artificial clot analogues to focus on the stiffness. However, the efficacy of thrombectomy can be affected by other mechanical properties of thrombi such as friction, 19 platelet activation,20,21 and Neutrophil Extracellular Traps. 22 Besides, the experiments were performed in in-vitro systems, which may not represent the complexities encountered in actual clinical settings.
Conclusions
The efficacy of thrombectomy was largely affected by the clot stiffness and each thrombectomy device shows specific efficacy in a discrete clot stiffness range. Mechanical thrombectomy with the new aspiration system with a unique hydro-separator technology resulted in higher thrombectomy performance than a commercially available stent retriever and aspiration catheter in this experimental model in a wider range of clot stiffness. Further in vivo or clinical studies should be performed to confirm the safety of the device and the efficacy observed in this study.
Supplemental Material
Supplemental material, sj-pdf-1-ine-10.1177_15910199211015060 for A new aspiration device equipped with a hydro-separator for acute ischemic stroke due to challenging soft and stiff clots by Naoki Kaneko, Mahsa Ghovvati, Yutaro Komuro, Lea Guo, Kasra Khatibi, Lucido L Ponce Mejia, Hamidreza Saber, Nasim Annabi and Satoshi Tateshima in Interventional Neuroradiology
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: ST has been a consultant for Irvine Neurovascular, Balt USA, Cerenovus, Medtronic, Phenox GmbH, and Stryker. The other authors have no personal or financial interest in any of the materials or devices described in this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Naoki Kaneko https://orcid.org/0000-0002-3579-7908
Supplemental material: Supplemental material for this article is available online.
References
- 1.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 2.Turk AS, Siddiqui A, Fifi JT, et al. Aspiration thrombectomy versus stent retriever thrombectomy as first-line approach for large vessel occlusion (COMPASS): a multicentre, randomised, open label, blinded outcome, non-inferiority trial. Lancet 2019; 393: 998–1008. [DOI] [PubMed] [Google Scholar]
- 3.Nikoubashman O, Dekeyzer S, Riabikin A, et al. True first-pass effect. Stroke 2019; 50: 2140–2146. [DOI] [PubMed] [Google Scholar]
- 4.Zaidat OO, Castonguay AC, Linfante I, et al. First pass effect: a new measure for stroke thrombectomy devices. Stroke 2018; 49: 660–666. [DOI] [PubMed] [Google Scholar]
- 5.Ducroux C, Piotin M, Gory B, et al. First pass effect with contact aspiration and stent retrievers in the aspiration versus stent retriever (ASTER) trial. J Neurointerv Surg 2020; 12: 386–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abbasi M, Liu Y, Fitzgerald S, et al. Systematic review and meta-analysis of current rates of first pass effect by thrombectomy technique and associations with clinical outcomes. J Neurointerv Surg 2021; 13: 212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuki I, Kan I, Vinters HV, et al. The impact of thromboemboli histology on the performance of a mechanical thrombectomy device. AJNR Am J Neuroradiol 2012; 33: 643–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sporns PB, Hanning U, Schwindt W, et al. Ischemic stroke: histological thrombus composition and pre-interventional CT attenuation are associated with intervention time and rate of secondary embolism. Cerebrovasc Dis 2017; 44: 344–350. [DOI] [PubMed] [Google Scholar]
- 9.Maegerlein C, Friedrich B, Berndt M, et al. Impact of histological thrombus composition on preinterventional thrombus migration in patients with acute occlusions of the middle cerebral artery. Interv Neuroradiol 2018; 24: 70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nogueira RG, Levy EI, Gounis M, et al. The trevo device: preclinical data of a novel stroke thrombectomy device in two different animal models of arterial thrombo-occlusive disease. J Neurointerv Surg 2012; 4: 295–300. [DOI] [PubMed] [Google Scholar]
- 11.van der Marel K, Chueh JY, Brooks OW, et al. Quantitative assessment of device-clot interaction for stent retriever thrombectomy. J Neurointerv Surg 2016; 8: 1278–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeo LLL, Bhogal P, Gopinathan A, et al. Why does mechanical thrombectomy in large vessel occlusion sometimes fail? A review of the literature. Clin Neuroradiol 2019; 29: 401–414. [DOI] [PubMed] [Google Scholar]
- 13.Chueh JY, Wakhloo AK, Hendricks GH, et al. Mechanical characterization of thromboemboli in acute ischemic stroke and laboratory embolus analogs. AJNR Am J Neuroradiol 2011; 32: 1237–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fennell VS, Setlur Nagesh SV, Meess KM, et al. What to do about fibrin rich ‘tough clots’? Comparing the solitaire stent retriever with a novel geometric clot extractor in an in vitro stroke model. J NeuroIntervent Surg 2018; 10: 907–910. [DOI] [PubMed] [Google Scholar]
- 15.Annabi N, Zhang YN, Assmann A, et al. Engineering a highly elastic human protein-based sealant for surgical applications. Sci Transl Med 2017; 9: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaneko N, Komuro Y, Yokota H, et al. Stent retrievers with segmented design improve the efficacy of thrombectomy in tortuous vessels. J NeuroIntervent Surg 2019; 11: 119–122. [DOI] [PubMed] [Google Scholar]
- 17.Weafer FM, Duffy S, Machado I, et al. Characterization of strut indentation during mechanical thrombectomy in acute ischemic stroke clot analogs. J Neurointerv Surg 2019; 11: 891–897. [DOI] [PubMed] [Google Scholar]
- 18.Machi P, Jourdan F, Ambard D, et al. Experimental evaluation of stent retrievers' mechanical properties and effectiveness. J Neurointerv Surg 2017; 9: 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunning GM, McArdle K, Mirza M, et al. Clot friction variation with fibrin content; implications for resistance to thrombectomy. J NeuroIntervent Surg 2018; 10: 34–38. [DOI] [PubMed] [Google Scholar]
- 20.Douglas A, Fitzgerald S, Mereuta OM, et al. Platelet-rich emboli are associated with von Willebrand factor levels and have poorer revascularization outcomes. J NeuroIntervent Surg 2020; 12: 557–562. [DOI] [PubMed] [Google Scholar]
- 21.Johnson S, Chueh J, Gounis MJ, et al. Mechanical behavior of in vitro blood clots and the implications for acute ischemic stroke treatment. J Neurointerv Surg 2020; 12: 853–857. [DOI] [PubMed] [Google Scholar]
- 22.Ducroux C, Di Meglio L, Loyau S, et al. Thrombus neutrophil extracellular traps content impair tPA-induced thrombolysis in acute ischemic stroke. Stroke 2018; 49: 754–757. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ine-10.1177_15910199211015060 for A new aspiration device equipped with a hydro-separator for acute ischemic stroke due to challenging soft and stiff clots by Naoki Kaneko, Mahsa Ghovvati, Yutaro Komuro, Lea Guo, Kasra Khatibi, Lucido L Ponce Mejia, Hamidreza Saber, Nasim Annabi and Satoshi Tateshima in Interventional Neuroradiology