Abstract
Antiplatelet therapies are commonly used in neurointerventional procedures. However, specific guidelines for their use in these settings is lacking and it can often be difficult to balance the potential risks and benefits of these medications. Considering the continued growth and adoption of neurointerventional procedures, it is crucial to understand the properties of these agents in order to use them safely. Large-scale clinical trials are still needed to clarify many of these aspects for this emerging field. However, the existing literature already provides insight into which antiplatelet drugs are of benefit to the neurointerventionalist as well as their associated risks of ischemic and hemorrhagic complications. Hence, this review focuses on the applications of GPIIb/IIIA inhibitors to neurointerventional procedures.
Keywords: Platelets, hemorrhage, thrombectomy, stroke, aneurysm
Introduction
Antiplatelet use for neurointerventional procedures varies significantly between individuals and institutions. This is the result of a lack of randomised control trials (RCTs) and guidelines for the appropriate use of these agents. Despite this, a wealth of literature exists on this topic and further insights can be drawn from the use of antiplatelets in more established clinical contexts including acute ischemic stroke and percutaneous coronary intervention (PCI).
The aim of this review is to discuss and evaluate the use of antiplatelets for neurointerventional procedures including mechanical thrombectomy, stenting and coiling. We use this evidence to provide information on common dosing recommendations (Table 1) and indications, with the aim of supporting the neurointerventionalist in decision making for the use of peri-procedural antiplatelets while minimising the risk of ischemic and hemorrhagic complications. In addition, we also scrutinize the quality of the existing evidence, highlight gaps in the literature, and discusses the need for further studies and randomized control trials to tailor the use of antiplatelets depending on the clinical context and the patient.
Table 1.
Pharmacological and clinical information for glycoprotein IIb/IIIa inhibitors.
| Eptifibatide | Tirofiban | Abciximab | |
|---|---|---|---|
| Route | IV/IA | IV/IA | IV/IA |
| Loading/bolus dose | 180–200 µg/kg (IA/IV) (to repeat after 10 min) 9 , 12 , 59 | 0.4 µg/kg/min over 30 min (IV/IA) 60 , 61 or (low dose scheme) 0.25–0.5 mg in 1 ml/min (IA) 38 | 0.25 mg/kg (IA, IV) 35 , 44 , 45 , 54 |
| Maintenance dose | 0.5–2 µg/kg/min (IV) 37 , 45 | 0.10 µg/kg/min 45 , 60 , 61 | 0.125 µg/kg/min (IV) 44 , 45 |
| Binding affinity (KD) | + (120 nmol/l) 62 | ++ (15 nmol/l) 62 | ++++ (5 nmol/l) 62 |
| ≥80% platelet inhibition after IV bolus | 15 min 8 | 10 min 63 | 10 min 63 |
| Restoration to normal platelet activity | 4 h 64 | 3–4 h 64 | 48–72 h 64 |
| Dose adjustment in CKD | Yes 65 | Yes 65 | No 65 |
| Precautions: - Elective major surgery (to hold) |
2–4 h 3 | 2–4 h 3 | 12 h 66 |
| Thrombocytopenic event rate | + 67 | + 67 | ++++ 67 |
| Tips to prevent bleeding complications: | - single wall arterial entry | -single wall arterial entry | -single wall arterial entry |
| - early sheath removal (ACT <150 to 180) 36 | -early sheath removal (ACT <150 to 180) 36 | -early sheath removal (ACT <150 to 180) 36 | |
| Bridging to dual oral antiplatelets: - time to wait before measuring baseline ARU and PRU values (VerifyNow assay) |
48 h 68 | 48 h 68 | 14 days 68 |
These are common dosing regimens described in the literature, please use local guidelines and clinical expertise to guide practice.
IA: intra-arterial; IV: intra-venous; ACT: activated clotting time; CKD: chronic kidney disease; ARU: aspirin resistance unit; PRU: P2Y12 reaction units.
This narrative review focuses on gpIIbIIIa inhibitors while part I discusses P2Y12 inhibitors for neurointerventional procedures. 1
GPIIb/IIIa inhibitors in neurointerventional procedures
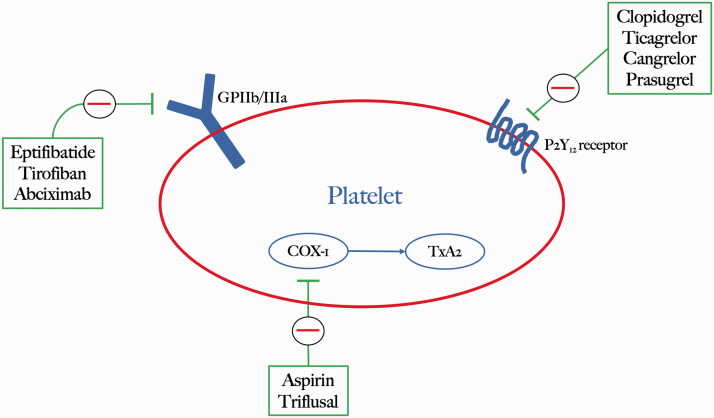
Platelets are activated following endothelial injury or from the rupture of an atherosclerotic plaque. Both events lead to activation of glycoprotein IIb/IIIa (GPIIb/IIIa) which is the most abundant integrin on the platelet surface, triggering platelet aggregation 2 (Figure 1). Antagonists of the platelet GPIIb/IIIa receptor prevent fibrinogen binding as well as the activation of von Willebrand Factor (vWF), fibronectin, and vitronectin in platelets. As a result, they suppress both the formation of platelet thrombi as well as the deposition of fibrin onto established thrombi. GPIIb/IIIa inhibitors must maintain ≥80% receptor occupancy to achieve sufficient therapeutic efficacy. 3
Figure 1.
Platelet activation pathways targeted by antiplatelet drugs including GP IIb/IIIa, P2Y12 and COX-1 inhibitors.
In cardiovascular studies, GPIIb/IIIa inhibitors have been administered in patients undergoing procedures, such as percutaneous coronary intervention (PCI), as an intravenous (IV) bolus followed by a continuous maintenance infusion. The purpose of this protocol is to use the initial bolus to reduce acute platelet activation, aggregation and thrombus formation. The maintenance infusion eliminates the potential for new thrombus formation until the patient is switched to an oral antiplatelet regimen. Intraoperatively, this approach prevents the thrombogenic property of stents and coils, without significantly increasing the rate of hemorrhagic complications. 4 Currently, there is no standard protocol for this in neurointerventional procedures which has resulted in significant variability in terms of dosing and administration methods.
Despite the lack of clear guidelines, GPIIb/IIIa inhibitors are increasingly being used to treat thromboembolic events, mainly in the setting of wide neck aneurysms, coil protrusions, stenting, double stenting and intimal injury after microcatheter displacement. 5 An older strategy involved combining thrombolysis (tissue plasminogen activator (tPA), urokinase) with contextual mechanical thrombus fragmentation. 6 This yielded a 44–75% recanalization rate with a high rate of re-occlusion. A meta-analysis by Brinjikji et al suggested that the GPIIb/IIIa inhibitors abciximab, tirofiban and eptifibatide were superior to thrombolytics in terms of recanalization rate (72% vs. 50%), perioperative morbidity (11% vs. 29%), and long-term morbidity (16% vs. 35%). 6
The use of GPIIb/IIIa inhibitors has also been evaluated in the setting of prophylaxis for thromboembolic complications, particularly in patients unable to receive dual antiplatelet therapy (DAPT) preoperatively. This approach has been encouraged due to the reduced risk of thrombosis with GPIIb/IIIa inhibitors after stent deployment compared to patients receiving a standard preprocedural loading dose of aspirin and clopidogrel. 7
Eptifibatide
Mechanism and pharmacokinetics
Eptifibatide 2 mg/ml (Integrilin®; Cor Therapeutics, South San Francisco, CA and Schering-Plough, Kenilworth, NJ) is a GPIIb/IIIa inhibitor which was first introduced in 1998. Its amino-acid chain was modelled based on the lysine-glycine-aspartic acid (K-G-D sequence) first described in the venom of the South-eastern pygmy rattlesnake (Sistrurus m. barbouri). It was chosen due to its specificity for the Arg-Gly-Asp sequence found in the GPIIb/IIIa receptor. 3 Eptifibatide sits in the binding pocket between the IIb and IIIa arms of the GPIIb/IIIa receptor, thus preventing the binding of fibrinogen, von Willebrand factor, and other adhesive ligands to GPIIb/IIIa. 3 When administered intravenously, eptifibatide inhibits platelet aggregation in a dose- and concentration-dependent manner.
Eptifibatide binds to GPIIb/IIIa with low affinity, compared to abciximab, which allows for rapid dissociation from the receptor. As a result, platelet aggregation inhibition with eptifibatide is reversible following cessation of treatment. The inhibition rate of platelet aggregation is ≥84% after 15 min following a bolus dose (180-mcg/kg bolus), with ≥90% inhibition at steady state. 8
Eptifibatide for neurointerventional procedures
In neurointervention, administration of eptifibatide has been reported both via intravenous and intra-arterial routes and can be given as a bolus and/or continuous infusion. It can also be used for therapeutic and prophylactic purposes. Infusions of eptifibatide have not been widely adopted, 4 hence, a practical approach to achieving thromboembolic prophylaxis is via a bolus of 200 µg/kg. 9 which can be administered intra-arterially at the start of the neurointerventional procedure (Table 1). This was adopted by Sedat et al. who suggested this is an effective approach to lower the rate of intraoperative thromboembolic complications (7%) during ruptured aneurysm embolization, without increasing the risk of haemorrhage (4%), compared to the use of heparin and/or aspirin (20% thromboembolic complication rate). 9 An alternative approach is to use an intravenous delivery of a single bolus of 180 µg/kg in unruptured aneurysms which also demonstrated a reasonably adequate safety profile. 10 In the prophylactic branch of patients undergoing aneurysm embolization, the rate of bleeding complications was 5.9% (2.4% intracranial hemorrhage, 3.5% groin hematoma requiring transfusions). 7
Rescue administration
Intra-arterial eptifibatide is able to achieve a high level of recanalization (77%), less pronounced in distal occlusions, with some risk of symptomatic intracranial hemorrhage (14%). 11 Patients receiving eptifibatide had a higher recanalization rate compared to those treated with abciximab (68.0–91.0% vs 58.0–74.0% respectively; P = 0.05). 8
Two approaches have been described for intra-arterial bolus regimens including a starting dose of 25% of the dose limit (90 µg/kg) 12 or an intra-arterial bolus of 180 µg/kg adjusted to body weight. 11 This bolus can be followed by an intravenous infusion at 1.5–3.75 mg/h (0.5 µg/kg/min), particularly when thrombi persist, and continued post-procedurally in a time window between 2 and 48 h. 13 Another feasible approach is with a 75–180 µg/kg bolus, followed by a 0.5–2 µg/kg/min infusion for the same time window. 14 , 15 However, the intra-arterial route may reduce the dose required for rescue treatment (<90 µg/kg vs 180 µg/kg), thus reducing the risk of bleeding complications. 13 Similarly, an intra-arterial route is often preferred in the setting of acute thrombosis as it delivers the drug to the clot more rapidly compared to the traditional intravenous route.
During acute stenting for mechanical thrombectomy, eptifibatide has similar rates of intracranial hemorrhagic complications and mortality relative to tirofiban. 16 In tandem occlusions, the rate of hemorrhagic complications was low irrespective of concomitant rt-PA administration. 17
Additional information
Platelet transfusions have limited utility in eptifibatide-related hemorrhage as new platelets are inhibited by the circulating drug. No reversal agents for this drug have been developed as of yet, 4 however, the use of intravenous desmopressin (0.3 µg/kg) and cryoprecipitate in these scenarios has been reported with encouraging results. 18 , 19 In patients with an eGFR below 50 mL/min, the infusion dose should be reduced to 1 µg/kg/min. The common dosing regimens for eptifibatide are summarized in Table 1.
Tirofiban
Mechanism and pharmacokinetics
Tirofiban (Aggrastat®; Merck, Deerfield, IL) was first injected into humans by Peerlinck et al. in 1993 and is a reversible, competitive inhibitor of the GPIIb/IIIa receptor. It is a synthetic nonpeptide mimetic of the Arg-Gly-Asp sequence, identified within the snake venom echistatin which antagonizes the activation of GPIIb/IIIa. 20
Tirofiban exerts its effects by preventing the binding of fibrinogen, and other ligands, to GPIIb/IIIa resulting in inhibition of the final step of thrombus formation. In addition to competing with fibrinogen and vWF, tirofiban can interact with the latent and active states of GPIIb/IIIa receptors allowing it to bind to both stimulated and non-stimulated platelets. Overall, tirofiban is highly specific for the GPIIb/IIIa receptors and binds with a higher affinity compared to eptifibatide. 21 Following tirofiban intravenous infusion at the recommended dose, 90% inhibition of platelet function is achieved by 30 min 22 in a dose- and concentration-dependent manner. The half-life of tirofiban is 1.5–2 h and restoration of normal hemostatic function following drug cessation is achieved within 3–4 h.
Tirofiban for neurointerventional procedures
In the context of mechanical thrombectomy, tirofiban provides no increased risk of intracranial hemorrhage or distal embolization. 23 Furthermore, three-month mortality is reduced in patients treated with tirofiban for posterior circulation stroke. 23 Even in patients undergoing mechanical thrombectomy for occlusion with underlying intracranial atherosclerotic stenosis, tirofiban is associated with more favourable functional outcomes, lower mortality and lower rates of re-occlusion compared to controls without tirofiban (or other antiplatelet agents). 24 Moreover, during acute intraprocedural stent thrombosis in the context of intracranial atherosclerotic stenosis, intra-arterial bolus administration of tirofiban followed by IV infusion was efficacious and safe with no significant increased risk of intracranial hemorrhage or death. 25 With regards to mechanical thrombectomy, intra-arterial administration of tirofiban was beneficial predominantly for large artery atherosclerosis strokes relative to those of cardio-embolic origin. 26 Conversely, a randomized control trial found no significant effect of tirofiban in AIS on long-term survival, dependency or death without increasing the risk of bleeding compared to using aspirin. 27 This further highlights the need for large scale trials investigating the use of tirofiban in neurointerventional procedures.
In neurointerventional procedures both intravenous and intra-arterial routes are described for prophylactic and rescue purposes. Intravenous injections for prophylaxis or rescue purposes are characterized by a 0.4 µg/kg/min loading dose of tirofiban over 30 min followed by a 0.10 µg/kg/min maintenance infusion. 28 , 29 An alternative protocol for flow-diverter deployment has been suggested by Chalouhi et al. 30 which involves pre-treatment with aspirin and clopidogrel followed by a 0.10 µg/kg/min maintenance infusion alone after deployment. This differs from the standard combination of a bolus plus maintenance infusion for 2 h.
Specifically for stent-assisted coiling, prophylactic administration of tirofiban via the intravenous route resulted in fewer thromboembolic events (3.37–3.91%) compared to the clopidogrel group (10.83–13.21%), without an increase in risk of intracranial hemorrhage. 31 However, according to a study by Chalouhi et al., the standard intravenous protocol (bolus + infusion) resulted in a higher rate of intracranial hemorrhage compared to the infusion (without a bolus) approach, 30 highlighting the need for further prospective studies to clarify these risks.
With regards to flow-diversion in unruptured aneurysms, pre-treatment with P2Y12-inhibitor agents (mainly aspirin and clopidogrel) 5–10 days pre-procedurally is often employed.
Tirofiban did not significantly increase the rate of symptomatic hemorrhage or thromboembolic complications in a study of 148 aneurysms treated with stent-assisted coiling and flow diverters. 32
The tirofiban protocol is also an excellent alternative to inhibit platelet aggregation in patients with subarachnoid hemorrhage when the use of a flow diverter was not anticipated. In particular, the reversibility of tirofiban, which can be achieved in 4 h, 33 can be useful in cases requiring invasive procedures such as ventriculostomy or rescue surgical clipping.
However, a meta-analysis by Cagnazzo et al suggested that tirofiban was associated with a similar rate of overall complications (17–23%) and aneurysm rebleeding (2.8–3%) compared the other antiplatelet therapies (aspirin + clopidogrel). 34
Rescue administration
Intra-arterial tirofiban injection dissolved 76–96% of thromboemboli during aneurysm embolization, 35 , 36 with a higher recanalization rate compared to those treated with abciximab. 37
According to a single-centre observational study by Zhao W et al, intra-arterial “low-dose” tirofiban was used to cope with thromboembolic complications and the authors advised using a 0.25–0.5 mg (total) dose at a rate of 1 ml/min (0.05 mg/ml) or 0.4 µg/kg/min for 1000 s (up to 1 mg in total). 25 , 38 This was not associated with an increased incidence of symptomatic intracranial hemorrhage. In summary, both intravenous and intra-arterial approaches have been described with similar clinical outcomes and complication rates. 39 However, the intra-arterial approach has advantages including a more efficient thrombolytic capacity with lower drug doses resulting in fewer systemic complications (Table 1). 29
Compared to abciximab, tirofiban appears to be a more cost-beneficial and effective approach for the immediate resolution of thromboemboli with a similar safety profile. 40
Additional information
Platelet transfusions are of limited benefit due to the inhibitory effects of the circulating drug 4 as is the case for other GPIIb/IIIa inhibitors as well. Hence, cessation of tirofiban is advised with concomitant bleeding. Intravenous desmopressin (0.3 µg/kg) and cryoprecipitate have been utilized for reversal with some success. 18 It should also be noted that precautions should be taken during administration of tirofiban in combination with enoxaparin due to an increased risk of cutaneous and oral bleeding events. 41
Abciximab
Mechanism and pharmacokinetics
Abciximab (ReoPro®; Centrocor, Malvern PA and jointly marketed with Eli Lilly, Indianapolis, IN) is an antibody segment which inhibits platelet activity and leucocyte adhesion in a dose-dependent manner by binding to GPIIb/IIIa, vitronectin and Mac-1 receptors. Blockade of vitronectin receptors prevents smooth muscle cell adhesion and migration, thereby reducing intimal proliferation. Abciximab may also inhibit activated MAC-1 receptors, thus reducing the recruitment of monocytes and polymorphonuclear leucocytes at the site of vascular injury. Inhibition of platelet aggregation is proportionate to the degree of GPIIb/IIIa receptor blockade. 42
Abciximab administered as a 0.25 mg/kg intravenous bolus blocks >80% of GPIIb/IIIa receptors at 10 min. 43 Continuous infusion (0.125 µg/kg/min or 10 µg/min) can be administered after the bolus to maintain adequate platelet inhibition. 42 The peak concentration is reached at 30 min and the half-life for abciximab ranges from 8 to 12 h. Following drug cessation, platelet function generally recovers within 48 h and hemostatic restoration is reached in 72 h. 42
Abciximab for neurointerventional procedures
Both intravenous and intra-arterial routes have been described for abciximab. Intravenous administration of abciximab has been adopted in the context of high risk coronary angioplasty. This is based on the results of the EPIC trial which described a 0.25 mg/kg bolus dose delivered over a 5-min period followed by an infusion of 10 µg/min for 12 h. 35 In the context of neurointerventional procedures specifically, the same 0.25 mg/kg bolus dose has been described but with a 0.125 mg/kg maintenance infusion, 44 and this regimen was recently recommended in a DELPHI consensus statement, based on an international online survey amongst INR practitioners. 45 With regards to the intra-arterial route, a rescue bolus ranging from 5 to 15 mg or 0.125 mg/kg has also been described for thromboembolic complications.46–48 Taken together, there is significant variability in abciximab regimens used in neurointervention, both in terms of the initial bolus and subsequent continuous infusion. The regimens outlined in Table 1 reflect the highest quality, but limited, evidence available at present, consisting of the EPIC trial results and DELPHI consensus. Nonetheless, future prospective studies and RCTs will need to clarify the risks versus benefits of this protocol specific to neurointerventional procedures.
With regards to risk of hemorrhage, previous trials (ADMIRAL, GUSTO IV-ACS and TARGET), 42 have demonstrated that the rate of minor hemorrhage associated with abciximab is between 3 and 12.1%, which is greater than for tirofiban (2.8%). 49 The risk of major bleeding is 0.6–0.7%, similar to that of eptifibatide (1.7%) and tirofiban (0.9%). 50 Following extension of the abciximab infusion to 48 h, the incidence of major bleeding increases to 1%. In the setting of acute ischemic stroke, a Cochrane review of three trials found that abciximab significantly increased the risk of intracranial hemorrhage without improving mortality or disability. 51 Similarly, a recent meta-analysis highlighted that abciximab increases the rate of symptomatic intracranial hemorrhage during AIS. Although the use of low-dose abciximab in AIS is controversial, 52 using a half dose (0.125 mg/kg) with no maintenance administration followed by oral aspirin (150 mg) and clopidogrel (75 mg) appears to be safe and effective for acute stenting during AIS. 53 This emphasises the need for further RCTs to clarify the risks and benefits of abciximab for neurointerventional procedures and that the risk of bleeding with abciximab may be a significant concern relative to other antiplatelet agents.
Rescue administration
Rescue administration (intravenous/intra-arterial) is associated with an overall reperfusion rate of between 63 and 69%. 44 , 54 From a technical perspective, administration through a microcatheter selectively placed in the vicinity of the thromboembolus is considered a safe and effective route. 55 , 56 Even when adopted together with prophylactic antiaggregation (ASA) or anticoagulation (heparin), Abciximab doesn’t appear to worsen the risk of intracranial hemorrhage or extracranial bleeding complications during coil embolization. 54
Additional information
Thrombocytopenia (platelet count <100 × 109/l) is one of the most common side effects associated with abciximab which is estimated to be around 2%. 57 This is significantly higher than the 0.5% rate associated with tirofiban according to the ADMIRAL, GUSTO IV-ACS and TARGET clinical trials. 50 Severe thrombocytopenia (platelet count <50 × 109/l) accounts for 0.9–2% of these cases of thrombocytopenia compared to 0.1% in the tirofiban group. In clinical practice, thrombocytopenia may, rarely, be profound and associated with hemorrhagic complications. To prevent thrombocytopenia, platelet counts should be monitored before and during treatment. Abciximab should be stopped and appropriate treatment initiated when platelet counts decrease by ≥25% and/or to <100 × 109/l. 42
The recent removal of abciximab from some healthcare systems has resulted in the choice of GPiib/iiia inhibitors being limited to either eptifibatide or tirofiban. However, there is a growing body of evidence for the use of cangrelor in INR procedures as well, which is a P2Y12 inhibitor antiplatelet.
Transitioning to oral antiplatelets
The recent international online survey DELPHI consensus by Ospel and colleagues (2020) on flow-diverter and stent assisted coiling in ruptured aneurysms, recommended that GPIIbIIIa inhibitor infusions can be transitioned to oral agents within 24 h post-procedure for conscious patients. This period of intravascular antiplatelet use can be prolonged in intubated patients due to the poor reliability of nasogastric administration of oral antiplatelet agents. 45 A similar DELPHI consensus by Goyal et al. 58 was unable to reach a consensus view on the peri-procedural use of, and transitioning between, antiplatelet agents for acute intracranial stenting. This further highlights the need for prospective studies to clarify these contentious issues.
Conclusion
The emerging field of neurointerventional procedures will benefit from improved understandings of antiplatelet drugs, their mechanisms, dosages, efficacies, risks and applications. The literature outlined in this review can be used as a tool, together with clinical expertise, to inform the use of GPIIb/IIIa inhibitors in the neurointerventional setting. The main limitation of this review and its recommendations is the heterogeneity in the existing evidence which ranges from case studies to RCTs with significant gaps in the literature. As the practice of interventional neuroradiological procedures is rapidly growing, it is crucial to draw insights from the existing evidence covered here to inform antiplatelet practice. Nonetheless, continued investigations and RCTs will be necessary to clarify the risks and benefits of these drugs and to develop evidence-based protocols for common INR procedures.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Davide Simonato https://orcid.org/0000-0003-3601-9136
Leonard LL Yeo https://orcid.org/0000-0002-4249-0402
Pervinder Bhogal https://orcid.org/0000-0002-5514-5237
References
- 1.Borchert B, Simonato D, Hickman CR, et al. P2Y12 inhibitors for the neurointerventionalist. Under Review 2021.
- 2.Murugappan S, Shankar H, Kunapuli SP. Platelet receptors for adenine nucleotides and thromboxane A2. Semin Thromb Hemost 2004; 30: 411–418. [DOI] [PubMed] [Google Scholar]
- 3.Tcheng JE. Clinical challenges of platelet glycoprotein IIb/IIIa receptor inhibitor therapy: bleeding, reversal, thrombocytopenia, and retreatment. Am Heart J 2000; 139: S38–S45. [DOI] [PubMed] [Google Scholar]
- 4.Dornbos D, Katz JS, Youssef P, et al. Glycoprotein IIb/IIIa inhibitors in prevention and rescue treatment of thromboembolic complications during endovascular embolization of intracranial aneurysms. Neurosurgery 2018; 82: 268–277. [DOI] [PubMed] [Google Scholar]
- 5.Bruening R, Mueller-Schunk S, Morhard D, et al. Intraprocedural thrombus formation during coil placement in ruptured intracranial aneurysms: treatment with systemic application of the glycoprotein IIb/IIIa antagonist tirofiban. Ajnr Am J Neuroradiol 2006; 27: 1326–1331. [PMC free article] [PubMed] [Google Scholar]
- 6.Brinjikji W, Luetmer PH, Comstock B, et al. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. Am J Neuroradiol 2015; 36: 811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryu C-W, Park S, Shin HS, et al. Complications in stent-assisted endovascular therapy of ruptured intracranial aneurysms and relevance to antiplatelet administration: a systematic review. Am J Neuroradiol 2015; 36: 1682–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah I, Khan SO, Malhotra S, et al. Eptifibatide: the evidence for its role in the management of acute coronary syndromes. Core Evid 2009; 4: 49–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sedat J, Chau Y, Gaudard J, et al. Administration of eptifibatide during endovascular treatment of ruptured cerebral aneurysms reduces the rate of thromboembolic events. Neuroradiology 2015; 57: 197–203. [DOI] [PubMed] [Google Scholar]
- 10.Yi HJ, Gupta R, Jovin TG, et al. Initial experience with the use of intravenous eptifibatide bolus during endovascular treatment of intracranial aneurysms. Am J Neuroradiol 2006; 27: 1856–1860. [PMC free article] [PubMed] [Google Scholar]
- 11.Memon MZ, Natarajan SK, Sharma J, et al. Safety and feasibility of intraarterial eptifibatide as a revascularization tool in acute ischemic stroke. J Neurosurg 2011; 114: 1008–1013. [DOI] [PubMed] [Google Scholar]
- 12.Harrington RA. Design and methodology of the PURSUIT trial: evaluating eptifibatide for acute ischemic coronary syndromes. Platelet glycoprotein IIb-IIIa in unstable angina: receptor suppression using integrilin therapy. Am J Cardiol 1997; 80: 34B–38B. [DOI] [PubMed] [Google Scholar]
- 13.Ramakrishnan P, Yoo AJ, Rabinov JD, et al. Intra-arterial eptifibatide in the management of thromboembolism during endovascular treatment of intracranial aneurysms: case series and a review of the literature. Intervent Neurol 2013; 2: 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qureshi AI, Hussein HM, Janjua N, et al. Postprocedure intravenous eptifibatide following intra-arterial reteplase in patients with acute ischemic stroke. J Neuroimag 2008; 18: 50–55. [DOI] [PubMed] [Google Scholar]
- 15.Bath PM, Woodhouse LJ, Appleton JP, et al. Antiplatelet therapy with aspirin, clopidogrel, and dipyridamole versus clopidogrel alone or aspirin and dipyridamole in patients with acute cerebral ischaemia (TARDIS): a randomised, open-label, phase 3 superiority trial. Lancet 2018; 391: 850–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Movva H, Rabah R, Tekle W, et al. There is no difference in safety and efficacy with tirofiban or eptifibatide for patients undergoing treatment of large vessel occlusion and underlying intracranial atherosclerosis. Interdiscipl Neurosurg 2021; 23: 100927. [Google Scholar]
- 17.Osteraas ND, Crowley RW, Panos N, et al. Eptifibatide use following emergent carotid stenting in acute anterior circulation ischemic stroke with tandem occlusion. J Stroke Cerebrovasc Dis 2020; 29: 105021. [DOI] [PubMed]
- 18.Li YF, Spencer FA, Becker RC. Comparative efficacy of fibrinogen and platelet supplementation on the in vitro reversibility of competitive glycoprotein IIb/IIIa (alphaIIb/beta3) receptor-directed platelet inhibition. Am Heart J 2001; 142: 204–210. [DOI] [PubMed] [Google Scholar]
- 19.Reiter R, Jilma-Stohlawetz P, Horvath M, et al. Additive effects between platelet concentrates and desmopressin in antagonizing the platelet glycoprotein IIb/IIIa inhibitor eptifibatide. Transfusion 2005; 45: 420–426. [DOI] [PubMed] [Google Scholar]
- 20.Peerlinck K, De Lepeleire I, Goldberg M, et al. MK-383 (L-700,462), a selective nonpeptide platelet glycoprotein IIb/IIIa antagonist, is active in man. Circulation 1993; 88: 1512–1517. [DOI] [PubMed] [Google Scholar]
- 21.Topol EJ, Byzova TV, Plow EF. Platelet GPIIb-IIIa blockers. Lancet 1999; 353: 227–231. [DOI] [PubMed] [Google Scholar]
- 22.McClellan KJ, Goa KL. A review of its use in acute coronary syndromes. Drugs 1998; 56: 1067–1080. [DOI] [PubMed] [Google Scholar]
- 23.Yang M, Huo X, Gao F. Low-dose rescue tirofiban in mechanical thrombectomy for acute cerebral large-artery occlusion. Eur J Neurol 2020; 27: 1056–1061. [DOI] [PubMed]
- 24.Yan Z, Shi Z, Wang Y, et al. Efficacy and safety of low-dose tirofiban for acute intracranial atherosclerotic stenosis related occlusion with residual stenosis after endovascular treatment. J Stroke Cerebrovasc Dis 2020; 29: 104619. [DOI] [PubMed] [Google Scholar]
- 25.Sun L, Zhang J, Song Y, et al. Safety and efficacy of tirofiban in rescue treatment for acute intracranial intraprocedural stent thrombosis. Front Neurol 2020; 11: 492. [DOI] [PMC free article] [PubMed]
- 26.Sun C, Li X, Zhao Z, et al. Safety and efficacy of tirofiban combined with mechanical thrombectomy depend on ischemic stroke etiology. Front Neurol 2019; 10: 1100. [DOI] [PMC free article] [PubMed]
- 27.Torgano G, Zecca B, Monzani V, et al. Effect of intravenous tirofiban and aspirin in reducing short-term and long-term neurologic deficit in patients with ischemic stroke: a double-blind randomized trial. Cerebrovasc Dis 2010; 29: 275–281. [DOI] [PubMed] [Google Scholar]
- 28.Kim S, Choi J-H, Kang M, et al. Safety and efficacy of intravenous tirofiban as antiplatelet premedication for stent-assisted coiling in acutely ruptured intracranial aneurysms. Am J Neuroradiol 2016; 37: 508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoon CH, Lee H-W, Kim YS, et al. Preliminary study of tirofiban infusion in coil embolization of ruptured intracranial aneurysms. Neurosurgery 2018; 82: 76–84. [DOI] [PubMed] [Google Scholar]
- 30.Chalouhi N, Jabbour P, Daou B, et al. A new protocol for anticoagulation with tirofiban during flow diversion. Neurosurgery 2016; 78: 670–674. [DOI] [PubMed] [Google Scholar]
- 31.Zi-Liang W, Xiao-Dong L, Tian-Xiao L, et al. Intravenous administration of tirofiban versus loading dose of oral clopidogrel for preventing thromboembolism in stent-assisted coiling of intracranial aneurysms. Int J Stroke 2017; 12: 553–559. [DOI] [PubMed] [Google Scholar]
- 32.Samaniego EA, Shaban A, Ortega-Gutierrez S, et al. Stroke mechanisms and outcomes of isolated symptomatic basilar artery stenosis. Stroke Vasc Neurol 2019; 4: 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith J, Humphrey H, Rao SV. Chapter 42 – thrombus pharmacotherapy. In: Topaz O. (ed) Cardiovascular thrombus. Cambridge, MA: Academic Press, pp.587–603. [Google Scholar]
- 34.Cagnazzo F, di Carlo DT, Cappucci M, et al. Acutely ruptured intracranial aneurysms treated with flow-diverter stents: a systematic review and meta-analysis. Am J Neuroradiol 2018; 39: 1669–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.EPIC Investigators. Use of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high-risk coronary angioplasty. N Engl J Med 1994; 330: 956–961. [DOI] [PubMed] [Google Scholar]
- 36.Stavropoulos SW, Shlansky-Goldberg RD. Use of antiplatelet inhibitors in peripheral vascular interventions. Semin Intervent Radiol 2005; 22: 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von MA, Wille H, Schoenhoefer PS. ESPRIT trial. Lancet 2006; 368: 448–449. [DOI] [PubMed] [Google Scholar]
- 38.Zhao W, Che R, Shang S, et al. Low-dose tirofiban improves functional outcome in acute ischemic stroke patients treated with endovascular thrombectomy. Stroke 2017; 48: 3289–3294. [DOI] [PubMed] [Google Scholar]
- 39.Brinjikji W, Morales-Valero SF, Murad MH, et al. Rescue treatment of thromboembolic complications during endovascular treatment of cerebral aneurysms: a meta-analysis. Am J Neuroradiol 2015; 36: 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeong HW, Jin S-C. Intra-arterial infusion of a glycoprotein IIb/IIIa antagonist for the treatment of thromboembolism during coil embolization of intracranial aneurysm: a comparison of abciximab and tirofiban. Am J Neuroradiol 2013; 34: 1621–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen M, Théroux P, Borzak S, et al. ACUTE II Investigators. Randomized double-blind safety study of enoxaparin versus unfractionated heparin in patients with non-ST-segment elevation acute coronary syndromes treated with tirofiban and aspirin: the ACUTE II study. The Antithrombotic Combination Using Tirofiban and Enoxaparin. Am Heart J 2002; 144: 470–477. [DOI] [PubMed]
- 42.Ibbotson T, McGavin JK, Goa KL. Abciximab: an updated review of its therapeutic use in patients with ischaemic heart disease undergoing percutaneous coronary revascularisation. Drugs 2003; 63: 1121–1163. [DOI] [PubMed] [Google Scholar]
- 43.Valgimigli M, Campo G, Tebaldi M, et al. Abciximab: a reappraisal of its use in coronary care. Biologics 2008; 2: 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones RG, Davagnanam I, Colley S, et al. Abciximab for treatment of thromboembolic complications during endovascular coiling of intracranial aneurysms. Am J Neuroradiol 2008; 29: 1925–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ospel JM, Brouwer P, Dorn F, et al. Antiplatelet management for stent-assisted coiling and flow diversion of ruptured intracranial aneurysms: a DELPHI consensus statement. Am J Neuroradiol 2020; 41: 1856–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Linfante I, Etezadi V, Andreone V, et al. Intra-arterial abciximab for the treatment of thrombus formation during coil embolization of intracranial aneurysms. J Neurointerv Surg 2010; 2: 135–138. [DOI] [PubMed] [Google Scholar]
- 47.Park JH, Kim JE, Sheen SH, et al. Intraarterial abciximab for treatment of thromboembolism during coil embolization of intracranial aneurysms: outcome and fatal hemorrhagic complications. JNS 2008; 108: 450–457. [DOI] [PubMed] [Google Scholar]
- 48.Fiorella D, Albuquerque FC, Han P, et al. Preliminary experience using the neuroform stent for the treatment of cerebral aneurysms. Neurosurgery 2004; 54: 6–17; discussion 16. [DOI] [PubMed] [Google Scholar]
- 49.Topol EJ, Moliterno DJ, Herrmann HC, TARGET Investigators. Do Tirofiban and ReoPro Give Similar Efficacy Trial et al. Comparison of two platelet glycoprotein IIb/IIIa inhibitors, tirofiban and abciximab, for the prevention of ischemic events with percutaneous coronary revascularization. N Engl J Med 2001; 344: 1888–1894. [DOI] [PubMed] [Google Scholar]
- 50.Suleiman M, Gruberg L, Hammerman H, et al. Comparison of two platelet glycoprotein IIb/IIIa inhibitors, eptifibatide and abciximab: outcomes, complications and thrombocytopenia during percutaneous coronary intervention. J Invasive Cardiol 2003; 15: 319–323. [PubMed] [Google Scholar]
- 51.Ciccone A, Motto C, Abraha I, et al. Glycoprotein IIb‐IIIa inhibitors for acute ischaemic stroke. Stroke 2014; 45: e155–e156. [DOI] [PubMed] [Google Scholar]
- 52.Zhu X, Cao G. Safety of glycoprotein IIb-IIIa inhibitors used in stroke-related treatment: a systematic review and meta-analysis. Clin Appl Thromb Hemost 2020; 26: 1–11. [DOI] [PMC free article] [PubMed]
- 53.Delgado F, Oteros R, Jimenez-Gomez E, et al. Half bolus dose of intravenous abciximab is safe and effective in the setting of acute stroke endovascular treatment. J NeuroIntervent Surg 2019; 11: 147–152. [DOI] [PubMed] [Google Scholar]
- 54.Ries T, Siemonsen S, Grzyska U, et al. Abciximab is a safe rescue therapy in thromboembolic events complicating cerebral aneurysm coil embolization: single center experience in 42 cases and review of the literature. Stroke 2009; 40: 1750–1757. [DOI] [PubMed] [Google Scholar]
- 55.Duncan IC, Fourie PA. Catheter-directed intra-arterial abciximab administration for acute thrombotic occlusions during neurointerventional procedures. Interv Neuroradiol 2002; 8: 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mounayer C, Piotin M, Baldi S, et al. Intraarterial administration of abciximab for thromboembolic events occurring during aneurysm coil placement. Am J Neuroradiol 2003; 24: 2039–2043. [PMC free article] [PubMed] [Google Scholar]
- 57.Merlini PA, Rossi M, Menozzi A, et al. Thrombocytopenia caused by abciximab or tirofiban and its association with clinical outcome in patients undergoing coronary stenting. Circulation 2004; 109: 2203–2206. [DOI] [PubMed] [Google Scholar]
- 58.MG, KO, Me J, et al. A DELPHI consensus statement on antiplatelet management for intracranial stenting due to underlying atherosclerosis in the setting of mechanical thrombectomy. Neuroradiology 2020; 63: 627–632. [DOI] [PubMed] [Google Scholar]
- 59.Gilchrist IC, O’Shea JC, Kosoglou T, et al. Pharmacodynamics and pharmacokinetics of higher-dose, double-bolus eptifibatide in percutaneous coronary intervention. Circulation 2001; 104: 406–411. [DOI] [PubMed] [Google Scholar]
- 60.Cho YD, Lee JY, Seo JH, et al. Intra-arterial tirofiban infusion for thromboembolic complication during coil embolization of ruptured intracranial aneurysms. Eur J Radiol 2012; 81: 2833–2838. [DOI] [PubMed] [Google Scholar]
- 61.Kang H-S, Kwon BJ, Roh HG, et al. Intra-arterial tirofiban infusion for thromboembolism during endovascular treatment of intracranial aneurysms. Neurosurgery 2008; 63: 230–238. [DOI] [PubMed] [Google Scholar]
- 62.Schneider DJ. Anti-platelet therapy: glycoprotein IIb-IIIa antagonists. Br J Clin Pharmacol 2011; 72: 672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Danzi GB, Capuano C, Sesana M, et al. Variability in extent of platelet function inhibition after administration of optimal dose of glycoprotein IIb/IIIa receptor blockers in patients undergoing a high-risk percutaneous coronary intervention. Am J Cardiol 2006; 97: 489–493. [DOI] [PubMed] [Google Scholar]
- 64.Schrör K. The pharmacology of cilostazol. Diabete Obes Metab 2002; 4: S14–S19. [DOI] [PubMed] [Google Scholar]
- 65.Anderson JR, Riding D. Glycoprotein IIb/IIIa inhibitors in patients with renal insufficiency undergoing percutaneous coronary intervention. Cardiol Rev 2008; 16: 213–218. [DOI] [PubMed] [Google Scholar]
- 66.O’Riordan JM, Margey RJ, Blake G, et al. Antiplatelet agents in the perioperative period. Arch Surg 2009; 144: 69–76. [DOI] [PubMed] [Google Scholar]
- 67.Said SM, Hahn J, Schleyer E, et al. Glycoprotein IIb/IIIa inhibitor-induced thrombocytopenia: diagnosis and treatment. Clin Res Cardiol 2007; 96: 61–69. [DOI] [PubMed] [Google Scholar]
- 68.Wahed A, Quesada A, Dasgupta A. Hematology and coagulation: a comprehensive review for board preparation, certification and clinical practice. Cambridge, MA: Academic Press, 2019. [Google Scholar]