Abstract
Significance: Sepsis is a major public health concern, with high mortality and morbidity, especially among patients undergoing trauma. It is characterized by a systemic inflammatory response syndrome (SIRS) occurring in response to infection. Although classically associated with pathogens, many patients with SIRS do not have infection. The variability of the disease course cannot be fully explained by our current understanding of its pathogenesis. Thus, other factors are likely to play key roles in the development and progression of SIRS/sepsis.
Recent Advances: Circulating levels of damage-associated molecular patterns (DAMPs) seem to correlate with SIRS/sepsis morbidity and mortality. Of the known DAMPs, those of mitochondrial (mt) origin have been of particular interest, since their DNA (mtDNA) and formyl peptides (mtFPs) resemble bacterial DNA and peptides, and hence, when released, may be recognized as “danger signals.”
Critical Issues: mtDAMPs released after tissue injury trigger immune responses similar to those induced by pathogens. Thus, they can result in systemic inflammation and organ damage, similar to that observed in SIRS/sepsis. We will discuss recent findings on the roles of mtDAMPs, particularly regarding the less recognized mtFPs, in the activation of inflammatory responses and development of SIRS/sepsis.
Future Directions: There are no established methods to predict the course of SIRS/sepsis, but clinical studies reveal that plasma levels of mtDAMPs may correlate with the outcome of the disease. We propose that non-pathogen-initiated, mtDAMPs-induced SIRS/sepsis events need further studies aimed at early clinical recognition and better treatment of this disease.
Keywords: neutrophils, injury, mitochondrial DAMPs, mitochondrial formyl peptides, innate immunity
Introduction
At least 1.7 million adults develop sepsis each year according to the Centers for Disease Control and Prevention, and this type of infection kills more than 250,000 Americans a year. Sepsis is a major public health concern requiring $38.2 billion or 8.8% of total U.S. hospital costs in 2017. Moreover, sepsis-related costs are rising (73). Based on worldwide data, more than 31 million cases of sepsis and 19 million cases of “severe sepsis” (i.e., sepsis plus organ dysfunction) require intensive care each year (32). Sepsis is also the leading cause of mortality and critical illness in the world, especially in low-income countries where the mortality of sepsis is 50% or higher (32, 95).
The results of the second International Sepsis Definitions Conference defined sepsis as systemic inflammatory response syndrome (SIRS) associated with presumed or confirmed infection (14). “Severe sepsis” indicated additional development of life-threatening organ dysfunction, and “septic shock” implied the additional development of hemodynamic instability. However, the symptoms of sepsis are often non-specific and highly variable, in many cases depending on susceptibility, physiologic reserves, and reaction of the host.
The “classic” signs are tachypnea, temperature >38.3°C (or <36°C), and increased heart rate. Changes in non-specific markers of inflammation, such as white blood cell count >12 × 109/L or leukopenia <4 × 109/L, presence of immature white blood cell forms, or elevated C reactive protein levels, are also common. These changes can then be followed by organ dysfunction and septic shock (71). On the molecular level, there is commonly a so-called “cytokine storm” (30). This is classically described as being initiated by pattern recognition receptors (PRRs) sensing pathogenic patterns such as lipopolysaccharides. However, equally important may be PRR sensing of endogenous molecules such as mitochondrial damage-associated molecular patterns (mtDAMPs), which can turn on the same signal cascades, leading to vast pro-inflammatory cytokine production followed by organ damage, coagulation, and apoptosis, and the vicious circle is completed (15).
In 2016, the summary from the third International Consensus Definitions for Sepsis has proposed a new definition, named Sepsis-3, and described it as “life-threatening organ dysfunction caused by a dysregulated host response to infection” (108, 109, 112). The new definition discarded the use of the term “SIRS” (a diagnostic standard for the past 25 years) in the identification of sepsis and eliminated the term “severe sepsis.” The authors recommended the use of Sequential Organ Failure Assessment (SOFA) scoring to assess the severity of organ dysfunction in a potentially septic patient. The SOFA scoring has often been used in clinical trials. Due to its complexity, however, it is sometimes reduced to the so-called “quick (q)SOFA” score, which includes three clinical parameters: respiratory rate ≥22/min, change in mental status, and a systolic blood pressure ≤100 mmHg.
Nonetheless, these new definitions have limitations, particularly in the early detection of sepsis. Hence, they have encountered criticism (13, 103). It is still not clear whether SIRS criteria or qSOFA score has more prognostic accuracy for in-hospital mortality, thus this needs more research. The Sepsis-3 definition neglects the early (clinical) identification of sepsis before the development of organ failure. Moreover, it relies on descriptions of body responses with no clear biochemical identification of the causes of sepsis. Last, many health care facilities still use older sepsis definitions as drivers of emergency room and intensive care unit protocols.
Damage-Associated Molecular Patterns
Although there has been considerable improvement in the understanding of sepsis pathogenesis in recent years, its classical definition—an exacerbated inflammatory response to pathogens—cannot fully explain some of the abnormal physiological alterations and the unpredictable course of the disease. Moreover, 30% of patients with sepsis-like syndromes are never proven to have infections (48, 94, 130, 131). Therefore, much attention has been turned to other factors that can contribute to the evolution and persistence of the inflammatory state in patients with sepsis.
During infection, the inflammatory response is initiated by recognition of exogenous microbial ligands known as pathogen-associated molecular patterns (PAMPs), by specific PRRs. The PRRs are predominantly expressed on immune cells, but they also exist on resident cells in tissues or even vascular endothelial cells (ECs) (91), and their activation induces the production of pro-inflammatory cytokines, typically beginning with tumor necrosis factor alpha (TNF-α) and interleukin-1 (IL-1) (83). After Matzinger's suggestion that the immune system is also triggered by endogenous “danger” signals released by the body's own injured tissues, many of these endogenous ligands were found to promote immune response (81).
These danger signals, known as DAMPs, are passively released by dying or damaged cells, although some DAMPs can be actively secreted as well. Similar to PAMPs, they can activate the immune system through the activation of classical PRRs. Also, several other non-PRRs recognize DAMPs, including ion channels and G-protein-coupled receptors (89). Activation of both PRRs and non-PRRs by DAMPs promotes sterile inflammation, which is independent of pathogen infection (37).
Numerous endogenous molecules have been identified as DAMPs, and some of them have been shown to have a role in the pathogenesis and prognostic of sepsis. For instance, the first identified DAMP, the high-mobility group box 1 (HMGB1) (133), acts as a late mediator of sepsis (121). High plasma levels of HMGB1 are associated with severity and mortality in sepsis (4, 43). In a murine model of sepsis, targeting HMGB1 increased survival and improved clinical outcomes (118). Heat-shock proteins (HSP), also defined as DAMPs, have a role in the pathophysiology of sepsis as well. Extracellular HSP60 and HSP72 levels are significantly higher in patients with septic shock, and these increased HSP levels have been shown to correlate with worse outcomes (140).
Mitochondrial Damage-Associated Molecular Patterns
Mitochondria are cellular components that produce energy in the form of adenosine triphosphate (ATP). The ATP itself is one of the DAMPs. Injury-induced release of mitochondria and their content increase local ATP levels that can enhance bacterial killing by macrophages in sepsis, acting through P2X7 and P2X4 receptors (18, 19). Mitochondria also produce reactive oxygen species (ROS), which can kill invaders such as bacteria.
Evolutionist Sagan (100a) originally suggested that roughly 2 billion years ago, an anaerobic eukaryotic cell engulfed an aerobic prokaryote, forming an endosymbiotic relationship with the prokaryote, thus creating the organelle that we now recognize as a mitochondrion (26, 100a). Originally, mitochondria contained many molecules common to bacteria simply because they originated from bacteria, but they have adapted to living within cells by exporting most of their genetic material to the nucleus.
Under physiological conditions, mitochondrial components are contained within the cell and although they can be released by cellular injury (62) there was no evidence of active release of mtDAMPs until we recently showed that they can be secreted in extracellular vesicles by monocytes stimulated with lipopolysaccharide (62).
Our group has been interested in DAMPs originating from mitochondria and in their role in injury-related sepsis. We and others have been studying mtDAMPs for more than 10 years and here we overview recent research on mtDAMPs. Though several components of mitochondria may act as DAMPs, including transfactor A, mitochondrial (TFAM), ATP, cardiolipin, cytochrome c, succinate, and mtRNA (72), we will focus on two particular mitochondrial molecules that remain very similar to those of bacteria and thus are of great interest to our group: mitochondrial DNA (mtDNA) and particularly the less recognized mitochondrial N-formyl peptides (mtFPs). We will discuss their ability to induce immune stimulation, immune suppression, and sepsis-like responses. Although mtDAMPs' role in innate immunity is well established (137), their possible function in adaptive immunity has not been well investigated.
mtDNA and Its Sensors: PRRs
Human mtDNA is a circular, double-stranded, highly unmethylated CpG motifs-containing molecule that comprises 37 genes. These genes are encoding 2 ribonucleic acids (RNAs), 22 transfer RNAs, and 13 subunits of the mitochondrial respiratory chain complexes (3). In contrast to nuclear DNA, mtDNA has conserved low-methylated CpG motifs, characteristic of bacterial and viral DNA, and thus can be recognized by classical “foreign” DNA-sensing PRRs such as Toll-like receptor 9 (TLR9), NOD-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome, and stimulatory of interferon genes (STING) (27, 38, 47, 49).
In “resting” cells, TLR9 is located in the endoplasmic reticulum (70) but after activation by CpG DNA it is translocated to the membranes of endosomes and later to lysosomal compartments, where it binds its ligand, CpG DNA, and recruits the intracellular adapter protein myeloid differentiation factor-88 (MyD88). This is followed by activation of nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinases (MAPKs) and induction of inflammatory responses (2, 67). TLR9/MyD88 also activates IL-1 receptor-associated kinase 4 (IRAK4), which activates IRAK1, IRAK2, TNF receptor-associated factor 3, and inhibitory-κB kinase α and finally triggers interferon regulatory factor 7 to induce type I interferon (58, 110).
Activation of NLRP3 inflammasome by mtDNA leads to recruitment and activation of caspase-1, which controls maturation and release of interleukins such as IL-1β and IL-18 whose pro-inflammatory activities regulate host responses to infection and injury (107). And finally, endoplasmic reticulum-resident STING is activated by cyclic guanosine monophosphate-adenosine monophosphate (cGAMP), which is generated after interaction between mtDNA and peri-mitochondrial cyclic GMP-AMP synthase (cGAS). The cGAS-cGAMP-STING signaling pathway leads to interferon regulatory factor 3-dependent expression of type I interferon, known to have antiviral activity (74).
mtFPs and Formyl Peptide Receptors
Unlike eukaryotic cells and similar to bacteria, mitochondria require an N-terminal formyl methionine (fMet) residue to initiate protein synthesis. Formylation of the methionyl group is catalyzed by formyl transferases (Fmt) using formyl tetrahydrofolic acid (formyl-THF) as the formyl donor. In eukaryotes, most of the proteins have their formyl group removed after translation by peptide deformylases (36). Nevertheless, fMet is the amino acid coded by the AUG start codon; therefore, nearly all proteins in the prokaryotic domain have fMet as their N-terminal amino acid (75).
In mitochondria, formylated peptides are synthetized by mitoribosomes located in the mitochondrial matrix. There is no leader sequence in the mitochondrial mRNA to guide mitoribosome binding. Instead, the binding of N-terminal fMet to the mRNA start codon is the initiator of translation, justifying the essential role of fMet in mitochondrial peptide synthesis (6). Thus, mtFPs can be potent chemoattractants for immune cells and platelets that express formyl peptide receptors (FPRs) (20, 101, 104), although, as we have shown, only some mtFPs act in this way (55) as discussed later.
The FPRs are a group of well-conserved G-protein-coupled receptors that play an important role in host defense and inflammatory responses. In humans, three FPRs have been identified, FPR1, FPR2/ALX, and FPR3. These receptors are found in a variety of cells, with the highest expression in neutrophils for FPR1 and FPR2, and monocytes/macrophages for FPR3 (143). The FPRs are activated by a variety of structurally and chemically unrelated ligands, and N-formyl-peptides are the only ligand group common to all three human receptors (17, 46, 143).
FPR1 was the first FPR to be cloned and sequenced and it is the most studied receptor among the three of them (22). The primary ligands for FPR1 are bacterial and mitochondrial N-formylated peptides, which are bound with an affinity that is 100-fold higher than the identical non-formylated versions of those peptides. Based on studies with FPR1 agonists, N-formyl-methionyl-leucyl-phenylalanine (fMLF, also called fMLP), a prototypic representative of bacterial chemotactic factor, was identified as the most potent one (111). Gene-knockout (KO) and single nucleotide polymorphism studies revealed that FPR1 can play a dual role in the pathogenesis of various diseases (128). At the cellular level, polymorphonuclear neutrophil (PMN) stimulation via FPR1 induces release of calcium from intracellular stores as well as chemotaxis, degranulation, ROS, and cytokine production (22, 23), all of which will be discussed later.
Compared with FPR1, FPR2/ALX is more promiscuous and binds fMLF with a much lower affinity (45). This suggests that it may become activated at, rather than at a distance from, inflammatory sites. FPR2 is also capable of binding to lipids and lipid-associated proteins, such as lipoxin A4, serum amyloid A, and oxidized low-density lipoprotein (LDL) (46, 69). Depending on the ligand, FPR2 can induce either pro-inflammatory or anti-inflammatory effects. This dual effect may be mostly due to different dimerization states of the receptors after ligand binding (16).
FPR3 is the least studied FPR, and its physiological function remains poorly understood. Contrary to FPR1 and FPR2, FPR3 is not expressed in PMN. FPR3 is also relatively insensitive to formylated peptides and the endogenous 21–amino acid acetylated amino-terminal peptide F2L is its most specific ligand described to date (84, 95).
The FPRs have also been identified in many species, including rodents, rabbits, and horse, with distinct functional responses to formylated peptides observed (143). In mice, their genome encodes at least seven functional genes for FPRs (125). Gene Fpr1 is clearly the murine equivalent of human FPR1, based on homology, cell expression, and physiological function (44). Human FPR2 has two murine equivalents: gene Fpr2 encodes the ALX receptor specific for lipoxin A4, and gene Fpr3 encodes a receptor, which binds formylated peptides, serum amyloid A, and other similar ligands (46, 69).
It is possible that the murine receptor encoded by the gene Fpr2 also mimics the human FPR3, since both bind F2L (35). Despite the high homology between mouse and human FPR1, murine Fpr1 binds fMLF with a 100-fold lower affinity than does the human receptor (44) and the affinity seems to change considerably depending on the mouse strain (unpublished data). Nevertheless, murine Fpr1 is the primary receptor for N-formylated peptides in mice (44).
mtDAMPs in Pathophysiology of SIRS/Sepsis
Similar to bacteria that may reach the circulation, mtDAMPs may be found in blood after organ injury, trauma, or even a major surgery (102, 124, 147). When mtDAMPs are released into circulation, they are recognized by the immune system, driving inflammatory responses (22, 134) and there are increasing numbers of reports indicating that mtDAMPs play an important role in human diseases (7, 39).
We and others have shown that patients undergoing trauma have higher levels of plasma mtDNA compared with healthy subjects (65, 102, 141, 147). Increased plasma mtDNA levels are usually observed within 90 min after a serious injury, such as traffic collisions or falls, and they originate from liver injury, muscle crush injury or bone fracture, and may stay elevated for at least 24 h after trauma (147). The levels of mtDNA in the blood of such patients could be thousands of times higher compared with healthy controls (147). Damage to cells that are rich in mitochondria, such as liver cells, may release more mtDNA into circulation. Plasma mtDNA concentration has been found to be significantly elevated on the first day after trauma and much higher in the non-survivors group (141).
Severe trauma is often followed by uncontrolled inflammation (i.e., SIRS) and end-organ damage, with close associations between mtDNA levels and post-injury complications (50, 65, 113). Plasma levels of circulating mtDNA are also significantly increased in severe sepsis/septic shock patients and are much higher than in patients with postoperative inflammation or after traumatic injury (105, 124, 141). Plasma concentration of mtDNA was proposed to be an independent predictor for post-traumatic SIRS (40).
The concept that mtDNA catalyzes sterile inflammation after trauma currently seems to be well accepted but has not yet led to changes in clinical management. The aims would be to confirm mtDNA as a predictor of severity of SIRS/multiple organ failure to optimize the clinical treatment and possibly find therapeutic agents preventing inflammatory effects of mtDNA. Importantly, mtDNA might be a better biomarker than lactate concentration or even SOFA score for predicting the mortality of patients with sepsis after admission in the emergency room (64), hence further studies are of importance.
Contrary to mtDNA, only a limited number of publications can be found on the role of mtFPs in sepsis. Wenceslau et al. suggested that, by acting through FPRs, mtFPs promote inflammation and vascular dysfunction, contributing to the development of sepsis (135). Using a rat model, they showed that mtFPs causes sepsis-like syndrome and cardiovascular collapse as well as hyperthermia, vascular leakage, blood clotting, and lung injury (136). They also applied mtFPs intratracheally and observed PMN migration to the lungs assisted with tracheal, bronchial, and bronchiolar contraction, which was all blocked by a selective antagonist of FPR2, WRW4 (Trp-Arg-Trp-Trp-Trp-Trp-NH2) (139). They concluded that mtFPs released during bacterial infection and trauma/SIRS may induce FPR activation, followed by sepsis-like symptoms and leading to acute lung injury (ALI).
Dorward et al. clearly demonstrated increased levels of mtFPs in bronchoalveolar lavage fluid (BALF) and serum of patients with acute respiratory distress syndrome (ARDS), and they documented FPR1-dependent PMN activation and chemotaxis, both in vivo and in vitro (23). They evaluated whether mtFPs play a role in the regulation of pulmonary migration by applying formyl-methionyl-isoleucyl-threonine (fMIT) to the lungs of wild type (WT) and Fpr1-KO mice. Compared with untreated WT mice, they showed that WT mice treated with cyclosporin H (CsH; FPR1 antagonist) or Fpr1-KO mice had a decreased inflammatory response and reduced PMN migration toward the lungs and, hence, lesser lung injury.
They further validated these results in a mouse sterile lung injury model, in which they intratracheally administered hydrochloric acid to the lungs of WT and Fpr1-KO mice and found a reduced number of PMN in BALF of Fpr1-KO mice, although the total number of circulating PMN was unchanged (23). The authors concluded that FPR1 plays an important role in PMN migration toward the lungs and thus in lung damage in sterile lung injury and suggested that FPR1 is a potential therapeutic target in sterile ALI.
The mtDAMPs can also induce strong inflammatory responses and sepsis-like symptoms. We have studied their roles and found that many of the signaling pathways that mtDAMPs activate are similar to those activated in SIRS/sepsis. In our systematic approach to recognizing the role and effects of mtDAMPs, we investigated them in both clinical and laboratory settings.
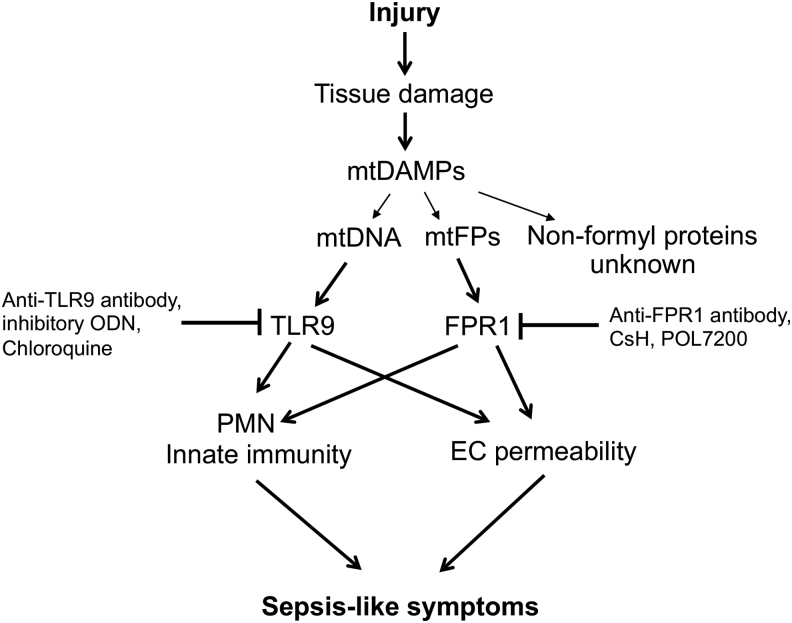
We confirmed the presence of mtDAMPs in patients with trauma, showed exaggerated inflammatory responses triggered by mtDAMPs in animals treated with mtDAMPs, and validated these observations in in vitro experiments. In the latter experiments, we identified the individual roles of mtDNA and mtFPs by blocking their specific receptors or using synthetics mitochondrial f-peptides. Our main findings are outlined in Figure 1 and will be delineated in the next sections. We will show how our studies, though still ongoing, help shed light on the complex pathology of SIRS/sepsis.
FIG. 1.
A scheme depicting the effects of mtDAMPs released after injury on activation of various receptors and their respective signaling pathways, leading to sepsis-like symptoms. Ab, antibody; mtDAMPs, mitochondrial damage-associated molecular patterns.
mtDAMPs Induce Systemic Inflammation and Organ Damage
In general, mtDAMPs that we applied in our experiments were prepared by using frozen liver samples. First, we prepared mitochondria; then, we sonicated them as described elsewhere (96, 147). For in vivo experiments, mtDAMPs prepared from 10% of a whole liver were administered intraperitoneally unless otherwise noted. Thus, mtDAMPs contained mtFPs, mtDNA, and non-formylated proteins.
Early on, we showed that mtDAMPs applied via the tail vein (i.v.) of rats induced ALI, including oxidative damage as observed by immunohistochemistry (Fig. 2A, B). Other markers of inflammatory responses, that is, increased pulmonary albumin permeability, edema, pulmonary IL-6 accumulation, and PMN infiltration into the airways, were also detected (Fig. 2C–H). Moreover, i.v. administration of mtDAMPs increased levels of the pro-inflammatory cytokines, TNF-α, and IL-6 (Fig. 2I, J) in BALF. Matrix metalloproteinase-8 (MMP-8) levels were increased in rat lungs (Fig. 2K) and liver (Fig. 2L), consistent with increased PMN infiltration into lungs (Fig. 2H) (147). These data confirm the potentially harmful pro-inflammatory effects of mtDAMPs in animal models and parallel clinical observations.
FIG. 2.
mtDAMPs cause systemic inflammation and organ injury in rats. Tail vein injection of mtDAMPs induced lung injury in rats (A, B) and oxidative damage, demonstrated by 4-hydroxy-2-noneal staining (C, D). mtDAMPs also increased pulmonary permeability (E), lung wet/dry weight (F), accumulation of IL-6 in lung (G), and PMN infiltration into the airways (H). Moreover, mtDAMPs augmented TNF-α (I) and IL-6 (J) levels in lung lavage fluids (BALF), and MMP-8 levels in the lungs (K) and liver (L). All studies were done at least three times, *p < 0.05, ANOVA/post-hoc. Adapted from Zhang et al. (147). BALF, bronchoalveolar lavage fluid; IL, interleukin; MMP-8, matrix metalloproteinase-8; PMN, polymorphonuclear neutrophil; TNF-α, tumor necrosis factor alpha. Color images are available online.
The results from another study suggested that mtDNA might contribute to the initiation of sterile SIRS and lung injury in rats through the activation of the TLR9/NF-κB axis and the induction of pro-inflammatory cytokine expression (145).
mtDAMPs Stimulate PMN
The role of MAPK signaling pathways in the production of inflammatory cytokines is well characterized, and MAPK activities correlate with SIRS/sepsis severity (29, 90, 144). It has also been documented that pro-inflammatory cytokine and MMP levels in BALF and blood are upregulated in sepsis and ALI (33, 116). The MMPs comprise a family of proteases that are widely expressed in developmental, physiological, and pathological processes. They also play broad functions in inflammation and tissue repair (92), often by virtue of their ability to activate other mediators.
Other studies strongly suggest that MMPs are involved in the pathogenesis of sepsis (68, 126, 129). MMP-8 levels in serum are significantly higher in patients with severe sepsis than in healthy controls and higher in non-survivors than in survivors (68). The PMN activation linked to MMP release has also been implicated in the development of sepsis-induced ALI, and MMP inhibition has improved survival in some animal models of sepsis (117). The PMN activation also results in degranulation of MMPs with subsequent extracellular matrix and basement membrane degradation (76). This process has also been implicated in the development of sepsis-induced ALI (117).
Following on from that prior work, we showed that mtDAMPs, indeed, activate p38 and p42/44 (ERK1/2) MAPKs in human PMN (Fig. 3A, B) (147). Moreover, we showed that mtDAMPs induced MMP-8 release from PMN (Fig. 3C, D), which was inhibited by antibodies against FPR1 or by the FPR1 antagonist, CsH. Again, this indicated that mtFPs present in mtDAMPs played a key role in that process. When activated with mtDAMPs, PMN also produce IL-8, a pro-inflammatory chemokine (Fig. 3E, F).
FIG. 3.
mtDAMPs activate PMN. mtDAMPs induce phosphorylation of p38 (A) and p44/42 (ERK1/2) (B) MAPKs in a dose-dependent manner. mtDAMPs activate MMP-8 also in a dose-dependent manner (C) via FPR1 (D). mtDAMPs increase IL-8 production after 4 and as long as 24 h stimulation (E, F). αFPR1 denotes anti-FPR1 antibody treatment. */** denote p < 0.05 versus control. ***denotes p < 0.05 versus control or mtDAMPs. Adapted from Zhang et al. (147). ERK, extracellular signal-regulated kinase; FPR, formyl peptide receptor; MAPK, mitogen-activated protein kinase.
Additional experiments showed that mtDNA or unmethylated CpG oligodeoxynucleotides (ODN; short synthetic DNA fragments that are common in mitochondrial and bacterial DNA, and that can activate TLR9) did not induce the production of IL-8, unless fMLF was applied at the same time. Similarly, mtFPs alone did not elicit IL-8 production, indicating that both mtFPs and mtDNA are required to stimulate production of this important chemokine (147). Phosphorylation and thus activation of p38 and p42/44, which can proceed or follow ROS formation, also triggers neutrophil extracellular trap (NET) formation (41, 59).
mtDNA Induces NETs
The formation of NETs was originally described by Brinkmann in 2004 (10) and has subsequently been investigated by his (8, 9) and other groups (57, 97, 99). Pathogen-activated release of PMN granule proteins such as myeloperoxidase, neutrophil elastase, and cathepsin G occurs along with the release of chromatin. Together, they form extracellular net-like structures that engulf and kill bacteria (10, 127).
Over the past few years NETs have been identified as important participants in the development of sepsis and post-surgical systemic inflammation (12, 21, 106). The NETs can play a dual role in inflammation and other pathophysiological conditions, being either beneficial (i.e., by trapping and eliminating bacteria, fungi, or viruses from the circulation or at infective sites) or detrimental (i.e., by eliciting harmful inflammation and tissue or organ damage in conditions such as sepsis) (88, 100, 123).
Our and another group's data suggest that NETs are formed not only as a defensive response to invading pathogens but also as a response to sterile triggers, such as mtDAMPs (52, 82, 114).
Specifically, we have demonstrated that mtDNA induces human PMN to undergo “NETosis” (Fig. 4A, B) (52). We further showed that this NET formation was TLR9-dependent, as it was completely blocked by the inhibitory ODN TTAGGG that blocks TLR9 signaling (5, 52, 56). It remains to be determined whether mtDNA is the only component of mtDAMPs involved in inducing this effect or whether there are also roles for mtFPs or other mtDAMPs in NETs formation.
FIG. 4.
mtDAMPs induce NETs formation. (A) Untreated PMN do not form NETs. (B) Fifty micrograms per milliliter of mtDNA induced NET formation. Magnification 40 × . DAPI, a strong fluorescent stain of DNA. Adapted from Itagaki et al. (52). DAPI, 4′,6-diamidino-2-phenylindole; mtDNA, mitochondrial DNA; NETs, neutrophil extracellular traps. Color images are available online.
The mtDNA released by tissue/cellular damage after injury or infection (98) has also been reported to induce NETs with a variety of other clinical secondary effects. These include deep vein thrombosis that may contribute to organ injury (34). Moreover, NET formation itself may further increase mtDNA levels. Interestingly, ex vivo generation of NETs has been shown to be downregulated in PMN isolated from patients with sepsis compared with controls (42). It is not clear whether in vivo NETs formation in these patients is also impaired due to sepsis, and whether therefore those PMN kill pathogens less effectively via NETs. Thus clearly, further investigation is necessary in this area.
mtDAMPs Increase EC Permeability
Although sterile SIRS and sepsis may have different etiologies, their pathophysiology overlaps. Thus, each can result in multi-system organ dysfunction and potentially death (28). One of the common characteristics of those conditions is disruption of vascular endothelial barrier function. This can lead to loss of plasma volume, circulatory collapse, and shock or to organ dysfunction due to increased extracellular fluid or tissue edema (135, 136, 138). Thus, vascular leakage may occur early as a cause or later as an effect of such physiologic dysfunction.
Nonetheless, EC dysfunction has commonly been proposed as an early biomarker of sepsis (79, 115). Both bacteria and mtFPs can signal via FPRs expressed on vascular ECs, causing increased EC permeability and thus tissue hypoperfusion (54, 93). However, ECs also express other innate immune receptors, including TLR9, that can be activated by pathogen DNA or mtDNA, inducing inflammatory pathways such as NF-κB and MAPKs. These can modulate coagulation and EC permeability, all of which are involved in the pathology of sepsis (60).
Indeed, we demonstrated that mtDAMPs increased the permeability of EA.hy926 ECs, a cell line derived from the human umbilical cord EC (Fig. 5A) (25, 119). This effect was inhibited by treating mtDAMPs with proteases (Fig. 5B), suggesting that proteins present in the mtDAMPs play an important role in regulating vascular endothelial barrier function. The addition of PMN to this system did not have any effect on mtDAMPs-induced permeability change (Fig. 5C). We did show a role for mtDNA in EC permeability. Chloroquine, which blocks endosomal acidification and thus inhibits TLR9 response to DNA (132, 142), inhibited mtDAMPs-induced EA.hy926 permeability by 50% (Fig. 5D).
FIG. 5.
mtDAMPs induce endothelial permeability. (A–F) EC permeability was measured by the monitoring of changes in transendothelial electrical resistance. Increased capacitance correlates with permeability increase. (A) mtDAMPs increase EC permeability in a dose-dependent manner. (B) Protein components of mtDAMPs play important roles in the regulation of endothelial permeability change. (C) Addition of PMN to mtDAMPs did not affect mtDAMPs-induced permeability change. (D) mtDAMPs-induced endothelial permeability was reduced by 50% after treatment of PMN with chloroquine, suggesting involvement of mtDNA. (E) mtDNA-induced EC permeability was affected by the addition of PMN, implying crosstalk between the cells. (F) mtDNA-induced EC permeability increase was reduced by the addition of TLR9-specific inhibitory ODN. Adapted from Sun et al. (119). EC, endothelial cell; ODN, oligodeoxynucleotides; TLR9, toll-like receptor 9. Color images are available online.
The mtDNA alone also stimulated EC permeability, but contrary to mtDAMPs, co-incubation with PMN here prolonged mtDNA-induced EC permeability for several hours, suggesting a PMN-EC crosstalk (Fig. 5E). The mtDNA-induced EC permeability was blocked by the TLR9-specific inhibitory ODN TTAGGG, again implicating TLR9 in this process (Fig. 5F). Surprisingly, mtFPs had no effect on EA.hy926 EC permeability. This corresponded to a lack of fMLF effects on these cells and indicates an absence of FPRs (119).
We assume that our data, contradictory to those published (136, 138), originate from the phenotype of EA.hy926 cells that we used. This differs from that of primary ECs, which express FPR1 and respond to fMLP and mtFPs by undergoing cytoskeletal changes and increasing their permeability (131). In addition, we observed mtDAMPs-triggered phosphorylation of p38 and p44/42 (ERK1/2) MAPKs and calcium mobilization in EC (119). These pathways can be also involved in stimulating EC permeability. Finally, upregulation of HMGB1 expression, which we showed for the first time in EC exposed to sterile inflammation by mtDAMPs (119), implicates mtDAMPs acting via HMGB1 in an autocrine or paracrine environment that may increase adhesion and endothelial permeability.
mtFPs in Human PMN Activation
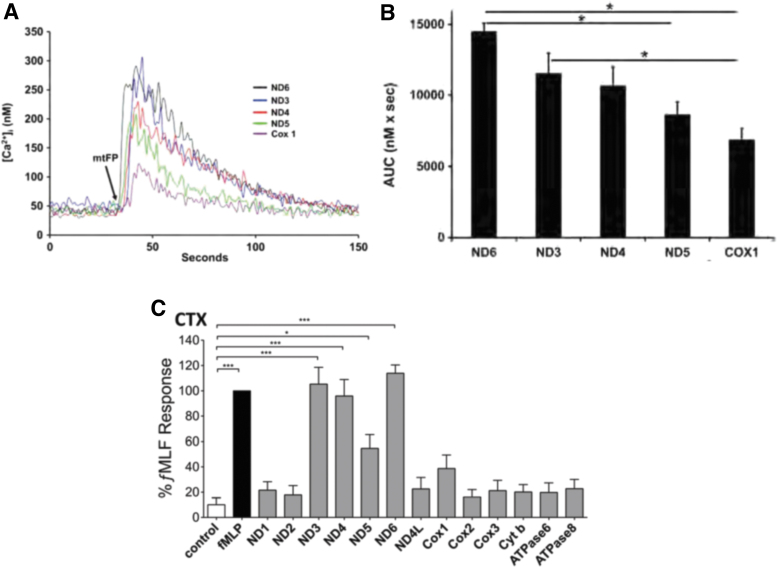
To evaluate their activity as agonists acting on human PMN via FPR1, we synthesized f-peptides based on the N-terminal 6–9 amino acids of the 13 human mitochondrial proteins encoded by mtDNA. These short peptide sequences share ∼70% amino acid identity between humans, mice, and pigs (by our unpublished analysis). To study the inflammatory ability of the native proteins, we therefore studied calcium depletion, chemotaxis, and other PMN functions induced by the action of mtFPs on PMN-FPR1 (55).
Interestingly, only 5 out of the 13 human mtFPs induced calcium depletion from PMN endoplasmic reticulum (ND6>ND3>ND4>ND5>COX1) and chemotaxis was induced by the same mtFPs in the same order of potency (Fig. 6A–C). This order strongly correlates with “BLOcks SUbstitution Matrix 62” (BLOSUM62) score, based on similarity to fMLF. BLOSUM62 score estimates sequence divergence of peptides or proteins based on evolutionary conservation (24). Here, analysis of PMN activation by mtFP closely reflected their evolutionary similarity to bacterial f-peptides and confirmed the minor divergence of human mtFPs from fMLF. Considering this low level of divergence, we think that in addition to their role in infection, mtFPs may play important roles in the activation of innate immune responses to injury and initiation of tissue repair.
FIG. 6.
mtFPs induce Ca2+ mobilization and PMN chemotaxis. Only 5 out of 13 mtFPs induce an increase in the intracellular Ca2+ levels, as analyzed by real traces (A) and the AUC (B) (*p < 0.05). These five mtFPs also induced PMN chemotaxis (C) (*p < 0.05, ***p < 0.001). Adapted from (55). AUC, area under the curve; mtFP, mitochondrial formyl peptide. Color images are available online.
mtFPs Induce ROS Production
Mitochondria are recognized as a major source of intracellular ROS generation (87), and mitochondria-derived ROS induce different biological responses depending on their levels. Low, moderate, and high concentrations of ROS generate metabolic signaling, inflammasome activation, and apoptosis/autophagy, respectively (31). By stimulation of nucleotide-binding domain leukine-rich repeat (NLR) and NLRP3 inflammasome, ROS induce secretion of pro-inflammatory cytokines such as IL-1β and IL-18 (1, 31, 47, 122).
We have also found that some of the six to seven residues of N-terminal human mtFPs we have synthesized also induce PMN ROS formation (unpublished data). In contrast, mtDNA has no effect on ROS generation (62). Interestingly, some synthetic oligonucleotides containing CpG-DNA that have been reported to activate TLR9 may also cause ROS production (63, 85). Whether ROS generation by other mechanisms induces higher expression of genes related to TLR9 pathways needs to be further investigated. However, it is clear that mtFPs released from the tissues/cells after injury may induce additional ROS production by intracellular mitochondrial machinery.
FPR Desensitization Enhances Susceptibility to Secondary Infection
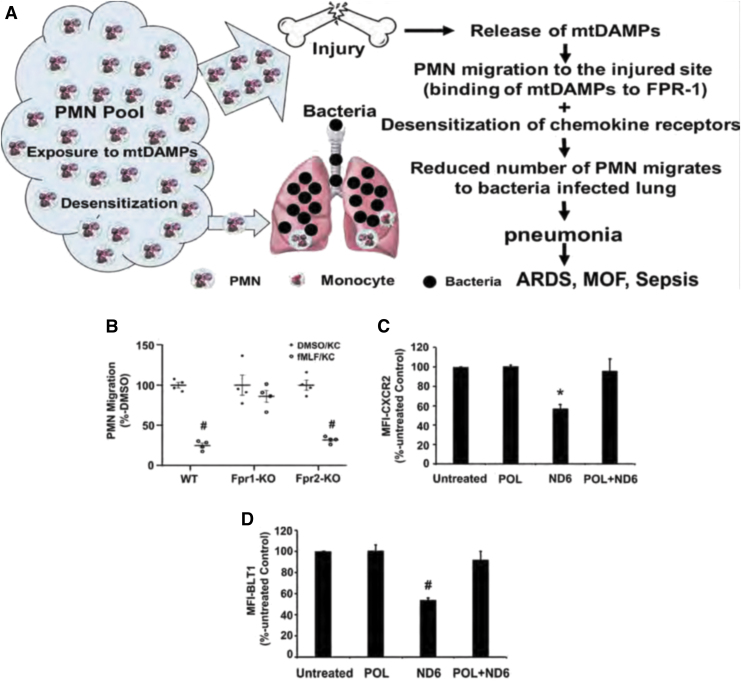
The role of mtFPs in clinical medicine has yet to be fully elucidated. Recently, however, we have shown that the release of mtDAMPs, especially mtFPs from tissues/cells after injury, can serve as an important PMN stimulus, causing them to migrate toward injury sites. This likely reflects both protection against bacterial invasion and the initiation of clearance of cellular debris required as a first step in healing (51). However, the binding of mtFPs to PMN FPR1 leads to desensitization and internalization of multiple PMN chemokine receptors, thus limiting the number of PMN available to migrate toward secondary infection site (Fig. 7A) (51, 53).
FIG. 7.
FPR1 desensitization after injury enhances susceptibility to secondary infection. (A) A scheme of injury-induced bacteria lung infection. mtDAMPs/mtFPs released from the injury site attract numerous PMN, making lungs more susceptible to pathogen infection due to the internalization of chemokine receptors on PMN, hence reducing PMN migration toward bacteria-infected lungs. This may lead to life-threatening ARDS and/or MOF. (B) fMLF-induced PMN heterologous desensitization of chemokine receptors is FPR1 specific. Bone marrow-derived PMN isolated from WT and Fpr2-KO mice were desensitized by exposure to fMLF and, hence, did not migrate to KC. The PMN from Fpr1-KO mice were protected from chemokine receptor desensitization by exposure to fMLF, thus the chemotaxis to KC was not affected. (C, D) Exposure of PMN to the most potent mtFP, ND6, reduced the expression of CXCR2 (C) (*p < 0.05 vs. untreated, POL, and POL+ND6) and BLT1 (D) (#p < 0.05 vs. untreated, POL, and POL+ND6). However, the pretreatment of PMN with POL7200 (POL), a specific antagonist of FPR1, rescued ND6-induced heterologous receptor desensitization/internalization of CXCR2 (C) and BLT1 (D). Cell-membrane expression levels were determined by flow cytometry. (B–D) were adapted from Itagaki et al. (51). ARDS, acute respiratory distress syndrome; BLT1, leukotriene B4 receptor-1; CXCR2, C-X-C motif chemokine receptor 2; fMLF, formyl-methionyl-leucyl-phenylalanine; KC, C-X-C chemokine family of keratinocyte cell-derived chemokine; KO, knockout; MOF, multiple organ failure; WT, wild type. Color images are available online.
The exposure of mouse PMN to fMLF activates FPR1 and then internalizes FPR1 and other chemokine receptors by homo- and heterologous receptor desensitization/internalization, reducing their availability on the cell membrane (22, 66). Accordingly, we have shown that the treatment of PMN isolated from WT and Fpr2-KO mice, but not from Fpr1-KO mice, with fMLF significantly reduces their migration toward the mouse chemokine KC (keratinocyte cell-derived chemokine, CXCL1) (Fig. 7B) (51).
The PMN from Fpr1-KO mice did not undergo chemokine receptor desensitization after exposure to FPs. Similarly, the exposure of human PMN to ND6, which potently mimics in vivo injury by activating FPR1 (55), reduced cell surface expression of C-X-C motif chemokine receptor 2 (CXCR2) (Fig. 7C) and of leukotriene B4 receptor-1 (BLT1) (Fig. 7D). POL7200, a specific antagonist of FPR1, reverted ND6 effects and abolished ND6-induced receptor desensitization and internalization (51). These results indicate that mtFPs may both activate and desensitize various PMN functions.
To recapitulate, mtFPs are released by injury. Although they attract and activate PMN, they may simultaneously suppress PMN chemotaxis toward infective sites where resident monocytes may have already encountered PAMPs and responded by secreting chemokines. Therefore, although counter-intuitive, blockade of FPR1 may prevent desensitization of other receptors and hence improve overall detection and management of pathogens. This may potentially diminish SIRS and protect the host against secondary infection after either tissue trauma or primary infection.
Both trauma and sepsis increase the risk for secondary infection (61, 86, 120). In search for possible future clinical applications, we have used well-established animal injury/lung infection mouse models (53). Administering mtDAMPs intraperitoneally (11) to emulate a physical injury, we have followed intratracheal administration of bacteria to mimic lung inoculation (53). Assuming that FPR1 plays an important role in the impaired bacterial clearance in the lung we delivered the FPR1-antagonist, CsH, which reduced the number of PMN that migrated toward mtDAMPs (injury sites) and improved bacterial clearance in the lung by preventing chemokine receptor internalization (Fig. 8A) (51). This approach will require careful timing and dosing of FPR1 antagonists to obtain beneficial effects in patients with trauma.
FIG. 8.
Proposed approaches into effective bacterial clearance in the lungs after injury. (A) An FPR1 antagonist (CsH) reduces injury-enhanced secondary lung infection. The mtDAMPs were administered intraperitoneally to mice to mimic injury followed by intratracheal instillation of bacteria to mimic bacterial lung infection. Bacteria were counted in lung homogenates. Adapted from Itagaki et al. (51). (B) Airway PMN instillation clears bacteria in lungs after injury. In the experimental in vivo model described in (A), intratracheal administration of exogenous PMN into mouse lungs leads to bacteria clearance in lungs. Adapted from Zhang et al. (146). CsH, cyclosporin H.
In another approach, to increase the number of PMN in bacteria-infected lungs after injury (hence presumably high levels of circulating mtFPs) we intratracheally administered exogenous human PMN and mitigated pulmonary infection (143). Exogenous PMN did not cause any damage to recipient animals in this study, whereas it dramatically alleviated pulmonary infection (Fig. 8B) (146). Again, this approach would require extensive additional work before attempting application in humans, but we presume that exogenous PMN could be beneficial for immune-compromised hosts (i.e., elderly) even where mtDAMPs do not play a major role as a primary condition.
Recently, we have shown that the binding of mtDNA to PMN TLR9 significantly reduces PMN migration toward chemokines (62). Since injury increases mtDNA in circulation, it will also contribute to susceptibility to sepsis by these means. Thus, both mtDAMPs, mtDNA and mtFPs, might worsen bacterial infection via reducing PMN chemotaxis in cases where tissue damage is induced by injury, chemotherapy (114), primary septic events (62), and after major surgeries (102).
Summary
The presented data clearly indicate that multiple components of mtDAMPs, at the very least mtFPs and mtDNA, are involved in the regulation of innate immunity. They are recognized as “non-self” and “danger signals,” much the same as pathogens such as bacteria and viruses.
After being released due to tissue trauma or primary inflammatory events, including organ damage resulting from SIRS/sepsis, mtDAMPs can trigger various signaling pathways in tissue resident cells and immune cells. Most notable among these are PMN, where mtDAMPs induce chemotaxis, as well as activate ROS generation, pro-inflammatory cytokine production, degranulation, cytoskeletal rearrangements, and NET formation. The exposure of PMN to mtDAMPs also induces desensitization of chemokine receptors. These responses, although depending on the intensity and circumstances, directly impact the occurrence, morbidity, and mortality in SIRS/sepsis.
Future Directions
We agree with other groups who emphasize three essential aspects of the approach to sepsis: early recognition and classification of severity, prevention of and support for organ dysfunction based on an optimal oxygen delivery, and treatment of the cause by control of the infective “source” (13, 103).
We believe that our studies on mtDAMPs both contain important knowledge regarding the fundamental mechanisms of SIRS/sepsis that can help in early disease recognition and also propose specific molecular solutions to aspects of inflammation that may be useful in clinical settings. Due to the similarities of PMN responses to PAMPs and DAMPs, immunological responses to those molecular patterns can be difficult to separate and identify.
Still, investigating new approaches may allow insights into this problem, making for a better clinical understanding of SIRS and sepsis. Both mtDNA and mtFPs can be measured and used as biomarkers, thus helping target patients who may benefit from specific treatment of downstream signaling pathways, such as TLR9, NLPR3, STING, or PRR1.
The reduction of plasma mtDNA and mtFPs by various methods, such as using DNase (to eliminate mtDNA effects) or deformylases (to remove a “formyl tag” from formylated proteins/peptides that makes them recognizable as bacteria-like “non-self”), might be considered as therapies for sepsis-like symptoms (77, 80). Also, specific receptor-blocking antibodies or new and potent FPR1 antagonists that can prevent binding of mtFPs released by injury to FPR1 may increase the number of PMN available to combat secondary infections via other chemokine receptors, making pneumonia, sepsis, and ARDS after trauma potentially preventable. All these approaches will require careful evaluation before they can be used clinically.
Abbreviations Used
- Ab
antibody
- ALI
acute lung injury
- ARDS
acute respiratory distress syndrome
- ATP
adenosine triphosphate
- AUC
area under the curve
- BALF
bronchoalveolar lavage fluid
- BLT1
leukotriene B4 receptor-1
- cGAMP
cyclic GMP-AMP dinucleotide
- cGAS
cyclic GMP-AMP synthase
- COX1
cyclooxygenase 1
- CsH
cyclosporin H
- CXCR2
C-X-C motif chemokine receptor 2
- DAPI
4′,6-diamidino-2-phenylindole
- EC
endothelial cell
- ERK
extracellular signal-regulated kinase
- fMet
formyl methionine
- fMIT
formyl-methionyl-isoleucyl-threonine
- fMLF
formyl-methionyl-leucyl-phenylalanine; also named fMLP
- FP
formyl peptide
- FPR
formyl peptide receptor
- HMGB1
high-mobility group box protein 1
- HSP
heat-shock proteins
- IL
interleukin
- KC
C-X-C chemokine family of keratinocyte cell-derived chemokine
- KO
knockout
- MAPK
mitogen-activated protein kinase
- MMP-8
matrix metalloproteinase-8
- MOF
multiple organ failure
- mtDAMPs
mitochondrial damage-associated molecular patterns
- mtDNA
mitochondrial DNA
- mtFPs
mitochondrial formyl peptides
- MyD88
myeloid differentiation factor-88
- ND3
mitochondrially encoded NADH dehydrogenase 3
- ND4
mitochondrially encoded NADH-ubiquinone oxidoreductase chain 4
- ND5
mitochondrially encoded NADH-ubiquinone oxidoreductase chain 5
- ND6
mitochondrially encoded NADH-ubiquinone oxidoreductase chain 6
- NETs
neutrophil extracellular traps
- NF-κB
nuclear factor-kappa B
- NLR
nucleotide-binding domain leucine-rich repeat
- NLRP3
NOD-like receptor family, pyrin domain containing 3
- ODN
oligodeoxynucleotides
- PAMP
pathogen-associated molecular pattern
- PMN
polymorphonuclear neutrophil
- PRR
pattern recognition receptor
- RNA
ribonucleic acid
- ROS
reactive oxygen species
- SIRS
systemic inflammatory response syndrome
- SOFA
Sequential Organ Failure Assessment
- THF
tetrahydrofolic acid
- TLR9
toll-like receptor 9
- TNF-α
tumor necrosis factor alpha
- WT
wild type
Author Disclosure Statement
No competing financial interests exist.
Funding Information
The work was funded by NIAID/NIH, grant number 1R03AI135346-01 (Kiyoshi Itagaki) and by the Department of Defense (DoD), grant number W81XWH-16-1-0464 (Carl J. Hauser).
References
- 1. Abais JM, Xia M, Zhang Y, Boini KM, and Li PL. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid Redox Signal 22: 1111–1129, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Akira S and Hoshino K. Myeloid differentiation factor 88-dependent and -independent pathways in toll-like receptor signaling. J Infect Dis 187 Suppl 2: S356–S363, 2003. [DOI] [PubMed] [Google Scholar]
- 3. Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJ, Staden R, and Young IG. Sequence and organization of the human mitochondrial genome. Nature 290: 457–465, 1981. [DOI] [PubMed] [Google Scholar]
- 4. Angus DC, Yang L, Kong L, Kellum JA, Delude RL, Tracey KJ, Weissfeld L, and Gen IMSI. Circulating high-mobility group box 1 (HMGB1) concentrations are elevated in both uncomplicated pneumonia and pneumonia with severe sepsis. Crit Care Med 35: 1061–1067, 2007. [DOI] [PubMed] [Google Scholar]
- 5. Avalos AM and Ploegh HL. Competition by inhibitory oligonucleotides prevents binding of CpG to C-terminal TLR9. Eur J Immunol 41: 2820–2827, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bianchetti R, Lucchini G, Crosti P, and Tortora P. Dependence of mitochondrial protein synthesis initiation on formylation of the initiator methionyl-tRNAf. J Biol Chem 252: 2519–2523, 1977. [PubMed] [Google Scholar]
- 7. Boyapati RK, Tamborska A, Dorward DA, and Ho GT. Advances in the understanding of mitochondrial DNA as a pathogenic factor in inflammatory diseases. F1000Res 6: 1–15, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brinkmann V, Goosmann C, Kuhn LI, and Zychlinsky A. Automatic quantification of in vitro NET formation. Front Immunol 3: 413, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brinkmann V, Laube B, Abu Abed U, Goosmann C, and Zychlinsky A. Neutrophil extracellular traps: how to generate and visualize them. J Vis Exp 1724, 2010. DOI: 10.3791/1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, and Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science 303: 1532–1535, 2004. [DOI] [PubMed] [Google Scholar]
- 11. Cai Y and Kimura S. Noninvasive intratracheal intubation to study the pathology and physiology of mouse lung. J Vis Exp e50601, 2013. DOI: 10.3791/50601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Camicia G, Pozner R, and de Larranaga G. Neutrophil extracellular traps in sepsis. Shock 42: 286–294, 2014. [DOI] [PubMed] [Google Scholar]
- 13. Carneiro AH, Povoa P, and Gomes JA. Dear Sepsis-3, we are sorry to say that we don't like you. Rev Bras Ter Intensiva 29: 4–8, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cecconi M, Evans L, Levy M, and Rhodes A. Sepsis and septic shock. Lancet 392: 75–87, 2018. [DOI] [PubMed] [Google Scholar]
- 15. Chousterman BG, Swirski FK, and Weber GF. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol 39: 517–528, 2017. [DOI] [PubMed] [Google Scholar]
- 16. Cooray SN, Gobbetti T, Montero-Melendez T, McArthur S, Thompson D, Clark AJL, Flower RJ, and Perretti M. Ligand-specific conformational change of the G-protein-coupled receptor ALX/FPR2 determines proresolving functional responses. Proc Natl Acad Sci U S A 110: 18232–18237, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Corminboeuf O and Leroy X. FPR2/ALXR agonists and the resolution of inflammation. J Med Chem 58: 537–559, 2015. [DOI] [PubMed] [Google Scholar]
- 18. Csoka B, Nemeth ZH, Szabo I, Davies DL, Varga ZV, Paloczi J, Falzoni S, Di Virgilio F, Muramatsu R, Yamashita T, Pacher P, and Hasko G. Macrophage P2X4 receptors augment bacterial killing and protect against sepsis. JCI Insight 3: e99431, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Csoka B, Nemeth ZH, Toro G, Idzko M, Zech A, Koscso B, Spolarics Z, Antonioli L, Cseri K, Erdelyi K, Pacher P, and Hasko G. Extracellular ATP protects against sepsis through macrophage P2X7 purinergic receptors by enhancing intracellular bacterial killing. FASEB J 29: 3626–3637, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Czapiga M, Gao JL, Kirk A, and Lekstrom-Himes J. Human platelets exhibit chemotaxis using functional N-formyl peptide receptors. Exp Hematol 33: 73–84, 2005. [DOI] [PubMed] [Google Scholar]
- 21. Denning NL, Aziz M, Gurien SD, and Wang P. DAMPs and NETs in Sepsis. Front Immunol 10: 2536, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dorward DA, Lucas CD, Chapman GB, Haslett C, Dhaliwal K, and Rossi AG. The role of formylated peptides and formyl peptide receptor 1 in governing neutrophil function during acute inflammation. Am J Pathol 185: 1172–1184, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dorward DA, Lucas CD, Doherty MK, Chapman GB, Scholefield EJ, Conway Morris A, Felton JM, Kipari T, Humphries DC, Robb CT, Simpson AJ, Whitfield PD, Haslett C, Dhaliwal K, and Rossi AG. Novel role for endogenous mitochondrial formylated peptide-driven formyl peptide receptor 1 signalling in acute respiratory distress syndrome. Thorax 72: 928–936, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eddy SR. Where did the BLOSUM62 alignment score matrix come from? Nat Biotechnol 22: 1035–1036, 2004. [DOI] [PubMed] [Google Scholar]
- 25. Edgell CJ, McDonald CC, and Graham JB. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci U S A 80: 3734–3737, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Emelyanov VV. Rickettsiaceae, rickettsia-like endosymbionts, and the origin of mitochondria. Biosci Rep 21: 1–17, 2001. [DOI] [PubMed] [Google Scholar]
- 27. Fang C, Wei X, and Wei Y. Mitochondrial DNA in the regulation of innate immune responses. Protein Cell 7: 11–16, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fang H, Jiang W, Cheng J, Lu Y, Liu A, Kan L, and Dahmen U. Balancing Innate Immunity and Inflammatory State via Modulation of Neutrophil Function: A Novel Strategy to Fight Sepsis. J Immunol Res 2015: 187048, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fattahi F, Kalbitz M, Malan EA, Abe E, Jajou L, Huber-Lang MS, Bosmann M, Russell MW, Zetoune FS, and Ward PA. Complement-induced activation of MAPKs and Akt during sepsis: role in cardiac dysfunction. FASEB J 31: 4129–4139, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ferrara JL, Abhyankar S, and Gilliland DG. Cytokine storm of graft-versus-host disease: a critical effector role for interleukin-1. Transplant Proc 25: 1216–1217, 1993. [PubMed] [Google Scholar]
- 31. Finkel T. Signal transduction by mitochondrial oxidants. J Biol Chem 287: 4434–4440, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fleischmann C, Scherag A, Adhikari NKJ, Hartog CS, Tsaganos T, Schlattmann P, Angus DC, and Reinhart K. Assessment of global incidence and mortality of hospital-treated sepsis current estimates and limitations. Am J Respir Crit Care Med 193: 259–272, 2016. [DOI] [PubMed] [Google Scholar]
- 33. Fligiel SEG, Standiford T, Fligiel HM, Tashkin D, Strieter RM, Warner RL, Johnson KJ, and Varani J. Matrix metalloproteinases and matrix metalloproteinase inhibitors in acute lung injury. Hum Pathol 37: 422–430, 2006. [DOI] [PubMed] [Google Scholar]
- 34. Fuchs Ta, Brill A, and Wagner DD. NET impact on deep vein thrombosis. Arterioscler Thromb Vasc Biol 32: 1777–1783, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gao JL, Guillabert A, Hu J, Le Y, Urizar E, Seligman E, Fang KJ, Yuan X, Imbault V, Communi D, Wang JM, Parmentier M, Murphy PM, and Migeotte I. F2L, a peptide derived from heme-binding protein, chemoattracts mouse neutrophils by specifically activating Fpr2, the low-affinity N-formylpeptide receptor. J Immunol 178: 1450–1456, 2007. [DOI] [PubMed] [Google Scholar]
- 36. Giglione C, Serero A, Pierre M, Boisson B, and Meinnel T. Identification of eukaryotic peptide deformylases reveals universality of N-terminal protein processing mechanisms. EMBO J 19: 5916–5929, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gong T, Liu L, Jiang W, and Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol 20: 95–112, 2020. [DOI] [PubMed] [Google Scholar]
- 38. Gray MW, Burger G, and Lang BF. The origin and early evolution of mitochondria. Genome Biol 2: 1018.1–1018.5, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Grazioli S and Pugin J. Mitochondrial damage-associated molecular patterns: from inflammatory signaling to human diseases. Front Immunol 9: 832, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gu X, Yao Y, Wu G, Lv T, Luo L, and Song Y. The plasma mitochondrial DNA is an independent predictor for post-traumatic systemic inflammatory response syndrome. PLoS One 8: e72834, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hakkim A, Fuchs TA, Martinez NE, Hess S, Prinz H, Zychlinsky A, and Waldmann H. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat Chem Biol 7: 75–77, 2011. [DOI] [PubMed] [Google Scholar]
- 42. Hashiba M, Huq A, Tomino A, Hirakawa A, Hattori T, Miyabe H, Tsuda M, and Takeyama N. Neutrophil extracellular traps in patients with sepsis. J Surg Res 194: 248–254, 2015. [DOI] [PubMed] [Google Scholar]
- 43. Hatada T, Wada H, Nobori T, Okabayashi K, Maruyama K, Abe Y, Uemoto S, Yamada S, and Maruyama I. Plasma concentrations and importance of high mobility group box protein in the prognosis of organ failure in patients with disseminated intravascular coagulation. Thromb Haemost 94: 975–979, 2005. [DOI] [PubMed] [Google Scholar]
- 44. He HQ, Liao D, Wang ZG, Wang ZL, Zhou HC, Wang MW, and Ye RD. Functional characterization of three mouse formyl peptide receptors. Mol Pharmacol 83: 389–398, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. He HQ, Troksa EL, Caltabiano G, Pardo L, and Ye RD. Structural determinants for the interaction of formyl peptide receptor 2 with peptide ligands. J Biol Chem 289: 2295–2306, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. He HQ and Ye RD. The formyl peptide receptors: diversity of ligands and mechanism for recognition. Molecules 22: 1–33, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. He Y, Hara H, and Núñez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci 41: 1012–1021, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Heenen S, Jacobs F, and Vincent JL. Antibiotic strategies in severe nosocomial sepsis: why do we not de-escalate more often? Crit Care Med 40: 1404–1409, 2012. [DOI] [PubMed] [Google Scholar]
- 49. Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, and Akira S. A Toll-like receptor recognizes bacterial DNA. Nature 408: 740–745, 2000. [DOI] [PubMed] [Google Scholar]
- 50. Hu Q, Ren J, Wu J, Li G, Wu X, Liu S, Wang G, Gu G, and Li J. Elevated levels of plasma mitochondrial DNA are associated with clinical outcome in intra-abdominal infections caused by severe trauma. Surg Infect 18: 610–618, 2017. [DOI] [PubMed] [Google Scholar]
- 51. Itagaki K, Kaczmarek E, Kwon WYMDPD, Chen L, Vlková B, Zhang Q, Riça I, Yaffe MB, Campbell Y, Marusich MF, Wang JM, Gong WH, Gao JL, Jung F, Douglas G, Otterbein LE, and Hauser CJ. Formyl peptide receptor-1 blockade prevents receptor regulation by mitochondrial danger-associated molecular patterns and preserves neutrophil function after trauma. Crit Care Med 48: e123–e132, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Itagaki K, Kaczmarek E, Lee YT, Tang IT, Isal B, Adibnia Y, Sandler N, Grimm MJ, Segal BH, Otterbein LE, and Hauser CJ. Mitochondrial DNA released by trauma induces neutrophil extracellular traps. PLoS One 10: e0120549, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Itagaki K, Rica I, Zhang J, Gallo D, DePrato M, Otterbein LE, and Hauser CJ. Intratracheal instillation of neutrophils rescues bacterial overgrowth initiated by trauma damage-associated molecular patterns. J Trauma Acute Care Surg 82: 853–860, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Joffre J, Hellman J, Ince C, and Ait-Oufella H. Endothelial responses in sepsis. Am J Respir Crit Care Med 202: 361–370, 2020. [DOI] [PubMed] [Google Scholar]
- 55. Kaczmarek E, Hauser CJ, Kwon WY, Rica I, Chen L, Sandler N, Otterbein LE, Campbell Y, Cook CH, Yaffe MB, Marusich MF, and Itagaki K. A subset of five human mitochondrial formyl peptides mimics bacterial peptides and functionally deactivates human neutrophils. J Trauma Acute Care Surg 85: 936–943, 2018. [DOI] [PubMed] [Google Scholar]
- 56. Kaminski JJ, Schattgen SA, Tzeng T-C, Bode C, Klinman DM, and Fitzgerald KA. Synthetic oligodeoxynucleotides containing suppressive TTAGGG motifs inhibit AIM2 inflammasome activation. J Immunol 191: 3876–3883, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kaplan MJ and Radic M. Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol 189: 2689–2695, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kawagoe T, Sato S, Matsushita K, Kato H, Matsui K, Kumagai Y, Saitoh T, Kawai T, Takeuchi O, and Akira S. Sequential control of Toll-like receptor-dependent responses by IRAK1 and IRAK2. Nat Immunol 9: 684–691, 2008. [DOI] [PubMed] [Google Scholar]
- 59. Keshari RS, Verma A, Barthwal MK, and Dikshit M. Reactive oxygen species-induced activation of ERK and p38 MAPK mediates PMA-induced NETs release from human neutrophils. J Cell Biochem 114: 532–540, 2013. [DOI] [PubMed] [Google Scholar]
- 60. Khakpour S, Wilhelmsen K, and Hellman J. Vascular endothelial cell Toll-like receptor pathways in sepsis. Innate Immun 21: 827–846, 2015. [DOI] [PubMed] [Google Scholar]
- 61. Koch RM, Kox M, Rahamat-Langendoen JC, Timmermans K, van der Hoeven JG, and Pickkers P. Increased risk for secondary infections in trauma patients with viral reactivation. Intensive Care Med 42: 1828–1829, 2016. [DOI] [PubMed] [Google Scholar]
- 62. Konecna B, Park J, Kwon W-Y, Vlkova B, Zhang Q, Huang W, In Kim H, Yaffe MB, Otterbein LE, Itagaki K, and Hauser CJ. Monocyte exocytosis of mitochondrial damps in sepsis suppresses neutrophil chemotaxis. J Trauma Acute Care Surg 90: 46–53, 2020. [DOI] [PubMed] [Google Scholar]
- 63. Krogmann AO, Lüsebrink E, Steinmetz M, Asdonk T, Lahrmann C, Lütjohann D, Nickenig G, and Zimmer S. Proinflammatory stimulation of toll-like receptor 9 with high dose CpG ODN 1826 impairs endothelial regeneration and promotes atherosclerosis in mice. PLoS One 11: e0146326, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kung CT, Hsiao SY, Tsai TC, Su CM, Chang WN, Huang CR, Wang HC, Lin WC, Chang HW, Lin YJ, Cheng BC, Su BY, Tsai NW, and Lu CH. Plasma nuclear and mitochondrial DNA levels as predictors of outcome in severe sepsis patients in the emergency room. J Transl Med 10: 130, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lam NYL, Rainer TH, Chiu RWK, Joynt GM, and Lo YMD. Plasma mitochondrial DNA concentrations after trauma. Clin Chem 50: 213–216, 2004. [DOI] [PubMed] [Google Scholar]
- 66. Lämmermann T and Kastenmüller W. Concepts of GPCR-controlled navigation in the immune system. Immunol Rev 289: 205–231, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, Lien E, Nilsen NJ, Espevik T, and Golenbock DT. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol 5: 190–198, 2004. [DOI] [PubMed] [Google Scholar]
- 68. Lauhio A, Hästbacka J, Pettilä V, Tervahartiala T, Karlsson S, Varpula T, Varpula M, Ruokonen E, Sorsa T, and Kolho E. Serum MMP-8, -9 and TIMP-1 in sepsis: high serum levels of MMP-8 and TIMP-1 are associated with fatal outcome in a multicentre, prospective cohort study. Hypothetical impact of tetracyclines. Pharmacol Res 64: 590–594, 2011. [DOI] [PubMed] [Google Scholar]
- 69. Lee HY, Oh E, Kim SD, Seo JK, and Bae YS. Oxidized low-density lipoprotein-induced foam cell formation is mediated by formyl peptide receptor 2. Biochem Biophys Res Commun 443: 1003–1007, 2014. [DOI] [PubMed] [Google Scholar]
- 70. Leifer CA, Kennedy MN, Mazzoni A, Lee C, Kruhlak MJ, and Segal DM. TLR9 is localized in the endoplasmic reticulum prior to stimulation. J Immunol 173: 1179–1183, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lever A and Mackenzie I. Sepsis: definition, epidemiology, and diagnosis. BMJ Open 335: 879–883, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Li S, Hu Q, Huang J, Wu X, and Ren J. Mitochondria-derived damage-associated molecular patterns in sepsis: from bench to bedside. Oxid Med Cell Longev 2019: 6914849, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Liang L, Moore B, and Soni A. 2020. National Inpatient Hospital Costs: the Most Expensive Conditions by Payer, 2017. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb261-Most-Expensive-Hospital-Conditions-2017.jsp Accessed on March 10, 2021. [PubMed]
- 74. Liu S, Feng M, and Guan W. Mitochondrial DNA sensing by STING signaling participates in inflammation, cancer and beyond. Int J Cancer 139: 736–741, 2016. [DOI] [PubMed] [Google Scholar]
- 75. Mader D, Liebeke M, Winstel V, Methling K, Leibig M, Götz F, Lalk M, and Peschel A. Role of N-terminal protein formylation in central metabolic processes in Staphylococcus aureus. BMC Microbiol 13: 1–9, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Maitra SR, Bhaduri S, Valane PD, Tervahartiala T, Sorsa T, and Ramamurthy N. Inhibition of matrix metalloproteinases by chemically modified tetracyclines in sepsis. Shock 20: 280–285, 2003. [DOI] [PubMed] [Google Scholar]
- 77. Mallavia B, Liu F, Lefrancais E, Cleary SJ, Kwaan N, Tian JJ, Magnen M, Sayah DM, Soong A, Chen J, Saggar R, Shino MY, Ross DJ, Derhovanessian A, Lynch JP, 3rd, Ardehali A, Weigt SS, Belperio JA, Hays SR, Golden JA, Leard LE, Shah RJ, Kleinhenz ME, Venado A, Kukreja J, Singer JP, and Looney MR. Mitochondrial DNA stimulates TLR9-dependent neutrophil extracellular trap formation in primary graft dysfunction. Am J Respir Cell Mol Biol 62: 364–372, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. This reference has been deleted.
- 79. Martin-Fernandez M, Vaquero-Roncero LM, Almansa R, Gomez-Sanchez E, Martin S, Tamayo E, Esteban-Velasco MC, Ruiz-Granado P, Aragon M, Calvo D, Rico-Feijoo J, Ortega A, Gomez-Pesquera E, Lorenzo-Lopez M, Lopez J, Doncel C, Gonzalez-Sanchez C, Alvarez D, Zarca E, Rios-Llorente A, Diaz-Alvarez A, Sanchez-Barrado E, Andaluz-Ojeda D, Calvo-Vecino JM, Munoz-Bellvis L, Gomez-Herreras JI, Abad-Molina C, Bermejo-Martin JF, Aldecoa C, and Heredia-Rodriguez M. Endothelial dysfunction is an early indicator of sepsis and neutrophil degranulation of septic shock in surgical patients. BJS Open 4: 524–534, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Martinez-Quinones P, Komic A, McCarthy CG, Webb RC, and Wenceslau CF. Targeting endothelial barrier dysfunction caused by circulating bacterial and mitochondrial n-formyl peptides with deformylase. Front Immunol 10: 1270, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Matzinger P. An innate sense of danger. Semin Immunol 10: 399–415, 1998. [DOI] [PubMed] [Google Scholar]
- 82. McIlroy DJ, Jarnicki AG, Au GG, Lott N, Smith DW, Hansbro PM, and Balogh ZJ. Mitochondrial DNA neutrophil extracellular traps are formed after trauma and subsequent surgery. J Crit Care 29: 1133 e 1–e5, 2014. [DOI] [PubMed] [Google Scholar]
- 83. Medzhitov R. Origin and physiological roles of inflammation. Nature 454: 428–435, 2008. [DOI] [PubMed] [Google Scholar]
- 84. Migeotte I, Riboldi E, Franssen JD, Grégoire F, Loison C, Wittamer V, Detheux M, Robberecht P, Costagliola S, Vassart G, Sozzani S, Parmentier M, and Communi D. Identification and characterization of an endogenous chemotactic ligand specific for FPRL2. J Exp Med 201: 83–93, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mohamed W, Domann E, Chakraborty T, Mannala G, Lips KS, Heiss C, Schnettler R, and Alt V. TLR9 mediates S. aureus killing inside osteoblasts via induction of oxidative stress. BMC Microbiol 16: 230, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Morgan AS. Risk factors for infection in the trauma patient. J Natl Med Assoc 84: 1019–1023, 1992. [PMC free article] [PubMed] [Google Scholar]
- 87. Murphy MP. How mitochondria produce reactive oxygen species. Biochem J 417: 1–13, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew AA, Phoon MC, Van Rooijen N, and Chow VT. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol 179: 199–210, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Newton K and Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol 4: 1–19, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. O'Sullivan AW, Wang JH, and Redmond HP. NF-κB and P38 MAPK inhibition improve survival in endotoxin shock and in a cecal ligation and puncture model of sepsis in combination with antibiotic therapy. J Surg Res 152: 46–53, 2009. [DOI] [PubMed] [Google Scholar]
- 91. Opitz B, Hippenstiel S, Eitel J, and Suttorp N. Extra- and intracellular innate immune recognition in endothelial cells. Thromb Haemost 98: 319–326, 2007. [PubMed] [Google Scholar]
- 92. Parks WC, Wilson CL, and López-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol 4: 617–629, 2004. [DOI] [PubMed] [Google Scholar]
- 93. Peters K, Unger RE, Brunner J, and Kirkpatrick CJ. Molecular basis of endothelial dysfunction in sepsis. Cardiovasc Res 60: 49–57, 2003. [DOI] [PubMed] [Google Scholar]
- 94. Phua J, Ngerng WJ, See KC, Tay CK, Kiong T, Lim HF, Chew MY, Yip HS, Tan A, Khalizah HJ, Capistrano R, Lee KH, and Mukhopadhyay A. Characteristics and outcomes of culture-negative versus culture-positive severe sepsis. Crit Care 17: R202, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Rabiet MJ, Macari L, Dahlgren C, and Boulay F. N-formyl peptide receptor 3 (FPR3) departs from the homologous FPR2/ALX receptor with regard to the major processes governing chemoattractant receptor regulation, expression at the cell surface, and phosphorylation. J Biol Chem 286: 26718–26731, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Raoof M, Zhang Q, Itagaki K, and Hauser CJ. Mitochondrial peptides are potent immune activators that activate human neutrophils via FPR-1. J Trauma 68: 1328–1332, 2010. [DOI] [PubMed] [Google Scholar]
- 97. Ravindran M, Khan MA, and Palaniyar N. Neutrophil extracellular trap formation: physiology, pathology, and pharmacology. Biomolecules 9: 1–15, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Riley JS and Tait SW. Mitochondrial DNA in inflammation and immunity. EMBO Rep 21: 1–17, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Saffarzadeh M, Cabrera-Fuentes HA, Veit F, Jiang D, Scharffetter-Kochanek K, Gille CG, Rooijakkers SHM, Hartl D, and Preissner KT. Characterization of rapid neutrophil extracellular trap formation and its cooperation with phagocytosis in human neutrophils. Discoveries (Craiova) 2: e19, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, Lohmeyer J, and Preissner KT. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One 7: e32366, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100a. Sagan L. On the origin of mitosing cells. J Theor Biol 14: 255–274, 1967. [DOI] [PubMed] [Google Scholar]
- 101. Salamah MF, Ravishankar D, Vaiyapuri R, Moraes LA, Patel K, Perretti M, Gibbins JM, and Vaiyapuri S. The formyl peptide fMLF primes platelet activation and augments thrombus formation. J Thromb Haemost 17: 1120–1133, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sandler N, Kaczmarek E, Itagaki K, Zheng Y, Otterbein L, Khabbaz K, Liu D, Senthilnathan V, Gruen RL, and Hauser CJ. Mitochondrial DAMPs are released during cardiopulmonary bypass surgery and are associated with postoperative atrial fibrillation. Heart Lung Circ 27: 122–129, 2018. [DOI] [PubMed] [Google Scholar]
- 103. Sartelli M, Kluger Y, Ansaloni L, Hardcastle TC, Rello J, Watkins RR, Bassetti M, Giamarellou E, Coccolini F, Abu-Zidan FM, Adesunkanmi AK, Augustin G, Baiocchi GL, Bala M, Baraket O, Beltran MA, Jusoh AC, Demetrashvili Z, De Simone B, de Souza HP, Cui Y, Davies RJ, Dhingra S, Diaz JJ, Di Saverio S, Dogjani A, Elmangory MM, Enani MA, Ferrada P, Fraga GP, Frattima S, Ghnnam W, Gomes CA, Kanj SS, Karamarkovic A, Kenig J, Khamis F, Khokha V, Koike K, Kok KYY, Isik A, Labricciosa FM, Latifi R, Lee JG, Litvin A, Machain GM, Manzano-Nunez R, Major P, Marwah S, McFarlane M, Memish ZA, Mesina C, Moore EE, Moore FA, Naidoo N, Negoi I, Ofori-Asenso R, Olaoye I, Ordoñez CA, Ouadii M, Paolillo C, Picetti E, Pintar T, Ponce-de-Leon A, Pupelis G, Reis T, Sakakushev B, Kafil HS, Sato N, Shah JN, Siribumrungwong B, Talving P, Tranà C, Ulrych J, Yuan KC, and Catena F. Raising concerns about the Sepsis-3 definitions. World J Emerg Surg 13: 1–9, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Schiffmann E, Corcoran BA, and Wahl SM. N formylmethionyl peptides as chemoattractants for leucocytes. Proc Natl Acad Sci U S A 72: 1059–1062, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Schneck E, Edinger F, Hecker M, Sommer N, Pak O, Weissmann N, Hecker A, Reichert M, Markmann M, Sander M, and Koch C. Blood levels of free-circulating mitochondrial DNA in septic shock and postsurgical systemic inflammation and its influence on coagulation: a secondary analysis of a prospective observational study. J Clin Med 9: 2056, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Schneck E, Mallek F, Schiederich J, Kramer E, Markmann M, Hecker M, Sommer N, Weissmann N, Pak O, Michel G, Hecker A, Padberg W, Boening A, Sander M, and Koch C. Flow cytometry-based quantification of neutrophil extracellular traps shows an association with hypercoagulation in septic shock and hypocoagulation in postsurgical systemic inflammation-a proof-of-concept study. J Clin Med 9: 1–19, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Schroder K and Tschopp J. The inflammasomes. Cell 140: 821–832, 2010. [DOI] [PubMed] [Google Scholar]
- 108. Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, Rubenfeld G, Kahn JM, Shankar-Hari M, Singer M, Deutschman CS, Escobar GJ, and Angus DC. Assessment of clinical criteria for sepsis for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315: 762–774, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, Angus DC, Rubenfeld GD, and Singer M. Developing a newdefinition and assessing newclinical criteria for Septic shock: for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315: 775–787, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, and Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science 300: 1148–1151, 2003. [DOI] [PubMed] [Google Scholar]
- 111. Showell HJ, Freer RJ, Zigmond SH, Schiffmann E, Aswanikumar S, Corcoran B, and Becker EL. The structure-activity relations of synthetic peptides as chemotactic factors and inducers of lysosomal enzyme secretion for neutrophils. J Exp Med 143: 1154–1169, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Signer HB. Responses to injury. Brain Control Responses Trauma 464: 104–107, 1994. [Google Scholar]
- 113. Simmons JD, Lee YL, Mulekar S, Kuck JL, Brevard SB, Gonzalez RP, Gillespie MN, and Richards WO. Elevated levels of plasma mitochondrial DNA DAMPs are linked to clinical outcome in severely injured human subjects. Ann Surg 258: 591–596, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Singel KL, Grzankowski KS, Khan ANMNH, Grimm MJ, D'Auria AC, Morrell K, Eng KH, Hylander B, Mayor PC, Emmons TR, Lénárt N, Fekete R, Környei Z, Muthukrishnan U, Gilthorpe JD, Urban CF, Itagaki K, Hauser CJ, Leifer C, Moysich KB, Odunsi K, Dénes Á, and Segal BH. Mitochondrial DNA in the tumour microenvironment activates neutrophils and is associated with worse outcomes in patients with advanced epithelial ovarian cancer. Br J Cancer 120: 207–217, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Skibsted S, Jones AE, Puskarich MA, Arnold R, Sherwin R, Trzeciak S, Schuetz P, Aird WC, and Shapiro NI. Biomarkers of endothelial cell activation in early sepsis. Shock 39: 427–432, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Solan PD, Dunsmore KE, Denenberg AG, Odoms K, Zingarelli B, and Wong HR. A novel role for matrix metalloproteinase-8 in sepsis. Crit Care Med 40: 379–387, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Steinberg J, Halter J, Schiller HJ, Dasilva M, Landas S, Gatto LA, Maisi P, Sorsa T, Rajamaki M, Lee HM, and Nieman GF. Metalloproteinase inhibition reduces lung injury and improves survival after cecal ligation and puncture in rats. J Surg Res 111: 185–195, 2003. [DOI] [PubMed] [Google Scholar]
- 118. Stevens NE, Chapman MJ, Fraser CK, Kuchel TR, Hayball JD, and Diener KR. Therapeutic targeting of HMGB1 during experimental sepsis modulates the inflammatory cytokine profile to one associated with improved clinical outcomes. Sci Rep 7: 1–14, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Sun S, Sursal T, Adibnia Y, Zhao C, Zheng Y, Li H, Otterbein LE, Hauser CJ, and Itagaki K. Mitochondrial DAMPs increase endothelial permeability through neutrophil dependent and independent pathways. PLoS One 8: e59989, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Sundar KM and Sires M. Sepsis induced immunosuppression: implications for secondary infections and complications. Indian J Crit Care Med 17: 162–169, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Sundén-Cullberg J, Norrby-Teglund A, Rouhiainen A, Rauvala H, Herman G, Tracey KJ, Lee ML, Andersson J, Tokics L, and Treutiger CJ. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit Care Med 33: 564–573, 2005. [DOI] [PubMed] [Google Scholar]
- 122. Swanson KV, Deng M, and Ting JPY. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol 19: 477–489, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Tan C, Aziz M, and Wang P. The vitals of NETs. J Leukoc Biol 2020. [Epub ahead of print]; DOI: 10.1002/JLB.3RU0620-375R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Thurairajah K, Briggs GD, and Balogh ZJ. The source of cell-free mitochondrial DNA in trauma and potential therapeutic strategies. Eur J Trauma Emerg Surg 44: 325–334, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Tiffany HL, Gao JL, Roffe E, Sechler JMG, and Murphy PM. Characterization of Fpr-rs8, an atypical member of the mouse formyl peptide receptor gene family. J Innate Immunity 3: 519–529, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Tong Y, Yu Z, Chen Z, Zhang R, Ding X, Yang X, Niu X, Li M, Zhang L, Billiar TR, Pitt BR, and Li Q. The HIV protease inhibitor Saquinavir attenuates sepsis-induced acute lung injury and promotes M2 macrophage polarization via targeting matrix metalloproteinase-9. Cell Death Dis 12: 1–14, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, and Zychlinsky A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog 5: e1000639, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Vacchelli E, Le Naour J, and Kroemer G. The ambiguous role of FPR1 in immunity and inflammation. Oncoimmunology 9: e1760061-1–e176006 1-3, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Vanlaere I and Libert C. Matrix metalloproteinases as drug targets in infections caused by gram-negative bacteria and in septic shock. Clin Microbiol Rev 22: 224–239, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Vincent JL. The clinical challenge of sepsis identification and monitoring. PLoS Med 13: e1002022, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, and Reinhart K. International study of the prevalence and outcomes of infection in intensive care units. JAMA 302: 2323–2329, 2009. [DOI] [PubMed] [Google Scholar]
- 132. Wagner H. The immunobiology of the TLR9 subfamily. Trends Immunol 25: 381–386, 2004. [DOI] [PubMed] [Google Scholar]
- 133. Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, and Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science 285: 248–251, 1999. [DOI] [PubMed] [Google Scholar]
- 134. Weinberg SE, Sena LA, and Chandel NS. Mitochondria in the regulation of innate and adaptive immunity. Immunity 42: 406–417, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Wenceslau CF, McCarthy CG, Goulopoulou S, Szasz T, NeSmith EG, and Webb RC. Mitochondrial-derived N-formyl peptides: novel links between trauma, vascular collapse and sepsis. Med Hypotheses 81: 532–535, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Wenceslau CF, McCarthy CG, Szasz T, Goulopoulou S, and Clinton Webb R. Mitochondrial N-formyl peptides induce cardiovascular collapse and sepsis-like syndrome. Am J Physiol Heart Circ Physiol 308: 768–777, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Wenceslau CF, McCarthy CG, Szasz T, Spitler K, Goulopoulou S, Webb RC; Working Group on DiCD. Mitochondrial damage-associated molecular patterns and vascular function. Eur Heart J 35: 1172–1177, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Wenceslau CF, McCarthy CG, and Webb RC. Formyl peptide receptor activation elicits endothelial cell contraction and vascular leakage. Front Immunol 7: 1–5, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Wenceslau CF, Szasz T, McCarthy CG, Baban B, NeSmith E, and Webb RC. Mitochondrial N-formyl peptides cause airway contraction and lung neutrophil infiltration via formyl peptide receptor activation. Pulm Pharmacol Ther 37: 49–56, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Wheeler DS, Lahni P, Odoms K, Jacobs BR, Carcillo JA, Doughty LA, and Wong HR. Extracellular heat shock protein 60 (Hsp60) levels in children with septic shock. Inflamm Res 56: 216–219, 2007. [DOI] [PubMed] [Google Scholar]
- 141. Yamanouchi S, Kudo D, Yamada M, Miyagawa N, Furukawa H, and Kushimoto S. Plasma mitochondrial DNA levels in patients with trauma and severe sepsis: time course and the association with clinical status. J Crit Care 28: 1027–1031, 2013. [DOI] [PubMed] [Google Scholar]
- 142. Yasuda H, Leelahavanichkul A, Tsunoda S, Dear JW, Takahashi Y, Ito S, Hu X, Zhou H, Doi K, Childs R, Klinman DM, Yuen PST, and Star RA. Chloroquine and inhibition of Toll-like receptor 9 protect from sepsis-induced acute kidney injury. Am J Physiol Renal Physiol 294: F1050–F1058, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Ye RD, Boulay F, Ji MW, Dahlgren C, Gerard C, Parmentier M, Serhan CN, and Murphy PM. International union of basic and clinical pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol Rev 61: 119–161, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Zhang H, Moochhala SM, and Bhatia M. Endogenous hydrogen sulfide regulates inflammatory response by activating the ERK pathway in polymicrobial sepsis. J Immunol 181: 4320–4331, 2008. [DOI] [PubMed] [Google Scholar]
- 145. Zhang JZ, Liu Z, Liu J, Ren JX, and Sun TS. Mitochondrial DNA induces inflammation and increases TLR9/NF-κB expression in lung tissue. Int J Mol Med 33: 817–824, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 146. Zhang Q, Kwon WY, Vlkova B, Rica I, Kaczmarek E, Park J, Kim HI, Konecna B, Jung F, Douglas G, Otterbein LE, Hauser CJ, and Itagaki K. Direct Airway instillation of neutrophils overcomes chemotactic deficits induced by injury. Shock 2020. [Epub ahead of print]; DOI: 10.1097/SHK.0000000000001691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, and Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464: 104–107, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]