The spectrum of fungal diseases that affect humans is broad, ranging from asymptomatic superficial mycoses to deep systemic diseases due to opportunistic or primary fungal pathogens. 1 Recently, the COronaVIrus Disease 2019 (COVID-19) pandemic has highlighted mucormycosis as an important opportunistic fungal disease, especially in patients with uncontrolled diabetes mellitus and prolonged, high-dose corticosteroid use. 2
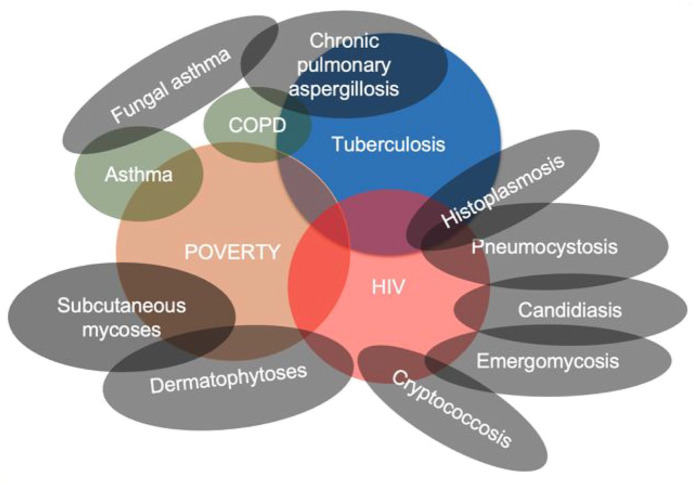
Fungal diseases substantially contribute to the burden of diseases in Africa, mainly driven by heavy affliction of poverty, tuberculosis (TB) and human immunodeficiency virus (HIV) (Figure 1).3,4 Recent estimates of the burden of key fungal diseases are summarized in Table 1.
Figure 1.
Major drivers of fungal diseases in Africa.
COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus.
Table 1.
Previous estimates of the burden of fungal diseases in Africa.
| Fungal disease | Burden | Author/reference |
|---|---|---|
| PCP, HIV-associated | The pooled prevalence of 15.4%; highest among inpatients, 22.4% | Wasserman et al. 5 |
| Oesophageal candidiasis, HIV-associated | Pooled prevalence of 12%; 34.1% pre-ART and 8.7% in ART era | Olum et al. 6 |
| Tinea capitis in African children | Pooled prevalence of 23%; about 138 million cases annually | Bongomin et al. 7 |
| Cryptococcal meningitis, HIV-associated | In a modelling study, of 223,100 incident cases of cryptococcal meningitis globally in 2014, 73% (162,500 cases) occurred in sub-Saharan Africa; of 181,100 global death cases due to cryptococcal meningitis, 135,900 (75%) occurred in sub-Saharan Africa | Rajasingham et al. 8 |
| Fungal asthma | The prevalence of fungal sensitization was ~3–52% (pool prevalence of 28%) and ~23.3%, due to Aspergillus species; prevalence of allergic bronchopulmonary aspergillosis of 1.6–21.2% | Kwizera et al. 9 |
| CPA | 1247 cases of CPA reported between 1976 and 2021, 61.3% had a history of active/treated TB, 2.3% had HIV and 1.5% had diabetes mellitus | Olum et al. 10 |
| Histoplasmosis | 470 cases of histoplasmosis between 1952 and 2017; 38% of the cases had HIV infection | Oladele et al. 11 |
ART, anti-retroviral therapy, CPA, chronic pulmonary aspergillosis; HIV, human immunodeficiency virus; PCP, pneumocystis pneumonia; TB, tuberculosis.
The overarching aim of the special collection was to provide a state-of-the-art overview of our current understanding of various aspects of fungal diseases in Africa. It was overwhelmingly successful with a total of 14 high-quality submissions summarized below.
In this issue, two articles further highlighted the burden of histoplasmosis in Africa. Kuate et al. 12 bring new insights into the burden of triple co-infection with histoplasmosis, TB and HIV in sub-Saharan Africa (SSA). Pulmonary histoplasmosis is often misdiagnosed as smear negative pulmonary TB due to similarities in their clinical and radiological presentations. 11 On the contrary, HIV, which is highly prevalent in SSA, is the most important risk factor for both disseminated histoplasmosis and TB. Ekeng et al. 13 summarized 44 cases of histoplasmosis among African children. Despite close to 55% of the cases being disseminated histoplasmosis, HIV was only reported in 6.8% of these children and most of the cases were due to Histoplasma capsulatum var. duboisii, the aetiologic agent of African histoplasmosis. These two articles therefore shine a light on the need for awareness among clinicians and the need to enhance laboratory diagnostic capacity for invasive fungal diseases in SSA and a need for a unified algorithm for pulmonary infections with similar presentation.
In line with the above, Osaigbovo and Bongomin 14 discussed the available point-of-care tests (POCTs) for invasive fungal infections (IFIs) which are designed to detect their respective fungal antigens or antibodies and barriers to their uptake, including cost, lack of evidence to back up policy recommendations and lack of awareness among health care providers. The authors suggested a blueprint strategy to increase availability and accessibility in SSA, including increasing awareness about IFIs and corresponding POCTs, research, integrating the diagnosis of IFIs into existing vertical disease programmes, country adoption of the World Health Organization’s Essential Diagnostics List, advocacy and improving POC diagnostics and supply chains.
Bongomin and Otu 15 showed that a deterioration in the symptoms component of the St. George’s Respiratory Questionnaire (SGRQ) and worsening of patients’ self-assessment domain may be associated with clinical recurrence of chronic pulmonary aspergillosis (CPA). However, they recommended diagnosis of recurrent CPA using a combination of clinical history, SGRQ scores, chest imaging and a workup to exclude other causes of the patients’ symptoms. Also regarding CPA, Oladele et al. 16 highlighted the need to standardize Aspergillus-specific IgG diagnostic cut-off values to enhance diagnosis of CPA among Nigerians. The authors reported a lower optimal diagnostic cut-off value (0.821) than the manufacturer’s recommended cut-off value (1.0) for the Bordier Aspergillus IgG antibody test, highlighting the ethnic differences in antibody response in CPA.
In Uganda, Kwizera et al. 17 found a high prevalence (60%) of Aspergillus fumigatus skin positivity in apparently healthy non-atopic individuals in Uganda with skin positivity being more in younger individuals. They proposed a revised definition of a suitable cut-off wheal size in healthy adults and not to use skin prick testing (SPT) alone to diagnose A. fumigatus sensitivity. Again in Uganda, Njovu et al. 18 established that approximately 71% of patients with clinical signs of pulmonary TB were positive for pulmonary fungal pathogens (PFPs) and about 4% had a co-infection of PFPs and Mycobacterium tuberculosis. PFPs and M. tuberculosis were isolated in people with HIV. The findings re-emphasize the need to have routine mycoses diagnostic tests in patients with suspected TB infection.
In Nigeria, Campbell et al. 19 found that A. fumigatus constituted only 4.3% of the 117 isolates of Aspergillus species in the 168 soil samples tested contrary to other studies done in Nigeria and in other parts of the world. Importantly, all the isolates did not exhibit triazole resistance. In Tanzania, Mushi et al. 20 identified Candida albicans as the most predominant Candida species isolated from 325 oral swabs from HIV-uninfected children aged between 2 and 15 months with low resistance to fluconazole. However, some C. albicans isolates were resistant to fluconazole, voriconazole and posaconazole; hence, continuous monitoring of susceptibility is required for effective management of oral candidiasis in children. In Senegal, Deh et al. 21 foregrounded the possibility of a highly inflammatory tinea capitis due to Microsporum audouinii as they described a case of a nine-year-old HIV negative schoolgirl who was diagnosed with a severe form of kerion celsi. The infection completely regressed after 2 months following evacuation of 50 ml of pus and treatment with oral terbinafine 125 mg per day and ketoconazole-based shampoo.
As part of the ongoing effort of the Global Action for Fungal Infections (GAFFI) to estimate the burden of serious fungal infections in each country, we received two submissions. Lakoh et al. 22 found that serious fungal infections affect a total of 4.92% of the population in Sierra Leone, and the annual burden of fungal infections among people with HIV was 2.9%. This was attributable to the late-stage presentation of people with HIV to care and high burden of advanced HIV disease in the country. Second, Huseynov et al. 23 estimated that fungal diseases affect an estimated 2.3% of the population in Azerbaijan. The need to improve diagnostic capabilities for IFIs of Azerbaijan was highlighted. In the same vein, Diongue et al. 24 from Senegal pointed out that despite the low frequency of IFIs for which more than half is cryptococcosis, the risk factors for IFIs are prevalent across Senegal. However, it was reported that fluconazole is the only systemic antifungal available in the country, yet it has no activity on many fungi such as Aspergillus spp. responsible for IFIs in Senegal.
Finally, Otu et al. 25 spotlight the importance and reliability of digital platforms and the case-based learning approach in fostering clinical reasoning skills and cascading knowledge to health professionals on clinical mycology based on their experience with online clinical mycology case competition amid the COVID-19 social distance restrictions hosted by the Medical Mycology Society of Nigeria.
In conclusion, these articles provide new insights into the burden and challenges with diagnosis and treatment of fungal diseases in Africa. There is a need to put emphasis on fungal diseases and channelling of more resources towards their prevention, diagnosis and management to improve outcomes.
Footnotes
Author contributions: Felix Bongomin: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Winnie Kibone: Writing – original draft; Writing – review & editing.
Jerom Okot: Writing – original draft; Writing – review & editing.
Lauryn Nsenga: Writing – original draft; Writing – review & editing.
Ronald Olum: Writing – original draft; Writing – review & editing.
Joseph Baruch Baluku: Writing – original draft; Writing – review & editing.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Felix Bongomin  https://orcid.org/0000-0003-4515-8517
https://orcid.org/0000-0003-4515-8517
Ronald Olum  https://orcid.org/0000-0003-1289-0111
https://orcid.org/0000-0003-1289-0111
Joseph Baruch Baluku  https://orcid.org/0000-0002-5852-9674
https://orcid.org/0000-0002-5852-9674
Contributor Information
Felix Bongomin, Department of Medical Microbiology & Immunology, Faculty of Medicine, Gulu University, P.O. Box 166, Gulu, Uganda; Non-communicable and Infectious Diseases Research (NIDER) Platform, Kampala, Uganda.
Winnie Kibone, School of Medicine, Makerere University, Kampala, Uganda.
Jerom Okot, Department of Medical Microbiology & Immunology, Faculty of Medicine, Gulu University, Gulu, Uganda.
Lauryn Nsenga, School of Medicine, Kabale University, Kabale, Uganda.
Ronald Olum, Non-communicable and Infectious Diseases Research (NIDER) Platform, Kampala, UgandaSchool of Medicine, Makerere University, Kampala, Uganda.
Joseph Baruch Baluku, Non-communicable and Infectious Diseases Research (NIDER) Platform, Kampala, Uganda; Division of Pulmonology, Kiruddu National Referral Hospital, Kampala, Uganda; Makerere University Lung Institute, Kampala, Uganda.
References
- 1. Richardson MD, Warnock DW. Fungal infection: Diagnosis and management. 4th ed. Oxford: Blackwell, 2012, p. 28. [Google Scholar]
- 2. Moorthy A, Gaikwad R, Krishna S, et al. SARS-CoV-2, uncontrolled diabetes and corticosteroids – an unholy trinity in invasive fungal infections of the maxillofacial region? A retrospective, multi-centric analysis. J Maxillofac Oral Surg 2021; 20: 418–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Driemeyer C, Falci DR, Oladele RO, et al. The current state of clinical mycology in Africa: a European Confederation of Medical Mycology and International Society for Human and Animal Mycology survey. The Lancet Microbe. Epub ahead of print 18 January 2022. DOI: 10.1016/S2666-5247(21)00190-7. [DOI] [PubMed] [Google Scholar]
- 4. Bongomin F, Adetona Fayemiwo S. Epidemiology of fungal diseases in Africa: a review of diagnostic drivers. Curr Med Mycol 2021; 7: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wasserman S, Engel ME, Griesel R, et al. Burden of pneumocystis pneumonia in HIV-infected adults in sub-Saharan Africa: a systematic review and meta-analysis. BMC Infect Dis 2016; 16: 482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Olum R, Baluku JB, Okidi R, et al. Prevalence of HIV-associated esophageal candidiasis in sub-Saharan Africa: a systematic review and meta-analysis. Trop Med Health 2020; 48: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bongomin F, Olum R, Nsenga L, et al. Estimation of the burden of tinea capitis among children in Africa. Mycoses 2021; 64: 349–363. [DOI] [PubMed] [Google Scholar]
- 8. Rajasingham R, Smith RM, Park BJ, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 2017; 17: 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kwizera R, Musaazi J, Meya DB, et al. Burden of fungal asthma in Africa: a systematic review and meta-analysis. PLoS ONE 2019; 14: e0216568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Olum R, Osaigbovo II, Baluku JB, et al. Mapping of chronic pulmonary aspergillosis in Africa. J Fungi 2021; 7: 790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oladele RO, Ayanlowo OO, Richardson MD, et al. Histoplasmosis in Africa: an emerging or a neglected disease? PLoS Negl Trop Dis 2018; 12: e0006046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuate MPN, Ekeng BE, Kwizera R, et al. Histoplasmosis overlapping with HIV and tuberculosis in sub-Saharan Africa: challenges and research priorities. Ther Adv Infect Dis 2021; 8: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ekeng BE, Edem K, Akintan P, et al. Histoplasmosis in African children: clinical features, diagnosis and treatment. Ther Adv Infect Dis 2022; 9: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Osaigbovo II, Bongomin F. Point of care tests for invasive fungal infections: a blueprint for increasing availability in Africa. Ther Adv Infect Dis 2021; 8: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bongomin F, Otu A. Utility of St. George’s Respiratory Questionnaire in predicting clinical recurrence in chronic pulmonary aspergillosis. Ther Adv Infect Dis 2021; 8: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oladele RO, Otu AA, Balogun OJ, et al. Standardization of Aspergillus IgG diagnostic cutoff in Nigerians. Ther Adv Infect Dis 2021; 8: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kwizera R, Bongomin F, Olum R, et al. Prevalence of Aspergillus fumigatus skin positivity in adults without an apparent/known atopic disease in Uganda. Ther Adv Infect Dis 2021; 8: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Njovu IK, Musinguzi B, Mwesigye J, et al. Status of pulmonary fungal pathogens among individuals with clinical features of pulmonary tuberculosis at Mbarara University Teaching Hospital in Southwestern Uganda. Ther Adv Infect Dis 2021; 8: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Campbell CA, Osaigbovo II, Oladele RO. Triazole susceptibility of Aspergillus species: environmental survey in Lagos, Nigeria and review of the rest of Africa. Ther Adv Infect Dis 2021; 8: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mushi MF, Loi N, Mshana SE. Oral candidiasis in HIV-uninfected pediatric population in areas with limited fungal diagnosis: a case study from a tertiary hospital, Tanzania. Ther Adv Infect Dis 2021; 8: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Deh A, Diongue K, Diadie S, et al. Kerion celsi due to Microsporum audouinii: a severe form in an immunocompetent girl. Ther Adv Infect Dis 2021; 8: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lakoh S, Orefuwa E, Kamara MN, et al. The burden of serious fungal infections in Sierra Leone: a national estimate. Ther Adv Infect Dis 2021; 8: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huseynov RM, Javadov SS, Osmanov A, et al. The burden of serious fungal infections in Azerbaijan. Ther Adv Infect Dis 2021; 8: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Diongue K, Diallo MA, Seck MC, et al. The evidence for unavailability of systemic antifungals in Senegal. Ther Adv Infect Dis 2021; 8: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Otu A, Oladele RO, Orefuwa E. Closing the knowledge gap in mycology in Nigeria by leveraging e-learning: perspectives from the field. Ther Adv Infect Dis 2021; 8: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]