Abstract
Diabetic kidney disease (DKD) is a leading cause of morbidity and mortality among people living with diabetes, and is one of the most important causes of end stage renal disease worldwide. In order to reduce progression of DKD, important management goals include treatment of hypertension, glycaemia and control of cardiovascular risk factors such as lipids, diet, smoking and exercise. Use of angiotensin converting enzyme inhibitors or angiotensin receptor blockers has an established role in prevention of progression of DKD. A number of other agents such as endothelin-1 receptor antagonists and bardoxolone have had disappointing results. Recent studies have, however, suggested that newer antidiabetic agents such as sodium-glucose transporter-2 inhibitors (SGLT-2i) and glucagon-like peptide-1 analogues have specific beneficial effects in patients with DKD. Indeed most recent guidance suggest that SGLT-2i drugs should be used early in DKD, irrespective of glucose control. A number of pathways are hypothesised for the development and progression of DKD, and have opened up a number of newer potential therapeutic targets. This article aims to discuss management of DKD with respect to seminal trials from the past, more recent trials informing the present and potential new therapeutic options that may be available in the future.
Keywords: chronic kidney disease, sodium glucose transporter-2 inhibitors, type 2 diabetes
Introduction
In 2015, 415 million people worldwide were estimated to have diabetes mellitus; by 2040 is it suggested that this will rise to 642 million. 1 A commensurate rise in renal disease associated with diabetes has been seen. Around 3% of people with newly diagnosed diabetes have evidence of diabetic kidney disease (DKD). The condition is the single most common cause of end stage renal disease (ESRD) in developed countries, accounting for 25–45% of all patients entering dialysis programmes. 2 Between 1990 and 2012, the number of deaths attributed to DKD rose by 94%. 3
DKD is a major cause of mortality and morbidity in people with diabetes. The condition is characterised by the presence of progressive renal impairment and the detection of albuminuria. In addition to renal morbidity, people who develop DKD suffer an increased cardiovascular mortality and morbidity, and well as increased risk of other microvascular complications. 4
In this review, we outline the pharmacotherapy of DKD especially in Type 2 diabetes (T2D), focussing on well-established evidence based therapies, newly identified agents and possible future interventions.
Risk factors, prevention, and management of DKD
Definition of DKD and screening
DKD is a clinical diagnosis made based on the presence of albuminuria and/or reduced eGFR, in a person with an established diagnosis of Type 1 diabetes (T1D) or T2D. 5 Table 1 outlines the various methods of measuring albuminuria and their normal ranges using the nomenclature recommended by the latest Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guidelines. These refer to normal to mildly increased, moderately increased and severely increased albuminuria to replace normo, micro- and macroalbuminuria, respectively. 6 In clinical practice recommendations by American Diabetes Association (ADA), normal albumin creatinine ratio (ACR) is defined as < 30 mg/g, and high urinary albumin excretion as ⩾ 30 mg/g. 7
Table 1.
Various urine albumin collections for defining albuminuria.
| New terminology for albuminuria as per KDIGO guidelines | Previous terminology | Urine ACR (mg/mmol) | Urine ACR (mg/g) | AER (mg/24 h) |
|---|---|---|---|---|
| Normal to mildly increased | Normal | <3 | <30 | <30 |
| Moderately increased a | Microalbuminuria | 3–30 | 30–300 | 30–300 |
| Severely increased b | Macroalbuminuria | >30 | >300 | >300 |
ACR, albumin creatinine ratio; AER, Albumin excretion rate; KDIGO, Kidney Disease: Improving Global Outcomes.
Relative to young-adult level.
Including nephrotic syndrome (AER usually > 2200 mg/24 h (ACR > 2200 mg/g; > 220 mg/mmol)).
The most convenient screening method appears to be a single (preferably early morning) urine sent for ACR, and an estimated glomerular filtration rate (eGFR), taken at least annually. If urinary ACR is abnormal, it should be repeated to confirm. Arbitrary cut offs for albuminuria are described, although it is clear that the value is a continuous variable, and may have significant day-to-day differences in an individual person. 8 The presence of mildly elevated ACR, however, does appear to be an early precursor of progression to severely increased albuminuria in many patients, although some may regress to normal albumin excretion due to therapeutic intervention. 9
A broader term of chronic kidney disease (CKD) in people with diabetes is used to describe people with diabetes who have a reduced eGFR below 60 ml/min/1.73 m2, irrespective of cause. If albuminuria is not present is such patients, then DKD may be less likely, and alternative diagnoses should be considered. 10 CKD in the absence of albuminuria in an elderly person with diabetes is less likely to progress to ESRD. 11 Other reasons to consider non-diabetic renal disease in people with diabetes and CKD are the absence of retinopathy, rapid deterioration in renal function, active urinary sediment, nephrotic range albuminuria (ACR > 220 mg/mmol), or symptoms/signs of other systemic disease.
Risk factors for the development of DKD
Risk factors for DKD are outlined in Table 2. Risk factors amenable to intervention include blood pressure, dyslipidaemia, smoking, and hyperglycaemia.
Table 2.
Risk factors for the development of diabetic kidney disease.
| Modifiable | Hypertension Hyperglycaemia Dyslipidaemia Smoking |
| Non-modifiable | Age Ethnicity Male Sex Genetic factors – ACE, APOC1, APOE, HSPG2, eNOS, VEGF, TGFβ1, PPARγ among others |
Hyperglycaemia is an important risk factor for the development of DKD and occurs as a result of insulin resistance and/or decreased insulin production. Hyperglycaemia results in complex interplay of downstream signals and increase in reactive oxygen species (ROS) either directly by activation of ROS processes and impairment of antioxidant function, or indirectly via activation of aldose reductase and production of advanced glycation end products. Hyperglycaemia is also associated with insulin resistance, production of protein kinase C and transforming growth factor β which results in DKD. 12
Over 22 years of follow-up of the Diabetes Control and Complications Trial (DCCT) cohort, which compared intensive versus standard glucose control in people with T1D, showed that intensive control can attenuate risk for the development of DKD very significantly. 13 An approximately 50% risk reduction in development of CKD stage 3 (eGFR < 60 mls/min/1.73 m2) was seen. In patients with T2D, however, the evidence is not so clear cut. Ten year follow-up of the United Kingdom Prospective Diabetes Study (UKPDS) study cohort showed that, while risk for the development of microalbuminuria was reduced by 33% in the intensively treated cohort, there was no significant difference in the rate of development of CKD or ESRD. 14
The Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial in 2008 studied the effects of intensive glucose control on vascular outcome and concluded that intensive control reduced the incidence of combined macrovascular and microvascular events, primarily due to reduction in incidence of nephropathy. 15 By contrast, the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study published at the same time noted that use of intensive glucose lowering therapy as compared to standard therapy was associated with increased mortality and did not significantly reduce major cardiovascular events. 16 There appears to be a concordance between the prevalence of retinopathy and renal dysfunction in patients with diabetes; in a study of 232 patients, retinopathy progression appeared to be associated with renal lesions and the development of end stage kidney disease ESRD. 17 In patients with T2D, diabetic retinopathy also appears to be a prognostic factor for the progression of CKD, hence may be a predictive tool of the clinical course of DKD. 18
Hypertension is a much stronger risk factor for the development and progression of DKD. In the UKPDS hypertension study, treated to a target of < 150/85 mmHg versus a standard target of < 180/105 mmHg led to a 37% risk reduction in microvascular complications. 19 Each 10 mmHg increase in blood pressure led to a 15% increase in risk of CKD stage 3 (eGFR < 60 ml/min/1.73 m2), onset of albuminuria or doubling of serum creatinine.
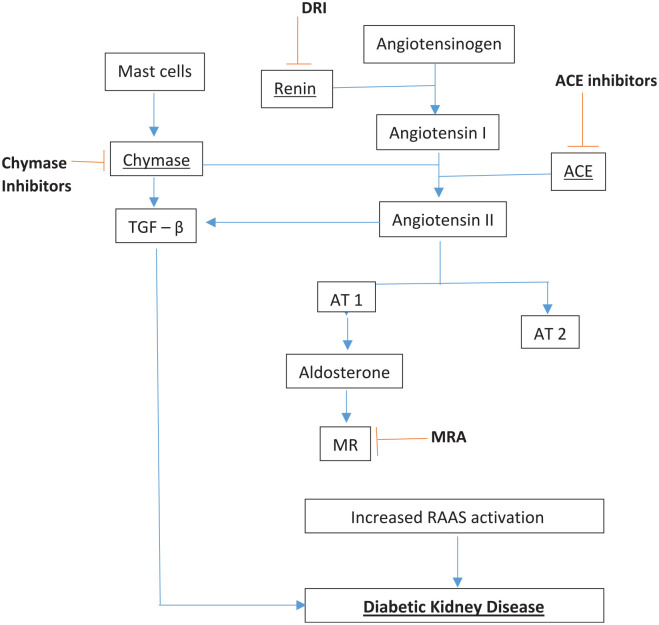
Renin angiotensin aldosterone system (RAAS) blockade in DKD
Figure 1 outlines the RAAS, and potential sites at which it may be inhibited for treatment of DKD. The cornerstone of management of DKD involves treatment of hypertension, primarily using RAAS blocking agents such as angiotensin converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARBs). ACEI promote sodium and water excretion by inhibition of aldosterone and also cause vasodilatation of renal arterioles, while ARBs cause sodium and water excretion by binding to angiotensin receptors. 20 There is strong randomised trial evidence that ACEI and ARBs delay the progression of DKD.
Figure 1.
Renin angiotensin aldosterone system, its role in diabetic kidney disease and sites of inhibition.
ACE Inhibitors, Angiotensin converting enzyme inhibitor; ARB, Angiotensin receptor blocker; AT-1, angiotensin II type 1 receptor; AT 2, angiotensin II type 2 receptor; DRI, direct renin inhibitor; MR, Mineralocorticoid receptor; MRA, Mineralocorticoid receptor antagonist; TGF–β, transforming growth factor-beta.
Angiotensin converting enzyme inhibitors (ACEI)
The Collaborative Study Group (CSG) Captopril Trial in 1993 demonstrated efficacy of captopril in patients with T1D and DKD, suggesting that captopril protected against deterioration in renal function in people with T1D, over and above its blood pressure lowering effect. 21 In patients with T1D and overt nephropathy, glomerular barrier size-selectivity progressively deteriorates with time and is effectively improved by ACEI. 22 By contrast, however, a prospective investigation in 2000 of the patients enrolled in the Ramipril Efficacy in Nephropathy (REIN) study, patients with T2D and nephropathy did not benefit significantly with ramipril; indeed renal function declined faster compared to use of other antihypertensives. 23 However, the Heart Outcome Prevention Evaluation (HOPE) study showed significant benefit of ramipril in cardiovascular mortality and morbidity, especially in people with T2D. 24 Further evidence for beneficial effects of ACEI in T2D was suggested in the Bergamo Nephrologic Diabetes Complications Trial (BENEDICT) in 2004, where use of trandolapril in patients with diabetes without albuminuria, prevented the onset of microalbuminuria. 25 Furthermore, the Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication (DREAM) trial in 2008 concluded that ramipril did not improve cardiorenal outcomes during 3-year follow-up. 26 In the BENEDICT-B trial in 2011, in patients with T2D and microalbuminuria, trandolapril resulted in normoalbuminuria in half of the patients which translated into significant cardioprotection. 27
In normotensive diabetic nephropathy, a Bayesian network meta-analysis for ACEI or ARBs revealed monotherapy with enalapril seemed to be the most efficacious intervention for reduction of albuminuria. 28 In a meta-analysis on major cardiovascular events (MACE), ACEI significantly reduced the risk of total MACE but did not have a significant effect on ESRD, doubling of serum creatinine or all-cause mortality. 29
Angiotensin receptor blockers (ARBs)
Use of ARBs in T2D with DKD have shown even stronger evidence of benefit. In the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) study in 2001, use of losartan as compared to placebo was associated with a reduction in ESRD, doubling of serum creatinine, a 35% decrease in proteinuria, and reduction in the rate for hospitalisation for heart failure (hHF). 30 The Irbesartan Diabetic Nephropathy Trial (IDNT) trial in 2001 showed that irbesartan protected against the progression of diabetic nephropathy, and the renoprotective effect was also independent of the antihypertensive effect. 31 A further study in 2002, the MicroAlbuminuria Reduction with VALsartan (MARVAL) trial, showed that for a similar level of blood pressure reduction, valsartan lowered urine albumin excretion rate (UAER) more effectively than amlodipine. 32 Similarly, the Incipient to Overt: Angiotensin II Blocker, Telmisartan, Investigation on Type 2 Diabetic Nephropathy (INNOVATON) study in 2007 was set out to investigate the efficacy of telmisartan in preventing transition from incipient to overt nephropathy in Japanese patients with T2D. 33 There was a dose dependent beneficial effect of telmisartan in not only reducing transition of microalbuminuria to macroalbuminuria, but also induced remission of microalbuminuria. In patients with T2D and normoalbuminuria, The Randomised Olmesartan And Diabetes MicroAlbuminuria Prevention (ROADMAP) study in 2011 suggested olmesartan delayed the onset of microalbuminuria. 34
In clinical practice, ACEI and ARBs are used interchangeably in DKD, although ARBs may be better tolerated in terms of cough and other adverse effects. 35 Overall, in patients with T2D and proteinuria found that ACEI/ARBs may reduce ESRD and slow the progression of nephropathy but do not demonstrate a decrease all-cause or cardiovascular mortality. 36
Combined ACEI and ARB therapy
ACEI can be bypassed by chymases leading to increased angiotensin II production. In addition, ARBs do not completely block the RAAS. As single blockade does not completely block the RAAS, it has been hypothesised that ‘dual blockade’ might provide some benefits in DKD. 37
In the ONTARGET study in 2008, the combination of Telmisartan and Ramipril was associated with more adverse events without an increase in benefit as compared to monotherapy. 38
In DKD, dual blockade was able to reduce proteinuria compared with single agent blockade, but did not provide long-term renal benefit in the VA nephron D study in 2014, which was stopped early due to increased adverse events including hyperkalaemia and cardiorenal events. 39 A meta-analysis of 32 studies on the combination treatment of ACEI and ARB in diabetic nephropathy concluded that dual therapy reduced proteinuria but there was no additional benefit in reducing ESRD in severe cases and there was increased incidence of adverse reactions, hence should be used with caution in this group. 40
Increased adverse reactions of dual therapy has been a limiting factor their use. Risks may be mitigated by measures such as improved education regarding hyperkalaemia or use of potassium binders to reduce the risk of hyperkalaemia. Given that low-dose ACEI and ARBs leads to more effective RAAS blockade with fewer adverse events, and with the advent of new RAAS inhibitors, RCTs to re-evaluate the role of dual RAAS blockade for cardiorenal protection with individualised therapy should be considered. 41
The chymase inhibitor, fulacimstat has been tested to determine whether it would offer additional benefits over ACEI. In a randomised trial in patients with T2D and DKD, however, the drug did not reduce albuminuria as compared to placebo. 42
Direct renin inhibitors (DRIs)
One of the rationales for developing direct renin inhibitors was to target the rate limiting step in the RAAS pathway. The ALTITUDE study evaluated the use of aliskiren in addition to RAAS blockade to reduce cardiovascular and renal events in patients with T2D and CKD, CVD or both was terminated prematurely. 43 There was no benefit seen in terms of cardiovascular or renal outcomes as compared to placebo, and a significant number of adverse effects such as hyperkalaemia, hypotension, and renal dysfunction occurred.
Hyporeninaemia may be seen in people with diabetes, and proponents for the use of DRIs in DKD suggest that identification of patients responsive to DRIs using plasma renin activity may be warranted, as this subgroup may benefit from DRI use. 44
A meta-analysis of the dual blockade of the RAAS in DKD suggested dual inhibition is superior to monotherapy for short-term indices such as reduction in blood pressure and urine protein excretion but did not translate to improvement in longer outcomes such as progression to ESRD, all-cause mortality or cardiovascular mortality. 45
Aldosterone breakthrough and the use of mineralocorticoid receptor antagonists (MRA)
The benefits of ACEI and ARBs in CKD has been ascribed to reduction in systemic and intraglomerular blood pressure and proteinuria, but there has been increasing focus on benefits of reduction in aldosterone due to the deleterious effect of overactivation of mineralocorticoid receptors (MR) by aldosterone in kidney and heart disease ultimately resulting in inflammation and fibrosis. 46
It has been noted that after long term use of ACEI or ARB, ‘aldosterone breakthrough’ may occur, in which aldosterone levels may rise to pre-treatment levels.47–49 In a study of patients with T1D and DKD on losartan, after 3 years the decline in eGFR was almost double in patients with aldosterone escape than without. 48 Aldosterone breakthrough may increase cardiovascular or renal mortality, and post hoc analysis of the AMADEO study (losArtan versus telMisArtan in hypertensive type-2 DiabEtic patients with Overt nephropathy) concluded that aldosterone breakthrough in T2D with DKD on ARB is a relatively frequent occurrence. 50 A Cochrane review in 2006 concluded that although the survival benefits of ACEI in DKD is known, survival benefits of ACEI with ARB are not known due to lack of direct comparison studies. Renal and toxicity profiles were similar. 51
Mineralocorticoid receptor antagonists (MRA) lower albuminuria but data showing prevention of ESRD have been lacking. Side effects including hyperkalaemia have previously prevented long-term studies in DKD, but treatment strategies with potassium binders, aldosterone synthase inhibitors and non-steroidal MRA have been developed for clinical testing. 37
A recent study of the non-steroid MRA, finerenone in the FIDELIO-DKD trial (Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease) has shown promising results. Finerenone, achieved the primary outcome (kidney failure, a sustained decrease of at least 40% of eGFR from baseline or death from renal causes) in 17.8% patients as compared to 21.1% in placebo (HR: 0.82, CI: 073 to 0.93, p = 0.001). 52
In the finerenone group, secondary CV outcomes were lower but hyperkalaemia related discontinuation was higher. Overall finerenone lowered risk of CKD progression and CV events. A further study by the same investigators found that finerenone reduced the risk of CV and renal failure outcomes in patients with and without a history of CVD as compared to placebo. 53
In the FIGARO-DKD study, fineronone therapy improved CV outcomes as compared to placebo in patients with T2D, CKD stages 2–4 with moderately elevated albuminuria or CKD stages 1 and 2 with severely elevated albuminuria. 54
Hence, finerenone may represent an important therapeutic option in DKD and non-diabetic kidney disease.
Newer drugs in DKD
A wealth of cardiovascular outcome trials (CVOTs) in people with T2D and DKD have been published, and have informed clinical practice.
Sodium Glucose Transporter-2 inhibitors (SGLT-2i)
SGLT-2i block glucose reabsorption in the proximal renal tubule, thereby promoting glycosuria, reducing in plasma glucose and inducing weight loss. As renal function declines, however, less glucose is filtered leading to an attenuated glucose lowering effect in patients with CKD. 55 A number of studies show that despite this reduced glucose lowering effect, SGLT-2i drugs offer a significant benefit in patients with DKD.
Two randomised controlled trials have reported use of SGLT-2i drugs in patients with DKD. The CREDENCE study used canagliflozin versus placebo in 4401 albuminuric patients with T2D and CKD. 56 Patients were included down to an eGFR of >30 ml/min/1.73 m2 (mean eGFR 56.2 ml/min/1.73 m2), and median ACR was 927 mg/g. Canagliflozin led to a 34% risk reduction in the renal specific composite of doubling of baseline creatinine, ESRD or death from renal causes (HR 0.66 (0.53–0.81); p < 0.001). In addition, canagliflozin reduced three-point major adverse cardiovascular events (3p-MACE) by 20% (HR 0.80 (0.67–0.95); p < 0.01) and hHF by 39% (HR 0.61 (0.47–90.8); p < 0.001). The numbers needed to treat (NNT) to prevent one circumstance of doubling of serum creatinine, ESRD or death from renal or cardiovascular cause was 21. Efficacy was highest in the eGFR range 45–60 ml/min/1.73 m2 and urinary ACR > 1000 mg/g.
The DAPA-CKD trial included patients with non-diabetic renal disease. In patients with eGFR 25–75 ml/min/1.73 m2 and albuminuria, dapagliflozin demonstrated a 44% reduction in the composite renal outcome of doubling of serum creatinine, ESRD or death from renal causes (HR 0.56 (0.45–0.68); p < 0.001). 57 The NNT was 19.
Meta-analysis confirms favourable effects of SGLT2i on the renal composite of doubling of serum creatinine (eGFR 40% decline), RRT initiation or renal related death (RR 0.63 (0.56–0.71) even in the presence of CVD or multiple risk factors (RR 0.67 (0.59–0.76) 58
Based on these studies, canagliflozin and dapagliflozin are now licenced for use for renal and cardiovascular protection in DKD and can be initiated in patients with T2D and macroalbuminuria, and eGFR > 30 ml/min/1.73 m2 (>15 ml/min/1.73 m2 for dapagliflozin) as add on to ACEI, irrespective of glucose control.
With regards to adverse effects, the commonest adverse event is genital mycotic infections, which may commonly occur early in treatment. 59 Urinary tract infections (UTIs) are less frequent. An important, but rare, adverse effect is that of euglycaemic ketoacidosis. 60 Careful patient education around ‘sick day rules’ (cessation of the drug during intercurrent illness), avoidance prior to surgery and avoidance of ketogenic diets is required. 61
Renoprotective effects of SGLT-2i are thought to be mediated by tubuloglomerular feedback, natriuresis and glucose induced osmotic diuresis which reduce intraglomerular pressure. 62
Cardiovascular outcome trials (CVOTs) have shown significant reduction in MACE in patients with T2D, and a meta-analysis of four key CVOTs (EMPAREG-OUTCOME, CANVAS, DECLARE, CREDENCE) indicate a major role of renal function in determining the extent of MACE prevention, where-in beneficial effects may be greater in patients with DKD. There may, therefore, be a case for early use of SGLT2i in order to slow progression of DKD and prevent MACE. 63
Although various studies have looked at renal outcomes, due to the heterogeneity in the end-points it is important to unify major adverse renal events (MARE) which has been proposed and this can be used to design future clinical trial focussed on DKD.64,65 However, there remains a difficulty in building an evidence base for judicious use of certain classes of drugs in groups of patients at a low risk of adverse events, for whom a matched placebo group may not reach pre-specified end-points at a sufficient rate.
Glucagon-like peptide-1 receptor agonists (GLP-1RA)
GLP-1RAs have shown some cardiovascular and renal benefits, although not all in class. Indeed, GLP-1RA which are incretin-mimetics (exendin-4 analogues such as exenatide/lixisenatide) have not demonstrated improved outcomes in CVOTs,66,67 whereas human GLP-1RA (albiglutide, liraglutide, dulaglutide, semaglutide) have shown some benefit.68,69
GLP-1RAs have shown promising results in CVOTs. Meta-analysis of the seven large GLP-1 trials of 56,004 patients, showed a 12% reduction in 3p-MACE. 70 Composite renal outcome was reduced by 17% for all GLP1-RA, mainly due to a reduction in new macroalbuminuria.
The cardiovascular and renal benefits of GLP-1RA agents may be linked to their direct action on blood pressure, glucose and weight, but also an effect on improving endothelial dysfunction and inflammation. 71 They may cause an initial drop in eGFR on commencement, which usually plateaus. They are licenced for use at eGFR > 15 ml/min/1.73 m2.
Comparing GLP1-RA and SGLT-2 inhibitors in patients with T2DM and CKD, SGLT-2 inhibitors were associated with a decreased risk of cardiovascular and renal events (RR (95% CI); 0.85 (0.75–0.96) and 0.68 (0.59–0.78), respectively), but GLP-1RA were not (RR 0.91 (0.80–1.04) and 0.86 (0.72–1.03), respectively). 72
A further network meta-analysis found that both groups reduced cardiovascular and renal outcomes with notable differences in risks and benefits. The absolute benefits of these drugs vary according to risk of cardiorenal outcomes and warrants individual risk assessment. 73
Given the different mechanism of action and beneficial effects seen with SGLT-2 inhibitors and GLP1-RA, the synergistic effects of using both groups has been explored. The long-term results of DURATION- 8 (once weekly exenatide plus dapagliflozin) further demonstrates the efficacy and safety of combination therapy as seen with other studies where GLP1-RA was added to existing SGLT-2 inhibitors treatment such as AWARD-10 (dulaglutide plus SLGT-2 inhibitor), SUSTAIN-9 (semaglutide plus SGLT-2 inhibitor). 69 Further studies are required to elucidate the effect of combination therapy of SGLT-2 inhibitor and GLP-1RA on renal events. One such trial is DECADE (NTR6839) which aims to determine the albuminuria-lowering effect of once weekly exenatide plus dapagliflozin. 74
Table 3 summarises some of the landmark clinical trials.
Table 3.
Landmark clinical trials.
| Name of the trial (year) [Ref] | Patient population | Intervention | No. recruited and follow-up | Result |
|---|---|---|---|---|
| UKPDS 33 (1998) 75 | Newly diagnosed T2D | Intensive treatment (sulphonylurea/insulin) vs standard glycaemic control | 3867 patients recruited and median follow-up of 10 years | Intensive vs standard control 11% HbA1c reduction (7% vs 7.9%) over 10 years follow-up, with RR 0.67 for microalbuminuria at 12 years follow-up |
| RENAAL (2001) 30 | T2D, nephropathy (ACR > 300 g/L and creatinine 115–265 micromol/L) | Losartan or placebo | 1513 patients recruited and median follow-up of 3.4 years | Losartan reduced the incidence of a doubling of the serum creatinine (risk reduction 25%, p = 0.006) and ESRD (risk reduction 28%, p = 0.002) but had no effect on rate of death. |
| ADVANCE (2008) 15 | T2D | Intensive glycaemic treatment vs standard control | 11,140 patients recruited and median follow-up of 5 years | Intensive control versus standard control (HbA1c 6.5% vs 7.3%) and former was associated with significant reduction in renal events, including new or worsening nephropathy (HR: 0.79, CI: 0.66–0.93, p = 0.006) and new onset microalbuminuria (HR: 0.91, CI: 0.85–0.98. |
| ROADMAP (2011) 34 | T2D with normoalbuminuria | Olmesartan vs placebo | 4447 patients recruited and median follow-up of 3.2 years | Primary outcome (time to the first onset of microalbuminuria) was 722 days vs 576 days for olmesartan vs placebo. Microalbuminuria developed in 8.2% in olmesartan group vs 9.8% in placebo group. |
| ALTITUDE (2012) 43 | T2D with microalbuminuria, macroalbuminuria or cardiovascular disease on ACEI/ARB | Aliskiren (300 mg) vs placebo | 8561 patients recruited and median follow-up of 32.9 months | Primary end point (a composite of time to cardiovascular death or a first occurrence of cardiac arrest with resuscitation, nonfatal MI, nonfatal stroke, unplanned hospitalisation for heart failure, ESRD, death attributable to kidney failure, or the need for RRT with no dialysis or transplantation or a doubling of the serum creatinine level) occurred in 18.3 vs 17.1% I aliskiren vs placebo (HR: 1.08, 95% CI: 0.98–1.2, p = 0.12) with significantly higher risk of hyperkalemia and hypotension in the former group. |
| LEADER (2017) 69 | T2D, high risk of cardiovascular disease | Liraglutide vs placebo | 9340 patients recruited and median follow-up of 3.84 years | The secondary renal outcomes (a composite of new-onset persistent macroalbuminuria, persistent doubling of the serum creatinine, ESRD, or death due to renal disease) occurred in 268 of 4668 vs 337 of 4672 (HR 0.78, 95% CI 0.67–0.92: p = 0.003) |
| SONAR (2019) 76 | T2D, eGFR 25-75 ml/min/1.73 m2 and ACR 300 – 5000 mg/g on ACEI/ARB | Atrasentan or placebo | 2640 responders were randomised and median follow-up of 2.2 years | The primary end point (a composite of doubling of the serum creatinine, ESRD) was 6.9 vs 7% for the atrasentan vs placebo (HR 0.65, 95% CI 0.49–0.88, p = 0.0047) |
| CREDENCE (2019) 56 | T2D, eGFR 30–90 ml/min/1.73 m2 and ACR 300–5000 mg/g, on ACEI/ARB | Canagliflozin vs placebo | 4401 patients recruited and median follow-up of 2.62 years | 30% lower relative risk of the primary outcome (a composite of ESRD, a doubling of the serum creatinine level, or death from renal or cardiovascular causes): 43.2 vs. 61.2 per 1000 patient-years (HR: 0.70, 95% CI: 0.59–0.82: p = 0.00001) |
| DAPA-CKD (2020) 57 | T2D, eGFR 25–75 ml/min/1.73 m2 and ACR 200–5000 mg/g | Dapagliflozin vs placebo | 4304 patients recruited and median follow-up of 2.4 years | The primary outcome event (a composite of sustained decline in the eGFR of at least 50%, ESRD, or death from renal or cardiovascular causes) occurred in 9.2% vs 14.5% (HR: 0.61, 95% CI: 0.51–0.72: p < 0.001) |
| FIDELIO-DKD (2020) 52 | T2D, eGFR 25–60 ml/min/1.73 m2 and ACR 30 – 300 mg/g and diabetic retinopathy or eGFR 25–75 ml/min/1.73 m2 and ACR 300 – 5000 mg/g. | Finerenone or placebo | 5734 patients recruited and median follow-up of 2.6 years | The primary composite outcome event (kidney failure, a sustained decrease of at least 40% in the eGFR from baseline, or death from renal causes) occurred in 17.8% in the finerenone group vs 21.1% in the placebo group (HR: 0.82, 95% CI: 0.73–0.93, p = 0.001) |
| FIGARO_DKD (2021) 54 | T2D, eGFR 25–90 ml/min/1.73 m2 and ACR 30–300 mg/g and diabetic retinopathy or eGFR ⩾ 60 ml/min/1.73 m2 and ACR 300–5000 mg/g. | Finerenone or placebo | 7437 patients recruited and median follow-up of 3.4 years | The primary outcome event (a composite of death from cardiovascular causes, nonfatal MI, nonfatal stroke, or hospitalisation for heart failure) occurred in 12.4% in the finerenone group vs 14.2% in the placebo group (HR: 0.87, 95% CI: 0.76–0.98, p = 0.03) |
ACEI, Angiotensin converting enzyme inhibitor; ACR, Albumin creatinine ratio; ARB, Aldosterone receptor blocker; CI, confidence interval; CKD, chronic kidney disease; eGFR: estimated glomerular filtration rate; ESRD, End stage renal disease; FIDELIO-DKD, Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease; HR, Hazard ratio; MI, Myocardial infarction; RENAAL, Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan; RR, Relative risk; RRT, Renal replacement therapy; T2D, Type 2 diabetes mellitus; UKPDS, United Kingdom Prospective Diabetes Study.
Other agents
Darbepoietin
Erythropoietin (EPO) is a glycoprotein hormone induced by hypoxia, is essential for erythropoiesis. In animal studies, EPO was identified as a direct renal protective factor, protecting kidney from hyperglycaemia induced damage and proteinuria. 77 Studies have alluded to EPO’s extra-erythropoietic actions including anti-apoptotic, anti-proliferative, anti-inflammatory and anti-angiogenic effects in pancreatic islets. Recombinant human EPO is protective against development of diabetes in mouse models. 78 In another animal study, potential role of erythropoietin beta in reducing progression of DKD was suggested most likely due to the anti-inflammatory and anti-oxidative effect. 79 It has also been suggested that polymorphisms of the gene encoding EPO may be risk markers for DKD. 80
Darbepoietin alfa in patients with DKD and moderate anaemia was tested in the TREAT study. 81 The drug did not reduce the primary composite outcomes including ESRD, and was associated with an increased risk of stroke. 81
Bardoxolone
Bardoxolone methyl is a therapeutic agent based on the scaffold of the natural product oleanolic acid. It attenuates inflammation by activating the Kelch-like ECH-associated protein 1 (Keap1)/nuclear factor erythroid 2-related factor 2 (Nrf2) system. 82
Bardoxolone has been tested in DKD in the Bardoxolone Methyl Evaluation in Patients With Chronic Kidney Disease and Type 2 Diabetes (BEACON) trial, a phase 3 RCT in 2185 patients with T2D and stage 4 CKD. The trial was terminated due to increase in heart failure in patients randomised to bardoxolone. In the post hoc analysis, bardoxolone significantly decreased albuminuria. 83
A subsequent phase 2 study evaluated patients with DKD without any evidence of heart failure. Results were promising as bardoxolone significantly increased eGFR, with a significant intergroup difference of 6.64 ml/min/1.73 m2 (p = 0.008). 84
It remains to be seen whether bardoxolone can be used safely in a subset of patients with DKD. A phase 3 study titled AYAME is evaluating bardoxolone in DKD stage 3 and 4 to evaluate the time of onset of a > 30% decrease in eGFR from baseline or end-stage kidney disease, with results expected in March 2022. 82
Endothelin a receptor antagonists
Endothelin-1 (ET-1) is as a potent vasoactive factor which binds to two receptors, endothelin type A (ETA) and type B (ETB). CKD results in increased ET-1 production, which is implicated in deterioration of renal function due to promotion of pro-fibrotic pathways. 85
The ASCEND study, in which participants were assigned to avosentan, an ETA receptor antagonist (25–50 mg) or placebo in addition to ACEI or ARB, avosentan did significantly reduce albuminuria, but due to fluid overload in the avosentan group, the trial was prematurely terminated. 86
A subsequent study, the SONAR trial tested atrasentan, a ETA receptor antagonist in patients with T2D and DKD, on major renal outcomes. They used an enrichment design to exclude patients who developed fluid retention during the early period to minimise the risk of heart failure. Atrasentan was noted to be renoprotective in carefully selected patients without a history of severe peripheral oedema or admission due to heart failure with DKD, and showed a lower risk of doubling of serum creatinine or ESRD compared to placebo. The study was terminated, however, due to lower than expected event rate. 76
A recent systematic review and meta-analysis confirmed that selective ETA receptor antagonists (atrsentan and avosentan) but not bosentan (dual ETA/B receptor antagonist) effectively lowers albuminuria, blood pressure and improves lipid profiles along with composite endpoints in DKD. In particular, atrasentan 0.75 mg provided maximal renal protection with minimal adverse effects, although vigilant monitoring of heart failure is required. 87
Bariatric and metabolic surgery
There is emerging evidence on the role of bariatric and metabolic surgery in diabetes and CKD. The renoprotective effects of metabolic surgery in DKD appear independent of weight loss and improvements of glycaemia. 88 Various observational and RCTs seem to indicate that bariatric and metabolic surgery as an adjunct to medical therapy may decrease progression of DKD. 89
In terms of assessing the risk of incident microvascular complications, in a retrospective cohort study, bariatric and metabolic surgery was associated with decreased microvascular complication with adjusted hazard ratio for nephropathy being 0.63, 0.51–0.78, p < 0.001. 90
In the Microvascular Outcomes after Metabolic Surgery (MOMS) study in patients with T2D and class I obesity, at 2 years follow-up, Roux-en-Y gastric bypass (RYGB) was more effective than best medical treatment for achieving remission of albuminuria and stage G1 to G3 and A2 to A3 CKD. 91 Bariatric and metabolic surgery has the potential to slow or arrest DKD in obese patients and further studies are required including the effect of sleeve gastrectomy on DKD. OBESE-DKD (NCT04626323) is a phase 2 study to look into the safety and efficacy of RYGB surgery versus best medical treatment in the progression of DKD in obese patients with T2D.
Possible future therapies
Attempts to elucidate the pathogenic pathways in DKD continue, with a view to developing potential future interventions in DKD. Figure 2 outlines some of the hypothesised pathogenic mechanisms contributing to DKD, and possible interventions.
Figure 2.
Pathogenesis of diabetic kidney disease with potential interventions.
ACEI, Angiotensin converting enzyme inhibitor; ARA, Adiponectin receptor agonist; ARB, Angiotensin receptor blocker; DRI, Direct renin inhibitor; GLP1-RA, Glucagon like peptide 1 receptor agonists; JAK/STAT, Janus kinase -signal transducer and activator of transcription; JAK/STAT Inh, Janus kinase -signal transducer and activator of transcription Inhibitor; Keap1/Nrf2, Kelch-like ECH-associated protein 1 -nuclear factor E2-related factor 2; MAPK/ERK, Mitogen-activated protein kinases/extracellular signal-regulated kinase; MiRNA, microRNA; MRA, Mineralocorticoid receptor antagonist; NADPH oxidase, nicotinamide adenine dinucleotide phosphate oxidase; NF-κB, Nuclear Factor kappa-light-chain-enhancer of activated B cells; PI3 K/AKT, phosphatidylinositol 3kinase /protein kinase B; SGLT2i, Sodium glucose co-transporter 2 inhibitors; TGFβ1 I, transforming growth factor-beta 1 inhibitor.
Potassium binders
Hyperkalaemia is common in patients with CKD, especially those on RAAS inhibition. The occurrence of hyperkalaemia increases mortality within a day of the event. 92 Patiromer, a potassium-binding polymer, binds potassium through the gastrointestinal tract and increases faecal excretion of potassium. The AMETHYST-DN study showed that the use of patiromer twice daily in patients with DKD using ACEI, ARB or both with mild to moderate hyperkalaemia, resulted in statistically significant decreases in potassium level after 1 month of treatment. 93
Another potassium-binding resin that has garnered interest is sodium zirconium cyclosilicate (SZC), a highly selective cation exchanger. In a multicentre phase 3 trial in patients with hyperkalaemia (serum potassium 5.0–6.5 mmol/L) with sub group analyses for patients with diabetes, concluded that as compared to placebo, SZC resulted in significant reduction in potassium levels at 48 hours. 94
The advantage of such agents may be that they enable maximal RAAS blockade without discontinuation or due to hyperkalaemia. 95
Anti-fibrotic therapy
Transforming growth factor beta-1 (TGF-β1) has been implicated in the pathogenesis of DKD. 96 It has been demonstrated that diabetes aggravates post-ischaemic renal fibrosis through persistent activation of TGF-β1 and sonic hedgehog (Shh) signalling, and insulin administration to control hyperglycaemia was not adequate to prevent progression of renal fibrosis. 97
Pirfenidone, an oral compound has been found to inhibit TGF-β1 production. In a small RCT of patients with DKD (eGFR 25–75 ml/min/1.73 m2), pirfenidone was thought to show promising results. 98 Apart from its anti-fibrotic role, pirfenidone has been investigated for its action on gut microbiome diversity. A recent study hypothesised that changes in gut microbiota between diabetic and lean mice allow insights into role of gut microbes in DKD and potentially beneficial effects of pirfenidone. They concluded that gut microbial dysbiosis was partially reversed by long acting pirfenidone therapy. 99
Pentoxifylline is a nonspecific phosphodiesterase inhibitor is another anti-fibrotic agent known for its anti-inflammatory, anti-proliferative, anti-proteinuric and rheologic properties. Recent studies have investigated the role of pentoxifylline in Klotho signalling. 100 The PREDIAN trial was an open label RCT to determine whether pentoxifylline reduced albuminuria, in addition to RAAS blockade. Addition of pentoxifylline resulted in a smaller decrease in eGFR and reduction in albuminuria. 101 Larger, more definitive clinical trials are, however, required.
Advanced glycation end product scavengers
Advanced glycation end products (AGEs) are accelerated in diabetes, and the interaction of AGEs with receptors (RAGEs) activates an inflammatory cascade which is implicated in diabetes complications. 102 AGE-RAGE activation transduces pathways including PI3 K/AKT, MAPK/ERK, JAK2-STAT and NADPH oxidase provoking oxidative stress and inflammation in the renal cell. 103 Blocking AGE-RAGE signalling has been hypothesised as beneficial for DKD. Target cells include podocytes, mesangial, glomerular, endothelial, myofibroblastic cells and the Nrf2/ARE/GLO-1 pathway. 104 Limitations of synthetic and natural inhibitors of glycation include adverse effects and high cost, and lack of dose dependent standardisation for their safety and efficacy. 103
Pyridoxamine, a natural form of Vitamin B6 appears to be an effective inhibitor of AGE via scavenging of methylglyoxal, and has been demonstrated to be beneficial in DKD in small studies, although further studies are needed to elucidate its role. 105
Janus kinase-signal transducer and activator of transcription (JAK-STAT) inhibitors
Increased signalling of the JAK-STAT pathway is seen across the spectrum of DKD. Baricitinib, an oral selective reversible inhibitor of JAK 1 and 2 has been approved for use in rheumatoid arthritis 106 and is being evaluated in the management of severe Covid-19 infections. 107 A phase 2 RCT demonstrated that baricitinib reduced albuminuria in patients with DKD but was associated with a higher frequency of anaemia. 108 Nifuroxazide, an oral antibiotic for acute infectious diarrhoea is a potent and effective STAT3 inhibitor. In rats with diabetic-induced nephropathy, nifuroxazide mitigated the inflammatory burden and hindered the development and progression of diabetic nephropathy. 109
Adiponectin receptor agonists
Adiponectin is an adipokine which improves insulin sensitivity. It also appears to have anti-inflammatory, anti-oxidative and anti-fibrotic effects, and animal studies a beneficial effect of AdipoRon, an orally synthetic adiponectin receptor agonist in DKD. 110 AdipoRon, appears to be a safe and attractive treatment option in DKD. 111 Although there have been extensive preclinical studies, translation to clinical studies in humans is awaited.
Hydrogen sulphide (H2S)
Impaired H2S signalling may play a role in pathogenesis of DKD. H2S may ameliorate RAAS activation, oxidative stress and inflammation and also reduce renal fibrosis. In-vitro an in-vivo experimental studies using H2S donors have demonstrated the beneficial effects of H2S donors on animal models of DKD. 112
Metallic organic framework (MOF)
Metallic organic framework (MOF), a novel class of crystalline porous material have been analysed at a nanoscale as therapeutic agents. Chromium containing MOF synthesised using nanochelating technology after 8 weeks of treatment in diabetic rats decreased insulin resistance and improved creatinine clearance. 113
Ethyl vanillin (EVA)
Ethyl vanillin (EVA), an analogue of vanillin, is a common food additive and has antioxidant activity. In vivo and in vitro studies demonstrated that EVA may protect against DKD through inhibition of oxidative stress and apoptosis and the protective effect might be associated with the regulation of the activation of Nrf2. 114
MicroRNAs (miRNAs)
MicroRNAs are small RNA molecules that are endogenously produced act as post-transcriptional suppressors of gene expression, and a review article discussed the use of different types of miRNAs as an anti-fibrotic treatment and role in DKD. 115 Although targeting fibrosis has been investigated as treatment for DKD, no approved therapeutic agent has been found but there is a potential for miRNA-based treatment in the future for DKD.
Dimethylamino parthenolide (DMAPT)
In mice, orally administered DMAPT (orally bioavailable analogue of parthenolide, a sesquiterpene lactone naturally present in plants) showed reduction in albuminuria, and significantly reduced glomerulosclerosis and tubulointerstitial fibrosis. 116
A review article summarised recent preclinical studies that had identified novel therapeutic renal and extra-renal targets in DKD including reduction of oxidative stress, inflammation, and haemodynamic changes. 117 The novel renal targets not discussed previously, included nicotinamide adenine dinucleotide phosphate oxidase 5 (NOX5), monocyte chemoattractant protein 1 – chemokine receptor 2 (MCP1-CCR2) and cannabinoid receptor type 1 and 2 (CB1R/CB2R), and extra-renal targets such as targeting mesenchymal stem cells (MSCs), extracellular vesicles (EVs) and microbiota were reviewed with strategies to improve translation of this research to humans.
Currently there are 34 phase 2 and 3 trials registered on clinicaltrials.gov on DKD/diabetic nephropathy that are recruiting, active but not recruiting and not yet recruiting patients. 118 To mention the ones not discussed previously, they include studies to investigate combination therapy of Dapagliflozin with AZD997 (a novel, selective mineralocorticoid receptor modulator) and another study with AZD5718 (a novel, potent, and selective FLAP inhibitor that inhibits the LTB4 production) which are currently recruiting. A study involving CLBS201, a CD34+ cell investigational product for administration into the renal arteries, to improve or slow the decline of renal function in patients with T2D and CKD.Whether ezetimibe inhibits intestinal cholesterol absorption and affects albuminuria and kidney fat accumulation is being looked into in the DiaKidZ study and several studies with fenofibrate, a PPAR alpha agonist in patients with diabetic nephropathy and patients with type 1 diabetes T1D. N-Acetylcysteine (NAC) has anti-oxidative effects and there is a study focused on the possible ameliorating effect of NAC on diabeties nephropathy in patients with T2D. Inhibition of the TRPC5-Rac-1 pathway to prevent podocyte damage using a TRPC5 channel inhibitor in diabetic nephropathy. Roflumilast, a selective PDE 4 inhibitor with anti-inflammatory and anti-fibrotic effects, added to standard therapy for diabetic nephropathy to investigate its possible role in diabetic nephropathy is currently registered as a trial but not yet recruiting. Other studies on cotadutide (dual receptor agonist with balanced GLP1 and glucagon activity), and on mesenchymal stromal cells (MSCs) which might improve complications from diabetes is being investigated in NEPSTROM study in patients with T2D and CKD, and IL-33 mononoclonal antibody with dapagliflozin and a monoclonal antibody to block vascular endothelial growth factor-B (VEGF-B) in patients with DKD.
Conclusions
DKD remains an important microvascular complication of diabetes accounting for significant morbidity and mortality. Established management has been control of hypertension with ACEI/ARB drugs, improvement of glucose control and management of cardiovascular risk factors. Recent pivotal studies have shifted the paradigm to use SGLT-2i drugs in all patients with T2D and established DKD. In addition, finerenone may be used in certain circumstances.
There is a huge amount of ongoing research into novel therapeutic agents in this condition, in the hope that they may offer further interventions to reduce the burden of DKD mortality and morbidity.
Footnotes
Author contributions: Ritwika Mallik: Conceptualisation; Project administration; Writing – original draft; Writing – review & editing.
Tahseen A. Chowdhury: Conceptualisation; Project administration; Supervision; Writing – original draft; Writing – review & editing.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Tahseen A. Chowdhury  https://orcid.org/0000-0001-8878-2331
https://orcid.org/0000-0001-8878-2331
Contributor Information
Ritwika Mallik, Department of Diabetes and Metabolism, The Royal London Hospital, London, UK.
Tahseen A. Chowdhury, Department of Diabetes and Metabolism, The Royal London Hospital, 7th Floor, John Harrison House, Whitechapel, London E1 1BB, UK.
References
- 1. IDF Diabetes Atlas. 9th ed. 2019, https://www.diabetesatlas.org/en/ (accessed 5 May 2021).
- 2. Reutens AT. Epidemiology of diabetic kidney disease. Med Clin North Am 2013; 97: 1–18. [DOI] [PubMed] [Google Scholar]
- 3. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol 2013; 24: 302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Parving HH, Mauer M, Ritz E. Diabetic nephropathy. In: Brenner B. (ed.) Brenner and Rector’s the kidney. 8th ed. Philadelphia, PA: Elsevier, 2016, p. 1265. [Google Scholar]
- 6. Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int 2020; 98: S1–S115. [DOI] [PubMed] [Google Scholar]
- 7. American Diabetes Association. Microvascular complications and foot care: standards of medical care in diabetes-2021 [published correction appears in Diabetes Care 2021; 44: 2186–2187]. Diabetes Care 2021; 44: S151–S167. [DOI] [PubMed] [Google Scholar]
- 8. Thomas MC, Brownlee M, Susztak K, et al. Diabetic kidney disease. Nat Rev Dis Primers 2015; 1: 15018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marshall SM. Natural history and clinical characteristics of CKD in type 1 and type 2 diabetes mellitus. Adv Chronic Kidney Dis 2014; 21: 267–272. [DOI] [PubMed] [Google Scholar]
- 10. Pham TT, Sim JJ, Kujubu DA, et al. Prevalence of nondiabetic renal disease in diabetic patients. Am J Nephrol 2007; 27: 322–328. [DOI] [PubMed] [Google Scholar]
- 11. Pugliese G, Solini A, Bonora E, et al. Chronic kidney disease in type 2 diabetes: lessons from the Renal Insufficiency and Cardiovascular Events (RIACE) Italian Multicentre Study. Nutr Metab Cardiovasc Dis 2014; 24: 815–822. [DOI] [PubMed] [Google Scholar]
- 12. Stanton RC. Role of glucose metabolism and mitochondrial function in diabetic kidney disease. Curr Diab Rep 2021; 21: 6. [DOI] [PubMed] [Google Scholar]
- 13. Nathan DM. and DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care 2014; 37: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359: 1577–1589. [DOI] [PubMed] [Google Scholar]
- 15. ADVANCE Collaborative Group, Patel A, MacMahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560–2572. [DOI] [PubMed] [Google Scholar]
- 16. Action to Control Cardiovascular Risk in Diabetes Study Group,Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358: 2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamanouchi M, Mori M, Hoshino J, et al. Retinopathy progression and the risk of end-stage kidney disease: results from a longitudinal Japanese cohort of 232 patients with type 2 diabetes and biopsy-proven diabetic kidney disease. BMJ Open Diabetes Res Care 2019; 7: e000726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hsing SC, Lee CC, Lin C, et al. The severity of diabetic retinopathy is an independent factor for the progression of diabetic nephropathy. J Clin Med 2020; 10: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Retnakaran R, Cull CA, Thorne KI, et al. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 2006; 55: 1832–1839. [DOI] [PubMed] [Google Scholar]
- 20. McGrath K, Edi R. Diabetic kidney disease: diagnosis, treatment, and prevention. Am Fam Physician 2019; 99: 751–759. [PubMed] [Google Scholar]
- 21. Lewis EJ, Hunsicker LG, Bain RP, et al. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group [published correction appears in N Engl J Med 1993; 330: 152]. N Engl J Med 1993; 329: 1456–1462. [DOI] [PubMed] [Google Scholar]
- 22. Ruggenenti P, Mosconi L, Sangalli F, et al. Glomerular size-selective dysfunction in NIDDM is not ameliorated by ACE inhibition or by calcium channel blockade. Kidney Int 1999; 55: 984–994. [DOI] [PubMed] [Google Scholar]
- 23. Ruggenenti P, Perna A, Gherardi G, et al. Chronic proteinuric nephropathies: outcomes and response to treatment in a prospective cohort of 352 patients with different patterns of renal injury. Am J Kidney Dis 2000; 35: 1155–1165. [DOI] [PubMed] [Google Scholar]
- 24. Sleight P. The HOPE study (heart outcomes prevention evaluation). J Renin Angiotensin Aldosterone Syst 2000; 1: 18–20. [DOI] [PubMed] [Google Scholar]
- 25. Ruggenenti P, Fassi A, Ilieva AP, et al. Preventing microalbuminuria in type 2 diabetes. N Engl J Med 2004; 351: 1941–1951. [DOI] [PubMed] [Google Scholar]
- 26. DREAM Trial Investigators,Dagenais GR, Gerstein HC, et al. Effects of ramipril and rosiglitazone on cardiovascular and renal outcomes in people with impaired glucose tolerance or impaired fasting glucose: results of the Diabetes REduction Assessment with ramipril and rosiglitazone Medication (DREAM) trial. Diabetes Care 2008; 31: 1007–1014. [DOI] [PubMed] [Google Scholar]
- 27. Ruggenenti P, Fassi A, Ilieva A, et al. Effects of verapamil added-on trandolapril therapy inhypertensive type 2 diabetes patients with microalbuminuria: the BENEDICT-B randomized trial. J Hypertens 2011; 29: 207–216. [DOI] [PubMed] [Google Scholar]
- 28. Ye H, Huo Z, Ye P, et al. Comparative proteinuria management of different angiotensin-converting enzyme inhibitors or angiotensin receptor blockers for normotensive patients with CKD: a Bayesian network meta-analysis. PeerJ 2020; 8: e8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu X, Ma L, Li Z. Effects of renin-angiotensin system blockers on renal and cardiovascular outcomes in patients with diabetic nephropathy: a meta-analysis of randomized controlled trials. J Endocrinol Invest 2020; 43: 959–972. [DOI] [PubMed] [Google Scholar]
- 30. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–869. [DOI] [PubMed] [Google Scholar]
- 31. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345: 851–860. [DOI] [PubMed] [Google Scholar]
- 32. Viberti G, Wheeldon NM. and MicroAlbuminuria Reduction With VALsartan (MARVAL) Study Investigators. Microalbuminuria reduction with valsartan in patients with type 2 diabetes mellitus: a blood pressure-independent effect. Circulation 2002; 106: 672–678. [DOI] [PubMed] [Google Scholar]
- 33. Makino H, Haneda M, Babazono T, et al. Prevention of transition from incipient to overt nephropathy with telmisartan in patients with type 2 diabetes. Diabetes Care 2007; 30: 1577–1578. [DOI] [PubMed] [Google Scholar]
- 34. Haller H, Ito S, Izzo JL, Jr, et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med 2011; 364: 907–917. [DOI] [PubMed] [Google Scholar]
- 35. Mallat SG. What is a preferred angiotensin II receptor blocker-based combination therapy for blood pressure control in hypertensive patients with diabetic and non-diabetic renal impairment? Cardiovasc Diabetol 2012; 11: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Coleman CI, Weeda ER, Kharat A, et al. Impact of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers on renal and mortality outcomes in people with type 2 diabetes and proteinuria. Diabet Med 2020; 37: 44–52. [DOI] [PubMed] [Google Scholar]
- 37. Frimodt-Møller M, Persson F, Rossing P. Mitigating risk of aldosterone in diabetic kidney disease. Curr Opin Nephrol Hypertens 2020; 29: 145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. ONTARGET Investigators,Yusuf S, Teo KK, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358: 1547–1559. [DOI] [PubMed] [Google Scholar]
- 39. Fried LF, Emanuele N, Zhang JH, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy [published correction appears in N Engl J Med 2014; 158: A7255]. N Engl J Med 2013; 369: 1892–1903. [DOI] [PubMed] [Google Scholar]
- 40. Ren F, Tang L, Cai Y, et al. Meta-analysis: the efficacy and safety of combined treatment with ARB and ACEI on diabetic nephropathy. Ren Fail 2015; 37: 548–561. [DOI] [PubMed] [Google Scholar]
- 41. Mei M, Zhou Z, Zhang Q, et al. Dual blockade of the renin-angiotensin system: a strategy that should be reconsidered in cardiorenal diseases. Nephron 2021; 145: 99–106. [DOI] [PubMed] [Google Scholar]
- 42. Rossing P, Strand J, Avogaro A, et al. Effects of the chymase inhibitor fulacimstat in diabetic kidney disease-results from the CADA DIA trial. Nephrol Dial Transplant 2021; 36: 2263–2273. [DOI] [PubMed] [Google Scholar]
- 43. Parving HH, Brenner BM, McMurray JJ, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012; 367: 2204–2213. [DOI] [PubMed] [Google Scholar]
- 44. Massolini BD, Contieri SSG, Lazarini GS, et al. Therapeutic renin inhibition in diabetic nephropathy: a review of the physiological evidence. Front Physiol 2020; 11: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Feng Y, Huang R, Kavanagh J, et al. Efficacy and safety of dual blockade of the renin-angiotensin-aldosterone system in diabetic kidney disease: a meta-analysis. Am J Cardiovasc Drugs 2019; 19: 259–286. [DOI] [PubMed] [Google Scholar]
- 46. Bauersachs J, Jaisser F, Toto R. Mineralocorticoid receptor activation and mineralocorticoid receptor antagonist treatment in cardiac and renal diseases. Hypertension 2015; 65: 257–263. [DOI] [PubMed] [Google Scholar]
- 47. Sato A, Hayashi K, Naruse M, et al. Effectiveness of aldosterone blockade in patients with diabetic nephropathy. Hypertension 2003; 41: 64–68. [DOI] [PubMed] [Google Scholar]
- 48. Schjoedt KJ, Andersen S, Rossing P, et al. Aldosterone escape during blockade of the renin-angiotensin-aldosterone system in diabetic nephropathy is associated with enhanced decline in glomerular filtration rate. Diabetologia 2004; 47: 1936–1939. [DOI] [PubMed] [Google Scholar]
- 49. Sato A, Saruta T. Aldosterone breakthrough during angiotensin-converting enzyme inhibitor therapy. Am J Hypertens 2003; 16: 781–788. [DOI] [PubMed] [Google Scholar]
- 50. Moranne O, Bakris G, Fafin C, et al. Determinants and changes associated with aldosterone breakthrough after angiotensin II receptor blockade in patients with type 2 diabetes with overt nephropathy. Clin J Am Soc Nephrol 2013; 8: 1694–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Strippoli GF, Bonifati C, Craig M, et al. Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists for preventing the progression of diabetic kidney disease. Cochrane Database Syst Rev 2006; 2006: CD006257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bakris GL, Agarwal R, Anker SD, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020; 383: 2219–2229. [DOI] [PubMed] [Google Scholar]
- 53. Filippatos G, Anker SD, Agarwal R, et al. Finerenone and cardiovascular outcomes in patients with chronic kidney disease and type 2 diabetes. Circulation 2021; 143: 540–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pitt B, Filippatos G, Agarwal R, et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med 2021; 385: 2252–2263. [DOI] [PubMed] [Google Scholar]
- 55. Fioretto P, Zambon A, Rossato M, et al. SGLT2 inhibitors and the diabetic kidney. Diabetes Care 2016; 39(Suppl. 2): S165–S171. [DOI] [PubMed] [Google Scholar]
- 56. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295–2306. [DOI] [PubMed] [Google Scholar]
- 57. Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020; 383: 1436–1446. [DOI] [PubMed] [Google Scholar]
- 58. Lo KB, Gul F, Ram P, et al. The effects of SGLT2 inhibitors on cardiovascular and renal outcomes in diabetic patients: a systematic review and meta-analysis. Cardiorenal Med 2020; 10: 1–10. [DOI] [PubMed] [Google Scholar]
- 59. Thong KY, Yadagiri M, Barnes DJ, et al. Clinical risk factors predicting genital fungal infections with sodium-glucose cotransporter 2 inhibitor treatment: the ABCD nationwide dapagliflozin audit. Prim Care Diabetes 2018; 12: 45–50. [DOI] [PubMed] [Google Scholar]
- 60. Peters AL, Buschur EO, Buse JB, et al. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care 2015; 38: 1687–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. SGLT-2 inhibitor in people with type 2 diabetes: an educational resource for health professionals, https://abcd.care/sites/abcd.care/files/site_uploads/Resources/Position-Papers/SGLT2-inhibitors-ABCD.pdf (accessed 17 February 2021).
- 62. Van Bommel EJM, Muskiet MHA, van Baar MJB, et al. The renal hemodynamic effects of the SGLT2 inhibitor dapagliflozin are caused by post-glomerular vasodilatation rather than pre-glomerular vasoconstriction in metformin-treated patients with type 2 diabetes in the randomized, double-blind RED trial. Kidney Int 2020; 97: 202–212. [DOI] [PubMed] [Google Scholar]
- 63. Giugliano D, De Nicola L, Maiorino MI, et al. Preventing major adverse cardiovascular events by SGLT-2 inhibition in patients with type 2 diabetes: the role of kidney. Cardiovasc Diabetol 2020; 19: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Prischl FC, Rossing P, Bakris G, et al. Major adverse renal events (MARE): a proposal to unify renal endpoints. Nephrol Dial Transplant 2021; 36: 491–497. [DOI] [PubMed] [Google Scholar]
- 65. García-Carro C, Vergara A, Bermejo S, et al. How to assess diabetic kidney disease progression? From albuminuria to GFR. J Clin Med 2021; 10: 2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373: 2247–2257. [DOI] [PubMed] [Google Scholar]
- 67. Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017; 377: 1228–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019; 394: 121–130. [DOI] [PubMed] [Google Scholar]
- 69. Mann JFE, Ørsted DD, Brown-Frandsen K, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med 2017; 377: 839–848. [DOI] [PubMed] [Google Scholar]
- 70. Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol 2019; 7: 776–785. [DOI] [PubMed] [Google Scholar]
- 71. Nauck MA, Meier JJ, Cavender MA, et al. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation 2017; 136: 849–870. [DOI] [PubMed] [Google Scholar]
- 72. Yamada T, Wakabayashi M, Bhalla A, et al. Cardiovascular and renal outcomes with SGLT-2 inhibitors versus GLP-1 receptor agonists in patients with type 2 diabetes mellitus and chronic kidney disease: a systematic review and network meta-analysis. Cardiovasc Diabetol 2021; 20: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Palmer SC, Tendal B, Mustafa RA, et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ 2021; 372: m4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jabbour SA, Frías JP, Ahmed A, et al. Efficacy and safety over 2 years of exenatide plus dapagliflozin in the DURATION-8 study: a multicenter, double-blind, phase 3, randomized controlled trial. Diabetes Care 2020; 43: 2528–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group [published correction appears in Lancet 1999; 354: 602]. Lancet 1998; 352: 837–853. [PubMed] [Google Scholar]
- 76. Heerspink HJL, Parving HH, Andress DL, et al. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial [published correction appears in Lancet 2019; 393: 1936]. Lancet 2019; 393: 1937–1947. [DOI] [PubMed] [Google Scholar]
- 77. She J, Yuan Z, Wu Y, et al. Targeting erythropoietin protects against proteinuria in type 2 diabetic patients and in zebrafish. Mol Metab 2018; 8: 189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Choi D, Schroer SA, Lu SY, et al. Erythropoietin protects against diabetes through direct effects on pancreatic beta cells. J Exp Med 2010; 207: 2831–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Eren Z, Günal MY, Arı E, et al. Pleiotropic and renoprotective effects of erythropoietin beta on experimental diabetic nephropathy model. Nephron 2016; 132: 292–300. [DOI] [PubMed] [Google Scholar]
- 80. Li H, Xu H, Li Y, et al. Associations between erythropoietin polymorphisms and risk of diabetic microvascular complications. Oncotarget 2017; 8: 112675–112684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361: 2019–2032. [DOI] [PubMed] [Google Scholar]
- 82. Kanda H, Yamawaki K. Bardoxolone methyl: drug development for diabetic kidney disease. Clin Exp Nephrol 2020; 24: 857–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rossing P, Block GA, Chin MP, et al. Effect of bardoxolone methyl on the urine albumin-to-creatinine ratio in patients with type 2 diabetes and stage 4 chronic kidney disease. Kidney Int 2019; 96: 1030–1036. [DOI] [PubMed] [Google Scholar]
- 84. Nangaku M, Kanda H, Takama H, et al. Randomized clinical trial on the effect of bardoxolone methyl on GFR in diabetic kidney disease patients (TSUBAKI study). Kidney Int Rep 2020; 5: 879–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kohan DE, Barton M. Endothelin and endothelin antagonists in chronic kidney disease. Kidney Int 2014; 86: 896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mann JF, Green D, Jamerson K, et al. Avosentan for overt diabetic nephropathy. J Am Soc Nephrol 2010; 21: 527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhou Y, Chi J, Huang Y, et al. Efficacy and safety of endothelin receptor antagonists in type 2 diabetic kidney disease: a systematic review and meta-analysis of randomized controlled trials. Diabet Med 2021; 38: e14411. [DOI] [PubMed] [Google Scholar]
- 88. Scheurlen KM, Probst P, Kopf S, et al. Metabolic surgery improves renal injury independent of weight loss: a meta-analysis. Surg Obes Relat Dis 2019; 15: 1006–1020. [DOI] [PubMed] [Google Scholar]
- 89. Martin WP, White J, López-Hernández FJ, et al. Metabolic surgery to treat obesity in diabetic kidney disease, chronic kidney disease, and end-stage kidney disease; what are the unanswered questions? Front Endocrinol (Lausanne) 2020; 11: 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Singh P, Adderley N, Subramanian A, et al. The impact of bariatric surgery on incident microvascular complications in patients with type 2 diabetes: a matched controlled population-based retrospective cohort study. Diabetes Care 2021; 44: 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Cohen RV, Pereira TV, Aboud CM, et al. Effect of gastric bypass vs best medical treatment on early-stage chronic kidney disease in patients with type 2 diabetes and obesity: a randomized clinical trial. JAMA Surg 2020; 155: e200420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med 2009; 169: 1156–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Bakris GL, Pitt B, Weir MR, et al. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN randomized clinical trial [published correction appears in JAMA 2015; 314: 731. Dosage error in article text]. JAMA 2015; 314: 151–161. [DOI] [PubMed] [Google Scholar]
- 94. Packham DK, Rasmussen HS, Lavin PT, et al. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med 2015; 372: 222–231. [DOI] [PubMed] [Google Scholar]
- 95. Zhang Y, Xu R, Wang F, et al. Effects and safety of a novel oral potassium-lowering drug-sodium zirconium cyclosilicate for the treatment of hyperkalemia: a systematic review and meta-analysis. Cardiovasc Drugs Ther 2021; 35: 1057–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Reeves WB, Andreoli TE. Transforming growth factor beta contributes to progressive diabetic nephropathy. Proc Natl Acad Sci U S A 2000; 97: 7667–7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kim DJ, Kang JM, Park SH, et al. Diabetes aggravates post-ischaemic renal fibrosis through persistent activation of TGF-β1 and Shh signalling. Sci Rep 2017; 7: 16782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sharma K, Ix JH, Mathew AV, et al. Pirfenidone for diabetic nephropathy. J Am Soc Nephrol 2011; 22: 1144–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Singh H, Miyamoto S, Darshi M, et al. Gut microbial changes in diabetic db/db mice and recovery of microbial diversity upon pirfenidone treatment. Microorganisms 2020; 8: 1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Donate-Correa J, Tagua VG, Ferri C, et al. Pentoxifylline for renal protection in diabetic kidney disease. a model of old drugs for new horizons. J Clin Med 2019; 8: 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Navarro-González JF, Mora-Fernández C, Muros de, Fuentes M, et al. Effect of pentoxifylline on renal function and urinary albumin excretion in patients with diabetic kidney disease: the PREDIAN trial. J Am Soc Nephrol 2015; 26: 220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Singh VP, Bali A, Singh N, et al. Advanced glycation end products and diabetic complications. Korean J Physiol Pharmacol 2014; 18: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Younus H, Anwar S. Prevention of non-enzymatic glycosylation (glycation): implication in the treatment of diabetic complication. Int J Health Sci (Qassim) 2016; 10: 261–277. [PMC free article] [PubMed] [Google Scholar]
- 104. Shen CY, Lu CH, Wu CH, et al. The development of maillard reaction, and advanced glycation end product (AGE)-receptor for AGE (RAGE) signaling inhibitors as novel therapeutic strategies for patients with AGE-related diseases. Molecules 2020; 25: 5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ramis R, Ortega-Castro J, Caballero C, et al. How does pyridoxamine inhibit the formation of advanced glycation end products? The role of its primary antioxidant activity. Antioxidants (Basel) 2019; 8: 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Mogul A, Corsi K, McAuliffe L. Baricitinib: the second FDA-approved JAK inhibitor for the treatment of rheumatoid arthritis. Ann Pharmacother 2019; 53: 947–953. [DOI] [PubMed] [Google Scholar]
- 107. Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with covid-19. N Engl J Med 2021; 384: 795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Tuttle KR, Brosius FC, III, Adler SG, et al. JAK1/JAK2 inhibition by baricitinib in diabetic kidney disease: results from a phase 2 randomized controlled clinical trial. Nephrol Dial Transplant 2018; 33: 1950–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Said E, Zaitone SA, Eldosoky M, et al. Nifuroxazide, a STAT3 inhibitor, mitigates inflammatory burden and protects against diabetes-induced nephropathy in rats. Chem Biol Interact 2018; 281: 111–120. [DOI] [PubMed] [Google Scholar]
- 110. Kim Y, Lim JH, Kim MY, et al. The adiponectin receptor agonist AdipoRon ameliorates diabetic nephropathy in a model of type 2 diabetes. J Am Soc Nephrol 2018; 29: 1108–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kim Y, Park CW. Mechanisms of adiponectin action: implication of adiponectin receptor agonism in diabetic kidney disease. Int J Mol Sci 2019; 20: 1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Sun HJ, Wu ZY, Cao L, et al. Hydrogen sulfide: recent progression and perspectives for the treatment of diabetic nephropathy. Molecules 2019; 24: 2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Fakharzadeh S, Kalanaky S, Argani H, et al. Ameliorative effect of a nano chromium metal-organic framework on experimental diabetic chronic kidney disease. Drug Dev Res 2021; 82: 393–403. [DOI] [PubMed] [Google Scholar]
- 114. Tong Y, Liu S, Gong R, et al. Ethyl vanillin protects against kidney injury in diabetic nephropathy by inhibiting oxidative stress and apoptosis. Oxid Med Cell Longev 2019; 2019: 2129350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Sakuma H, Hagiwara S, Kantharidis P, et al. Potential targeting of renal fibrosis in diabetic kidney disease using MicroRNAs. Front Pharmacol 2020; 11: 587689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Klein J, Caubet C, Camus M, et al. Connectivity mapping of glomerular proteins identifies dimethylaminoparthenolide as a new inhibitor of diabetic kidney disease. Sci Rep 2020; 10: 14898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Barutta F, Bellini S, Corbetta B, et al. The future of diabetic kidney disease management: what to expect from the experimental studies? J Nephrol 2020; 33: 1151–1161. [DOI] [PubMed] [Google Scholar]
- 118. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/home (accessed 23 September 2021).