Abstract
Little information is known about the cisgender women who seek and initiate pre-exposure prophylaxis (PrEP) for HIV prevention in the United States. Adherence Enhancement Guided by Individualized Texting and Drug Levels was a 48-week single-arm open-label demonstration study of daily oral tenofovir disoproxil fumaratel emtricitabine (TDF/FTC) in cisgender women ≥ 18 years old at risk for HIV. Participants were surveyed at screening and enrollment about sociodemographics, HIV risk perception and behaviors, and PrEP perspectives and aggregated into three risk groups according to HIV sexual risk behavior: being in a serodiscordant partnership (SD), engaging in sex work (SW), and having partners with unknown HIV status at risk for HIV (UP). One hundred sixty-seven women presented for screening with n = 31 screen failures. Of the 162 women completing enrollment, mean age was 40 (standard deviation 11), with 41% non-Hispanic Black, 22% non-Hispanic White, and 19% Latina. Compared with those who screened ineligible, enrolled participants were more likely to have heard of PrEP, had higher HIV risk perception, and reported higher perceived PrEP efficacy. Sixty-four women (47%) were categorized as SD, 21 (15%) as SW, and 51 (38%) as UP. The SW were more likely to report higher levels of drinking and drug use (p = 0.002) and history of intimate partner violence in the past year (p < 0.001) compared with SD and UP. Among cisgender women enrolled, there were significant differences between the three risk groups by demographics, HIV risk behavior, and PrEP perspectives, suggesting that interventions to successfully implement PrEP in US women may need to be tailored by HIV risk group.
Clinical Trial Registration number: NCT02584140.
Keywords: pre-exposure prophylaxis (PrEP), cisgender women, HIV prevention, risk behaviors
Introduction
In the United States, cisgender women made up 19% of the nearly 38,000 new HIV infections that were diagnosed in 2018 with Black and Latina cisgender women disproportionately affected by HIV.1 In California, cisgender women made up ∼11% of the 4700 new diagnoses in 2018, of which 25% were Black, 38% Latina, and 38% White, and the rate of Black women living with an HIV diagnosis was 9.3 times than that of White women and for Latina women it was 1.7 times that of White women.2 However, risk in cisgender women has been challenging to characterize and thus deserves more attention to prevent HIV among cisgender women.
The use of pre-exposure prophylaxis (PrEP) has been shown to reduce new infections in cisgender women and other priority populations with adequate adherence.3–7 The Centers for Disease Control and Prevention estimate that 468,000 cisgender women living in the United States may meet eligibility criteria to take and, thus, benefit from PrEP.8 However, the rates of PrEP uptake are particularly low among cisgender women, reported between 5% and 20% of all PrEP prescriptions, with Black and Latina women each accounting for 10% of PrEP initiation while comprising the majority of new HIV infections in cisgender women.9,10
Most of the research on PrEP in cisgender women has been conducted in sub-Saharan and East Africa. Even though PrEP has been approved by the US FDA since 2012, there are still limited data about women currently taking PrEP in the United States. Although there have been more studies in recent years, data from US cisgender women have come largely from focus groups and surveys11–16 and only one published clinical trial.17 Overall, qualitative and quantitative research has found that among US cisgender women, PrEP awareness is low but after education, many women are interested in taking it.
In focus groups of 26 urban Black women, most wanted to take PrEP due to concerns about condom failure and most women preferred a pill to an intravaginal gel.11 A study from a family planning clinic found that 60% of women would consider taking a daily pill, with PrEP acceptability associated with being Black and ever trading sex.13 Finally, in a cross-sectional study of emergency department patients with chief complaints indicative of HIV risk, nearly three-quarters were heterosexual women with PrEP-eligible patients more likely to be interested in learning more about PrEP.16
Data from a US-based phase 2 clinical trial of maraviroc-containing regimens as PrEP similarly underscores PrEP interest among cisgender women. Among 188 cisgender women, there were no new HIV infections and all regimens were safe and well tolerated.17 The study was limited, because women enrolled may have been low-risk for HIV, it was not powered for efficacy, and the study was not designed specifically with a focus on cisgender women. In a substudy that added 26 in-depth interviews to participant survey data from participants, women had positive opinions of PrEP, with 76% saying they would recommend PrEP to others and 59% agreeing that PrEP would be good for anyone.
However, only one-third reported a clear intention to use PrEP after the study finished.18 Further, fewer than 20% felt they had more personal control over HIV prevention with PrEP than with condoms.
Conceptualizing HIV risk, cisgender women at risk for HIV infection due to sexual behaviors who could benefit from PrEP can be broadly considered in three primary risk categories: (1) women in serodiscordant partnerships, (2) sex workers, and (3) women who have partners of unknown HIV status with increased risk for HIV. The third category captures women who are in sexual networks with partners who have higher rates or HIV and sexually transmitted infections (STIs) and where there may be lower awareness of partner status or risk factors. As a result, women in this risk group can be difficult to identify, and thus behavioral health and social and structural factors that increase a women's risk for acquiring HIV may need to be considered.
More than 60% of women at substantial risk for and with HIV have histories of trauma/abuse.19,20 Women who experience intimate partner violence (IPV) can use PrEP in a discreet manner, which offers them autonomy over their sexual health.21 In addition, substance use, trauma, and depression have all been associated with higher sexual risk behaviors such as condomless sex.22,23 Black and Latina women also may have increased HIV risk due to social and structural issues such as racism, poverty, unstable housing, inadequate health insurance, and IPV.24,25 Women seeking and initiating PrEP may have some of these risk factors but have overcome self-silencing and prioritized their health to access PrEP.26 Learning who these women are, what their HIV risk factors are, what they think and know about PrEP, and why they want to take it may help inform future PrEP implementation efforts.
The objectives of this analysis were to evaluate PrEP perspectives and HIV risk perception among cisgender women seeking PrEP and characterize sociodemographics and characteristics associated with HIV risk of the women who enrolled in a US PrEP demonstration project based on HIV sexual risk category.
Methods
Participants and recruitment
Adherence Enhancement Guided by Individualized Texting and Drug Levels (AEGiS) is a 48-week open-label clinical trial designed to evaluate PrEP adherence in HIV-uninfected cisgender women ≥18 years old at substantial risk for HIV in Los Angeles and San Diego counties. Substantial risk was defined as having an HIV-infected partner for at least 4 weeks, engaging in sex work (SW), having taken post-exposure prophylaxis (PEP) in the past year, having a bacterial STI in the past 6 months, or having a partner with unknown HIV status at increased risk for HIV due to injection drug use, bisexual behavior, sex for goods, recent incarceration, from a region with HIV prevalence >1%, or IPV. Additional inclusion criteria included being English or Spanish speaking, HIV-uninfected by fourth generation antigen/antibody assay or antibody assay plus HIV nucleic acid test, and having creatinine clearance >60 mL/min.27
Women interested in PrEP were recruited through flyers and advertisements as well as providers at testing sites, HIV and women's health clinics, and community-based organizations. There were five study sites, with four in Los Angeles—AIDS Project LA, To Help Everyone Health and Wellness Center (THE), Harbor-UCLA and USC—and one study site in San Diego at the Antiviral Research Center at UCSD. Informed consent was obtained at screening with additional visits at weeks 0, 4, 12, 24, 36, and 48 during which HIV testing, safety labs and STI testing (only at weeks 0, 24, and 48) were done in addition to a self-administered computer-assisted survey instrument (CASI). Participants received $10 at screening and $50 for all subsequent completed study visits.
Measurements
Women were aggregated into three HIV risk groups according to sexual HIV risk in the following order: (1) being in a serodiscordant partnership (SD); (2) engaging in SW; and (3) having partners with unknown HIV status at risk for HIV (UP). At their screening and baseline visits, women completed a CASI, which included questions about demographics, HIV risk perception, PrEP awareness, perceived PrEP efficacy, reasons for wanting to take PrEP, preferences for delivery method, substance use, depression, HIV literacy, IPV, and main partner characteristics. A main partner was defined as someone with whom a person has a regular sexual relationship.
The STI testing at baseline and weeks 24 and 48 included syphilis (serum RPR and if positive confirmatory treponemal test) as well as nucleic acid amplification testing of urine and swabs of pharynx, rectum, and vagina (if urine not performed) for chlamydia and gonorrhea (Hologic Aptima).
HIV risk perception was assessed by using the Perceived Risk of HIV Scale.28 For perceived PrEP efficacy, women were asked how effective they thought PrEP was in preventing HIV when someone takes it as prescribed by using a Visual Analogy Scale from 0% to 100%. For motivation to take PrEP, a list of reasons was provided, from which women were asked to select their primary reason for taking PrEP. These were further classified into self- and other-motivated reasons. In terms of preferences for HIV medications, women were asked about preferences for different methods including a daily pill, intermittent pill, microbicide gel, intravaginal ring, injection, and implant by using a 5-point Likert scale.29
Problems resulting from drug and alcohol use were assessed by using the Drug Abuse Screening Test-10 (DAST-10)30 and the Alcohol Use Disorders Identification Test (AUDIT),31 respectively. The severity of depressive symptoms over the past 2 weeks was assessed by using the Patient Health Questionnaire (PHQ)-9.32 HIV literacy was evaluated by using the HIV Knowledge Questionnaire (KQ)-18.33 IPV was assessed by asking about history of physical, sexual, and emotional abuse in the past year.
Statistical analysis
Statistical methods included descriptive analyses and Fisher's exact test for categorical variables or Wilcoxon rank-sum test for continuous variables to assess differences between individuals who enrolled and those who screened ineligible. Multivariable logistic regression analysis was conducted to identify covariates of successful enrollment. HIV risk groups were compared by using Fisher's exact test for categorical variables or Wilcoxon rank-sum test for continuous variables to determine differences by sociodemographics and risk factors associated with HIV. Statistical software R (version 3.6.1) was used for the analysis (www.r-project.org).
Ethical considerations
The research protocol was approved by the relevant Institutional Review Boards at University of California Los Angeles, Harbor-UCLA Medical Center, University of Southern California and University of California San Diego. The study was registered at clinicaltrials.gov.
Results
PrEP screening population and demographics
Between June 2016 and September 2018, 167 cisgender women were screened with 162 completing a screening survey. There were n = 26 screen failures due to not presenting for baseline visit (n = 21), not meeting risk criteria (n = 7), and having unacceptable labs per study protocol (n = 3). Mean age was 40 years (standard deviation 11) with 41% Non-Hispanic Black and 19% Latina. Forty-five percent completed high school/general education diploma or less, 69% earned less than $2000 monthly, and 47% reported unemployment or inability to work. Almost 49% reported a history of IPV in the past year. The most common reasons for wanting to take PrEP were protection from acquiring HIV (65%) and being in a serodiscordant relationship (26%).
Differences by enrollment status
Of the 162 participants who completed a screening survey, 136 enrolled and 26 participants screen-failed. When comparing the two groups, women who enrolled were more likely to have heard of PrEP (p = 0.023), had higher HIV risk perception (p = 0.002), and reported higher perceived PrEP efficacy (p = 0.022). In a multivariable logistic regression, having heard of PrEP [odds ratio (OR): 6.12, 95% confidence interval (CI): 1.8–20.6, p = 0.0035] and reporting higher HIV risk perception (OR: 1.1, 95% CI: 1.0–1.2, p = 0.048) remained statistically significant. In addition, older age was found to be associated with successful enrollment (OR: 1.1, 95% CI: 1.0–1.1, p = 0.049). There were no differences by demographics, HIV risk group, and prior PEP/PrEP use, where they had heard about PrEP, PrEP delivery preference, or reason for wanting to take PrEP (Table 1).
Table 1.
Screening Demographics and HIV Risk Factors by Enrollment Status
| Enrolled n = 136 (%) | Screened ineligible n = 26 (%) | p | |
|---|---|---|---|
| Age mean years (standard deviation) | 40 (11) | 39 (14) | 0.39 |
| Race/ethnicity | 0.36 | ||
| Non-Hispanic White | 30 (22%) | 5 (19%) | |
| Non-Hispanic Black | 52 (38%) | 14 (54%) | |
| Latina | 26 (19%) | 5 (19%) | |
| Other | 28 (21%) | 2 (8%) | |
| Education—≤high school | 61 (45%) | 12 (48%) | 0.69 |
| Income—<$2000 per month | 74 (68%) | 16 (76%) | 0.94 |
| Employment—unemployed/unable to work | 60 (47%) | 12 (48%) | 0.73 |
| Relationship status—single | 53 (40%) | 14 (56%) | 0.76 |
| HIV risk group | 0.23 | ||
| HIV+ partner | 64 (47%) | 9 (41%) | |
| Exchange sex | 21 (15%) | 1 (5%) | |
| Partner with unknown HIV status at risk for HIV | 51 (38%) | 12 (54%) | |
| IPV last year—yes to any | 56 (46%) | 16 (67%) | 0.075 |
| Heard of PrEP—yes | 79 (64%) | 9 (38%) | 0.023 |
| HIV risk perception—mean score (standard deviation) | 27 (6) | 22 (6) | 0.002 |
| Perceived PrEP efficacy—mean VAS score (standard deviation) | 85 (17) | 77 (17) | 0.022 |
| Motivation to take PrEP—self-focused | 79 (63%) | 18 (78%) | 0.23 |
No differences by prior PEP/PrEP use, where heard of PrEP, PrEP delivery preference, or reason for wanting to take PrEP.
p Values of ≤ 0.05 are in bold.
IPV, intimate partner violence; PEP, post-exposure prophylaxis; PrEP, pre-exposure prophylaxis; VAS, Visual Analogy Scale.
Baseline demographics and HIV risk factors by HIV risk group
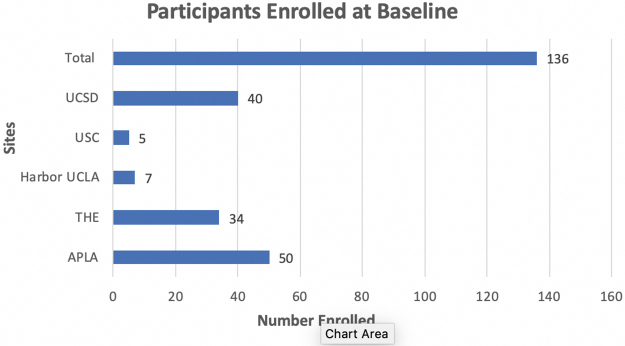
One hundred thirty-six women attended a baseline visit (Fig. 1). In terms of HIV risk group, n = 64 (47%) were in serodiscordant relationships, n = 21 (15%) engaged in exchange sex, and n = 51 (38%) reported partners with unknown risk behaviors. Overall, individuals who engaged in SW were found to have important differences in behaviors that confer HIV risk compared with SD and UP women. The SW had significantly more sex partners (p < 0.001; although baseline STI prevalence was not higher) and were more likely to report problem drinking (p = 0.002) and drug use (p = 0.001) as well as recent IPV history (p < 0.001). In addition, SW had higher HIV literacy scores compared with the other two groups based on KQ-18 results (p = 0.023).
FIG. 1.
Baseline enrollment numbers by site.
Protection from HIV was the most commonly reported reason to take PrEP in nearly all SW (95%) and most women with unknown risk partners versus only one-third of women with partners living with HIV (p < 0.001; Table 2). There were no differences in depression scores, HIV risk perception, perceived PrEP efficacy, and PrEP delivery preference.
Table 2.
Baseline Demographics and Risk Factors by HIV Risk Category
| Serodiscordant n = 64 (%) | SW n = 21 (%) | Unknown partner HIV status n = 51 (%) | p | |
|---|---|---|---|---|
| Age, mean (standard deviation) | 39 (10) | 43 (11) | 40 (12) | 0.41 |
| Race/ethnicity | 0.003 | |||
| Non-Hispanic White | 14 (22%) | 7 (33%) | 9 (18%) | |
| Non-Hispanic Black | 16 (25%) | 10 (48%) | 26 (51%) | |
| Latina | 13 (20%) | 1 (5%) | 12 (24%) | |
| Other | 21 (33%) | 3 (14%) | 4 (8%) | |
| Education—≤high school | 29 (45%) | 10 (48%) | 22 (43%) | 0.95 |
| Income—<$2000 per month | 31 (63%) | 13 (76%) | 30 (70%) | 0.6 |
| Employment—unemployed/unable to work | 27 (44%) | 14 (78%) | 19 (39%) | 0.016 |
| Number of sex partners for past 3 months, median (IQR) | 1 (1–1) | 10 (4–17) | 2 (1–3) | <0.001 |
| STI diagnosed at baseline—positive | 5 (8%) | 4 (20%) | 3 (6%) | 0.17 |
| Reporting current main partner—yes | 51 (80%) | 10 (48%) | 42 (82%) | 0.01 |
| AUDIT—high risk | 1 (2%) | 3 (15%) | 1 (2%) | 0.002 |
| DAST—severe/substantial risk | 3 (5%) | 8 (40%) | 2 (4%) | 0.001 |
| IPV past year—yes to any | 15 (28%) | 16 (76%) | 25 (52%) | <0.001 |
| HIV knowledge, mean score (standard deviation) | 14 (2.9) | 15 (2.7) | 12 (4.2) | 0.023 |
| Motivation to take PrEP—self-focused | 19 (33%) | 20 (95%) | 40 (85%) | <0.001 |
No group differences in depression scores, perceived PrEP efficacy, HIV risk perception, and PrEP delivery preference.
p Values of ≤ 0.05 are in bold.
AUDIT, Alcohol Use Disorders Identification Test; DAST, Drug Abuse Screening Test; IQR, interquartile range; STI, sexually transmitted infection; SW, sex work.
Main partner characteristics
One hundred three participants reported having a main partner. Less than 15% of partners were reported to be abusive or recently incarcerated, and 60% reported circumcision. Nearly 79% were aware of their partner's HIV status, with SD women significantly more likely to report her partner being tested for HIV (98%) and knowing if her main partner was living with HIV (92%) compared with SW (60%/70%) and UP (62%/64%) women (p < 0.001). In the 51 women reporting a partner living with HIV, 96% thought their partner took antiretroviral therapy (ART) and 70% thought they had undetectable viral loads. The SW and UP women compared with SD more frequently suspected partner infidelity with a woman SD (60% and 45% vs. 6%, p < 0.001).
Discussion
Cisgender women screening for a PrEP demonstration project in Southern California were epidemiologically from groups at increased risk of HIV acquisition. Those who went on to enroll had higher HIV risk perception and PrEP awareness than those who did not. Women who enrolled were predominantly in serodiscordant relationships; however, many had partners with unknown HIV risk, and almost one in six engaged in SW. There were significant differences between the three HIV risk groups by sociodemographics, PrEP motivations, and HIV risk factors.
Self-perceived HIV risk is critical to PrEP consideration and uptake but may be particularly difficult to assess for cisgender women, as risk is based primarily on known or unknown partner behavior.34 In our study, women who screened ineligible reported lower self-perceived HIV risk. Although this finding may suggest appropriate self-sorting behavior by those at lower risk for HIV, we have limited data to determine whether these women indeed had lower objective HIV transmission risk. Moreover, they were less likely to have heard of PrEP and think PrEP was efficacious. These findings may imply that cisgender women need more PrEP education and greater sexual risk awareness to increase their interest in PrEP since identifying risk in women can be challenging.
As IPV has been identified as a risk factor for HIV acquisition, it follows that nearly 50% of women seeking PrEP would report a recent history of IPV. Although not statistically significant, women who screen-failed were slightly more likely to report IPV compared with those who successfully enrolled in the study. This result supports a finding from one of the family planning survey studies in which individuals with a history of IPV had lower PrEP acceptability.13
Since history of IPV is an identifiable factor that can portend HIV risk, as opposed to other suspected partner behavior, this preliminary finding needs to be evaluated further as it could have important implications for framing PrEP discussions in women with IPV histories (i.e., discrete, alternative method to condoms, protection and safety as benefits).35
There continues to be a debate around whether partners of individuals living with HIV on ART should be offered and take PrEP given the science behind treatment as prevention.36,37 Our findings would suggest that at the very least, the discussion is warranted given women with HIV-infected partners reported that only 70% had achieved viral load suppression, which is likely an inflated approximation as previous studies have found that primary partners tend to overestimate partner viral load status.38 However, these women also reported overall strong relationships with relatively minimal experienced IPV and low concern for partner infidelity.
The decision to start PrEP in a cisgender women in serodiscordant couples should, thus, be individualized based on patient-specific factors, such as safety surrounding conception, feeling empowered about sexual health, and concern of partner's adherence to ART among many others. Although less than half of the women enrolled in AEGiS reported being in a serodiscordant relationship, more than three-quarters of women reported having main partners. Discussing main partner relationships with women who do not report relationships with individuals living with HIV is still important to fully understand the extent of HIV risk and need for ongoing PrEP and additional HIV prevention strategies including partner testing.
Individuals who engage in SW were found to have significant differences from the other two HIV risk groups, with the highest levels of HIV transmission risk as well as behaviors associated with increased HIV acquisition including higher levels of drinking and drug use and history of IPV. These women were also the most difficult to recruit and enroll and made up the smallest percentage of women who enrolled in the study. Our experience and results suggest that there may be a need to employ innovative approaches to reach women who engage in exchange sex, and they may require additional support to address syndemic factors as well as biomedical HIV prevention strategies.
Our study has several limitations. We had a relatively small sample size limiting our ability to draw meaningful conclusions when making comparisons between groups. In addition, our results from a study conducted in Los Angeles and San Diego might not be generalizable to cisgender women in other geographic locations due to different risk factors driving HIV transmission. Finally, we did not control for possible confounders including age and race which may have impacted our results.
Similar to many aspects of HIV treatment and prevention, PrEP implementation will not follow a “one size fits all” model. Based on important differences observed in sociodemographics, PrEP perspectives, and HIV risk factors, we need to empower cisgender women with PrEP knowledge and sexual risk awareness to engage women in PrEP and tailor interventions by primary HIV risk group that will support intersecting needs to increase PrEP uptake.
Acknowledgments
The authors would like to thank the participants for volunteering for this study. They would also like to acknowledge the efforts of the study staff at the study sites (Yvette Longdurian, Elizabeth Lampley, Deedee Pacheco, Leticia Muttera, Susan Alvarado, Javona Wright, Ramiro Correa, Esther Lim, Kelly Walsh, Deanne Fenton, Leah Burke).
Authors' Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by S.J., F.H., R.K., and E.E. The first draft of the article was written by J.B., and all authors commented on previous versions of the article. All authors read and approved the final article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by the California HIV/AIDS Research Program grants EI11-SD-005B and E111-LA-002B. Additional funding includes NIH grants: AI 36214 (CFAR Clinical Investigation and Biostatistics Core); TR001444 (KL2 to JB). This work was also supported by the UCLA Center for HIV Identification, Prevention, and Treatment Services (CHIPTS) NIMH grant P30MH58107; the UCLA Center for AIDS Research (CFAR) grant 5P30AI028697; and the UCLA Clinical Translational Science Institute (CTSI) Grant UL1TR001881. Study drug was provided by Gilead Sciences.
References
- 1. HIV and Women: HIV Diagnoses. 2021. Available at: https://www.cdc.gov/hiv/group/gender/women/diagnoses.html (Last accessed May 25, 2021).
- 2. Local Data: HIV in California. 2021. Available at: https://aidsvu.org/local-data/united-states/west/california/#demographics-2018 (Last accessed May 25, 2021).
- 3. Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012;367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grant RM, Anderson PL, McMahan V, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: A cohort study. Lancet Infect Dis 2014;14:820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010;363:2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012;367:423–434. [DOI] [PubMed] [Google Scholar]
- 7. McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): Effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 2016;387:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smith DK, Van Handel M, Wolitski RJ, et al. Vital signs: Estimated percentages and numbers of adults with indications for preexposure prophylaxis to prevent HIV acquisition—United States, 2015. J Mississippi State Med Assoc 2015;56:364–371. [PubMed] [Google Scholar]
- 9. Bush S, Magnuson D, Rawlings K, et al. Racial characteristics of FTC/TDF for pre-exposure prophylaxis (PrEP) users in the US. Boston, MA: ASM Microbe Conference, 2016. [Google Scholar]
- 10. Mera R, McCallister S, Mayer G, Magnuson D, Rawlings K. Truvada (TVD) for HIV pre-exposure prophylaxis (PrEP) utilization in the United States (2013–2015). Durban, South Africa: 21st International AIDS Conference, 2016. [Google Scholar]
- 11. Flash CA, Stone VE, Mitty JA, et al. Perspectives on HIV prevention among urban Black Women: A potential role for HIV pre-exposure prophylaxis. AIDS Patient Care STDs 2014;28:635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Auerbach JD, Kinsky S, Brown G, Charles V. Knowledge, attitudes, and likelihood of pre-exposure prophylaxis (PrEP) use among US women at risk of acquiring HIV. AIDS Patient Care STDs 2015;29:102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garfinkel DB, Alexander KA, McDonald-Mosley R, Willie TC, Decker MR. Predictors of HIV-related risk perception and PrEP acceptability among young adult female family planning patients. AIDS Care 2017;29:751–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Calabrese SK, Willie TC, Galvao RW, et al. Current US guidelines for prescribing HIV pre-exposure prophylaxis (PrEP) disqualify many women who are at risk and motivated to use PrEP. J Acquir Immune Defic Syndr 2019;81:395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Calabrese SK, Galvao RW, Dovidio JF, et al. Contraception as a potential gateway to pre-exposure prophylaxis: US women's pre-exposure prophylaxis modality preferences align with their birth control practices. AIDS Patient Care STDs 2020;34:132–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kulie P, Castel AD, Zheng Z, et al. Targeted screening for HIV pre-exposure prophylaxis eligibility in two emergency departments in Washington, DC. AIDS Patient Care STDs 2020;34:516–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gulick RM, Wilkin TJ, Chen YQ, et al. Safety and Tolerability of maraviroc-containing regimens to prevent HIV infection in women: A phase 2 randomized trial. Ann Intern Med 2017;167:384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Amico R, Ramirez C, Caplan M, et al. US Women in the HPTN069/ACTG 5305 Phase 2 PrEP Study: A Substudy Evaluating Acceptability and Experiences. Chicago, IL: HIV Research for Prevention, 2016. [Google Scholar]
- 19. Cohen M, Deamant C, Barkan S, et al. Domestic violence and childhood sexual abuse in HIV-infected women and women at risk for HIV. Am J Public Health 2000;90:560–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Willie TC, Stockman JK, Overstreet NM, Kershaw TS. Examining the impact of intimate partner violence type and timing on pre-exposure prophylaxis awareness, interest, and coercion. AIDS Behav 2018;22:1190–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Braksmajer A, Senn TE, McMahon J. The potential of pre-exposure prophylaxis for women in violent relationships. AIDS Patient Care STDs 2016;30:274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brown MJ, Masho SW, Perera RA, Mezuk B, Pugsley RA, Cohen SA. Sex disparities in adverse childhood experiences and HIV/STIs: Mediation of psychopathology and sexual behaviors. AIDS Behavior 2017;21:1550–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee K, Hutton HE, Lesko CR, et al. Associations of drug use, violence, and depressive symptoms with sexual risk behaviors among women with alcohol misuse. Womens Health Issues 2018;28:367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brody LR, Stokes LR, Kelso GA, et al. Gender role behaviors of high affiliation and low self-silencing predict better adherence to antiretroviral therapy in women with HIV. AIDS Patient Care STDs 2014;28:459–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kelso GA, Cohen MH, Weber KM, Dale SK, Cruise RC, Brody LR. Critical consciousness, racial and gender discrimination, and HIV disease markers in African American women with HIV. AIDS Behav 2014;18:1237–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Flash CA, Dale SK, Krakower DS. Pre-exposure prophylaxis for HIV prevention in women: Current perspectives. Int J Womens Health 2017;9:391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. CDC. US Public Health Service: Pre-exposure Prophylaxis for the Prevention of HIV infection in the United States—2017 Update: A clinical practice guideline. 2018. Available at: https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf (Last accessed March 2, 2019).
- 28. Napper LE, Fisher DG, Reynolds GL. Development of the perceived risk of HIV scale. AIDS Behav 2012;16:1075–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eisingerich AB, Wheelock A, Gomez GB, Garnett GP, Dybul MR, Piot PK. Attitudes and acceptance of oral and parenteral HIV preexposure prophylaxis among potential user groups: A multinational study. PLoS One 2012;7:e28238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Skinner HA. The drug abuse screening test. Addict Behav 1982;7:363–371. [DOI] [PubMed] [Google Scholar]
- 31. Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption—II. Addiction 1993;88:791–804. [DOI] [PubMed] [Google Scholar]
- 32. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carey MP, Schroder KE. Development and psychometric evaluation of the brief HIV Knowledge Questionnaire. AIDS Educ Prev 2002;14:172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aaron E, Blum C, Seidman D, et al. Optimizing delivery of HIV preexposure prophylaxis for women in the United States. AIDS Patient Care STDs 2018;32:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Willie TC, Keene DE, Kershaw TS, Stockman JK. “You Never Know What Could Happen”: Women's perspectives of pre-exposure prophylaxis in the context of recent intimate partner violence. Womens Health Issues 2020;30:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med 2016;375:830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rodger AJ, Cambiano V, Bruun T, et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA 2016;316:171–181. [DOI] [PubMed] [Google Scholar]
- 38. Stolte IG, de Wit JB, van Eeden A, Coutinho RA, Dukers NH. Perceived viral load, but not actual HIV-1-RNA load, is associated with sexual risk behaviour among HIV-infected homosexual men. AIDS (London, England) 2004;18:1943–1949. [DOI] [PubMed] [Google Scholar]