Abstract
Purpose
There is no study on the role of three-dimensional compressed sensing time of flight MR angiography (3D-CS-TOF) in the management of the WEB device. We evaluated the efficacy of 3-tesla 3D-CS-TOF for the management and follow-up of the WEB device implantations.
Materials and methods
Seventy-three aneurysms of 69 patients treated with the WEB device were retrospectively examined. Morphological parameters and embolization results of the aneurysms were assessed and compared on 3D-CS-TOF, CTA, and DSA images.
Results
Occluded, neck remnant, and recurrent aneurysms were observed in 61 (83.6%), 7 (9.6%), and 5 (6.8%) aneurysms, respectively. Inter- and intra-reader agreement values related to aneurysm size measurements were perfect. Aneurysms size, age, and proximal vessel tortuosity were negatively correlated with the visibility of the aneurysms and parent vessels on 3D-CS-TOF images (p = 0.043; p = 0.032; p < 0.001, respectively). Subarachnoid hemorrhage and age are associated with 3D-CS-TOF artifacts (p = 0.031; p = 0.005, respectively). 3D-CS-TOF findings are in perfect agreement with DSA or CT angiography (CTA) results (p < 0.001).
Conclusion
According to our results, 3D-CS-TOF can be an easy, fast, and reliable alternative for the management or follow-up of WEB assisted embolization.
Keywords: WEB, TOF, compressed sensing, cerebral aneurysm, DSA, non-contrast MR angiography
Introduction
Treatment of a wide neck or bifurcation aneurysms is a challenge from the endovascular perspective. The Woven EndoBridge (WEB) device is the first FDA-approved intra-saccular flow diverter (disruptor) intended to treat wide-neck or bifurcation aneurysms.1–3 This promising and new technique has provided a paradigm change in the endovascular treatment of wide-necked or bifurcation type cerebral aneurysms. 4
DSA is the gold standard for identifying, characterizing, and follow-up in the evaluation of cerebral aneurysms. 5 However, DSA has many risks, including radiation exposure, groin hematoma, high-cost, contrast-media-related reactions, experience requirement, and/or contrast-induced nephrotoxicity. 6 Time of flight (TOF) MR angiography (TOF-MRA) has become a promising and reliable approach because of its fast and completely non-invasive nature. 7 Also, it does not contain ionizing radiation and does not require medication or contrast-media administration.
The three-dimensional (3D) TOF-MRA technique with a compressed sensing (CS) algorithm has revealed new ways of accelerating image acquisition in 3-tesla MR units. 8 It uses random under-sampling. 9 Preliminary studies with a small-scale number of subjects have verified a good correlation between 3D-CS-TOF and DSA for detecting intracranial aneurysms and arterial stenosis.9–11
We focused on evaluating cerebral aneurysms treated with the WEB device by 3-tesla 3D-CS-TOF in this study. According to our knowledge, detailed information about the role of 3D-CS-TOF imaging in evaluating patients scheduled for WEB device implantation and/or the post-operative period is unknown.
Materials and methods
Study population
Local ethics committee approval was obtained for this observational study. Informed consent was obtained from all subjects before all imaging sessions and interventions. The clinical and imaging data of all cases treated with the WEB (Terumo, Aliso Viejo, California, USA) device within six years were reviewed retrospectively. All endovascular treatments were performed by the same experienced interventional neuroradiologist (with a seven-year experience for the WEB implantation). Therapeutic decisions were made by multidisciplinary consensus between interventional neuroradiologists and neurovascular surgeons of our hospital, based on recent literature. Risks, benefits, alternatives, potential complications, and different therapeutic approaches about aneurysm treatment techniques of wide-neck or bifurcation aneurysms had been extensively discussed with the patients and their families before treatments.
Inclusion criteria
A history of aneurysm embolization with the WEB device in our hospital,
Presence of 3D-CS-TOF examination pre- and post-operative period,
A follow-up of at least 50 days after the WEB implantation.
Exclusion criteria
Absence of intracranial aneurysm,
Narrow-neck, blister-like, fusiform, or dissecting type aneurysm,
Insufficient MR image quality to evaluate intracranial vasculature due to artifacts,
Patients with MRI contraindications (e.g., claustrophobia, pacemaker),
Presence of an interval >1 month between preoperative 3D-CS-TOF exam and the WEB implantation,
Pediatric cases.
Imaging protocols
All patients underwent 3-tesla non-contrast-material enhanced 3D-CS-TOF MRA in the preoperative period. All MR examinations were performed on a 3-tesla MR scanner (Skyra or Trio, Siemens Healthcare, Erlangen, Germany) using a dedicated birdcage (at least a 20-channel) head coil. After the acquisition of localizer images, isotropic high-resolution 3D-CS-TOF data was obtained for the study. Later, isotropic 3D T1W and T2W sequences were performed to evaluate the whole cranium accurately. Most of the 3D-CS-TOF exams were made on the Skyra MR unit because the acquisition time was less on this unit. Acquisition parameters of the 3-tesla MR exams were given in Table 1.
Table 1.
Three-tesla MRI acquisition details of the study.
| Sequences/ parameters | 3D-MPRAGE | 3D-SPACE |
3D-CS-TOF (Skyra) |
3D-CS-TOF (Trio) | 3D-FLAIR |
|---|---|---|---|---|---|
| TR/TE (ms) | 2130/3.45 | 3000/579 | 21/3.43 | 21/4.24 | 6000/405 |
| TI (ms) | 1100 | – | – | – | 2100 |
| Slice thickness (mm) | 0.8 | 0.6 | 0.3 | 0.4 | 0.9 |
| FOV (mm) | 230 × 230 | 240 × 240 | 220 × 200 | 384 × 384 | 230 × 230 |
| Acquisition time (minute) | 5 | 5 | 3 | 8 | 9 |
| NEX | 1 | 2 | 1 | 1 | 1 |
| Number of slices | 240 | 240 | 180 | 240 | 192 |
| Flip angle (°) | 8 | 100 | 18 | 18 | – |
| Imaging plane | Sagittal | Sagittal | Axial | Axial | Sagittal |
| PAT factor | 2 | 2 | 4 | 4 | – |
| Voxel size (mm) | 0.8 × 0.8 × 0.8 | 0.6 × 0.6 × 0.6 | 0.3 × 0.3 × 0.3 | 0.4 × 0.4 × 0.4 | 0.9 × 0.9 × 0.9 |
| FA mode | – | T2 variant | – | – | – |
3D-CS-TOF: 3D compress-sensing TOF MR-angiography; TI: time of inversion; 3D-SPACE: three-dimensional sampling perfection with application-optimized contrasts using different flip angle evolutions; 3D-MPRAGE: 3D T1W magnetization prepared rapid acquisition gradient-echo; 3D-FLAIR: 3D fluid-attenuated inversion-recovery; NEX: number of excitations; FOV: field of view; PAT: parallel acquisition technique.
DSA examinations (including rotational 3D angiographies in case of inconclusive findings on 2D DSA images), and endovascular procedures were performed using mono or biplane flat-panel angiography units (GE Medical Systems, Milwaukee, Wisconsin, USA). Before the WEB implantations, diagnostic DSA images were not acquired routinely on the patients. If the patients had a DSA exam obtained elsewhere, they were evaluated before the procedure. Antiplatelet therapy was not started before the procedures.
For therapeutic interventions, a standard 6-Fr 90-cm long-sheath and common femoral approach were used for all the cases. Patients were fully heparinized with a 5000 IU bolus dose of heparin. After the 5 or 6 Fr Sofia guide-catheter (Microvention, USA), the WEB device is introduced through an appropriate microcatheter. We used 0.014-inch microwires with a soft tip for the interventions. The size and type of the WEB device were chosen according to the measurement of the aneurysm width and height on 3D-CS-TOF and/or DSA findings. 1000 IU doses of heparin were given per hour until the procedure was finished. After carefully advanced into the aneurysmal sac and detachment of the WEB device, the final position and the lack of any complications were documented on post-deployment angiographic runs. Heparin was discontinued but not reversed at the end of the intervention. Dual anti-platelets therapy was given in patients with the WEB sagging to a parent vessel based on thromboembolism risk for three months.
Evaluated parameters and image analyses
Clinical and imaging findings of the patients were retrospectively reviewed. Observers 1 (with a 15-year experience) and 2 (with a six-year experience) rotated and evaluated the DSA, 3D-rotational angiography, and 3D-CS-TOF images. The volume-rendering technique (VRT) was used for the evaluation of the 3D-CS-TOF and 3D-rotational angiography data to reduce the overlay of peri-aneurysmal structures. Two or more aneurysms were accepted as multiple aneurysms.
Finally, all 3D-CS-TOF and DSA findings were interpreted by the observers; and maximum width, dome height, and neck diameters of the aneurysms were determined by consensus. These measurements are called consensus or DSA results of maximum width, dome height, and neck diameter of the aneurysms. Discrepancies related to the measurements were resolved with agreement by all authors. Also, the following scorings based on 3D-CS-TOF data were added to the study sheet.
Presence of complex-shaped aneurysm scores (2-point scale)
Grade 0: smooth and saccular aneurysm with spherical shaped,
Grade 1: aneurysms with wall protrusions, multiple lobes, complex shape, or baby sacs.
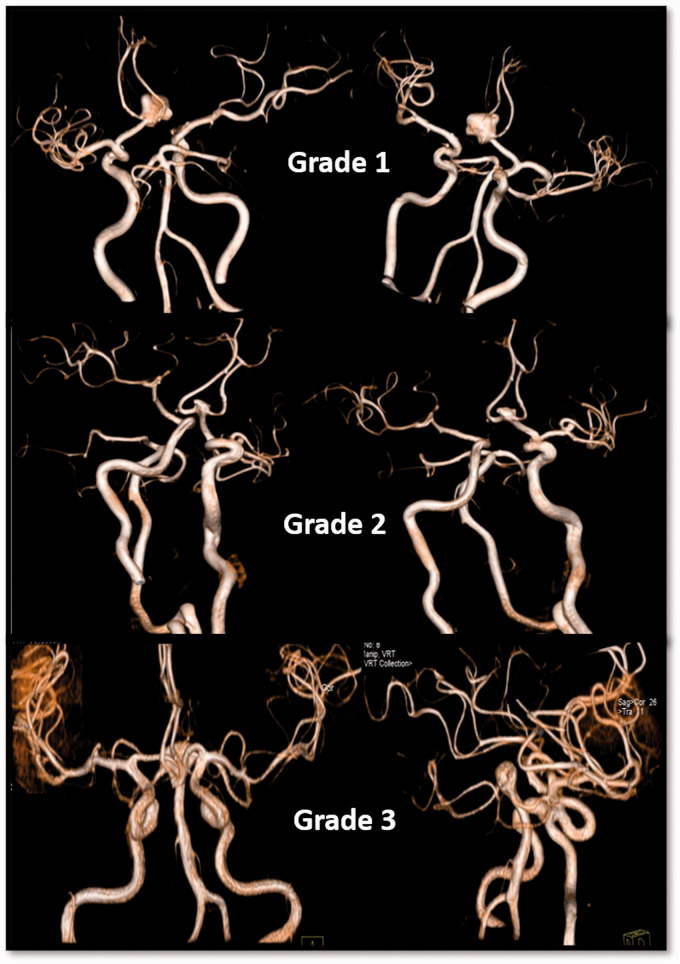
Tortuosity scores of proximal arteries (3-point scale)
Grade 1: no tortuosity,
Grade 2: mild tortuosity,
Grade 3: prominent tortuosity (Figure 1).
Figure 1.
The tortuosity scores of the proximal arteries and aneurysm complexity scores of three patients (upper row: grade 1 tortuosity and complex aneurysm, middle row: grade 2 tortuosity and complex aneurysm, lower row: grade 3 tortuosity and simple type basilary aneurysm).
Artifact scores of the 3D-CS-TOF on source images (3-point scale)
Grade 1: minimal,
Grade 2: moderate,
Grade 3: prominent.
Visibility scores of the aneurysm and perianeurysmal arteries (3-point scale)
Grade 1: minimal (prominent signal loss in the aneurysm and perianeurysmal arteries),
Grade 2: moderate (aneurysm and surrounding arteries are visible, but there are artifacts or blurring),
Grade 3: excellent (good diagnostic quality without clearly visible artifacts/blurring).
Delineation of parent vessels (3-point scale) scale should be as follows:
Grade 0: poor (parent vessels slightly visible, but not adequate for diagnosis),
Grade 1: acceptable (parent vessels roughly visible, diagnosable images),
Grade 2: excellent (appearances of parent vessels equal to DSA).
Follow-up visits were performed after the 6th, 12th months, and every year. The 3D-CS-TOF exams were planned for these visits. Also, at least one CTA (>64-detector) or DSA exam was performed 6 or 12 months after the intervention. Additional CTA or DSA was only performed if there was any suspicion of a recurrence or incomplete occlusion of the aneurysmal sac in parallel with the literature.4,5
On follow-up exams, all aneurysms were scored for degree of occlusion after the embolization procedures (score 0: residue/recurrence, score 1: neck-remnant/stable, score 2: improvement/complete occlusion) based on 3D-CS-TOF (outcome scoring) by the observer 1. Three months later, the same outcome scorings were repeated by the observers based on all data (CTA, 3D-CS-TOF, and DSA images). The last outcome scorings were accepted as a gold standard test for statistical comparisons of follow-up results. Two patients died while on follow-up. Therefore, follow-up results of these aneurysms were not included in outcome analyses.
Statistical analyses
The normal distribution of variables was evaluated using the Shapiro-Wilk test. According to the normality test result, the Mann-Whitney U test was used between the group comparisons. The agreement limits between DSA and 3D-CS-TOF were calculated with the Bland Altman method. The intraclass correlation coefficient (ICC) was used for the assessment of the agreements. A Spearman correlation test evaluated correlations. Data were analyzed statistically using SPSS version 21.0 software (SPSS IBM Inc., Chicago, IL, USA). A value of p < 0.05 was accepted as statistically significant.
Results
Seven patients were excluded from the study due to motion artifacts or pediatric age. The final number of the patients is 69, who are treated with the WEB device and examined with 3-tesla 3D-CS-TOF.
Multiple aneurysms were detected in 18 (26%) patients. The incidence of multiple aneurysms was significantly higher in women (11 females) than in men (7 males) (p = 0.002) (Figure 2). Most of the aneurysms were 5-9 mm diameter (60%) (Table 2). Aneurysms were located more frequently on the left side (Table 3; Figures 3 and 4). Ruptured aneurysms were mostly placed in middle or anterior cerebral artery location (37% for both). The A1 segment of nine (36%) patients with Acom aneurysm was absent or hypoplastic.
Figure 2.
Perioperative (a, b), first-year postoperative (c) DSA, preoperative 3D-CS-TOF (d), and first-year postoperative (e) 3D-CS-TOF images of a 54‐year‐old female with bilateral MCA bifurcation aneurysms (arrows). WEB device was placed in the symptomatic right MCA aneurysm, uneventfully (red arrows). Total occlusion was observed in postoperative images (red arrows in (c) and (e)). Size or morphological difference was not observed in the left MCA bifurcation aneurysm on the follow-up 3D-CS-TOF image (white arrow, (e)). This aneurysm treated with a single flow-diverter device uneventfully in a separate session (not shown here).
Table 2.
Patient characteristics and clinical data for the patients (73 aneurysms of 69 patients).
| Parameters | Values | % |
|---|---|---|
| Median age (range) | 55 (18–83) years | - |
| Sex (male/female) | 40/29 cases | 58/42 |
| Side (left/right/bilateral) | 27/17/29 cases | 37/23/40 |
| Intervention time after preoperative TOF (median) | 4 (range: 1–99) day(s) | – |
| Postoperative CS-TOF date after intervention (median) | 120 (range: 51–1501) day(s) | – |
| Presence/absence of subarachnoid hemorrhage | 43/30 aneurysms | 58.9/41.1 |
| Presence/absence of complex shape aneurysm | 65/8 aneurysms | 89.1/10.9 |
| Presence of contralateral A1 (absent/present/doubtful) | 13/53/3 | 18.8/76.8/4.3 |
| Localizations of the aneurysms | ||
| Internal carotid artery terminus (ICA-bifurcation) | 7 | 9.6 |
| Distal anterior cerebral artery (DACA) | 2 | 3 |
| Anterior communicating artery (ACOM) | 25 | 34.2 |
| Middle cerebral artery (MCA) | 27 | 40 |
| Basilar artery | 4 | 5 |
| Posterior communicating artery (PCOM) | 6 | 8 |
| Paraophthalmic segment | 1 | 1 |
| Vertebral artery | 1 | 1 |
| Aneurysm sizes | ||
| ≤4 mm | 18 | 25 |
| 5–9 mm | 44 | 60 |
| 10–14 mm | 11 | 15 |
Table 3.
Localization, side, and presence of anterior communicating artery (Acom) number of the aneurysms.
|
Aneurysm side/location |
Left (%) | Right (%) | Basilar (%) | Acom (%) |
|
27 (37) |
17 (23.3) |
4 (5.5) |
25 (34.2) |
|
| Presence of Acom artery | Absent (%) | Present (%) | Equivocal (%) | Aneurysmatic (%) |
| 9 (12.3) | 30 (50.7) | 2 (2.7) | 25 (34.2) |
Figure 3.
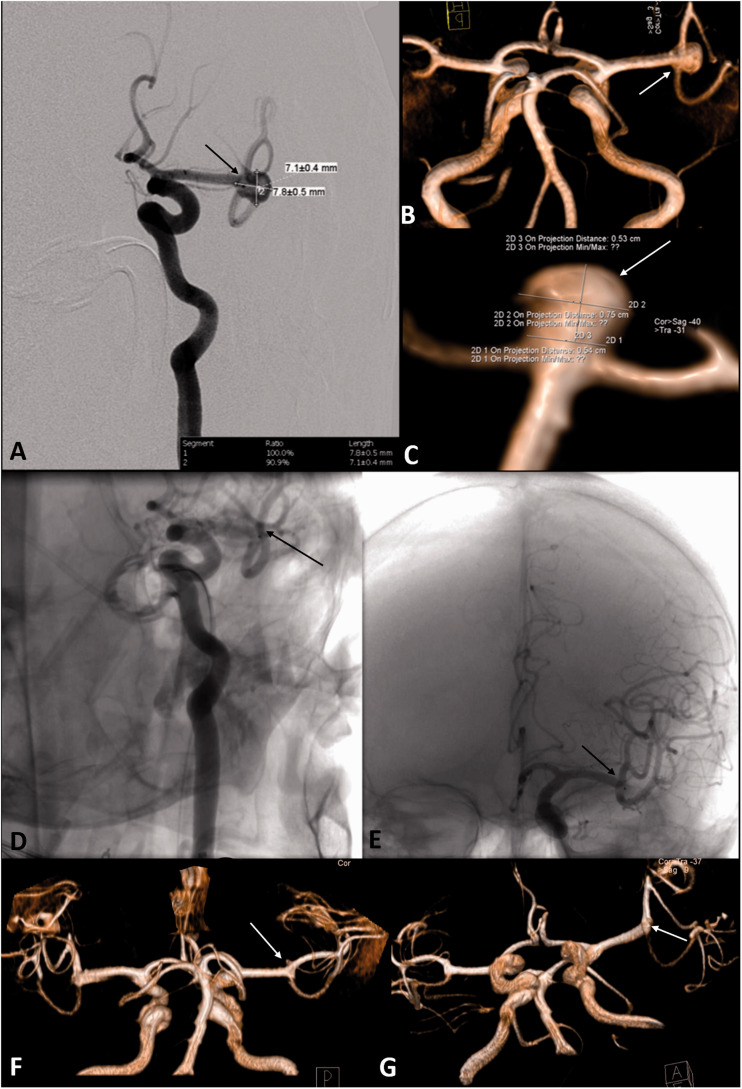
A 36‐year‐old female with a left ICA terminus aneurysm (diameter: 3 mm). Preoperative 3D-CS-TOF ((a) to (c)) and perioperative DSA ((d) to (f)) images clearly showed the characteristics and sizes of the aneurysm (arrows). The aneurysms can be recognized well in all images. 3D-CS-TOF findings are in perfect agreement with the DSA results in this case. After WEB implantation, total occlusion was observed on DSA images ((g) to (i)). No residual or recurrent aneurysm was detected in 3D-CS-TOF images obtained one year later (j). On 3D-CS-TOF images, there are artifacts around the proximal marker of the WEB (arrow in (j)). These artifacts did not hinder the diagnosis.
Figure 4.
A 73‐year‐old female with a non-ruptured left MCA bifurcation aneurysm (diameters: 7 × 8 mm). DSA (a) and 3D-CS-TOF ((b) and (c)) images showed that the aneurysm morphology, clearly (arrows). After WEB device implantation, complete aneurysm occlusion was observed, immediately (arrows in (d) and (e)). On first-year follow-up 3D-CS-TOF images, there is no recurrence, stenosis, or branch loss (arrows in F and G).
Persistent aneurysm filling during the interventions was observed in 5 cases. The intraoperative rupture was detected during the WEB device's placement in 3 ruptured aneurysms of 2 cases with subarachnoid hemorrhage history. In these eight aneurysms, coil placements (WEB assisted or primary coil embolization) were performed, and complete occlusion was achieved in all cases in the same procedures.
Based on consensus readings, median maximum width, dome height, neck diameter, and mean neck to width ratio of the aneurysms were 7 (2:14) mm, 6 (2:19) mm, 4 (2:9) mm, and 0.7 (0.56-0.84), respectively. The equivalents of these measurements on 3D-CS-TOF images were given in Table 4.
Table 4.
Median diameters (maximum width, dome height), necks sizes and mean neck to body ratios of the aneurysms in all readings.
|
Median (Min: Max) |
Mean ± (SD) |
|||
|---|---|---|---|---|
| Readings | Width (mm) | Height (mm) | Neck size (mm) | Neck to body ratio |
| Observer 1 F | 7 (3:14) | 7 (3:20) | 4 (2:8) | 0.68 ± 0.16 |
| Observer 1S | 7 (3:12) | 8 (3:19) | 5 (2:8) | 0.63 ± 0.33 |
| Observer 2 | 7 (2:12) | 7 (3:18) | 4 (2:8) | 0.63 ± 0.17 |
| Consensus | 7 (2:14) | 6 (2:19) | 4 (2:9) | 0.70 ± 0.14 |
Obs1F: first readings of the observer 1, Obs1S: second readings of the observer 1, Obs2: readings of the observer 2, Consensus: final decisions based on digital subtraction angiography.
There is a strong positive correlation between visibility and parent vessel delineation scores (r = 0.905, p < 0.001). In patients with high artifact scores, parent vessel delineation scores were generally low. There was an inverse relationship between these two parameters (r=-0.520, p = 0.001). Similarly; in patients with high proximal vessel tortuosity scores, visibility scores of perianeurysmal arteries and the aneurysm were generally low. There was an inverse relationship between these scores (r = −0.490, p < 0.001). There is a statistically significant positive correlation between age and artifact scores (r = 0.360, p = 0.005).
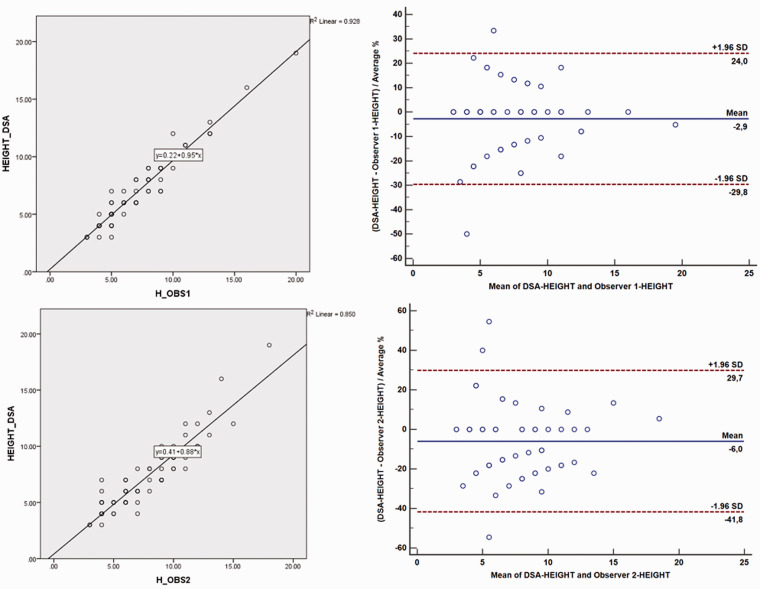
Inter- and intra-reader agreement values related to aneurysm size measurements were perfect for the readings (Table 5). The 3D-CS-TOF evaluations of the more experienced reader (observer 1) were more compatible with the results of consensus readings than observer 2. In general, the worst agreement values were the neck-to-body ratio measurements (Table 5). The method comparisons between consensus (DSA) and 3D-CS-TOF results related to height/width/neck measurements and neck-to-body ratios of the aneurysms were demonstrated in Diagrams 1 to 4.
Table 5.
Inter- and intra-observer agreement values of aneurysm sizes assessment readings of the patients.
|
Evaluated parameters |
|
|
|
|
| Aneurysm width (mm) | ICC | p value | Lower bound (95%CI) | Upper bound (95%CI) |
| DSA vs. Obs1F readings | 0.96 | <0.001 | 0.934 | 0.976 |
| DSA vs. Obs2 readings | 0.918 | <0.001 | 0.863 | 0.951 |
| Obs1F vs. Obs2 readings | 0.934 | <0.001 | 0.934 | 0.961 |
| Obs1F vs. Obs1S readings | 0.925 | <0.001 | 0.865 | 0.959 |
| Aneurysm height (mm) | ICC | p value | Lower bound (95%CI) | Upper bound (95%CI) |
| DSA vs. Obs1F readings | 0.981 | <0.001 | 0.969 | 0.989 |
| DSA vs. Obs2 readings | 0.959 | <0.001 | 0.932 | 0.975 |
| Obs1F vs. Obs2 readings | 0.96 | <0.001 | 0.932 | 0.977 |
| Obs1F vs. Obs1S readings | 0.961 | <0.001 | 0.93 | 0.979 |
| Neck to body ratio | ICC | p value | Lower bound (95%CI) | Upper bound (95%CI) |
| DSA vs. Obs1F readings | 0.467 | 0.008 | 0.116 | 0.679 |
| DSA vs. Obs2 readings | 0.497 | 0.004 | 0.162 | 0.698 |
| Obs1F vs. Obs2 readings | 0.588 | 0.001 | 0.298 | 0.759 |
| Obs1F vs. Obs1S readings | 0.848 | <0.001 | 0.725 | 0.916 |
| Neck size (mm) | ICC | p value | Lower bound (95%CI) | Upper bound (95%CI) |
| DSA vs. Obs1F readings | 0.95 | <0.001 | 0.918 | 0.97 |
| DSA vs. Obs2 readings | 0.834 | <0.001 | 0.724 | 0.901 |
| Obs1F vs. Obs2 readings | 0.806 | <0.001 | 0.669 | 0.886 |
| Obs1F vs. Obs1S readings | 0.777 | <0.001 | 0.597 | 0.877 |
ICC: intraclass correlation; DSA: consensus measurements based on all images including digital subtraction angiography; Obs1F: first CS-TOF readings results of the observer 1; Obs1S: second CS-TOF readings results of the observer 1; Obs2: CS-TOF readings results of the observer 2.
Diagram 1.
Method comparisons (DSA vs. 3D-CS-TOF MR angiography) of two measurements by two observers related to width parameter presented as regression analysis (scatterplots in column 1), Bland and Altman plot where differences are presented as percentage (column 2). The representation of the limit of agreements (dotted line), from −1.96 s to +1.96 s. The scatterplot shows a significant correlation for aneurysmal dimension measurements between 3D-CS-TOF and DSA.
Diagram 2.
Method comparisons (DSA vs. 3D-CS-TOF MR angiography of two measurements by two observers related to height parameter presented as regression analysis (column 1), Bland and Altman plot where differences are presented as percentage (column 2). The representation of the limit of agreements (dotted line), from −1.96 s to +1.96 s.
Diagram 3.
Method comparisons (DSA vs. 3D-CS-TOF MR angiography of two measurements by two observers related to the ratio of neck to the body parameter presented as regression analysis (column 1), Bland and Altman plot where differences are presented as a percentage (column 2). The representation of the limit of agreements (dotted line), from −1.96 s to +1.96 s.
Diagram 4.
Method comparisons (DSA vs. 3D-CS-TOF MR angiography of two measurements by two observers related to neck parameter presented as regression analysis (column 1), Bland and Altman plot where differences are presented as percentage (column 2). The representation of the limit of agreements (dotted line), from −1.96 s to +1.96 s.
There were statistically significant positive correlations between aneurysms number and sex; aneurysm height, delineation of the parent vessel scores and visibility scores; tortuosity scores, artifact scores, and age (Table 6). There were statistically significant negative correlations between age, tortuosity scores, artifact scores, and visibility scores; tortuosity scores, artifact scores, age, and delineation of parent vessel scores; aneurysm height, aneurysm width measurements, and artifact scores (Table 6). A positive correlation was observed between the presence of SAH and artifact scores (r = 0.253, p = 0.031). There were statistically significant negative correlations between outcome scores and aneurysm sizes (Table 7). Also, there were inverse but non-significant correlations between complex type aneurysms and outcome/artifact scores (Table 8). Details of the artifact, visibility, and proximal vessel tortuosity scores were given in Table 9.
Table 6.
Spearman's rho correlation analyses of the parameters.
| Aneurysm number | Sex | |
| Correlation Coefficient | .362** | |
| p value | 0.002 | |
| Visibility scores | Aneurysm height | |
| Correlation Coefficient | .258* | |
| p value | 0.043 | |
| Age | ||
| Correlation Coefficient | −.270* | |
| p value | 0.032 | |
| Tortuosity scores | ||
| Correlation Coefficient | −.365** | |
| p value | 0.001 | |
| Artifact scores | ||
| Correlation Coefficient | −.490** | |
| p value | 0.000 | |
| Delineation of parent vessel scores | ||
| Correlation Coefficient | .905** | |
| p value | 0.000 | |
| Delineation ofparent vessel scores | Age | |
| Correlation Coefficient | −.329** | |
| p value | 0.009 | |
| Tortuosity scores | ||
| Correlation Coefficient | −.335** | |
| p value | 0.004 | |
| Artifact scores | ||
| Correlation Coefficient | −.520** | |
| p value | 0.000 | |
| Age | Tortuosity scores | |
| Correlation Coefficient | .319* | |
| p value | 0.011 | |
| Artifact scores | ||
| Correlation Coefficient | .360** | |
| p value | 0.005 | |
| SAH | Artifact scores | |
| Correlation Coefficient | .253* | |
| p value | 0.031 | |
Notes = **: Correlation is significant at the 0.01 level (2-tailed), *: Correlation is significant at the 0.05 level (2-tailed). Correlation coefficient = 1 means a perfect positive correlation and the value = -1 means a perfect negative correlation, SAH: Subarachnoid hemorrhage.
Table 7.
Spearman's rho correlation analysis between outcome (follow-up occlusion) scores and aneurysm size measurements.
| Outcome scores | |||
|---|---|---|---|
| Variables | Aneurysm width, consensus session |
Correlation coefficient | −.294* |
| p value | 0.013 | ||
| Aneurysm height, consensus session |
Correlation coefficient | −.425** | |
| p value | 0.000 | ||
| Aneurysm neck, consensus session |
Correlation coefficient | −.377** | |
| p value | 0.001 | ||
| Aneurysm width, first session of observer 1 |
Correlation coefficient | −.346** | |
| p value | 0.007 | ||
| Aneurysm height, first session of observer 1 |
Correlation coefficient | −.401** | |
| p value | 0.002 | ||
| Aneurysm neck, first session of observer 1 |
Correlation coefficient | −.437** | |
| p value | 0.000 | ||
| Aneurysm width, second session of observer 1 |
Correlation coefficient | −.509** | |
| p value | 0.000 | ||
| Aneurysm height, second session of observer 1 |
Correlation coefficient | −.434** | |
| p value | 0.003 | ||
| Aneurysm neck, second session of observer 1 |
Correlation coefficient | −0.267 | |
| p value | 0.072 | ||
| Aneurysm width, readings of observer 2 |
Correlation coefficient | −.337** | |
| p value | 0.009 | ||
| Aneurysm height, readings of observer 2 |
Correlation coefficient | −.381** | |
| p value | 0.003 | ||
| Aneurysm neck, readings of observer 2 |
Correlation coefficient | −0.223 | |
| p value | 0.089 | ||
Correlation coefficient (Spearman's rho) = 1 means a perfect positive correlation and the value = −1 means a perfect negative correlation.
*Correlation is significant at the 0.05 level (2-tailed). **Correlation is significant at the 0.01 level (2-tailed).
Table 8.
Spearman's rho correlation analyses of the parameters.
| SAHArtifact scores | Presence of multiple aneurysms | |
| Correlation Coefficient | 0.129 | |
| p value | 0.276 | |
| Visibility scores | ||
| Correlation Coefficient | −0.111 | |
| p value | 0.350 | |
| Outcome scores | ||
| Correlation Coefficient | −0.008 | |
| p value | 0.945 | |
| Aneurysm width | ||
| Correlation Coefficient | −0.102 | |
| p value | 0.392 | |
| Neck size of the aneurysm | ||
| Correlation Coefficient | −0.026 | |
| p value | 0.825 | |
| Complex type aneurysm | ||
| Correlation Coefficient | 0.063 | |
| p valueComplex type aneurysm | 0.5 | |
| Correlation Coefficient | −0.069 | |
| p value | 0.560 | |
| Outcome | Complex type aneurysm | |
| Correlation Coefficient | −0.155 | |
| p value | 0.190 | |
Notes = Correlation coefficient = 1 means a perfect positive correlation and the value = -1 means a perfect negative correlation, SAH: Subarachnoid hemorrhage.
Table 9.
Numbers of the scorings of the patients (total aneurysm number: 73).
| Number (%) | |||
|---|---|---|---|
| Score 1 | Score 2 | Score 3 | |
| Delineation ofparent vessel | 1 (1.4) | 20 (27.4) | 52 (71.2) |
| Visibility | 1 (1.4) | 21 (28.8) | 51 (69.9) |
| Tortuosity | 45 (61.6) | 25 (34.2) | 3 (4.1) |
| Artefact | 43 (58.9) | 24 (32.9) | 6 (8.2) |
| Recurrence | Neck-remnant | Improvement | |
| Outcome CS-TOF | 5 (6.8) | 7 (9.6) | 61 (83.6) |
| Outcome consensus | 5 (6.8) | 7 (9.6) | 61 (83.6) |
Periprocedural complications were observed in 8 patients (11%). In 5 cases (7%), there were permanent neurologic deficits due to thromboembolism, a result of partial sagging into the perianeurysmal artery. In all patients, thromboembolic complications were observed in the first week after the intervention.
The median follow-up period of this study was 11 months (range: 50-2012 days after the interventions). Residue/recurrent, stable/neck-remnant, and healed/occluded aneurysms were observed in 5 (6.8%), 7 (9.6%), and 61 (83.6%) aneurysms, respectively (Table 9; Figure 5). The median diameter of the residual aneurysms was 5.5 mm. The post-operative 3D-CS-TOF findings are in perfect agreement with the consensus (DSA) results (p < 0.001).
Figure 5.
In the CS-TOF images of the 53-year-old female with headache, a saccular wide-neck aneurysm with a diameter of 6 mm was observed in the left MCA trifurcation (arrows in (a) to (c)). This aneurysm is embolized by the WEB device (arrows in (d) and (e)). After embolization, contrast material entrapment or stagnation is observed in the aneurysm on late-phase DSA image (arrow, (f)). No significant recurrence or remnant is observed on the first- ((g) and (h)) and second (i) years postoperative CS-TOF images (arrows). However, neck-remnant is seen on postoperative sixth-year CS-TOF image (arrow in (j)). Also, a small posttraumatic arteriovenous fistula was detected around R-MCA M3-4 segment on this image (broken arrow in (j)). Residue aneurysm due to WEB compaction was confirmed on postoperative sixth year DSA images (arrows in (k) to (m)). The arteriovenous fistula was confirmed also on CTA and DSA images (not shown here).
Discussion
Morphological features of the aneurysm and parent arteries play a key role in treatment planning, preserving parent vessel patency without adjunctive stenting and antiplatelet therapy, and deciding on the WEB treatment or other options.1–3 In this context, accurate and reliable measurements of the aneurysms and perianeurysmal arteries, and determination of aneurysm shape are crucial. Therefore, a fast and high-resolution MRA technique is a vital requirement.11,12
In this work, we proposed a non-contrast MRA with the CS technique, representing high-resolution 3D-TOF-MRA images with detailed arterial structures and aneurysms. 3D-CS-TOF is a new and promising CS technique for accelerating non-contrast MRA acquisition without compromising image quality. 13
In the literature, 3D-CS-TOF could provide excellent intra- and inter-rater agreement values with DSA in the assessment of intracranial and cervical arteries, as observed in our study.6,10,11 We found no noticeable differences between intra- and inter-observer variability for both high-resolution 3D-CS-TOF and CTA/DSA based size measurements of the aneurysms. Our results were highly correlated or agreed with previous CS-TOF studies.6,11
We showed that 3D-CS-TOF is very successful in the management and follow-up of WEB implantations. Also, this study demonstrates the feasibility of applying the 3D-CS-TOF to evaluate the residue aneurysms. Clipped and/or small residue aneurysms are often difficult to detect and evaluate on CTA or DSA images. 14 Multiple viewing angles provided by 3D-CS-TOF data with VRT technique are useful for the demonstration of post-clip residues and quick deletion of the overlapping vessels, as observed in our study. Size change or blood filling in the aneurysm during the follow-up period are the significant indicators of residue or progression. 6 Besides, isotropic 3D-SPACE acquisitions are useful for quick and accurate morphological evaluation of perianeurysmal soft-tissues in patients with clips or stents according to our experiences.
We observed that artifact and tortuosity scores increased with increasing age (Table 6). The conventional TOF technique is sensitive to turbulent or slow flow. 5 This phenomenon is less in 3D-CS-TOF than the conventional TOF method. 9 Therefore, 3D-CS-TOF is more potent for the determination of small or tortuous arteries, luminal narrowing/dilatations, and residue/complex aneurysms of the intracranial arteries. Also, we detected an inverse correlation between tortuosity scores and visibility and the delineation of parent vessel scores. We think that these results develop due to turbulent, complicated, or slow flow, which affects the quality of 3D-CS-TOF images negatively. Besides, patients with SAH had higher artifact scores and lower visibility scores (Tables 6 and 8). The reason for higher artifact scores in patients with SAH may be the hyperintensity of SAH around the aneurysm and movement due to cooperation difficulty.
Evaluation of flow-diverter (FD) or stented aneurysm by the conventional TOF or CE-MRA remains a challenge due to radiofrequency shielding and susceptibility artifacts related to stents or FD.5,15 3D-CS-TOF may be a problem-solving method for these situations because of its irregular under-sampling design. 9 Multiplanar source images of the 3D-CS-TOF technique are very useful for a stent or FD evaluation according to our observations.
A1 segment of nine (36%) patients with Acom aneurysm was absent or hypoplastic in this study. The 3D-CS-TOF easily demonstrated the shortest and safest pathway to achieve the aneurysm in these cases. We think that this feature of the 3D-CS-TOF is critical in reducing endovascular intervention time, morbidity/mortality, and radiation exposure.
The preferred imaging techniques for cerebral aneurysms assessment in daily clinical practice are CTA, contrast-enhanced MR angiography (CE-MRA), or DSA. CE-MRA was associated with several complications, such as nephrogenic systemic fibrosis, gadolinium accumulation, and anaphylactic shock. 15 Limitations or disadvantages of CTA and DSA are exposure to radiation, the use of iodinated contrast agents, anaphylactic reactions, and impaired accuracy in the presence of vascular calcifications, clips, coils, or perianeurysmal bones. 9 Cerebral CTA reconstructions are also hampered by the challenge of separating contrast-filled veins. 6 Besides, DSA has additional risks for peri- and post-procedural complications such as groin hematoma (which may require further interventions), pain, thromboembolic complications, radiation-related problems, and increased cost related to these risks. 5 However, the use of DSA is traditionally the gold standard for a precise assessment of the geometry, localization, other vascular pathologies, and size of the aneurysm.14,16
The high-resolution 3D-CS-TOF has several advantages, including a completely non-invasive nature, lack of ionizing radiation, and faster than silent MRA technique.13,15 However, it has several disadvantages. 3D-CS-TOF is slower than CE-MRA and more sensitive to motion or turbulent flow. It has a bigger pixel-size than DSA, limited anatomical coverage, relatively long acquisition duration, and post-processing time.5,17 Also, the acute-to-subacute phase of intra-saccular thrombus or high-density cysts may mimic residual aneurysm in 3D-CS-TOF data. 5 However, these are not essential problems for experienced readers. As another drawback, 3-tesla TOF-MRA acquisitions may cause WEB device migration in the first 3 months after the WEB implantation (especially in patients with wide-neck aneurysms with a high-flow) according to our experiences.
There are some limitations to the present study. First, our study was retrospective. We have not assessed conventional TOF-MRA or CE-MRA for head-to-head comparisons due to ethical reasons and imaging-time/specific-absorption-rate restrictions. Forty-three percent of the patients did not complete at least a one-year follow-up. This situation may affect our occlusion rates, and we could not adequately address the follow-up findings and results of these patients. Also, the images were evaluated by the readers, who were aware of the study purposes and knew that at least one embolized aneurysm with WEB was present in the patients; this could have resulted in a bias toward 3D-CS-TOF like other studies. Our results and experiences from a single tertiary referral hospital. This might bias the interpretation of imaging or demographic findings.
Conclusions
This is the first study that exclusively focuses on the role of the high-resolution 3D-CS-TOF in the evaluation of WEB implantations. We found that the high-resolution 3D-CS-TOF is a fast, non-invasive, reliable, and effective method for the management and follow-up of the WEB implantations. More comprehensive, prospective, and long-term follow-up studies are necessary to evaluate the role of the high-resolution 3D-CS-TOF.
Acknowledgements
The authors wish to thank Tolga Cukur, M Ozgur Ozates, and Meltem Y Erol for assistance with data acquisition and manuscript preparation.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
ORCID iDs: Oktay Algin https://orcid.org/0000-0002-3877-8366
Gıyas Ayberk https://orcid.org/0000-0002-9258-4001
References
- 1.Al Saiegh F, Hasan D, Mouchtouris N, et al. Treatment of acutely ruptured cerebral aneurysms with the woven EndoBridge device: experience post-FDA approval. Neurosurgery 2020; 87: E16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.- van Rooij SBT, Peluso JP, Sluzewski M, et al. The new low-profile WEB 17 system for the treatment of intracranial aneurysms: first clinical experiences. AJNR Am J Neuroradiol 2018; 39: 859–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.- Bhogal P, Udani S, Cognard C, et al. Endosaccular flow disruption: where are we now? J Neurointerv Surg 2019; 11: 1024–1025. [DOI] [PubMed] [Google Scholar]
- 4.- Lescher S, Du Mesnil de Rochemont R, Berkefeld J. Woven endobridge (WEB) device for endovascular treatment of complex unruptured aneurysms from a single-center experience. Neuroradiology 2016; 58: 383–390. [DOI] [PubMed] [Google Scholar]
- 5.- De Leacy R, Yaniv G, Nael K. Cerebral aneurysm follow-up: how standards have changed and why a perspective on the optimal follow-up frequency and imaging modality type treated cerebral aneurysms. Endovasc Today 2019; 18: 80–84. [Google Scholar]
- 6.- Kim HJ, Yoon DY, Kim ES, et al. Intraobserver and interobserver variability in CT angiography and MR angiography measurements of the size of cerebral aneurysms. Neuroradiology 2017; 59: 491–497. [DOI] [PubMed] [Google Scholar]
- 7.- HaiFeng L, YongSheng X, YangQin X, et al. Diagnostic value of 3D time-of-flight magnetic resonance angiography for detecting intracranial aneurysm: a meta-analysis. Neuroradiology 2017; 59: 1083–1092. [DOI] [PubMed] [Google Scholar]
- 8.- Kılıc T, Cukur T, Algin O, et al. Joint partial fourier and compressed sensing reconstruction for accelerated time-of-flight MR angiography. In: 26th signal processing and communications applications conference (SIU), Izmir, Turkey, 2018, pp.1–4,
- 9.- Lu SS, Qi M, Zhang X, et al. Clinical evaluation of highly accelerated compressed sensing time-of-flight MR angiography for intracranial arterial stenosis. AJNR Am J Neuroradiol 2018; 39: 1833–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.- Zhang X, Cao YZ, Mu XH, et al. Highly accelerated compressed sensing time-of-flight magnetic resonance angiography may be reliable for diagnosing head and neck arterial steno-occlusive disease: a comparative study with digital subtraction angiography. Eur Radiol 2020; 30: 3059–3065. [DOI] [PubMed] [Google Scholar]
- 11.- Lin Z, Zhang X, Guo L, et al. Clinical feasibility study of 3D intracranial magnetic resonance angiography using compressed sensing. J Magn Reson Imaging 2019; 50: 1843–1851. [DOI] [PubMed] [Google Scholar]
- 12.- Goyal N, Hoit D, DiNitto J, et al. How to WEB: a practical review of methodology for the use of the woven EndoBridge. J Neurointerv Surg 2020; 12: 512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.- Zhou Z, Han F, Yu S, et al. Accelerated noncontrast-enhanced 4-dimensional intracranial MR angiography using golden-angle stack-of-stars trajectory and compressed sensing with magnitude subtraction. Magn Reson Med 2018; 79: 867–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.- Kumar S, Gaikwad SB, Mishra NK. 3D rotational angiography in follow-up of clipped intracranial aneurysms. ISRN Radiol 2014; 2014: 935280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.- Ozpeynirci Y, Braun M, Pala A, et al. WEB-only treatment of ruptured and unruptured intracranial aneurysms: a retrospective analysis of 47 aneurysms. Acta Neurochir 2019; 161: 1507–1513. [DOI] [PubMed] [Google Scholar]
- 16.- Herzberg M, Forbrig R, Schichor C, et al. Preoperative digital subtraction angiography in incidental unruptured intracranial aneurysms: how much is too much? Clin Neuroradiol 2018; 28: 429–435. [DOI] [PubMed] [Google Scholar]
- 17.- Oishi H, Fujii T, Suzuki M, et al. Usefulness of silent MR angiography for intracranial aneurysms treated with a flow-diverter device. AJNR Am J Neuroradiol 2019; 40: 808–814. [DOI] [PMC free article] [PubMed] [Google Scholar]