Abstract
Medical robots provide enhanced dexterity, vision, and safety for a broad range of procedures. In this article, we present a handheld, robotic device capable of performing peripheral catheter insertions with high accuracy and repeatability. The device utilizes a combination of ultrasound imaging, miniaturized robotics, and machine learning to safely and efficiently introduce a catheter sheath into a peripheral blood vessel. Here, we present the mechanical design and experimental validation of the device, known as VeniBot. Additionally, we present results on our ultrasound deep learning algorithm for vessel segmentation, and performance on tissue-mimicking phantom models that simulate difficult peripheral catheter placement. Overall, the device achieved first-attempt success rates of 97 ± 4% for vessel punctures and 89 ± 7% for sheath cannulations on the tissue mimicking models (n = 240). The results from these studies demonstrate the viability of a handheld device for performing semi-automated peripheral catheterization. In the future, the use of this device has the potential to improve clinical workflow and reduce patient discomfort by assuring a safe and efficient procedure.
Keywords: venipuncture, medical robotics, ultrasound, machine vision, deep learning, automation, catheterization
1 Introduction
Medical robots enable enhanced precision, safety, and efficacy in various medical procedures that would otherwise be limited by human vision and dexterity [1,2]. Improvements in medical imaging, robotic control, and miniaturized computing have paved the way for the development of complex medical robots. These devices and technology have been implemented largely in the surgical field where accurate motion control and improved vision are key for achieving successful results [1,3–5]. However, outside of the operating room, robotics has seen limited clinical use. One such task where positioning guidance and improved vision is particularly useful is in performing percutaneous cannulation of vessels in order to advance peripheral catheters. Peripheral catherization, performed for either blood sampling, fluid delivery, or endovascular intervention, is a ubiquitous and often necessary step for both the treatment and diagnosis of the patient [6,7]. Furthermore, timely administration of a peripheral intravenous catheter (PIVC) is critical in emergency situations where delays or difficulties can affect morbidity and mortality [8,9]. Oftentimes, however, performing PIVC, even in the most routine procedures, can be made difficult and prolonged due to patients with small, rolling, or nonvisible blood vessels. These vessel characteristics are typically seen in elderly, pediatric, and chronically ill populations [10–14]. Success rates for these patients with difficult PIVC can even fall below 50% [11,15,16] and take an upwards of 1 h to successfully access the vessel [17]. Pediatric patients also present a notable challenge. A 20-month study at a U.S. pediatric hospital performed by Lininger reported only a 53% first-stick success rate in 249 pediatric patients, with an average of >2 attempts to gain venous access [16]. Furthermore, repeated failed attempts of placing a PIVC have also shown to increase the chances of injury such as phlebitis, thrombosis, and blood transmission infections [18,19]. In other cases, if peripheral vessels are not suitable or inaccessible for cannulation, more invasive and costly pathways, such as central venous access, are often necessary [20,21].
Despite recent advancements in robotics and medical imaging, clinical adoption of these technologies for venipuncture, particularly peripheral catherization, have remained limited. In this article, we present the design and evaluation of our handheld, portable, robotic device capable of performing peripheral catheterizations, known as VeniBot. This device couples two-dimensional (2D) ultrasound imaging with miniaturized robotic control to safely and efficiently cannulate peripheral blood vessels.
Previously, our lab developed a benchtop robotic venipuncture device capable of performing fully automated blood draws [22–24]. This version of the device had a 9 degree-of-freedom robotic system to position the needle manipulator and ultrasound probe on the patient's arm, and utilized near-infrared and ultrasound imaging to guide the needle to the target vessel. While this device was shown to work effectively in phantom arms and animal models [25], its large size requires it to be fixed to a benchtop for operation, limiting its practical use in ambulatory settings. To facilitate clinical translation and reduce device complexity, our lab has recently developed a miniaturized, handheld version of the benchtop device, named VeniBot [26]. The VeniBot is operated manually, in which the operator places and holds the device over the insertion site area, while the device identifies suitable vessels via ultrasound imaging and inserts an attached needle. In a first-in-human clinical pilot study, we demonstrated the device's safety and efficacy for performing routine venipuncture and blood sampling in 31 healthy adult volunteers [26].
In the present study, we present an enhanced version of the VeniBot (Fig. 1), capable of performing peripheral catheterizations as well as blood draws. Peripheral catherization requires both the successful puncture and simultaneous catherization of the vessel with a catheter sheath. In this article, we investigate whether the Venibot device can similarly be applied for peripheral catherization for the purposes of both fluid delivery and blood sampling. We report the results of our device performing PIVC placements on tissue-mimicking phantom arm models. Furthermore, we present our deep learning ultrasound vessel segmentation algorithm, as well as the image-guided needle insertion accuracy and catheter placement success rates. Section. 2 of this article describes the steps for device operation and the major components of the device. Section. 3 covers the experimental setup and study results that validate the efficacy and safety of the device in vitro. Finally, Sec. 4 concludes with a discussion on the capabilities and limitations of the device and future work.
Fig. 1.

VeniBot – Handheld device for peripheral catheterization. (a) Computer-aided design (CAD) model. (b) Physical prototype of the device. Housing and frame components are fabricated utilizing both extrusion and stereolithography three-dimensional printing techniques.
2 System Design
The device consists of three main components: 1) the robotic needle manipulator, 2) the ultrasound imaging probe, and 3) the host processor. An overview of all major device components can be seen in Fig. 2. To summarize, ultrasound B-mode 2D imaging is used to identify any suitable blood vessels for cannulation. Once identified and segmented, the vessel coordinate position is fed into the kinematics and control algorithms. The control software then sends commands to each motor to perform the necessary needle manipulations for a successful catherization. The following sections highlight the role and major functions of each component seen in Fig. 2. Sec. 2.1 looks closer at what is done at each step of device operation.
Fig. 2.

Internal components and mechanisms of the VeniBot device. The device features a 2D ultrasound probe combined with a needle manipulator unit. This robotic system comprises 3 degrees-of-freedom for performing the venipuncture: 1) the alignment motor (Zm) for vertical motion, 2) the insertion-angle motor (θm) for adjusting the angle-of-insertion, and 3) the insertion motor (Injm) responsible for needle translation. A needle clip containing the PIVC is loaded into the device and held in place via a neodymium magnet. A custom ultrasound gel clip is attached to the transducer head to provide acoustic coupling. A blood flash sensor is located within the catheter sheath push to detect if blood flow is present through the catheter sheath postpuncture.
2.1 End-to-End Workflow.
Device operation, from start to finish, consists of five phases: 1) ultrasound imaging, 2) vessel segmentation, 3) needle alignment, 4) needle insertion and 5) catheter placement. A breakdown of each operational step can be seen in Fig. 3. The device is designed to be operated in a handheld configuration, with the user holding the device over the general insertion site area during the procedure. Once positioned, the device will then instruct the user if any vessels have been identified from the current ultrasound image. A green LED indicator will prompt the operator to stop scanning when the target vessel is found and aligned with the needle trajectory path. Once aligned with the target vessel, the user can initiate needle insertion via a button press on the device. From the tracked vessel coordinates, the necessary kinematics are computed and commands are sent to the needle manipulator to carry out the procedure. The vertical (Zm) linear translation and insertion-angle (θm) rotation values are calculated from calibration constants and determined from the current vessel depth (Zu) imaged by the ultrasound probe (Fig. 3, step 3). For example, θm is linearly related to the vessel depth; a deeper vessel depth results (Zu) in a steeper angle-of-insertion. Once aligned and initiated, the needle is inserted at 30 mm/s and halts progression when either: a puncture has been confirmed from the force sensor or the needle tip reaches its desired final position. Once a blood flash is confirmed by the device, the angle-of-insertion is lowered and the catheter sheath is simutaneously inserted forward while the guide needle is retracted back. This unique operation is explained further in Sec. 2.4. Finally, with the catheter sheath secured in the vessel, the device can then be removed from the site and the guide needle disposed.
Fig. 3.

Major operational steps and protocol. Step 1: 2D ultrasound imaging provides a cross-sectional view of the target vessel. Step 2: Vessel segmentation is accomplished using a deep learning algorithm. A best-fit ellipse is applied to the resulting semantic segmentation to identify the vessel wall and vessel center coordinates. Step 3: The device aligns the needle trajectory such that the needle tip will intersect the vessel center at the ultrasound imaging plane. Step 4: The needle is inserted forward. Closed loop feedback control makes adjustments to any changes in movement from the vessel or device. Once vessel puncture is confirmed, the insertion is halted. Successful puncture is confirmed via motor position encoders, ultrasound needle tip confirmation, and force-sensing at the needle tip. Step 5: Once punctured, the catheter sheath is inserted into the vessel while the guide needle is retracted back simultaneously. Once complete, the device is removed while the catheter sheath remains in place.
2.2 Ultrasound Imaging.
The device contains an attached ultrasound imaging probe to image and track suitable vessels for cannulation in the patient's upper forearm. An Interson SP-L01 2D ultrasound probe, operated at a 15 MHz frequency, is used to acquire 2D B-mode images of peripheral blood vessels. This probe is lightweight, USB powered, and does not require an external controller to function, making it ideal for handheld use. A custom ultrasound gel clip, seen in Fig. 2, is attached to the probe head and contains a hydrogel material, which provides acoustic coupling between the ultrasound transducer and the patient's forearm.
The probe is configured in a short-axis orientation, which provides a cross-sectional view of the target vessel to appear as an ellipsoid. From the 2D ultrasound image stream, the device employs a deep learning segmentation algorithm to identify any vessel structures located in the image in real-time. This is described further in Sec. 3.1. If multiple vessels are found in the image, the algorithm will choose a single vessel based on a weighted sum of criteria, including: 1) the prediction confidence and 2) the cross-sectional area. However, the operator still gives final say on confirming the vessel identified by the software. This weighted sum algorithm is defined in Eq. (1).
| (1) |
= The alternative option (imaged vessel).
= The selected vessel based on highest performance.
= Relative weight of importance of the criterion .
= Performance value of alternative when compared to criterion . Performance values are normalized based on all possible values i for each criteria j.
After selection, a best-fit ellipsoid algorithm is applied to the chosen vessel segmentation, providing the vessel center coordinate and its surrounding walls. This vessel coordinate position is determined relative to the transducer head; this is the top pixel layer in the ultrasound image. These vessel center coordinates are then used to determine the motor kinematics to assure the needle tip reaches the target vessel center.
2.3 Force Sensor.
A force sensor, fixed within the needle manipulator, is used to record forces along the needle axis during insertion (seen more clearly in Fig. 4). Experienced phlebotomists note that they can haptically “feel” when the needle has punctured the initial vessel wall due to the sudden drop-in force after the initial puncture. By incorporating a force sensor into the device, we aim to integrate this method of vessel puncture detection. Determining the moment of puncture via force sensing is especially useful to ensure the needle insertion is halted just after the puncture is detected, preventing dangerous back-wall vessel punctures. This device incorporates a Honeywell FSS series 0-5 N force sensor, suitable for identifying minute discrepancies in force changes. The results section below looks at the effectiveness of the force sensor at determining the moment of vessel puncture.
Fig. 4.

PIVC cannulation mechanism. CAD model depicting the method for achieving PIVC cannulation. (a) Once the needle has punctured the vessel, a solenoid latch is raised, allowing the guide needle and needle clip to retract backwards (green arrow). A constant force spring combined with a rotary damper assures a slow, constant velocity of the guide needle. (b) As the guide needle is retracted backwards, the catheter sheath remains in place, allowing the guide needle to slowly retract out. Subsequently, the entire system is then inserted forward (blue arrow) at a velocity equal to the guide needle retraction (green arrow). (c) Guide needle fully retracted and at rest. Once the guide needle has been retraced out completely, the device is removed, and the catheter sheath is free to remain cannulated in the vessel.
2.4 Needle Manipulator.
The needle manipulator consists of a 3 degree-of-freedom robotic system that is attached to the ultrasound imaging unit. This manipulator is responsible for performing the necessary alignments and translations of the needle and catheter sheath to assure a successful cannulation. These 3 degrees-of-freedom include a: 1) vertical (Zm) linear translation, 2) insertion-angle (θm) rotation, and 3) needle insertion (Injm) linear translation. The degrees-of-freedom and their movements are highlighted in Fig. 2. Vertical movement (Zm) is achieved using a DC motorized linear stage. The angle-of-insertion is achieved using a Maxon 0.75 W DC motor (part #: 337880) in a worm-to-spur gear configuration. Needle insertion is accomplished using a linear motor (LM1247 Faulhaber Quickshaft). Unlike traditional rotary motors, this unique, linear-translation motor can achieve high speed and force outputs while still maintaining high placement accuracy and quiet movement, making it particularly ideal for our application. This translation is achieved by driving a multipole magnetic forcer rod via a high-powered magnetic coil fixed in a nonmetallic housing frame.
In order to perform PIVC venipunctures, an additional step of inserting the catheter sheath while retracting the guide needle out must be accomplished. To achieve this, a vessel cannulation mechanism has been integrated into the needle manipulator unit (Fig. 4). This system accomplishes vessel cannulation by lowering the angle of insertion and then simultaneously retracting the guide needle back while keeping the catheter sheath in position. This way, the catheter sheath can be translated forward into the vessel while the guide needle is slowly retracted back. To initiate the guide needle retraction, a solenoid latch is engaged, resulting in the linear force spring pulling the guide needle back to its home position (Fig. 4). To prevent the spring from quickly retracting the guide needle, a rotary damper is included to reduce retraction velocity to ∼10 mm/s. It is important that the guide needle is not retracted out too fast; otherwise, the catheter sheath will buckle during insertion without the rigid support from the guide needle. Because of this, the guide needle's retraction velocity is kept equal to the catheter sheath's velocity. Once the guide needle has been fully retracted and the catheter sheath is in place, the device can then be safely removed from the patient's arm. Video S1, available in the Supplemental Materials on the ASME Digital Collection, demonstrates the needle manipulator performing a peripheral catherization on a phantom model.
2.5 Blood Flash Sensor.
A blood-flash refers to the blood that is initially seen in the cannula once the needle punctures the vessel. Clinicians use this as a method to confirm that the needle has successfully punctured the initial vessel wall and has reached its desired target [27]. In our device, we have incorporated a blood-flash sensor that can confirm that the vessel was punctured and blood flow is seen (Fig. 2). This is achieved using an infrared emitter and reflectance sensor that detects if any blood is present in the needle chamber. This is done by measuring changes in IR reflectance (blood present) from a normalized, baseline value (no blood present). A Fairchild QRE1113 sensor is used as our IR blood flash sensor. This blood-flash sensor acts as a method of confirming for the device that the insertion was successful. Initially, we investigated whether the blood-flash sensor could also contribute to the control programs responsible for halting needle progression by quickly confirming a successful puncture. However, after investigation, we concluded that there is too large of a delay between the blood flash detection and the moment of vessel puncture for a significant halt in needle progression to be made. From our phantom catheterization studies simulating blood flow, blood flash was detected by the device within an average of 1.2 ± 0.3 s after the puncture had occurred. In theory, this value would need to lie around ∼0.5 s in order to signal the needle insertion halting in time before a backwall punctured occurred. However, future designs will explore new methods for quickly confirming blood flow or vessel puncture in the device. Regardless, the current sensor still provides a reliable means of confirming a successful vessel puncture. From a blood flash study on phantom arms, we obtained successful blood-flash confirmations from the sensor in 95% (n = 40) of cases.
3 Results and Discussion
The following section covers the experimental studies performed to validate the needle placement accuracy and repeatability, ultrasound imaging, and peripheral catheterization success rates.
3.1 Deep Learning Ultrasound Vessel Segmentation.
Proper segmentation and tracking of the target blood vessel using ultrasound imaging is a key first step. Without reliable and accurate vessel tracking, the device would not be able to manipulate the needle to the correct location. Here, we utilize a deep learning convolutional neural network (CNN) to provide segmentations of blood vessels from raw ultrasound B-mode images [29]. The deep learning model for vessel segmentation is based on the U-Net architecture [30], which provides a pixel-level semantic labeling of each ultrasound image. This model is ideal for our application, due to its relatively fast processing time (∼25 FPS) and low requirement in training data. The model labels each pixel in the image as belonging to either the vessel class (pixel value = 1) or not (pixel value = 0). Much like the U-Net model, our model is comprised of both a contracting (encoding) and expanding (decoding) path to learn unique vessel features from training data (Fig. 5(a)).
Fig. 5.

Deep learning for ultrasound segmentation. (a) U-net model architecture. Raw ultrasound images are fed into the encoder or contracting path (boxes on left, in orange) as gray scale images of size: 256,256,2. Final output is a binary image depicting pixels as belonging to the vessel class (1 = white) or not (0 = black). (b) Intersection-over-union (IoU) results for the training and validation after 10 epochs. (c) Box plot showing the vessel center error between the model's predicted coordinate (x, y) and the true center coordinate.
The contracting path, similar to an encoder, is used to capture context via a compact feature map. A symmetric expanding path plays the role of a decoder, allowing for precise feature localization. The decoder retains spatial information despite the down sampling and max pooling that is performed during the encoder stage. For encoder down sampling, our model utilizes 2-stride convolutions using a 3 × 3 kernel and corresponding transpose convolutions for up sampling in the decoder. Each convolutional layer is then preceded by batch normalization and followed by nonlinear activation using a rectified linear unit (ReLU) [30].
This U-Net model was trained from ultrasound image sets of peripheral forearm blood vessels. A total of 37 video sequences were acquired by scanning a range of upper extremity vessels from 35 healthy volunteers. From this dataset, 12,600 frames were annotated using a Matlab-based annotation software tool to serve as the ground-truth for training the U-Net segmentation model. Of the total ultrasound dataset, 70% of the data was used for model training, 20% were used for validation, and the remaining 10% were set aside for testing. The intersection-over-union (IoU) metric was used to compare the model's segmentation capabilities between the training and validation data sets. Final training and validation results both achieved an IoU of 83% after 10 epochs (Fig. 5(b)). The near-identical training and validation IoU scores seen in the graph in Fig. 5(b) demonstrate that the model is performing adequately and with little overfitting. The error between the predicted and actual vessel center coordinate (x, y) was negligible, with errors at 0.03 ± 0.5 and -0.01 ± 0.2, respectively (Fig. 5(c)). Lastly, the model was able to achieve these segmentations at a consistent rate of 25 frames per second. These results indicate that the proposed deep learning approach is capable of reliable, real-time vessel segmentation.
3.2 Force-Guided Punctured Detection.
In these experiments, we evaluated needle insertion under force feedback guidance using phantom arms. Here, the device was tasked with cannulating vessels in phantom arm models using the real-time force profile sensed along the needle axis to determine vessel puncture. Phantom arms were comprised of a 3.35 and 1.50 mm inner diameter flexible silastic tubing, to simulate 2 different-sized blood vessels, surrounded by a gel-like porcine/agar gelatin concentration to simulate the surrounding hypodermis tissue [31].
Forces along the needle axis can be indicative of a successful vessel puncture, which is characterized as a sharp rise and drop in force. Utilizing this profile, we ran phantom experiments to evaluate the efficacy of a force guided system to halt needle insertion once this peak in force was detected. For these experiments, the device was positioned and aligned with the target vessel and the needle was continuously inserted at a constant rate of 15 mm/s (Fig. 6(a)). A peak detection algorithm was utilized to identify peaks in force during the insertion. This peak detection algorithm works by identifying any peaks in the incoming data and filters it based on a certain threshold and width constant. Once a peak was detected, a signal was outputted, the needle insertion was halted (Fig. 6(b)), and the final tip position was recorded relative to the imaged vessel center (Fig. 6(c)).
Fig. 6.
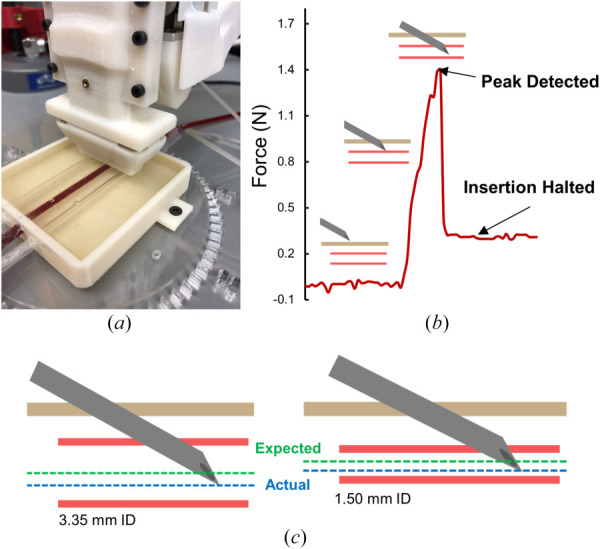
Force guided puncture detection. (a) Device positioned over the phantom arm box. (b) Example force profile during a needle insertion. (c) Final results of the force-guided puncture detection. For 3.35 mm and 1.50 mm diameter vessels, an average stopping error of 1.2 ± 0.9 mm and 0.7 ± 0.6 mms was achieved, respectively. Green line represents the expected final needle tip position, while blue line represents the average of actual final needle tip position.
A total of 20 trials on both 3.35 and 1.50 mm inner diameter vessels were conducted to assess the stopping error of the needle once a successful puncture was detected. For the 3.35 mm vessel, a successful peak in force was detected in each of the 20 trials, resulting in an average stopping error of 1.2 ± 0.9 mm (Table 1). For the 1.50 mm vessel, 16 of the 20 trials successfully detected a peak in force, resulting in an average stopping error of 0.7 ± 0.6 mm (Table 1). The four trials that failed were due to the algorithm not detecting any peak during the insertion. This was because the force profile did not peak, but instead plateaued at a max force. This resulted in a posterior vessel wall puncture, as the needle was not halted during insertion. Overall, these results indicate successful capabilities of the device at detecting the moment of vessel puncture utilizing force profiles alone. For smaller vessels of ∼1.50 mm diameter, typically seen in pediatric and infant populations, this force-based puncture detection method alone would not suffice. However, it should be noted that the final device utilizes not only force feedback data to determine vessel puncture, but also the extrapolated needle tip position from motor position encoders combined with ultrasound imaging information. The performance results of this entire system can be seen in the following section. To conclude, it may also be beneficial to investigate if these unique force profiles could better assist with needle tip guidance during insertion; this could provide the device with a more accurate, real-time, understanding of where the needle tip is with respect to its target position.
Table 1.
Force-guided puncture detection results. Stopping error is the difference between the final needle tip position and its target (vessel center).
| Vessel size ID (mm) | Success rate (%) | Stopping error (mm) |
|---|---|---|
| 3.35 | 100% (n = 20) | 1.2 ± 0.9 |
| 1.50 | 80% (n = 20) | 0.7 ± 0.6 |
3.3 Needle Positioning Accuracy and Repeatability.
Needle positioning experiments were conducted to demonstrate the needle placement accuracy and repeatability of the device over its given workspace. In these experiments, the device was repeatedly tasked with attempting to place the needle tip at the selected target position. The device was fixed in place over a water box used as a medium for acoustic coupling, providing cross-sectional images of the needle tip once in the imaging plane field-of-view. From the ultrasound image display, a target coordinate (Fig. 7(a), yellow arrow) was manually selected along the needle trajectory path. From there, the device would then attempt to send the needle tip to the selected target coordinate. Figure 7(a) shows an example image of the ultrasound with the original target (yellow arrow) and final needle tip position (gray). The needle tip's final position in the ultrasound image was extracted by determining the tip centroid via image processing. Coordinate positions for both the target and actual needle tip were determined relative to the ultrasound transducer head. Actual 2D coordinate positions were then compared to the desired target to determine the accuracy and standard deviation across multiple trials. From 25 consecutive trials, the device was able to reliably position the needle tip to within 0.2 +/- 0.1 mm of the desired target (Fig. 7(c)). For context, average median cubital vessel diameters for adults and infants are around 2–3 and 1 mm, respectively [28]. Given this, these results validate the capabilities of the device for accurate and reliable needle positioning for venipuncture procedures. The main source of placement error is most likely due to the minor differences that occur when loading the needle and needle clip into the device; one clip might have the needle slightly misaligned to either the left or right of the calibrated target. However, this issue can be remedied with future design enhancements that incorporate low-tolerance needle loading.
Fig. 7.

Accurate and reliable needle positioning. The device was tasked with positioning the needle tip to a target location selected from the ultrasound image display. (a) Example ultrasound image of the final needle tip position with respect to the desired target (yellow). Red line depicts the needle trajectory path through the ultrasound imaging plane. (b) Results from needle positioning trials (n = 25). All final needle tip positions (gray dots) centered around the desired target (0,0) coordinate. (c) Bar chart showing the mean and distribution of the Euclidean needle placement error from the center (0,0). This error is the average difference between all final needle positions and their desired target.
3.4 Phantom Arm Trials.
In the final set of experiments, we assessed the capability of the device in placing a catheter sheath in vessels on tissue-mimicking phantom arm models. To simulate difficult peripheral catheter placement, the device was tested on phantoms that have been constructed with varying tissue elastic moduli, vessel diameters, and vessel depths. In a previous paper, we demonstrated, via a factorial design experiment, that these three phantom arm properties were the most significant in affecting venipuncture success rates [23]. As such, these three properties (vessel diameter, depth, and tissue elasticity) were varied from low to high across a total of 16 different phantom arm models. The composition, fabrication, and mechanical properties of these phantom arms are described in further detail in Chen [31].
In this experiment (Fig. 8), the device performs the procedure on the phantom arm in entirety, including vessel tracking, needle alignment/insertion, and catheter placement. Figure 8(a) shows the device's first-attempt success rate for both vessel puncture and catheter sheath cannulation across 16 different phantom arms. A puncture is considered successful when: 1) the guide needle punctures only the initial vessel wall, and 2) blood flow into the blood-flash chamber is confirmed. A cannulation is considered successful when: 1) the catheter sheath is securely placed in the vessel, and 2) blood retrieval, via a line hookup, from the sheath is possible. Of the 16 cases, 15 venipunctures were attempted in each (240 total venipunctures). From Fig. 8(a), we see that the device performed best on phantoms with 3 mm vessel diameters, 3 mm depths, and a high surrounding tissue elasticity (100% first-attempt success, top right graph). Inversely, the device struggled when attempting catheter cannulations on deeper, smaller vessels with a low tissue elasticity (73%, bottom left graph). However, puncture success rates remained high (>90%) across all cases. Overall, the device achieved an average first-attempt success rate of 97 ± 4% for punctures and 89 ± 7% for cannulations. Considering reports indicate success rates of ∼50% for peripheral catheterizations in difficult venous access patients, the results from our study demonstrate significant improvement [11,15,16].
Fig. 8.

Device performance on tissue-mimicking phantom arms. (a) First-attempt success rates for both vessel puncture (blue) and catheter sheath cannulation (orange) performed on varying vessel sizes and tissue properties in phantom arms. Bar graphs show the average performance across 15 attempts for each of the 16 subcases (e.g., top left graph shows performance on phantom arms containing 2- and 3-mm ID vessels with a surrounding low elastic tissue modulus and a vessel depth of 3 mms). (b) First-attempt success rates on puncturing and cannulating vessels at various degrees of misalignment between the vessel and needle trajectory path. Top images show the experimental setup. Bottom graph depicts puncture and cannulation successes spanning misalignment angles of 0 to 45 deg. Percentages are the average of 10 attempts at each interval of 5 deg. Phantom arm box is composed of a high elastic (20 kPa) tissue and 3 mm diameter vessel at a 3 mm depth.
From our results, cannulation failures (in orange) typically occurred due to the catheter sheath buckling during insertion into the vessel lumen. This was caused by either: 1) the catheter sheath tip getting caught on the inner vessel wall, or 2) due to the guide needle not fully puncturing the vessel or slipping out during the lowering of the angle-of-insertion. Knowing this, future versions will attempt to compensate for this problem.
In a second experiment, we looked at the how vessel-to-needle misalignment could affect both the vessel puncture and catheter placement success rates. In practice, ideally, the needle trajectory path is parallel with the target vessel (0-degree misalignment, as seen in Fig. 8(b), left image). However, since the device is manually operated, it is possible that there will be misalignment between the target vessel and the needle trajectory. In this experiment, we tasked the device with placing a catheter sheath in the target vessel at varying angles of misalignment (Fig. 8(b)). We again observed the first-attempt success rate for both vessel puncture and cannulation. A total of 10 venipuncture attempts were made for each interval at 5 deg, ranging from 0 (no misalignment) to 45 (very high misalignment). The results (Fig. 8(b), lower graph) show that the device can reliably perform catheter placements up to ∼20 deg of misalignment. After that, successful catheter placements begin to fall to rates of ∼50% success. However, with proper device placement and operation, most misalignments between the device and target vessel will typically be less than 15 degrees in actual use. Therefore, the results shown here demonstrate the reliability of the device for performing catheter placements even during instances of high needle-to-vessel misalignment.
4 Discussion
Intravenous catherization is a common procedure that is often the first step in the process of diagnosing and treating patients. However, proper identification and catheterization of a suitable vessel can be challenging in patients with difficult venous access. In this article, we present VeniBot, a handheld robotic device capable of both identifying suitable blood vessels for cannulation and placing a peripheral intravenous catheter for blood sampling or fluid delivery. Here, we demonstrated high first-attempt success rates in a series of phantom arm studies that mimicked difficult peripheral catheter placement.
In these studies, we focused on peripheral catherization of the upper forearm, and as such, designed the phantom arm models to simulate these environments. Typical median cubital vessels range from 2-3 mm [28]. From our own ultrasound data sets of upper extremity blood vessels, we also confirmed vessel diameters ranging anywhere from 2 to 5 mm in inner diameter. Therefore, we chose synthetic vessels with an inner diameter of 2 and 3 mm. Additionally, although this device was designed and intended for peripheral catherization, its modular design could allow it to be configured for other catherization procedures, such as arterial access procedures. Certain changes and updates would need to be made to allow for a deeper insertion into arterial vessels. Furthermore, needle bending during insertion was not a significant issue for the peripheral venipunctures that we performed. However, when considering vascular access for deeper vessels, such as arteries or deep veins, compensation for needle bending may need to be addressed in the future.
The current device operates by having the user first place the device over the general insertion site area. From there, the deep learning algorithm segments any vessels found in the ultrasound image and selects one based on size and prediction confidence. However, the device has no internal confirmation if the selected vessel is suitable for cannulation; it simply compares the vessel size and segmentation accuracy to neighboring vessels. In short, the operator must still confirm the segmented vessels from the ultrasound image. In the pursuit of making a more autonomous device, future work would involve developing additional capability for intelligent vessel selection. For instance, anatomical mapping of neighboring vessels could allow the device to choose a target based on established clinical rules and best practices.
Maintaining sterility between the device and patient in between procedures is also a major concern. Currently, the two points of contact that the device and patient make are at: 1) the ultrasound acoustic gel clip to skin contact and, 2) the needle puncture. To address this issue of sterility, our custom-made gel clips are disposed of after each procedure. Furthermore, each gel clip is sterilized after assembly in an alcohol-water solution bath. As for the needle, the needle and needle clips are pre-assembled before the procedure and are only opened and exposed immediately before the procedure, similar to typical venipuncture procedures.
From the phantom study (Fig. 8), the device achieved high success rates in both puncture (97%) and sheath cannulation (89%). However, sheath cannulations in models with small vessels and low-elasticity tissue suffered the greatest failure rates (73%). This was due to the catheter sheath either buckling and/or slipping out of the vessel postpuncture. In order to address this challenge and to obtain more consistent results, future work will involve exploring alternative means of achieving peripheral catherization. An example of this would be to redesign the insertion mechanism so that it punctures the vessel at a 90-deg right angle. This angle-of-attack, coupled with accurate robotic insertion, should greatly reduce vessel rolling and still be able to halt needle progression to prevent a double vessel wall puncture. This method of peripheral catheterization would also require a special catheter sheath that could bend at high angles such that it can remain secured in the vessel lumen without kinking.
Lastly, the device is powered and controlled via a wire connection to a nearby PC, requiring management of wire placement during operation. In the pursuit of making a device suitable for clinical use, future work would involve adapting the device to a battery powered, Bluetooth enabled version that contains embedded, on-board processing. Additionally, steady hand placement of the device during the procedure is necessary to achieve consistent results. Compensation for hand motion could be incorporated via an additional degree-of-freedom that would allow the needle trajectory to adjust in a side-to-side motion to compensate for small movements caused by either the operator or patient. As of now, this “needle-to-vessel” alignment mechanism has been designed and constructed in our lab, but it has yet to be implemented in the current version of the device.
5 Conclusion
Here, we have presented the design and evaluation of VeniBot, a handheld robotic device for peripheral catherization. This technology has the potential to reduce complications for patients and improve clinical workflow by providing a safe, fast, and reliable means of obtaining venous access.
Supplementary Material
Supplementary Video 1
Acknowledgment
The authors acknowledge and thank all the previous contributors from the Biomedical Engineering Department at Rutgers University that have worked on past iterations of the device, including: Nicholas Demaio, Hill Chang, Michael Masino, and Brandon Jones. The authors also thank the NIH for providing financial support (Nos. R01EB020036 and F31HL149219) to this project, as well as the Rutgers TechAdvance (Grant No. TA2019-0318).
Funding Data
Biomedical Engineering Department at Rutgers University (No. 642662; Funder ID: 10.13039/100011132).
NIH for providing financial support (Nos. R01EB020036 and F31HL149219; Funder ID: 10.13039/100000002).
Rutgers TechAdvance (Grant No. TA2019-0318; Funder ID: 10.13039/100011132).
References
- [1]. Shademan, A. , Decker, R. S. , Opfermann, J. D. , Leonard, S. , Krieger, A. , and Kim, P. C. W. , 2016, “ Supervised Autonomous Robotic Soft Tissue Surgery,” Sci. Transl. Med., 8(337), p. 337ra64. 10.1126/scitranslmed.aad9398 [DOI] [PubMed] [Google Scholar]
- [2]. Moustris, G. P. , Hiridis, S. C. , Deliparaschos, K. M. , and Konstantinidis, K. M. , 2011, “ Evolution of Autonomous and Semi-Autonomous Robotic Surgical Systems: A Review of the Literature,” Int. J. Med. Robot. Comput. Assist. Surg., 7(4), pp. 375–392. 10.1002/rcs.408 [DOI] [PubMed] [Google Scholar]
- [3]. Edwards, T. L. , Xue, K. , Meenink, H. C. M. , Beelen, M. J. , Naus, G. J. L. , Simunovic, M. P. , Latasiewicz, M. , Farmery, A. D. , de Smet, M. D. , and MacLaren, R. E. , 2018, “ First-in-Human Study of the Safety and Viability of Intraocular Robotic Surgery,” Nat. Biomed. Eng., 2(9), pp. 649–656. 10.1038/s41551-018-0248-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Fagogenis, G. , Mencattelli, M. , Machaidze, Z. , Rosa, B. , Price, K. , Wu, F. , Weixler, V. , Saeed, M. , Mayer, J. E. , and Dupont, P. E. , 2019, “ Autonomous Robotic Intracardiac Catheter Navigation Using Haptic Vision,” Sci. Robot., 4(29), p. eaaw1977. 10.1126/scirobotics.aaw1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Daudelin, J. , Jing, G. , Tosun, T. , Yim, M. , Kress-Gazit, H. , and Campbell, M. , 2018, “ An Integrated System for Perception-Driven Autonomy With Modular Robots,” Sci. Robot., 3(23), p. 31. 10.1126/scirobotics.aat4983 [DOI] [PubMed] [Google Scholar]
- [6]. Alexandrou, E. , Ray-Barruel, G. , Carr, P. J. , Frost, S. , Inwood, S. , Higgins, N. , Lin, F. , Alberto, L. , Mermel, L. , and Rickard, C. M. , 2015, “ International Prevalence of the Use of Peripheral Intravenous Catheters,” J. Hosp. Med., 10(8), pp. 530–533. 10.1002/jhm.2389 [DOI] [PubMed] [Google Scholar]
- [7].National Center for Health Statistics, National Hospital Ambulatory Medical Care Survey: 2016. Emergency Department Summary Tables, Report. [PubMed]
- [8]. Sampalis, J. S. , Lavoie, A. , Williams, J. I. , Mulder, D. S. , and Kalina, M. , 1993, “ Impact of on-Site Care, Prehospital Time, and Level of in-Hospital Care on Survival in Severely Injured Patients,” J. Trauma - Inj. Infect. Crit. Care, 34(2), p. 252. 10.1097/00005373-199302000-00014 [DOI] [PubMed] [Google Scholar]
- [9]. Parker, S. I. A. , Benzies, K. M. , and Hayden, K. A. , 2017, “ A Systematic Review: Effectiveness of Pediatric Peripheral Intravenous Catheterization Strategies,” J. Adv. Nurs,., 73(7), pp. 1570–1582. 10.1111/jan.13211 [DOI] [PubMed] [Google Scholar]
- [10]. Armenteros-Yeguas, V. , Gárate-Echenique, L. , Tomás-López, M. A. , Cristóbal-Domínguez, E. , Moreno-de Gusmão, B. , Miranda-Serrano, E. , and Moraza-Dulanto, M. I. , 2017, “ Prevalence of Difficult Venous Access and Associated Risk Factors in Highly Complex Hospitalised Patients,” J. Clin. Nurs., 26(23–24), pp. 4267–4275. 10.1111/jocn.13750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Rauch, D. , Dowd, D. , Eldridge, D. , MacE, S. , Schears, G. , and Yen, K. , 2009, “ Peripheral Difficult Venous Access in Children,” Clin. Pediatr. (Phila)., 48(9), pp. 895–901. 10.1177/0009922809335737 [DOI] [PubMed] [Google Scholar]
- [12]. Walsh, G. , 2008, “ Difficult Peripheral Venous Access: Recognizing and Managing the Patient at Risk,” J. Assoc. Vasc. Access, 13(4), pp. 198–203. 10.2309/java.13-4-7 [DOI] [Google Scholar]
- [13]. Kuensting, L. L. , DeBoer, S. , Holleran, R. , Shultz, B. L. , Steinmann, R. A. , and Venella, J. , 2009, “ Difficult Venous Access in Children: Taking Control,” J. Emerg. Nurs., 35(5), pp. 419–424. 10.1016/j.jen.2009.01.014 [DOI] [PubMed] [Google Scholar]
- [14]. Carr, P. J. , Rippey, J. C. R. , Cooke, M. L. , Trevenen, M. L. , Higgins, N. S. , Foale, A. S. , and Rickard, C. M. , 2019, “ Factors Associated With Peripheral Intravenous Cannulation First-Time Insertion Success in the Emergency Department. A Multicentre Prospective Cohort Analysis of Patient, Clinician and Product Characteristics,” BMJ Open, 9(4), p. e022278. 10.1136/bmjopen-2018-022278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Carr, P. J. , Rippey, J. C. R. , Budgeon, C. A. , Cooke, M. L. , Higgins, N. , and Rickard, C. M. , 2016, “ Insertion of Peripheral Intravenous Cannulae in the Emergency Department: Factors Associated With First-Time Insertion Success,” J. Vasc. Access., 17(2), pp. 182–190. 10.5301/jva.5000487 [DOI] [PubMed] [Google Scholar]
- [16]. Lininger, R. A. , 2003, “ Pediatric Peripheral i.v. Insertion Success Rates,” Pediatr. Nurs., 29(5), pp. 351–354.https://pubmed.ncbi.nlm.nih.gov/14651305/ [PubMed] [Google Scholar]
- [17]. Witting, M. D. , 2012, “ IV Access Difficulty: Incidence and Delays in an Urban Emergency Department,” J. Emerg. Med., 42(4), pp. 483–487. 10.1016/j.jemermed.2011.07.030 [DOI] [PubMed] [Google Scholar]
- [18]. Miliani, K. , Taravella, R. , Thillard, D. , Chauvin, V. , Martin, E. , Edouard, S. , and Astagneau, P. , on behalf of the CATHEVAL Study Group 2017, “ Peripheral Venous Catheter-Related Adverse Events: Evaluation From a Multicentre Epidemiological Study in France (the CATHEVAL Project),” PLoS One, 12(1), p. e0168637. 10.1371/journal.pone.0168637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Lefrant, J.-Y. , Muller, L. , De La Coussaye, J.-E. , Prudhomme, M. , Ripart, J. , Gouzes, C. , Peray, P. , Saissi, G. , and Eledjam, J.-J. , 2002, “ Risk Factors of Failure and Immediate Complication of Subclavian Vein Catheterization in Critically Ill Patients,” Intensive Care Med., 28(8), pp. 1036–1041. 10.1007/s00134-002-1364-9 [DOI] [PubMed] [Google Scholar]
- [20]. Eddins, J. , Horattas, C. , Trupiano, J. , Hopkins, S. , Pasini, D. , Martino, C. , and Murty, A. , 2002, “ Changing Concepts in Long-Term Central Venous Access: Catheter Selection and Cost Savings,” J. Vasc. Access Devices, 7(1), pp. 47–48. 10.1016/S1083-0081(02)70563-3 [DOI] [PubMed] [Google Scholar]
- [21]. Sou, V. , McManus, C. , Mifflin, N. , Frost, S. A. , Ale, J. , and Alexandrou, E. , 2017, “ A Clinical Pathway for the Management of Difficult Venous Access,” BMC Nurs., 16(1), p. 64. 10.1186/s12912-017-0261-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Balter, M. L. , Leipheimer, J. M. , Chen, A. I. , Shrirao, A. , Maguire, T. J. , and Yarmush, M. L. , 2018, “ Automated End-to-End Blood Testing at the Point-of-Care: Integration of Robotic Phlebotomy With Downstream Sample Processing,” Technology, 06(02), pp. 59–66. 10.1142/S2339547818500048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Chen, A. I. , Balter, M. L. , Maguire, T. J. , and Yarmush, M. L. , 2020, “ Deep Learning Robotic Guidance for Autonomous Vascular Access,” Nat. Mach. Intell., 2(2), pp. 104–115. 10.1038/s42256-020-0148-7 [DOI] [Google Scholar]
- [24]. Balter, M. L. , Chen, A. I. , Fromholtz, A. , Gorshkov, A. , Maguire, T. J. , and Yarmush, M. L. , 2016, “ System Design and Development of a Robotic Device for Automated Venipuncture and Diagnostic Blood Cell Analysis,” IEEE International Conference on Intelligent Robots and Systems , Daejeon, Korea, Oct. 9–14, Institute of Electrical and Electronics Engineers Inc., pp. 514–520. 10.1109/IROS.2016.7759102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Balter, M. L. , Chen, A. I. , Maguire, T. J. , and Yarmush, M. L. , 2017, “ Adaptive Kinematic Control of a Robotic Venipuncture Device Based on Stereo Vision, Ultrasound, and Force Guidance,” IEEE Trans. Ind. Electron., 64(2), pp. 1626–1635. 10.1109/TIE.2016.2557306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Leipheimer, J. M. , Balter, M. L. , Chen, A. I. , Pantin, E. J. , Davidovich, A. E. , Labazzo, K. S. , and Yarmush, M. L. , 2020, “ First-in-Human Evaluation of a Hand-Held Automated Venipuncture Device for Rapid Venous Blood Draws,” Technology, pp. 1–10. 10.1142/S2339547819500067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Dhingra, N. , Diepart, M. , Dziekan, G. , Khamassi, S. , Otaiza, F. , and Wilburn, S. , 2010, WHO Guidelines on Drawing Blood: Best Practices in Phlebotomy, World Health Organization, Geneva, Switzerland, pp. 1–105. [PubMed] [Google Scholar]
- [28]. Mukai, K. , Nakajima, Y. , Nakano, T. , Okuhira, M. , Kasashima, A. , Hayashi, R. , Yamashita, M. , Urai, T. , and Nakatani, T. , 2020, “ Safety of Venipuncture Sites at the Cubital Fossa as Assessed by Ultrasonography,” J. Patient Saf., 16(1), pp. 98–105. 10.1097/PTS.0000000000000441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Smistad, E. , and Løvstakken, L. , 2016, “ Vessel Detection in Ultrasound Images Using Deep Convolutional Neural Networks,” Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics, Springer Verlag, Berlin, pp. 30–38. [Google Scholar]
- [30].MICCAI, 2015, Lecture Notes in Computer Science, Vol. 9351, Springer, Cham, Berlin. [Google Scholar]
- [31]. Chen, A. I. , Balter, M. L. , Chen, M. I. , Gross, D. , Alam, S. K. , Maguire, T. J. , and Yarmush, M. L. , 2016, “ Multilayered Tissue Mimicking Skin and Vessel Phantoms With Tunable Mechanical, Optical, and Acoustic Properties,” Med. Phys., 43(6; Part 1), pp. 3117–3131. 10.1118/1.4951729 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Video 1


