Abstract
The adipose tissue (AT) has a major role in contributing to obesity-related pathologies through regulating systemic immunometabolism. The pathogenicity of the AT is underpinned by its remarkable plasticity to be reprogrammed during obesity, in the perspectives of tissue morphology, extracellular matrix (ECM) composition, angiogenesis, immunometabolic homoeostasis and circadian rhythmicity. Dysregulation in these features escalates the pathogenesis conferred by this endometabolic organ. Intriguingly, the potential to be reprogrammed appears to be an Achilles’ heel of the obese AT that can be targeted for the management of obesity and its associated comorbidities. Here, we provide an overview of the reprogramming processes of white AT (WAT), with a focus on their dynamics and pleiotropic actions over local and systemic homoeostases, followed by a discussion of potential strategies favouring therapeutic reprogramming. The potential involvement of AT remodelling in the pathogenesis of COVID-19 is also discussed.
Keywords: adipose tissue, cellular senescence, chronic inflammation, circadian rhythm, COVID-19, obesity
Introduction
Obesity, now a global epidemic, is a multifaceted metabolic disease characterised by excessive adiposity and adipose tissue (AT) dysfunction [1,2]. It is estimated that more than 1.9 billion adults worldwide are currently living with obesity or are overweight [3]. Obesity is a significant driver of non-communicable diseases and is consistently associated with increased all-cause mortality across different ethnicities [4,5]. On the surface, obesity is a manifestation of chronic overnutrition, especially the overconsumption of ultraprocessed foods, which is compounded by sedentary lifestyles and misaligned diurnal eating patterns/windows [6–8]. However, excessive adiposity per se, even in the absence of other full-fledged pathology, has profound implications on cardiometabolic risk over the course of life span [9,10].
The white AT (WAT), due to its ability in buffering excessive energy and regulating systemic metabolic health, is closely associated with the pathogenesis of obesity-related morbidities and is at the heart of obesity research. In addition to adipocytes, the AT is a heterogeneous organ that harbours diverse cell populations, including immune cells, stromal/stem cells, fibroblasts, endothelial cells (ECs), and smooth muscle cells, which orchestrate AT and systemic homoeostasis [11,12]. The role of WAT goes beyond an energy sink that handles energy surplus. In fact, the crux of the matter in obesity is the pathologic reprogramming of WAT, a multifaceted process that involves the changes in intra- and extra-cellular signalling, cell functionality and identity, cell–cell communications, and extracellular composition. Specifically, the plasticity of WAT allows it to be functionally reprogrammed in response to repeated episodes of feasting through the recruitment of immune cells, secretion of pro-inflammatory adipokines, and remodelling of extracellular matrix (ECM). As a consequence of the maladaptive reprogramming, the WAT develops an inflammatory and fibrotic phenotype associated with cellular senescence, escalating systemic low-grade inflammation and metabolic impairments [13–15].
On the other side of the coin, AT can also engage in thermogenesis or energy burning through uncoupling the oxidative phosphorylation and ATP production, where protons are shuttled through uncoupling protein-1 (UCP1) to generate heat [16]. These thermogenic AT depots, namely the brown or beige AT, are recruited and activated during cold exposure or pharmaceutical induction of adrenergic receptors [17,18]. An appreciated amount of the classical interscapular brown AT, characterised by homogenous multilocular adipocytes and constitutively high expression of UCP1, is present in rodents and human newborns and acts as a defensive mechanism for cold stress [19]. Whereas in the human adults, the interscapular depot is diminished, but alternative thermogenic AT depots consisting of both brown and beige adipocytes [20] are identified around the cervical, supraclavicular, paraspinal and suprarenal regions using the combined position positron emission tomography and computed tomography (PET-CT) [21–23]. Although it remains debatable whether brown/beige AT contributes to a meaningful amount of energy expenditure, hence facilitates weight loss [24], the fact that these depots benefit cardiometabolic health beyond thermogenesis and regulate systemic metabolism [25–29] grants the therapeutic potential of reprogramming the AT into an energy-burning ‘furnace’, which has been eloquently reviewed elsewhere [30]. In this review, we will outline the various facets behind the pathological reprogramming of AT, particularly of the WAT (Figure 1), whose multifarious and dynamic roles during obesity development and progression are discussed.
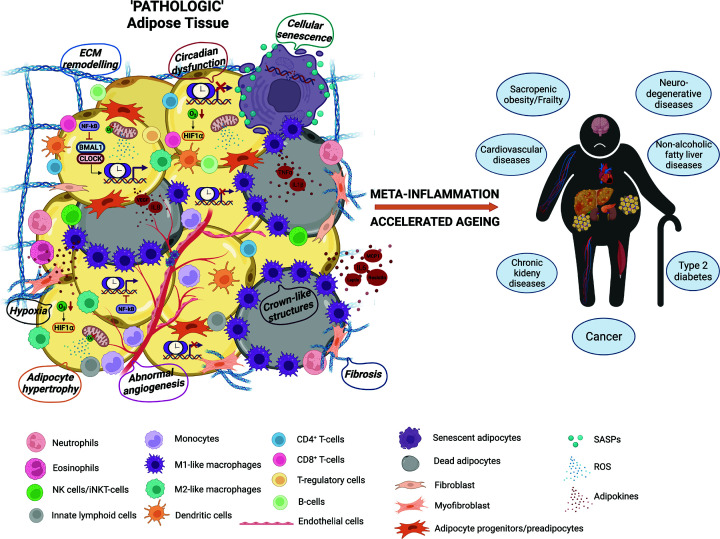
Figure 1. Pathologic reprogramming of WAT as the major culprit of obesity-associated diseases.
As a highly plastic endocrine organ, the AT undergoes extensive remodelling which involves hyperplasia/hypertrophy, fibrosis, angiogenesis and hypoxic response, chronic inflammation, cellular senescence and clock dysfunction. These abnormalities intertwine and escalate meta-inflammation and premature ageing, which ultimately manifest as obesity-related morbidities.
Physical reprogramming of AT
The transition from a lean to an obese phenotype is accompanied by the dimensional expansion of AT, where both hypertrophy (increase in cell size) and hyperplasia (increase in cell numbers) are employed in a depot-specific manner to accommodate the overwhelming energy reserve. Insights regarding the relative contribution of hypertrophy and hyperplasia have been offered by the AdipoChaser mice, in which a high-fat diet (HFD) triggers hypertrophy initially but preferentially induces de novo adipogenesis of the visceral depot in the long term [31]. In rodents, the shift towards hyperplasia likely occurs once the enlarged adipocytes exceed certain threshold volumes. But the story becomes more complicated in humans. It has been suggested that the numbers of adipocytes are similar between normal-weight individuals and patients with obesity, whereas the size of adipocytes becomes distinguishably larger in obesity [32]. Spalding et al. reported that the number of one’s adipocytes is established during childhood and adolescence, which remains stable through a constant rate of turnover and is resistant to change upon weight fluctuations in adulthood [33]. However, people with obesity are also found to reach a higher level of adipocyte number threshold earlier in their life [33]. Interestingly, an subsequent overfeeding trial demonstrated that the abdominal subcutaneous AT (SAT) and mid-thigh SAT respond to a nutritional challenge by hypertrophy and hyperplasia respectively [34]. Nevertheless, hypertrophy in both visceral AT (VAT) and SAT has been suggested as a significant risk factor for cardiometabolic derangements across different ethnicities [35,36]. In fact, hypertrophy, especially in the visceral depot, is recognised as a maladaptive response of WAT, which is accompanied by down-regulation of adipogenesis and insulin sensitivity and but heightened cellular stress and inflammatory profile [37,38]. However, the morphological reprogramming of AT may also exert important physiological and adaptive effects. Smaller adipocytes, compared with their larger counterparts, have been positively associated with inflammatory markers and obesity comorbidities [39,40], supporting that size of adipocytes per se does not contribute to AT dysfunction [40,41]. Instead, the reduced AT expandability and buffering capacity appear to underlie pathological reprogramming [42]. Collectively, both hypertrophy and hyperplasia could mediate human obesity, but the former mode of expansion entails deleterious consequences. What are the consequences of going bigger?
Remodelling of ECM
The ability of WAT to enlarge is facilitated by its ECM, which consists of proteins, polysaccharides, and proteoglycans and offers a flexible mechanical and structural scaffold for AT and a biochemical framework for cell–cell communication [43]. Functional and epigenetic reprogramming of the ECM is crucial for maintaining the microenvironment homoeostasis of AT, hence influencing systemic metabolism and inflammatory milieu [44]. In contrast, AT fibrosis, due to an abnormal build-up of ECM constituents, down-regulates the plasticity of adipocytes and propagates inflammatory signals [45]. However, the link between AT fibrosis and clinical manifestations in humans is yet well-defined and is influenced by AT depot and population ethnicity. Fibrosis in VAT, the depot that is more closely related to obesity complications due to proximity to internal organs [46], seems to be an adaptive response to limit its expansion while promoting hyperplasia and preventing tissue dysfunction. Studies comparing the degree of fibrosis in human AT have suggested an inverse relationship between VAT fibrosis and symptomatology and progression of type 2 diabetes (T2D) and non-alcoholic fatty liver disease, although these might have been confounded by medication use [47,48]. On the other hand, fibrosis in SAT is positively associated with pre-diabetes and insulin resistance (IR), with the relationship being more prominent in Chinese ethnic background [49,50]. In addition, SAT fibrosis has been negatively associated with weight loss after bariatric surgery [51]. Notably, the other study found that the physical stiffness of AT, but not the surrogate marker of fibrosis (collagen), is associated with patients’ clinical presentation [52]. Therefore, more comprehensive methods that include both biochemical and physical measures shall be adopted when assessing AT fibrosis in future.
Fibrosis of WAT is intricately linked to an imbalance between the degradation and synthesis of ECM components. Among these components, collagen proteins, which are primarily secreted from adipocytes, have been extensively investigated. Up-regulation of collagen expression, specifically collagen VI, has been observed in both human and murine obesity and is positively correlated with pathogenic ECM remodelling [53]. In particular, the visceral adipocytes develop a fibroblast-like transcriptome characterised by overt expressions of collagen VI, fibronectin and pro-fibrotic cytokine transforming growth factor-β (Tgfb) in response to HFD [54]. The fibrotic signal in AT is also amplified by suppressing the adipogenic potential, where adipocyte progenitors are rewired into myofibroblast cell fates [55]. Collagen VI knockout mice are protected from genetic and diet-associated weight gain and ectopic fat deposition, accompanied by better insulin sensitivity despite having larger adipocyte size. Similarly, adipocyte-specific overexpression of endotrophin, a cleavage product of collagen VI, induces ECM fibrosis and inflammatory responses, while neutralisation of endotrophin attenuates AT inflammation and confers protection against HFD-induced IR and dyslipidaemia [56]. Mechanistically, deletion of collagen VI reduces the rigidity of ECM through regulating lumican, decorin and elastin, and thereby protects the adipocytes from mechanical stress and necrotic death during expansion, which would otherwise contribute to the formation of ‘crown-like’ structures (CLSs) and an inflammatory signature [53]. Furthermore, the ECM has been reported to reprogramme adipocyte metabolism and contribute to the phenotypic differences between visceral and subcutaneous depots. Culturing visceral adipocytes in subcutaneous ECM rescues HFD-induced IR and promotes adipogenesis, whereas visceral ECM dampens glucose uptake and expression of adipogenic genes in subcutaneous adipocytes [57]. Notably, in brown AT, reduced expressions of Tgfb and its downstream collagen genes are associated with an impairment in brown AT thermogenesis [58]. These findings indicate that the AT resident cells and ECM components act synergistically to facilitate the physiological and pathological AT reprogramming.
Angiogenesis and hypoxia
What prompts the bigger AT to go harder at the same time? Current thinking suggests that dysregulated angiogenesis and associated hypoxia due to a crowded AT niche are the main initiating factors of fibrosis. The AT is among one of the most vascularised tissues with each adipocyte surrounded by a capillary network [59], much like a ‘mesh squishy ball’. Angiogenesis is required to meet the oxygen demand, to deliver the nutrients and hormones, and to remove waste products of the tissue. Recruitment of new blood vessels is initiated by up-regulation of angiogenic factors, including leptin, vascular endothelial growth factor (VEGF) and TGFβ, released from adipocytes, stromal cells, and immune cells. Note that as much as 50% of the AT secretome represents angiogenic modulators [60]. SAT taken from young people with obesity but are otherwise healthy has significantly altered expressions of genes involved in angiogenesis and ECM remodelling, rather than those implicated in inflammatory processes [61]. This implies that the abnormal angiogenesis, together with ECM remodelling, precedes inflammatory cascades in AT. It is likely that angiogenesis is an attempt made by the AT during acute expansion to appropriately reprogramme and accommodate energy influx, while over time the failure to do so subsequently leads to tissue dysfunction. In support of this view, gene expression of VEGF is up-regulated upon 3 days of HFD, while impaired AT capilarisation and abnormal tissue levels of VEGF are associated with human chronic obesity [62], especially among those with IR [63,64]. Administration of anti-angiogenic agents, such as TNP-470, before the development of diet-induced obesity (DIO), has been shown to be anti-obesity [65], implying that initiation of angiogenesis in the lean fat may serve as an essential prerequisite for obesogenesis upon overnutrition. However, the antidiabetic medication thiazolidinedione exerts its anti-inflammatory benefit through promoting capillary density, which helps support the healthy expansion of adipocytes, alleviating hypoxia and inflammation [66]. Likewise, overexpression of VEGF in white and brown AT exhibits increased vascular density of AT and attenuated hypoxia, accompanied with a relative enrichment of anti-inflammatory M2 macrophages, followed by amelioration of DIO and IR [67]. Furthermore, activation of VEGFB/VEGF receptor (VEGFR)-1 signalling confers protection against obesity-induced metabolic complications and AT inflammation through restoring insulin sensitivity and optimal vasculature in VAT, as well as inducing expression of Ucp1 in SAT, implying the role of angiogenesis in supporting beiging [68]. Nevertheless, higher vascular density and an increased expression of VEGFR2 have been observed in VAT compared with the subcutaneous depot, which is coupled with enhanced AT inflammation and hypoxia in participants with obesity, suggesting that angiogenesis mediated by VEGFA/VEGFR2 signalling is associated with a pathogenic visceral microenvironment [69]. Further investigation is required to resolve the mechanisms contributing to the pathologic angiogenesis in obesity as well as the influences of different AT niche and metabolic signals in mediating the physiological roles of angiogenic responses.
In contrast with the oncogenic vascularisation where oxygen insufficiency triggers angiogenesis [70], hypoxia of AT fails to stimulate an angiogenic response, but instead, elicits a reprogramming process mainly mediated by hypoxia-inducible factor-1α (HIF1α) [71]. This is evidenced by the observation that the HIF1α level is significantly increased in VAT after 4 days of HFD, followed by an up-regulation of fibrotic proteins [71]. Furthermore, overexpressing HIF1α induces AT dysfunction, which is reflected by systemic glucose intolerance and an increased immune cell filtration and enhanced fibrosis of AT primarily through up-regulating lysyl oxidase (LOX). The deleterious role of hypoxia is further demonstrated that inhibition of HIF1α, either by treatment with PX-478 or AT-specific deletion, alleviates AT fibrosis and dysfunction and rescues metabolic deterioration and HFD-induced pathologies [72]. The influence of hypoxia on AT fibrotic potential is also detailed by a recent study using an in vitro three-dimensional AT model in which hypoxia leads to a fibrillar assembly of fibronectin, which is a major trigger of aberrant deposition of ECM [73], as well as imposing mechanical stress to adipocytes through the formation of actin stress fibres that amplifies the synthesis of the fibrillar matrix [74]. Interestingly, increased energy expenditure and up-regulated expressions of Ucp1 and Pgc1a in the SAT are also observed in the PX-478-treated mice, demonstrating the AT beiging is mobilised upon inhibiting the AT hypoxia response [75]. Further investigation is needed to unravel whether beiging is secondary to or a direct target of HIF1α inhibition.
Abnormal hypoxia signalling observed in rodents is also mirrored by human AT during obesity, where a reduced oxygen partial pressure (PO2) and/or overexpression of HIF1α in SAT, specifically in AT-derived macrophages (ATMs), have been identified in individuals with metabolically unhealthy obesity compared with lean individuals, although the clinical significance of AT hypoxia is debatable [76–79]. In contrast, Goossens et al. reported that PO2 is paradoxically high in the obese AT [80]. However, the AT PO2 observed in individuals with obesity ranged from 20 to 90 mmHg and overlapped with that of lean subjects. Given that there is no defined cutoff for diagnosing AT hypoxia in humans, it can be argued that higher AT PO2 in participants with obesity is not entirely attributed to physiologically relevant hyperoxia, but instead, to higher adiposity. Intriguingly, the authors also reported that adipocytes of the obese AT have an impaired oxygen uptake and consumption, which is paralleled by mitochondrial dysfunction and reduced AT capillarisation [80]. Although this finding has been recently disputed by Lee et al., who elegantly demonstrated an increased but futile oxygen consumption of adipocytes due to adenosine nucleotide translocase-2 (ANT2)-mediated uncoupled respiration, thereby inducing a relative state of cellular hypoxia [72]. Nevertheless, these findings highlight that oxygen flux within the adipocytes also regulates tissue homoeostasis. Indeed, exposing adipocytes to a hypoxic condition has been shown to alter the adipokine profile and insulin signalling [81,82], but whether the in vitro oxygen deprivation reflects a physiological level of hypoxia is of concern. Furthermore, AT PO2 is inversely associated with genes involving ECM remodelling and inflammatory markers, including interleukin-6 (IL6) and tumour necrosis factor (TNF), while expression of VEGF is positively related to AT oxygenation [77]. Additionally, expression of CD248 in response to hypoxia is positively associated with pathways enriched in blood vessel development, ECM organisation and inflammatory cascades [83]. This is further demonstrated that AT-specific CD248 knockout attenuates hypoxic response and HFD-induced inflammation and fibrosis, accompanied by improvements in adiponectin synthesis and glucose homoeostasis [83]. Collectively, these findings underlie the interdependence between hypoxia and defective angiogenesis in which they work in synergy to favour a pro-fibrotic and pro-inflammatory microenvironment. Ultimately, a pathologic ECM remodelling is coupled with AT immune activation, where inflammation, fibrosis and impaired angiogenesis interact reciprocally to provoke obesity-induced AT dysfunction [2].
Reprogramming by immune modulation
Unlike acute inflammation defined by heat, redness, pain and swelling, the diseased state of obesity is underlaid by chronic sterile inflammation associated with metabolic pathologies or ‘meta-inflammation’ [84], where obesity stimulates a re-composition of the immune architecture within the AT [85]. Importantly, AT inflammation is the dominant contributor to systemic inflammation, thus creating a malignant environment that favours chronic diseases [86,87]. The metabolic and immune regulations of AT are often intertwined, in that an increased and sustained insulin signalling in chronic overnutrition pushes the adipocyte storage capacity beyond the limit, leading to cellular stress and dysfunctional lipid homoeostasis. The failure of buffering excessive lipids by the insulin-resistant AT, coupled with gut dysbiosis, contributes to rising levels of circulating free fatty acids (FFAs) and lipopolysaccharides, which in turn induce nuclear factor (NF)-kB signalling mediated by pattern recognition receptors (PRRs), specifically toll-like receptors on ATMs and adipocytes [88,89]. Induction of PPRs and NF-kB signalling primes NLR family pyrin domain containing - (NLRP3) inflammasome activation, which has a substantial role in AT dysfunction and meta-inflammation [90].
Local expansion and infiltration of ATM are among the phenotypic signatures of an inflamed AT. Specifically, macrophages comprise 4–10% of AT cellularity in lean state, but can increase up to 50% during obesity. Moreover, they are the dominant producers of pro-inflammatory adipokines and hence, major drivers of AT pro-inflammatory reprogramming [91–93]. Such expansion of ATM population is also accompanied by a phenotypic switch from an immune-modulatory M2 state to an immune-active M1 state positive for CD11c, hence leading to up-regulation of pro-inflammatory signalling but the suppressed resolution [94]. Note that conventional dendritic cells (cDCs) in AT also express CD11c but has a tolerogenic phenotype supporting an anti-inflammatory VAT microenvironment [95], hence the definitive roles of DCs and M1 ATMs require careful interpretations. Obesity-induced phenotypic shift and population accrual are also observed in other immune cell types, including a predominance of CD8+ T cells, especially those expressing metallothionein, and Th1 cells over T regulatory cells and Th2 cells [96,97]. These cells in turn aggravate IR and macrophage infiltration [98,99]. Moreover, ablating and restoring the pro- and anti-inflammatory cell lineages respectively have been demonstrated to relieve HFD-induced inflammation and restore insulin sensitivity [100,101].
Recent advances in single-cell RNA sequencing (scRNA-seq) have offered insights into novel AT immune populations, including the previously uncharacterised group 3-innate lymphoid cells (ILC3s), type 2B cDCs and inflammatory macrophages (IMs), and their extensive interactomes, which underpin the AT inflammatory milieu [102]. Specifically, obesity induces expansion and reprogramming of AT-resident cDCs and ILC3s and migratory monocytes, which, under the influence of pro-inflammatory adipokine and hypoxia signalling, preferentially differentiate into IMs and lipid-associated macrophages (LAMs) to avidly produce IL-1B and TNF in the obese fat [102]. Moreover, an enrichment of ILC1s that predominantly produce interferon-γ (IFNγ) in the omental fat is associated with human diabesity, and injection of AT ILC1s leads to VAT fibrosis through promoting M1-like ATM expansion and macrophage TGFβ-Smad3 signalling [103]. Although our understanding regarding AT immunity is largely attributed by studies delineating single cell lineage, it is important to recognise that AT is an active immune reservoir where different immune cell populations work synergistically and additively while interacting with surrounding adipocytes, ECM components and stromal cells to orchestrate the metabolic–immune homoeostasis [104–106]. Hence the extensive cellular cross-talk confers AT immune cells with the remarkable ability to be reprogrammed in response to surrounding niche and nutritional status. Such a dynamic cellular landscape has been elaborated by studies identifying heterogeneous immune populations performing distinct AT-specific physiological and homoeostatic roles [107,108], whose functionalities shift during obesity progression [109]. For example, in contrast with the conventional invariant natural killer T (iNKT) cells that are mainly involved in pro-inflammatory and carcinogenic responses, AT-resident iNKT cells, under the influence of FFA-enriched niche, acquire a unique phenotype that lacks PLZF expression but is positive for T-bet, GATA3 and E4NP4. These PLZFlow iNKT cells have major regulatory roles in lean mice through promoting ATM M2 polarisation and T-regulatory cell expansion mediated by the adipo-cytokines IL10 and IL2, conferring protection against DIO and adipose inflammation [110,111]. Subsequently, by using scRNA-seq, two subsets of AT iNKT cells distinguished by expression of NK1.1 are identified in the obese AT, where NK1.1− and NK1.1+ cells act on the opposites of a spectrum by producing IL10 and IFNγ respectively [112]. Paradoxically, during the lean state, IFNγ produced by NK1.1+ cells perform an anti-inflammatory role by signalling NK cells to protect against pathologic ATM expansion [112]. Similarly, ILC3s, which is a novel cell population only found in human AT, have homoeostatic roles in lean fat through interacting with preadipocytes and adipose ECs but amplify AT inflammation through expressing IL6 [102]. Elucidating the mechanisms behind the fine-tuning of AT immune cell functions by different nutritional status will shed light on new therapies to reprogramme the AT microenvironment.
There is emerging evidence regarding the non-canonical functions of AT immune cells beyond immune-modulation, including angiogenesis and browning/beiging [105,113–115]. In addition to their anti-inflammatory properties, the M2-like ATMs are activated during a cold challenge to secrete the signalling protein SLIT3, which promotes the AT sympathetic nerve fibres to release norepinephrine and to nudge the white adipocytes into thermogenic adipocytes [116]. Similarly, tissue-resident γδ T cells, which are required for T regulatory cell accumulation, have crucial involvement in adaptive thermogenesis and brown AT innervation [58,117]. Conversely, sympathetic neuron-associated macrophages (Cx3cr1+ SAMs) that are recruited to the nerve bundles of WAT during obesity can degrade norepinephrine, which is otherwise crucial for lipolysis and thermogenesis [113]. However, the Cx3cr1+ macrophages are reportedly IL27 producers, where administration of IL27 protects against IR and obesity through its direct actions on Ucp1high adipocytes [118]. Such functional discrepancies of ATMs add another layer of complexity to the heterogeneity of AT immune populations. Future research aiming to leverage this cellular heterogeneity could offer new tools in favouring an adaptive AT immuno-reprogramming.
Indeed, ATM accumulation and CLS formation are hallmarks of AT dysfunction, the recruitment of phagocytic ATMs forming CLS can also be considered an adaptive response by AT to eliminate necrotic debris and to prevent loss of homoeostasis. In contrast with the scRNA-seq study reporting the pro-inflammatory role of LAMs, these CLS-containing LAMs expressing CD9 and Trem-2 also exert beneficial influences over AT remodelling during obesity by counteracting hypertrophy and preventing adipocyte death, while LAM ablation leads to enhanced weight gain and metabolic impairments [119]. Nevertheless, significant increases in LAMs and ATMs undergoing lipid-handling reprogramming have been identified using single-nucleus RNA sequencing in the VAT of obese mice [120], but it is yet to be defined whether such a population shift is a depot-dependent adaptation against the lipid burden or is at the expense of AT functionality. Moreover, abolishing the AT inflammatory signalling has been shown to dampen angiogenesis and inhibit AT browning, which is accompanied by fibrosis and IR, despite a reduced weight gain and an increased M2 ATM polarisation upon HFD [121]. Fasting and calorie restriction (CR), which are potential strategies against obesity comorbidities, are also found to be associated with an enrichment of ATM population during the intervention, specifically phagocytic macrophages, whose roles may include activation of non-canonical lipolysis and clearance of cell debris [122–124]. Again, these results underline the pleiotropic and dynamic roles of AT inflammation across different biological scenarios, and further understanding regarding the temporal control of the AT immune landscape during disease progression is needed.
Reprogramming by cell cycle arrest and senescence
In addition to inducing pathologic AT reprogramming, obesity per se is also a major driver of biological ageing and ageing-associated diseases [125]. In fact, the Mouse Ageing Cell Atlas has shown that ageing is invariably associated with increased cellular and genetic signatures of senescence and inflammation across the 23 tissues/organs, including multiple depots of AT, where ageing-associated senescence also seems to arise the earliest [126–128]. Specifically, an abnormal accumulation of cellular senescence is a common denominator and contributor of ageing- and obesity-associated metabolic pathologies, while removal of senescence burden mitigates HFD-induced metabolic dysfunction, rejuvenate physical resilience, and extend healthy lifespan [129,130]. How do cells become senescent in the first place?
Mechanistically, cell cycle arrest instigated by DNA damage response (DDR) is coupled with and exacerbated by aberrant immune–metabolic responses in obesity, characterised by meta-inflammation, mitochondrial dysfunction and oxidative stress [131,132]. In particular, dysregulated ROS production is integrative to the initiation and maintenance of DDR and cellular senescence through p38 mitogen-activated protein kinase (MAPK) and p21-MAPK14-TGFβ1 signalling pathways [133,134]. Once become senescent, these cells adopt senescence-associated secretory phenotypes (SASPs), which foster inflammatory and fibrotic signalling, as well as propagating senescence in surrounding cells [135]. Note that the pro-inflammatory SASPs are also implicated in immunosurveillance and clearance of precancerous cells, and functionalities of SASPs are shifted to be anti-inflammatory and anti-fibrotic under appropriate spatial and temporal influences [136]. Moreover, the recent proteomic study elucidates the heterogeneous phenotypes of SASPs, which are largely driven by the initial senescence stimuli and the surrounding tissue microenvironment [137]. Hence, defining the AT-specific inducers of cellular senescence and the signature of SASPs are fundamental for understanding their roles in obesity and its associated diseases.
Cellular senescence of AT is one of the pathologic hallmarks of chronological ageing and obesity. Indeed, an obese AT is similar to an ‘ageing’ one, both of which are characterised by a hypertrophic morphology and a pro-inflammatory and pro-fibrotic secretome [138]. In the rodent model of diabesity, increased senescence markers, including p53 and γ-H2AX, of AT are coupled with an imbalance between pro- and anti-inflammatory adipokines and an increased number of pro-inflammatory ATMs [139]. Specifically, the visceral depot seems to be more susceptible to be senescent and expresses higher levels of p53, p21 and p16 during DIO or ageing [140,141]. More importantly, the ‘ageing’ AT can play a dominant role in instigating metabolic diseases since removal or implantation of the senescent AT rescues or exacerbates HFD-induced pre-diabetes respectively. Mice with AT-specific deficiency of p53 also exhibited better maintenance of immune homoeostasis and adipocyte insulin sensitivity when fed a Westernised diet, underscoring the senescence machinery in the pathologic reprogramming [139]. More direct evidence comes from the observation that VAT-specific clearance of cells expressing p21 (p21high), which attenuates AT senescence-associated β-galactosidase (SABG) activity, is sufficient to protect against DIO-induced systemic IR without affecting pancreatic β-cell function, indicating a causal role of the senescent AT in the T2D pathogenesis [142]. Interestingly, the obesity-associated AT senescence also induces anxiety-like behaviours and hypothalamic senescence, probably mediated by the actions of SASPs [143]. Given that the arcuate nucleus of the hypothalamus is the master regulator of appetite and hence the risk of weight gain [144], hypothalamic senescence in obesity may have spillover damage on appetite regulation and facilitates overeating, establishing a vicious cycle of weight gain through the AT–brain cross-talk. But such a hypothesis warrants further investigation.
The associations between AT cellular senescence and disease risk profile are also consistently shown in human studies, where an increased senescence burden in AT has been observed in patients with obesity, pre-diabetes/T2D and pre-frailty [13,145–147]. Importantly, subcutaneous adipocytes in participants with obesity and hyperinsulinemia, which are thought to be post-mitotic and hence less prone to be senescent, are capable of re-entering the cell cycle, evidenced by the expression of cell cycle markers cyclin A2/D1, and thereby proceeding towards senescence. Specifically, such cell cycle reprogramming is characterised by an absence of mitosis and increased nuclear content, known as endoreplication, which is otherwise advantageous for cells during regeneration and compensatory hypertrophy. However, under chronic insulin signalling, these hypertrophic adipocytes fail to be functionally adaptative and acquire senescence phenotypes that pave the way for AT inflammation and metabolic dysfunction. Moreover, in contrast with the animal findings where senescence is often observed in the visceral depot, the authors reported that cellular senescence in humans has a minor role in reshaping the omental AT compared with the subcutaneous depot [13]. It is illuminating to observe how insulin may serve more of a mitotic stimulus than an anabolic signal during metabolic dysfunction, but questions remain on what triggers the adipocytes to respond to insulin by re-entering the cell cycle in a depot-specific manner and whether the downstream targets and signalling cascades of insulin have altered in the senescent cells. Although most evidence has pointed that eliminating the senescence burden is a promising strategy to improve systemic metabolic profile, it is also likely that the pre-senescence signalling helps maximise the storage capacity of the subcutaneous depot, hence alleviating the hypertrophic burden on VAT and preventing systemic lipotoxicity and ectopic fat deposition [148]. Also, adipocytes without growth arrest are more hypertrophied and susceptible to necrotic death. Therefore, defining the dynamics of adaptive and pathologic senescence in obesity may be of relevance in the therapeutical manipulation of AT, especially for the ageing populations.
In addition to adipocytes, senescence is also implicated in AT immune cells, preadipocytes/adipocyte progenitors and ECs. Indeed, by leveraging transcriptomics at the single-cell level, the accumulation of senescent p21high cells in DIO mice are shown to be mostly derived from preadipocytes, ATM and ECs, whose genes are enriched in inflammatory pathways but depleted for angiogenesis [142]. A healthy expansion of AT requires appropriate recruitment of adipocyte progenitors with a proliferative potential to favour hyperplasia over hypertrophy, which is linked to IR and AT mal-reprogramming. However, the reduced adipogenic potential observed in ageing or obesity is not due to a diminished pool of progenitors, but instead, to an increased senescence burden on adipocyte precursors [146]. Importantly, a single injection of senescent preadipocytes is sufficient to induce physical deterioration lasting up to 6 months in young healthy mice [129]. Moreover, these senescent progenitors, which are positive for SABG, γ-H2AX and cyclin-dependent kinase (CDK) inhibitors, have been shown to facilitate AT homing of macrophages and repress the expression of adipogenic regulators peroxisome proliferator-activated receptor-γ (Pparg) and CCAAT/enhancer-binding protein-α (C/ebpa) in non-senescent progenitors through the actions of SASPs. Conversely, ablation of senescent progenitors through targeting the p38MAPK-p16Ink4a pathway or JAK signalling restores adipogenesis, inflammageing and enhances cold-induced beiging of WAT due to increased recruitment of beige precursors [149,150]. Interestingly, elimination of cellular senescence is also associated with preserved fat mass during ageing, where age-dependent lipodystrophy is otherwise implicated in metabolic and physical impairments in the old mice [151,152]. Whether the senescent adipocyte precursors can be a potential target in congenital lipodystrophy, a rare genetic disorder associated with a near-absence of SAT and systemic metabolic dysfunction [153], remains to be clarified. Furthermore, cellular senescence of AT mesenchymal stem cells in the elderly or those with obesity is also associated with a dampened cellular repair and angiogenic response and a reduced expression of Sirtuin-1 (SIRT1), a positive regulator of anti-inflammatory response and AT beiging/browning [145,154,155].
As discussed earlier, a sufficient vascularisation through angiogenesis is critical for supporting adaptive reprogramming of AT, where the angiogenic response is dependent on the extensive cross-talk between adipocytes and adipose tissue endothelial cells (ATECs) [156]. To note, individuals with obesity suffer from ATEC senescence characterised by γ-H2AX expression, and such senescent signature is found more prominent in VAT than SAT, potentially due to differences in depot micro-environment. Importantly, the senescent ECs in the obese VAT also exhibit an up-regulated gene expressions of monocyte chemoattractant protein-1 (MCP1), IL8 and VEGFR2 that encourage VAT angiogenesis and inflammation [69]. Therefore, cellular senescence may be one of the mechanisms explaining the discrepancies in the literature reporting the roles of angiogenesis in obesity, where a senescence-driven angiogenic response reflects a pathological reprogramming of AT vasculature. Intriguingly, both senescence and VEGF signalling reprogramme ECs towards glycolytic metabolism, suggestive of a similar metabolic response to pro-senescence and pro-angiogenic signals [157]. Hence, how senescent ATECs mediate dysfunctional angiogenesis in an obese AT is of future research interest.
ATECs are also involved in AT lipid transport and homoeostasis through the expression of fatty acid transporters and CD36 and secretion of PPARγ ligands, where activation of PPARγ is shown to promote AT angiogenesis and adipogenic differentiation of pre-adipocytes [158,159]. In fact, it has been revealed that senescent ATEC is associated with blunted lipid uptake in response to PPARγ, which may precipitate higher circulating FFAs and ectopic lipid deposition. Instead, these senescent ATEC have higher nuclear translocation of NF-kB and Forkhead box protein O1 (FOXO1), demonstrating that the anti-inflammatory and insulin-sensitising effects of PPARγ do not translate in the context of cellular senescence, which may deserve consideration in pharmaceutical development [160]. The pathologic influence of senescent ECs is also underpinned by a recent study, which showed that global EC senescence induces adipocyte oxidative stress and systemic IR through down-regulating adipocyte insulin signalling and propagating WAT senescence. Interestingly, the WAT is the most susceptible to the deleterious impact of systemic EC senescence compared with other organs [161]. Future efforts are required to define the physiological role of ATEC senescence in obesity complications and the cross-talk between ATEC and other metabolic organs.
Reprogramming by circadian rhythm
Several lines of evidence supporting the positive associations between chronic shift work or (social) jet lag and incidence of metabolic diseases have led to an increasing appreciation of the contribution of circadian misalignment in the pathogenesis of obesity and the influences of clock genes in AT homoeostasis [162–164]. The circadian clock system is a multi-oscillatory network comprising a master clock in the suprachiasmatic nuclei (SCN) [165], and peripheral clocks running autonomously in all other body tissues, which can be entrained by SCN through electrical, endocrine and metabolic pathways, although the precise mechanisms are yet to be defined [166].
The molecular machinery of the circadian system has been elegantly reviewed here [167,168]. Briefly, our internal timekeeper is based on transcription–translation feedback loops consisting of circadian locomotor output cycles kaput (CLOCK)–brain and muscle ARNT-like 1 (BMAL1) heterodimers and their target clock genes Period (PER 1-3), Cryptochrome (CRY 1-2), REV-ERB and retinoic acid-related orphan receptor (ROR). Accumulation of these clock proteins then serve as repressors of CLOCK-BMAL1 and hence their own transcriptions, generating oscillations in gene expressions and creating an antiphase relationship between BMAL1 and PER/CRY expressions. The circadian oscillations of CLOCK–BMAL1 heterodimer then regulate the transcription rhythmicity of clock-controlled genes containing E-box sequences. Despite operating on a common feedback mechanism, these core clock machinery regulate crucial but diverse pathways in a tissue-specific manner, where there are very few overlapping clock gene outputs between different organs [169,170].
One of the pioneering studies investigating the association between obesity and circadian dysfunction comes from the observation of Clock mutant mice, where global Clock mutation leads to hyperphagia, obesity and metabolic pathologies independent of diet. Specifically, the weight gain of Clock mutant mice is largely attributed to visceral adiposity, which is accompanied by AT hypertrophy [171]. By contrast, administration of Rev-Erbα agonist reduces adiposity and mitigates systemic IR and inflammation in DIO mice [251]. Furthermore, AT-specific deletion of Bmal1 or Cry also promotes significant weight gain and higher adiposity, which is accompanied by the formation of hypertrophic adipocytes and CLS during HFD [172,173], implying that dysfunctional clock operation is possibly the pathogenic driver and accelerator of metabolic abnormalities. Indeed, genetically obese (ob/ob or KK-Ay) or DIO mice have reportedly altered or dampened oscillations of clock genes in VAT, where the abnormal AT rhythmicity precedes the development of morbid obesity and metabolic dysfunctions [174,175]. Although there are inconsistencies regarding the influences of obesity on human AT clock machinery, the number of rhythmic genes in SAT is shown to be reduced by more than fourfold in patients with obesity and T2D compared with lean individuals, which is coupled with an abnormal oscillatory transcriptome implicated in AT metabolic and immune dysfunction [176,177].
In fact, genes involved in AT homoeostasis and functionality, including fatty acid metabolism, adipogenesis and thermogenesis, also demonstrate robust circadian oscillations driven by the core clock machineries, underscoring the extensive interconnections among metabolic, immune and circadian pathways in AT [178–180]. In WAT, expression of transcripts encoding the lipogenic enzymes (elongation of long-chain fatty acids family member 6 (Elovl6) and stearoyl-CoA desaturase enzyme 1 (Scd1)) and lipolytic enzymes (hormone-sensitive lipase (Lipe) and adipose triglyceride lipase (Pnpla2)) are under the direct influences of core clock components BMAL1 and CLOCK, which confer the diurnal variability in lipid turnover and serum lipid profile [181,182]. The core clock component Rev-Erbα also functions as a potent transcription regulator in WAT and directly down-regulates transcriptions of lipoprotein lipase [183], fibroblast growth factor-21 (FGF21) cofactor [184], and enzymes involved in triglyceride synthesis [185]. A similar suppression on adipogenesis and fatty acid metabolism by Per2 through its direct interaction with PPARγ in WAT has also been suggested [186].
Additionally, NF-κB, the master regulator of AT inflammatory cascade, represses BMAL1/CLOCK transcriptional activity, thus causing circadian clock dysfunction in response to inflammation [187]. A recent study further suggests that chromatin binding by BMAL1 has profound influences over genes involved in AT reprogramming processes, including inflammation, ECM remodelling and hypoxia response [175]. Specifically, there is a repositioning of BMAL1 genome-wide occupancy in the obese VAT, such that an up-regulated production of pro-inflammatory adipokines is observed. These observations highlight the interconnections between circadian and immune reprogramming of AT, which is also evidenced by the close proximity of BMAL1 to the NF-κB consensus motif [175].
Similar to its white counterpart, the brown AT is profoundly influenced by circadian reprogramming, and ∼8% of the protein-coding genes in brown AT are clock-controlled [188]. Specifically, uptake of fatty acids and glucose by brown AT demonstrates strong diurnal rhythmicity, and up to 12-fold differences between highest and lowest FA uptake within a day–night cycle are found [189,190]. These variations coincide with the oscillating expressions of lipoprotein lipase, angiopoietin-like 4, glucose transporter-4, and UCP1, which are also in antiphase with REV-ERBA and BMAL1 [191,192]. Mechanistically, BMAL1 has a suppressive effect on brown adipogenesis and brown adipocyte commitment, which is mediated by enhanced activity of TGFβ relative to the bone morphogenetic protein signalling [193]. Surprisingly, brown AT-specific deletion of Bmal1 significantly down-regulates tissue metabolism and thermogenesis, which is accompanied by greater weight gain during HFD despite an up-regulation in Ucp1 [194]. Further investigation unravelling the impact of clock machinery in regulating sympathetic innervation and UCP1-independent thermogenesis of brown AT is needed. Similarly, Per2, the transcriptional suppressor of BMAL1, is found to be indispensable for UCP1-dependent cold-induced thermogenesis, where Per2 mutation leads to cold intolerance and defected brown AT metabolism [195]. Collectively, an intact and optimised circadian system is critical for adaptive reprogramming of both white and brown depots, and an improved understanding of circadian regulation of AT could yield novel obesity therapeutics. Intriguingly, despite enhancing systemic circadian rhythms in DIO mice through small molecules has shown to alleviate metabolic disease burden [196], the benefit of specifically targeting AT circadian rhythmicity in obesity is unexplored.
AT reprogramming as therapeutic tools
The mainstream anti-obesity therapeutics have been centred around manipulation of appetite regulation and hence the food intake component in the energy balance equation. Indeed, such approaches have recently offered significant breakthroughs in tackling the obesity crisis, especially in the case of glucagon-like peptide-1 analogues [197]. On the other hand, sympathomimetics, such as phentermine, are prescribed to induce thermogenesis and lipolysis through direct stimulation of the β-adrenergic receptors [198]. However, the prevailing unwanted effects of anti-obesity medications, including cardiovascular events and gastrointestinal discomfort, render intolerability and safety concerns [199]. Advances in recent obesity therapeutics have been articulated by Muller et al. [200]. Here, we will broadly summarise the therapeutics that modulate AT reprogramming, thus targeting the root cause of metabolic dysfunction, in addition to their proposed benefits on weight loss or maintenance (Figure 2).
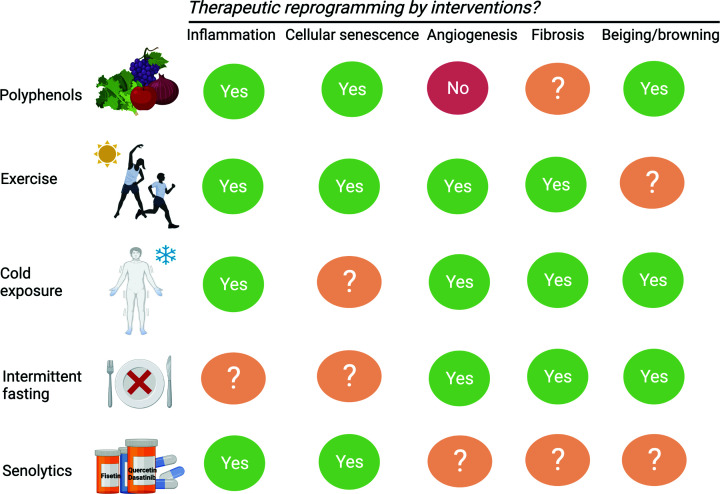
Figure 2. Interventions favouring adaptive reprogramming of AT.
Nutritional, pharmacological and lifestyle interventions demonstrating efficacy in rectifying metabolic diseases through modulating the phathological reprogramming processes. The mechanistic targets of each potential intervention are illustrated.
Involvement of AT senescence burden in obesity has made senolytics, which are mostly naturally occurring polyphenols targeting senescent cells, effective in rescuing AT dysfunction and its associated complications. Importantly, senolytics, especially when used in combination, target multiple cell damage and pro-survival pathways, including inhibition of HIF1A, PI3K (phosphatidylinositol 3-kinase)/Akt (protein kinase B)/mTOR (mammalian target of rapamycin) and B-cell lymphoma 2 (Bcl2) anti-apoptotic signalling, hence inducing apoptosis in senescent cells without off-target effects [201]. The combination of quercetin and dasatinib restores insulin sensitivity and subcutaneous adipogenesis in obese mice, accompanied with suppressed SASPs and reduced monocyte infiltrate in AT, although the resident ATM populations are unaltered by senolytics [129,130]. By leveraging mass cytometry, the senescent adipocyte progenitors, CD4+/CD8+ T cells, NK cells and ATECs in AT, but not ATMs expressing p16, are identified as the cellular targets of the senolytic polyphenol [202]. In fact, expressions of p16 and SABG activity are transiently elevated in macrophages when reprogramming towards an M2-like phenotype [203], which again, demonstrates the high specificity of senolytics in targeting bona fide senescence. In addition to their senotherapeutic actions, many of the polyphenols, such as epigallocatechin gallate, curcumin and resveratrol derived from green tea, turmeric and grapes respectively also demonstrate potent anti-inflammatory and insulin-sensitising properties as well as ameliorating the pathologic reprogramming of AT and extending life span [204–206]. These are achieved at least partially through influencing the gut microbiota and its metabolites, which are implicated in reshaping the AT during metabolic stress [207–209]. Note that resveratrol has shown to decrease expression of angiotensin-converting enzyme-2 (ACE2), which is the cellular entry point of SARS-CoV-2, in human SAT, but the clinical implications of resveratrol on COVID-19 are yet to be examined [210]. Moreover, capsaicin and capsinoids derived from chilli peppers have been shown to protect against DIO and dyslipidaemia through inducing expression of beiging factors in WAT and enhancing the sympathetic tone and vascularity of brown AT [211–213]. Importantly, scRNA-seq study has shown that adipocyte progenitors expressing transient receptor potential vanilloid 1 (Trpv1), the molecular target of capsaicin, are predominantly responsible for cold-induced thermogenesis and beiging through differentiating into brown and beige adipocytes [214], suggesting the mechanistic role of capsaicin as a browning/beiging agent. Benefits of these natural compounds are also supported by human dietary interventions reporting improvements in metabolic profile and body weight control by adopting a plant-based Mediterranean dietary patten [215,216].
In addition to pharmaceutical approaches, exercise has shown to alleviate the senescent and inflammatory signature of WAT [140,217,218]. Interestingly, transplantation of SAT taken from exercised mice is sufficient to benefit systemic glucose homoeostasis in sedentary mice [219,220]. Moreover, improvements in AT vasculature and glucose homoeostasis are also evident in insulin-resistant individuals [221], implying an adaptive reprogramming of AT by exercise. Paradoxically, despite an increased body temperature, exercise leads to enhanced sympathetic activity and beiging/browning of murine AT as evidenced by the up-regulation of key marker genes and the presence of multilocular cells in the subcutaneous depot [222,223]. Although the effect of habitual exercise on human brown AT activity is controversial [224,225], which can be confounded by concurrent cold challenges during winter swimming, the beneficial adaptations of a ‘trained’ AT can justify exercise as an adjunct anti-obesity therapy. Specifically, in addition to inducing beiging/browning, cold exposure modulates monocyte activation and angiogenesis, and importantly, alleviates the inflammatory and fibrotic phenotype of AT, where recruitment of the PRDM16 (PR domain containing 16)–GTF2IRD1 (general transcription factor II-I repeat domain-containing protein 1) complex by cold exposure suppresses pro-inflammatory gene expressions and pro-fibrotic signalling in the adipocytes [226–228]. Nevertheless, whether the reduction in pro-inflammatory transcripts is attributed to cold-induced beiging/browning or cold exposure per se is unclear. The anti-inflammatory effect of cold exposure merits human investigations.
Daily CR is among the lifestyle modifications showing efficacy in delaying or preventing the onset of obesity and its comorbidities [3,229]. Yet, CR per se has not shown to be superior to prolonged intermittent fasting (IF) [230]. In fact, it is the fasting component that underlies the benefit of CR, where fasting without food reduction recapitulates the metabolic adaptations to CR [231]. Indeed, IF has been shown to benefit metabolic profile and promote adaptive reprogramming of AT, including up-regulation of mitochondrial metabolism and Ucp1 expression and improvements in pro-fibrotic and pro-inflammatory burden [231–234]. Interestingly, markers of M1-like ATM are elevated in the SAT of women with overweight or obesity subject to IF [123], which may imply AT inflammation as a prerequisite or a priming signal for beneficial adaptations. Future trials with longer intervention periods could disentangle the differential roles of AT immuno-reprogramming during nutritional challenges. Specifically, time-restricted feeding (TRF), which implements a daily feeding period of ≤12 h and is designed for synchronising food intake with diurnal rhythms in metabolism, has shown to reverse DIO-induced complications and alleviate the burden of metabolic syndrome in individuals with overweight or obesity, despite a lack of dietary restriction and drastic weight loss [235–237]. In fact, a 10-h TRF significantly induces adipocyte VEGF expression and AT angiogenesis, which is accompanied by resistance to obesity and AT inflammation upon HFD. Interestingly, such fasting-induced reprogramming is abolished by deletion of hepatic FGF21 signalling, underpinning the liver–AT cross-talk in regulating metabolic homoeostasis [238]. Given that circadian misalignment dictates and accelerates AT pathologic reprogramming [239,240], further efforts are needed to confirm the potential of TRF as part of the toolkit for therapeutically reprogramming AT in humans. Additionally, it remains unclear whether one’s circadian pattern or chronotype influences the physiological responses of AT during TRF, and whether the misaligned AT clocks underlies the discrepancies in risks of obesity observed between the ‘morning larks’ and the ‘night owls’ [241–243]. Currently, bariatric surgery remains the ‘gold standard’ for treating morbid obesity with a high success rate that cannot be outrun by lifestyle or pharmaceutical interventions alone [200]. Further trials aiming to investigate the synergy of combining interventions that can therapeutically reprogramme AT and target appetite regulation could offer new avenues for non-invasive metabolic risk management.
Implications of AT remodelling in COVID-19
In addition to managing chronic diseases, therapeutic reprogramming of AT may have wider implications on the current COVID-19 pandemic, considering that the AT has shown to be an infection reservoir of the SARS-CoV-2 and potentiates the systemic ‘cytokine storm’ of COVID-19 by adopting a pro-inflammatory phenotype upon viral infection [244]. Notably, infection of AT by SARS-CoV-2 has been found in male patients with overweight or obesity, and such infection is associated with adipose dysfunction characterised by reduced adiponectin and adipsin production, contributing to systemic IR and hyperglycaemia and impaired de novo lipogenesis [245,246]. Although the relationship between obesity and adipose expression of ACE2 is debatable [246,247], the insulin-resistant and obese AT is associated with T-cell exhaustion due to programmed death-ligand 1 (PD-L1) overexpression [248], altered methylation of ACE2 [249]. In addition, there is an up-regulated expression of glucose-related protein-78 (GRP78) in the obese AT, which acts as a co-receptor to facilitate the interaction between SARS-CoV-2 and ACE2 [250]. Collectively, these studies suggest a greater infection susceptibility of the ‘sick’ fat. Interestingly, dietary and lifestyle interventions, including exercise and cold exposure, have been shown to reduce adipose expressions of GRP78 and neuropilin-1 (NRP1), which is a known SARS-CoV-2 entry factor [249–251]. However, it is probably that SARS-CoV-2 may also manoeuvre the AT niche and exploit it as a pathogenic hub favouring virus replication and transmission, and such viral reprogramming of AT needs to be resolved. Given the dominant role of AT in regulating metabolic homoeostasis and the latter one is a strong predictor of infection outcomes [252], the benefits of ‘educating’ the AT as an adjunct to COVID-19 prevention and vaccination warrants longitudinal examinations.
Conclusion
Our knowledge of obesity as a metabolic disease has been evolved from a central focus on appetite and energy homoeostasis to a growing appreciation on peripheral influences by metabolic organs, specifically the pathologic reprogramming of the WAT. This is in turn, intricately linked to the central ‘lipostat’ [253] and systemic homoeostasis. Moreover, our ability to sequence at the single cell and single nucleus resolution and profile metabolites with an increasing level of sophistication has offered novel insights into the potential of targeting the dysfunctional AT as therapeutic alternatives. However, the multifaceted and dynamic nature of the AT reprogramming poses challenges in understanding the temporal course leading to the ‘sick AT’ during the onset and progression of obesity. Such challenges are further escalated by the heterogeneity and plasticity of AT cell populations, which are extraordinary in the sense that they can be readily reprogrammed by the tissue microenvironment and subsequently re-establish a new homoeostatic set-point. Such that, the AT can still retain its obesogenic memory after weight loss and HFD reversal, leading to an uncoupling of adiposity and metabolic dysfunction and facilitating weight rebound and cycling, which in turn, disrupts immuno-metabolic homeostasis [254–256]. Moreover, questions remain on the triggers leading to the ‘obese and sick’ AT and whether these events vary between individuals based on their baseline risk factors, chronotypes and the gut microbiome. Understanding the interindividual differences in the AT architecture and illuminating the evolving functional landscape of AT and its cell populations during obesity progression will help develop tailored management and promote better adaptations to interventions. Lastly, it is important to realise that these reprogramming processes alone cannot be definitively labelled as either ‘pathologic’ or ‘adaptive’; instead, the reprogramming potential of AT should be considered as an evolutionarily conserved mechanism accommodating environmental and metabolic fluctuations and a powerful anti-obesity tool to be leveraged.
Acknowledgements
Cartoons in Figures 1 and 2 were created with BioRender.com.
Abbreviations
- ACE2
angiotensin-converting enzyme-2
- Akt
Protein kinase B
- AT
adipose tissue
- ATEC
adipose tissue endothelial cell
- ATM
adipose tissue macrophage
- Bcl2
B-cell lymphoma 2
- BMAL
brain and muscle ARNT-like 1
- cDC
conventional dendritic cell
- CLOCK
circadian locomotor output cycles kaput
- CLS
crown-like structure
- CR
calorie restriction
- CRY
cryptochrome
- DDR
DNA damage response
- EC
endothelial cell
- ECM
extracellular matrix
- Elovl6
elongation of long-chain fatty acids family member 6
- FFA
free fatty acid
- FGF21
fibroblast growth factor-21
- GRP78
glucose-related protein-78
- GTF2IRD1
general transcription factor II-I repeat domain-containing protein 1
- HFD
high-fat diet
- HIF1α
hypoxia-inducible factor-1α
- IF
intermittent fasting
- IFNγ
interferon-γ
- IL
interleukin
- ILC
innate lymphoid cell
- iNKT
invariant natural killer cell
- IR
insulin resistance
- JNK
c-Jun N-terminal kinase
- LAM
lipid-associated macrophage
- Lipe
hormone-sensitive lipase
- MAPK
mitogen-activated protein kinase
- mTOR
mammalian target of rapamycin
- NF-kB
nuclear factor κ-light-chain-enhancer of activated B cells
- NLRP3
NLR family pyrin domain containing-3
- PD-L1
programmed death-ligand 1
- PER
period
- PI3K
phosphatidylinositol 3-kinase
- Pnpla2
adipose triglyceride lipase
- PO2
partial pressure of oxygen
- PPARγ
peroxisome proliferator-activated receptor-γ
- PRR
pattern recognition receptor
- PRDM16
PR domain containing 16
- ROR
retinoic acid-related orphan receptor
- SABG
senescence-associated β-galactosidase
- SASP
senescence-associated secretory phenotype
- SAT
subcutaneous adipose tissue
- scRNA-seq
single-cell RNA sequencing
- Scd1
stearoyl-CoA desaturase enzyme 1
- T2D
type 2 diabetes
- TGFβ
transforming growth factor-β
- TNF
tumour necrosis factor
- TRF
time-restricted feeding
- UCP1
uncoupling protein-1
- VAT
visceral adipose tissue
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
- WAT
white adipose tissue
Data Availability
The data are available from the corresponding author upon reasonable request.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC)—Excellent Young Scientists Fund (Hong Kong and Macau) [grant number 81922079 (to X.H.)].
Open Access
Open access for this article was enabled by the participation of Chinese University of Hong Kong in an all-inclusive Read & Publish agreement with Portland Press and the Biochemical Society under a transformative agreement with JULAC.
Author Contribution
Yue Qi wrote the original draft and contributed to the figures. Xiaoyan Hui revised and edited the manuscript.
References
- 1.Allison D.B., Downey M., Atkinson R.L., Billington C.J., Bray G.A., Eckel R.H.et al. (2008) Obesity as a disease: a white paper on evidence and arguments commissioned by the Council of The Obesity Society. Obesity 16, 1161–1177 10.1038/oby.2008.231 [DOI] [PubMed] [Google Scholar]
- 2.Crewe C., An Y.A. and Scherer P.E. (2017) The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J. Clin. Invest. 127, 74–82 10.1172/JCI88883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO (2021) Obesity and overweight. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight#:∼:text=Worldwide%20obesity%20has%20nearly%20tripled,%2C%20and%2013%25%20were%20obese [Google Scholar]
- 4.Di Angelantonio E., Bhupathiraju S.N., Wormser D., Gao P., Kaptoge S., de Gonzalez A.B.et al. (2016) Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 388, 776–786 10.1016/S0140-6736(16)30175-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhaskaran K., dos-Santos-Silva I., Leon D.A., Douglas I.J. and Smeeth L. (2018) Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3.6 million adults in the UK. Lancet Diabetes Endocrinol. 6, 944–953 10.1016/S2213-8587(18)30288-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tobias D.K. and Hall K.D. (2021) Eliminate or reformulate ultra-processed foods? Biological mechanisms matter. Cell Metab. 33, 2314–2315 10.1016/j.cmet.2021.10.005 [DOI] [PubMed] [Google Scholar]
- 7.Gupta N.J., Kumar V. and Panda S. (2017) A camera-phone based study reveals erratic eating pattern and disrupted daily eating-fasting cycle among adults in India. PLoS ONE 12, e0172852 10.1371/journal.pone.0172852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gill S. and Panda S. (2015) A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 22, 789–798 10.1016/j.cmet.2015.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mongraw-Chaffin M., Foster M.C., Anderson C.A.M., Burke G.L., Haq N., Kalyani R.R.et al. (2018) Metabolically healthy obesity, transition to metabolic syndrome, and cardiovascular risk. J. Am. Coll. Cardiol. 71, 1857–1865 10.1016/j.jacc.2018.02.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Commodore-Mensah Y., Lazo M., Tang O., Echouffo-Tcheugui J.B., Ndumele C.E., Nambi V.et al. (2021) High burden of subclinical and cardiovascular disease risk in adults with metabolically healthy obesity: The Atherosclerosis Risk in Communities (ARIC) Study. Diabetes Care 44, 1657–1663 10.2337/dc20-2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosen E.D. and Spiegelman B.M. (2014) What we talk about when we talk about fat. Cell 156, 20–44 10.1016/j.cell.2013.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lenz M., Arts I.C.W., Peeters R.L.M., de Kok T.M. and Ertaylan G. (2020) Adipose tissue in health and disease through the lens of its building blocks. Sci. Rep. 10, 10433 10.1038/s41598-020-67177-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Q., Hagberg C.E., Silva Cascales H., Lang S., Hyvönen M.T., Salehzadeh F.et al. (2021) Obesity and hyperinsulinemia drive adipocytes to activate a cell cycle program and senesce. Nat. Med. 27, 1941–1953 10.1038/s41591-021-01501-8 [DOI] [PubMed] [Google Scholar]
- 14.Després J.P. and Lemieux I. (2006) Abdominal obesity and metabolic syndrome. Nature 444, 881–887 10.1038/nature05488 [DOI] [PubMed] [Google Scholar]
- 15.Klop B., Elte J.W.F. and Cabezas M.C. (2013) Dyslipidemia in obesity: mechanisms and potential targets. Nutrients 5, 1218–1240 10.3390/nu5041218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cannon B. and Nedergaard J. (2004) Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 10.1152/physrev.00015.2003 [DOI] [PubMed] [Google Scholar]
- 17.Cypess A.M., Weiner L.S., Roberts-Toler C., Elía E.F., Kessler S.H., Kahn P.A.et al. (2015) Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab. 21, 33–38 10.1016/j.cmet.2014.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cypess A.M., Chen Y.-C., Sze C., Wang K., English J., Chan O.et al. (2012) Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc. Natl. Acad. Sci. U.S.A. 109, 10001–10005 10.1073/pnas.1207911109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lidell M.E., Betz M.J., Leinhard O.D., Heglind M., Elander L., Slawik M.et al. (2013) Evidence for two types of brown adipose tissue in humans. Nat. Med. 19, 631–634 10.1038/nm.3017 [DOI] [PubMed] [Google Scholar]
- 20.Naja Therese, Peijs L., Daugaard S., Homøe P., Loft A.et al. (2013) A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab. 17, 798–805 10.1016/j.cmet.2013.04.011 [DOI] [PubMed] [Google Scholar]
- 21.Cypess A.M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A.B.et al. (2009) Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360, 1509–1517 10.1056/NEJMoa0810780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nedergaard J., Bengtsson T. and Cannon B. (2007) Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol. Endocrinol. Metab. 293, E444–E452 10.1152/ajpendo.00691.2006 [DOI] [PubMed] [Google Scholar]
- 23.van Marken Lichtenbelt W.D., Vanhommerig J.W., Smulders N.M., Drossaerts J.M.A.F.L., Kemerink G.J., Bouvy N.D.et al. (2009) Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 360, 1500–1508 10.1056/NEJMoa0808718 [DOI] [PubMed] [Google Scholar]
- 24.Fernández-Verdejo R., Marlatt K.L., Ravussin E. and Galgani J.E. (2019) Contribution of brown adipose tissue to human energy metabolism. Mol. Aspects Med. 68, 82–89 10.1016/j.mam.2019.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li M., Li L., Li B., Hambly C., Wang G., Wu Y.et al. (2021) Brown adipose tissue is the key depot for glucose clearance in microbiota depleted mice. Nat. Commun. 12, 4725 10.1038/s41467-021-24659-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitehead A., Krause F.N., Moran A., MacCannell A.D.V., Scragg J.L., McNally B.D.et al. (2021) Brown and beige adipose tissue regulate systemic metabolism through a metabolite interorgan signaling axis. Nat. Commun. 12, 1905 10.1038/s41467-021-22272-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becher T., Palanisamy S., Kramer D.J., Eljalby M., Marx S.J., Wibmer A.G.et al. (2021) Brown adipose tissue is associated with cardiometabolic health. Nat. Med. 27, 58–65 10.1038/s41591-020-1126-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kajimura S., Spiegelman B.M. and Seale P. (2015) Brown and beige fat: physiological roles beyond heat generation. Cell Metab. 22, 546–559 10.1016/j.cmet.2015.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu P., Hui X., Zheng Q., Gao Y., Jin L., Jiang W.et al. (2021) Mitochondrial uncoupling protein 1 antagonizes atherosclerosis by blocking NLRP3 inflammasome–dependent interleukin-1β production. Sci Adv. 7, eabl4024 10.1126/sciadv.abl4024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maurer S., Harms M. and Boucher J. (2021) The colorful versatility of adipocytes: white‐to‐brown transdifferentiation and its therapeutic potential in humans. FEBS J. 288, 3628–3646 10.1111/febs.15470 [DOI] [PubMed] [Google Scholar]
- 31.Wang Q.A., Tao C., Gupta R.K. and Scherer P.E. (2013) Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 19, 1338–1344 10.1038/nm.3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salans L.B., Cushman S.W. and Weismann R.E. (1973) Studies of human adipose tissue. Adipose cell size and number in nonobese and obese patients. J. Clin. Invest. 52, 929–941 10.1172/JCI107258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spalding K.L., Arner E., Westermark P.O., Bernard S., Buchholz B.A., Bergmann O.et al. (2008) Dynamics of fat cell turnover in humans. Nature 453, 783–787 10.1038/nature06902 [DOI] [PubMed] [Google Scholar]
- 34.Tchoukalova Y.D., Votruba S.B., Tchkonia T., Giorgadze N., Kirkland J.L. and Jensen M.D. (2010) Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc. Natl. Acad. Sci. U.S.A. 107, 18226–18231 10.1073/pnas.1005259107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weyer C., Foley J.E., Bogardus C., Tataranni P.A. and Pratley R.E. (2000) Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia 43, 1498–1506 10.1007/s001250051560 [DOI] [PubMed] [Google Scholar]
- 36.Veilleux A., Caron-Jobin M., Noël S., Laberge P.Y. and Tchernof A. (2011) Visceral adipocyte hypertrophy is associated with dyslipidemia independent of body composition and fat distribution in women. Diabetes 60, 1504–1511 10.2337/db10-1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vishvanath L. and Gupta R.K. (2019) Contribution of adipogenesis to healthy adipose tissue expansion in obesity. J. Clin. Invest. 129, 4022–4031 10.1172/JCI129191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Honecker J., Weidlich D., Heisz S., Lindgren C.M., Karampinos D.C., Claussnitzer M.et al. (2021) A distribution-centered approach for analyzing human adipocyte size estimates and their association with obesity-related traits and mitochondrial function. Int. J. Obes. (Lond.) 45, 2108–2117 10.1038/s41366-021-00883-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McLaughlin T., Deng A., Yee G., Lamendola C., Reaven G., Tsao P.S.et al. (2010) Inflammation in subcutaneous adipose tissue: relationship to adipose cell size. Diabetologia 53, 369–377 10.1007/s00125-009-1496-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fang L., Guo F., Zhou L., Stahl R. and Grams J. (2015) The cell size and distribution of adipocytes from subcutaneous and visceral fat is associated with type 2 diabetes mellitus in humans. Adipocyte 4, 273–279 10.1080/21623945.2015.1034920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mundi M.S., Karpyak M.V., Koutsari C., Votruba S.B., O’Brien P.C. and Jensen M.D. (2010) Body fat distribution, adipocyte size, and metabolic characteristics of nondiabetic adults. J. Clin. Endocrinol. Metab. 95, 67–73 10.1210/jc.2009-1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Virtue S. and Vidal-Puig A. (2008) It’s not how fat you are, it’βs what you do with it that counts. PLoS Biol. 6, e237 10.1371/journal.pbio.0060237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Divoux A. and Clément K. (2011) Architecture and the extracellular matrix: the still unappreciated components of the adipose tissue. Obes. Rev. 12, e494–e503 10.1111/j.1467-789X.2010.00811.x [DOI] [PubMed] [Google Scholar]
- 44.Pietiläinen K.H., Ismail K., Järvinen E., Heinonen S., Tummers M., Bollepalli S.et al. (2016) DNA methylation and gene expression patterns in adipose tissue differ significantly within young adult monozygotic BMI-discordant twin pairs. Int. J. Obes. (Lond.) 40, 654–661 10.1038/ijo.2015.221 [DOI] [PubMed] [Google Scholar]
- 45.Sun K., Tordjman J., Clément K. and Scherer P.E. (2013) Fibrosis and adipose tissue dysfunction. Cell Metab. 18, 470–477 10.1016/j.cmet.2013.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Després J.-P. and Lemieux I. (2006) Abdominal obesity and metabolic syndrome. Nature 444, 881–887 10.1038/nature05488 [DOI] [PubMed] [Google Scholar]
- 47.Muir L.A., Neeley C.K., Meyer K.A., Baker N.A., Brosius A.M., Washabaugh A.R.et al. (2016) Adipose tissue fibrosis, hypertrophy, and hyperplasia: Correlations with diabetes in human obesity. Obesity 24, 597–605 10.1002/oby.21377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leven A.-S., Gieseler R.K., Schlattjan M., Schreiter T., Niedergethmann M., Baars T.et al. (2021) Association of cell death mechanisms and fibrosis in visceral white adipose tissue with pathological alterations in the liver of morbidly obese patients with NAFLD. Adipocyte 10, 558–573 10.1080/21623945.2021.1982164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alba D.L., Farooq J.A., Lin M.Y.C., Schafer A.L., Shepherd J. and Koliwad S.K. (2018) Subcutaneous fat fibrosis links obesity to insulin resistance in Chinese Americans. J. Clin. Endocrinol. Metab. 103, 3194–3204 10.1210/jc.2017-02301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henninger A.M.J., Eliasson B., Jenndahl L.E. and Hammarstedt A. (2014) Adipocyte hypertrophy, inflammation and fibrosis characterize subcutaneous adipose tissue of healthy, non-obese subjects predisposed to type 2 diabetes. PloS ONE 9, e105262 10.1371/journal.pone.0105262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Divoux A., Tordjman J., Lacasa D., Veyrie N., Hugol D., Aissat A.et al. (2010) Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes 59, 2817–2825 10.2337/db10-0585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abdennour M., Reggio S., Le Naour G., Liu Y., Poitou C., Aron-Wisnewsky J.et al. (2014) Association of adipose tissue and liver fibrosis with tissue stiffness in morbid obesity: links with diabetes and BMI loss after gastric bypass. J. Clin. Endocrinol. Metab. 99, 898–907 10.1210/jc.2013-3253 [DOI] [PubMed] [Google Scholar]
- 53.Khan T., Muise E.S., Iyengar P., Wang Z.V., Chandalia M., Abate N.et al. (2009) Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol. Cell. Biol. 29, 1575–1591 10.1128/MCB.01300-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones J.E.C., Rabhi N., Orofino J., Gamini R., Perissi V., Vernochet C.et al. (2020) The adipocyte acquires a fibroblast-like transcriptional signature in response to a high fat diet. Sci. Rep. 10, 2380 10.1038/s41598-020-59284-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marcelin G., Silveira A.L.M., Martins L.B., Ferreira A.V.M. and Clément K. (2019) Deciphering the cellular interplays underlying obesity-induced adipose tissue fibrosis. J. Clin. Invest. 129, 4032–4040 10.1172/JCI129192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun K., Park J., Gupta O.T., Holland W.L., Auerbach P., Zhang N.et al. (2014) Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction. Nat Commun. 5, 3485 10.1038/ncomms4485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strieder-Barboza C., Baker N.A., Flesher C.G., Karmakar M., Patel A., Lumeng C.N.et al. (2020) Depot-specific adipocyte-extracellular matrix metabolic crosstalk in murine obesity. Adipocyte 9, 189–196 10.1080/21623945.2020.1749500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu B., Jin C., Zeng X., Resch J.M., Jedrychowski M.P., Yang Z.et al. (2020) γδ T cells and adipocyte IL-17RC control fat innervation and thermogenesis. Nature 578, 610–614 10.1038/s41586-020-2028-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cao Y. (2007) Angiogenesis modulates adipogenesis and obesity. J. Clin. Invest. 117, 2362–2368 10.1172/JCI32239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hocking S.L., Wu L.E., Guilhaus M., Chisholm D.J. and James D.E. (2010) Intrinsic depot-specific differences in the secretome of adipose tissue, preadipocytes, and adipose tissue-derived microvascular endothelial cells. Diabetes 59, 3008–3016 10.2337/db10-0483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matulewicz N., Stefanowicz M., Nikołajuk A. and Karczewska-Kupczewska M. (2017) Markers of adipogenesis, but not inflammation, in adipose tissue are independently related to insulin sensitivity. J. Clin. Endocrinol. Metab. 102, 3040–3049 10.1210/jc.2017-00597 [DOI] [PubMed] [Google Scholar]
- 62.Spencer M., Unal R., Zhu B., Rasouli N., McGehee R.E., Peterson C.A.et al. (2011) Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. J. Clin. Endocrinol. Metab. 96, E1990–E1998 10.1210/jc.2011-1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pasarica M., Sereda O.R., Redman L.M., Albarado D.C., Hymel D.T., Roan L.E.et al. (2009) Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 58, 718–725 10.2337/db08-1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tinahones F.J., Coín-Aragüez L., Mayas M.D., Garcia-Fuentes E., Hurtado-Del-Pozo C., Vendrell J.et al. (2012) Obesity-associated insulin resistance is correlated to adipose tissue vascular endothelial growth factors and metalloproteinase levels. BMC Physiol. 12, 4 10.1186/1472-6793-12-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bråkenhielm E., Cao R., Gao B., Angelin B., Cannon B., Parini P.et al. (2004) Angiogenesis inhibitor, TNP-470, prevents diet-induced and genetic obesity in mice. Circ. Res. 94, 1579–1588 10.1161/01.RES.0000132745.76882.70 [DOI] [PubMed] [Google Scholar]
- 66.Spencer M., Yang L., Adu A., Finlin B.S., Zhu B., Shipp L.R.et al. (2014) Pioglitazone treatment reduces adipose tissue inflammation through reduction of mast cell and macrophage number and by improving vascularity. PLoS ONE 9, e102190 10.1371/journal.pone.0102190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elias I., Franckhauser S., Ferré T., Vilà L., Tafuro S., Muñoz S.et al. (2012) Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes 61, 1801–1813 10.2337/db11-0832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robciuc M.R., Kivelä R., Williams I.M., de Boer J.F., van Dijk T.H., Elamaa H.et al. (2016) VEGFB/VEGFR1-induced expansion of adipose vasculature counteracts obesity and related metabolic complications. Cell Metab. 23, 712–724 10.1016/j.cmet.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Villaret A., Galitzky J., Decaunes P., Estève D., Marques M.-A., Sengenès C.et al. (2010) Adipose tissue endothelial cells from obese human subjects: differences among depots in angiogenic, metabolic, and inflammatory gene expression and cellular senescence. Diabetes 59, 2755 10.2337/db10-0398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weis S.M. and Cheresh D.A. (2011) Tumor angiogenesis: molecular pathways and therapeutic targets. Nat. Med. 17, 1359–1370 10.1038/nm.2537 [DOI] [PubMed] [Google Scholar]
- 71.Halberg N., Khan T., Trujillo M.E., Wernstedt-Asterholm I., Attie A.D., Sherwani S.et al. (2009) Hypoxia-inducible factor 1α induces fibrosis and insulin resistance in white adipose tissue. Mol. Cell. Biol. 29, 4467–4483 10.1128/MCB.00192-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee Y.S., Kim J.W., Osborne O., Oh D.Y., Sasik R., Schenk S.et al. (2014) Increased adipocyte O2 consumption triggers HIF-1α, causing inflammation and insulin resistance in obesity. Cell 157, 1339–1352 10.1016/j.cell.2014.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.To W.S. and Midwood K.S. (2011) Plasma and cellular fibronectin: distinct and independent functions during tissue repair. Fibrogenesis Tissue Rep. 4, 21 10.1186/1755-1536-4-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anvari G. and Bellas E. (2021) Hypoxia induces stress fiber formation in adipocytes in the early stage of obesity. Sci. Rep. 11, 21473 10.1038/s41598-021-00335-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun K., Halberg N., Khan M., Magalang Ulysses J. and Scherer Philipp E. (2013) Selective inhibition of hypoxia-inducible factor 1α ameliorates adipose tissue dysfunction. Mol. Cell. Biol. 33, 904–917 10.1128/MCB.00951-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cancello R., Henegar C., Viguerie N., Taleb S., Poitou C., Rouault C.et al. (2005) Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes 54, 2277–2286 10.2337/diabetes.54.8.2277 [DOI] [PubMed] [Google Scholar]
- 77.Cifarelli V., Beeman S.C., Smith G.I., Yoshino J., Morozov D., Beals J.W.et al. (2020) Decreased adipose tissue oxygenation associates with insulin resistance in individuals with obesity. J. Clin. Invest. 130, 6688–6699 10.1172/JCI141828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lawler H.M., Underkofler C.M., Kern P.A., Erickson C., Bredbeck B. and Rasouli N. (2016) Adipose tissue hypoxia, inflammation, and fibrosis in obese insulin-sensitive and obese insulin-resistant subjects. J. Clin. Endocrinol. Metab. 101, 1422–1428 10.1210/jc.2015-4125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Todorčević M., Manuel A.R., Austen L., Michailidou Z., Hazlehurst J.M., Neville M.et al. (2021) Markers of adipose tissue hypoxia are elevated in subcutaneous adipose tissue of severely obese patients with obesity hypoventilation syndrome but not in the moderately obese. Int. J. Obes. 45, 1618–1622 10.1038/s41366-021-00793-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goossens G.H., Bizzarri A., Venteclef N., Essers Y., Cleutjens J.P., Konings E.et al. (2011) Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation 124, 67–76 10.1161/CIRCULATIONAHA.111.027813 [DOI] [PubMed] [Google Scholar]
- 81.Arcidiacono B., Chiefari E., Foryst-Ludwig A., Currò G., Navarra G., Brunetti F.S.et al. (2020) Obesity-related hypoxia via miR-128 decreases insulin-receptor expression in human and mouse adipose tissue promoting systemic insulin resistance. EBioMedicine 59, 102912 10.1016/j.ebiom.2020.102912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hosogai N., Fukuhara A., Oshima K., Miyata Y., Tanaka S., Segawa K.et al. (2007) Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56, 901–911 10.2337/db06-0911 [DOI] [PubMed] [Google Scholar]
- 83.Petrus P., Fernandez T.L., Kwon M.M., Huang J.L., Lei V., Safikhan N.S.et al. (2019) Specific loss of adipocyte CD248 improves metabolic health via reduced white adipose tissue hypoxia, fibrosis and inflammation. EBioMedicine 44, 489–501 10.1016/j.ebiom.2019.05.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hotamisligil G.S. (2017) Inflammation, metaflammation and immunometabolic disorders. Nature 542, 177–185 10.1038/nature21363 [DOI] [PubMed] [Google Scholar]
- 85.Odegaard J.I. and Chawla A. (2013) The immune system as a sensor of the metabolic state. Immunity 38, 644–654 10.1016/j.immuni.2013.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Deng T., Lyon C.J., Bergin S., Caligiuri M.A. and Hsueh W.A. (2016) Obesity, inflammation, and cancer. Annu. Rev. Pathol. 11, 421–449 10.1146/annurev-pathol-012615-044359 [DOI] [PubMed] [Google Scholar]
- 87.Berg A.H. and Scherer P.E. (2005) Adipose tissue, inflammation, and cardiovascular disease. Circ. Res. 96, 939–949 10.1161/01.RES.0000163635.62927.34 [DOI] [PubMed] [Google Scholar]
- 88.Lee J.Y., Sohn K.H., Rhee S.H. and Hwang D. (2001) Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J. Biol. Chem. 276, 16683–16689 10.1074/jbc.M011695200 [DOI] [PubMed] [Google Scholar]
- 89.Shi H., Kokoeva M.V., Inouye K., Tzameli I., Yin H. and Flier J.S. (2006) TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 116, 3015–3025 10.1172/JCI28898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vandanmagsar B., Youm Y.-H., Ravussin A., Galgani J.E., Stadler K., Mynatt R.L.et al. (2011) The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 17, 179–188 10.1038/nm.2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cinti S., Mitchell G., Barbatelli G., Murano I., Ceresi E., Faloia E.et al. (2005) Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 46, 2347–2355 10.1194/jlr.M500294-JLR200 [DOI] [PubMed] [Google Scholar]
- 92.Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L. and Ferrante A.W. (2003) Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 10.1172/JCI200319246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nguyen M.T., Favelyukis S., Nguyen A.K., Reichart D., Scott P.A., Jenn A.et al. (2007) A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J. Biol. Chem. 282, 35279–35292 10.1074/jbc.M706762200 [DOI] [PubMed] [Google Scholar]
- 94.Lumeng C.N., Bodzin J.L. and Saltiel A.R. (2007) Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 10.1172/JCI29881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Macdougall C.E., Wood E.G., Loschko J., Scagliotti V., Cassidy F.C., Robinson M.E.et al. (2018) Visceral adipose tissue immune homeostasis is regulated by the crosstalk between adipocytes and dendritic cell subsets. Cell Metab. 27, 588–601, e584. 10.1016/j.cmet.2018.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McLaughlin T., Liu L.F., Lamendola C., Shen L., Morton J., Rivas H.et al. (2014) T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans. Arterioscler. Thromb. Vasc. Biol. 34, 2637–2643 10.1161/ATVBAHA.114.304636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vijay J., Gauthier M.-F., Biswell R.L., Louiselle D.A., Johnston J.J., Cheung W.A.et al. (2020) Single-cell analysis of human adipose tissue identifies depot- and disease-specific cell types. Nat. Metab. 2, 97–109 10.1038/s42255-019-0152-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Trim W., Turner J.E. and Thompson D. (2018) Parallels in immunometabolic adipose tissue dysfunction with ageing and obesity. Front. Immunol. 9, 169 10.3389/fimmu.2018.00169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nishimura S., Manabe I., Nagasaki M., Eto K., Yamashita H., Ohsugi M.et al. (2009) CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 15, 914–920 10.1038/nm.1964 [DOI] [PubMed] [Google Scholar]
- 100.Winer S., Chan Y., Paltser G., Truong D., Tsui H., Bahrami J.et al. (2009) Normalization of obesity-associated insulin resistance through immunotherapy. Nat. Med. 15, 921–929 10.1038/nm.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Patsouris D., Li P.-P., Thapar D., Chapman J., Olefsky J.M. and Neels J.G. (2008) Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 8, 301–309 10.1016/j.cmet.2008.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hildreth A.D., Ma F., Wong Y.Y., Sun R., Pellegrini M. and O’Sullivan T.E. (2021) Single-cell sequencing of human white adipose tissue identifies new cell states in health and obesity. Nat. Immunol. 22, 639–653 10.1038/s41590-021-00922-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang H., Shen L., Sun X., Liu F., Feng W., Jiang C.et al. (2019) Adipose group 1 innate lymphoid cells promote adipose tissue fibrosis and diabetes in obesity. Nat. Commun. 10, 3254 10.1038/s41467-019-11270-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu R. and Nikolajczyk B.S. (2019) Tissue immune cells fuel obesity-associated inflammation in adipose tissue and beyond. Front. Immunol. 10, 1587 10.3389/fimmu.2019.01587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Trim W.V. and Lynch L. (2021) Immune and non-immune functions of adipose tissue leukocytes. Nat. Rev. Immunol. 10.1038/s41577-021-00635-7 [DOI] [PubMed] [Google Scholar]
- 106.Spallanzani R.G., Zemmour D., Xiao T., Jayewickreme T., Li C., Bryce P.J.et al. (2019) Distinct immunocyte-promoting and adipocyte-generating stromal components coordinate adipose tissue immune and metabolic tenors. Sci. Immunol. 4, eaaw3658 10.1126/sciimmunol.aaw3658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hill D.A., Lim H.-W., Kim Y.H., Ho W.Y., Foong Y.H., Nelson V.L.et al. (2018) Distinct macrophage populations direct inflammatory versus physiological changes in adipose tissue. Proc. Natl. Acad. Sci. U.S.A. 115, E5096–E5105 10.1073/pnas.1802611115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cipolletta D., Feuerer M., Li A., Kamei N., Lee J., Shoelson S.E.et al. (2012) PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature 486, 549–553 10.1038/nature11132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Coats B.R., Schoenfelt K.Q., Barbosa-Lorenzi V.C., Peris E., Cui C., Hoffman A.et al. (2017) Metabolically activated adipose tissue macrophages perform detrimental and beneficial functions during diet-induced obesity. Cell Rep. 20, 3149–3161 10.1016/j.celrep.2017.08.096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lynch L., Nowak M., Varghese B., Clark J., Hogan Andrew E., Toxavidis V.et al. (2012) Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity 37, 574–587 10.1016/j.immuni.2012.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lynch L., Michelet X., Zhang S., Brennan P.J., Moseman A., Lester C.et al. (2015) Regulatory iNKT cells lack expression of the transcription factor PLZF and control the homeostasis of Treg cells and macrophages in adipose tissue. Nat. Immunol. 16, 85–95 10.1038/ni.3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lamarche N.M., Kane H., Kohlgruber A.C., Dong H., Lynch L. and Brenner M.B. (2020) Distinct iNKT cell populations use IFNγ or ER stress-induced IL-10 to control adipose tissue homeostasis. Cell Metab. 32, 243–258, e246. 10.1016/j.cmet.2020.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pirzgalska R.M., Seixas E., Seidman J.S., Link V.M., Sánchez N.M., Mahú I.et al. (2017) Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat. Med. 23, 1309–1318 10.1038/nm.4422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Qiu Y., Nguyen K.D., Odegaard J.I., Cui X., Tian X., Locksley R.M.et al. (2014) Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 157, 1292–1308 10.1016/j.cell.2014.03.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hui X., Gu P., Zhang J., Nie T., Pan Y., Wu D.et al. (2015) Adiponectin enhances cold-induced browning of subcutaneous adipose tissue via promoting M2 macrophage proliferation. Cell Metab. 22, 279–290 10.1016/j.cmet.2015.06.004 [DOI] [PubMed] [Google Scholar]
- 116.Wang Y.-N., Tang Y., He Z., Ma H., Wang L., Liu Y.et al. (2021) Slit3 secreted from M2-like macrophages increases sympathetic activity and thermogenesis in adipose tissue. Nat. Metab. 3, 1536–1551 10.1038/s42255-021-00482-9 [DOI] [PubMed] [Google Scholar]
- 117.Kohlgruber A.C., Gal-Oz S.T., Lamarche N.M., Shimazaki M., Duquette D., Koay H.-F.et al. (2018) γδ T cells producing interleukin-17A regulate adipose regulatory T cell homeostasis and thermogenesis. Nat. Immunol. 19, 464–474 10.1038/s41590-018-0094-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang Q., Li D., Cao G., Shi Q., Zhu J., Zhang M.et al. (2021) IL-27 signalling promotes adipocyte thermogenesis and energy expenditure. Nature 600, 314–318 10.1038/s41586-021-04127-5 [DOI] [PubMed] [Google Scholar]
- 119.Jaitin D.A., Adlung L., Thaiss C.A., Weiner A., Li B., Descamps H.et al. (2019) Lipid-associated macrophages control metabolic homeostasis in a Trem2-dependent manner. Cell 178, 686.e614–698.e614 10.1016/j.cell.2019.05.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sárvári A.K., Van Hauwaert E.L., Markussen L.K., Gammelmark E., Marcher A.-B., Ebbesen M.F.et al. (2021) Plasticity of epididymal adipose tissue in response to diet-induced obesity at single-nucleus resolution. Cell Metab. 33, 437–453, e435. 10.1016/j.cmet.2020.12.004 [DOI] [PubMed] [Google Scholar]
- 121.Zhu Q., An Y.A., Kim M., Zhang Z., Zhao S., Zhu Y.et al. (2020) Suppressing adipocyte inflammation promotes insulin resistance in mice. Mol. Metab. 39, 101010 10.1016/j.molmet.2020.101010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Weinstock A., Brown E.J., Garabedian M.L., Pena S., Sharma M., Lafaille J.et al. (2019) Single-cell RNA sequencing of visceral adipose tissue leukocytes reveals that caloric restriction following obesity promotes the accumulation of a distinct macrophage population with features of phagocytic cells. Immunometabolism 1, e190008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu B., Hutchison A.T., Thompson C.H., Lange K. and Heilbronn L.K. (2019) Markers of adipose tissue inflammation are transiently elevated during intermittent fasting in women who are overweight or obese. Obes. Res. Clin. Pract. 13, 408–415 10.1016/j.orcp.2019.07.001 [DOI] [PubMed] [Google Scholar]
- 124.Fazeli P.K., Zhang Y., O’Keefe J., Pesaresi T., Lun M., Lawney B.et al. (2020) Prolonged fasting drives a program of metabolic inflammation in human adipose tissue. Mol. Metab. 42, 101082 10.1016/j.molmet.2020.101082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tchkonia T., Morbeck D.E., Von Zglinicki T., Van Deursen J., Lustgarten J., Scrable H.et al. (2010) Fat tissue, aging, and cellular senescence. Aging Cell 9, 667–684 10.1111/j.1474-9726.2010.00608.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Almanzar N., Antony J., Baghel A.S., Bakerman I., Bansal I., Barres B.A.et al. (2020) A single-cell transcriptomic atlas characterizes ageing tissues in the mouse. Nature 583, 590–595 10.1038/s41586-020-2496-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang M.J., Pisco A.O., Darmanis S. and Zou J. (2021) Mouse aging cell atlas analysis reveals global and cell type-specific aging signatures. eLife 10, e62293 10.7554/eLife.62293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Smith U., Li Q., Rydén M. and Spalding K.L. (2021) Cellular senescence and its role in white adipose tissue. Int. J. Obes. 45, 934–943 10.1038/s41366-021-00757-x [DOI] [PubMed] [Google Scholar]
- 129.Xu M., Pirtskhalava T., Farr J.N., Weigand B.M., Palmer A.K., Weivoda M.M.et al. (2018) Senolytics improve physical function and increase lifespan in old age. Nat. Med. 24, 1246–1256 10.1038/s41591-018-0092-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Palmer A.K., Xu M., Zhu Y., Pirtskhalava T., Weivoda M.M., Hachfeld C.M.et al. (2019) Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell 18, e12950 10.1111/acel.12950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jurk D., Wilson C., Passos J.F., Oakley F., Correia-Melo C., Greaves L.et al. (2014) Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat. Commun. 5, 4172 10.1038/ncomms5172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kumari R. and Jat P. (2021) Mechanisms of cellular senescence: cell cycle arrest and senescence associated secretory phenotype. Front. Cell Dev. Biol. 9, 645593 10.3389/fcell.2021.645593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Passos J.F., Nelson G., Wang C., Richter T., Simillion C., Proctor C.J.et al. (2010) Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol. Syst. Biol. 6, 347–347 10.1038/msb.2010.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Freund A., Patil C.K. and Campisi J. (2011) p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 30, 1536–1548 10.1038/emboj.2011.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tchkonia T., Zhu Y., van Deursen J., Campisi J. and Kirkland J.L. (2013) Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J. Clin. Invest. 123, 966–972 10.1172/JCI64098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ito Y., Hoare M. and Narita M. (2017) Spatial and temporal control of senescence. Trends Cell Biol. 27, 820–832 10.1016/j.tcb.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 137.Basisty N., Kale A., Jeon O.H., Kuehnemann C., Payne T., Rao C.et al. (2020) A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 18, e3000599 10.1371/journal.pbio.3000599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Coppé J.-P., Desprez P.-Y., Krtolica A. and Campisi J. (2010) The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 5, 99–118 10.1146/annurev-pathol-121808-102144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Minamino T., Orimo M., Shimizu I., Kunieda T., Yokoyama M., Ito T.et al. (2009) A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat. Med. 15, 1082–1087 10.1038/nm.2014 [DOI] [PubMed] [Google Scholar]
- 140.Schafer M.J., White T.A., Evans G., Tonne J.M., Verzosa G.C., Stout M.B.et al. (2016) Exercise prevents diet-induced cellular senescence in adipose tissue. Diabetes 65, 1606–1615 10.2337/db15-0291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Varghese M., Griffin C., McKernan K., Eter L., Abrishami S. and Singer K. (2020) Female adipose tissue has improved adaptability and metabolic health compared to males in aged obesity. Aging 12, 1725–1746 10.18632/aging.102709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang L., Wang B., Gasek N.S., Zhou Y., Cohn R.L., Martin D.E.et al. (2021) Targeting p21Cip1 highly expressing cells in adipose tissue alleviates insulin resistance in obesity. Cell Metab. 34, 75.e78–89.e78 10.1016/j.cmet.2021.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ogrodnik M., Zhu Y., Langhi L.G.P., Tchkonia T., Krüger P., Fielder E.et al. (2019) Obesity-induced cellular senescence drives anxiety and impairs neurogenesis. Cell Metab. 29, 1061.e1068–1077.e1068 10.1016/j.cmet.2018.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Timper K. and Brüning J.C. (2017) Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis. Model Mech. 10, 679–689 10.1242/dmm.026609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Conley S.M., Hickson L.J., Kellogg T.A., McKenzie T., Heimbach J.K., Taner T.et al. (2020) Human obesity induces dysfunction and early senescence in adipose tissue-derived mesenchymal stromal/stem cells. Front. Cell Dev. Biol. 8, 197 10.3389/fcell.2020.00197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gustafson B., Nerstedt A. and Smith U. (2019) Reduced subcutaneous adipogenesis in human hypertrophic obesity is linked to senescent precursor cells. Nat. Commun. 10, 2757 10.1038/s41467-019-10688-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Justice J.N., Gregory H., Tchkonia T., LeBrasseur N.K., Kirkland J.L., Kritchevsky S.B.et al. (2018) Cellular senescence biomarker p16INK4a+ cell burden in thigh adipose is associated with poor physical function in older women. J. Gerontol. A Biol. Sci. Med. Sci. 73, 939–945 10.1093/gerona/glx134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tchkonia T., Thomou T., Zhu Y., Karagiannides I., Pothoulakis C., Jensen M.D.et al. (2013) Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 17, 644–656 10.1016/j.cmet.2013.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Berry D.C., Jiang Y., Arpke R.W., Close E.L., Uchida A., Reading D.et al. (2017) Cellular aging contributes to failure of cold-induced beige adipocyte formation in old mice and humans. Cell Metab. 25, 166–181 10.1016/j.cmet.2016.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xu M., Tchkonia T., Ding H., Ogrodnik M., Lubbers E.R., Pirtskhalava T.et al. (2015) JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc. Natl. Acad. Sci. U.S.A. 112, E6301–E6310 10.1073/pnas.1515386112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Xu M., Palmer A.K., Ding H., Weivoda M.M., Pirtskhalava T., White T.A.et al. (2015) Targeting senescent cells enhances adipogenesis and metabolic function in old age. eLife 4, e12997 10.7554/eLife.12997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu Z., Jin L., Yang J.-K., Wang B., Wu K.K.L., Hallenborg P.et al. (2018) The dysfunctional MDM2–p53 axis in adipocytes contributes to aging-related metabolic complications by induction of lipodystrophy. Diabetes 67, 2397–2409 10.2337/db18-0684 [DOI] [PubMed] [Google Scholar]
- 153.Magré J., Delépine M., Khallouf E., Gedde-Dahl T., Van Maldergem L., Sobel E.et al. (2001) Identification of the gene altered in Berardinelli–Seip congenital lipodystrophy on chromosome 11q13. Nat. Genet. 28, 365–370 10.1038/ng585 [DOI] [PubMed] [Google Scholar]
- 154.Hui X., Zhang M., Gu P., Li K., Gao Y., Wu D.et al. (2017) Adipocyte SIRT1 controls systemic insulin sensitivity by modulating macrophages in adipose tissue. EMBO Rep. 18, 645–657 10.15252/embr.201643184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Qiang L., Wang L., Kon N., Zhao W., Lee S., Zhang Y.et al. (2012) Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Pparγ. Cell 150, 620–632 10.1016/j.cell.2012.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nugroho D.B., Ikeda K., Barinda A.J., Wardhana D.A., Yagi K., Miyata K.et al. (2018) Neuregulin-4 is an angiogenic factor that is critically involved in the maintenance of adipose tissue vasculature. Biochem. Biophys. Res. Commun. 503, 378–384 10.1016/j.bbrc.2018.06.043 [DOI] [PubMed] [Google Scholar]
- 157.Sabbatinelli J., Prattichizzo F., Olivieri F., Procopio A.D., Rippo M.R. and Giuliani A. (2019) Where metabolism meets senescence: focus on endothelial cells. Front. Physiol. 10, 1523 10.3389/fphys.2019.01523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gogg S., Nerstedt A., Boren J. and Smith U. (2019) Human adipose tissue microvascular endothelial cells secrete PPARγ ligands and regulate adipose tissue lipid uptake. JCI Insight 4, e125914 10.1172/jci.insight.125914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gealekman O., Burkart A., Chouinard M., Nicoloro S.M., Straubhaar J. and Corvera S. (2008) Enhanced angiogenesis in obesity and in response to PPARgamma activators through adipocyte VEGF and ANGPTL4 production. Am. J. Physiol. Endocrinol. Metab. 295, E1056–E1064 10.1152/ajpendo.90345.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Briot A., Decaunes P., Volat F., Belles C., Coupaye M., Ledoux S.et al. (2018) Senescence alters PPARγ (peroxisome proliferator–activated receptor gamma)-dependent fatty acid handling in human adipose tissue microvascular endothelial cells and favors inflammation. Arterioscler. Thromb. Vasc. Biol. 38, 1134–1146 10.1161/ATVBAHA.118.310797 [DOI] [PubMed] [Google Scholar]
- 161.Barinda A.J., Ikeda K., Nugroho D.B., Wardhana D.A., Sasaki N., Honda S.et al. (2020) Endothelial progeria induces adipose tissue senescence and impairs insulin sensitivity through senescence associated secretory phenotype. Nat. Commun. 11, 481 10.1038/s41467-020-14387-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sun M., Feng W., Wang F., Li P., Li Z., Li M.et al. (2018) Meta-analysis on shift work and risks of specific obesity types. Obes. Rev. 19, 28–40 10.1111/obr.12621 [DOI] [PubMed] [Google Scholar]
- 163.Gao Y., Gan T., Jiang L., Yu L., Tang D., Wang Y.et al. (2020) Association between shift work and risk of type 2 diabetes mellitus: a systematic review and dose-response meta-analysis of observational studies. Chronobiol. Int. 37, 29–46 10.1080/07420528.2019.1683570 [DOI] [PubMed] [Google Scholar]
- 164.Parsons M.J., Moffitt T.E., Gregory A.M., Goldman-Mellor S., Nolan P.M., Poulton R.et al. (2015) Social jetlag, obesity and metabolic disorder: investigation in a cohort study. Int. J. Obes. (Lond.) 39, 842–848 10.1038/ijo.2014.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Patton A.P. and Hastings M.H. (2018) The suprachiasmatic nucleus. Curr. Biol. 28, R816–R822 10.1016/j.cub.2018.06.052 [DOI] [PubMed] [Google Scholar]
- 166.Albrecht U. (2012) Timing to perfection: the biology of central and peripheral circadian clocks. Neuron 74, 246–260 10.1016/j.neuron.2012.04.006 [DOI] [PubMed] [Google Scholar]
- 167.Takahashi J.S. (2017) Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 18, 164–179 10.1038/nrg.2016.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Brown L.S. and Doyle F.J. III (2020) A dual-feedback loop model of the mammalian circadian clock for multi-input control of circadian phase. PLoS Comput. Biol. 16, e1008459 10.1371/journal.pcbi.1008459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Storch K.-F., Lipan O., Leykin I., Viswanathan N., Davis F.C., Wong W.H.et al. (2002) Extensive and divergent circadian gene expression in liver and heart. Nature 417, 78–83 10.1038/nature744 [DOI] [PubMed] [Google Scholar]
- 170.Panda S., Antoch M.P., Miller B.H., Su A.I., Schook A.B., Straume M.et al. (2002) Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109, 307–320 10.1016/S0092-8674(02)00722-5 [DOI] [PubMed] [Google Scholar]
- 171.Turek F.W., Joshu C., Kohsaka A., Lin E., Ivanova G., McDearmon E.et al. (2005) Obesity and metabolic syndrome in circadian clock mutant mice. Science 308, 1043–1045 10.1126/science.1108750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chaix A., Lin T., Le H.D., Chang M.W. and Panda S. (2019) Time-restricted feeding prevents obesity and metabolic syndrome in mice lacking a circadian clock. Cell Metab. 29, 303–319, e304. 10.1016/j.cmet.2018.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Paschos G.K., Ibrahim S., Song W.-L., Kunieda T., Grant G., Reyes T.M.et al. (2012) Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat. Med. 18, 1768–1777 10.1038/nm.2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ando H., Kumazaki M., Motosugi Y., Ushijima K., Maekawa T., Ishikawa E.et al. (2011) Impairment of peripheral circadian clocks precedes metabolic abnormalities in ob/ob mice. Endocrinology 152, 1347–1354 10.1210/en.2010-1068 [DOI] [PubMed] [Google Scholar]
- 175.Maury E., Navez B. and Brichard S.M. (2021) Circadian clock dysfunction in human omental fat links obesity to metabolic inflammation. Nat. Commun. 12, 2388 10.1038/s41467-021-22571-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Otway D.T., Mäntele S., Bretschneider S., Wright J., Trayhurn P., Skene D.J.et al. (2011) Rhythmic diurnal gene expression in human adipose tissue from individuals who are lean, overweight, and type 2 diabetic. Diabetes 60, 1577–1581 10.2337/db10-1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Stenvers D.J., Jongejan A., Atiqi S., Vreijling J.P., Limonard E.J., Endert E.et al. (2019) Diurnal rhythms in the white adipose tissue transcriptome are disturbed in obese individuals with type 2 diabetes compared with lean control individuals. Diabetologia 62, 704–716 10.1007/s00125-019-4813-5 [DOI] [PubMed] [Google Scholar]
- 178.Christou S., Wehrens S.M.T., Isherwood C., Moller-Levet C.S., Wu H., Revell V.L.et al. (2019) Circadian regulation in human white adipose tissue revealed by transcriptome and metabolic network analysis. Sci. Rep. 9, 2641 10.1038/s41598-019-39668-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zvonic S., Ptitsyn A.A., Conrad S.A., Scott L.K., Floyd Z.E., Kilroy G.et al. (2006) Characterization of peripheral circadian clocks in adipose tissues. Diabetes 55, 962–970 10.2337/diabetes.55.04.06.db05-0873 [DOI] [PubMed] [Google Scholar]
- 180.Pivovarova O., Gögebakan Ö., Sucher S., Groth J., Murahovschi V., Kessler K.et al. (2016) Regulation of the clock gene expression in human adipose tissue by weight loss. Int. J. Obes. 40, 899–906 10.1038/ijo.2016.34 [DOI] [PubMed] [Google Scholar]
- 181.Shostak A., Meyer-Kovac J. and Oster H. (2013) Circadian regulation of lipid mobilization in white adipose tissues. Diabetes 62, 2195 10.2337/db12-1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Shostak A., Husse J. and Oster H. (2013) Circadian regulation of adipose function. Adipocyte 2, 201–206 10.4161/adip.26007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Delezie J., Dumont S., Dardente H., Oudart H., Gréchez-Cassiau A., Klosen P.et al. (2012) The nuclear receptor REV-ERBα is required for the daily balance of carbohydrate and lipid metabolism. FASEB J. 26, 3321–3335 10.1096/fj.12-208751 [DOI] [PubMed] [Google Scholar]
- 184.Jager J., Wang F., Fang B., Lim H.-W., Peed L.C., Steger D.J.et al. (2016) The nuclear receptor Rev-erbα regulates adipose tissue-specific FGF21 signaling. J. Biol. Chem. 291, 10867–10875 10.1074/jbc.M116.719120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Solt L.A., Wang Y., Banerjee S., Hughes T., Kojetin D.J., Lundasen T.et al. (2012) Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature 485, 62–68 10.1038/nature11030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Grimaldi B., Bellet M.M., Katada S., Astarita G., Hirayama J., Amin R.H.et al. (2010) PER2 controls lipid metabolism by direct regulation of PPARγ. Cell Metab. 12, 509–520 10.1016/j.cmet.2010.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Shen Y., Endale M., Wang W., Morris A.R., Francey L.J., Harold R.L.et al. (2021) NF-κB modifies the mammalian circadian clock through interaction with the core clock protein BMAL1. PLoS Genet. 17, e1009933 10.1371/journal.pgen.1009933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang R., Lahens N.F., Ballance H.I., Hughes M.E. and Hogenesch J.B. (2014) A circadian gene expression atlas in mammals: implications for biology and medicine. Proc. Natl. Acad. Sci. U.S.A. 111, 16219–16224 10.1073/pnas.1408886111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.van den Berg R., Kooijman S., Noordam R., Ramkisoensing A., Abreu-Vieira G., Tambyrajah L.L.et al. (2018) A diurnal rhythm in brown adipose tissue causes rapid clearance and combustion of plasma lipids at wakening. Cell Rep. 22, 3521–3533 10.1016/j.celrep.2018.03.004 [DOI] [PubMed] [Google Scholar]
- 190.van der Veen D.R., Shao J., Chapman S., Leevy W.M. and Duffield G.E. (2012) A diurnal rhythm in glucose uptake in brown adipose tissue revealed by in vivo PET-FDG imaging. Obesity 20, 1527–1529 10.1038/oby.2012.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lee P., Bova R., Schofield L., Bryant W., Dieckmann W., Slattery A.et al. (2016) Brown adipose tissue exhibits a glucose-responsive thermogenic biorhythm in humans. Cell Metab. 23, 602–609 10.1016/j.cmet.2016.02.007 [DOI] [PubMed] [Google Scholar]
- 192.Gerhart-Hines Z., Feng D., Emmett M.J., Everett L.J., Loro E., Briggs E.R.et al. (2013) The nuclear receptor Rev-erbα controls circadian thermogenic plasticity. Nature 503, 410–413 10.1038/nature12642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Nam D., Guo B., Chatterjee S., Chen M.H., Nelson D., Yechoor V.K.et al. (2015) The adipocyte clock controls brown adipogenesis through the TGF-β and BMP signaling pathways. J. Cell Sci. 128, 1835–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hasan N., Nagata N., Morishige J.-i., Islam M.T., Jing Z., Harada K.-i.et al. (2021) Brown adipocyte-specific knockout of Bmal1 causes mild but significant thermogenesis impairment in mice. Mol. Metab. 49, 101202 10.1016/j.molmet.2021.101202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chappuis S., Ripperger J.A., Schnell A., Rando G., Jud C., Wahli W.et al. (2013) Role of the circadian clock gene Per2 in adaptation to cold temperature. Mol. Metab. 2, 184–193 10.1016/j.molmet.2013.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.He B., Nohara K., Park N., Park Y.S., Guillory B., Zhao Z.et al. (2016) The small molecule nobiletin targets the molecular oscillator to enhance circadian rhythms and protect against metabolic syndrome. Cell Metab. 23, 610–621 10.1016/j.cmet.2016.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gribble F.M. and O’Rahilly S. (2021) Obesity therapeutics: the end of the beginning. Cell Metab. 33, 705–706 10.1016/j.cmet.2021.03.012 [DOI] [PubMed] [Google Scholar]
- 198.Garvey W.T., Ryan D.H., Look M., Gadde K.M., Allison D.B., Peterson C.A.et al. (2012) Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am. J. Clin. Nutr. 95, 297–308 10.3945/ajcn.111.024927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Müller T.D., Clemmensen C., Finan B., DiMarchi R.D. and Tschöp M.H. (2018) Anti-obesity therapy: from rainbow pills to polyagonists. Pharmacol. Rev. 70, 712–746 10.1124/pr.117.014803 [DOI] [PubMed] [Google Scholar]
- 200.Müller T.D., Blüher M., Tschöp M.H. and DiMarchi R.D. (2021) Anti-obesity drug discovery: advances and challenges. Nat. Rev. Drug Discov. 21, 201–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kirkland J.L. and Tchkonia T. (2020) Senolytic drugs: from discovery to translation. J. Intern. Med. 288, 518–536 10.1111/joim.13141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yousefzadeh M.J., Zhu Y., McGowan S.J., Angelini L., Fuhrmann-Stroissnigg H., Xu M.et al. (2018) Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 36, 18–28 10.1016/j.ebiom.2018.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hall B.M., Balan V., Gleiberman A.S., Strom E., Krasnov P., Virtuoso L.P.et al. (2017) p16(Ink4a) and senescence-associated β-galactosidase can be induced in macrophages as part of a reversible response to physiological stimuli. Aging 9, 1867–1884 10.18632/aging.101268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Konings E., Timmers S., Boekschoten M.V., Goossens G.H., Jocken J.W., Afman L.A.et al. (2014) The effects of 30 days resveratrol supplementation on adipose tissue morphology and gene expression patterns in obese men. Int. J. Obes. 38, 470–473 10.1038/ijo.2013.155 [DOI] [PubMed] [Google Scholar]
- 205.Boccellino M. and D'Angelo S. (2020) Anti-obesity effects of polyphenol intake: current status and future possibilities. Int. J. Mol. Sci. 21, 5642 10.3390/ijms21165642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wang S., Moustaid-Moussa N., Chen L., Mo H., Shastri A., Su R.et al. (2014) Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 25, 1–18 10.1016/j.jnutbio.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Virtue A.T., McCright S.J., Wright J.M., Jimenez M.T., Mowel W.K., Kotzin J.J.et al. (2019) The gut microbiota regulates white adipose tissue inflammation and obesity via a family of microRNAs. Sci. Transl. Med. 11, eaav1892 10.1126/scitranslmed.aav1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hui S., Liu Y., Huang L., Zheng L., Zhou M., Lang H.et al. (2020) Resveratrol enhances brown adipose tissue activity and white adipose tissue browning in part by regulating bile acid metabolism via gut microbiota remodeling. Int. J. Obes. (Lond.) 44, 1678–1690 10.1038/s41366-020-0566-y [DOI] [PubMed] [Google Scholar]
- 209.Li M., Li L., Li B., Hambly C., Wang G., Wu Y.et al. (2021) Brown adipose tissue is the key depot for glucose clearance in microbiota depleted mice. Nat. Commun. 12, 4725–4725 10.1038/s41467-021-24659-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.De Ligt M., Hesselink M.K.C., Jorgensen J., Hoebers N., Blaak E.E. and Goossens G.H. (2021) Resveratrol supplementation reduces ACE2 expression in human adipose tissue. Adipocyte 10, 408–411 10.1080/21623945.2021.1965315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Fuse S., Endo T., Tanaka R., Kuroiwa M., Ando A., Kume A.et al. (2020) Effects of capsinoid intake on brown adipose tissue vascular density and resting energy expenditure in healthy, middle-aged adults: a randomized, double-blind, placebo-controlled study. Nutrients 12, 2676 10.3390/nu12092676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Baskaran P., Krishnan V., Ren J. and Thyagarajan B. (2016) Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br. J. Pharmacol. 173, 2369–2389 10.1111/bph.13514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Osuna-Prieto F.J., Martinez-Tellez B., Segura-Carretero A. and Ruiz J.R. (2021) Activation of brown adipose tissue and promotion of white adipose tissue browning by plant-based dietary components in rodents: a systematic review. Adv. Nutr. 12, 2147–2156 10.1093/advances/nmab084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Shamsi F., Piper M., Ho L.-L., Huang T.L., Gupta A., Streets A.et al. (2021) Vascular smooth muscle-derived Trpv1+ progenitors are a source of cold-induced thermogenic adipocytes. Nat. Metab. 3, 485–495 10.1038/s42255-021-00373-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kastorini C.M., Milionis H.J., Esposito K., Giugliano D., Goudevenos J.A. and Panagiotakos D.B. (2011) The effect of Mediterranean diet on metabolic syndrome and its components: a meta-analysis of 50 studies and 534,906 individuals. J. Am. Coll. Cardiol. 57, 1299–1313 10.1016/j.jacc.2010.09.073 [DOI] [PubMed] [Google Scholar]
- 216.Bendall C.L., Mayr H.L., Opie R.S., Bes-Rastrollo M., Itsiopoulos C. and Thomas C.J. (2018) Central obesity and the Mediterranean diet: a systematic review of intervention trials. Crit. Rev. Food Sci. Nutr. 58, 3070–3084 10.1080/10408398.2017.1351917 [DOI] [PubMed] [Google Scholar]
- 217.Ziegler A.K., Damgaard A., Mackey A.L., Schjerling P., Magnusson P., Olesen A.T.et al. (2019) An anti-inflammatory phenotype in visceral adipose tissue of old lean mice, augmented by exercise. Sci. Rep. 9, 12069 10.1038/s41598-019-48587-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Christiansen T., Paulsen S.K., Bruun J.M., Ploug T., Pedersen S.B. and Richelsen B. (2010) Diet-induced weight loss and exercise alone and in combination enhance the expression of adiponectin receptors in adipose tissue and skeletal muscle, but only diet-induced weight loss enhanced circulating adiponectin. J. Clin. Endocrinol. Metab. 95, 911–919 10.1210/jc.2008-2505 [DOI] [PubMed] [Google Scholar]
- 219.Stanford K.I., Middelbeek R.J., Townsend K.L., Lee M.Y., Takahashi H., So K.et al. (2015) A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes 64, 2002–2014 10.2337/db14-0704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Takahashi H., Alves C.R.R., Stanford K.I., Middelbeek R.J.W., Nigro P., Ryan R.E.et al. (2019) TGF-β2 is an exercise-induced adipokine that regulates glucose and fatty acid metabolism. Nat. Metab. 1, 291–303 10.1038/s42255-018-0030-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Honkala S.M., Motiani P., Kivelä R., Hemanthakumar K.A., Tolvanen E., Motiani K.K.et al. (2020) Exercise training improves adipose tissue metabolism and vasculature regardless of baseline glucose tolerance and sex. BMJ Open Diabetes Res. Care 8, e000830 10.1136/bmjdrc-2019-000830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Stanford K.I., Middelbeek R.J.W. and Goodyear L.J. (2015) Exercise effects on white adipose tissue: beiging and metabolic adaptations. Diabetes 64, 2361–2368 10.2337/db15-0227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Stanford K.I. and Goodyear L.J. (2016) Exercise regulation of adipose tissue. Adipocyte 5, 153–162 10.1080/21623945.2016.1191307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Vosselman M.J., Hoeks J., Brans B., Pallubinsky H., Nascimento E.B., van der Lans A.A.et al. (2015) Low brown adipose tissue activity in endurance-trained compared with lean sedentary men. Int. J. Obes. (Lond.) 39, 1696–1702 10.1038/ijo.2015.130 [DOI] [PubMed] [Google Scholar]
- 225.Søberg S., Löfgren J., Philipsen F.E., Jensen M., Hansen A.E., Ahrens E.et al. (2021) Altered brown fat thermoregulation and enhanced cold-induced thermogenesis in young, healthy, winter-swimming men. Cell Rep. Med. 2, 100408 10.1016/j.xcrm.2021.100408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Li R.M., Chen S.Q., Zeng N.X., Zheng S.H., Guan L., Liu H.M.et al. (2017) Browning of abdominal aorta perivascular adipose tissue inhibits adipose tissue inflammation. Metab. Syndr. Relat. Disord. 15, 450–457 10.1089/met.2017.0074 [DOI] [PubMed] [Google Scholar]
- 227.Xue Y., Petrovic N., Cao R., Larsson O., Lim S., Chen S.et al. (2009) Hypoxia-independent angiogenesis in adipose tissues during cold acclimation. Cell Metab. 9, 99–109 10.1016/j.cmet.2008.11.009 [DOI] [PubMed] [Google Scholar]
- 228.Hasegawa Y., Ikeda K., Chen Y., Alba D.L., Stifler D., Shinoda K.et al. (2018) Repression of adipose tissue fibrosis through a PRDM16-GTF2IRD1 complex improves systemic glucose homeostasis. Cell Metab. 27, 180.e186–194.e186 10.1016/j.cmet.2017.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.NICE (2021) NICE impact cardiovascular disease prevention [National Institute for Health and Care Excellence]. https://www.nice.org.uk/Media/Default/About/what-we-do/Into-practice/measuring-uptake/CVD-prevention-impact-report/nice-impact-cvd-prevention.pdf [Google Scholar]
- 230.Mitchell S.J., Bernier M., Mattison J.A., Aon M.A., Kaiser T.A., Anson R.M.et al. (2019) Daily fasting improves health and survival in male mice independent of diet composition and calories. Cell Metab. 29, 221.e223–228.e223 10.1016/j.cmet.2018.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Pak H.H., Haws S.A., Green C.L., Koller M., Lavarias M.T., Richardson N.E.et al. (2021) Fasting drives the metabolic, molecular and geroprotective effects of a calorie-restricted diet in mice. Nat. Metab. 3, 1327–1341 10.1038/s42255-021-00466-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Patterson R.E. and Sears D.D. (2017) Metabolic effects of intermittent fasting. Annu. Rev. Nutr. 37, 371–393 10.1146/annurev-nutr-071816-064634 [DOI] [PubMed] [Google Scholar]
- 233.Dwaib H.S., AlZaim I., Eid A.H., Obeid O. and El-Yazbi A.F. (2021) Modulatory effect of intermittent fasting on adipose tissue inflammation: amelioration of cardiovascular dysfunction in early metabolic impairment. Front. Pharmacol. 12, 626313 10.3389/fphar.2021.626313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Harney D.J., Cielesh M., Chu R., Cooke K.C., James D.E., Stöckli J.et al. (2021) Proteomics analysis of adipose depots after intermittent fasting reveals visceral fat preservation mechanisms. Cell Rep. 34, 108804 10.1016/j.celrep.2021.108804 [DOI] [PubMed] [Google Scholar]
- 235.Sutton E.F., Beyl R., Early K.S., Cefalu W.T., Ravussin E. and Peterson C.M. (2018) Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 27, 1212.e1213–1221.e1213 10.1016/j.cmet.2018.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Chaix A., Zarrinpar A., Miu P. and Panda S. (2014) Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 20, 991–1005 10.1016/j.cmet.2014.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wilkinson M.J., Manoogian E.N.C., Zadourian A., Lo H., Fakhouri S., Shoghi A.et al. (2020) Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab. 31, 92.e105–104.e105 10.1016/j.cmet.2019.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Hua L., Li J., Feng B., Jiang D., Jiang X., Luo T.et al. (2021) Dietary intake regulates white adipose tissues angiogenesis via liver fibroblast growth factor 21 in male mice. Endocrinology 162, bqaa244 10.1210/endocr/bqaa244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Xiong X., Lin Y., Lee J., Paul A., Yechoor V., Figueiro M.et al. (2021) Chronic circadian shift leads to adipose tissue inflammation and fibrosis. Mol. Cell. Endocrinol. 521, 111110 10.1016/j.mce.2020.111110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hunter A.L., Pelekanou C.E., Barron N.J., Northeast R.C., Grudzien M., Adamson A.D.et al. (2021) Adipocyte NR1D1 dictates adipose tissue expansion during obesity. eLife 10, e63324 10.7554/eLife.63324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Sun X., Gustat J., Bertisch S.M., Redline S. and Bazzano L. (2020) The association between sleep chronotype and obesity among black and white participants of the Bogalusa Heart Study. Chronobiol. Int. 37, 123–134 10.1080/07420528.2019.1689398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ruiz-Lozano T., Vidal J., de Hollanda A., Canteras M., Garaulet M. and Izquierdo-Pulido M. (2016) Evening chronotype associates with obesity in severely obese subjects: interaction with CLOCK 3111T/C. Int. J. Obes. 40, 1550–1557 10.1038/ijo.2016.116 [DOI] [PubMed] [Google Scholar]
- 243.Yu J.H., Yun C.H., Ahn J.H., Suh S., Cho H.J., Lee S.K.et al. (2015) Evening chronotype is associated with metabolic disorders and body composition in middle-aged adults. J. Clin. Endocrinol. Metab. 100, 1494–1502 10.1210/jc.2014-3754 [DOI] [PubMed] [Google Scholar]
- 244.Martínez-Colón G.J., Ratnasiri K., Chen H., Jiang S., Zanley E., Rustagi A.et al. (2021) SARS-CoV-2 infects human adipose tissue and elicits an inflammatory response consistent with severe COVID-19. bioRxiv 2021.2010.2024.465626 [Google Scholar]
- 245.Reiterer M., Rajan M., Gómez-Banoy N., Lau J.D., Gomez-Escobar L.G., Ma L.et al. (2021) Hyperglycemia in acute COVID-19 is characterized by insulin resistance and adipose tissue infectivity by SARS-CoV-2. Cell Metab. 33, 2174.e2175–2188.e2175 10.1016/j.cmet.2021.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zickler M., Stanelle-Bertram S., Ehret S., Heinrich F., Lange P., Schaumburg B.et al. (2022) Replication of SARS-CoV-2 in adipose tissue determines organ and systemic lipid metabolism in hamsters and humans. Cell Metab. 34, 1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kruglikov I.L. and Scherer P.E. (2020) The role of adipocytes and adipocyte‐like cells in the severity of COVID‐19 infections. Obesity 28, 1187–1190 10.1002/oby.22856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Eljaafari A., Pestel J., Le Magueresse-Battistoni B., Chanon S., Watson J., Robert M.et al. (2021) Adipose-tissue-derived mesenchymal stem cells mediate PD-L1 overexpression in the white adipose tissue of obese individuals, resulting in T cell dysfunction. Cells 10, 2645 10.3390/cells10102645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Izquierdo A.G., Carreira M.C., Boughanem H., Moreno‐Navarrete J.M., Nicoletti C.F., Oliver P.et al. (2021) Adipose tissue and blood leukocytes ACE2 DNA methylation in obesity and after weight loss. Eur. J. Clin. Invest. 52, e13685 10.1111/eci.13685 [DOI] [PubMed] [Google Scholar]
- 250.Shin J., Toyoda S., Nishitani S., Fukuhara A., Kita S., Otsuki M.et al. (2021) Possible involvement of adipose tissue in patients with older age, obesity, and diabetes with SARS-CoV-2 infection (COVID-19) via GRP78 (BIP/HSPA5): significance of hyperinsulinemia management in COVID-19. Diabetes 70, 2745–2755 10.2337/db20-1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Soll D., Beer F., Spranger L., Li L., Spranger J. and Mai K. (2021) Effects of weight loss on adipose and muscular Neuropilin 1 mRNA expression in obesity: potential implication in SARS-CoV-2 infections? Obes. Facts 15, 90–98 10.1159/000520419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Zhu L., She Z.G., Cheng X., Qin J.J., Zhang X.J., Cai J.et al. (2020) Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing Type 2 diabetes. Cell Metab. 31, 1068.e1063–1077.e1063 10.1016/j.cmet.2020.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Schwartz M.W., Seeley R.J., Zeltser L.M., Drewnowski A., Ravussin E., Redman L.M.et al. (2017) Obesity pathogenesis: An Endocrine Society Scientific Statement. Endocr. Rev. 38, 267–296 10.1210/er.2017-00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Blaszczak A.M., Bernier M., Wright V.P., Gebhardt G., Anandani K., Liu J.et al. (2020) Obesogenic memory maintains adipose tissue inflammation and insulin resistance. Immunometabolism 2, e200023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Li X., Jiang L., Yang M., Wu Y.-W. and Sun J.-Z. (2018) Impact of weight cycling on CTRP3 expression, adipose tissue inflammation and insulin sensitivity in C57BL/6J mice. Exp. Ther. Med. 16, 2052–2059 10.3892/etm.2018.6399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Barbosa-da-Silva S., Fraulob-Aquino J.C., Lopes J.R., Mandarim-de-Lacerda C.A. and Aguila M.B. (2012) Weight cycling enhances adipose tissue inflammatory responses in male mice. PLoS ONE 7, e39837 10.1371/journal.pone.0039837 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available from the corresponding author upon reasonable request.